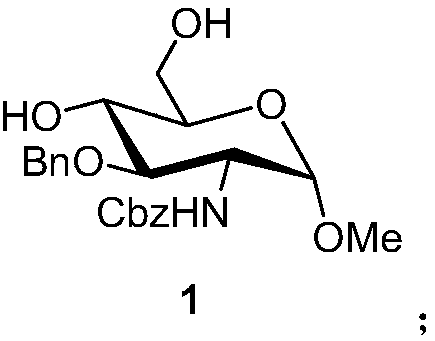
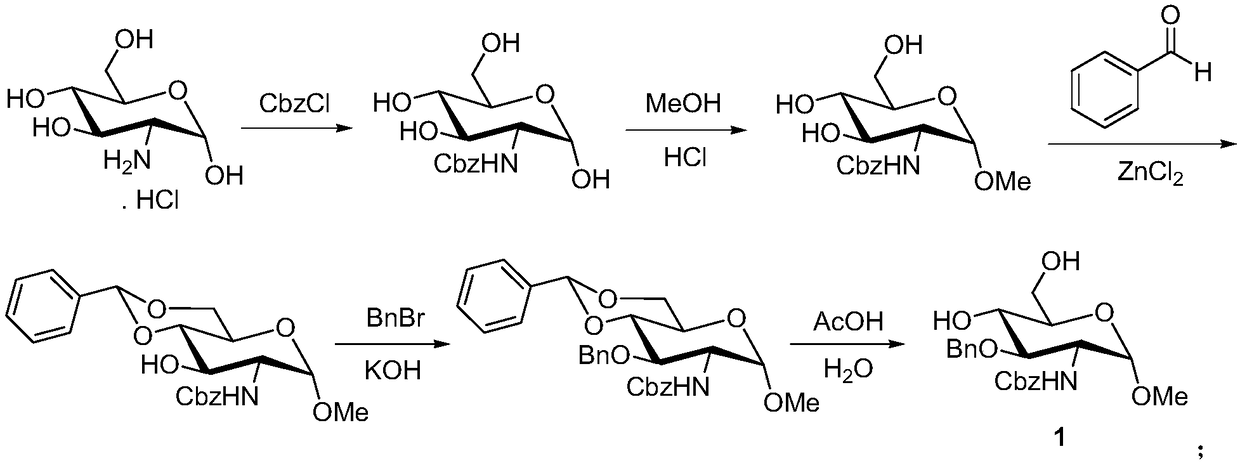
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33results about How to "Reflect the effect of green environmental protection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Three-in-one spunlaced composite non-woven fabric
InactiveCN102605557AOvercome expensiveGood flexibilityNon-woven fabricsPolymer scienceNonwoven fabric
The invention relates to a three-in-one spunlaced composite non-woven fabric, belonging to the technical field of non-woven fabrics, wherein the non-woven fabric comprises a substrate layer, a hygroscopic layer and a hydrophilic layer, which are integrated in spunlace, wherein the hygroscopic layer is located between the substrate layer and the hydrophilic layer; the substrate layer comprises chemical short fibers or degradable short fibers; the hygroscopic layer comprises wood pulp short fibers; and the hydrophilic layer comprises spunbonded non-woven fabrics. The three-in-one spunlaced composite non-woven fabric has the advantages that the non-woven fabric has good softness, excellent contact feel and great water aggregation performance as the chemical short fibers or degradable short fibers are used as the substrate layer, not only has excellent strength but also has great water aggregation performance as the spunbonded non-woven fabrics are used as the hydrophilic layer, and can play a good high-hygroscopic effect as the wood pulp short fibers are used as the hygroscopic layer; both the hygroscopic layer and the hydrophilic layer are degradable to reflect the green environmental effect; and the overall structure is simple and inexpensive to meet the disposable requirement.
Owner:CHANGSHU FEILONG NON WOVEN MACHINERY
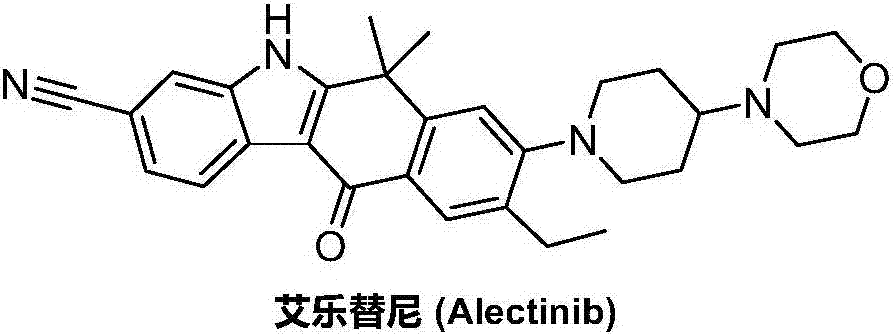
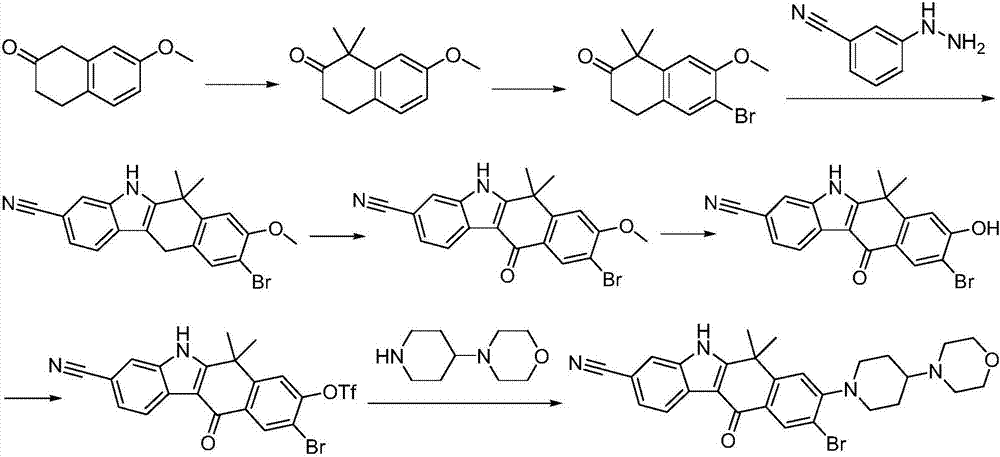
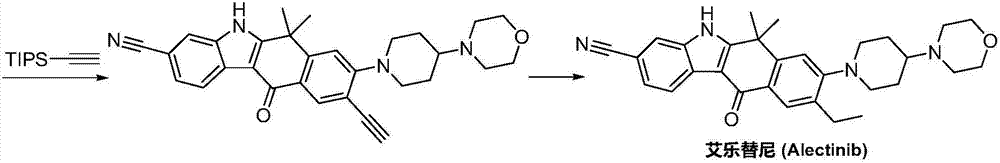
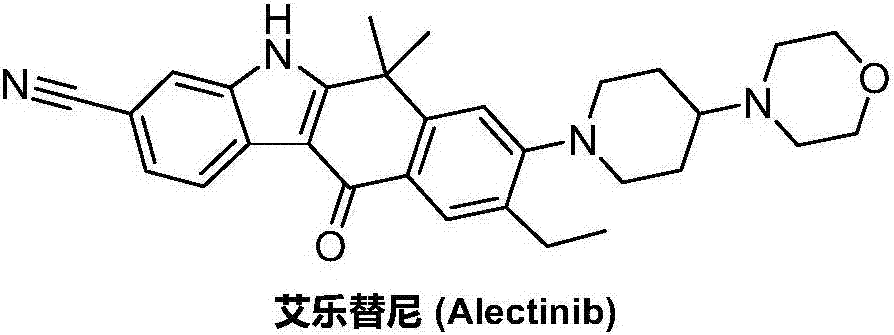
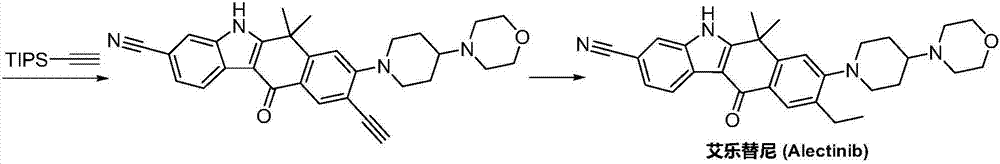
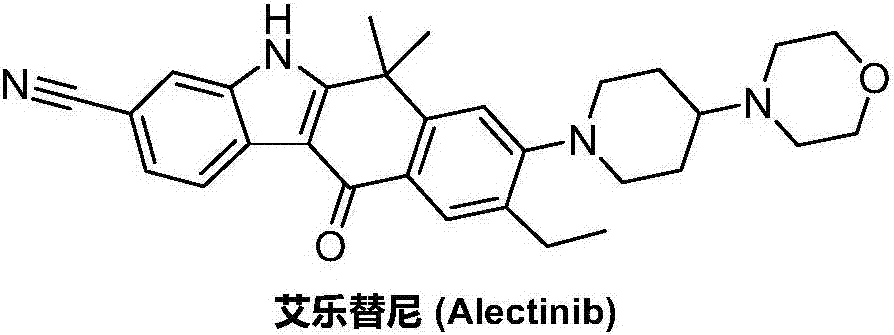
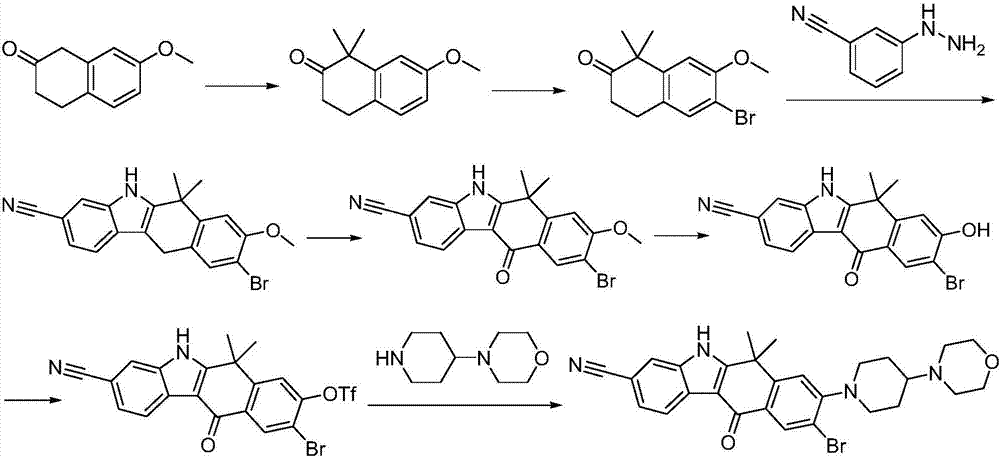
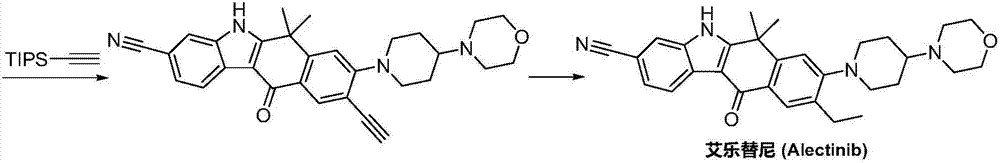
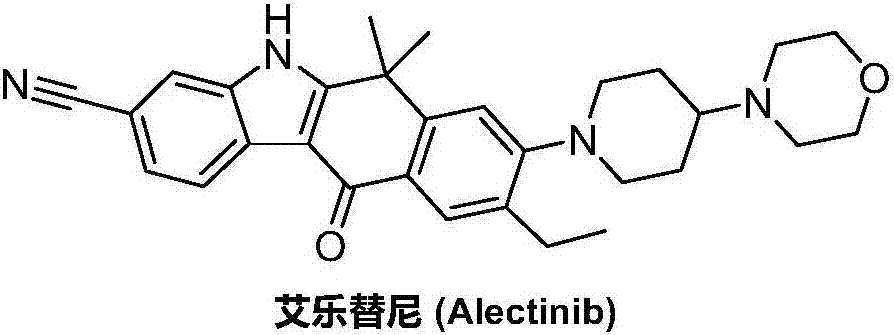
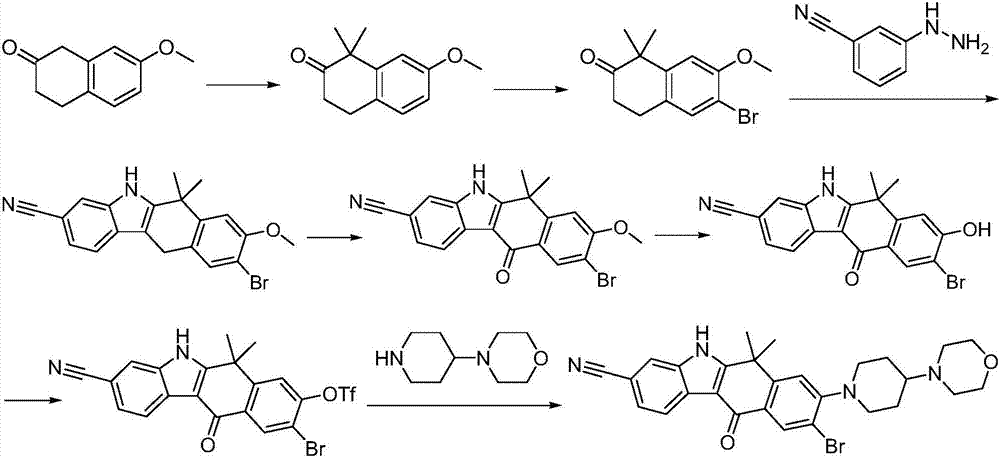
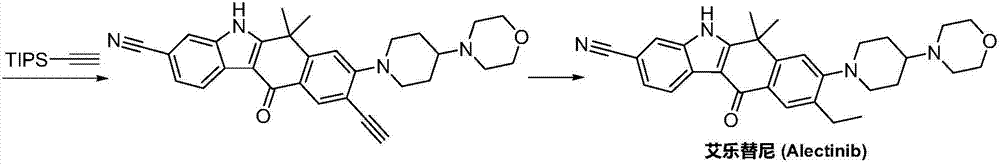
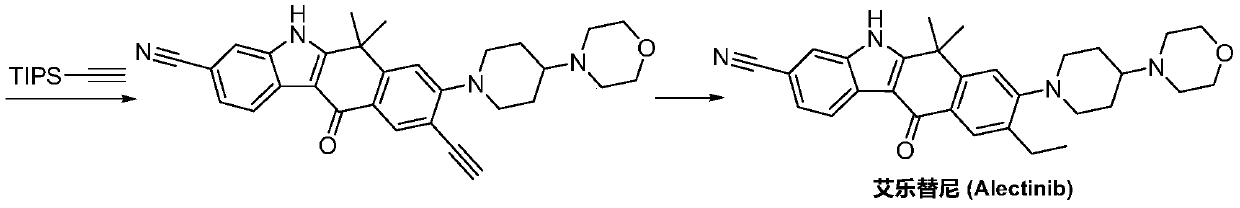
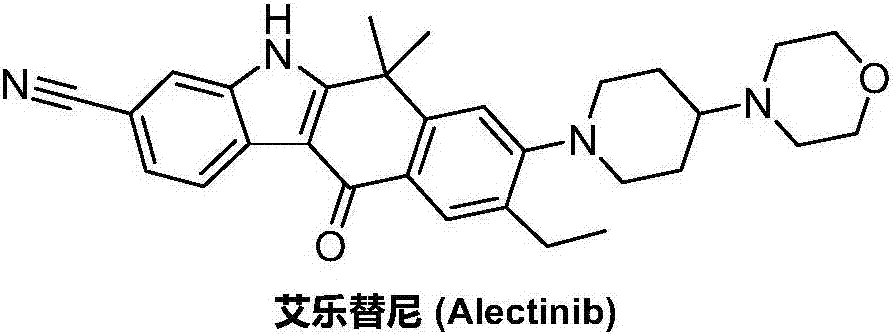
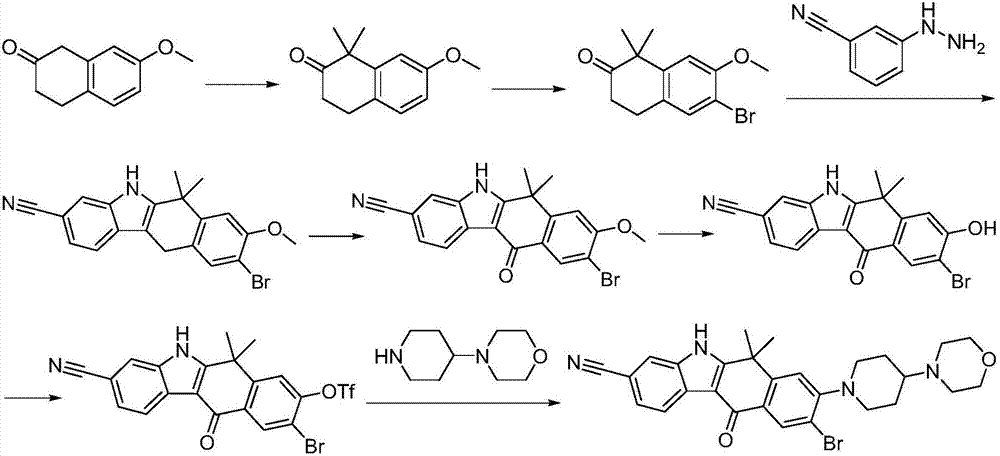
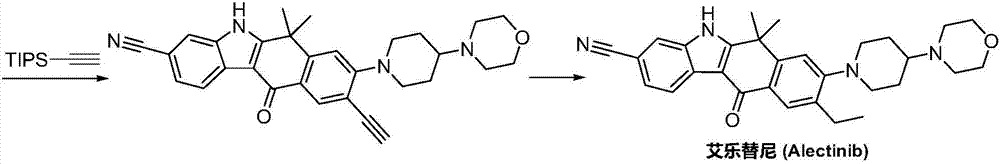
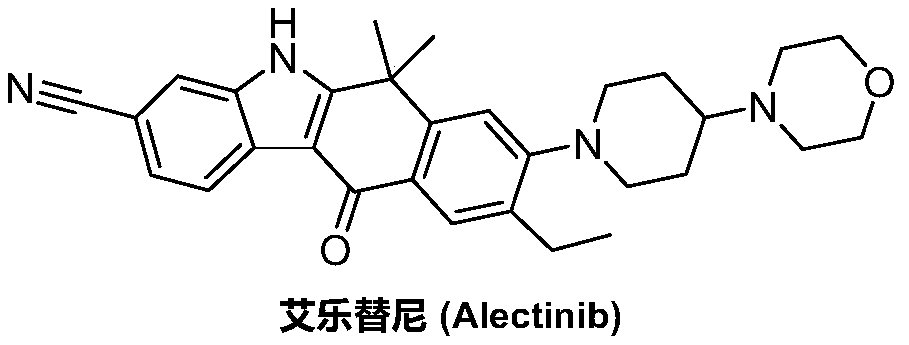
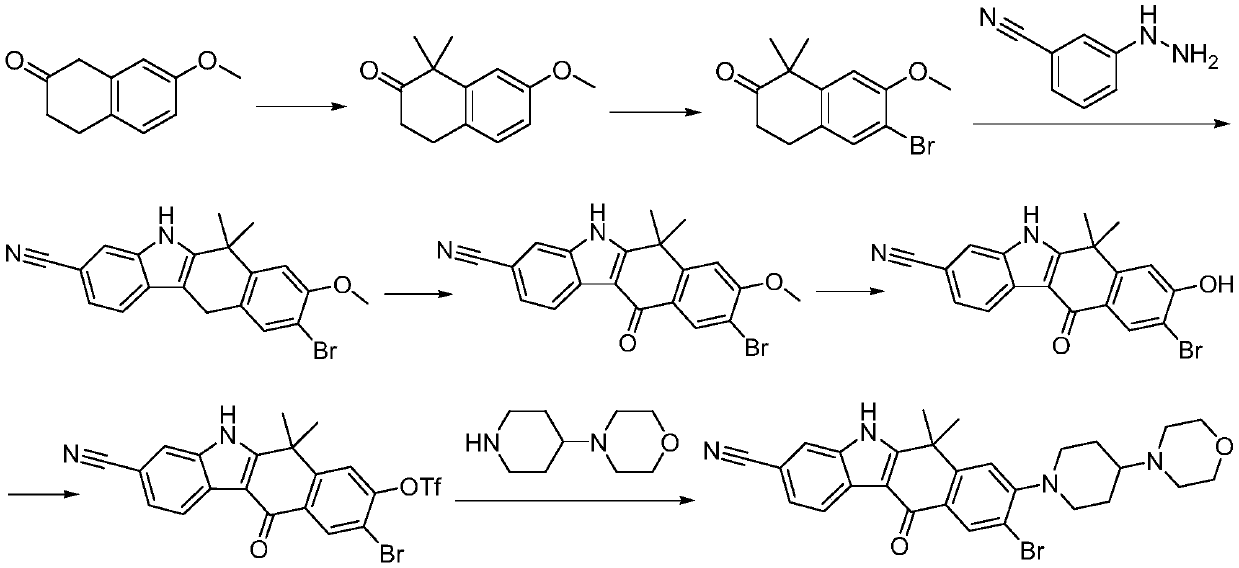
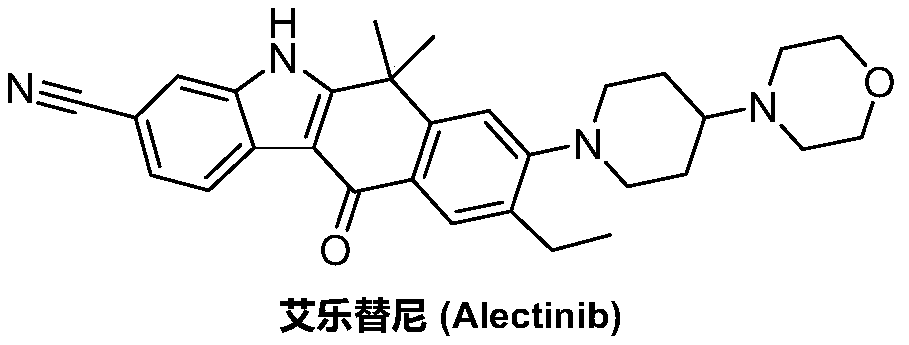
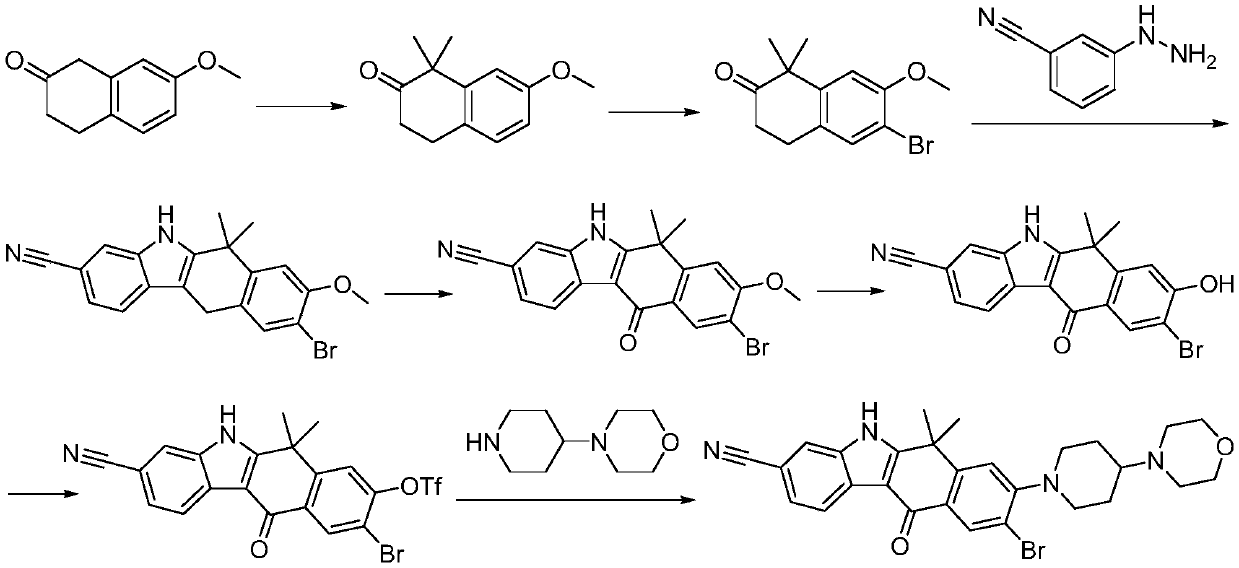
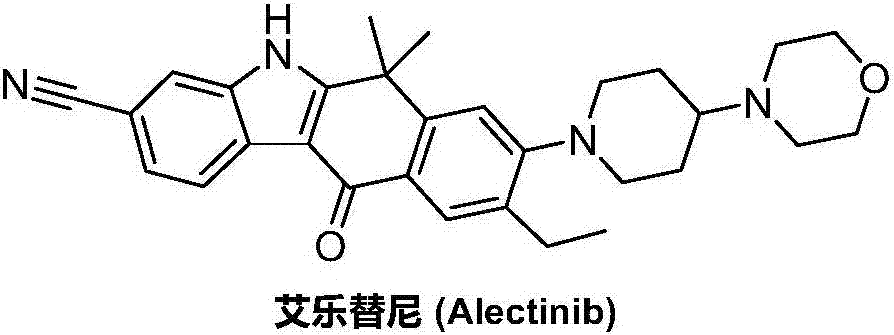
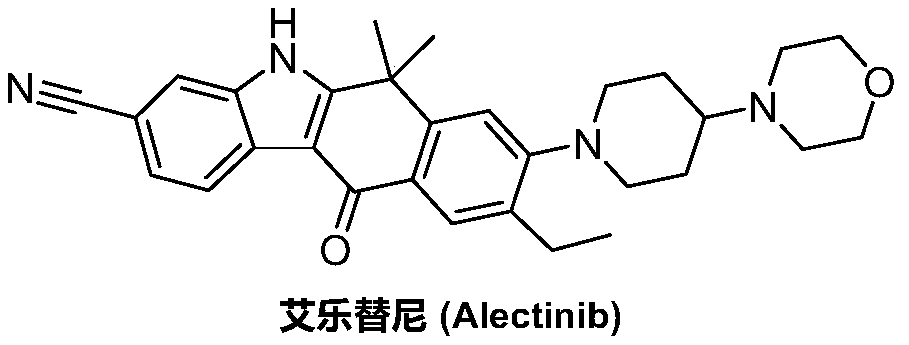
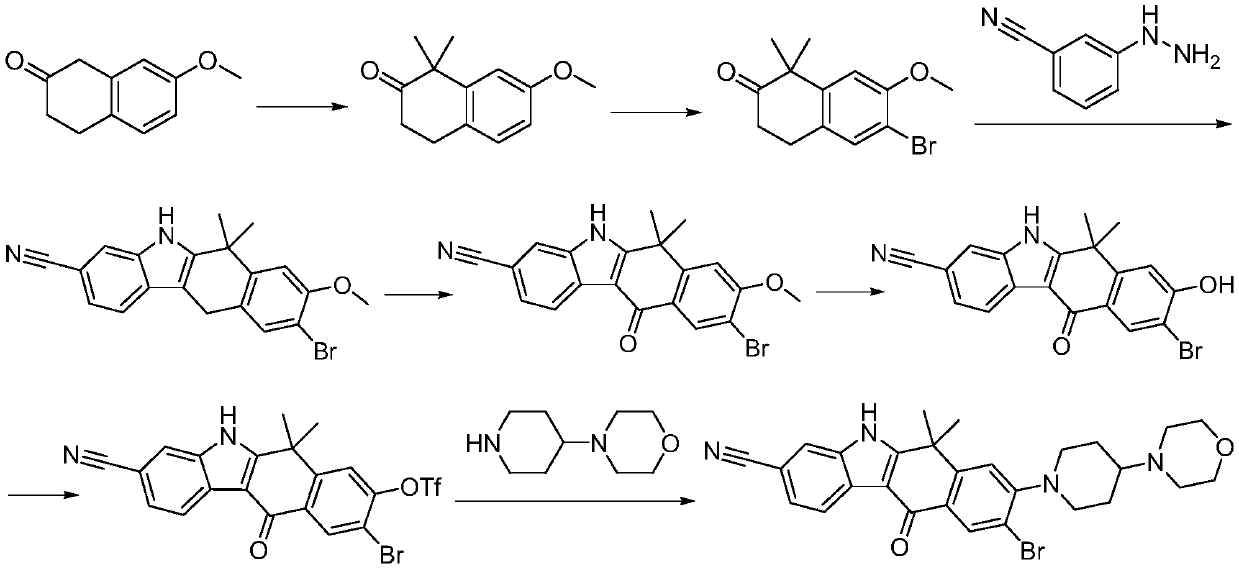
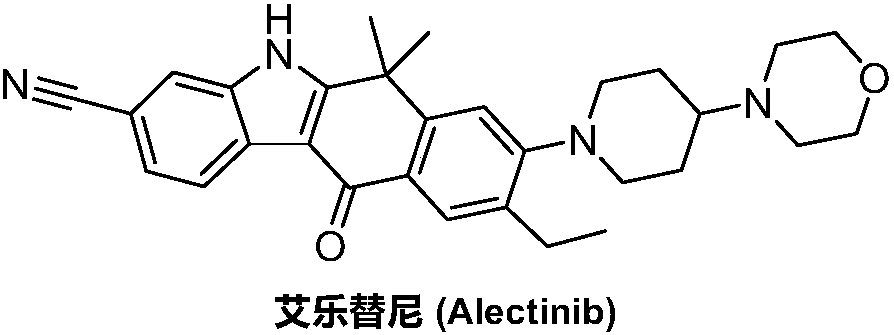
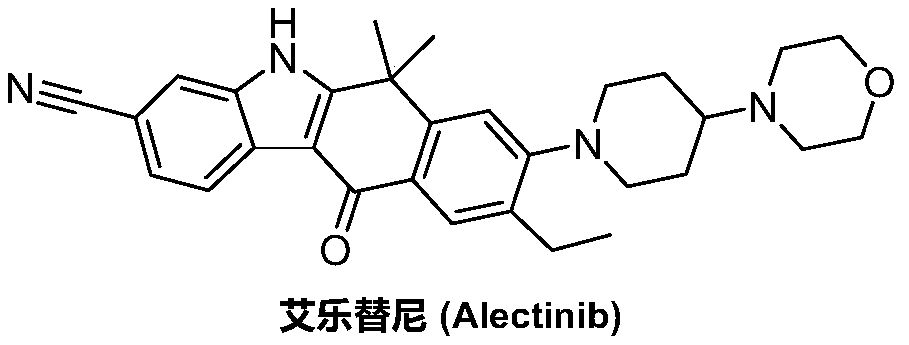
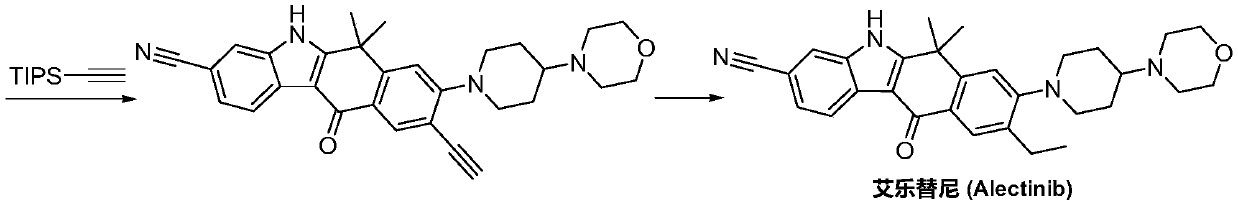
Method for preparing alectinib
InactiveCN107129488AReasonable and unique designSave raw materialsOrganic chemistryBromineTrifluoromethyl
Owner:HUNAN BOAODE BIOPHARML TECH DEV
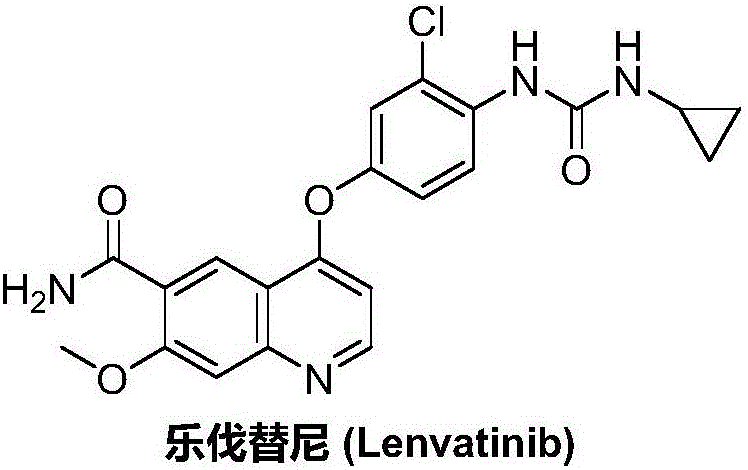
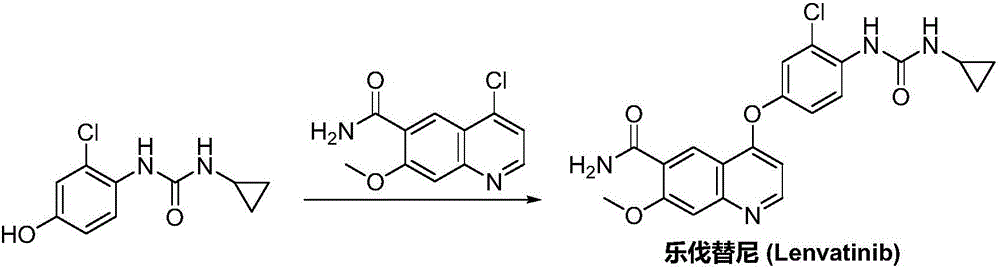
Lenvatinib synthesizing method
InactiveCN105801481AMeet the needs of useMild reaction conditionsOrganic chemistryLenvatinibBenzyl chloroformate
The invention discloses a lenvatinib synthesizing method.The method includes the steps that 4-amino-3-chlorophenol and benzyl chloroformate are subjected to amidation reaction, the obtained 4-(carbobenzoxy)amino-3-chlorophenol and 4-chlorine-7-methoxyquinoline-6-formamide are subjected to condensation reaction, the obtained 4-[3-chlorine-4-(carbobenzoxy)aminophenoxy]-7-methoxyquinoline-6-formamide and cyclopropylamine are subjected to amidation reaction, and the finished product lenvatinib is obtained.The method is short in process route step, operation is simplified, cost is low, and the method is environmentally friendly and suitable for industrial production.
Owner:湖南欧亚药业有限公司
Preparation method of alectinib
InactiveCN106995433AMeet the needs of useApplicable generationOrganic chemistryFischer indole synthesisMorpholine
The invention discloses a preparation method of alectinib. The preparation method comprises the following steps of using 2-(4-bromo-3-hydroxyphenyl)ethyl acetate as a raw material; performing trifluoromethanesulfonic acid etherification with trifluoromethyl sulfonic anhydride, so as to obtain a trifluoromethanesulfonic acid etherification compound; performing substitution reaction with the other raw material, namely 4-(4-piperidyl)morpholine, so as to obtain 2-{4-bromo-3-[4-morpholine-4-yl]piperidine-1-yl]phenyl}ethyl acetate, then performing dimethylation reaction and hydrolysis reaction to obtain 2-{4-bromo-3-[4-morpholine-4-yl]piperidine-1-yl]phenyl}-2-methyl propionate to be subjected to condensation reaction with malonic acid mono-tert-butyl ester, so as to obtain the 4-{4-bromo-3-[4-morpholine-4-yl]piperidine-1-yl]phenyl}-4-methyl-3-oxopentanoate tert-butyl; utilizing a typical Fischer indole synthesis method, enabling carbonyl and phenylhydrazine to cyclize under the acid catalyzing action to form indole nuclear parent; finally, performing cyclizing reaction, boric acidifying and catalytic coupling reaction, so as to prepare the alectinib. The preparation method has the advantages that the design of route method is reasonable, the price of raw material is low, the obtaining is easy, and the reaction condition is easily and effectively controlled.
Owner:HUNAN BOAODE BIOPHARML TECH DEV
Preparation method of Alectinib
ActiveCN106928184ALess impuritiesEasy to operateOrganic chemistry1H-indole-3-carboxylic acidIsopropyl
The invention discloses a preparation method of Alectinib. The method comprises the steps that tert-butyl 4-(4-ethyl-3-iodophenyl)-4-methyl-3-oxopentanoate and 4-(piperidin-4-yl)-morpholine are subjected to a substitution reaction; obtained tert-butyl 4-{4-ethyl-3-[4-(morpholine-4-yl)piperidin-1-yl]phenyl}-4-methyl-3-oxopentanoate is subjected to a cyclization reaction and hydrolysis reaction, obtained 6-cyano-2-{2-[4-ethyl-3-(4- morpholine-4-yl)piperidin-1-yl)phenyl]prop-2-yl}-1H-indole-3-carboxylic acid is subjected to the cyclization reaction, and the Alectinib is obtained. According to the method, the operation is simplified, the cost is low, and the method is an environment-friendly technical method and suitable for industrial production.
Owner:湖南润星制药有限公司
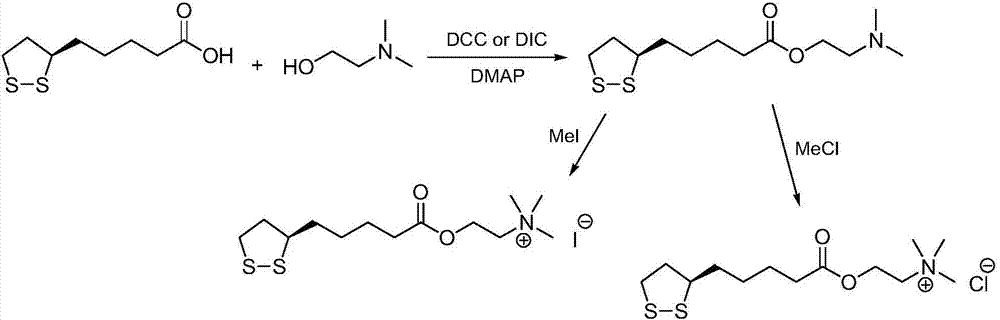
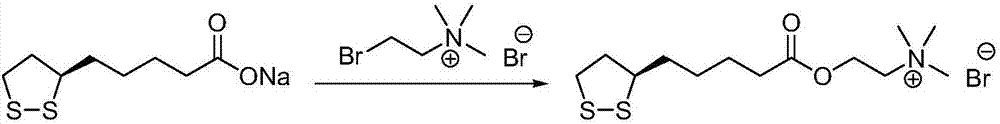
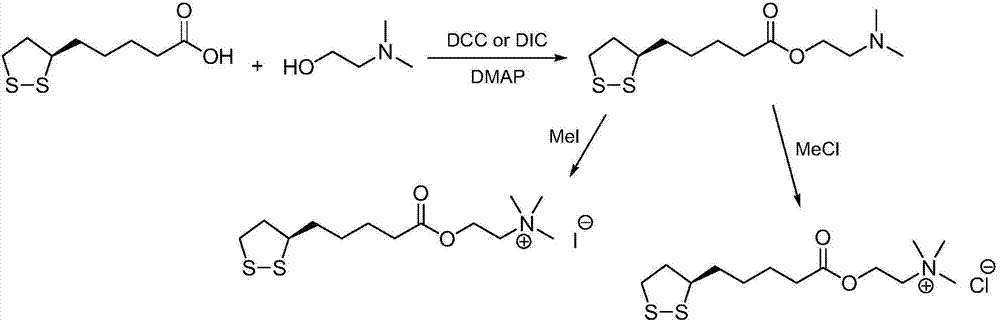
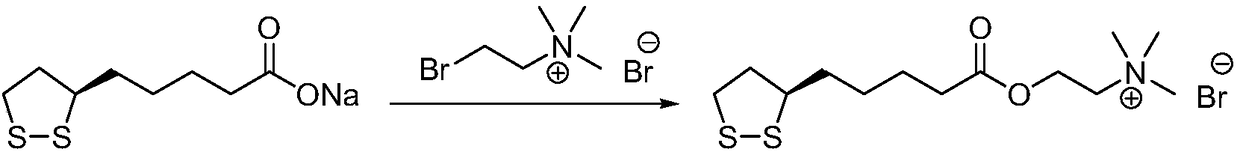
Preparation method of R-lipoic acid cholinesterase halide
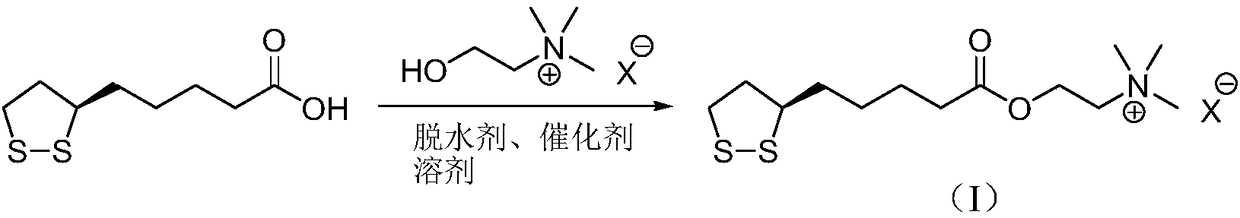
InactiveCN107089967ALess impurities in the reactionEasy to operateOrganic chemistry methodsChemical synthesisSolvent
The invention provides a preparation method of an R-lipoic acid cholinesterase halide and belongs to the technical field of medicinal chemical synthesis. R-lipoic acid-2-halogen ethyl ester I and trimethylamine react in a solvent to generate quaternary ammonium salt, so that an R-lipoic acid cholinesterase halide II is obtained. Impurities during reaction are fewer, and operations such as aftertreatment and purification are simplified; raw materials and the used reagent are easy to get and safe to use, and a reaction route is reasonable, so that the preparation method is applicable to industrial enlarged production; and no pollutant is produced in a preparation process, so that the green environmental protection effect is realized.
Owner:SUZHOU FUSHILAI PHARMA CO LTD
Preparation method of Alectinib intermediate
InactiveCN106928125AMeet the needs of useUse requirements applyOrganic chemistryMorpholineTriflic acid
The invention discloses a preparation method of an Alectinib intermediate, namely tert-butyl 4-{4-ethyl-3-[4-(morpholine-4-yl)piperidin-1-yl]phenyl}-4-methyl-3-oxopentanoate. The method comprises the steps that ethyl 2-(4-ethyl-3-methoxyphenyl)acetate and iodomethane are subjected to a bis-methylation reaction; obtained ethyl 2-(4-ethyl-3-methoxyphenyl)-2-methylpropanoate is subjected to a hydrolysis reaction; obtained ethyl 2-(4-ethyl-3-hydroxyphenyl) -2-methylpropanoate is subjected to a trifluoromethanesulfonic acid esterification reaction; obtained 5-[2-( ethyloxylcarbonyl)prop-2-yl]-2-ethylphenyl trifluoromethanesulfonate is subjected to a substitution reaction; obtained ethyl 2-{4-ethyl-3-[4-(morpholine-4-yl)piperidin-1-yl] phenyl} -2-methylpropanoate is subjected to a hydrolysis reaction; obtained 2-{4-ethyl-3-[4-(morpholine-4-yl)piperidin-1-yl] phenyl}-2-methyl propioric acid is subjected to a condensation reaction to obtain the Alectinib intermediate. According to the method, the operation is simplified, the cost is low, and the method is an environment-friendly technical method and suitable for industrial production.
Owner:HUNAN BOAODE BIOPHARML TECH DEV
A kind of preparation method of alectinib
Owner:湖南润星制药有限公司
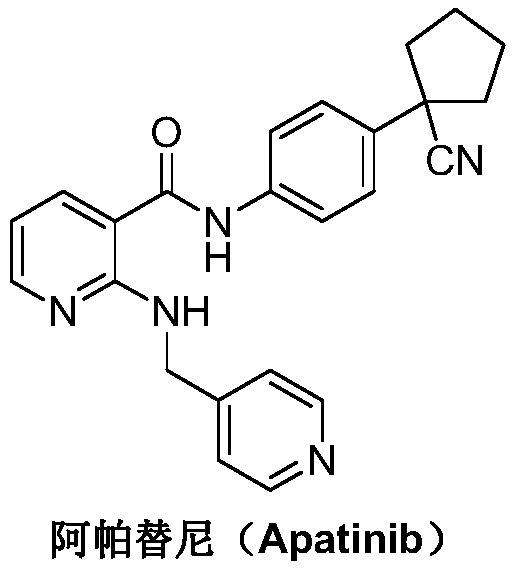
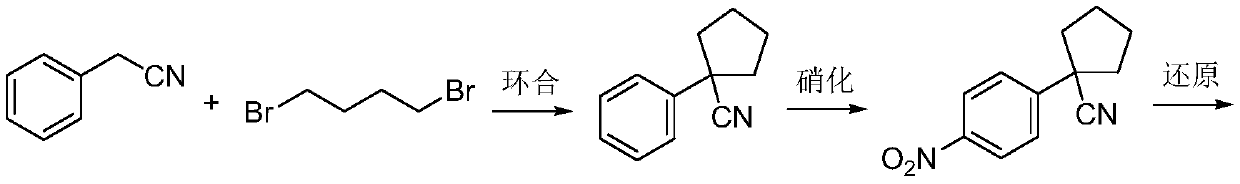
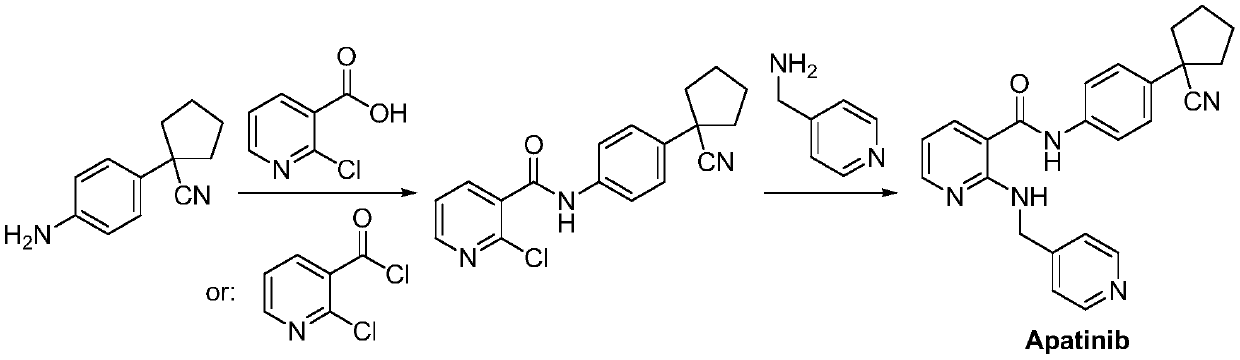
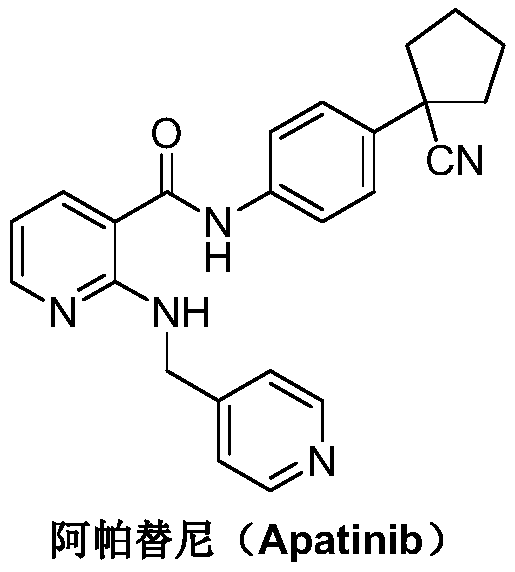
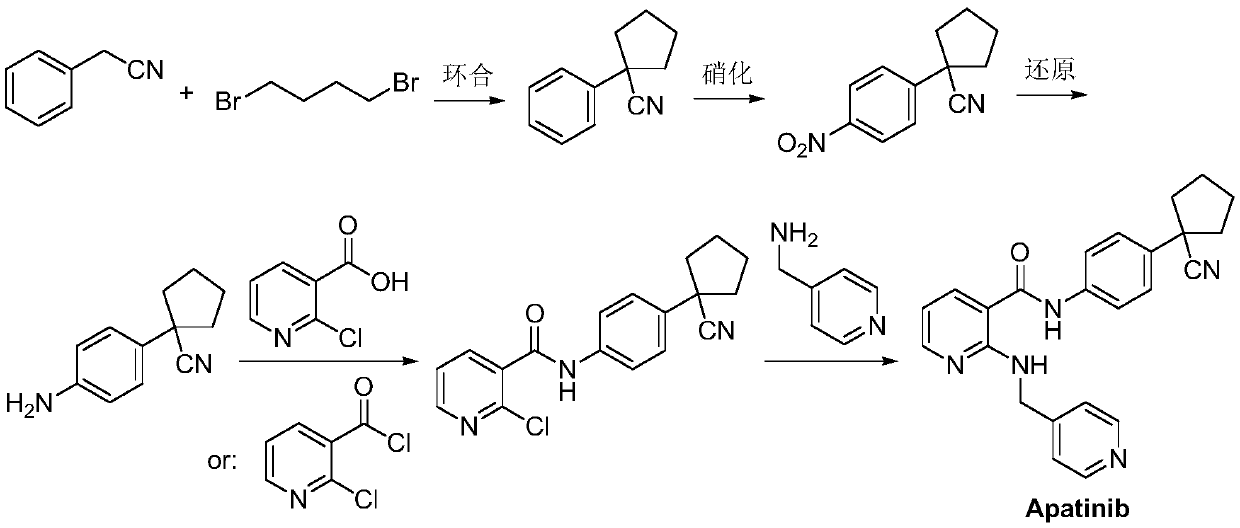
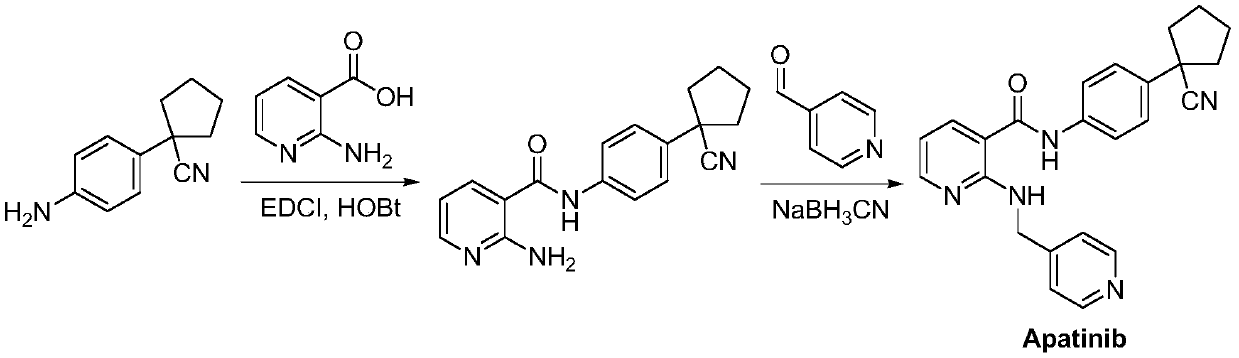
Preparation method for apatinib
ActiveCN109879805AAvoid expensiveMild reaction conditionsOrganic chemistryFormateTert-Butyloxycarbonyl protecting group
The invention relates to a preparation method for apatinib. The preparation method comprises the following steps: performing condensation reaction on 4-(tert-butoxycarbonyl group) alkyl phenylacetateand 1,4-butane dihalide, thereby acquiring 1-[4-(tert-butoxycarbonyl group) phenyl] alkyl cyclopentane formate; performing deprotection reaction on the acquired product, thereby acquiring 1-(4-aminophenyl) alkyl cyclopentane formate; performing amidation reaction on the acquired product and 2-chloronicotinoyl chloride, thereby acquiring 1-{4-[(2-chlorine pyridine-3-group) carbonyl amino] phenyl} alkyl cyclopentane formate; performing substitution reaction on the acquired product and 4-aminomethyl pyridine, thereby acquiring 1-{4-[(2-((4-pyridyl methyl) amino) pyridine-3-group) carbonyl amino]phenyl} alkyl cyclopentane formate; performing amidation reaction on the acquired product, thereby acquiring 1-{4-[(2-((4-pyridyl methyl) amino) pyridine-3-group) carbonyl amino] phenyl} cyclopentaneformamide; lastly, dehydrating, thereby acquiring an end product.
Owner:SUZHOU FUSHILAI PHARMA CO LTD
Preparation method of alectinib intermediate
ActiveCN106892860AMeet the needs of useUse requirements applyOrganic chemistryMorpholineTriflic acid
The invention discloses a preparation method of an alectinib intermediate, tert-butyl 4-{4-ethyl-3-[4-(morpholine-4-yl)piperidine-1-yl]phenyl}-4-methyl-3-oxovalerate. The preparation method comprises: subjecting ethyl 2-(4-ethyl-3-hydroxyphenyl)acetate and triflic anhydride to triflic acid esterification; subjecting the obtained 5-[(ethoxycarbonyl)methyl]-2-ethylphenyltriflate and 4-(4-piperidyl)morpholine to substitution reaction; subjecting the obtained ethyl 2-{4-ethyl-3-[4-(morpholine-4-yl)piperidine-1-yl]phenyl}acetate and methyl iodide to bis-methylation reaction; hydrolyzing the obtained ethyl 2-{4-ethyl-3-[4-(morpholine-4-yl)piperidine-1-yl]phenyl}-2-methylpropanoate; subjecting the obtained 2-{4-ethyl-3-[4-(morpholine-4-yl)piperidine-1-yl]phenyl}-2-methylpropanoic acid and mono-tert-butyl malonate to condensation reaction to obtain the alectinib intermediate. The preparation method is simple to perform and low in cost, is a green technique and is applicable to industrial production.
Owner:湖南润星制药有限公司
A kind of preparation method of alectinib
Owner:湖南润星制药有限公司
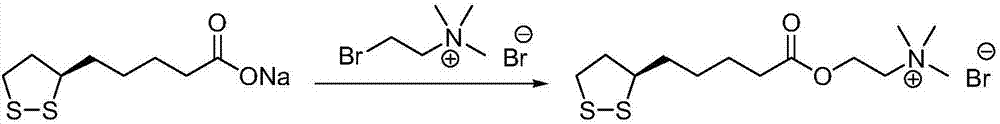
Method for preparing R-lipoic acid choline ester halide
ActiveCN106967044ALess impuritiesEasy to operateOrganic chemistry methodsChemical synthesisAfter treatment
The invention provides a method for preparing R-lipoic acid choline ester halide, and belongs to the technical field of chemical synthesis of drugs. The method comprises a method B or a method B, wherein the method A comprises performing an esterification reaction between R-lipoic acid and halogenated choline in a system composed of a dehydrating agent, a catalyst and a solvent to obtain R-lipoic acid choline ester halide I; and the method B comprises performing an esterification reaction between R-sulfenyloctanoylchloride and halogenated choline in a system composed of an acid-binding agent alkali and a solvent to obtain the R-lipoic acid choline ester halide I. The reaction of each step has fewerimpurities, and the operations of after treatment, purification and the like are simplified; the method is safe to use, and suitable for industrial large-scale production; and a green and environmental protection effect is reflected.
Owner:SUZHOU FUSHILAI PHARMA CO LTD
A kind of preparation method of alectinib
ActiveCN107033124BMeet the needs of useApplicable generationOrganic chemistryPropionateGreen environment
The invention discloses a preparation method for alectinib. The preparation method comprises the following steps: subjecting 2-4-{4-ethyl-3[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-2-methylethyl propionate to a reduction reaction; then subjecting prepared 2-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-2-methylpropionaldehyde and tert-butyl 2,2-dichloroacetate to an addition-rearrangement reaction; then subjecting obtained 3-chloro-4-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-4-methyl-tert-butyl 2-oxovalerate and m-aminobenzonitrile to a substitution reaction; then carrying out a cyclization reaction and a hydrolysis reaction on prepared 3-(3-cyanophenylamino)ethyl-4-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-4-methyl-tert-butyl 2-oxovalerate; and subjecting obtained 6-cyano-2-{2-[4-ethyl-3-(4-(morpholin-4-yl)piperidin-1-yl)phenyl]prop-2-yl}-1H-indole-3-carboxylic acid to a cyclization reaction so as to obtain alectinib. The method has the advantages of simple operation and low cost, and is a green environment-friendly process suitable for industrial production.
Owner:湖南润星制药有限公司
Preparation method of alectinib intermediate
The invention discloses a preparation method of 1,1-dimethyl-6-ethyl-7-[4-(morpholine-4-yl)piperidine-1-yl]-3,4-dihydro-2-naphthalenone which is an alectinib intermediate. The method comprises: carrying out a bromination reaction between 6-ethyl-3,4-dihydro-2-naphthalenone and a bromination reagent; allowing the obtained 6-ethyl-7-bromine-3,4-dihydro-2-naphthalenone and 4-(4-piperidyl)morpholine to undergo a substitution reaction; and allowing obtained 6-ethyl-7-[4-(morpholine-4-yl)piperidine-1-yl]-3,4-dihydro-2-naphthalenone and iodomethane to undergo a dimethylation reaction to obtain 1,1-dimethyl-6-ethyl-7-[4-(morpholine-4-yl)piperidine-1-yl]-3,4-dihydro-2-naphthalenone. The preparation method is relatively short in steps, simplified in operation, and low is cost, is a green eco-friendly technical method, and is suitable for industrial production.
Owner:HUNAN BOAODE BIOPHARML TECH DEV
A kind of preparation method of alectinib intermediate
Owner:湖南润星制药有限公司
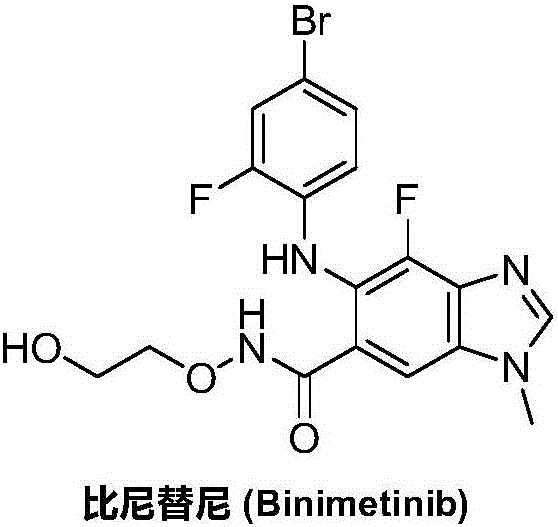
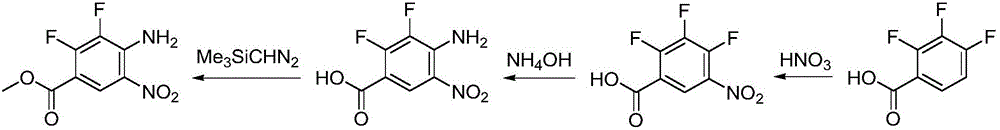
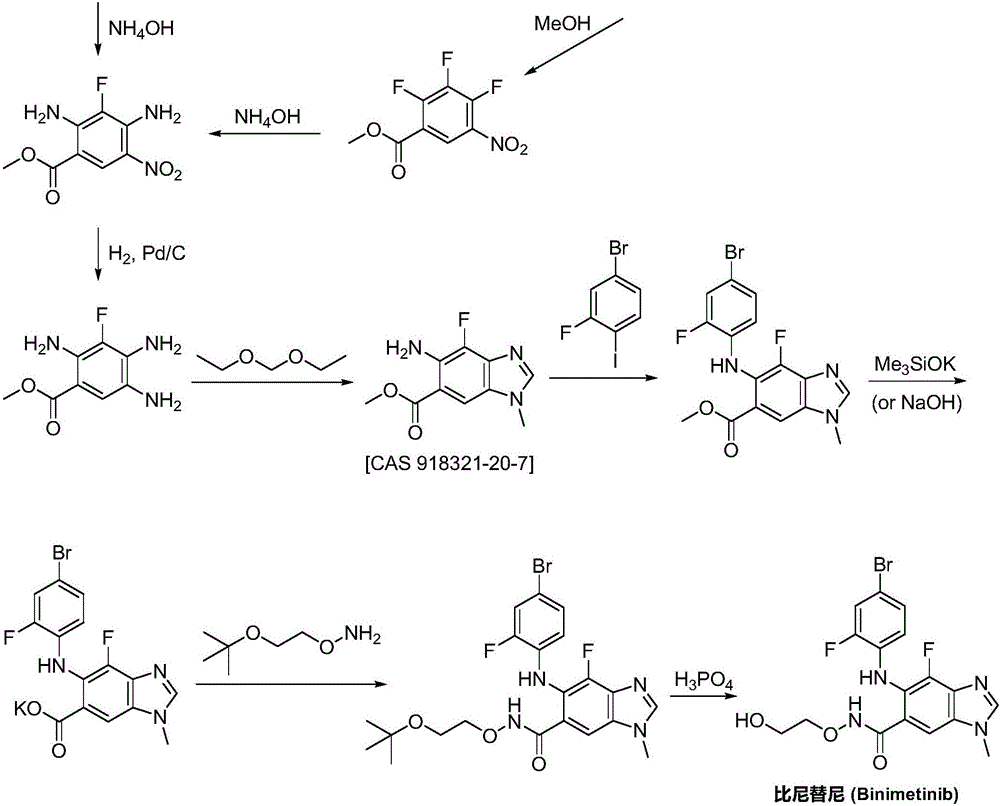
Synthesizing method for binimetinib
The invention discloses a synthesizing method for binimetinib. The method comprises the steps that 2,3,4-trifluoro-5-nitrobenzoic acid and O-(2-tert-butoxy ethyl)hydroxylamine are subjected to a condensation reaction, and obtained N-(2-tert-butoxy ethyoxyl)-2,3,4-trifluoro-5-nitrobenzamide and ammonium hydroxide are subjected to an ammonolysis reaction; obtained N-(2-tert-butoxy ethyoxyl)-2,4-diamino-3-fluoro-5-nitrobenzamide and formic acid are subjected to a ring-closure reaction in a Pearlman's catalyst; obtained N-(2-tert-butoxy ethyoxyl)-6-amino-7-fluoro-3H-benzimidazole-5-formamide and 4-bromo-2-fluoro-1-iodobenzene are subjected to a substitution reaction; obtained N-(2-tert-butoxy ethyoxyl)-6-[(4-bromo-2-fluoro-phenyl)amino]-7-fluoro-3H-benzimidazole-5-formamide is firstly subjected to a methylation reaction with methyl iodide and then subjected to a deprotection reaction with phosphoric acid, and binimetinib is obtained. The synthesizing method is short in route, operation is simplified, cost is low, and the synthesizing method is environmentally friendly and suitable for industrial production.
Owner:湖南欧亚药业有限公司
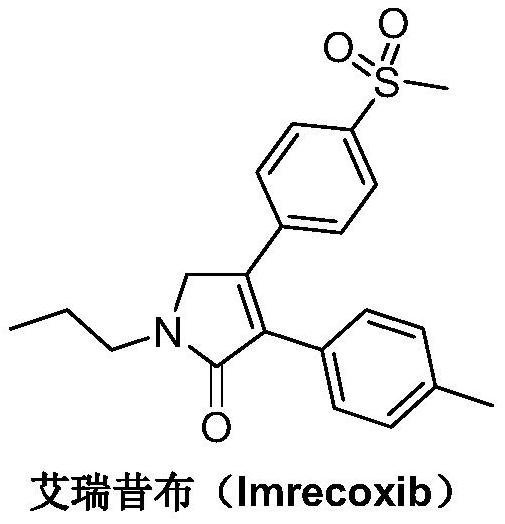
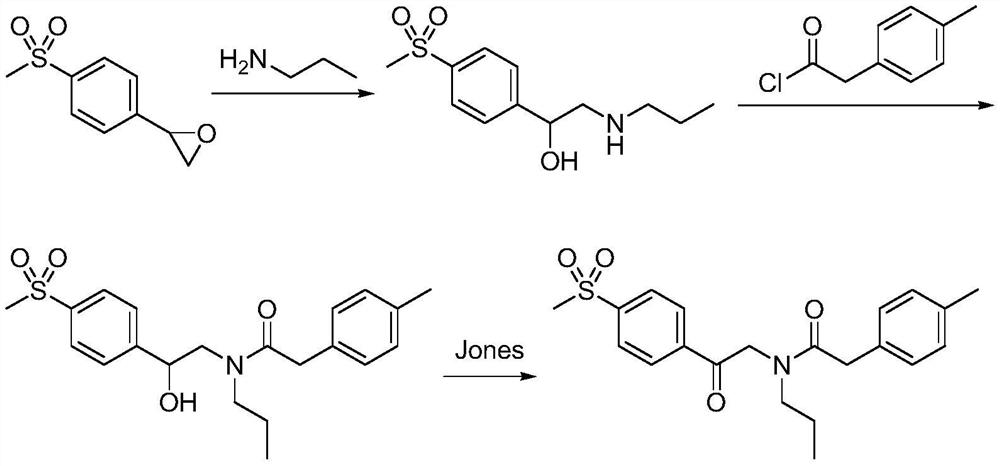
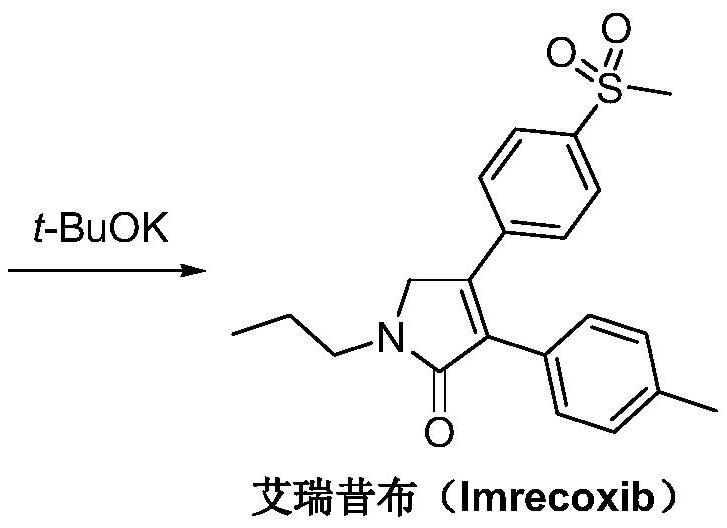
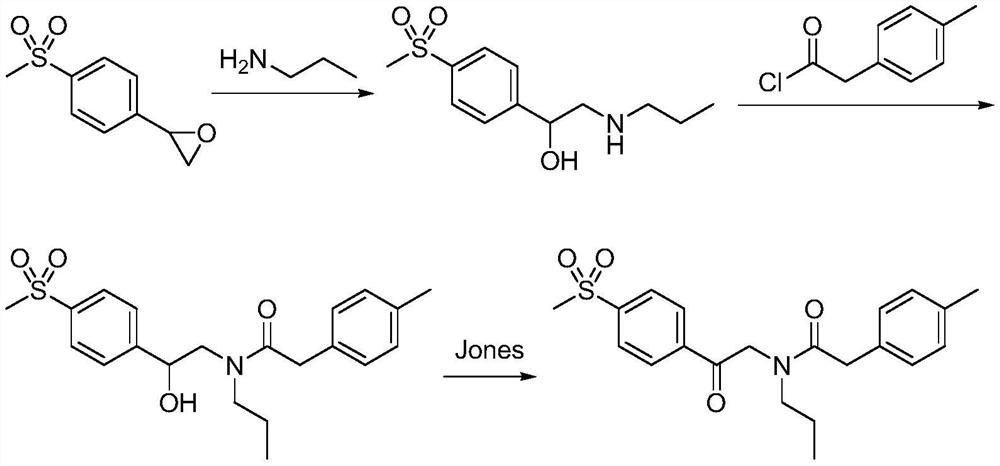
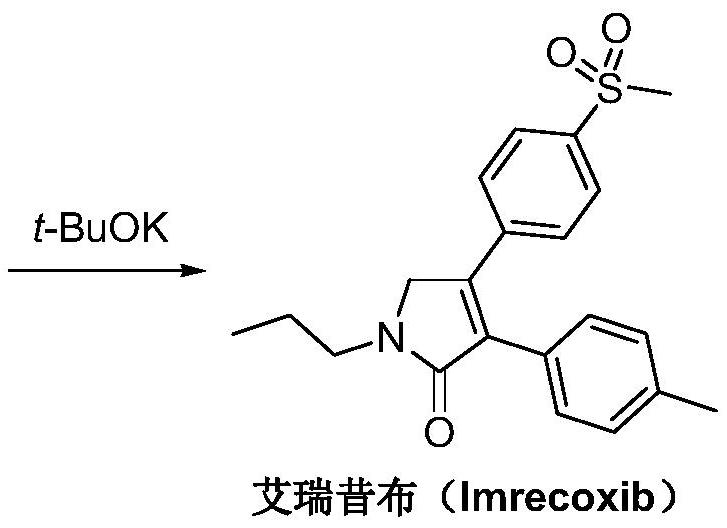
A kind of synthetic method of Erecoxib
ActiveCN108912030BMeet the needs of useSimplify and optimize reaction stepsOrganic chemistryPyrrolidinonesAcyl group
A kind of synthetic method of Erecoxib, step: with (E)-1-p-methylsulfonylphenyl-1-nitro-2-p-tolylethylene and isocyanoacetate in the mixture of alkali reagent and solvent The condensation cyclization reaction is carried out in the system; the obtained 3-p-methylphenyl-4-p-methylsulfonylphenylpyrrole-2-carboxylate is reduced at -78°C in a system of reducing agent and solvent Reaction; The obtained 3-p-methylphenyl-4-p-methylsulfonylphenylpyrrole-2-formaldehyde is oxidized in the system of hydrogen peroxide and solvent; the obtained 3-p-methylphenyl-4- Perform substitution reaction between p-methylsulfonylphenyl-3-pyrrolidin-2-one and 1-halopropane or hydrocarbon sulfonate propyl ester derivatives in a system of alkali reagent and solvent to obtain Erecoxib. The reaction steps are simplified and optimized, and the process operation is simple, which helps to reduce the cost; the impurities in the reaction are less and controllable, reflecting green environmental protection; the starting materials and the reagents used are easily available.
Owner:SUZHOU FUSHILAI PHARMA CO LTD
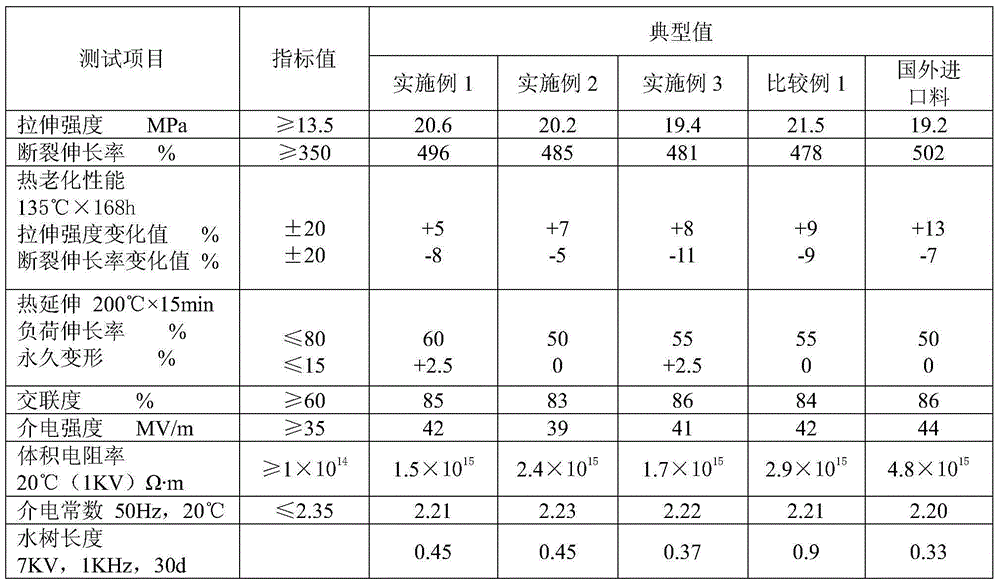
Medium-voltage water-tree-retardant crosslinked polyethylene cable material and preparation method thereof
The invention provides a medium-voltage water-tree-retardant crosslinked polyethylene cable material and a preparation method thereof and belongs to the technical field of wire cable materials and preparation of the wire cable materials. The medium-voltage water-tree-retardant crosslinked polyethylene cable material is prepared from raw materials as follows: 60-75 parts of polyolefin composite resin, 15-25 parts of a polyethylene silane-grafting material, 5-10 parts of water-tree-retardant composite catalytic master batch and 2-5 parts of a cross-linking agent. The polyolefin composite resin comprises raw materials as follows: 60-80 parts of low-density polyethylene and 20-40 parts of ethylene vinyl acetate copolymer; the water-tree-retardant composite catalytic master batch comprises raw materials as follows: 70-80 parts of low-density polyethylene, 3-5 parts of a catalyst, 10-15 parts of a nucleating agent, 2-5 parts of an antioxidant, 0.1-0.2 parts of a crosslinking agent and 3-5 parts of an assistant crosslinking agent. Formation of a water tree is delayed, surrounding water can be dispersed to a certain extent, formation of the water tree can be effectively inhibited, the overall physical property of the material is improved, and the material has excellent mechanical and physical properties and is environment-friendly.
Owner:CHANGSHU ZHONGLIAN PHOTOELECTRICITY NEW STUFF
A kind of synthetic method of filgotinib
ActiveCN104987333BWon't happenLess impuritiesOrganic chemistryChemical synthesisTert-Butyloxycarbonyl protecting group
The invention discloses a filgotinib synthetic method and belongs to the technical field of chemical synthesis of medicines. 6-chloro-2-aminopyridine and di-tert-butyl dicarbonate ester are subjected to condensation reaction to obtain 6-chloro-2-tert-butyloxycarbonyl aminopyridine; hydrolysis reaction is performed; the obtained 6-chloro-2-tert-butyloxycarbonyl aminopyridine and trifluorinated methyl sulfonic anhydride are subjected to trifluoromethanesulfonic acid esterification reaction; the obtained 2-tert-butyloxycarbonylamino-6-pyridyltrifluoromethanesulfonate and [(1,1-dioxo-4-thiomorpholinyl)methyl]benzo-4-boronic acid pinacol ester are subjected to condensation reaction to obtain a tert-butyl ester derivative; the tert-butyl ester derivative is treated by trifluoroacetic acid and subjected to de-protection; the obtained intermediate and ethoxycarbonyl isothiocyanate are subjected to isothiocyanate reaction; the obtained intermediate and hydroxylamine hydrochloride are subjected to ring closing reaction; the obtained intermediate and cyclopropane carbonyl chloride are subjected to amidation reaction to obtain the finished product. Operation is simplified; reagents are available; the concept of environment friendliness and environment protection is embodied.
Owner:SUZHOU FUSHILAI PHARMA CO LTD
The method for preparing r-lipoic acid choline ester halide
ActiveCN106967044BLess impuritiesEasy to operateOrganic chemistry methodsChemical synthesisAfter treatment
The invention provides a method for preparing R-lipoic acid choline ester halide, and belongs to the technical field of chemical synthesis of drugs. The method comprises a method B or a method B, wherein the method A comprises performing an esterification reaction between R-lipoic acid and halogenated choline in a system composed of a dehydrating agent, a catalyst and a solvent to obtain R-lipoic acid choline ester halide I; and the method B comprises performing an esterification reaction between R-sulfenyloctanoylchloride and halogenated choline in a system composed of an acid-binding agent alkali and a solvent to obtain the R-lipoic acid choline ester halide I. The reaction of each step has fewerimpurities, and the operations of after treatment, purification and the like are simplified; the method is safe to use, and suitable for industrial large-scale production; and a green and environmental protection effect is reflected.
Owner:SUZHOU FUSHILAI PHARMA CO LTD
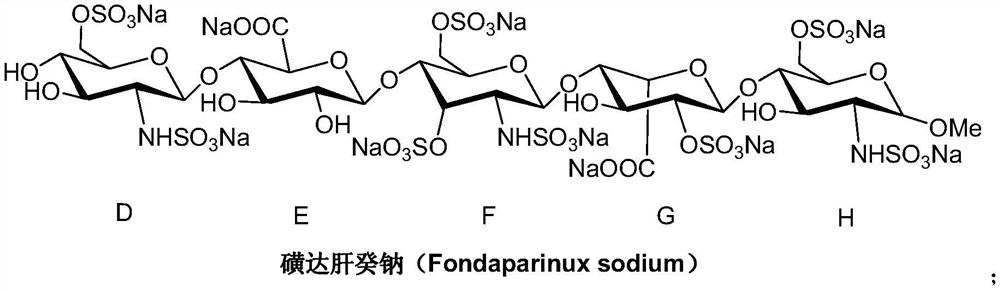
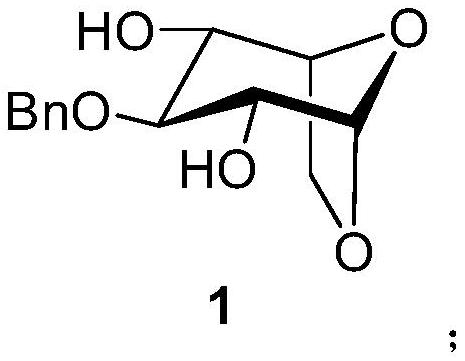
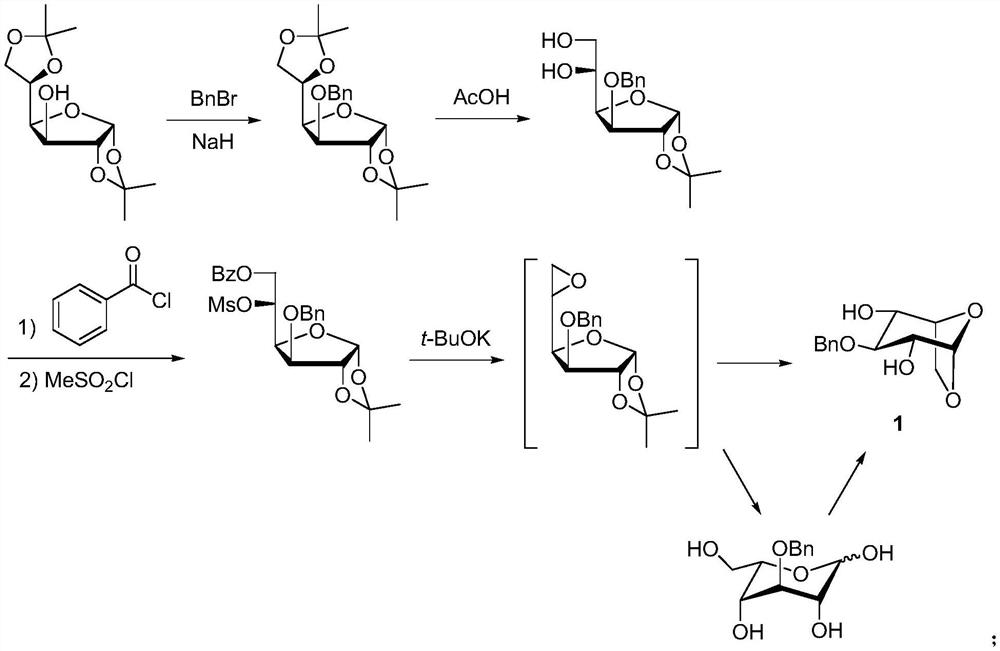
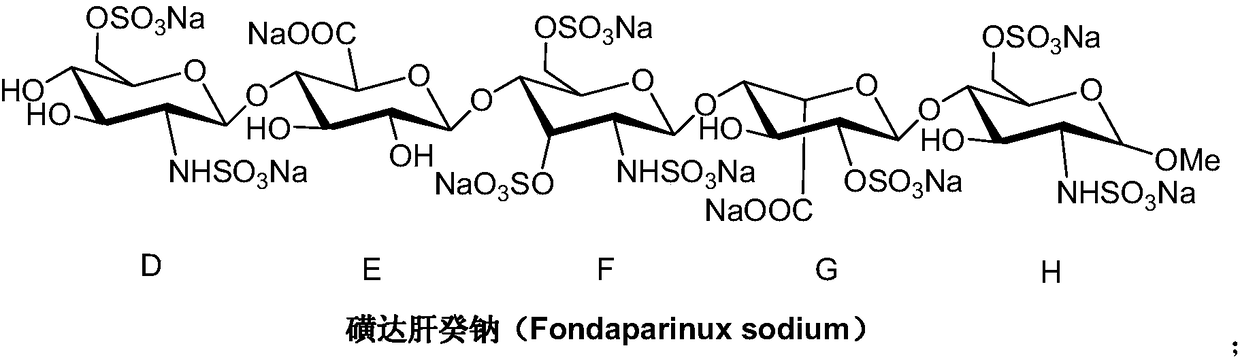
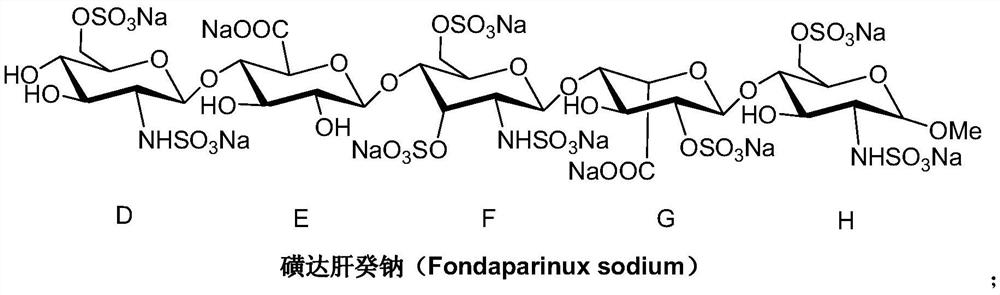
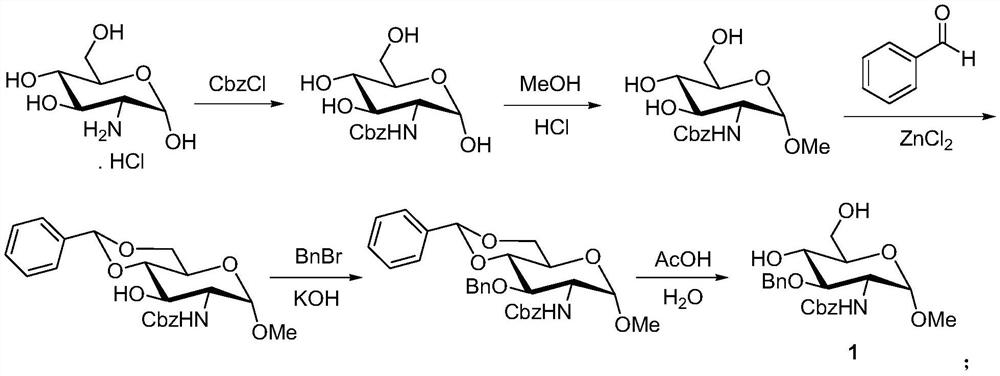
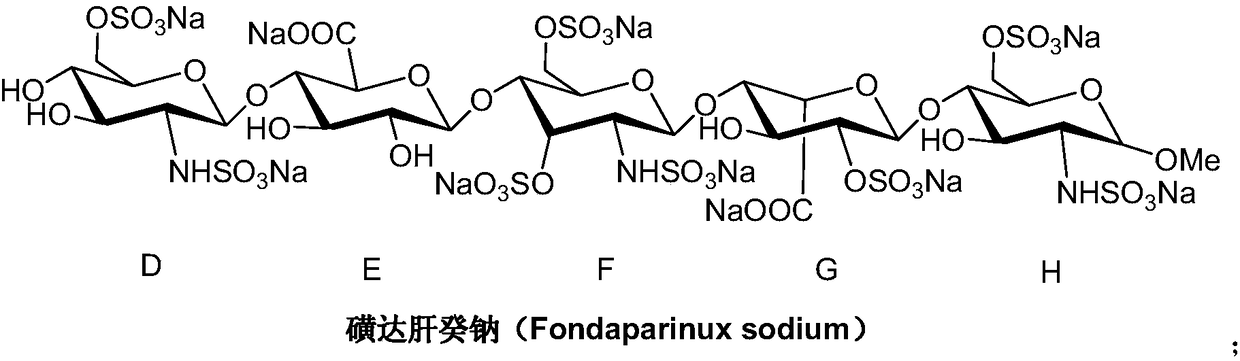
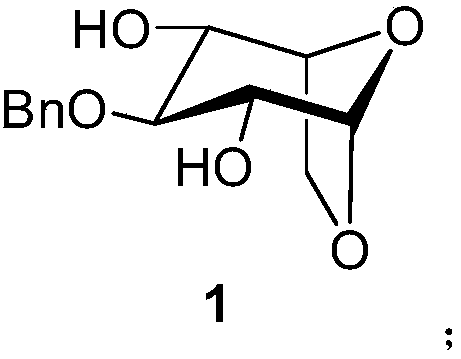
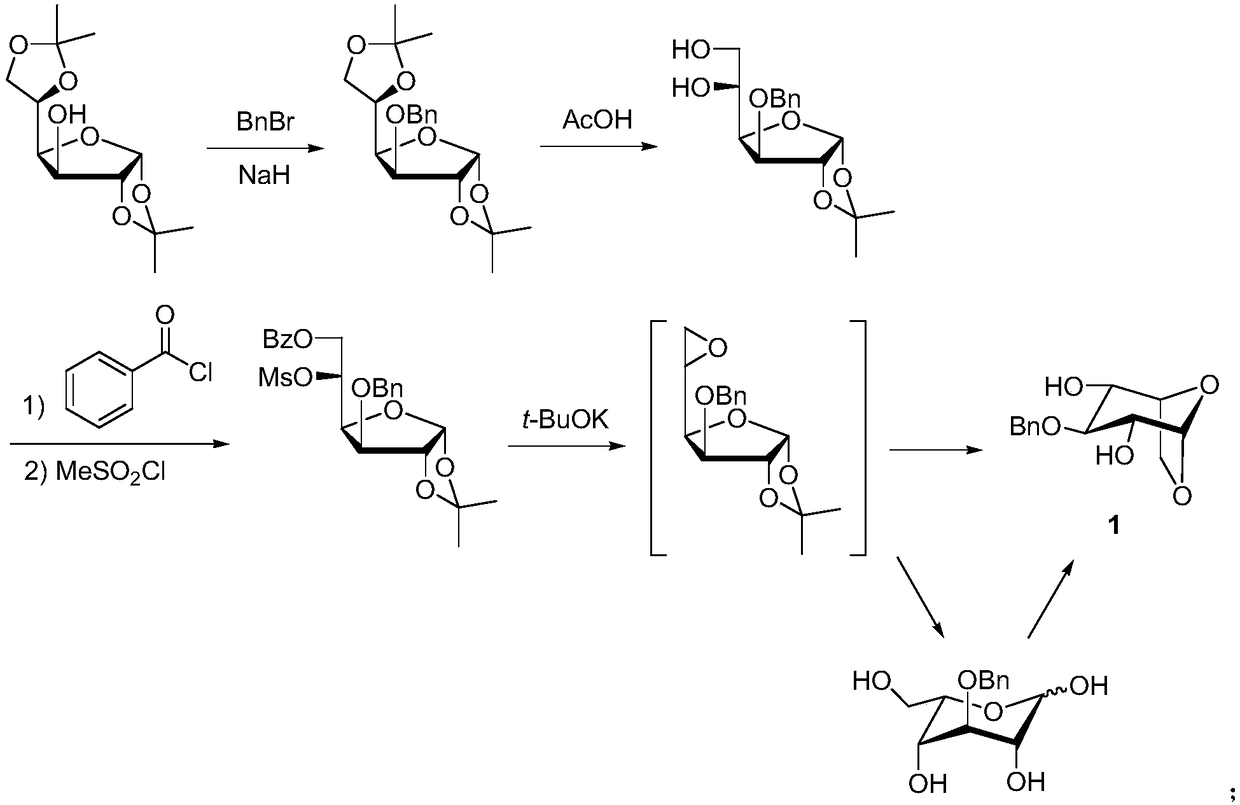
A kind of synthetic method of fondaparinux sodium monosaccharide intermediate
ActiveCN109438534BReduce generationConducive to purification and refiningSugar derivativesSugar derivatives preparationSulfonyl chlorideBenzoic acid
The invention discloses a method for synthesizing a monosaccharide intermediate of fondaparinux sodium, which uses 1,2-O-isopropylidene-3-O-benzyl-α-D-glucofuranose as a raw material, through mixing with benzene Esterification reaction of formic acid, esterification reaction with substituent sulfonic anhydride and / or substituent sulfonyl chloride, followed by hydrolysis reaction and addition of sulfuric acid aqueous solution to obtain fondaparinux sodium monosaccharide fragment intermediate 1,6-dehydration ‑3‑O‑Benzyl‑β‑L‑Idopyranose. The synthesis method has simple process, less side reaction impurities and high yield, and is suitable for process scale-up synthesis to meet the industrial production of fondaparinux sodium.
Owner:江苏美迪克化学品有限公司
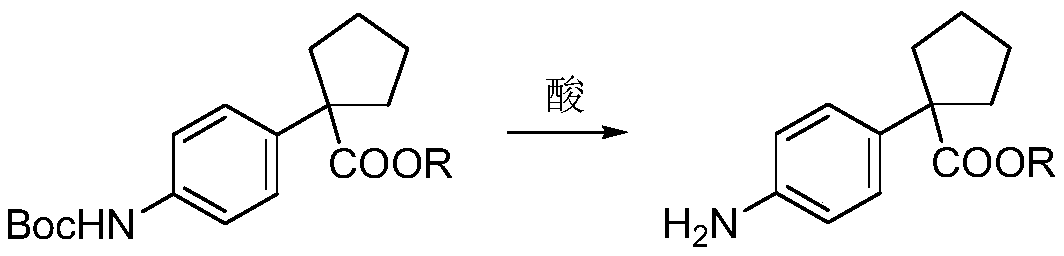
A kind of Apatinib intermediate and preparation method thereof
ActiveCN110003101BMeet preparation requirementsSimple processing methodOrganic chemistryChemical synthesisPhenylacetic acid
An Apatinib intermediate and its preparation method belong to the technical field of pharmaceutical chemical synthesis. The chemical name of the Apatinib intermediate is 1-{4-[(2-((4-pyridyl methyl)amino)pyridine-3-yl)carbonylamino]phenyl}cyclopentanecarboxylic alkyl ester. The preparation method comprises the following steps: 4-aminophenylacetic alkyl ester and 2-Chloronicotinyl chloride are subjected to an amidation reaction in a system of an acid-binding agent base and a solvent to obtain 4-[(2-chloropyridine-3-yl)carbonylamino]phenylacetic alkyl ester; the product and 4-aminomethylpyridineare subjected to a substitution reaction in a system of an acid-binding agent base and a solvent to obtain 4-{[2-((4-pyridylmethyl)amino)pyridine-3-yl]carbonylamino}phenylacetic alkyl ester; and theproduct and 1,4-dihalogenbutane are subjected to a condensation reaction to obtain a finished product. The technology is simple, is low-cost, is green and environmentally-friendly, and is suitable forindustrial production.
Owner:SUZHOU FUSHILAI PHARMA CO LTD
Preparation method of fondaparinux sodium monosaccharide intermediate
ActiveCN109369738AProduced noMeet industrial productionSugar derivativesSugar derivatives preparationGlucose polymersD-Glucose
The invention discloses a preparation method of a fondaparinux sodium monosaccharide intermediate. The preparation method includes using methyl glucose protected by C4 and C6 positions as a raw material to obtain methyl 3-O-benzyl-4,6-O-benzylidene-2-(benzyloxycarbonyl)amino-2-deoxy-alpha-D-glucopyranoside by methylation, amidation and benzylation, and then conducting dehydroxylation protection group protection reaction to obtain the fondaparinux sodium monosaccharide fragment intermediate methyl-3-O-benzyl-2-(benzyloxycarbonyl)amino-2-deoxy-alpha-D-glucopyranoside. The preparation method is simple in process, less in side reaction impurities, high in yield and suitable for process amplification preparation to meet the industrial production of fondaparinux sodium.
Owner:江苏美迪克化学品有限公司
A kind of preparation method of ederaris
ActiveCN108409740BMild reaction conditionsThe reaction conditions are mild and easy to controlOrganic chemistryAntineoplastic agentsCompound aChemical compound
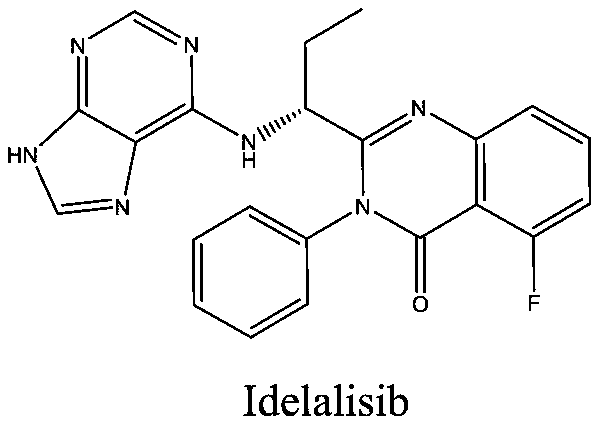
The invention belongs to the field of medicine synthesis and provides a novel idelalisib preparation method. The novel idelalisib preparation method includes steps: in an appropriate solvent, subjecting a compound A to nucleophilic substitution reaction with B in existence of an acid-binding agent to obtain an intermediate C; hydrolyzing the compound C into an intermediate D under appropriate alkali action; subjecting the intermediate D and a compound E to condensation to obtain an intermediate F; in an appropriate solvent, subjecting the intermediate F to ring closing reaction under a catalytic system of HMDS (hexamethyl disilazane) / lewis acid to obtain a final product namely idelalisib.
Owner:YANCHENG TEACHERS UNIV +1
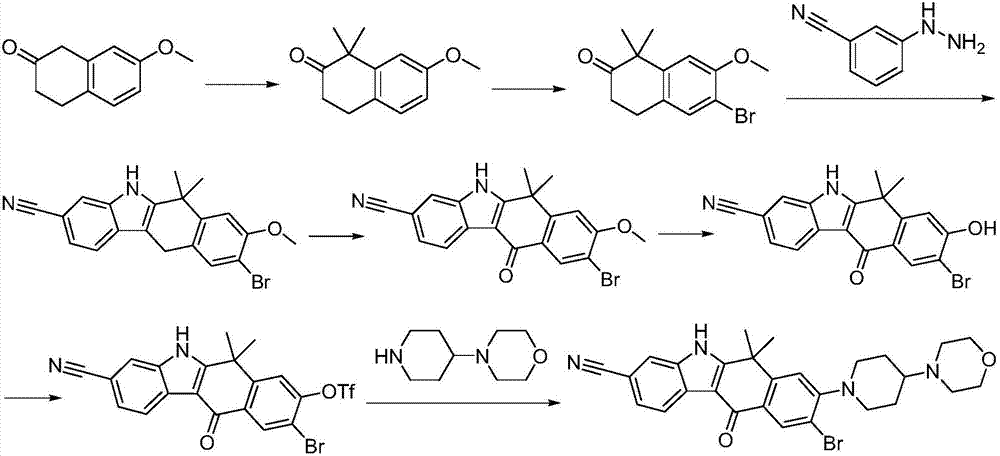
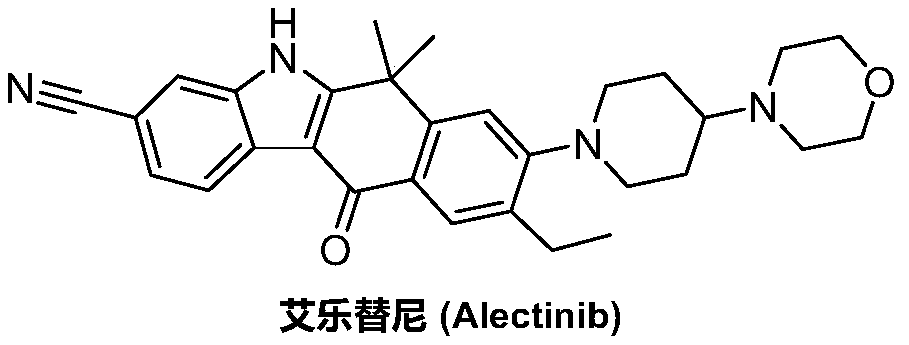
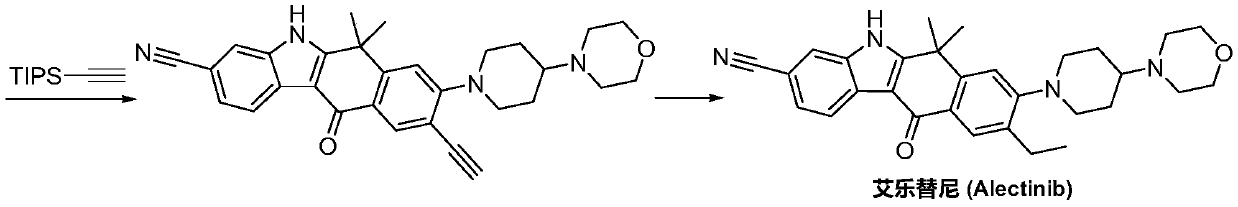
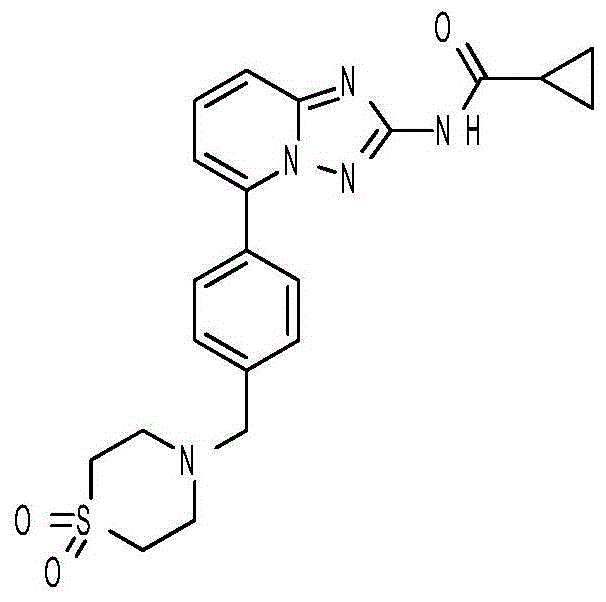
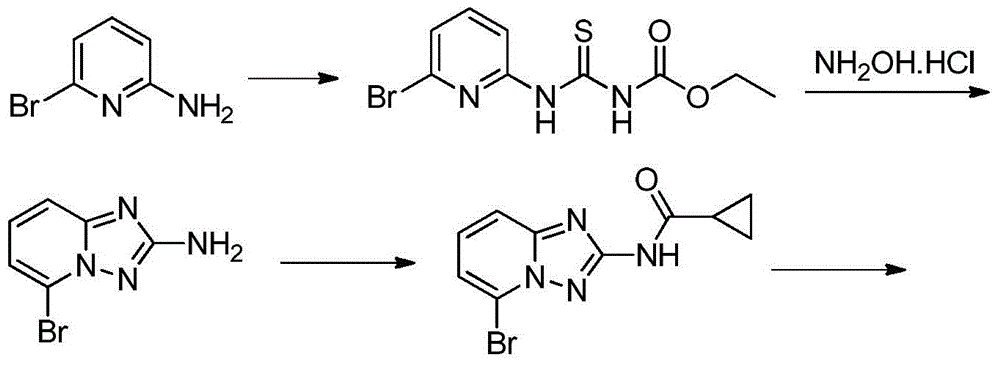
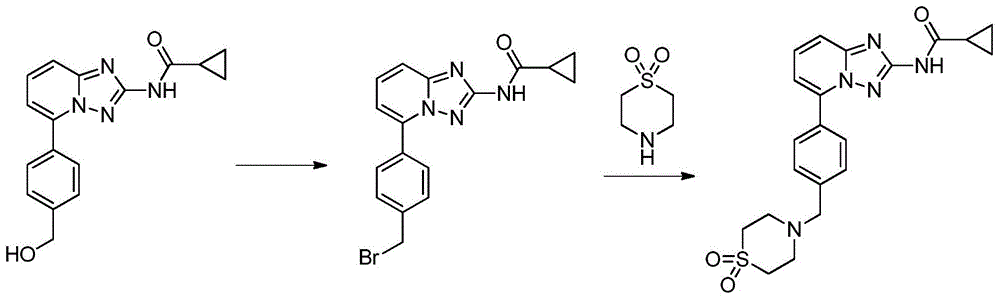
A kind of synthetic method of Alectinib
ActiveCN105777710BMeet the needs of useApplicable generationOrganic chemistryErlotinibSynthesis methods
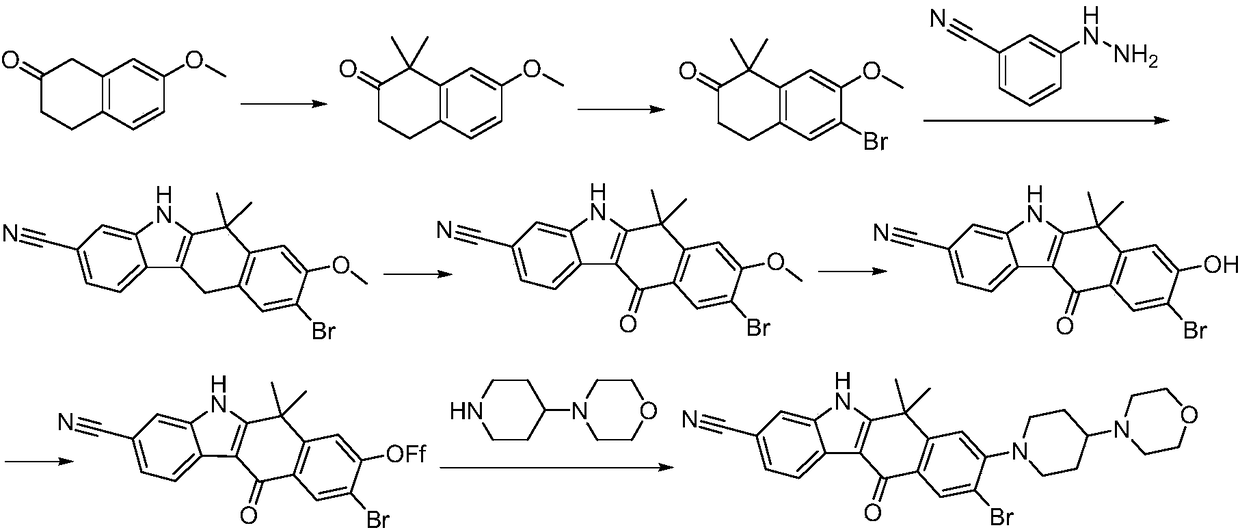
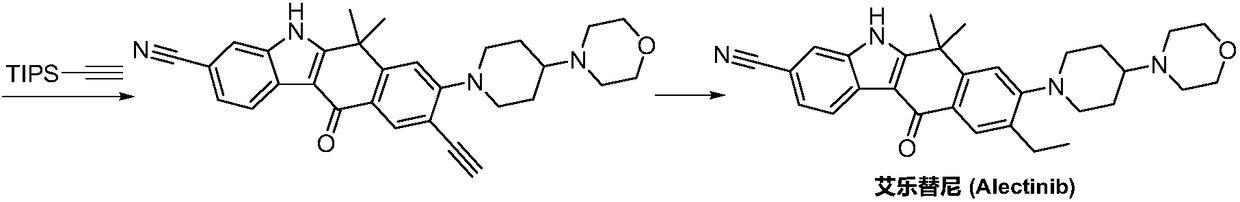
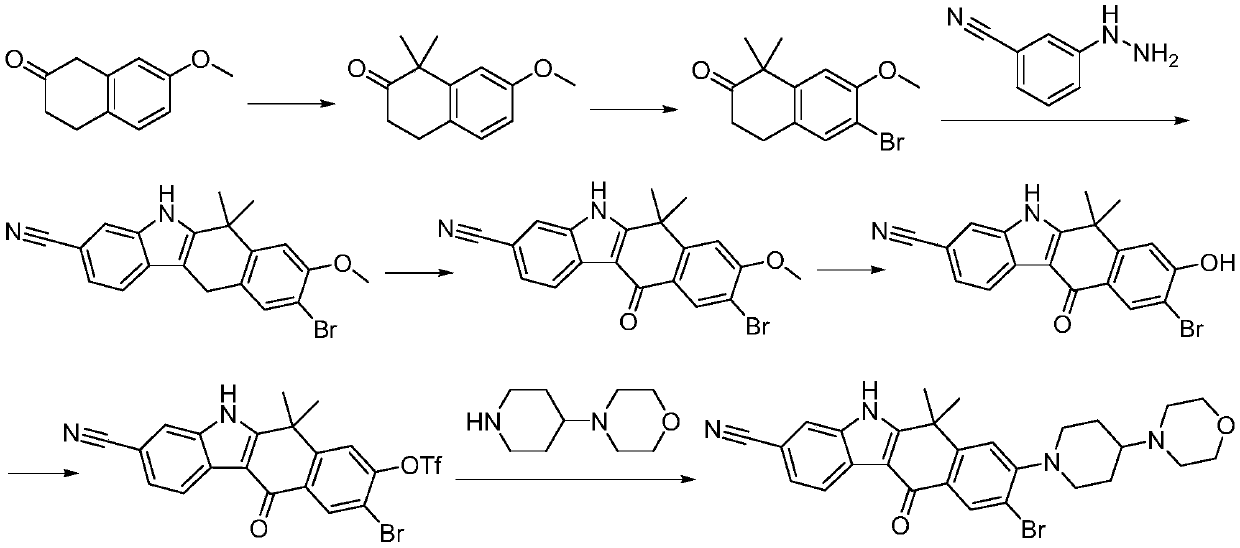
The invention discloses a synthesis method of Alectinib. The method comprises the following steps: carrying out a borating reaction between 6-bromo-3,4-dihydro-2-naphthalenone and n-butyl lithium and then an organic boron reagent; carrying out a catalytic coupling reaction between the obtained 3,4,-dihydro-2-naphthalenone-6-boric acid and bromoethane; carrying out a dimethylation reaction between the obtained 6-ethyl-3,4-dihydro-2-naphthalenone and iodomethane; carrying out a bromination reaction between the obtained 1,1-dimethyl-6-ethyl-3,4-dihydro-2-naphthalenone and a bromination reagent; carrying out a substitution reaction between the obtained 1,1-dimethyl-6-ethyl-7-bromo-3,4-dihydro-2-naphthalenone and 4-(4-piperidyl)morpholine; carrying out a cyclization reaction between the obtained 1,1-dimethyl-6-ethyl-7-[4-(morpholine-4-yl)piperidine-1-yl]-3,4-dihydro-2-naphthalenone and 3-cyanophenylhydrazine; and carrying out an oxidation reaction between the obtained 9-ethyl-6,6-dimethyl-8-[4-(morpholine-4-yl)piperidine-1-yl]-6,11-dihydro-5H-benzo[b]carbazole-3-formonitrile and dichlorodicyanobenzoquinone to obtain a finished product of Alectinib. The synthesis method has the advantages of relatively short route, simplified operation and relatively low cost and is a green and environment-friendly method suitable for industrial production.
Owner:湖南欧亚药业有限公司
The synthetic method of Erecoxib
ActiveCN108997188BMeet the needs of useUse requirements applyOrganic chemistryChemical synthesisAcetophenone
A method for synthesizing ericoxib belongs to the technical field of pharmaceutical chemical synthesis. Steps: Synthesis of 2-n-propylamino-1-p-methanesulfonyl acetophenone: N-n-propyl-β-hydroxy-4-methanesulfonyl phenethylamine in a system composed of oxidizing reagent, solvent and water The oxidation reaction is carried out to obtain 2-n-propylamino-1-p-methylsulfonyl acetophenone; synthesis of Irecoxib: combine the obtained 2-n-propylamino-1-p-methylsulfonyl acetophenone with p-methylsulfonyl acetophenone. Tolylacetate undergoes a condensation cyclization reaction in a system of alkali reagents and solvents to obtain ericoxib. The reaction steps are significantly simplified and optimized, and the process operation is simple, which helps to reduce costs; the impurities in the reaction are less, controllable, and no pollutants are produced, reflecting the green and environmentally friendly effect; the starting materials and reagents used are easy to obtain, and can be mass-produced To meet the demand for raw materials and be suitable for industrial production.
Owner:SUZHOU FUSHILAI PHARMA CO LTD
A kind of preparation method of fondaparinux sodium monosaccharide intermediate
ActiveCN109369738BProduced noMeet industrial productionSugar derivativesSugar derivatives preparationSide reactionBenzyl group
The invention discloses a method for preparing a monosaccharide intermediate of fondaparinux sodium. Using glucosamine protected at positions C4 and C6 as raw materials, methyl 3‑O is obtained through methylation, amidation and benzylation. ‑Benzyl‑4,6‑O‑benzylidene‑2‑(benzyloxycarbonyl)amino‑2‑deoxy‑α‑D‑glucopyranoside, and then undergo a dehydroxyl protecting group protection reaction to obtain fondaparin Sodium decyl monosaccharide fragment intermediate methyl 3‑O‑benzyl‑2‑(benzyloxycarbonyl) amino‑2‑deoxy‑α‑D‑glucopyranoside. The preparation method has simple process, less impurities in side reactions, and high yield, and is suitable for process scale-up preparation to meet the industrial production of fondaparinux sodium.
Owner:江苏美迪克化学品有限公司
The preparation method of Apatinib
ActiveCN109879805BAvoid expensiveMild reaction conditionsOrganic chemistryTert-Butyloxycarbonyl protecting groupPhenylacetic acid
Owner:SUZHOU FUSHILAI PHARMA CO LTD
Method for synthesizing fondaparinux sodium monosaccharide intermediate
ActiveCN109438534AReduce the formation of impuritiesImprove product quality and yieldSugar derivativesSugar derivatives preparationIdopyranoseEsterification reaction
The invention discloses a method for synthesizing a fondaparinux sodium monosaccharide intermediate. According to the method, a fondaparinux sodium monosaccharide fragment intermediate, namely 1,6-dehydro-3-O-benzyl-beta-L-idopyranose, is prepared by taking 1,2-O-isopropylidene-3-O-benzyl-alpha-D-glucofuranose as a raw material, subjecting the raw material to an esterification reaction with benzoic acid, subjecting the reaction product to the esterification reaction with substituted sulfonic anhydride and / or substituted sulfonyl chloride, then subjecting the reaction product to a hydrolysis reaction, and adding a sulfuric acid aqueous solution for a reaction. The synthesis method has the advantages of simple process, few side reaction impurities and high yield, and is suitable for amplification synthesis process to meet the industrial production of fondaparinux sodium.
Owner:江苏美迪克化学品有限公司
A kind of preparation method of alectinib
The invention discloses a preparation method of Alectinib. The method comprises the steps that tert-butyl 4-(4-ethyl-3-iodophenyl)-4-methyl-3-oxopentanoate is subjected to a cyclization reaction and a hydrolysis reaction; obtained 6-cyano-2-[2-(4-ethyl-3-iodophenyl) prop-2-yl]-1H-indole-3-carboxylic acid is subjected to the cyclization reaction; obtained 9-ethyl-6,6-dimethyl-8-iodo-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile and 4-(piperidin-4-yl)-morpholine are subjected to a substitution reaction, and the Alectinib is obtained. According to the method, the operation is simplified, the cost is low, and the method is an environment-friendly technical method and suitable for industrial production.
Owner:湖南润星制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com