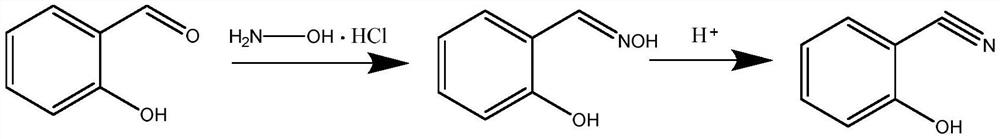
Method for preparing 2-hydroxy-benzonitril by adopting micro-flow field technology
A salicylonitrile and microfluidic field technology, applied in the field of organic compound preparation, can solve the problems of increased salicylaldehyde by-products, difficult to proceed in a forward direction, low reaction conversion rate, etc., and achieves less reaction impurities, shortened reaction time, Simple to use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation method of salicylonitrile comprises the steps:
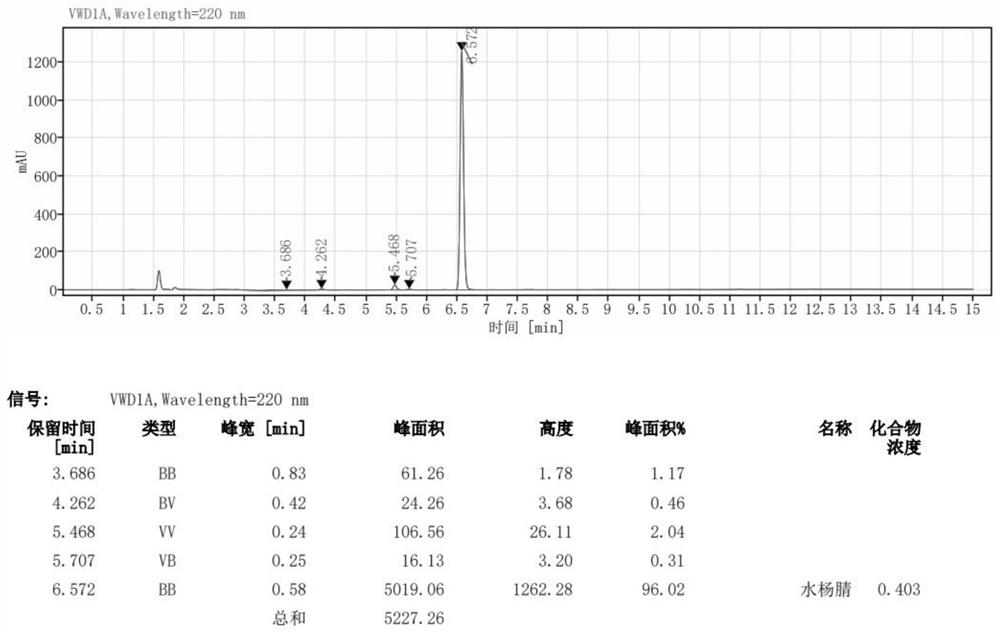
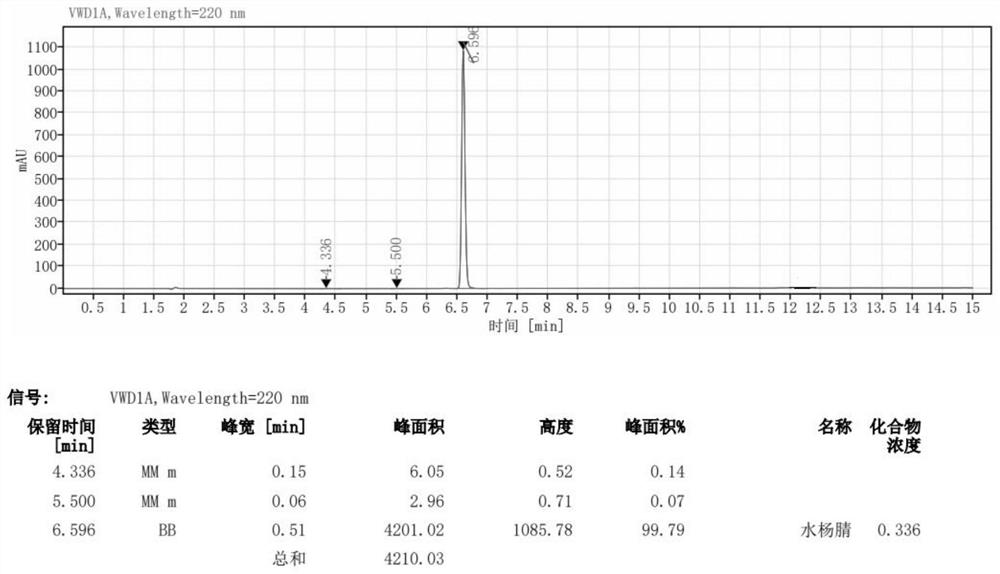
[0035] Use 100mL dimethyl sulfoxide (DMSO) to ultrasonically dissolve 20.0g (1.05eq) of hydroxylamine hydrochloride, add 33.47g (1.0eq) of salicylaldehyde after complete dissolution, and stir evenly. Single feed: pump the raw materials into the microchannel reactor, place the reactor in a constant temperature oil bath at 100°C, control the pumping speed so that the residence time is 10min, and adjust the pressure backup valve to make the system pressure 0.85MPa to make the system The fluid is kept flowing, the reactor outlet is sampled, washed with water, and extracted to obtain the crude salicylonitrile. Then recrystallized with xylene to obtain 31.14 g of white solid, with a yield of 95.38% and a purity of 99.79%.
Embodiment 2
[0037] The preparation method of salicylonitrile comprises the steps:
[0038] Use 250mL dimethyl sulfoxide (DMSO) to ultrasonically dissolve 50.0g (1.05eq) of hydroxylamine hydrochloride, add 83.68g (1.0eq) of salicylaldehyde after complete dissolution, and stir evenly. Single feed: pump the raw materials into the microchannel reactor, place the reactor in a constant temperature oil bath at 100°C, control the pumping speed so that the residence time is 10min, and adjust the pressure backup valve to make the system pressure 0.85MPa to make the system The fluid is kept flowing, the reactor outlet is sampled, washed with water, and extracted to obtain the crude salicylonitrile. Then recrystallized from xylene to obtain 77.45 g of white solid with a yield of 94.89% and a purity of 99.56%.
Embodiment 3
[0040] The preparation method of salicylonitrile comprises the steps:
[0041] Use 100mL dimethyl sulfoxide (DMSO) to ultrasonically dissolve 20.0g (1.05eq) of hydroxylamine hydrochloride, add 33.47g (1.0eq) of salicylaldehyde after complete dissolution, and stir evenly. Single-feed feed: pump the raw materials into the microchannel reactor, place the reactor in a 90°C constant temperature oil bath, control the pumping speed so that the residence time is 20min, and adjust the pressure backup valve to make the system pressure 0.65MPa to make the system The fluid is kept flowing, the reactor outlet is sampled, washed with water, and extracted to obtain the crude salicylonitrile. Then recrystallized from xylene to obtain 30.85 g of white solid, with a yield of 94.49% and a purity of 99.32%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com