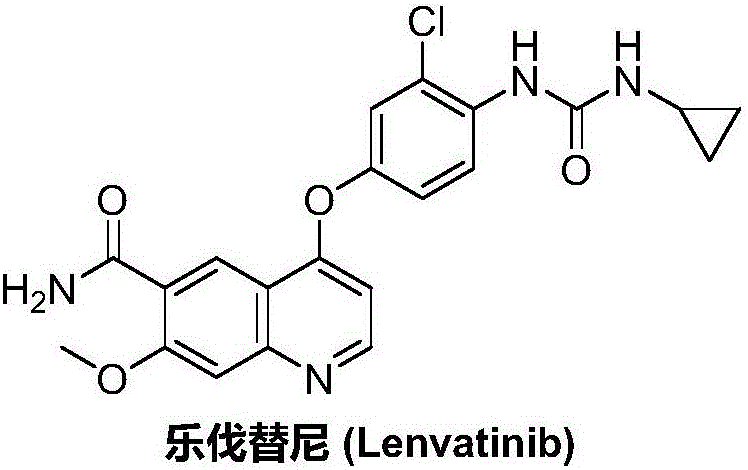
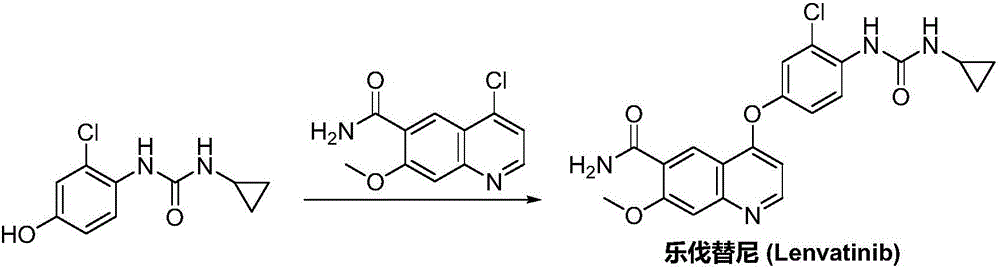
Lenvatinib synthesizing method
A lenvatinib and synthetic method technology, applied in the field of synthesis of new anti-tumor drug lenvatinib, can solve the problems of increasing reaction steps and purification operations, reducing yield, etc., to simplify operations, reduce side reactions, and technical Reasonable effect of the plan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A) Preparation of 4-(benzyloxycarbonyl)amino-3-chlorophenol:
[0033] 4-Amino-3-chlorophenol (10.0g, 0.07mol) was dissolved in tetrahydrofuran (75mL), stirred, benzyl chloroformate (14.3g, 0.08mol) was added, and N,N-diisopropylethylamine ( 36.0g, 0.28mol), the reaction mixture was stirred and reacted at 50°C for 8 hours, and the reaction was confirmed by TLC spotting. Evaporate and concentrate to dryness, and recrystallize from a methanol-isopropanol mixed solvent to obtain 4-(benzyloxycarbonyl)amino-3-chlorophenol as an off-white solid (17.0 g), with a yield of 88.0%. The reaction formula of this step is as follows:
[0034]
[0035] B) Preparation of 4-[3-chloro-4-(benzyloxycarbonyl)aminophenoxy]-7-methoxyquinoline-6-carboxamide:
[0036] 4-(Benzyloxycarbonyl)amino-3-chlorophenol (17.0g, 0.06mol) and 4-chloro-7-methoxyquinoline-6-carboxamide (18.1g, 0.08mol) were dissolved in dichloromethane ( 75 mL), sodium hydride (6.0 g, content 60%, 0.15 mol) was added, the ...
Embodiment 2
[0042] A) Preparation of 4-(benzyloxycarbonyl)amino-3-chlorophenol:
[0043] 4-Amino-3-chlorophenol (12.5g, 0.09mol) was dissolved in chloroform (90mL), stirred, benzyl chloroformate (18.6g, 0.11mol) was added, triethylamine (39.6g, 0.39mol) was added dropwise, The reaction mixture was stirred and reacted at 55°C for 7 hours, TLC was spotted to confirm the completion of the reaction, the reaction solution was concentrated to dryness by rotary evaporation, adjusted to neutrality by adding dilute hydrochloric acid, extracted by adding ethyl acetate, dried by magnesium sulfate, concentrated to dryness by rotary evaporation, methanol- Recrystallized from a mixed solvent of isopropanol to obtain 4-(benzyloxycarbonyl)amino-3-chlorophenol as an off-white solid (21.1 g), with a yield of 87.2%. The reaction formula of this step is the same as in Example 1;
[0044] B) Preparation of 4-[3-chloro-4-(benzyloxycarbonyl)aminophenoxy]-7-methoxyquinoline-6-carboxamide:
[0045] 4-(Benzyloxyc...
Embodiment 3
[0049] A) Preparation of 4-(benzyloxycarbonyl)amino-3-chlorophenol:
[0050] 4-Amino-3-chlorophenol (8.5g, 0.06mol) was dissolved in acetonitrile (60mL), stirred, added benzyl chloroformate (14.1g, 0.08mol), added 4-dimethylaminopyridine (36.2g, 0.30mol ), the reaction mixture was stirred and reacted at 60° C. for 6 hours, and TLC was spotted to confirm that the reaction was complete. The reaction solution was concentrated to dryness by rotary evaporation, adjusted to neutrality by adding dilute hydrochloric acid, extracted by adding ethyl acetate, dried over magnesium sulfate, and concentrated to dryness by rotary evaporation. Recrystallization from a mixed solvent of methanol-isopropanol gave 4-(benzyloxycarbonyl)amino-3-chlorophenol as an off-white solid (14.7 g), with a yield of 89.5%. The reaction formula of this step is the same as in Example 1;
[0051] B) Preparation of 4-[3-chloro-4-(benzyloxycarbonyl)aminophenoxy]-7-methoxyquinoline-6-carboxamide:
[0052] 4-(Benzyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com