Preparation method of R-lipoic acid cholinesterase halide
A lipoic acid choline and halide technology, which is applied in the field of pharmaceutical chemical synthesis, can solve problems such as preparation conditions and operations that are not given, and achieve the effects of safe use, simplified operation, and reasonable reaction route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
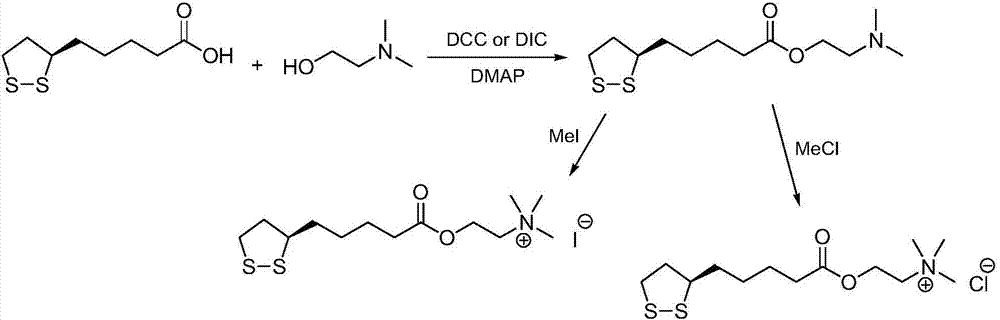
[0030] (1) Preparation of R-lipoic acid-2-chloroethyl ester:
[0031] Method A): Add R-lipoic acid (10.0g, 48.5mmol) and dichloromethane (200mL) into the reaction flask, stir to dissolve, add N,N'-dicyclohexylcarbodiimide (12.0g, 58.2mmol ) and 4-dimethylaminopyridine (1.2g, 9.7mmol), cooled in an ice bath, 2-chloroethanol (8.8g, 109.1mmol) was added dropwise, and the reaction mixture was reacted at 35°C for 18h. Post-treatment and purification, the crude product was recrystallized with a mixed solvent of ethyl acetate and n-hexane to obtain R-lipoic acid-2-chloroethyl ester, a light yellow solid (12.0g), with a yield of 92%, and the reaction formula was:
[0032]
[0033] (2) Preparation of R-lipoic acid choline ester chloride:
[0034] R-lipoic acid-2-chloroethyl ester (12.0g, 44.6mmol) and toluene (200mL) were added to the reaction flask, stirred and dissolved, cooled in an ice bath, trimethylamine aqueous solution (7.5mL, 16.9M, 126.8mmol) was added dropwise, The reac...
Embodiment 2
[0037] (1) Preparation of R-lipoic acid-2-bromoethyl ester:
[0038] Method A): Add R-lipoic acid (12.0g, 58.2mmol) and acetonitrile (250mL) into a reaction flask, stir to dissolve, add N,N'-carbonyldiimidazole (10.4g, 64.0mmol) and 2,6-bis Pyridine (0.6g, 5.8mmol) was cooled in an ice bath, 2-bromoethanol (21.8g, 174.5mmol) was added dropwise, and the reaction mixture was reacted at 20°C for 24h. After treatment and purification, the crude product was recrystallized with a mixed solvent of ethyl acetate and n-hexane to obtain R-lipoic acid-2-bromoethyl ester, a light yellow solid (15.7g), with a yield of 86%. The reaction formula is:
[0039]
[0040](2) Preparation of R-lipoic acid choline ester bromide:
[0041] R-lipoic acid-2-bromoethyl ester (15.5g, 49.5mmol) and acetonitrile (200mL) were added to the reaction flask, stirred to dissolve, cooled in an ice bath, and trimethylamine aqueous solution (6.5mL, 16.9M, 109.9mmol) was added dropwise, The reaction mixture was ...
Embodiment 3
[0044] (1) Preparation of R-lipoic acid-2-iodoethyl ester:
[0045] Method A): Add R-lipoic acid (15.0g, 72.7mmol) and methyl tert-butyl ether (300mL) into the reaction flask, stir to dissolve, add N,N'-diisopropylcarbodiimide (11.9 g, 94.5mmol) and pyridine (1.7g, 21.8mmol), cooled in an ice bath, 2-iodoethanol (18.8g, 109.1mmol) was added dropwise, and the reaction mixture was reacted at 50°C for 12h. After treatment and purification, the crude product was recrystallized with a mixed solvent of ethyl acetate and n-hexane to obtain R-lipoic acid-2-iodoethyl ester, a light yellow solid (23.0g), with a yield of 88%, and the reaction formula was:
[0046]
[0047] (2) Preparation of R-lipoic acid choline ester iodide:
[0048] Add R-lipoic acid-2-iodoethyl ester (23.0g, 63.8mmol) and N,N-dimethylformamide (250mL) into the reaction flask, stir to dissolve, cool in an ice bath, add trimethylamine aqueous solution (6.0mL , 16.9M, 101.4mmol), the reaction mixture was reacted at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com