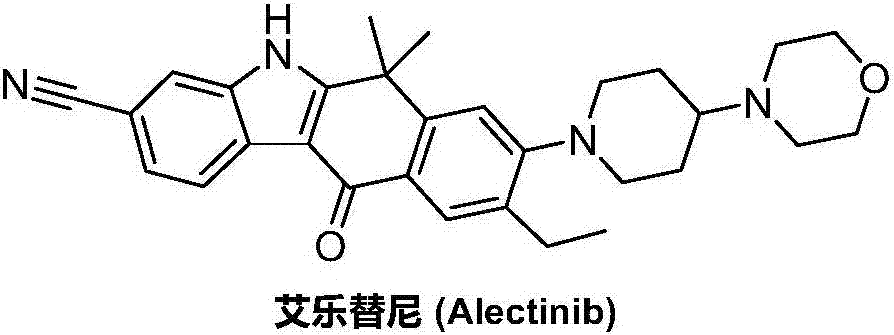
Preparation method of alectinib intermediate
A technology for intermediates and tinib, which is applied in the field of preparation of alectinib intermediates, can solve the problems of cumbersome operation, difficulty in obtaining, and low yield, and achieve the effects of reasonable technical scheme, simplified operation, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A) Preparation of 6-ethyl-7-bromo-3,4-dihydro-2-naphthone:
[0035] 6-Ethyl-3,4-dihydro-2-naphthalenone (4.5g, 25.9mmol) was dissolved in 1,2-dichloroethane (25mL), and N-bromosuccinimide (5.1 g, 28.7mmol), the reaction mixture was stirred and reacted at 30°C for 5 hours, TLC spotting confirmed the completion of the reaction, slowly added water (10mL), cooled to -10°C for crystallization for 3 hours, filtered, recrystallized from isopropanol, and obtained 6- Ethyl-7-bromo-3,4-dihydro-2-naphthone, off-white solid (6.3g), yield 95.5%.
[0036] B) Preparation of 6-ethyl-7-[4-(morpholin-4-yl)piperidin-1-yl]-3,4-dihydro-2-naphthone:
[0037] 6-Ethyl-7-bromo-3,4-dihydro-2-naphthone (6.0g, 23.7mmol) was dissolved in 1,4-dioxane (25mL), and 4-(4-piperidinyl ) morpholine (7.3g, 42.7mmol), sodium isopropoxide (4.3g, 52.2mmol), the reaction mixture was stirred and reacted at 90°C for 12 hours, TLC spot plate confirmed that the reaction was complete, the reaction solution was coo...
Embodiment 2
[0041] A) Preparation of 6-ethyl-7-bromo-3,4-dihydro-2-naphthone:
[0042]6-Ethyl-3,4-dihydro-2-naphthalenone (4.5g, 25.8mmol) was dissolved in tetrahydrofuran (25mL), and N-bromosuccinimide (5.4g, 30.3mmol) was added slowly, and the reaction The mixture was stirred and reacted at 35°C for 2 hours. After the reaction was confirmed by TLC spotting, water (15 mL) was slowly added, cooled to -10°C for crystallization for 5 hours, filtered, and recrystallized from isopropanol to obtain 6-ethyl-7-bromo- 3,4-Dihydro-2-naphthone, off-white solid (6.4g), yield 98%.
[0043] B) Preparation of 6-ethyl-7-[4-(morpholin-4-yl)piperidin-1-yl]-3,4-dihydro-2-naphthone:
[0044] 6-Ethyl-7-bromo-3,4-dihydro-2-naphthone (6.0g, 23.7mmol) was dissolved in 1,4-dioxane (25mL), and 4-(4-piperidinyl ) morpholine (7.3g, 42.9mmol), sodium isopropoxide (4.3g, 52.4mmol), the reaction mixture was stirred and reacted at 95°C for 18 hours, TLC spot plate confirmed that the reaction was complete, the reactio...
Embodiment 3
[0048] A) Preparation of 6-ethyl-7-bromo-3,4-dihydro-2-naphthone:
[0049] 6-Ethyl-3,4-dihydro-2-naphthalenone (4.5g, 25.8mmol) was dissolved in toluene (20mL), bromine water (4.4g, 27.5mmol) was slowly added, and the reaction mixture was stirred at 20°C for 6 hours , TLC spot plate confirmed that the reaction was complete, slowly added water (15mL), cooled to -10 ° C for 4 hours, filtered, and recrystallized from isopropanol to obtain 6-ethyl-7-bromo-3,4-dihydro- 2-Naphthone, off-white solid (6.5g), yield 99%.
[0050] B) Preparation of 6-ethyl-7-[4-(morpholin-4-yl)piperidin-1-yl]-3,4-dihydro-2-naphthone:
[0051] 6-Ethyl-7-bromo-3,4-dihydro-2-naphthone (6.0g, 23.7mmol) was dissolved in N,N-dimethylacetamide (25mL), and 4-(4-piperidine Base) morpholine (10.8g, 63.4mmol), sodium ethoxide (4.8g, 70.5mmol), the reaction mixture was stirred and reacted at 110°C for 8 hours, TLC was spotted to confirm that the reaction was complete, the reaction solution was cooled to room tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com