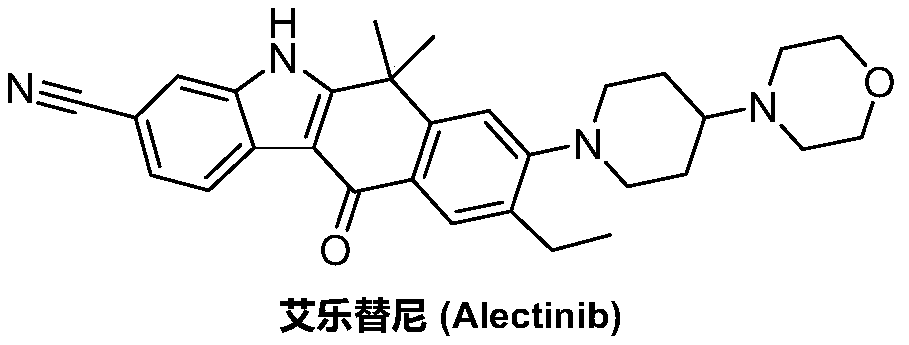
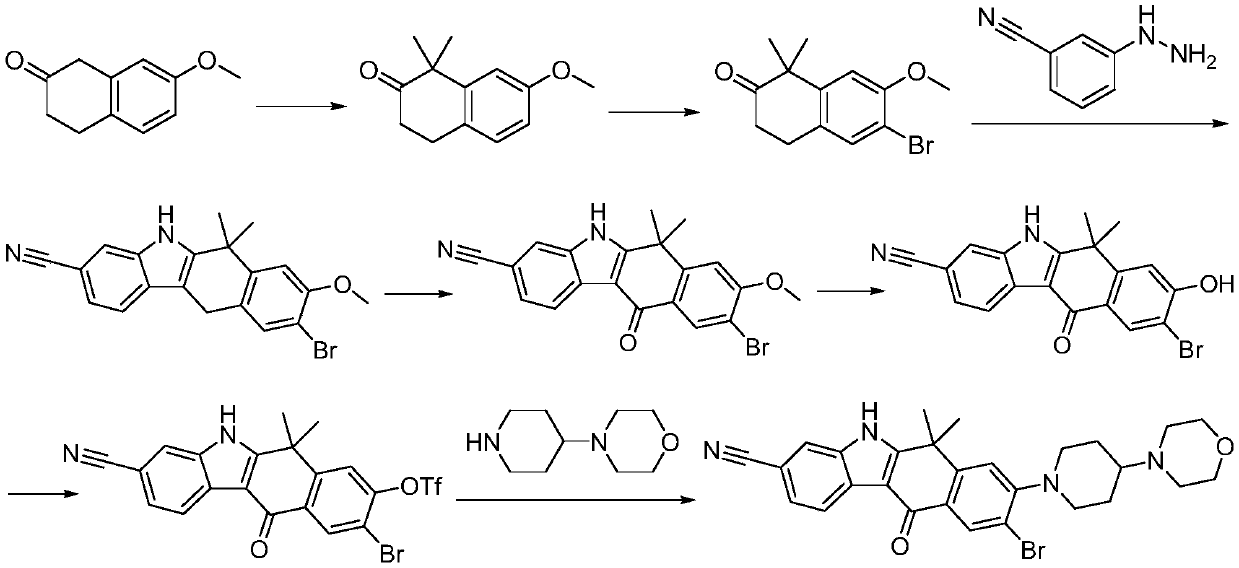
A kind of preparation method of alectinib
A technology of alectinib and ethyl, which is applied in the field of medicinal chemical synthesis, can solve the problems of unfavorable industrial production promotion, expensive starting materials, and the use of a large amount of solvents, and achieve effective control of reaction conditions, cheap raw materials, and easy access to raw materials. The effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A) Preparation of 2-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-2-methylpropanal:
[0038] 2-{4-Ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-2-methylpropanoic acid ethyl ester (5.0g, 12.9mmol) dissolved in tetrahydrofuran (80mL), cooled to -78°C, slowly added diisobutylaluminum hydride (18.0mmol) tetrahydrofuran solution dropwise, kept at -78°C and stirred for 2 hours, evaporated to dryness under reduced pressure, added dilute hydrochloric acid to adjust to neutral , extracted with ethyl acetate, washed with water and dried, evaporated to dryness under reduced pressure, and recrystallized from isopropanol to obtain 2-{4-ethyl-3-[4-(morpholin-4-yl)piperidine-1- Base]phenyl}-2-methylpropanal, pale yellow solid (4.1g), yield 92%.
[0039] B) Preparation of 3-chloro-4-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-4-methyl-2-oxopentanoic acid tert-butyl ester:
[0040] 2-{4-Ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-2-methylpropanal (4.0g, 11.6...
Embodiment 2
[0048] A) Preparation of 2-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-2-methylpropanal:
[0049] 2-{4-Ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-2-methylpropanoic acid ethyl ester (10.0g, 25.7mmol) was dissolved in 1 , 4-dioxane (120mL), cooled to -78°C, slowly added dropwise a solution of bis(2-methoxyethoxy)aluminum hydride (41.0mmol) in 1,4-dioxane, and kept warm Stir and react at -75°C for 3 hours, rotary evaporate to dryness under reduced pressure, adjust to neutrality by adding dilute hydrochloric acid, extract with ethyl acetate, wash with water and dry, rotary evaporate to dryness under reduced pressure, recrystallize from isopropanol to obtain 2-{4 -Ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-2-methylpropanal, pale yellow solid (8.0 g), yield 90%.
[0050] B) Preparation of 3-chloro-4-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-4-methyl-2-oxopentanoic acid tert-butyl ester:
[0051] 2-{4-Ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phen...
Embodiment 3
[0059] A) Preparation of 2-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-2-methylpropanal:
[0060] 2-{4-Ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-2-methylpropanoic acid ethyl ester (2.3g, 5.9mmol) was dissolved in methanol Methyl tert-butyl ether (30mL), cooled to -78°C, slowly added diisobutylaluminum hydride (7.5mmol) in methyl tert-butyl ether solution dropwise, kept at -68°C and stirred for 1 hour, then rotary evaporated under reduced pressure To dryness, add dilute hydrochloric acid to adjust to neutrality, extract with ethyl acetate, wash with water and dry, rotary evaporate to dryness under reduced pressure, and recrystallize from isopropanol to obtain 2-{4-ethyl-3-[4-(morpholine -4-yl)piperidin-1-yl]phenyl}-2-methylpropanal, light yellow solid (1.7g), yield 83%.
[0061] B) Preparation of 3-chloro-4-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-4-methyl-2-oxopentanoic acid tert-butyl ester:
[0062]2-{4-Ethyl-3-[4-(morpholin-4-yl)piperidin-1-y...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com