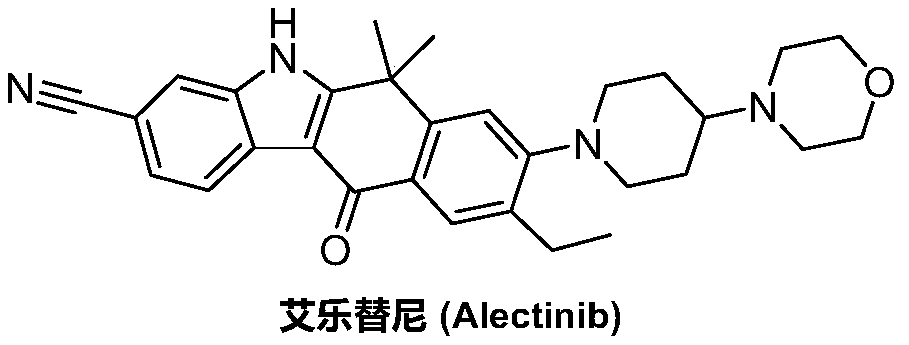
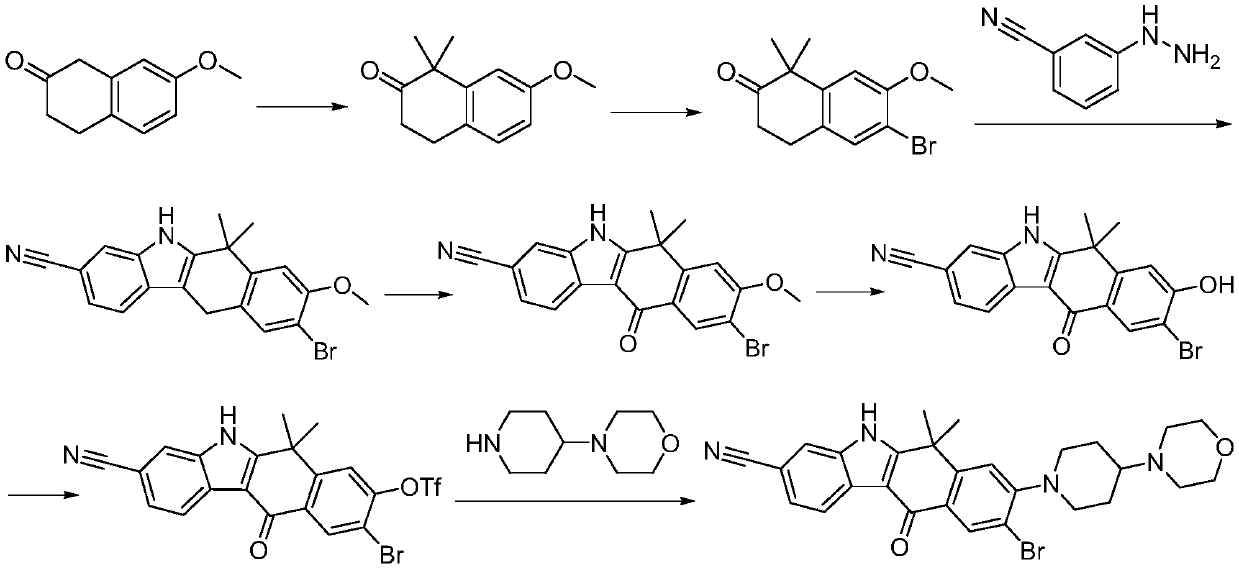
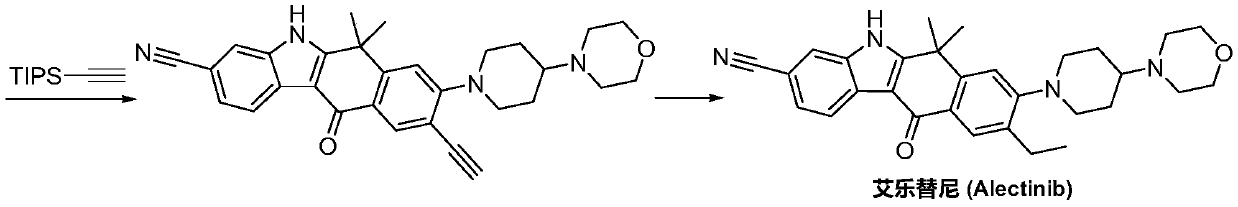
A kind of preparation method of alectinib intermediate
A technology for intermediates and tinib, which is applied in the field of preparation of alectinib intermediates, can solve the problems of short process flow, difficulty in obtaining, and low yield, and achieve the effects of reasonable technical scheme, simplified operation, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A) Preparation of 5-[(ethoxycarbonyl)methyl]-2-ethylphenyl trifluoromethanesulfonate:
[0042] 2-(4-ethyl-3-hydroxyphenyl) ethyl acetate (10.0g, 48.0mmol) was dissolved in triethylamine (9.7g, 95.9mmol), and trifluoromethanesulfonic anhydride (18.3g, 64.9mmol), stirred and reacted at 20°C for 2 hours. After post-treatment and purification, 5-[(ethoxycarbonyl)methyl]-2-ethylphenyl trifluoromethanesulfonate was obtained as a light yellow solid (15.8g ), the yield is 97%.
[0043] B) Preparation of ethyl 2-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}ethyl acetate:
[0044] 5-[(Ethoxycarbonyl)methyl]-2-ethylphenyl trifluoromethanesulfonate (15.0g, 44.1mmol) was dissolved in N,N-dimethylformamide (250mL), and 4- (4-piperidinyl)morpholine (16.9g, 99.3mmol), sodium methoxide (6.0g, 111.1mmol), the reaction mixture was stirred and reacted at 100°C for 12 hours, the reaction solution was cooled to room temperature, water (180mL) was added and cooled to Crystallize at 0°C for...
Embodiment 2
[0052] A) Preparation of 5-[(ethoxycarbonyl)methyl]-2-ethylphenyl trifluoromethanesulfonate:
[0053] Ethyl 2-(4-ethyl-3-hydroxyphenyl)acetate (12.0g, 57.6mmol) was dissolved in N,N-diisopropylethylamine (18.6g, 143.9mmol), and trifluoromethyl was slowly added dropwise Sulfonic acid anhydride (24.4g, 86.5mmol), stirred and reacted at 25°C for 1 hour, after post-treatment and purification, 5-[(ethoxycarbonyl)methyl]-2-ethylphenyl trifluoromethanesulfonate was obtained , Light yellow solid (18.6g), yield 95%.
[0054] B) Preparation of ethyl 2-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}ethyl acetate:
[0055] 5-[(Ethoxycarbonyl)methyl]-2-ethylphenyl trifluoromethanesulfonate (18.0g, 52.9mmol) was dissolved in N,N-dimethylformamide (350mL), and 4- (4-piperidinyl)morpholine (24.3g, 142.7mmol), sodium ethoxide (10.8g, 158.7mmol), the reaction mixture was stirred at 110°C for 6 hours, the reaction solution was cooled to room temperature, water (200mL) was added and cooled to Cr...
Embodiment 3
[0063] A) Preparation of 5-[(ethoxycarbonyl)methyl]-2-ethylphenyl trifluoromethanesulfonate:
[0064] 2-(4-Ethyl-3-hydroxyphenyl) ethyl acetate (2.0g, 9.6mmol) was dissolved in pyridine (1.2g, 15.2mmol), and trifluoromethanesulfonic anhydride (3.3g, 11.7mmol) was slowly added dropwise ), the reaction was stirred at 0°C for 4 hours, after post-treatment and purification, 5-[(ethoxycarbonyl)methyl]-2-ethylphenyl trifluoromethanesulfonate was obtained as a pale yellow solid (2.6g), The yield is 80%.
[0065] B) Preparation of ethyl 2-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}ethyl acetate:
[0066] 5-[(Ethoxycarbonyl)methyl]-2-ethylphenyl trifluoromethanesulfonate (2.5g, 7.3mmol) was dissolved in 1,4-dioxane (40mL), and 4-( 4-piperidinyl)morpholine (2.3g, 13.5mmol), sodium isopropoxide (1.2g, 14.6mmol), the reaction mixture was stirred at 90°C for 18 hours, the reaction solution was cooled to room temperature, water (30mL) was added and cooled Crystallize at 0°C for 3 hours...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com