Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
149results about How to "Maximize contact" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
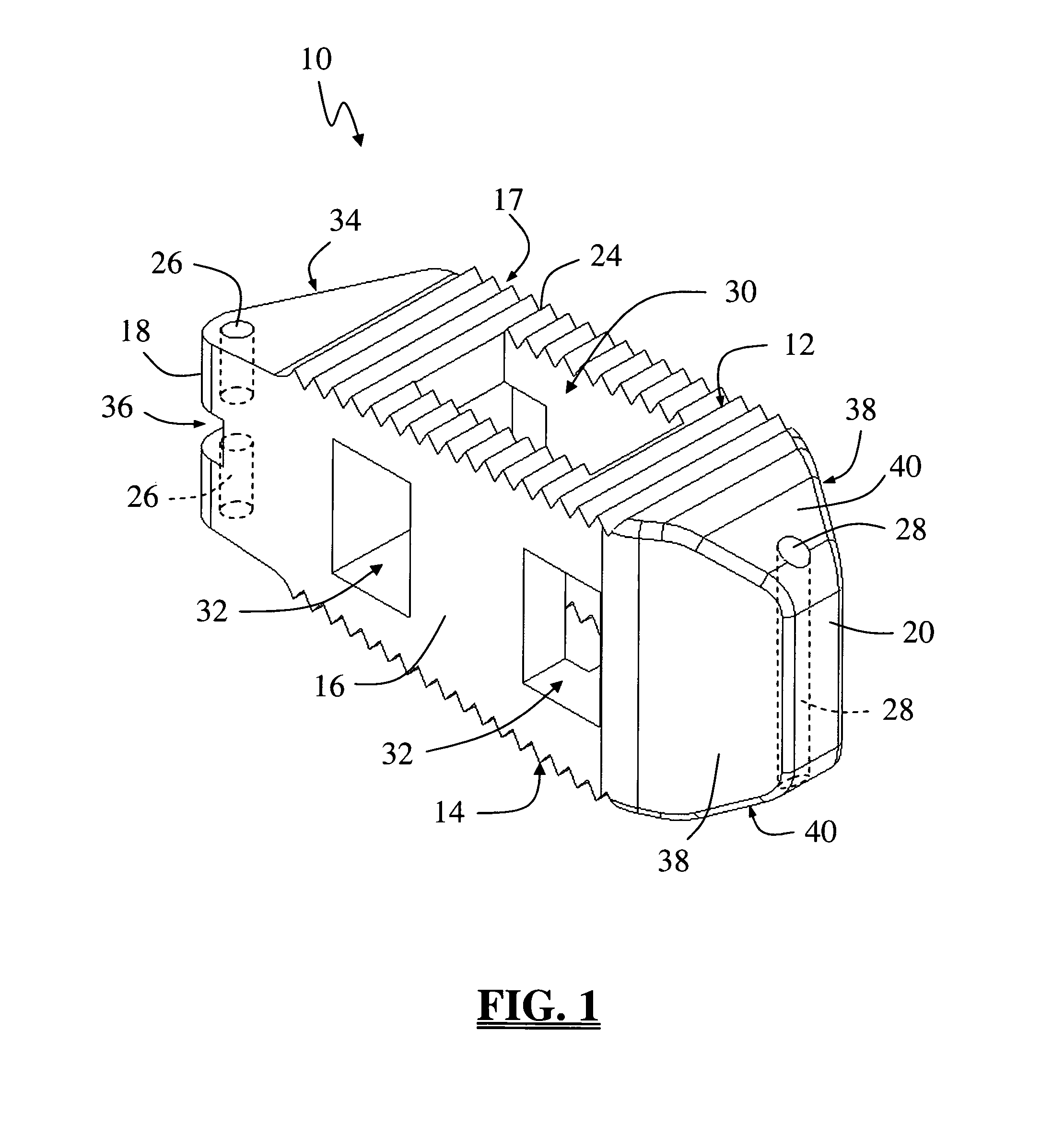
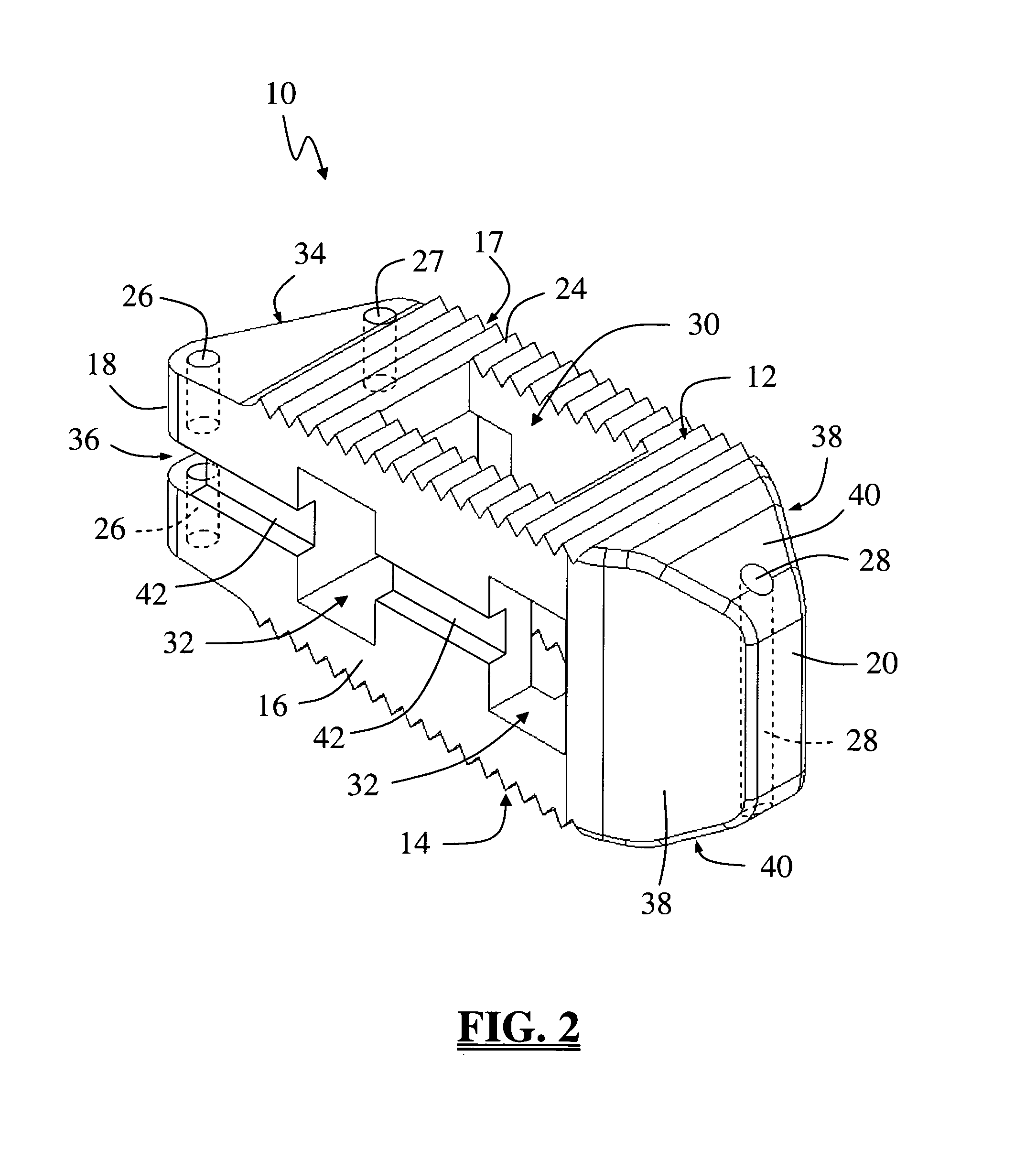
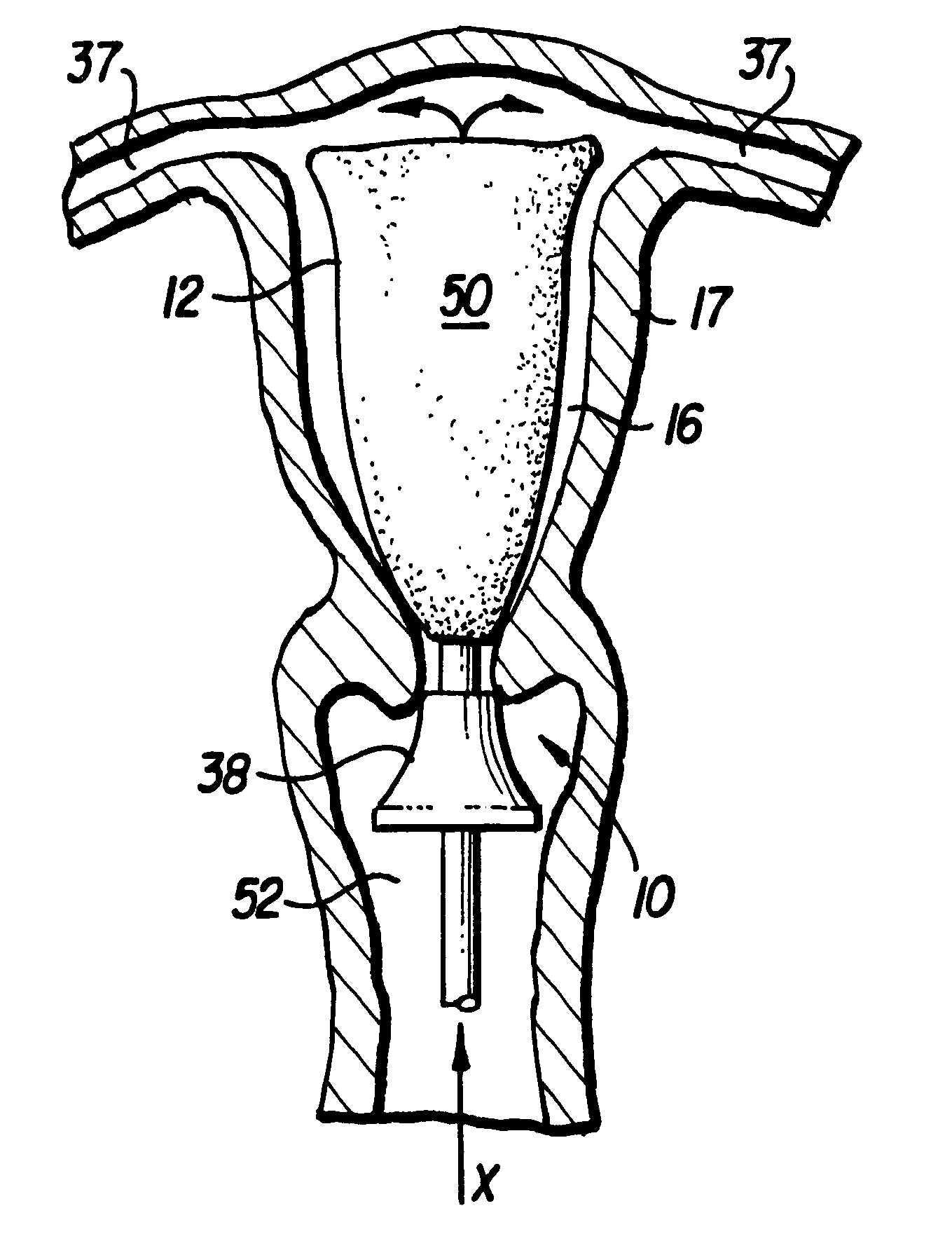
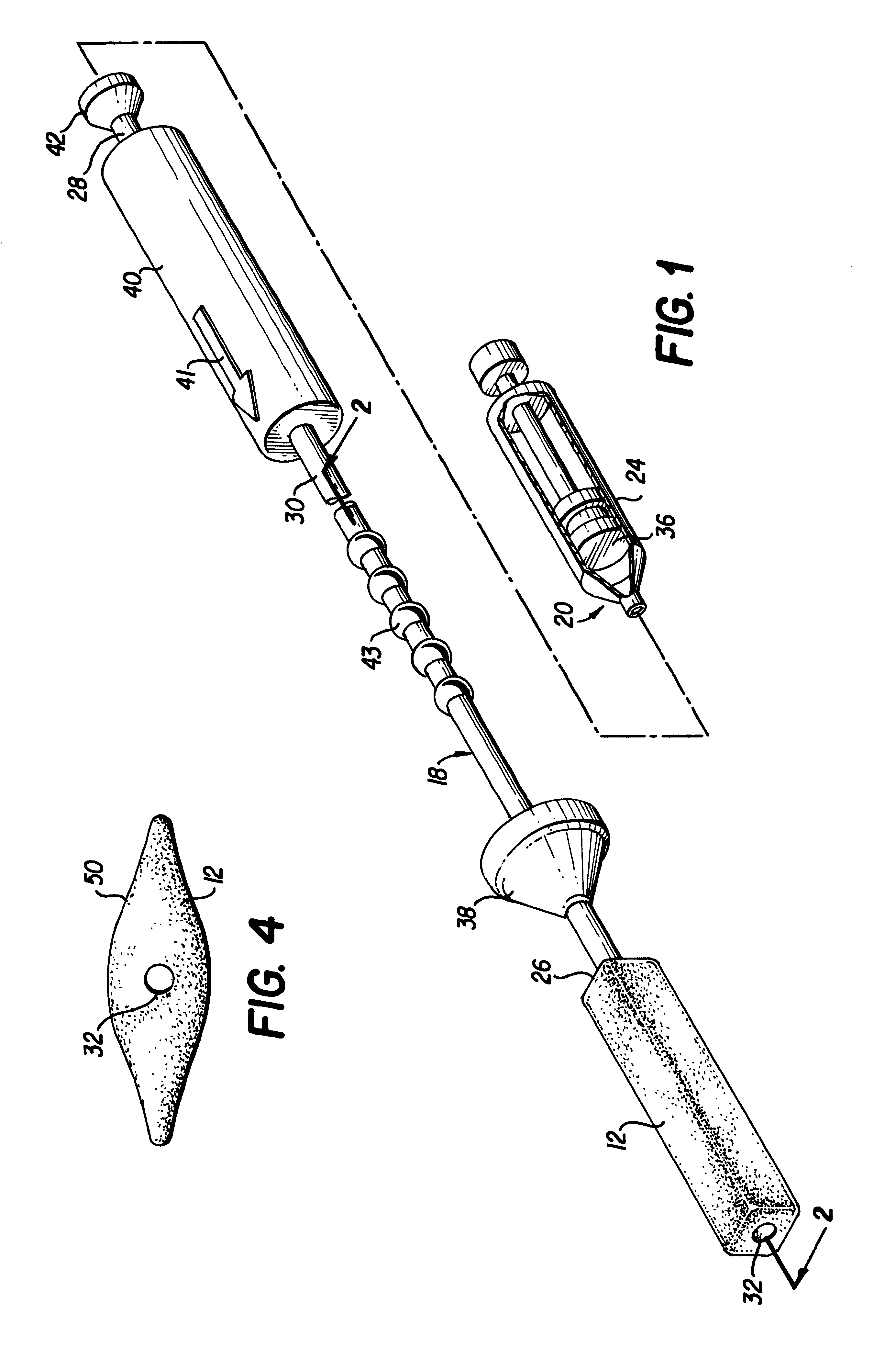
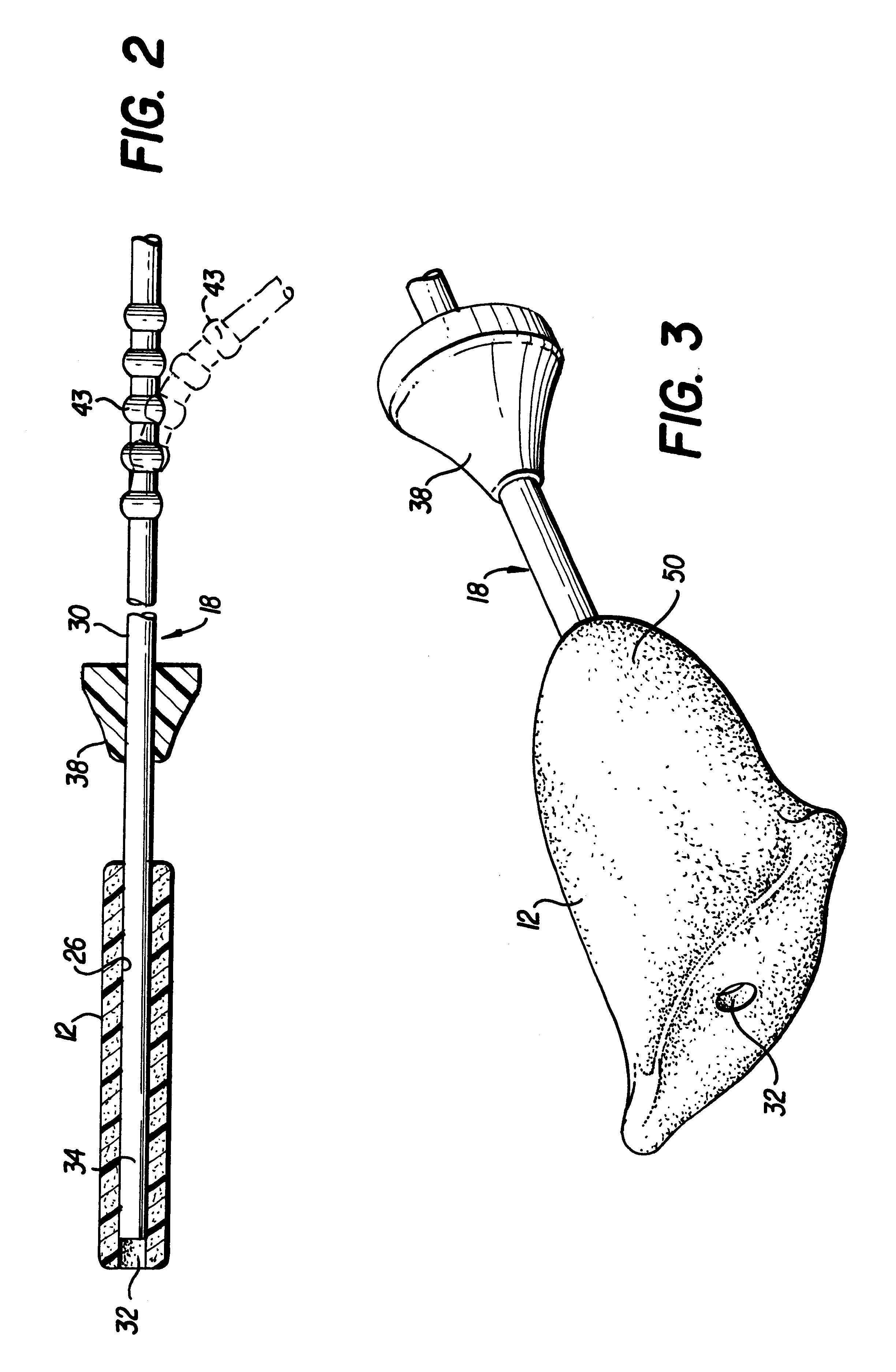
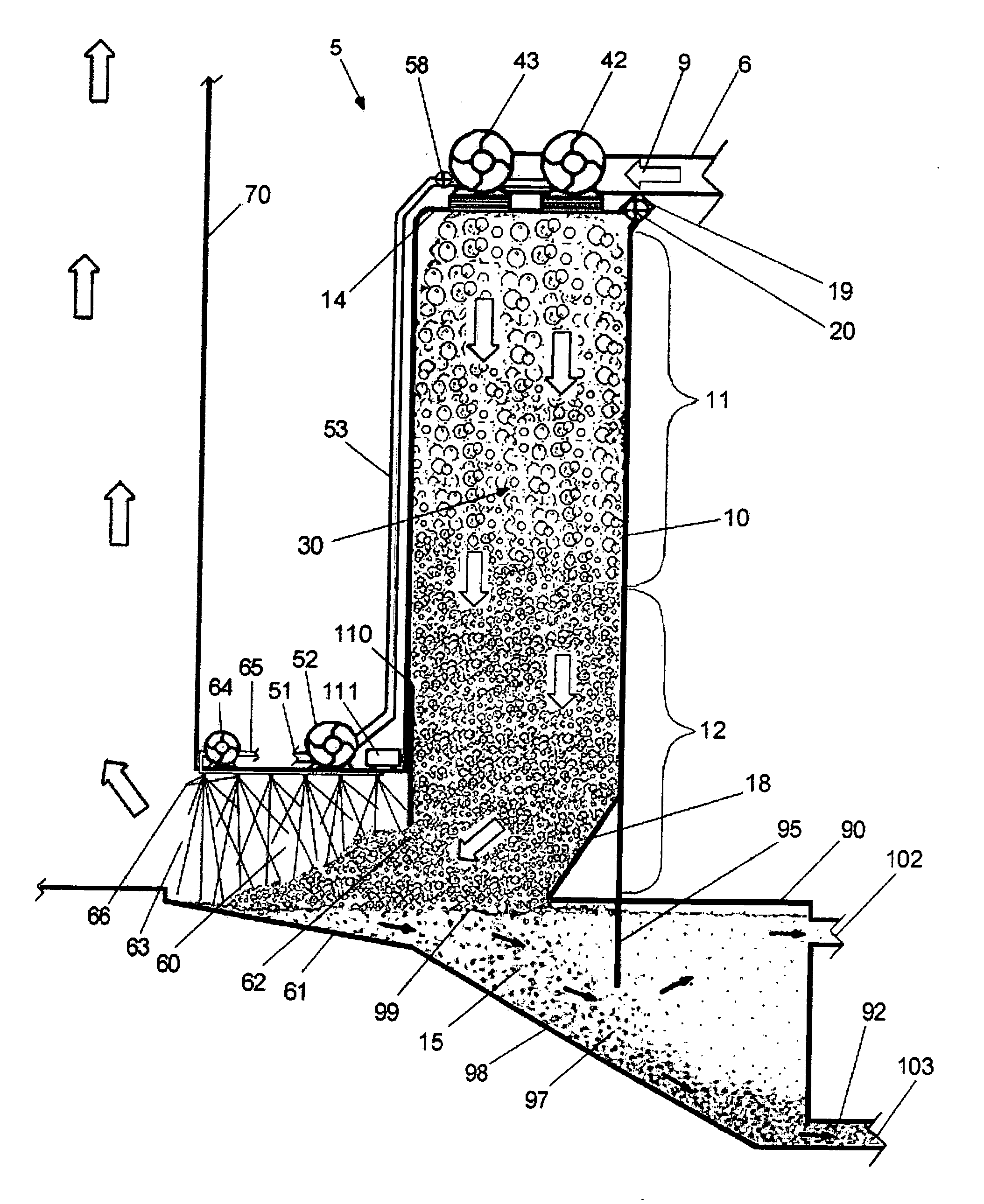
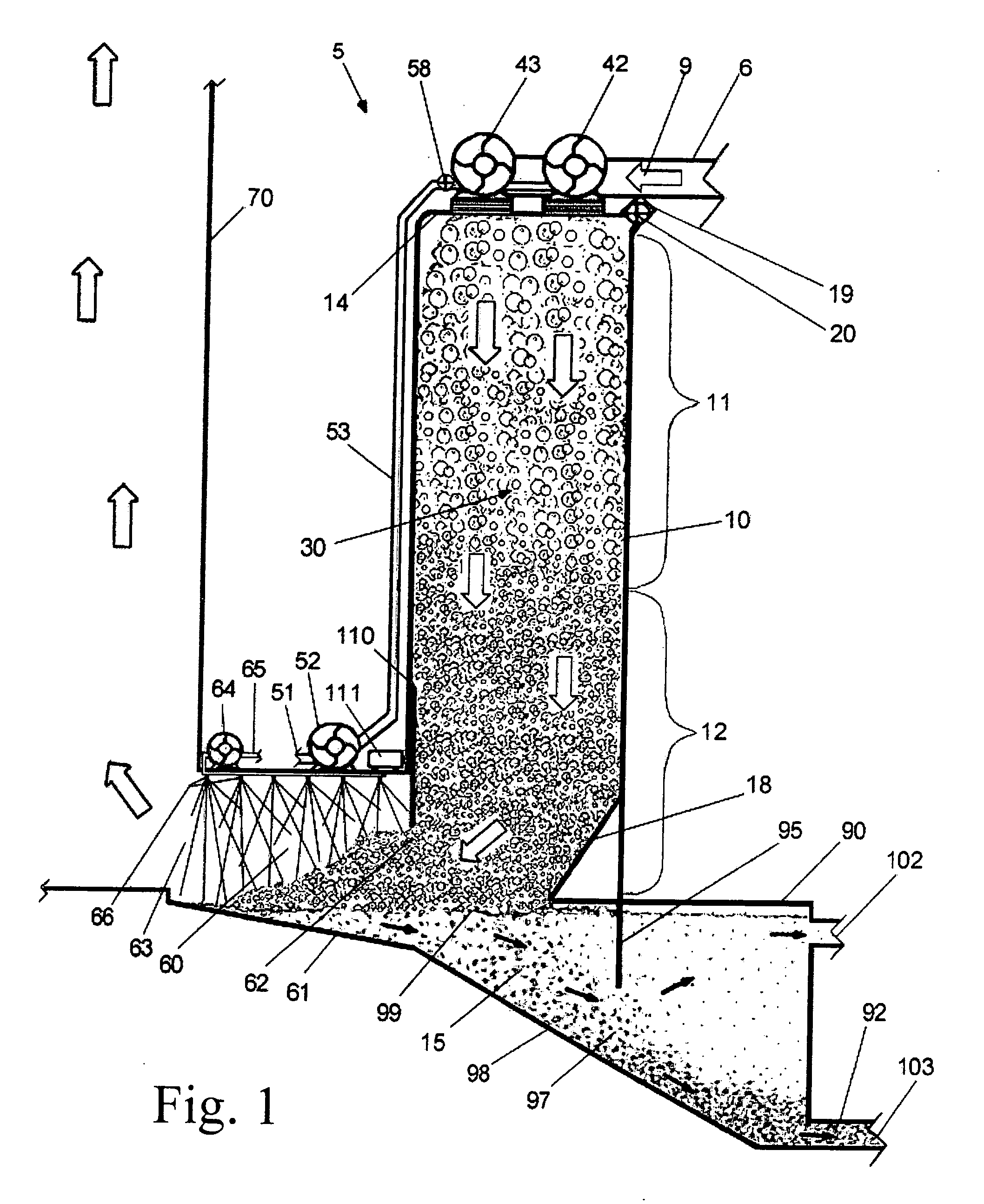
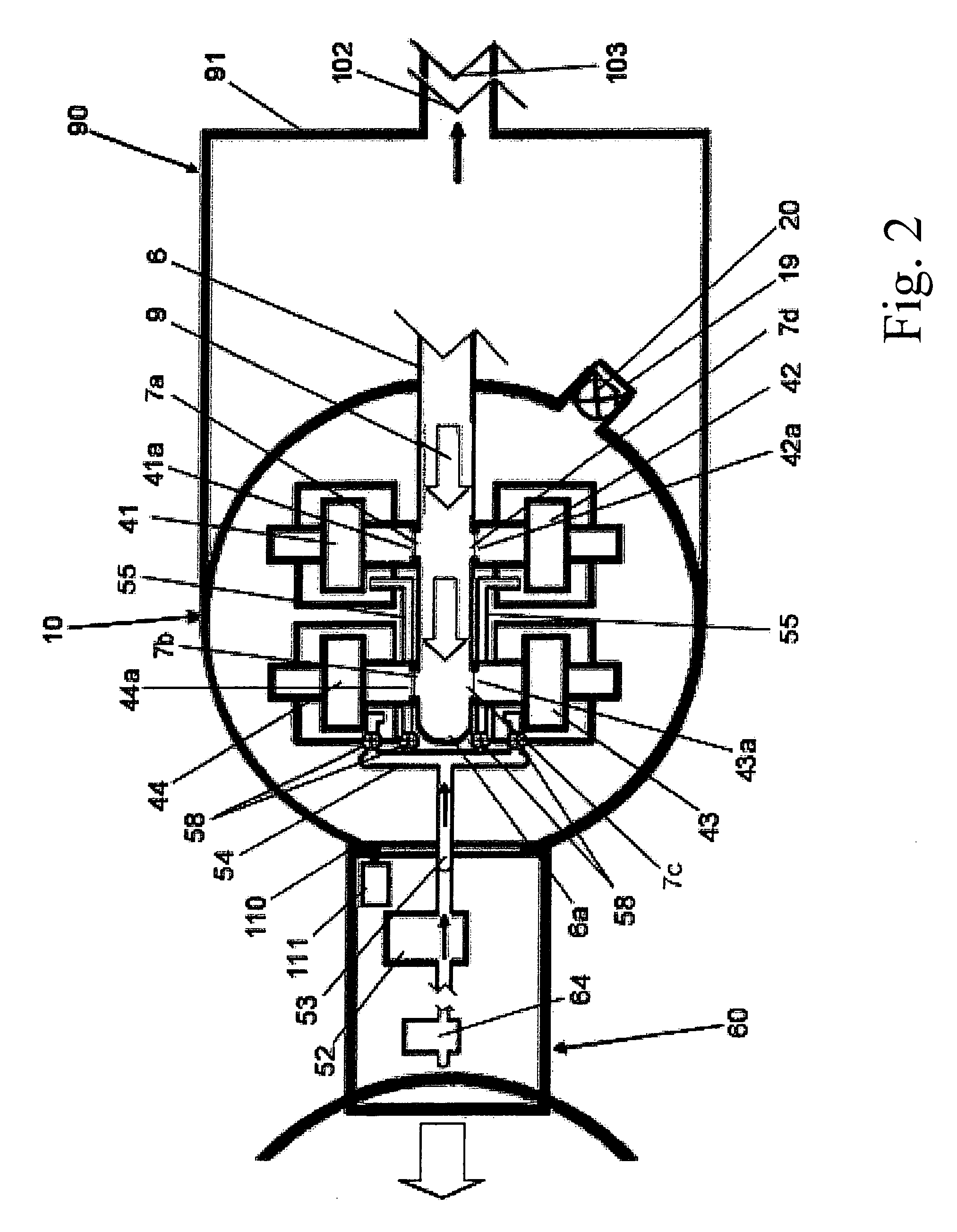
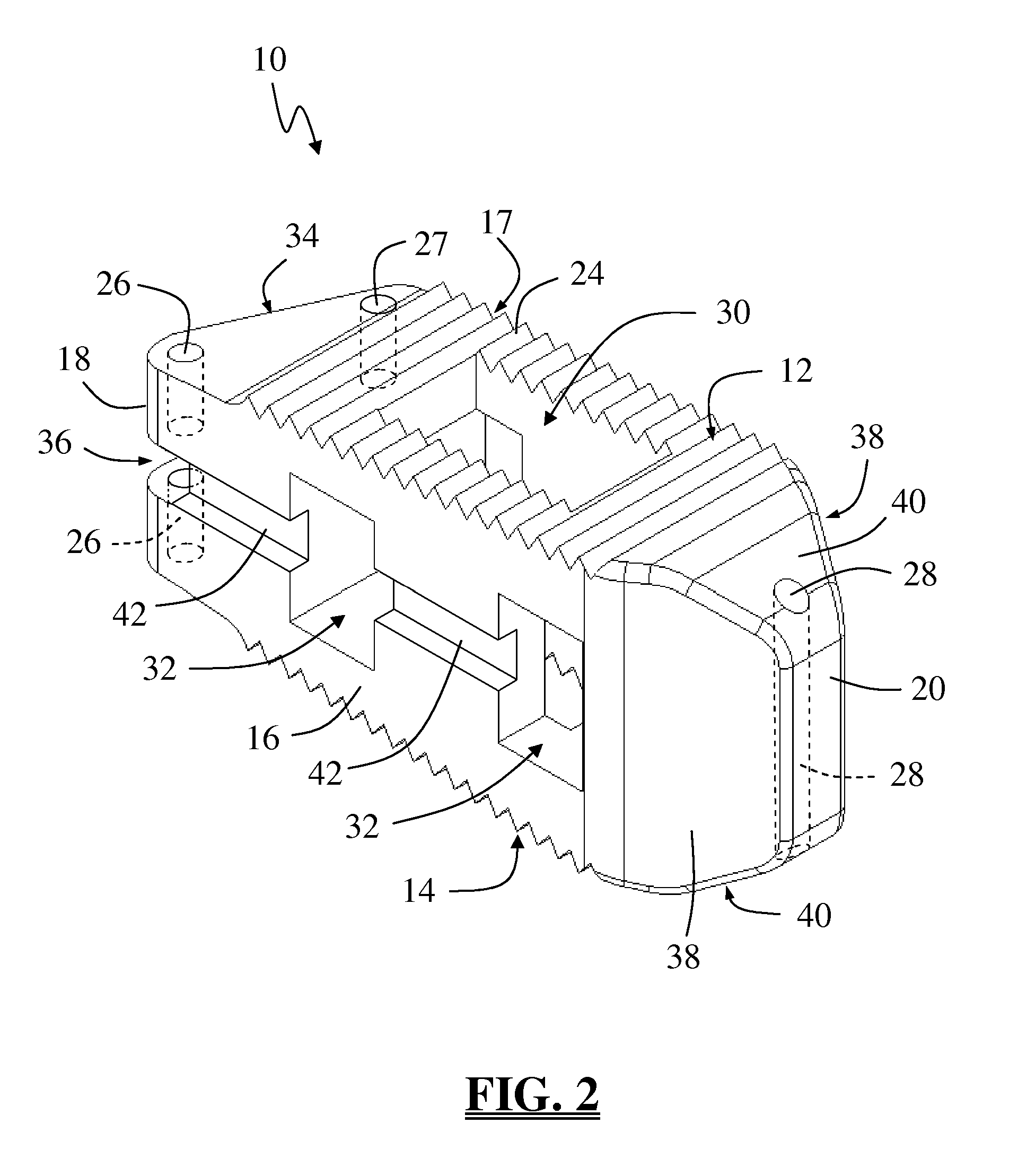
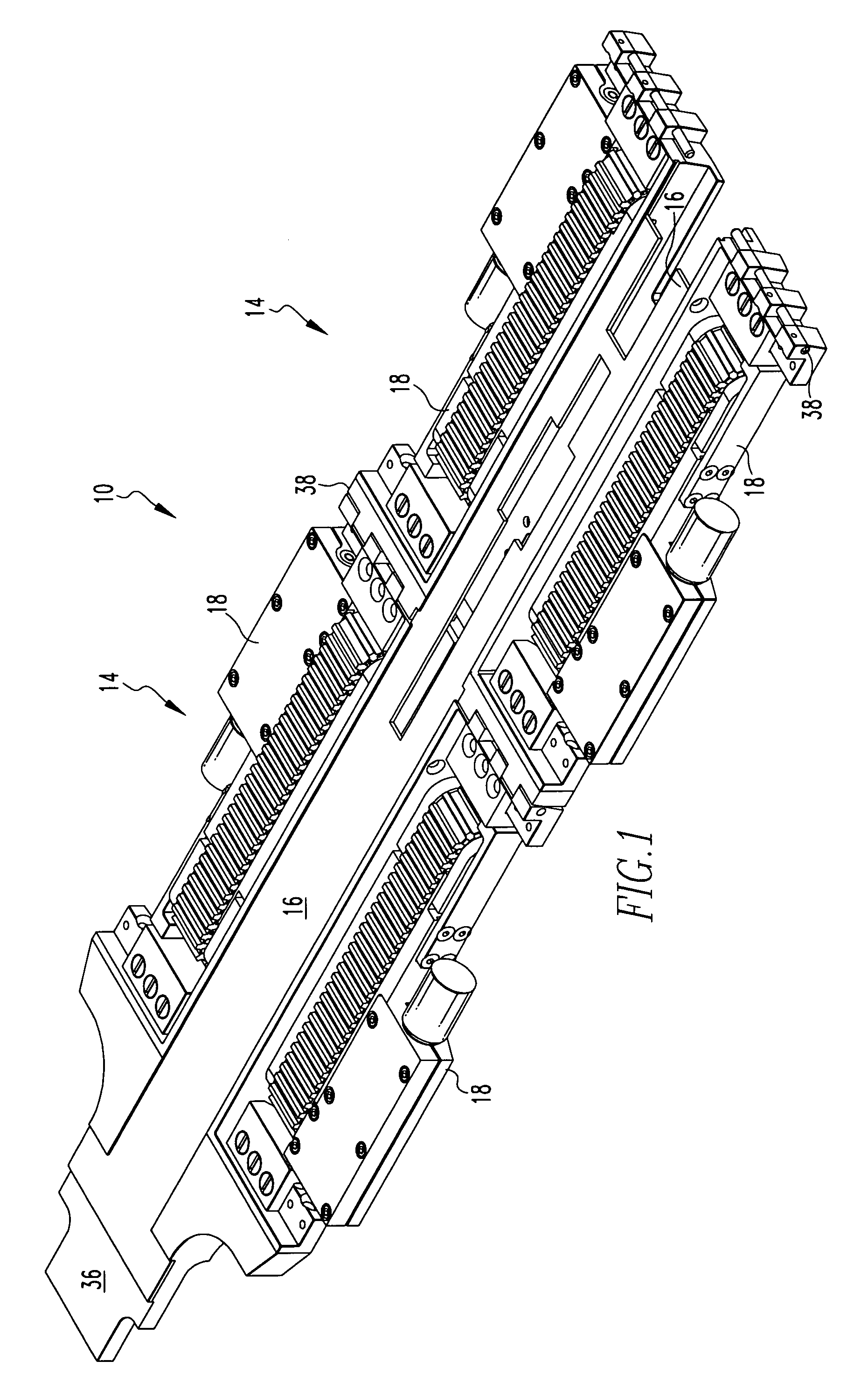
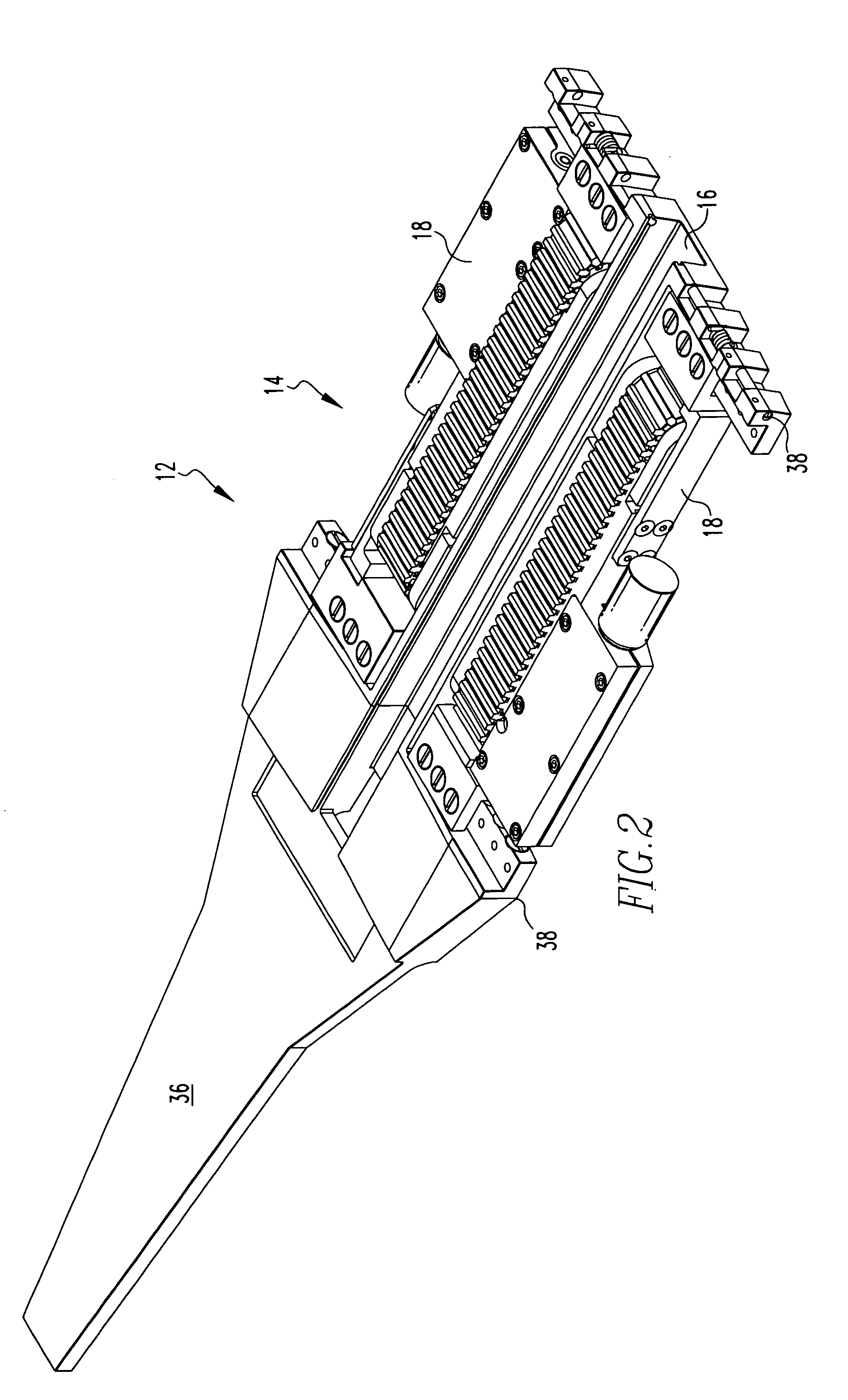
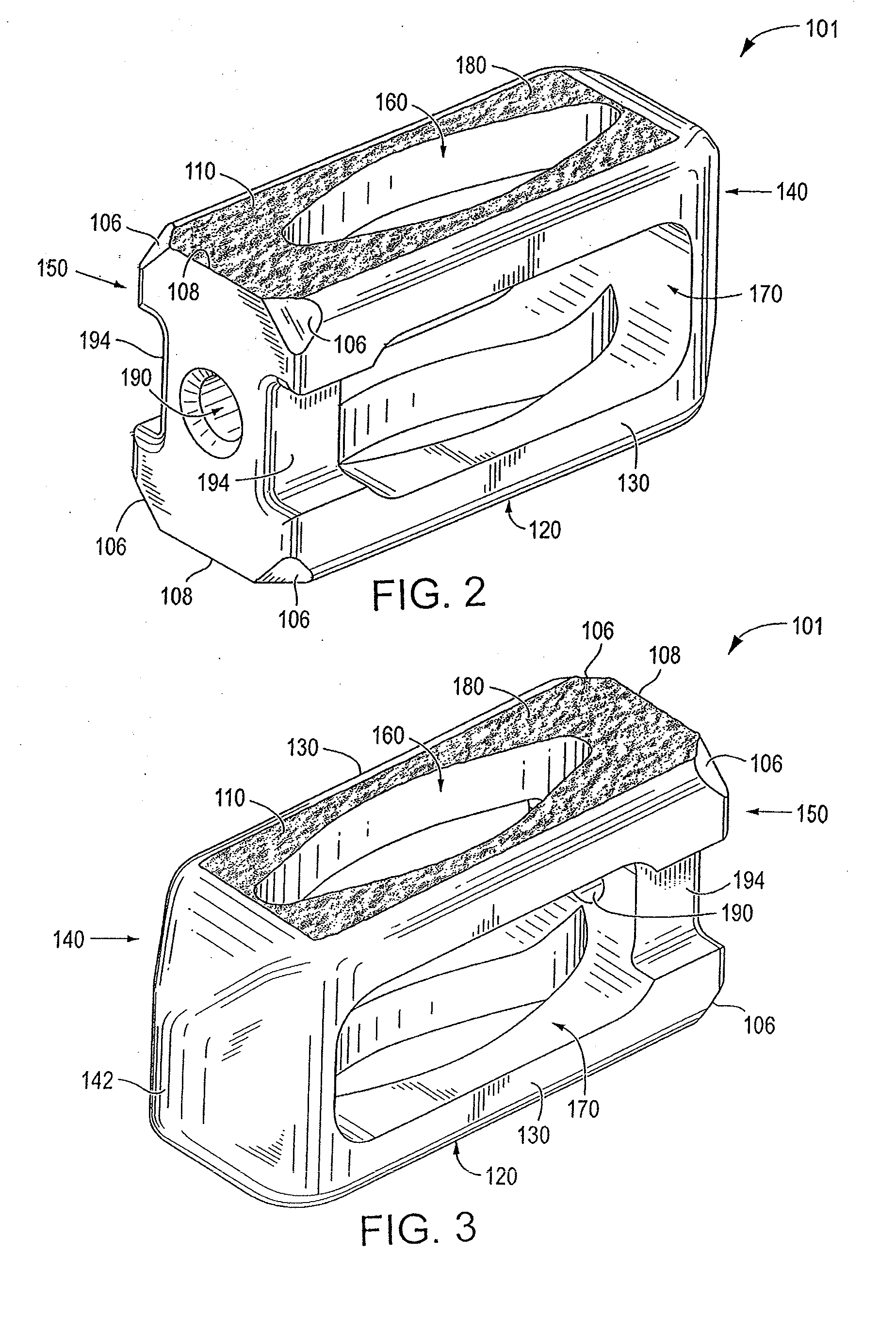
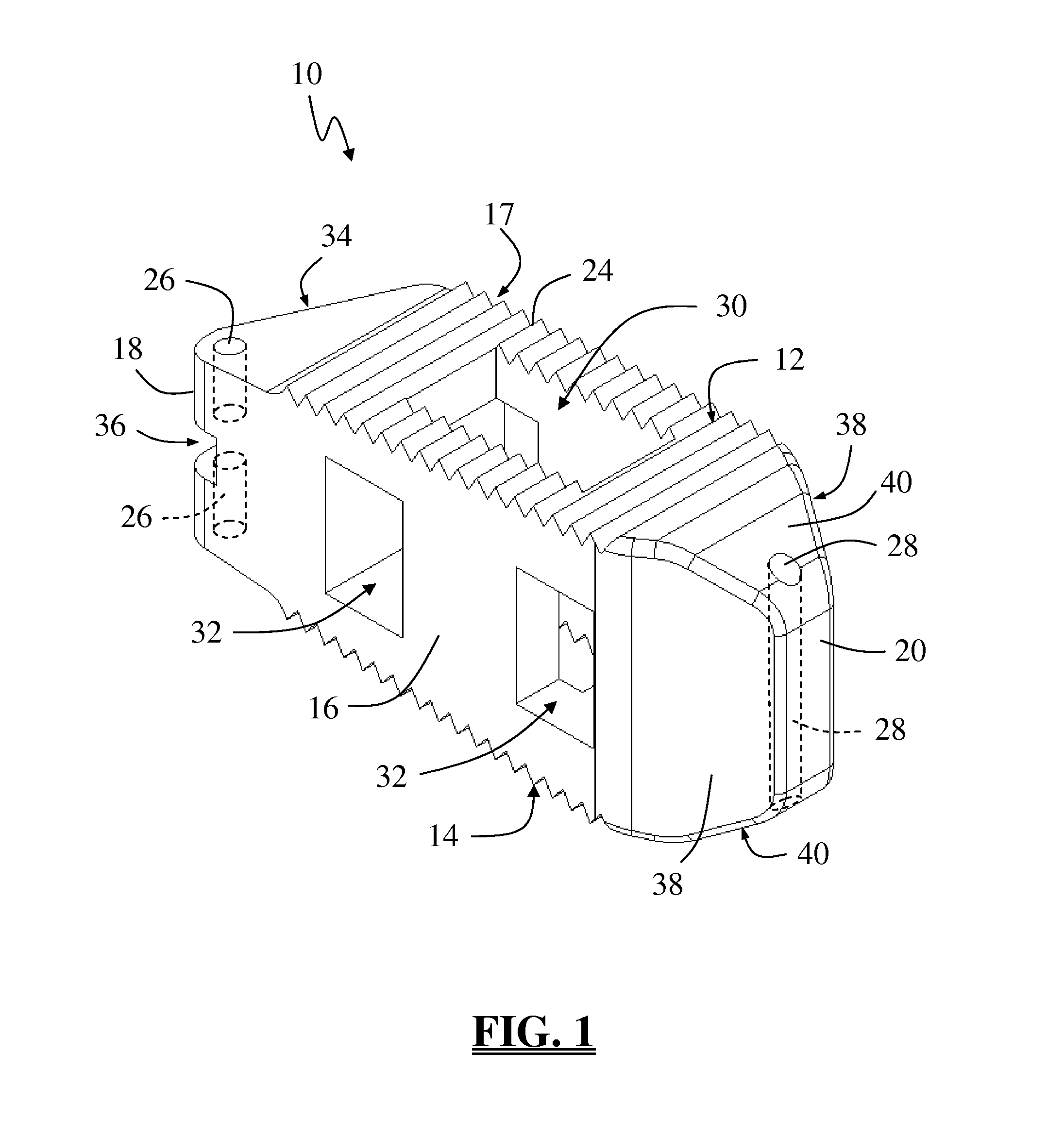
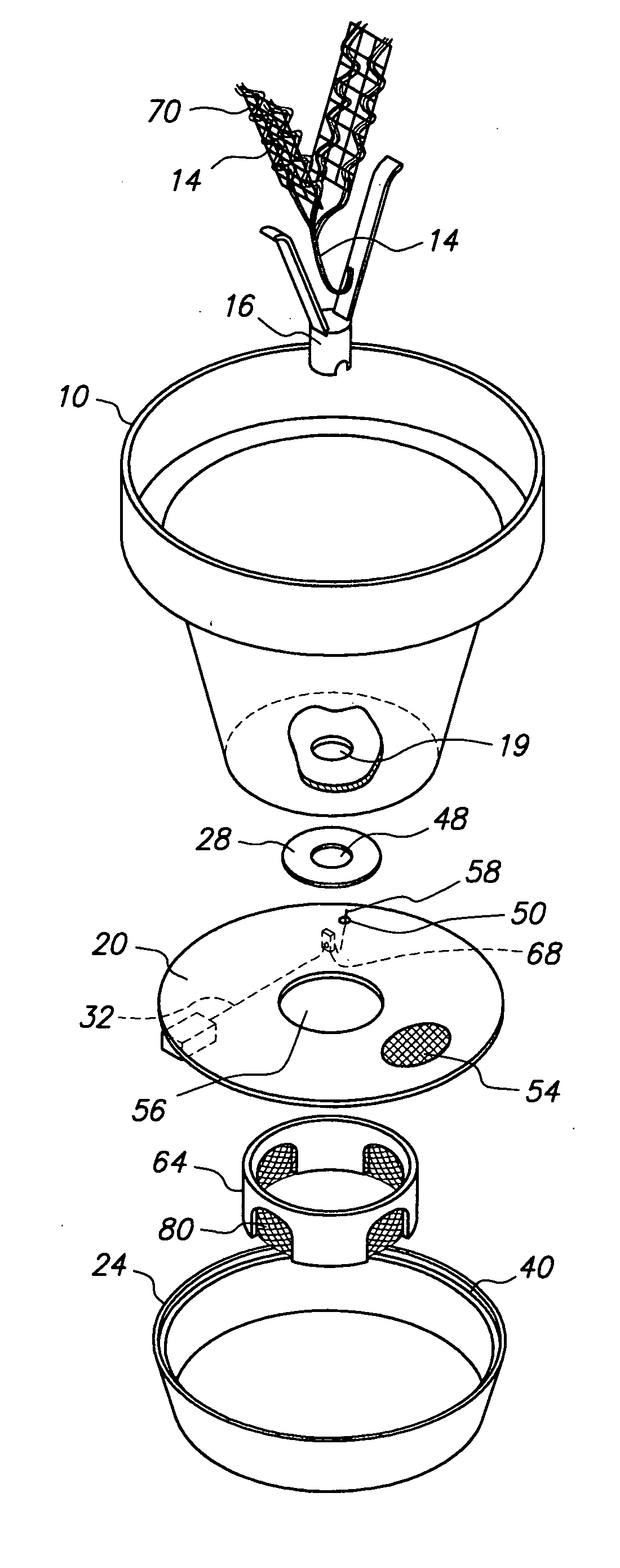
Spinal fusion implant and related methods
ActiveUS7867277B1Increase contactAvoids dural impingementBone implantJoint implantsIntervertebral discMedicine
A spinal fusion implant of non-bone construction to be introduced into any variety of spinal target sites. The spinal fusion implant of the present invention includes a top surface, a bottom surface, first and second lateral sides, a proximal (posterior) end and a distal (anterior) end. The spinal fusion implant of the present invention may be used to provide temporary or permanent fixation within an orthopedic target site. To do so, the spinal fusion implant may be introduced into a disc space while locked to a surgical insertion instrument and thereafter employed in the proper orientation and released. Once deposited in the disc space, the spinal fusion implant of the present invention effects spinal fusion over time as the natural healing process integrates and binds the implant.
Owner:NUVASIVE
Apparatus and method for delivering and deploying an expandable body member in a uterine cavity
InactiveUS6395012B1Maximize contactSufficient pressureStentsBalloon catheterEndometrial layerGynecology
An apparatus and method delivers an expandable body member into a uterine cavity of a uterus through a cervical opening and deploys the expandable body member in the uterine cavity. The expandable body member in a compressed state is inserted into the uterine cavity. A fluid is provided to the uterine cavity at a pressure sufficient to inflate the uterine cavity. The expandable body member expands from the compressed state to the expanded state. After expansion of the expandable body member and inflation of the uterine cavity, the fluid pressure is relieved thereby collapsing the uterine cavity about the expandable body member in the expanded state such that an endometrial layer of the uterus and a surface of the expandable body member substantially contact each other in a facially opposing relationship maximizing contact therebetween. The apparatus which performs the method includes an elongated tubular member having the expandable body member connected to a distal portion of the elongated tubular member and a fluid-providing device connected to a proximal portion of the elongated tubular member.
Owner:YOON INBAE +1
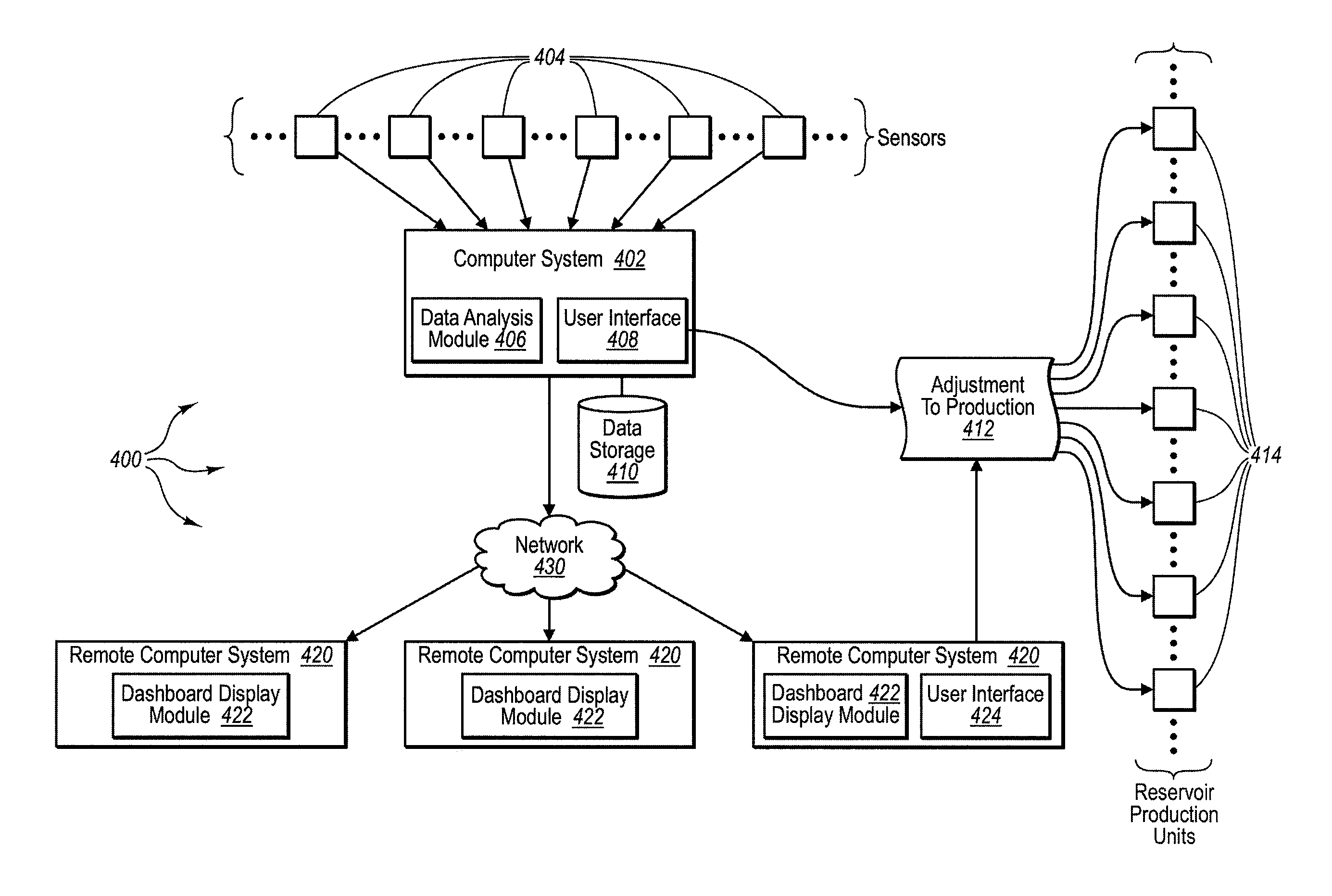
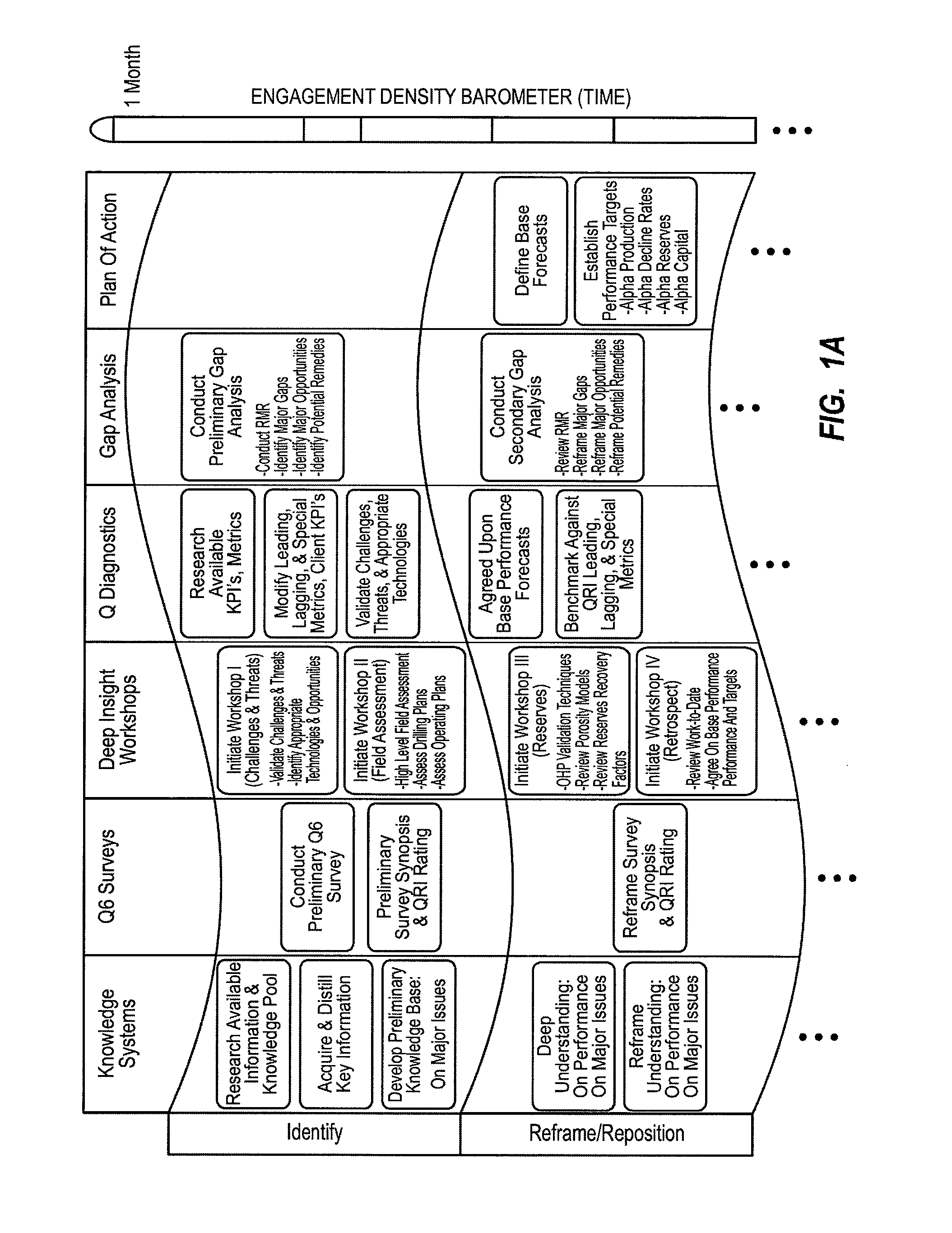
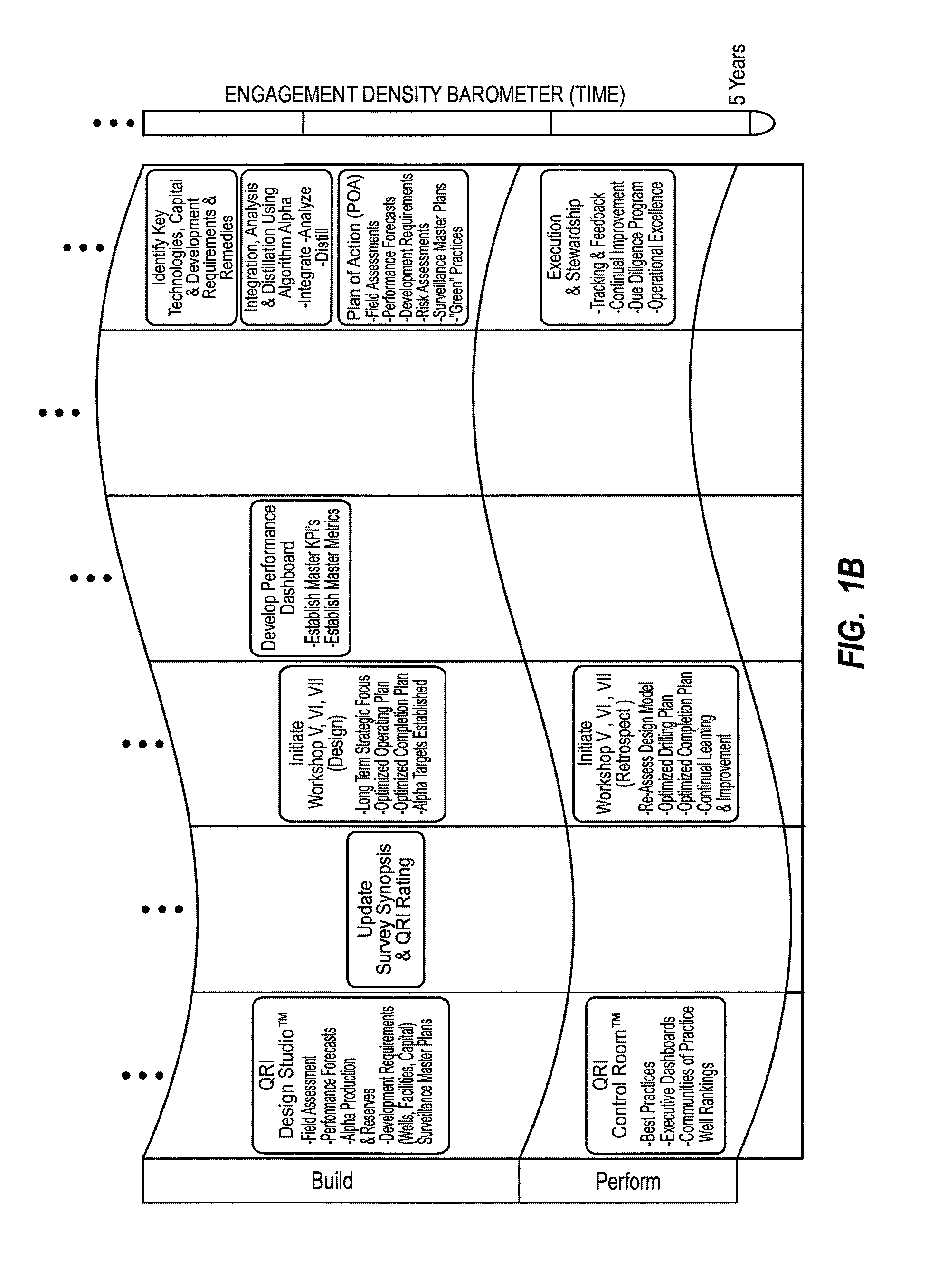
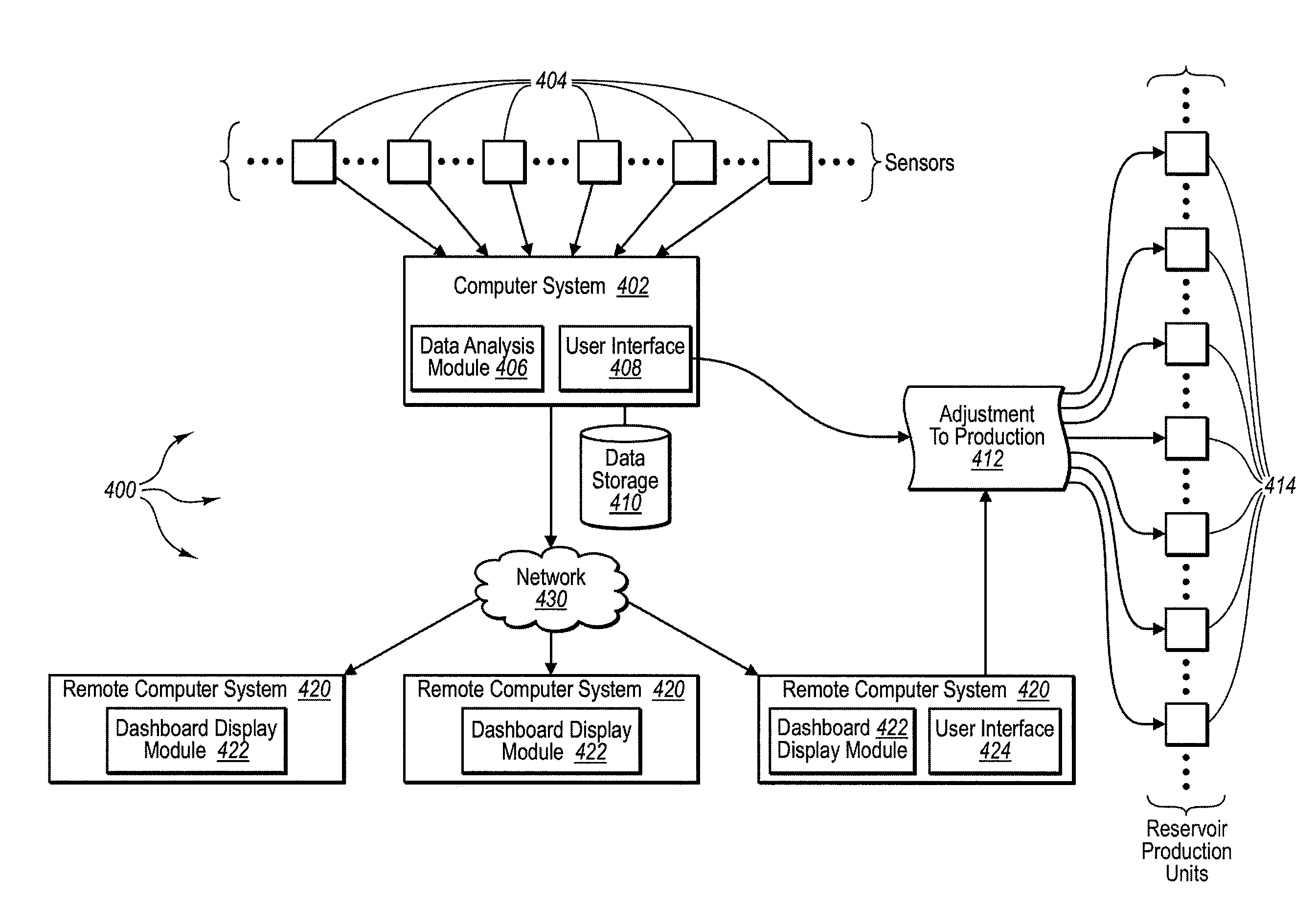
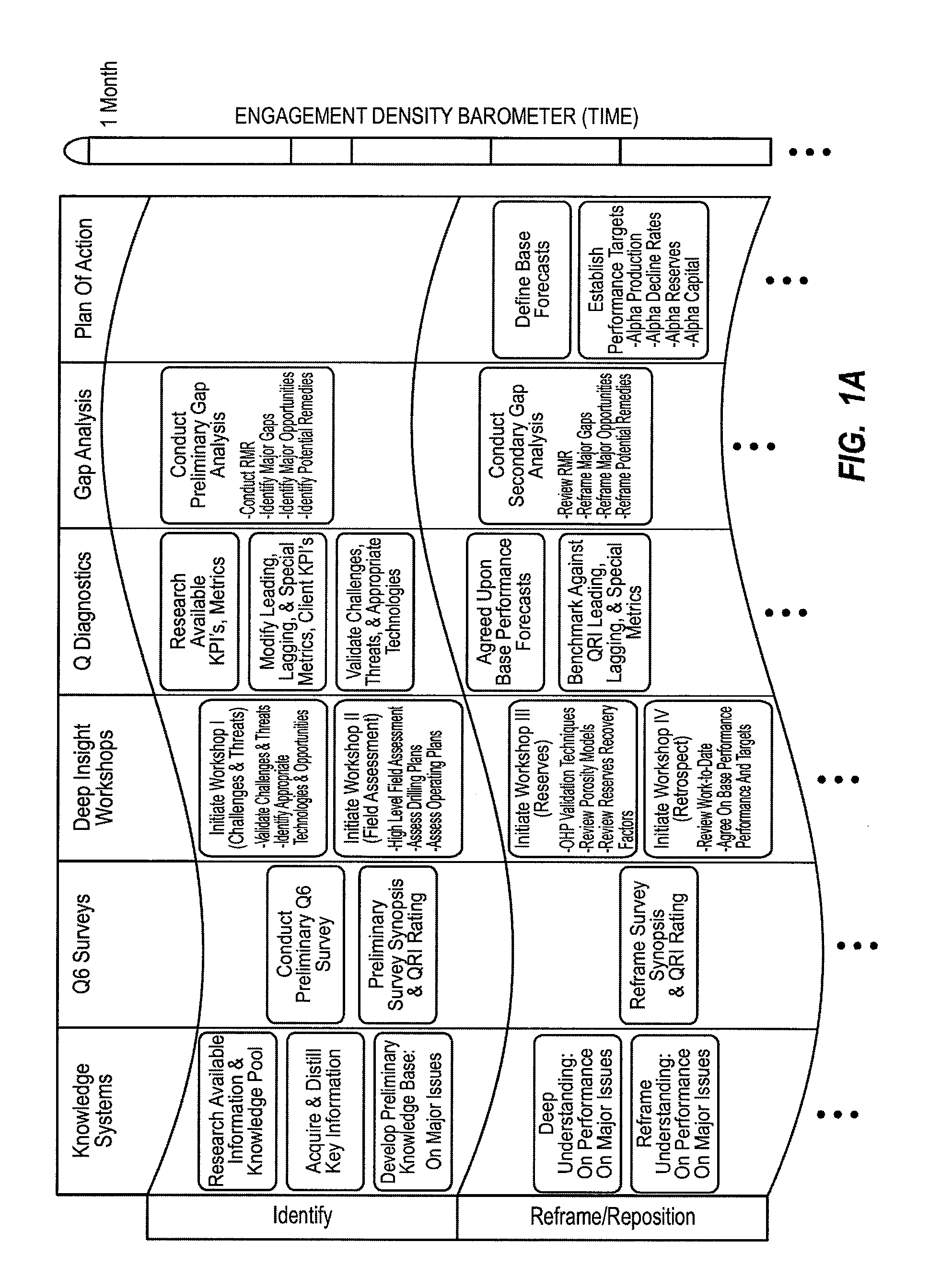
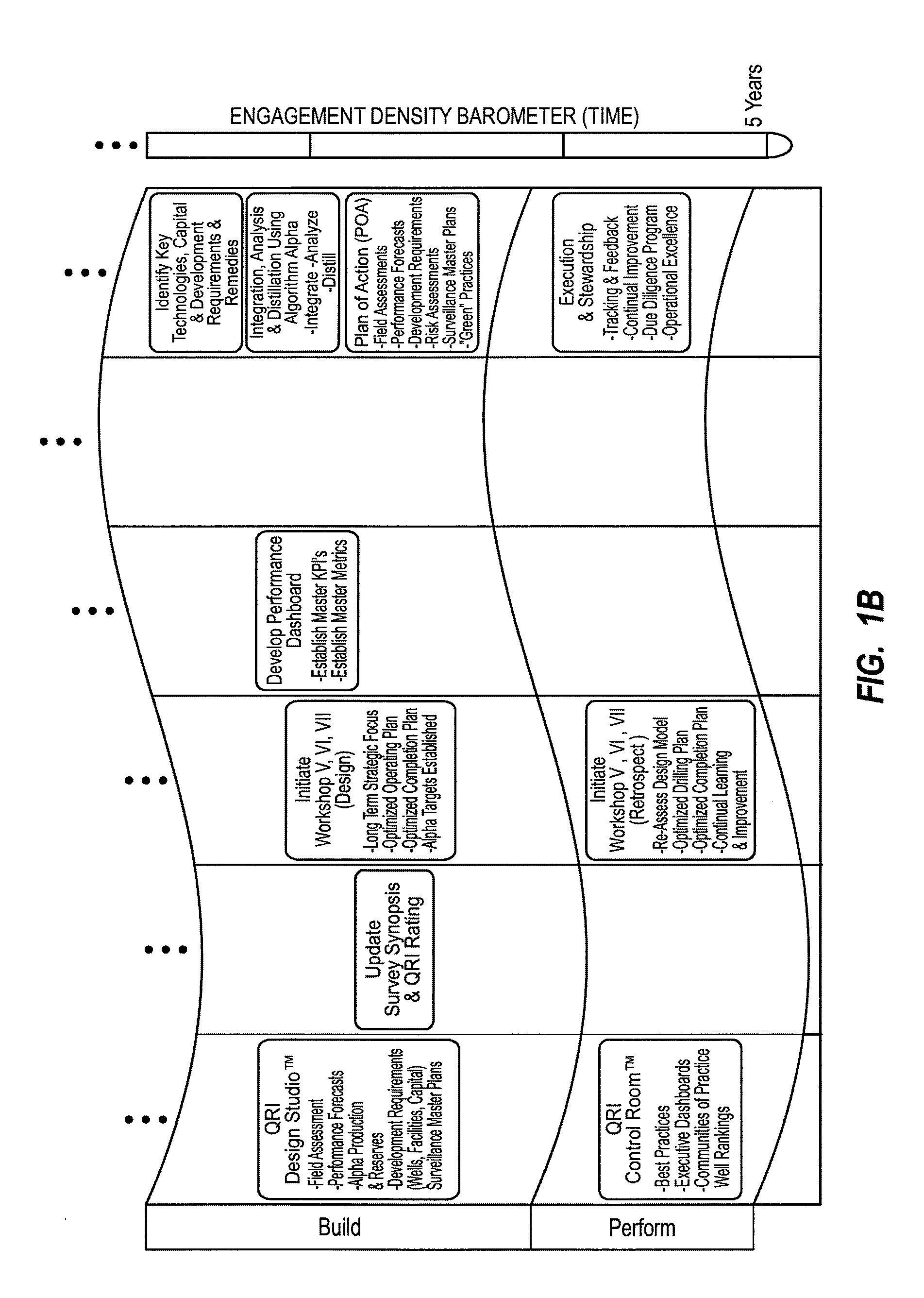
Method for dynamically assessing petroleum reservoir competency and increasing production and recovery through asymmetric analysis of performance metrics
InactiveUS7963327B1Increase short-term production rate and long-term recoveryAccurate assessmentElectric/magnetic detection for well-loggingSurveyProcess engineeringPetroleum reservoir
Methods for accurately assessing the condition of a petroleum reservoir and designing and implementing a plan of action to increase production and recovery of petroleum from the reservoir. Information is gathered using a unique set of metrics and information gathering techniques and analyzed in a targeted fashion by properly weighting the data in the context of the particular reservoir and goals of the producer. A reservoir rating is generated using asymmetric analysis of metrics and then used to formulate a plan of action. Production architecture (e.g., number, location and manner of constructing oil and injector wells) is then constructed according to the plan of action. Reservoir performance can be continuously monitored and used to verify production and recovery goals and / or provide triggers or alarms to alter production equipment.
Owner:QRI GROUP
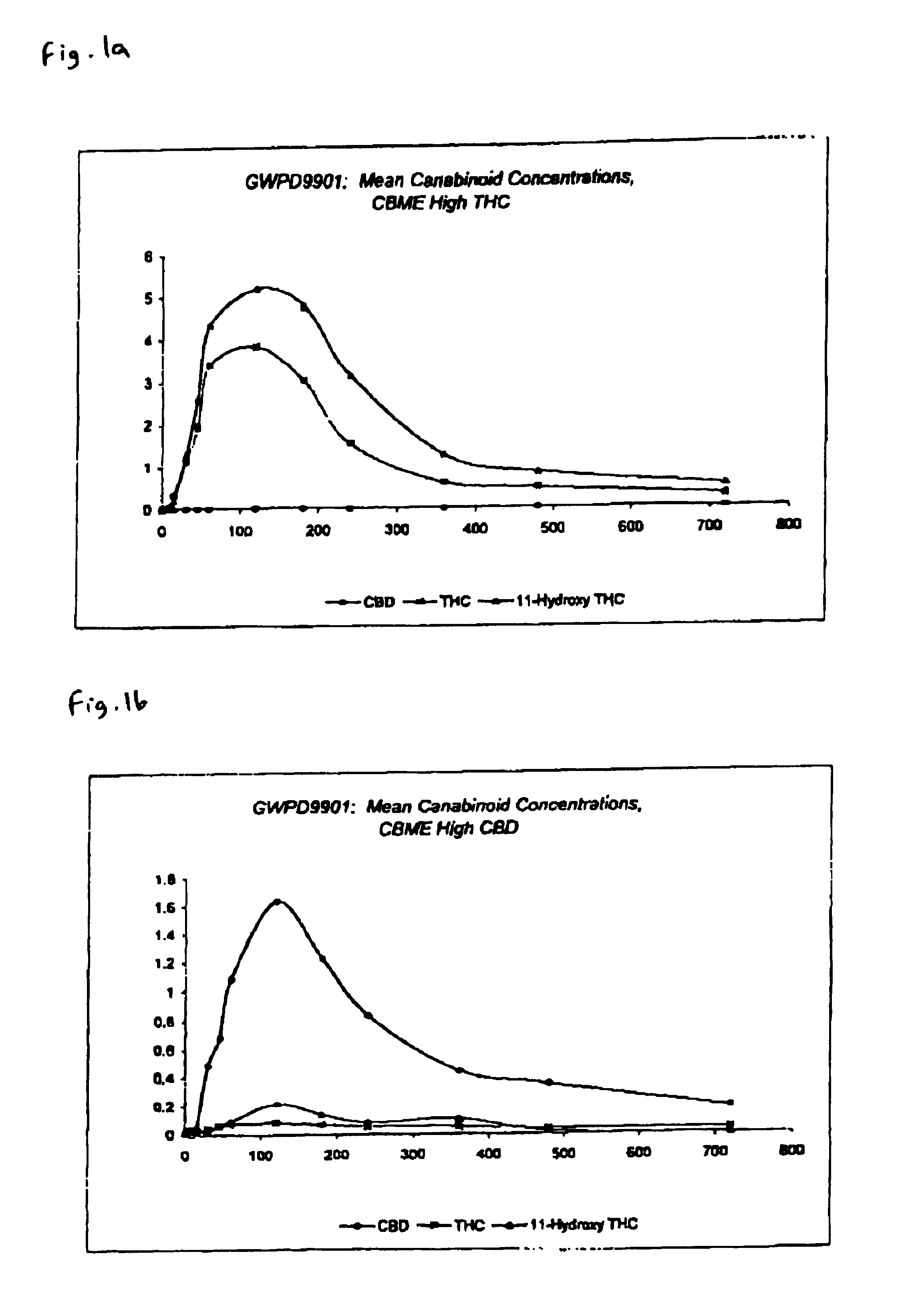
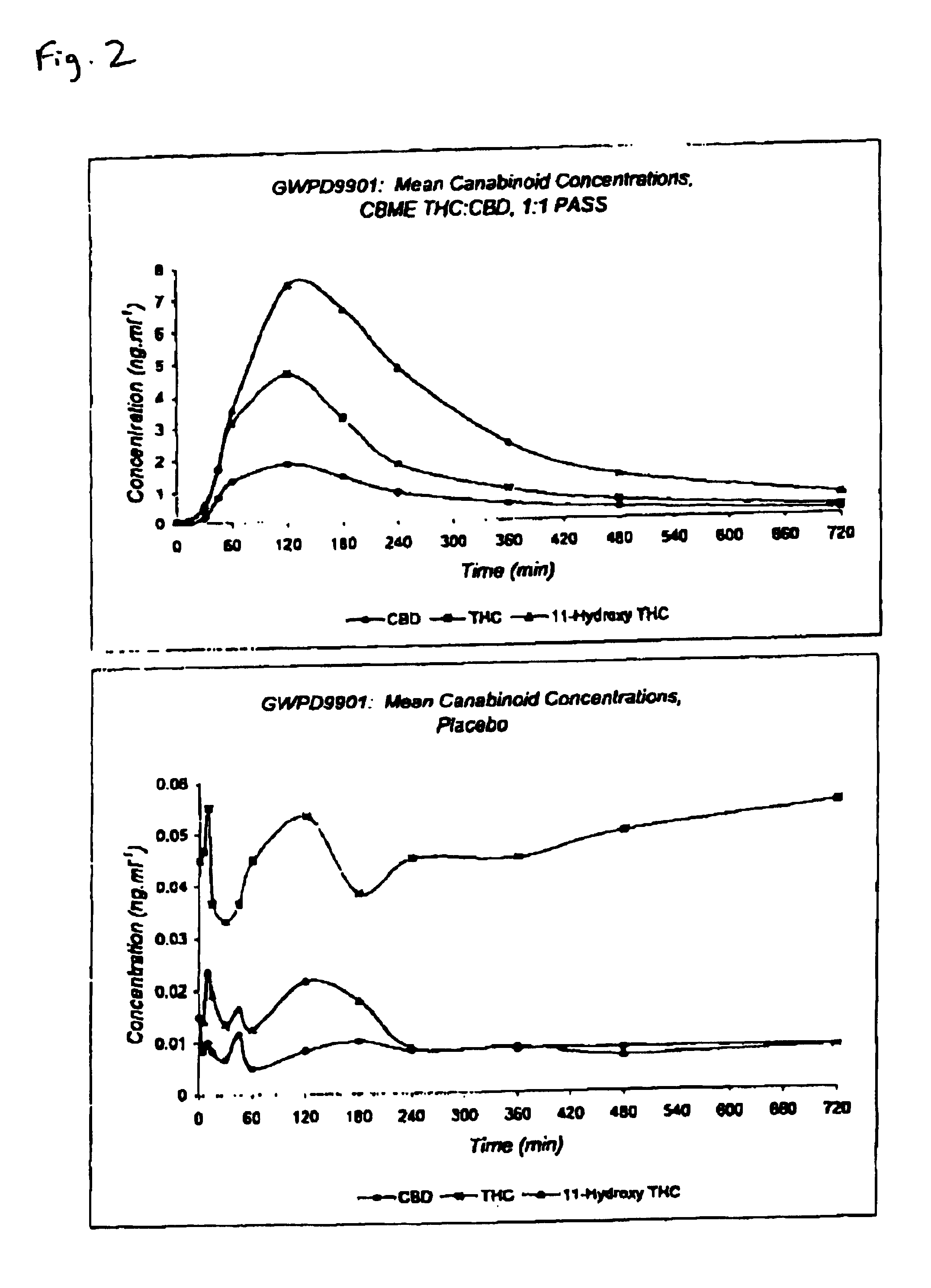
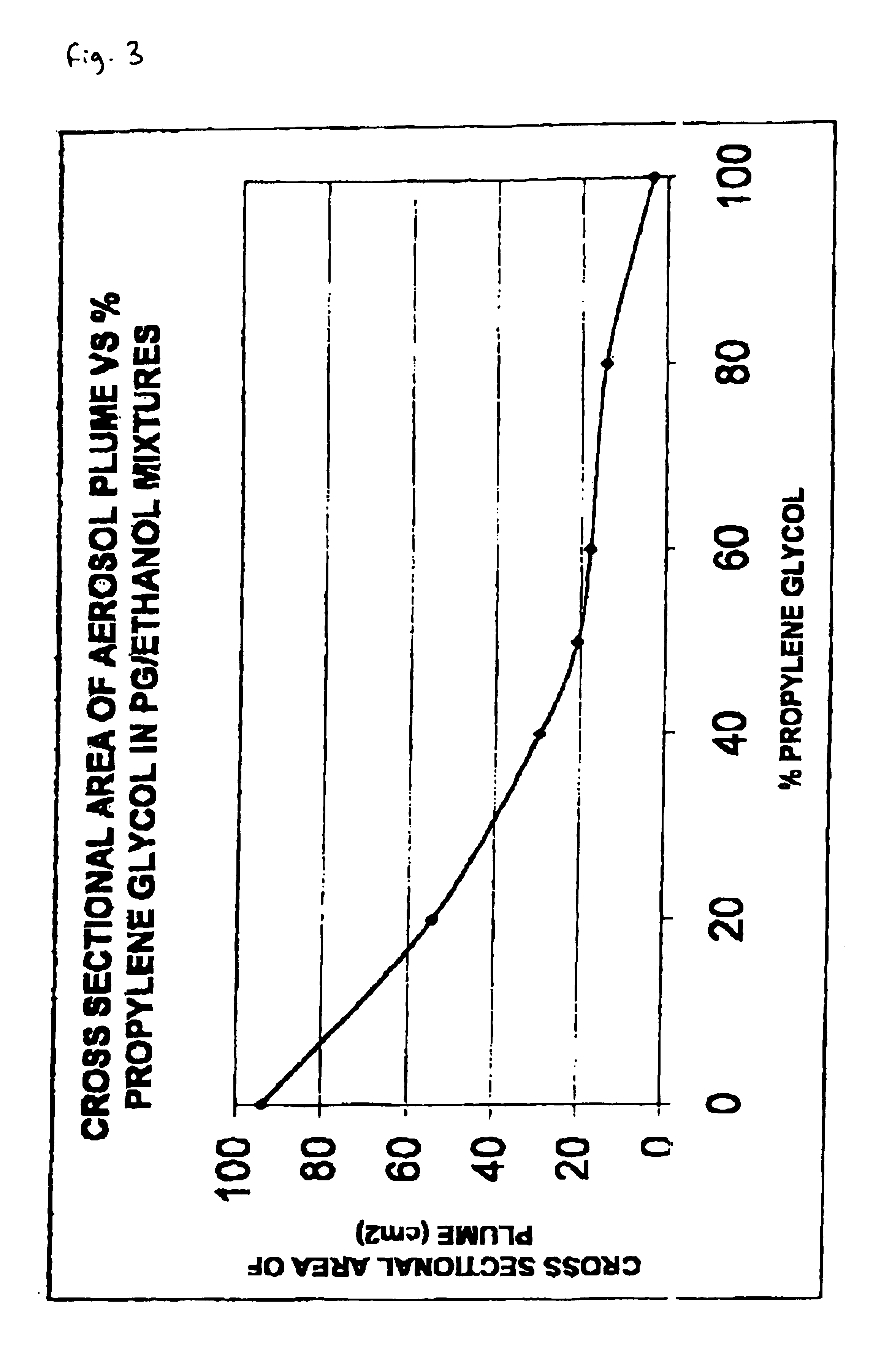
Pharmaceutical formulation
InactiveUS6946150B2Low degreeThe process is simple and effectiveBiocideNervous disorderPharmaceutical formulationCo solvent
The invention relates to pharmaceutical formulations, and more particularly to formulations containing cannabinoids for administration via a pump action spray. In particular, the invention relates to pharmaceutical formulations, for use in administration of lipophilic medicaments via mucosal surfaces, comprising: at least one lipophilic medicament, a solvent and a co-solvent, wherein the total amount of solvent and co-solvent present in the formulation is greater than 55% wt / wt of the formulation and the formulation is absent of a self emulsifying agent and / or a fluorinated propellant.
Owner:GW RES LTD
Method and means for capture and long-term sequestration of carbon dioxide
InactiveUS20090081096A1High heat of reactionHigh regeneration energyCombination devicesGas treatmentSolubilityAmbient pressure
The invention teaches a practical method of recovering CO2 from a mixture of gases, and sequestering the captured CO2 from the atmosphere for geologic time as calcium carbonate and provides a CO2 scrubber for carbon capture and sequestration. CO2 from the production of calcium oxide is geologically sequestered. A calcium hydroxide solution is produced from the environmentally responsibly-produced calcium oxide. The CO2 scrubber incorporates an aqueous froth to maximize liquid-to-gas surface area and time-of-contact between gaseous CO2 and the calcium hydroxide solution. The CO2 scrubber decreases the temperature of the liquid and the mixed gases, increases ambient pressure on the bubbles and vapor pressure inside the bubbles, diffuses the gas through intercellular walls from relative smaller bubbles with relative high vapor pressure into relative larger bubbles with relative low vapor pressure, and decreases the mean-free-paths of the CO2 molecules inside the bubbles, in order to increase solubility of CO2 and the rate of dissolution of gaseous CO2 from a mixture of gases into the calcium hydroxide solution.The CO2 scrubber recovers gaseous CO2 directly from the atmosphere, from post-combustion flue gas, or from industrial processes that release CO2 as a result of process. CO2 reacts with calcium ions and hydroxide ions in solution forming insoluble calcium carbonate precipitates. The calcium carbonate precipitates are separated from solution, and sold to recover at least a portion of the cost of CCS.
Owner:WESTEC ENVIRONMENTAL SOLUTIONS
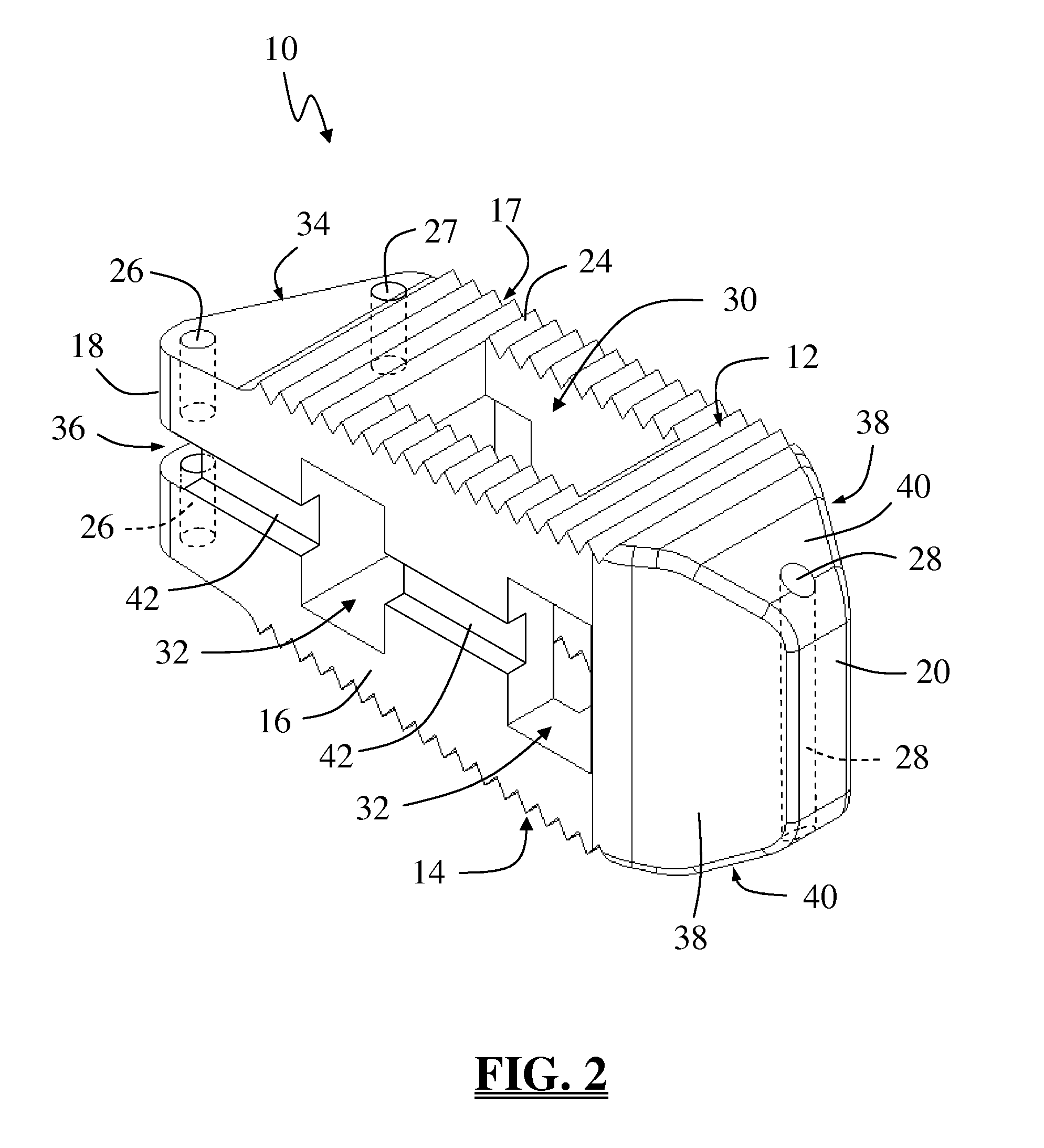
Spinal fusion implant and related methods
ActiveUS8623088B1Facilitates cortical ring contactImprove fitBone implantSpinal implantsSpinal columnPlastic surgery
A spinal fusion implant of non-bone construction to be introduced into any variety of spinal target sites. The spinal fusion implant of the present invention includes a top surface, a bottom surface, first and second lateral sides, a proximal (posterior) end and a distal (anterior) end. The spinal fusion implant of the present invention may be used to provide temporary or permanent fixation within an orthopedic target site. To do so, the spinal fusion implant may be introduced into a disc space while locked to a surgical insertion instrument and thereafter employed in the proper orientation and released. Once deposited in the disc space, the spinal fusion implant of the present invention effects spinal fusion over time as the natural healing process integrates and binds the implant.
Owner:NUVASIVE
Method for dynamically assessing petroleum reservoir competency and increasing production and recovery through asymmetric analysis of performance metrics
InactiveUS20110168391A1Increase short-term production rate and long-term recoveryAccurate assessmentElectric/magnetic detection for well-loggingSurveyProcess engineeringPetroleum reservoir
Owner:QRI GROUP
Method of converting pyrolyzable organic materials to biocarbon
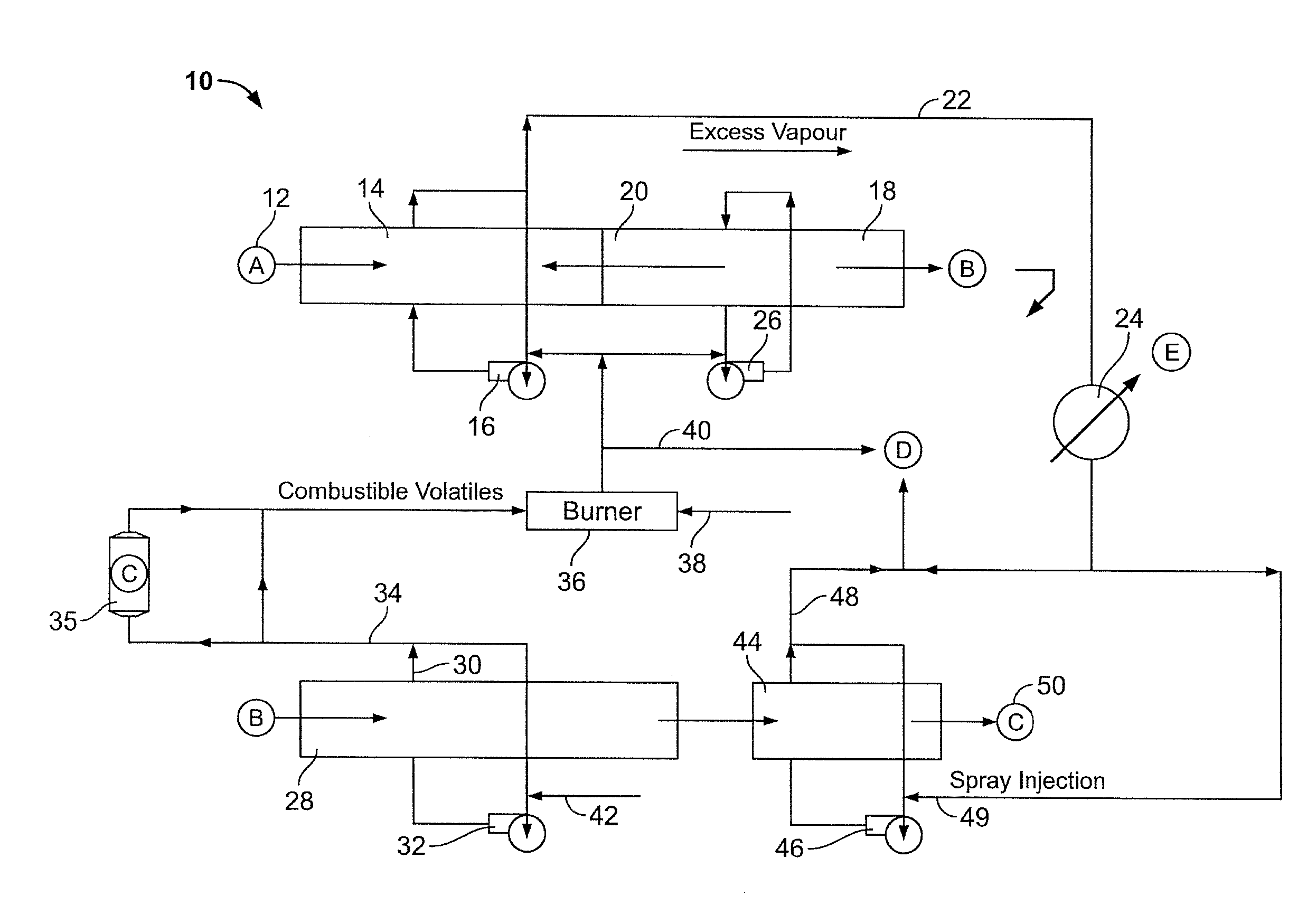
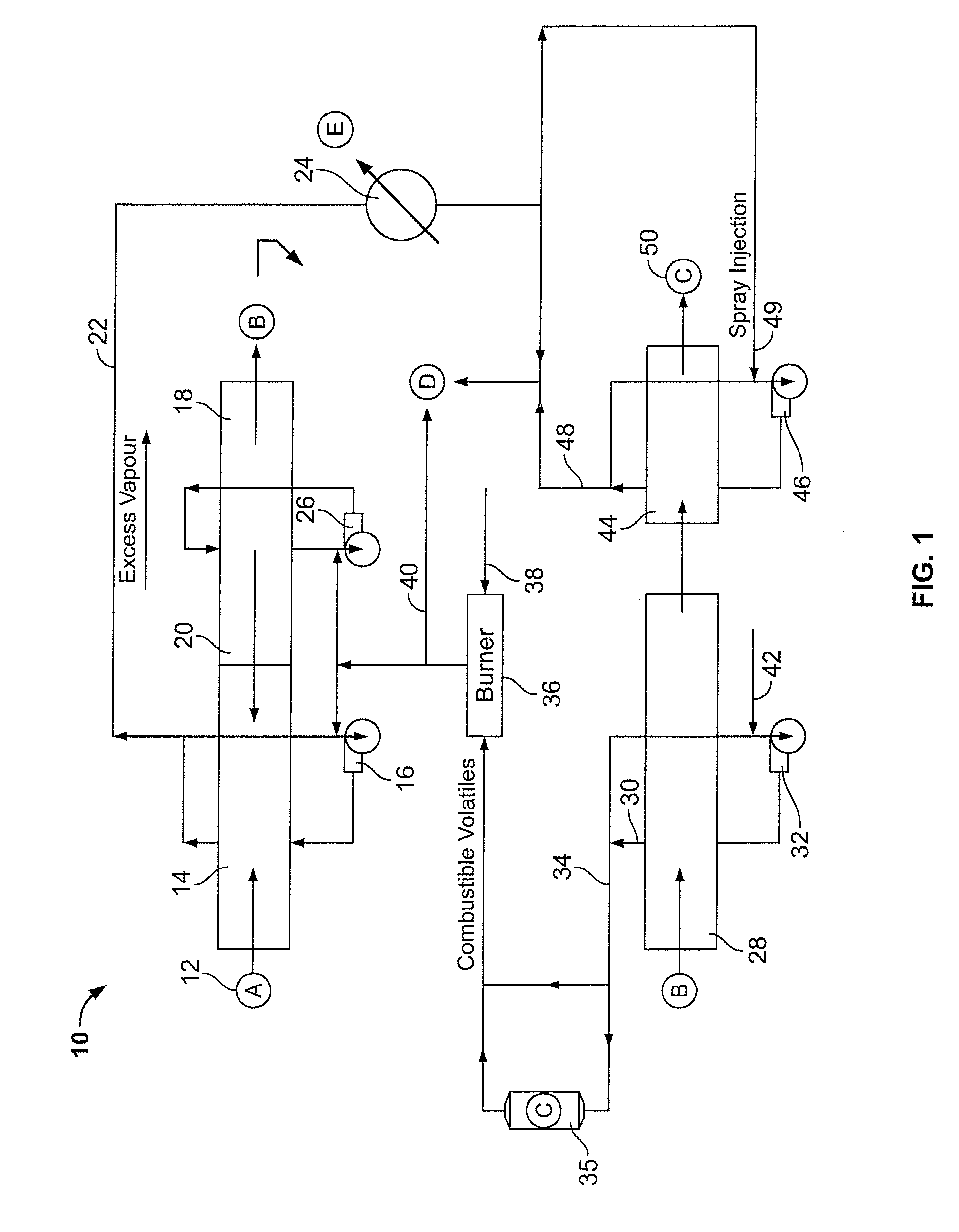
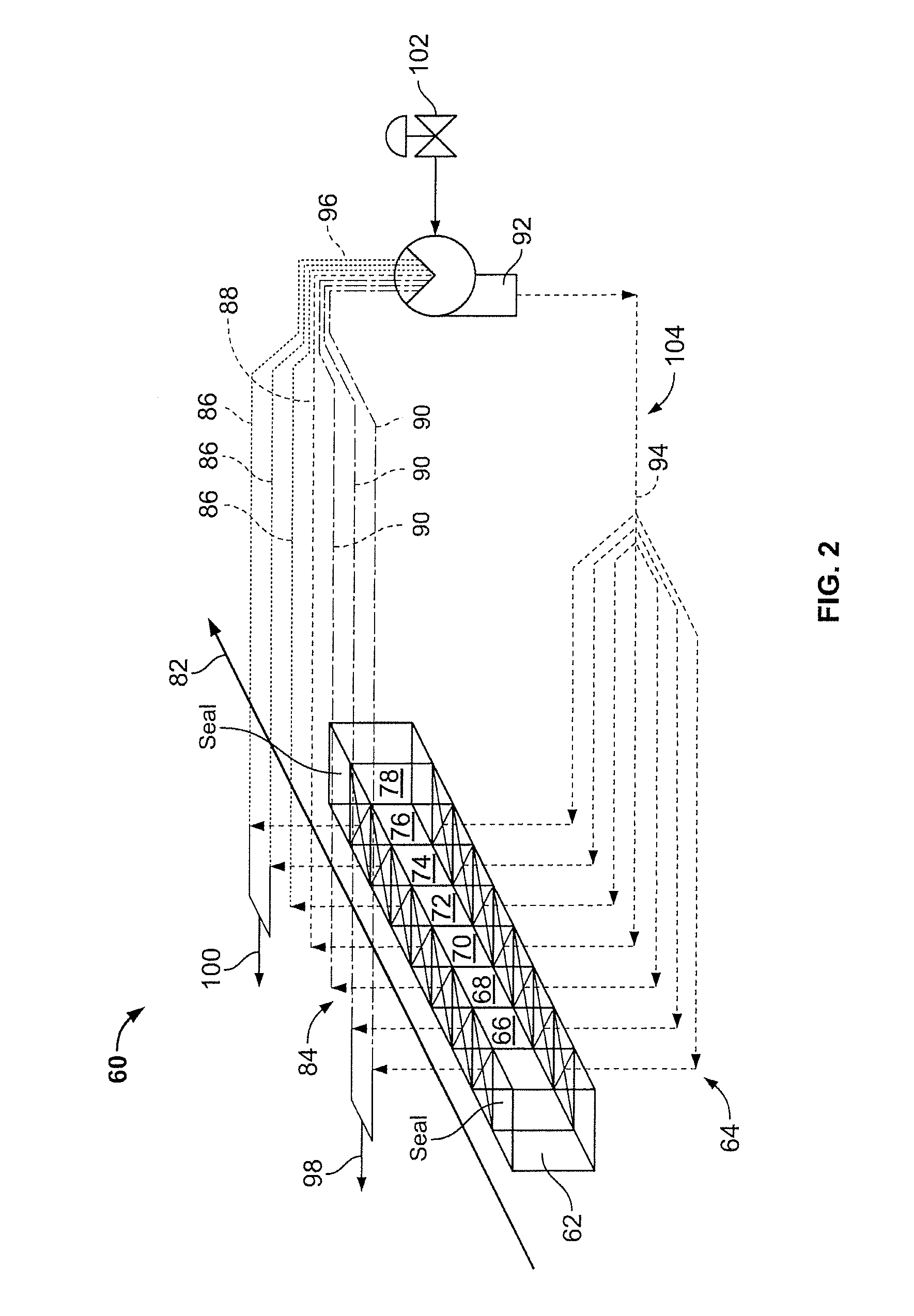
InactiveUS20100300866A1Simple methodWidespread benefit to the efficiency of the method and the biocarbon qualityCombustible gas coke oven heatingCarbon compoundsVolatilesCarbonization
A method of forming a pyrolysed biocarbon from a pyrolyzable organic material is delineated. The method involves the conversion of pyrolyzable organic materials to biocarbon for subsequent use. A carbonization circuit is employed with individual feedstock segments being advanced through the circuit. The method facilitates user manipulation of rate of advancement of the feedstock through the circuit, selective collation of volatiles from pyrolyzing feedstock, selective exposure of predetermined feedstock segments to collated volatiles as well as thermal recovery and redistribution as desired by the user. This results in the capacity for a customizable biocarbon product, the latter being an auxiliary feature of the methodology.
Owner:ALTERNA ENERGY
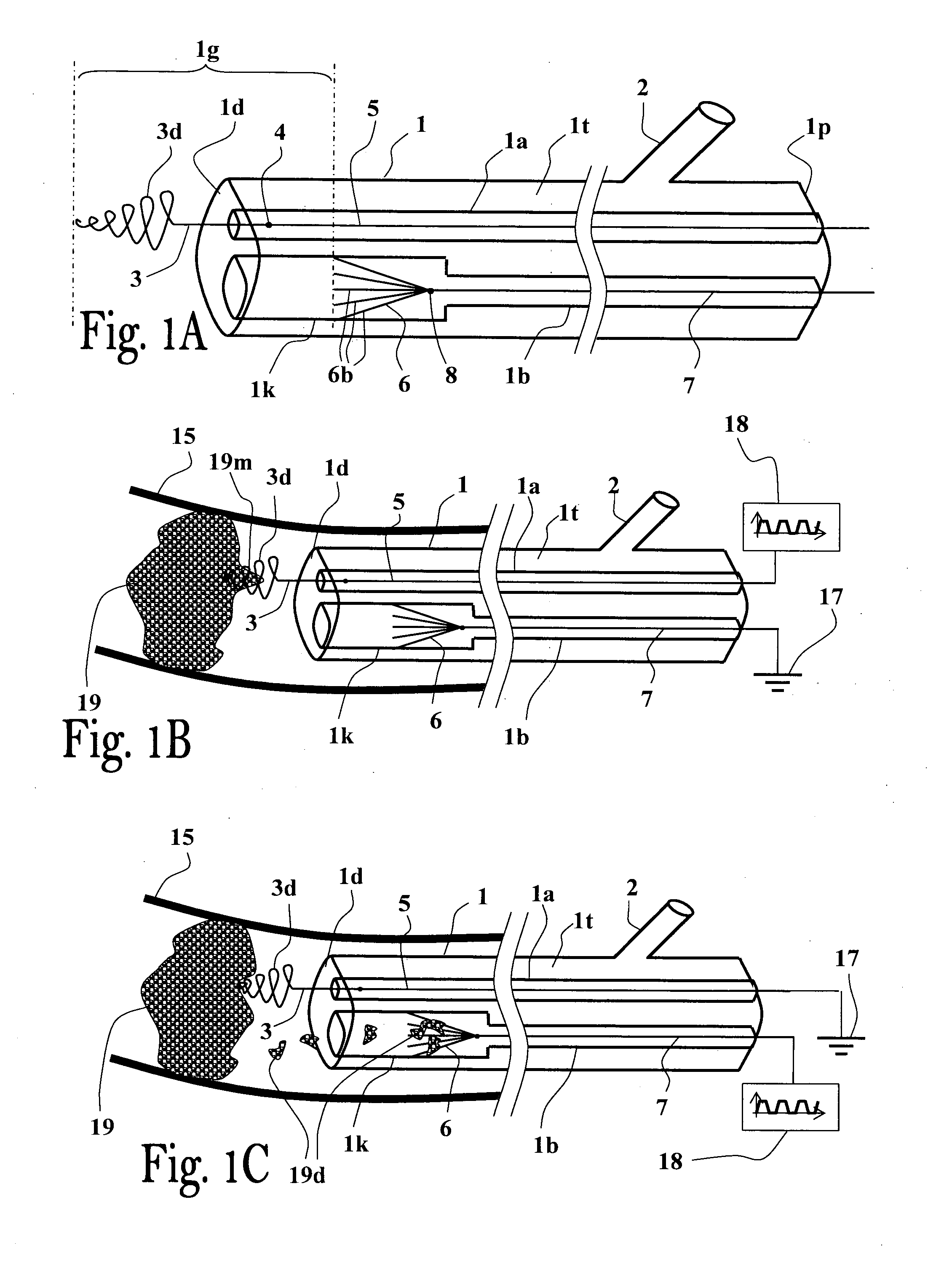
Method and apparatus for thrombus dissolution/thrombectomy by an electrode catheter device
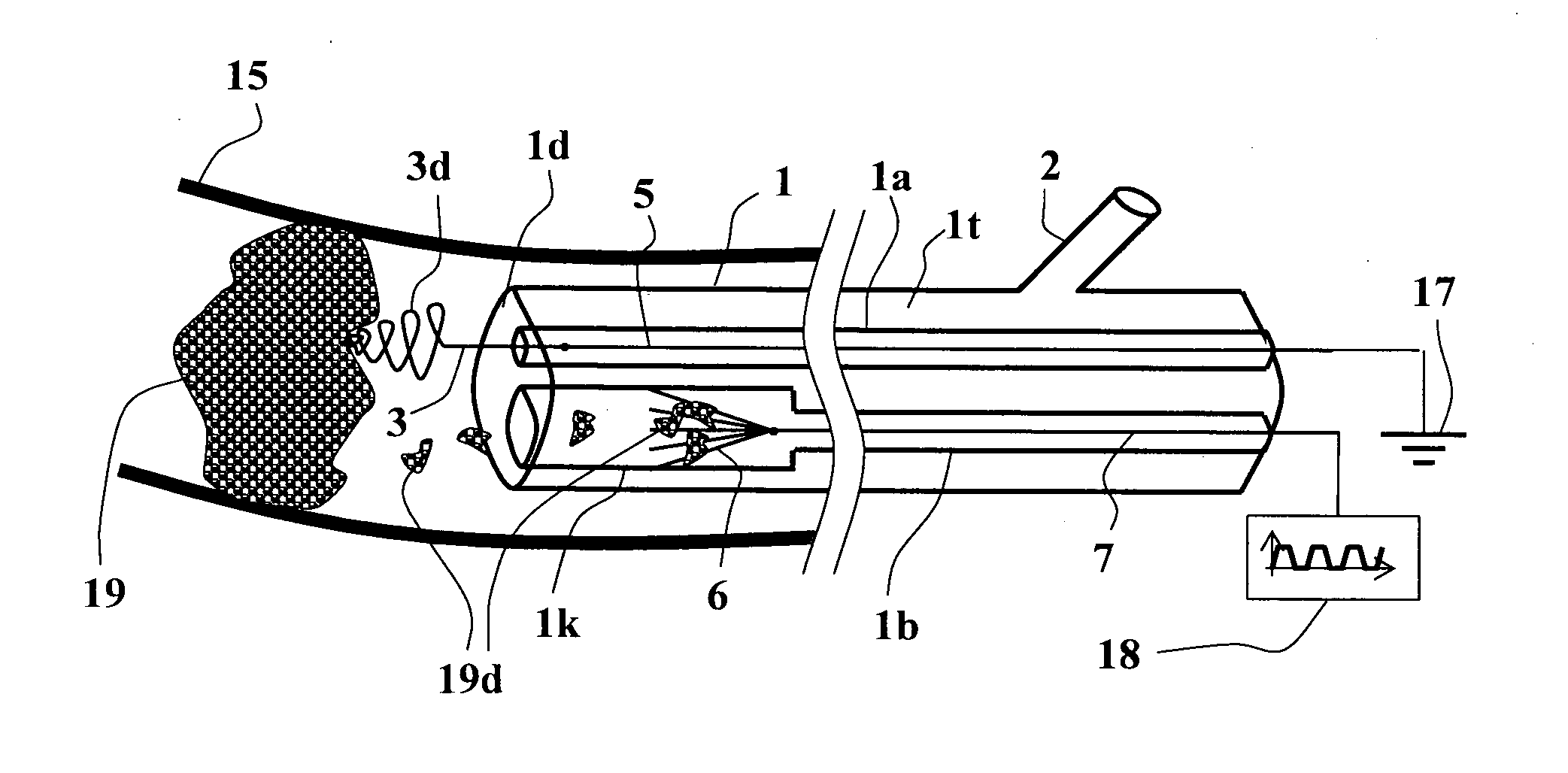
ActiveUS20110301594A1Eliminate damageEasy to useCatheterSurgical instrument detailsElectricityThrombus
The present invention generally relates to a flexible catheter device capable of being introduced into body passages, withdraw fluids therefrom or introduce fluids thereinto, and which includes electrodes configured to apply electrical signals in the body passage for carrying out thrombus dissolution and / or thrombectomy, wherein one of said electrodes is designed to contact the thrombus material and remove it or dissolve it, and wherein the electrical voltage signals are a unipolar pulsatile voltage signal.
Owner:MAGNETO THROMBECTOMY SOLUTIONS LTD
Process for converting a hydrocarbon to an oxygenate or a nitrile
InactiveUS7294734B2Maximize contactOrganic compound preparationCarboxylic acid esters preparationOxygenateOxygen
This invention relates to a process for converting a hydrocarbon reactant to a product comprising an oxygenate or a nitrile, the process comprising: (A) flowing a reactant composition comprising the hydrocarbon reactant, and oxygen or a source of oxygen, and optionally ammonia, through a microchannel reactor in contact with a catalyst to convert the hydrocarbon reactant to the product, the hydrocarbon reactant undergoing an exothermic reaction in the microchannel reactor; (B) transferring heat from the microchannel reactor to a heat exchanger during step (A); and (C) quenching the product from step (A).
Owner:VELOCYS CORPORATION
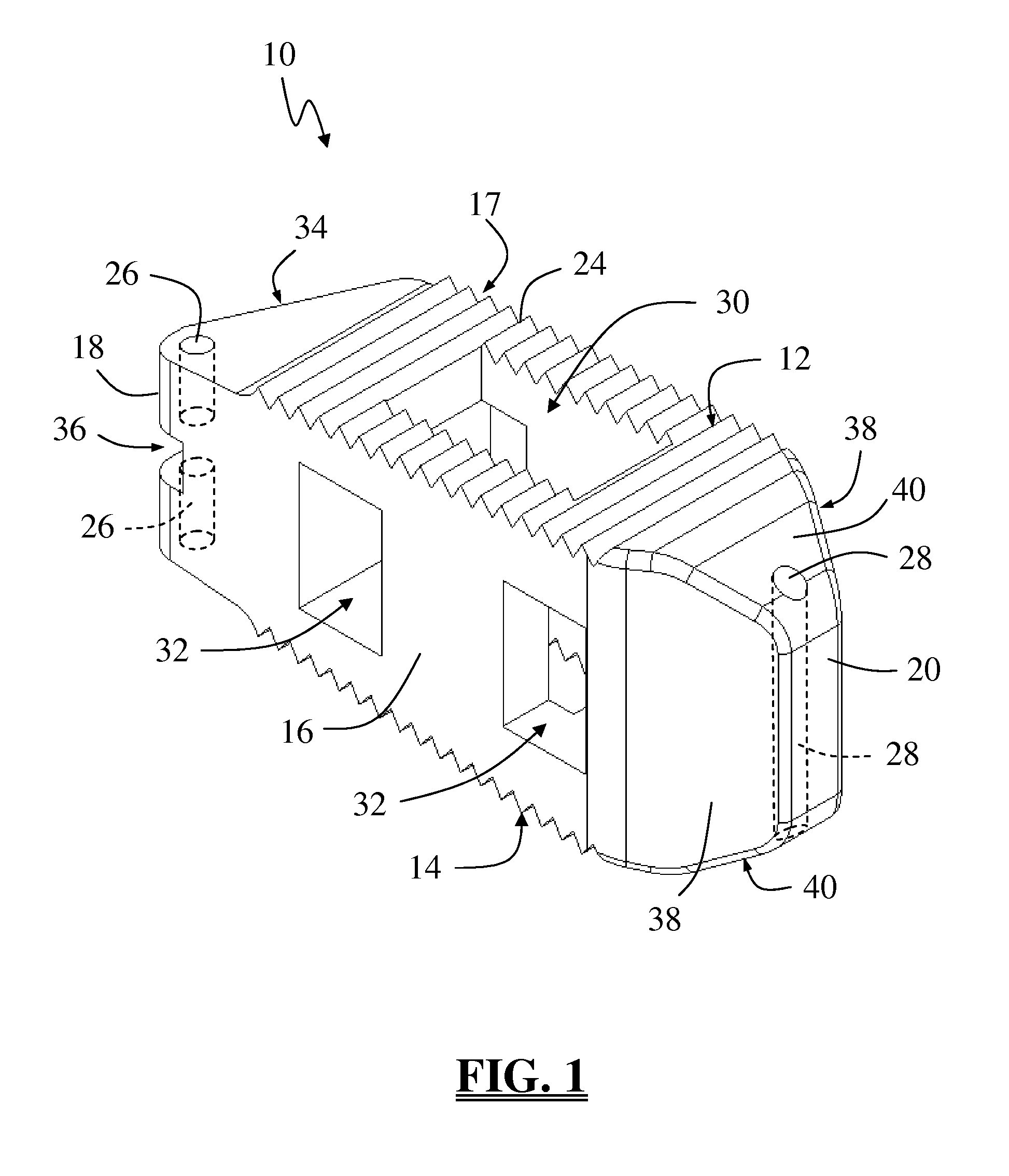
Remote controlled inspection vehicle utilizing magnetic adhesion to traverse nonhorizontal, nonflat, ferromagnetic surfaces
InactiveUS6889783B1Maximize contactEndless track vehiclesMagnetic holding devicesRemote controlActuator
A remote controlled inspection vehicle provides interchangeable modules, permitting the vehicle to be easily configured to perform a wide variety of tasks. The vehicle includes at least one frame module having a pair of drive modules on either side. Each drive module includes a continuous track surrounding a permanent magnet, and is dimensioned and configured to pivot around its longitudinal axis. The frame modules are dimensioned and configured to be hingedly secured to other frame modules, end effectors including various sensors for performing inspections, and tail units to assist in placing the vehicle in the desired environment.
Owner:SIEMENS ENERGY INC
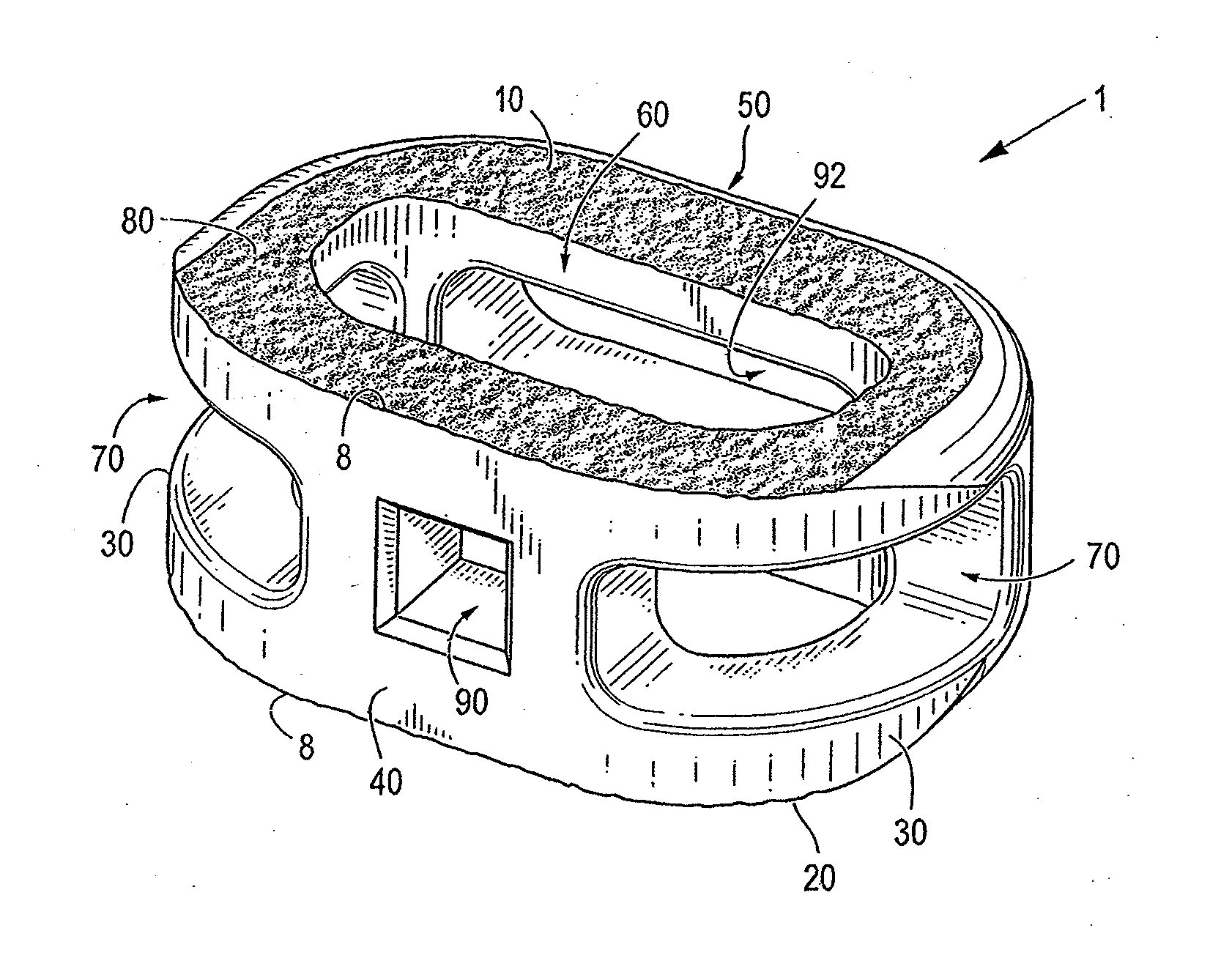
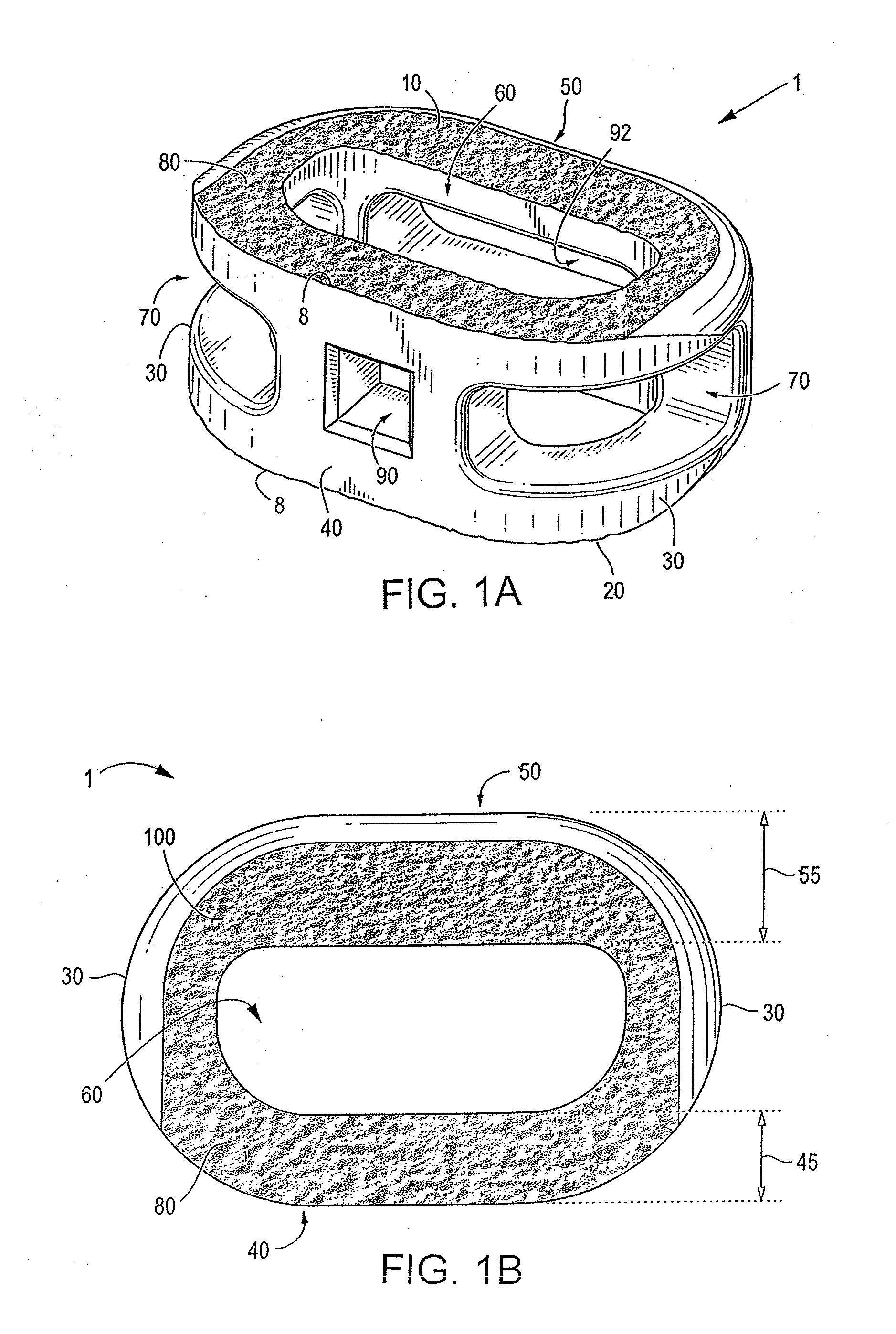
Spinal implant having a passage for enhancing contact between bone graft material and cortical endplate bone
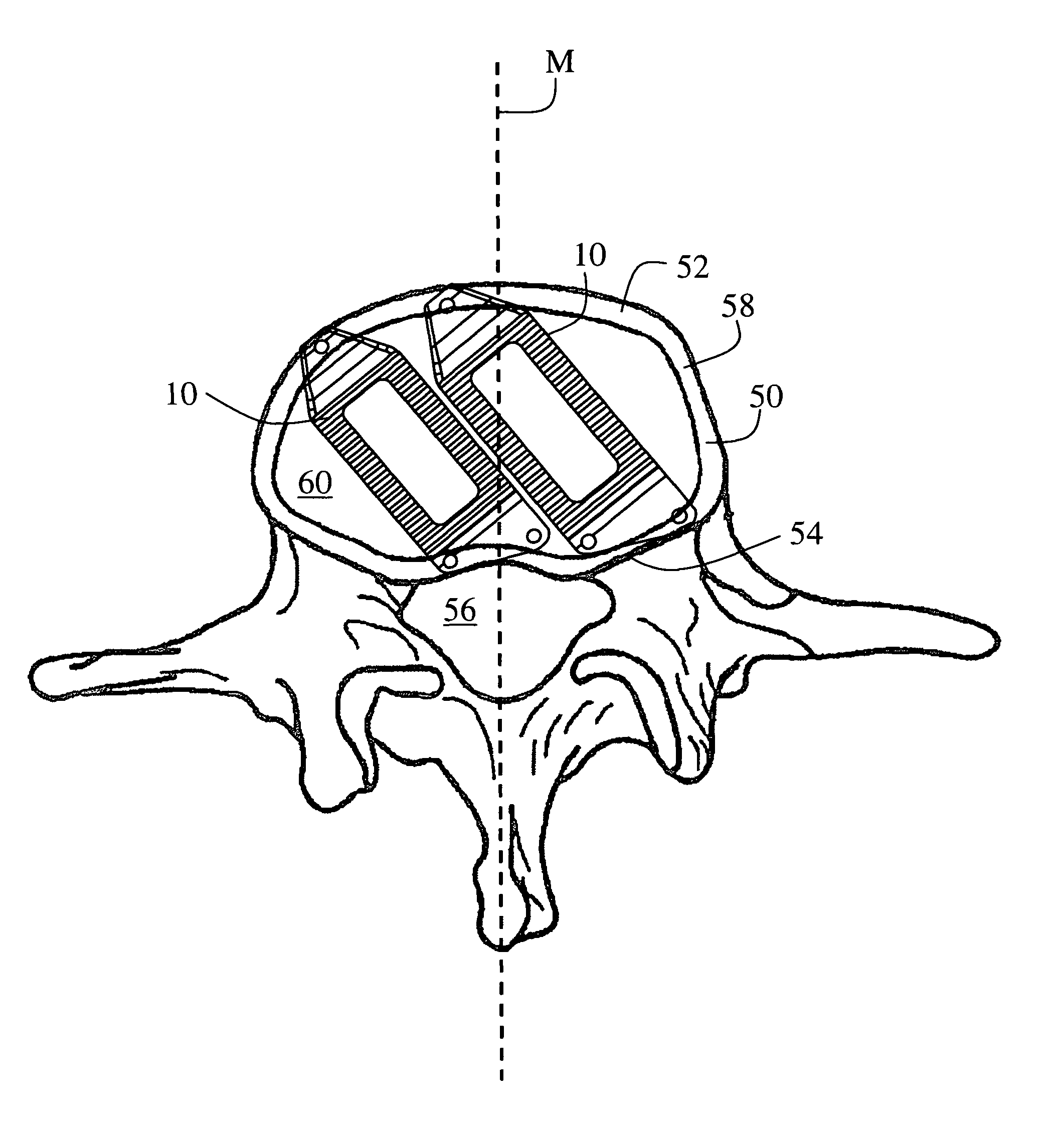
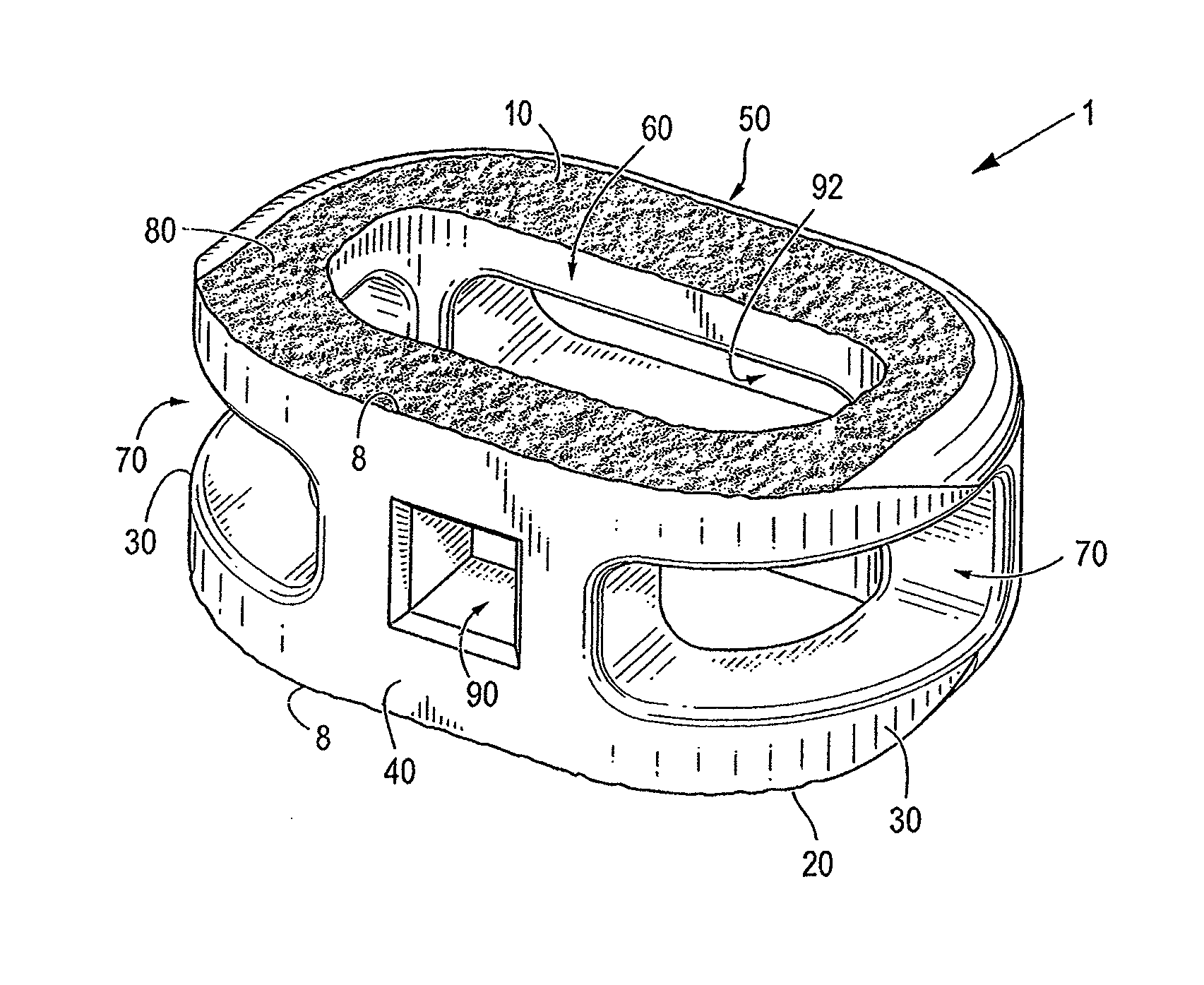
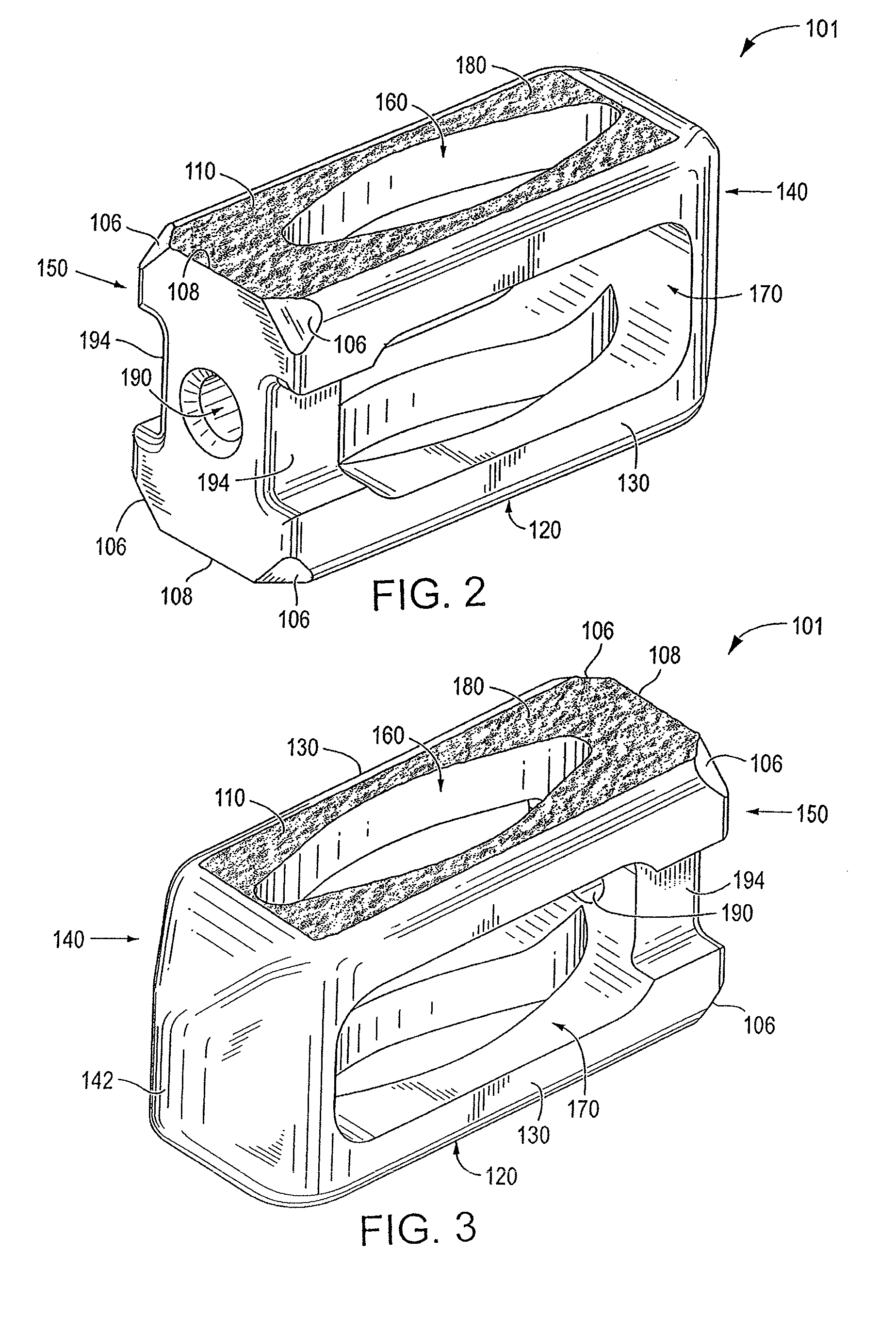
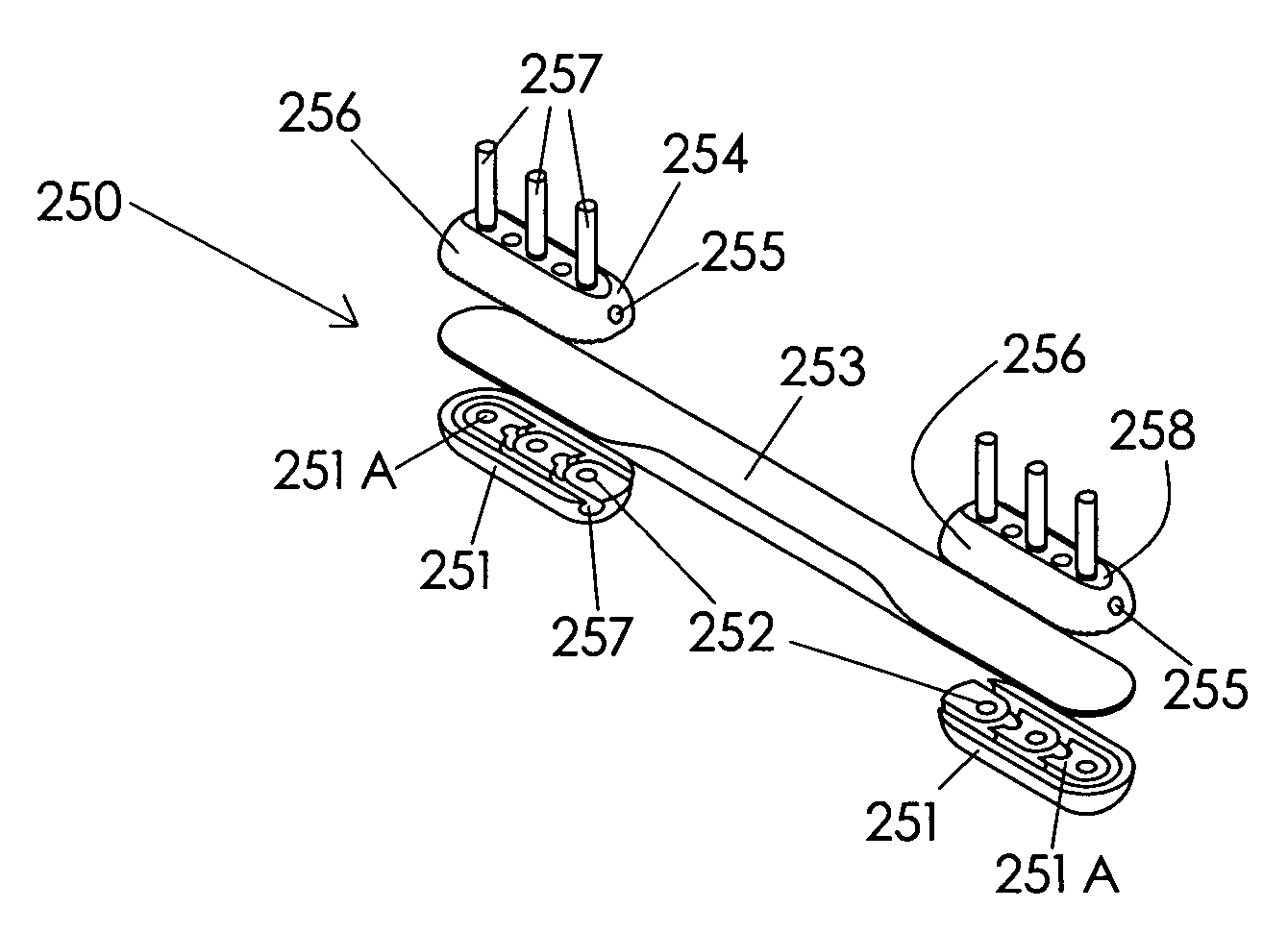
ActiveUS20120277876A1Maximizing the contact of a bone graft materialMaximize contactBone implantSpinal implantsVertebral boneBone growth
An interbody spinal implant including a body having a top surface, a bottom surface, opposing lateral sides, opposing anterior and posterior portions, and a substantially hollow center in communication with a vertical aperture, which are filled with a bone graft material. The dimensions, shape, and position of the vertical aperture facilitate contact between the bone graft material and vertebral endplate bone to support and enhance bone growth.
Owner:TITAN SPINE
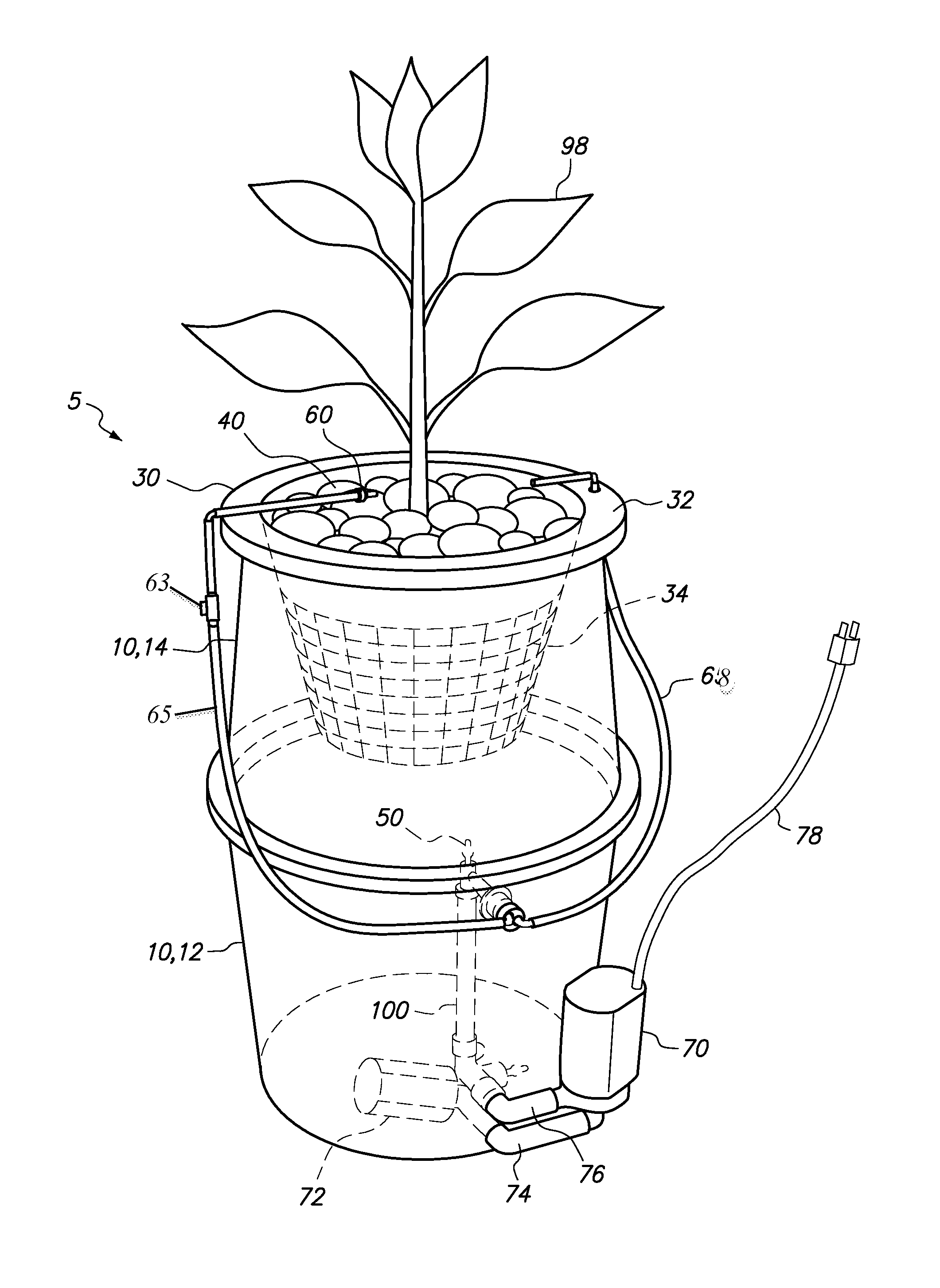
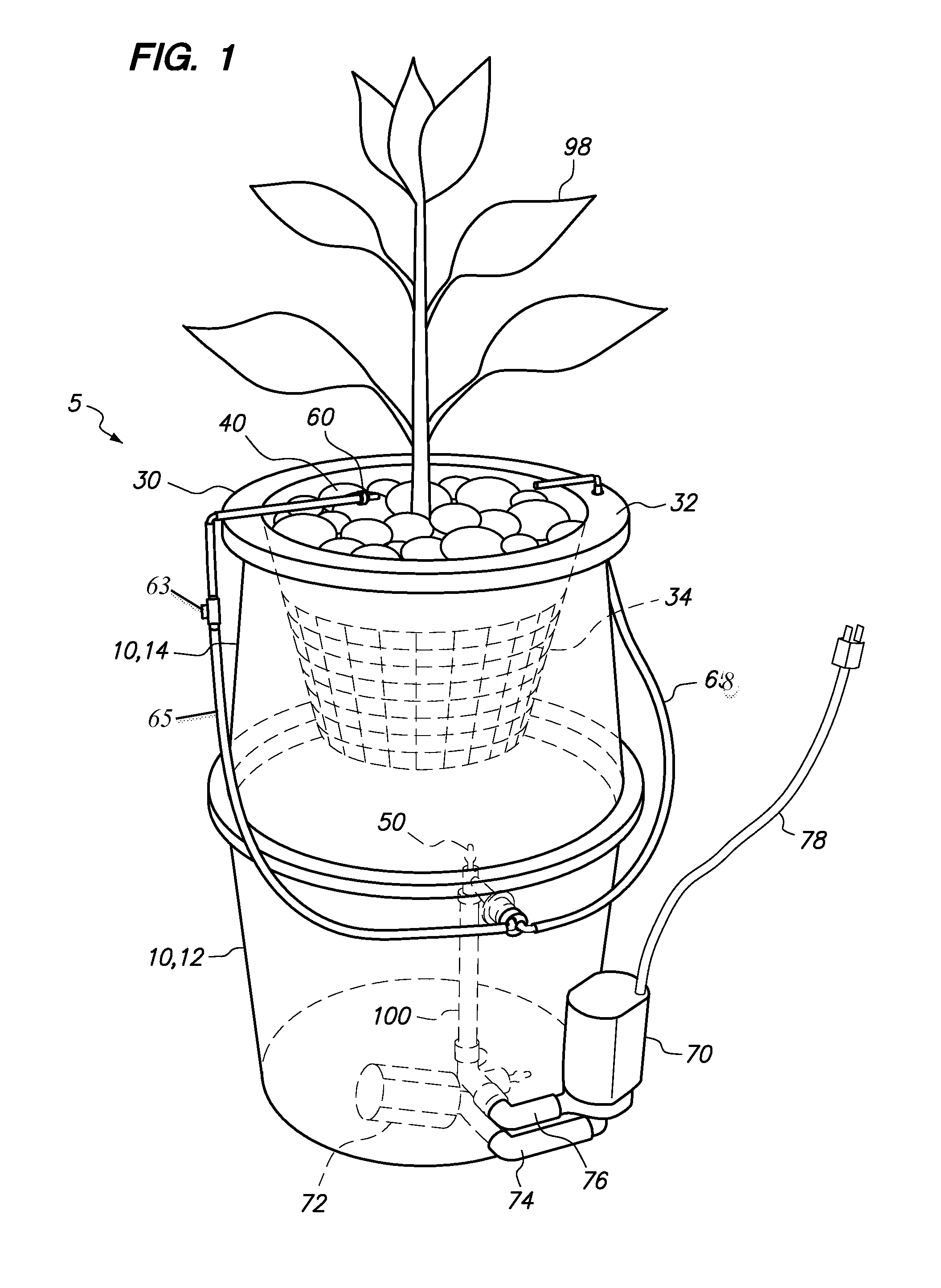
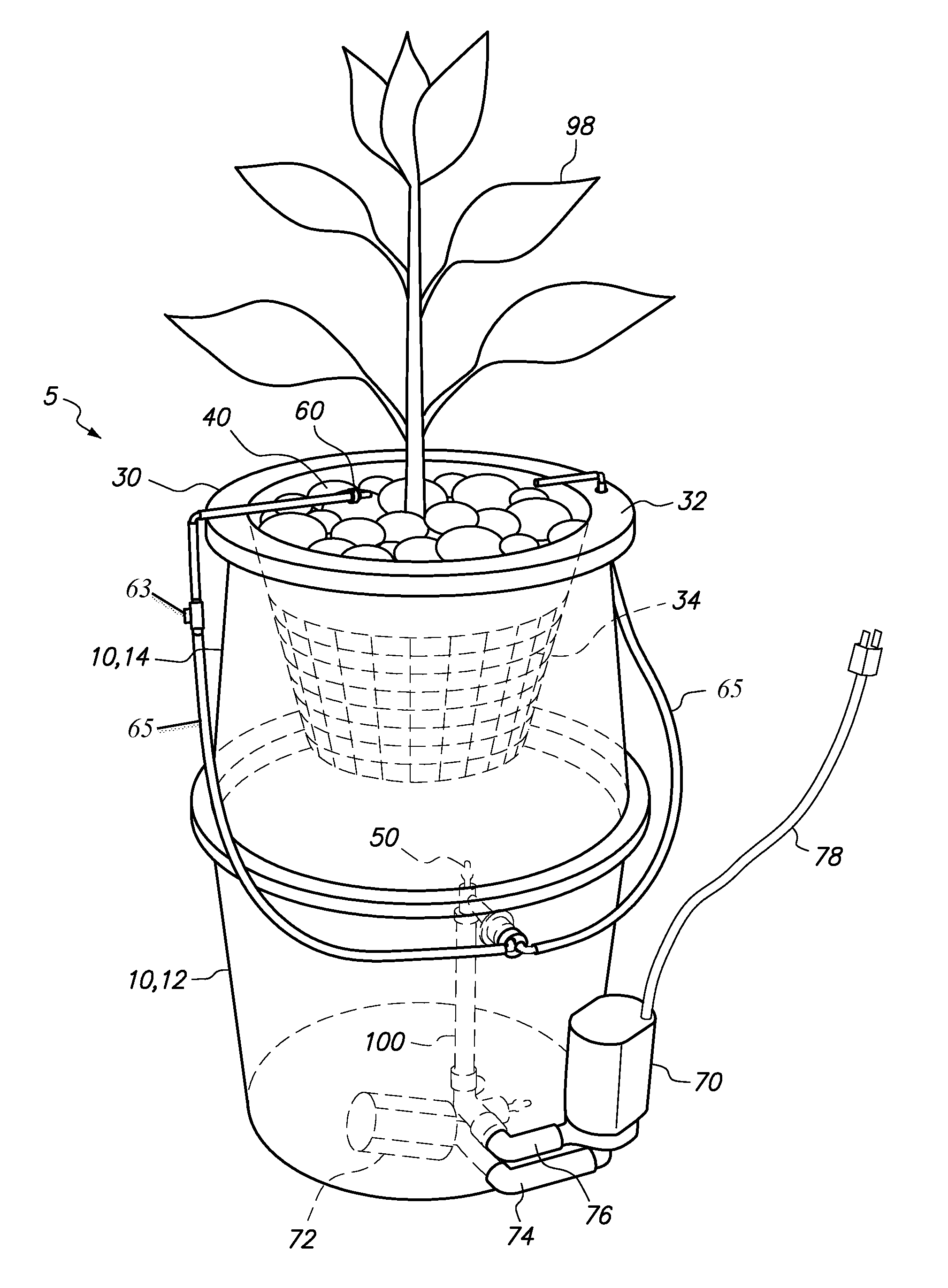
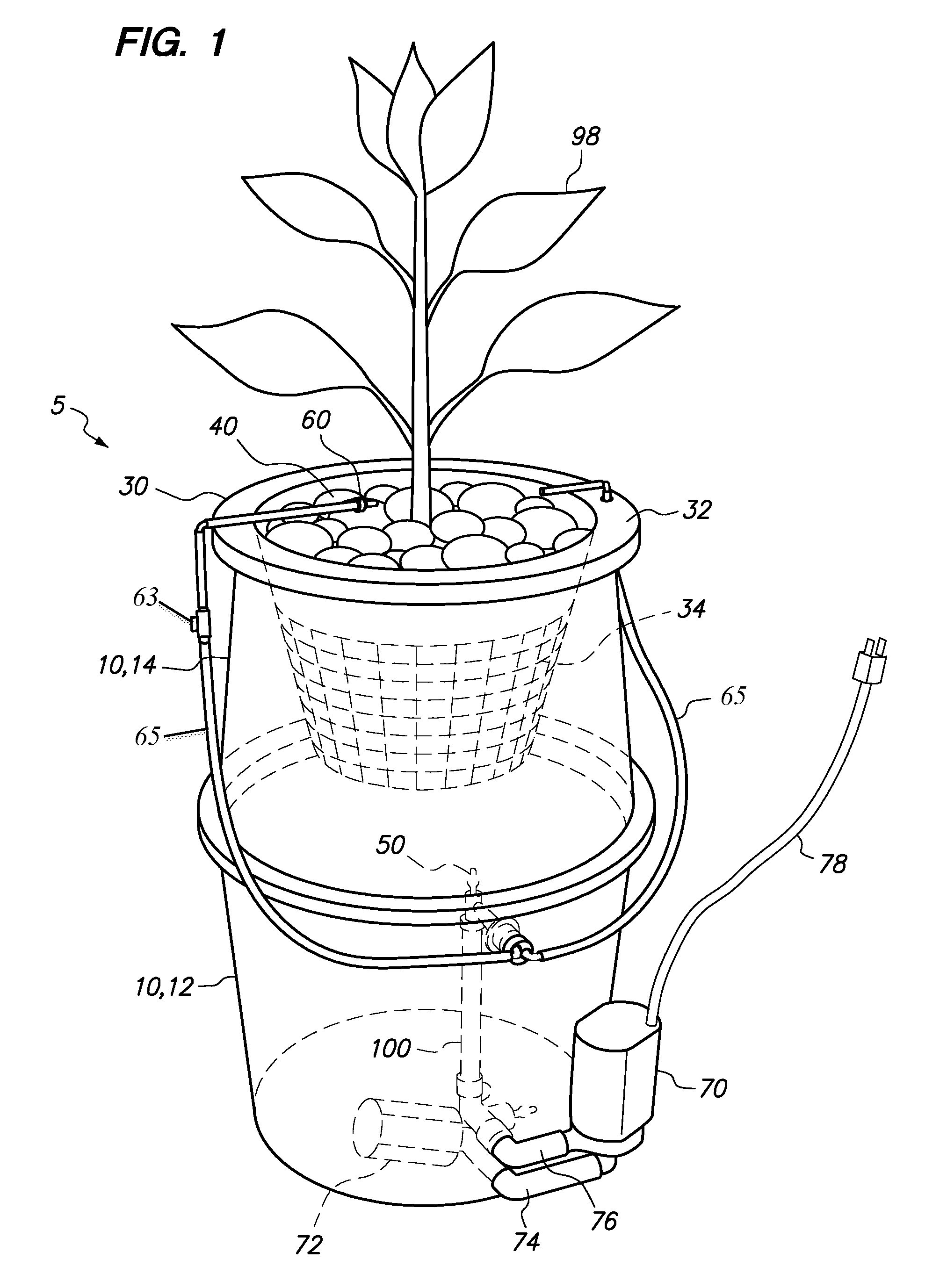
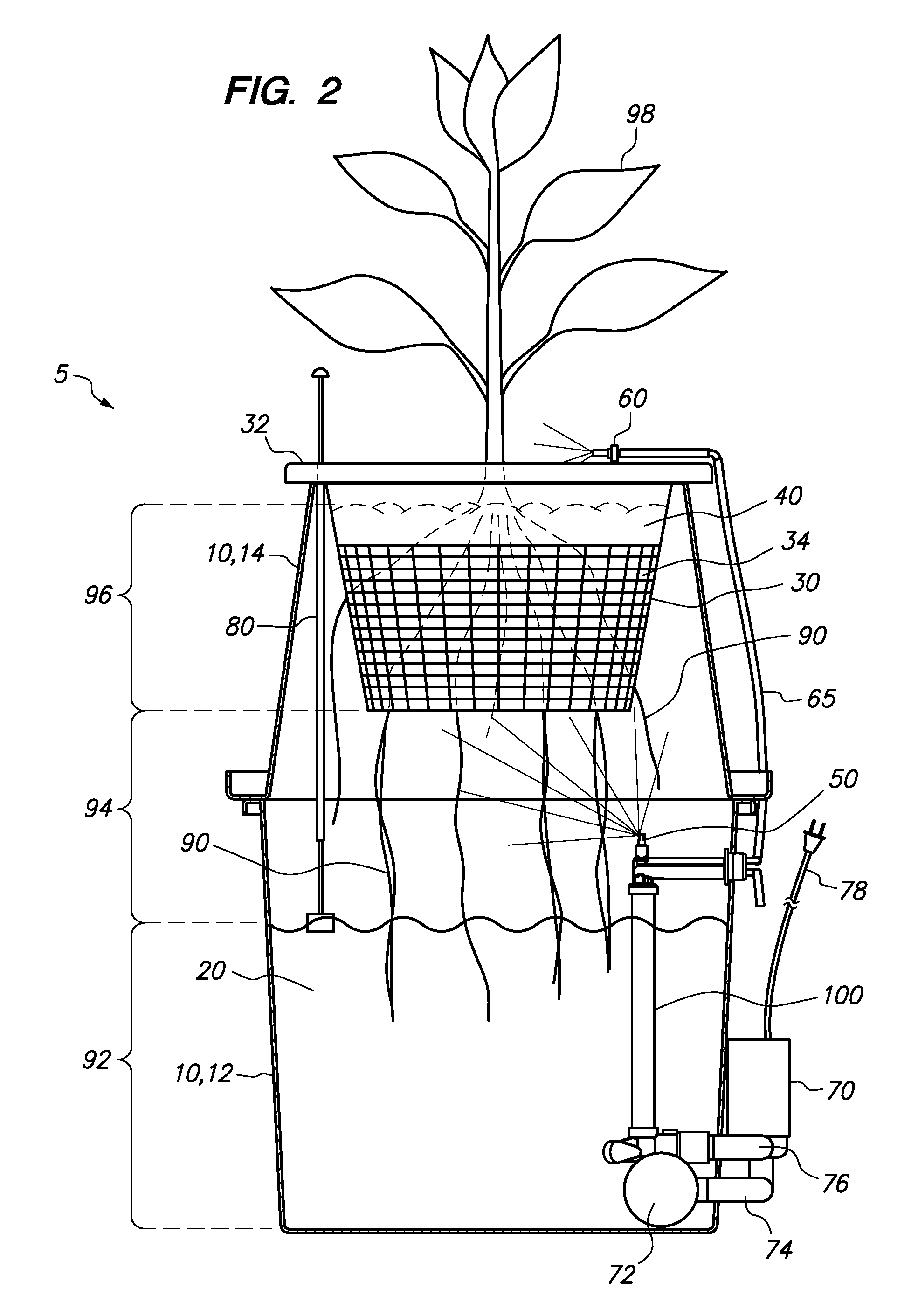
Hydroponic plant container with highly oxygenated nutrient solution using continuous air injection and continuous coriolis effect mixing
InactiveUS20130255152A1Increase dissolved oxygen levelsMaximize contactAgriculture gas emission reductionCultivating equipmentsPlant rootsNutrient solution
A hydroponic plant container with a reservoir of “highly oxygenated” nutrient solution that is the result of continuous direct air injection into the bottom of the reservoir, at a specific range of trajectories, along with continuous Coriolis effect mixing. The highly oxygenated nutrient solution is used to provide a deep water culture for the lower section of the plant roots, to saturate the middle section of the plant roots with fine droplets of the highly oxygenated nutrient solution, and to saturate the growing media and the upper section of the plants roots with a continuous drip of the highly oxygenated nutrient solution.
Owner:JOHNSON DAN +1
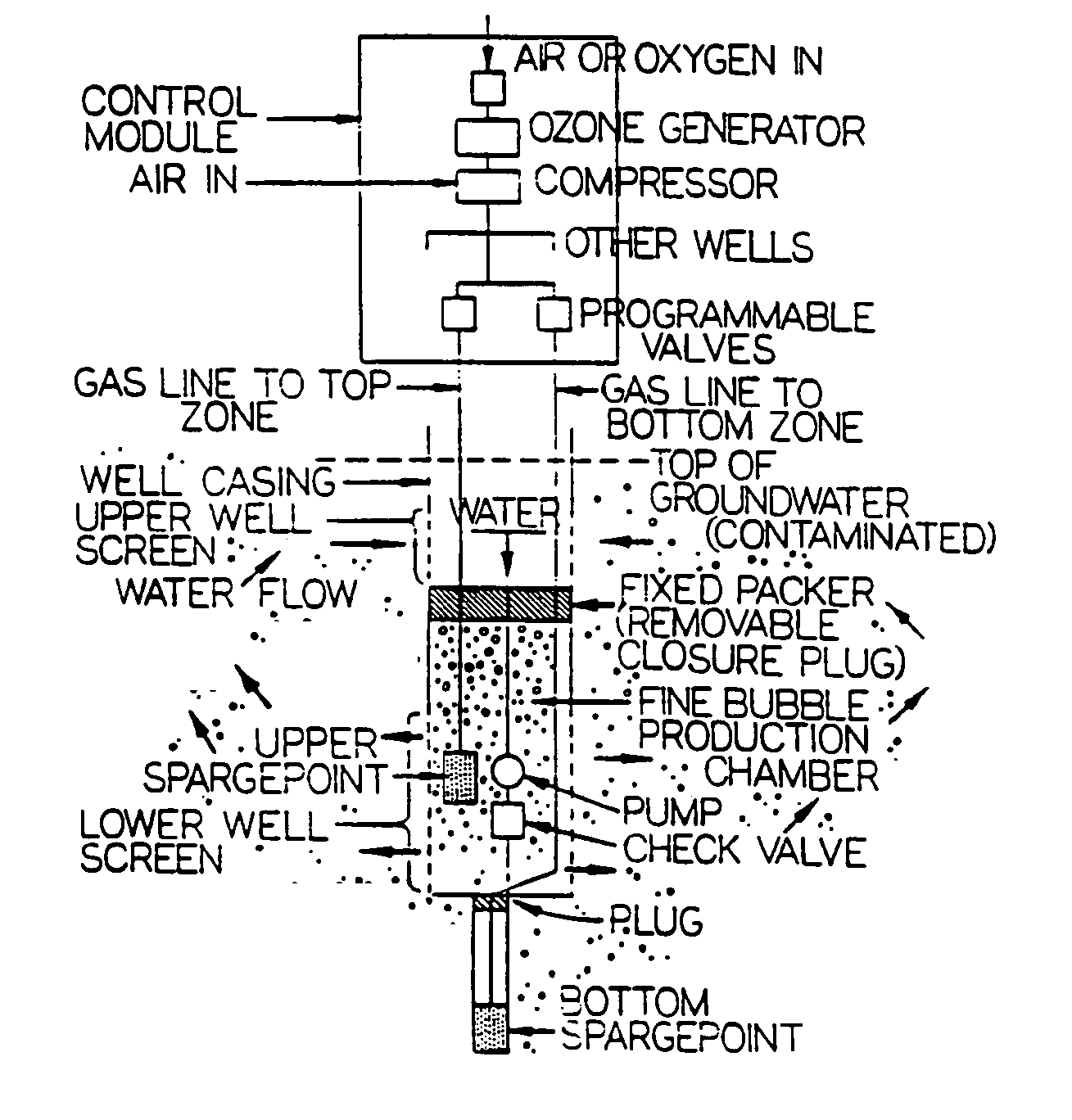
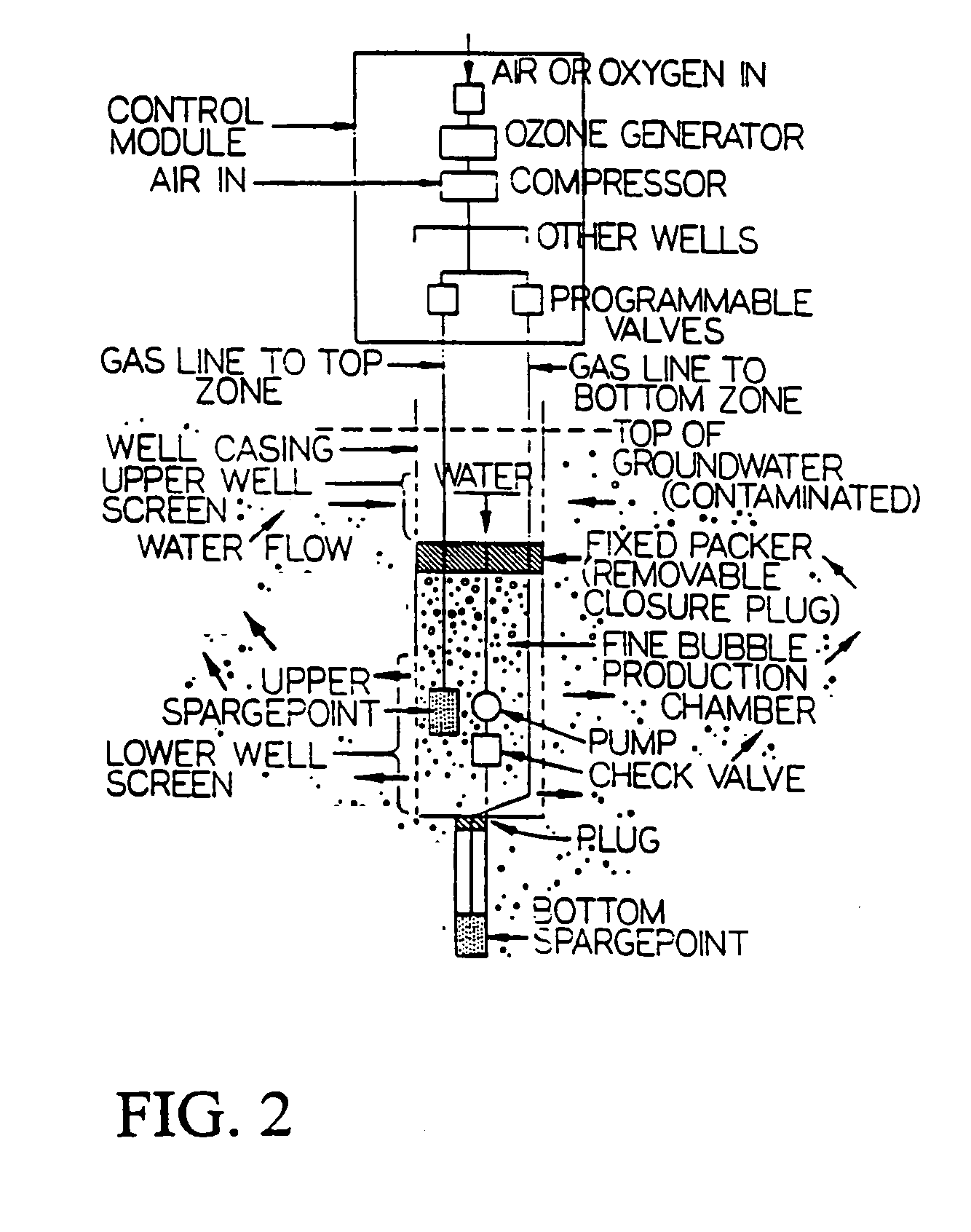
Gas-gas-water treatment system for groundwater and soil remediation
InactiveUS7022241B2Efficient removalReduce operating costsTreatment using aerobic processesMixing methodsDecompositionPetroleum product
A sparging system for in-situ groundwater remediation for removal of contamination including dissolved chlorinated hydrocarbons and dissolved hydrocarbon petroleum products including the use in injection wells of microfine bubble generators, matched to substrates of selected aquifer regions, for injection and distribution of said bubbles containing oxidizing gas through said aquifer and to selectively encapsulating gases including oxygen and ozone in duo-gas bubbles which, in the presence of co-reactant substrate material acting as a catalyst, are effective to encourage biodegradation of leachate plumes which contain biodegradable organics, or Criegee decomposition of leachate plumes containing dissolved chlorinated hydrocarbons.
Owner:KERFOOT TECH
Apparatus and method for selective processing of materials with radiant energy
InactiveUS7823366B2Maximize contactReduce capacityWrappers shrinkageMechanical working/deformationControl systemDna testing
Apparatus selectively processes a substrate using radiant energy. The substrate can consist of any target material having a portion to be processed using the radiant energy and a larger portion to be unprocessed. The apparatus includes a source of radiant energy (preferably a quantum cascade laser) that has a customizable spectrum that can be configured to be specifically absorbed only by the portion to be processed, and a control system for targeting the radiant energy only at the portion to be processed. Specific examples of the use of the apparatus and method are in the technologies of heat-shrinking polyethylene film, fusing toner to paper in a laser printer, heating reaction vessels in DNA testing, and temperature profiling bottle pre-forms.
Owner:DOUGLAS MACHINE LIABILITY
Process for converting a hydrocarbon to an oxygenate or a nitrile
InactiveUS20080031788A1Maximize contactOrganic compound preparationCarboxylic acid esters preparationHydrocotyle bowlesioidesOxygenate
This invention relates to a process for converting a hydrocarbon reactant to a product comprising an oxygenate or a nitrile, the process comprising: (A) flowing a reactant composition comprising the hydrocarbon reactant, and oxygen or a source of oxygen, and optionally ammonia, through a microchannel reactor in contact with a catalyst to convert the hydrocarbon reactant to the product, the hydrocarbon reactant undergoing an exothermic reaction in the microchannel reactor; (B) transferring heat from the microchannel reactor to a heat exchanger during step (A); and (C) quenching the product from step (A).
Owner:VELOCYS INC
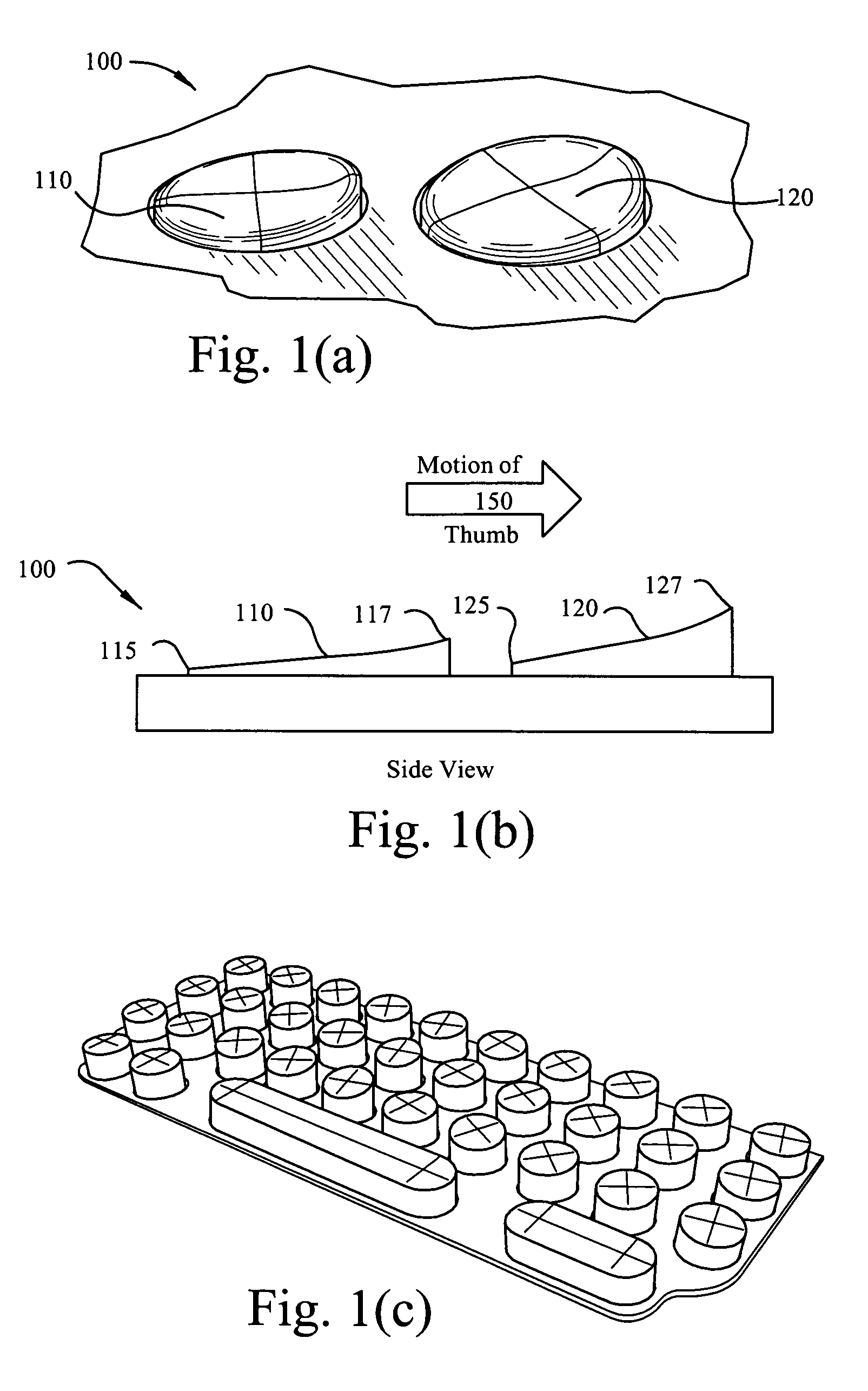
Keypad ergonomics
InactiveUS20060062626A1Easy to operateImprove gripDigital data processing detailsOther printing apparatusKey pressingEngineering
Owner:SYMBOL TECH INC
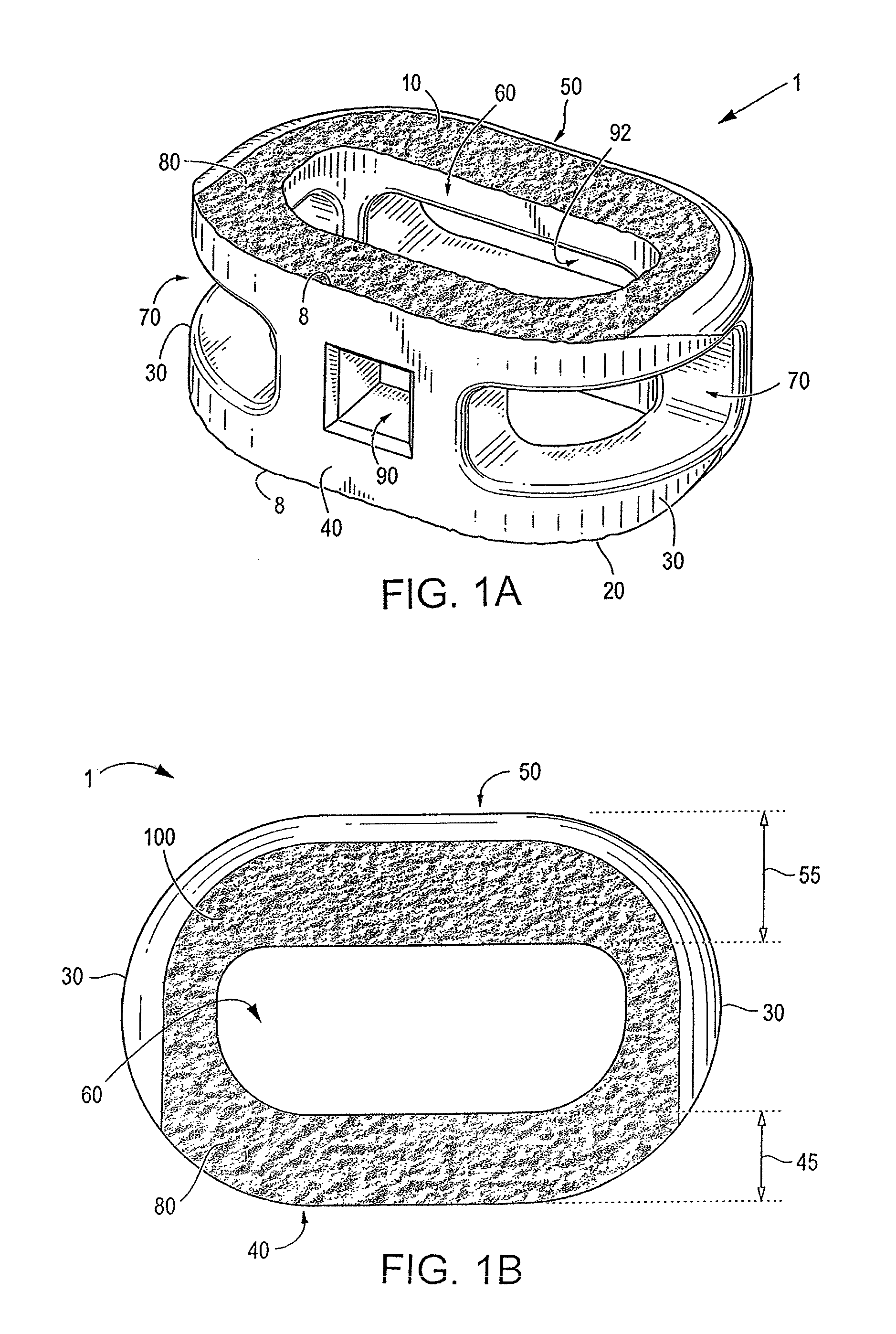
Spinal implant having a passage for enhancing contact between bone graft material and cortical endplate bone
ActiveUS8551176B2Maximizing the contact of a bone graft materialMaximize contactBone implantSpinal implantsBone growthSpinal implant
An interbody spinal implant including a body having a top surface, a bottom surface, opposing lateral sides, opposing anterior and posterior portions, and a substantially hollow center in communication with a vertical aperture, which are filled with a bone graft material. The dimensions, shape, and position of the vertical aperture facilitate contact between the bone graft material and vertebral endplate bone to support and enhance bone growth.
Owner:TITAN SPINE
Quantum dots tailored with electronically-active polymers
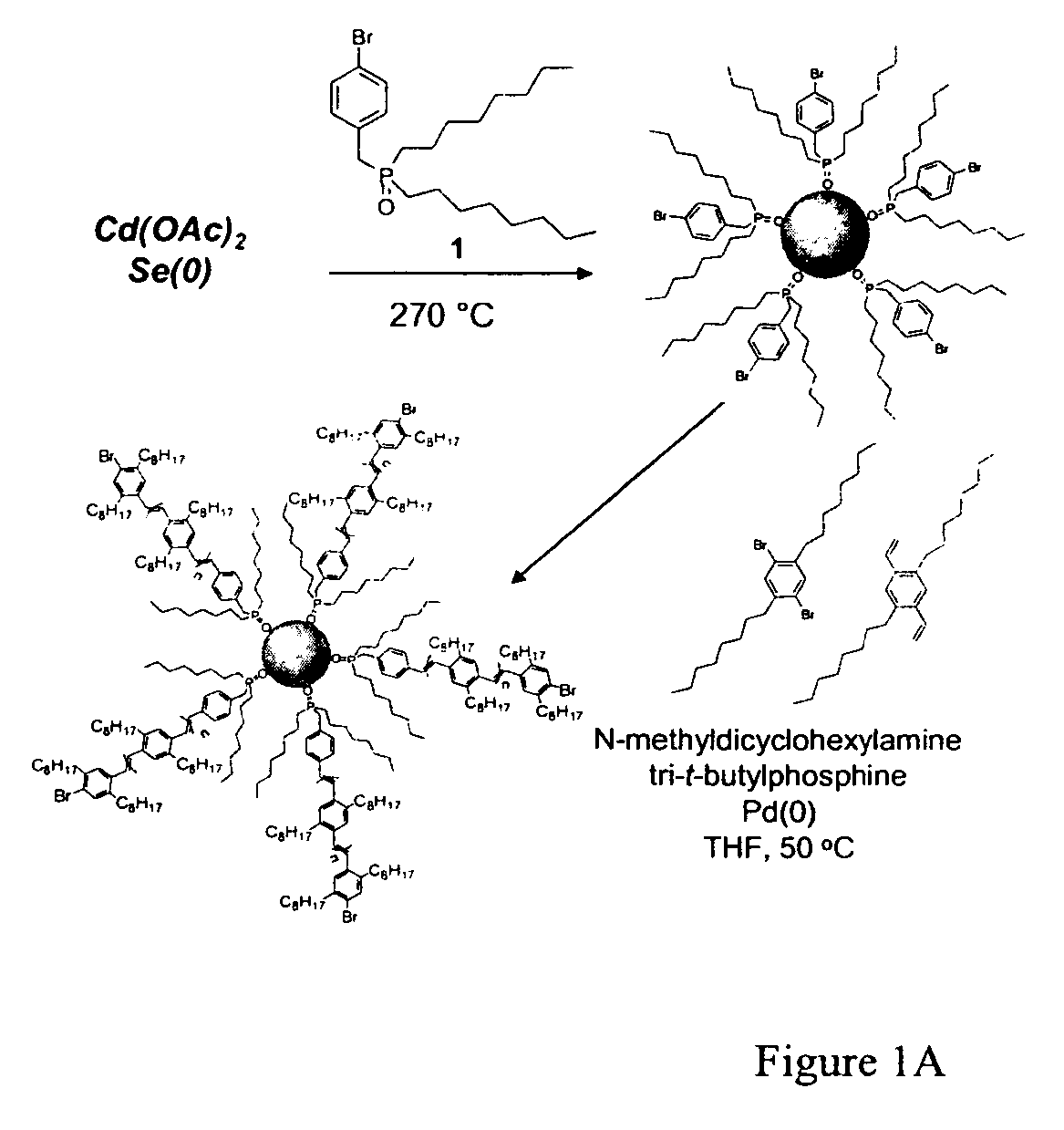
InactiveUS20060128845A1Maximize charge transportMaximize interfacial contactMaterial nanotechnologyNanoinformaticsPhotoluminescenceElectron
Cadmium selenide, and other quantum dot materials, can be integrated into thin films of poly(para-phenylene vinylene) (PPV) or other polymer compounds without aggregation of the nanocrystals. Solid-state photoluminescence spectra of composite materials prepared by these novel techniques reveal the effect of this greatly enhanced quantum dot-polymer interface relative to cases where the nanoparticles are aggregated, such that electronic communication and energy transfer between the nanoparticle and polymer components is made more efficient.
Owner:MASSACHUSETTS UNIV OF
Method for the production of natural botanical extracts
InactiveUS20050074519A1Short processing timeWithout sacrificing flavorAnimal feeding stuffTeaEnzymeProcess time
Methods for producing natural botanical extracts, such as natural vanilla extracts, with low processing times and high efficiencies are provided. The methods include a high temperature extraction step and, optionally, an enzymatic treatment step. The natural vanilla extracts or other botanical extracts produced by the methods may provide the same degree of flavoring at lower concentrations than conventionally produced natural extracts.
Owner:SENSIENT FLAVORS
Metal wiring structure for integration with through substrate vias
ActiveUS20100032809A1Improve carrying capacityImprove reliabilitySemiconductor/solid-state device detailsSolid-state devicesEngineeringDielectric layer
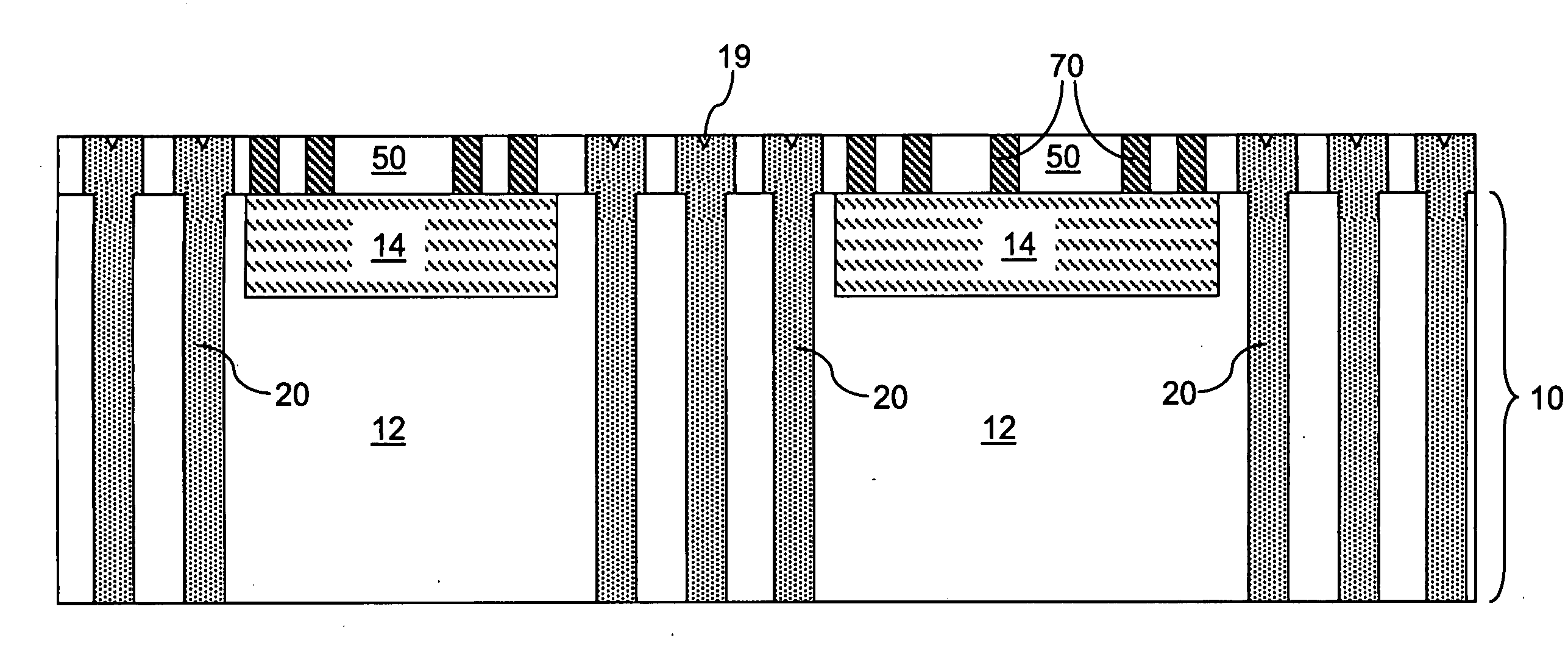
An array of through substrate vias (TSVs) is formed through a semiconductor substrate and a contact-via-level dielectric layer thereupon. A metal-wire-level dielectric layer and a line-level metal wiring structure embedded therein are formed directly on the contact-via-level dielectric layer. The line-level metal wiring structure includes cheesing holes that are filled with isolated portions of the metal-wire-level dielectric layer. In one embodiment, the entirety of the cheesing holes is located outside the area of the array of the TSVs to maximize the contact area between the TSVs and the line-level metal wiring structure. In another embodiment, a set of cheesing holes overlying an entirety of seams in the array of TSVs is formed to prevent trapping of any plating solution in the seams of the TSVs during plating to prevent corrosion of the TSVs at the seams.
Owner:GLOBALFOUNDRIES US INC
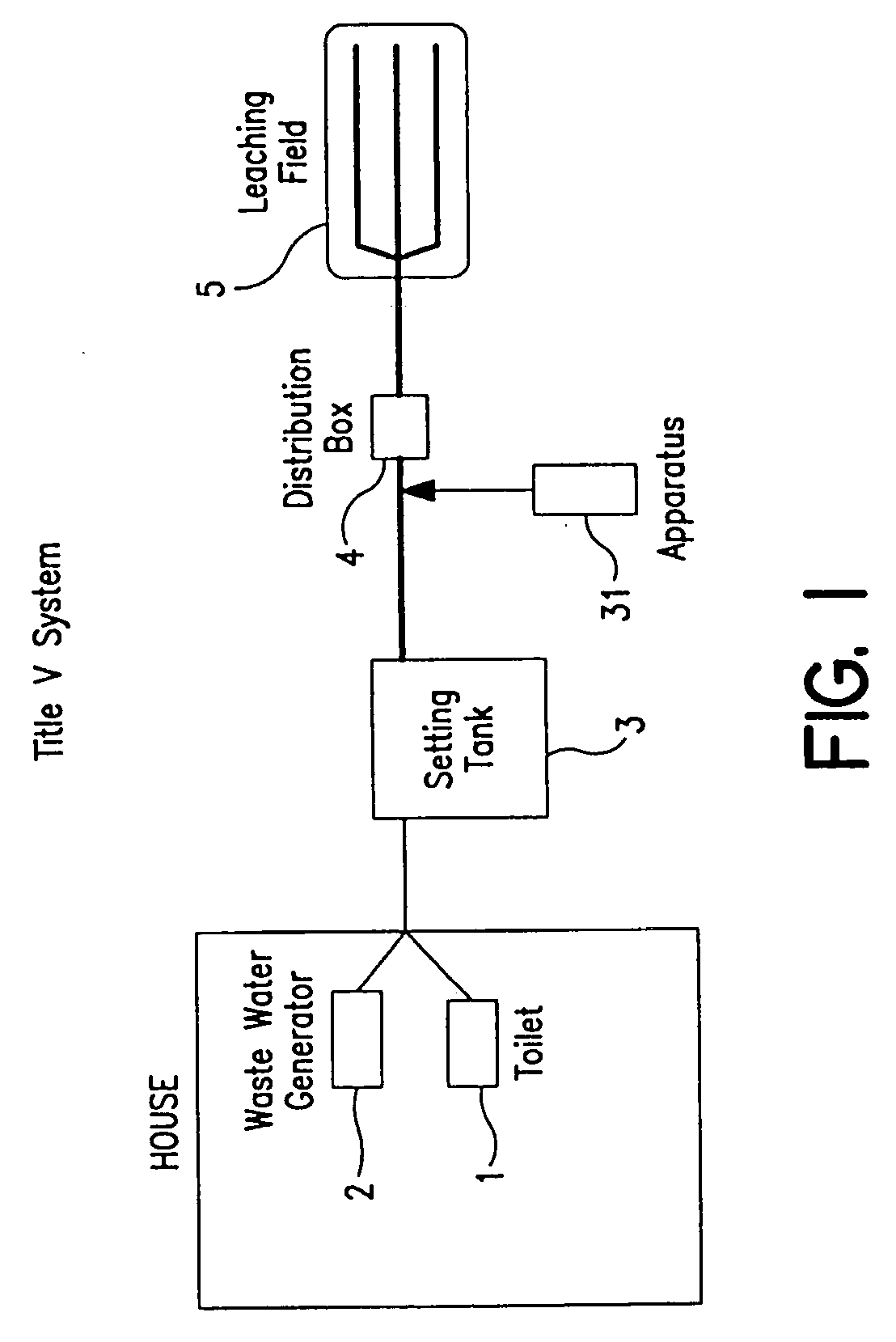
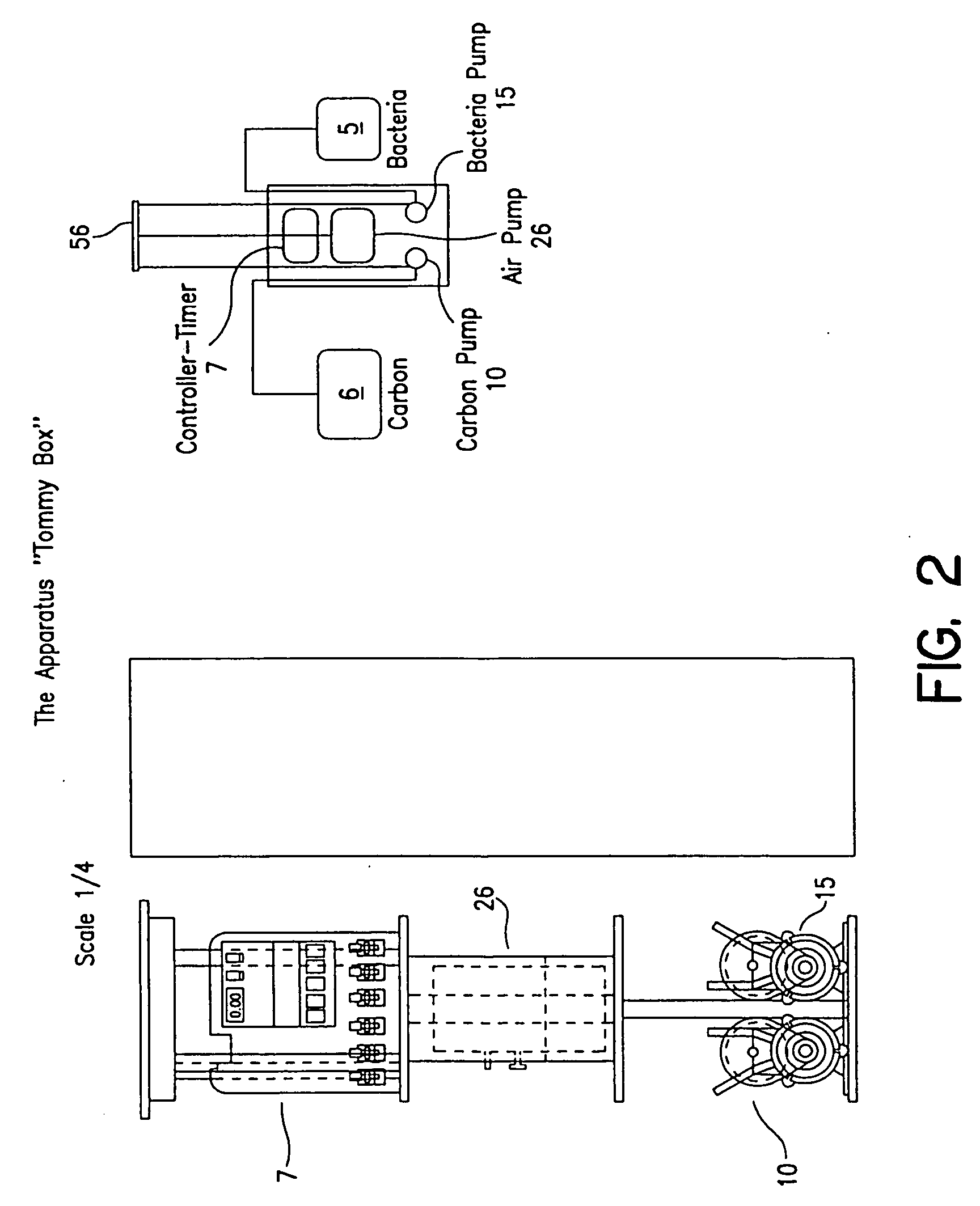
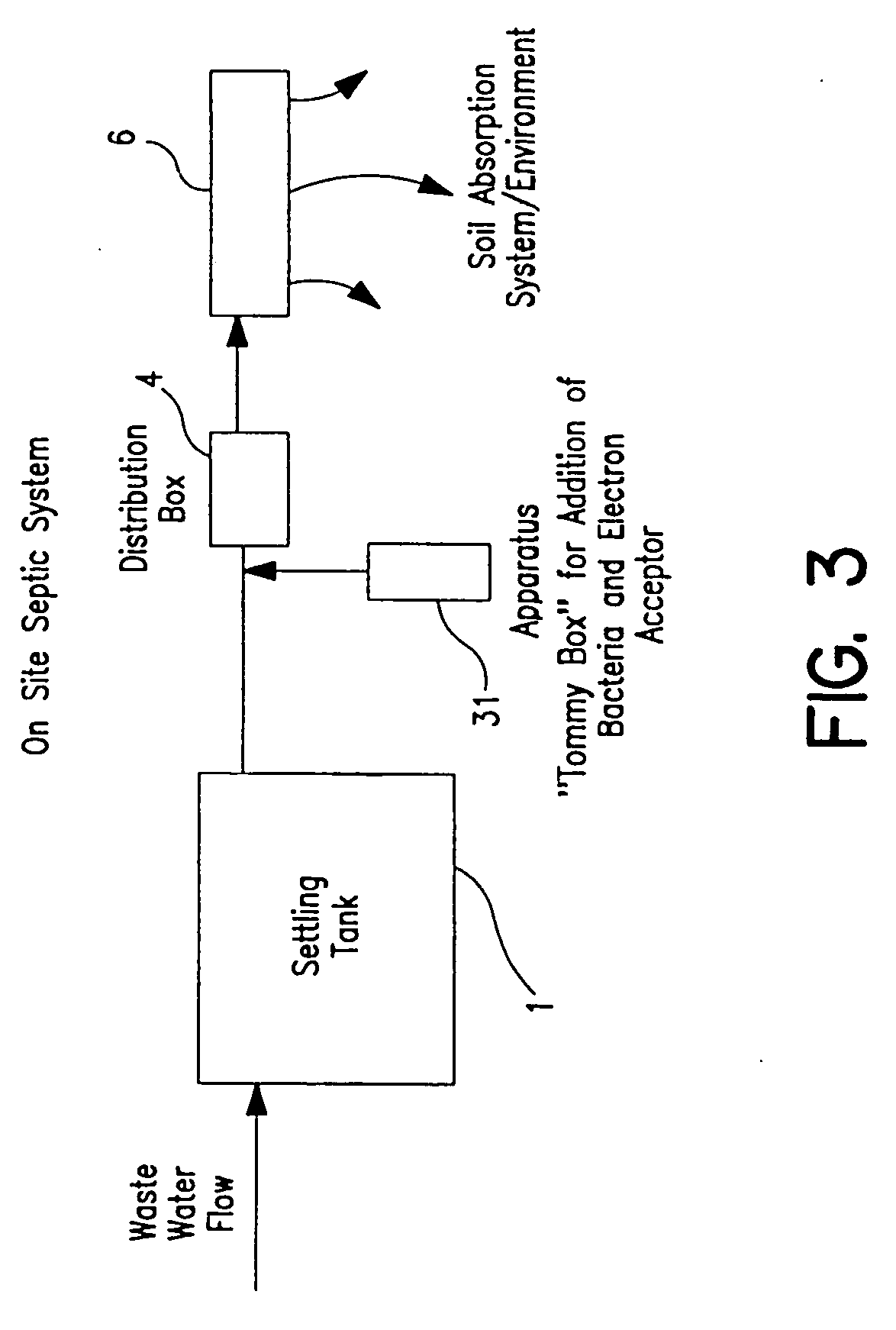
Process and apparatus for waste water treatment
InactiveUS20050145563A1Maximize contactEfficient mannerTreatment using aerobic processesBacteriaPollutantAnaerobic treatment
Systems for treating water containing unwanted contaminants. More particularly, the present invention relates to waste water treatment systems including biological media used to aerobically or anaerobically treat solid and liquid waste in water for large and small-scale waste water systems in a way that minimizes the size of the system required to output high-quality, environmentally suitable water that is depleted of ammonia, nitrites, nitrates and other contaminants.
Owner:BOYD STEVEN H +1
Apparatus and high speed process for making highly stretched film
InactiveUS6375781B1Maximize contactMinimize neck-inMechanical working/deformationLaminationElectrical and Electronics engineeringPre stretching
An apparatus and high speed process for heating and stretching a film having a bridle mechanism comprising a first heated roll and a second heated roll. The film is stretched as the film is transferred from the first heated roll to the second heated roll which rotates at a speed greater than the speed at which the first heated roll rotates. The film is fed into the bridle mechanism at a rate of about 1,500 fpm to about 2,500 fpm wherein it is stretched to a length that is up to about 450% greater than its initial, pre-stretched length. The increased contact between the large diameter heated rolls and the film and the short draw gap between the two heated rolls result in a highly stretched film having minimal neck-in.
Owner:ILLINOIS TOOL WORKS INC
Oxidation process using microchannel technology and novel catalyst useful in same
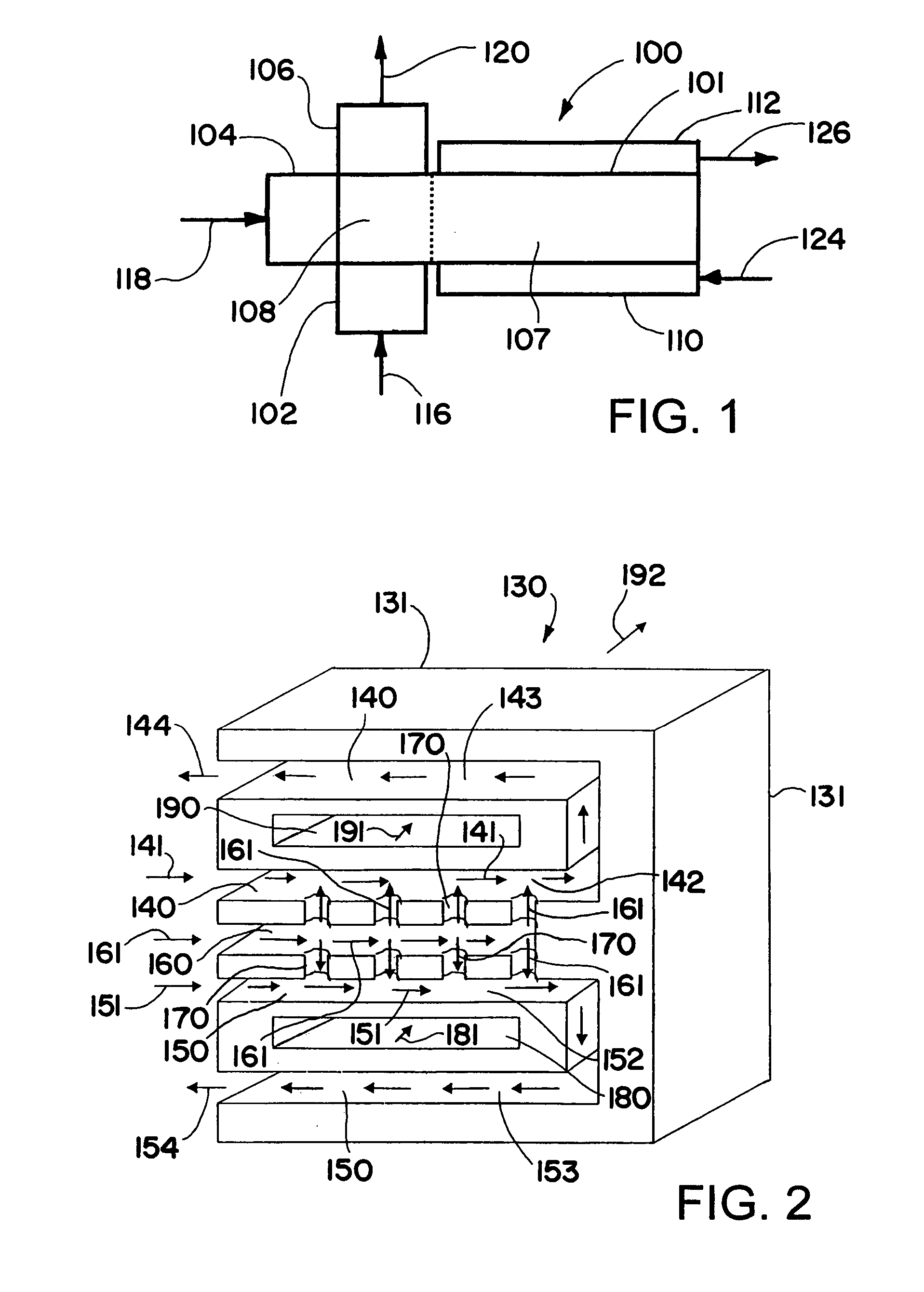
InactiveUS7226574B2Maximize contactMethane captureChemical/physical/physico-chemical microreactorsContact timeCarbon oxide
Owner:VELOCYS INC
Spinal fusion implant and related methods
ActiveUS20110112642A1Increase contactAvoids dural impingementBone implantJoint implantsIntervertebral discPlastic surgery
A spinal fusion implant of non-bone construction to be introduced into any variety of spinal target sites. The spinal fusion implant of the present invention includes a top surface, a bottom surface, first and second lateral sides, a proximal (posterior) end and a distal (anterior) end. The spinal fusion implant of the present invention may be used to provide temporary or permanent fixation within an orthopedic target site. To do so, the spinal fusion implant may be introduced into a disc space while locked to a surgical insertion instrument and thereafter employed in the proper orientation and released. Once deposited in the disc space, the spinal fusion implant of the present invention effects spinal fusion over time as the natural healing process integrates and binds the implant.
Owner:NUVASIVE
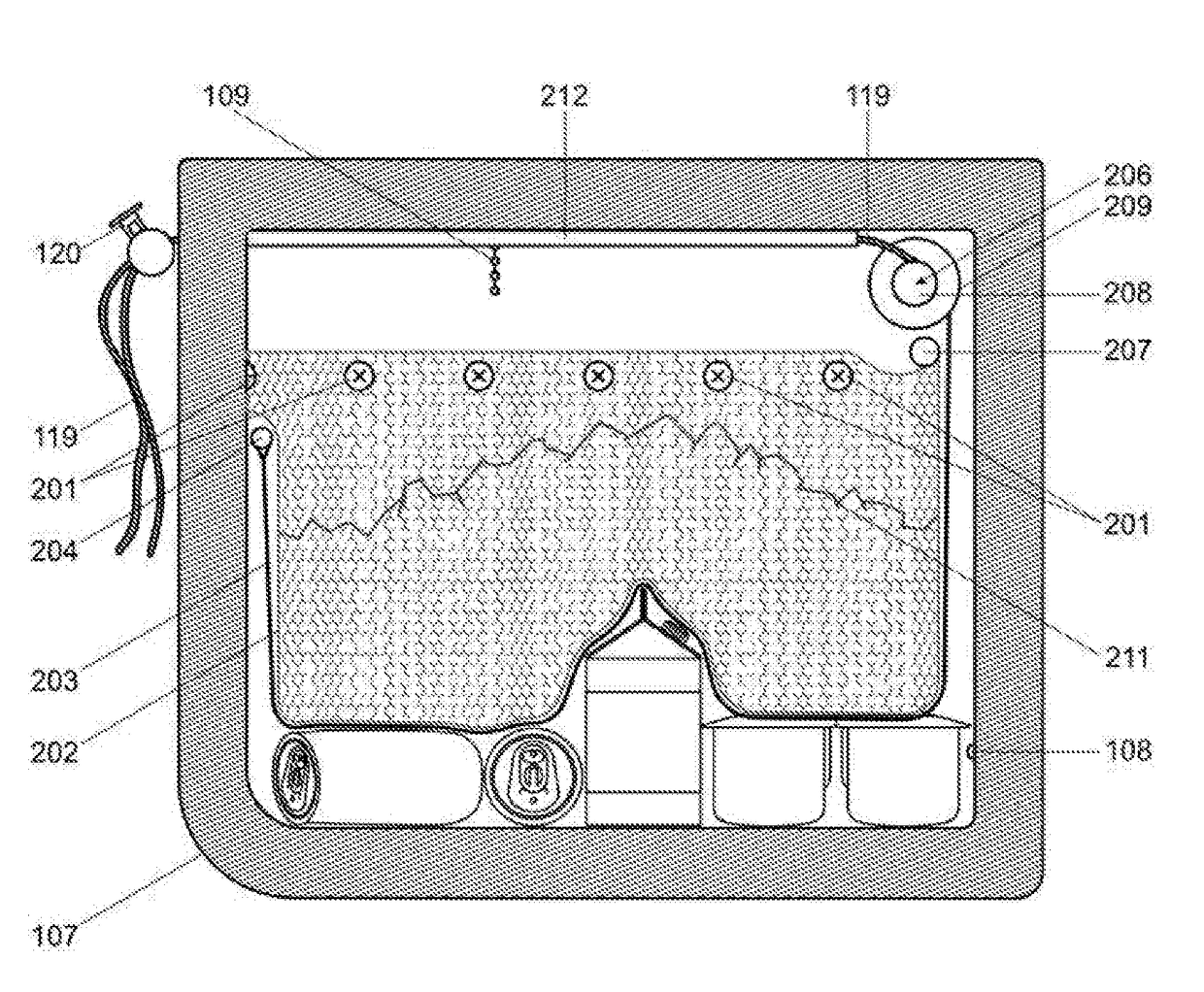
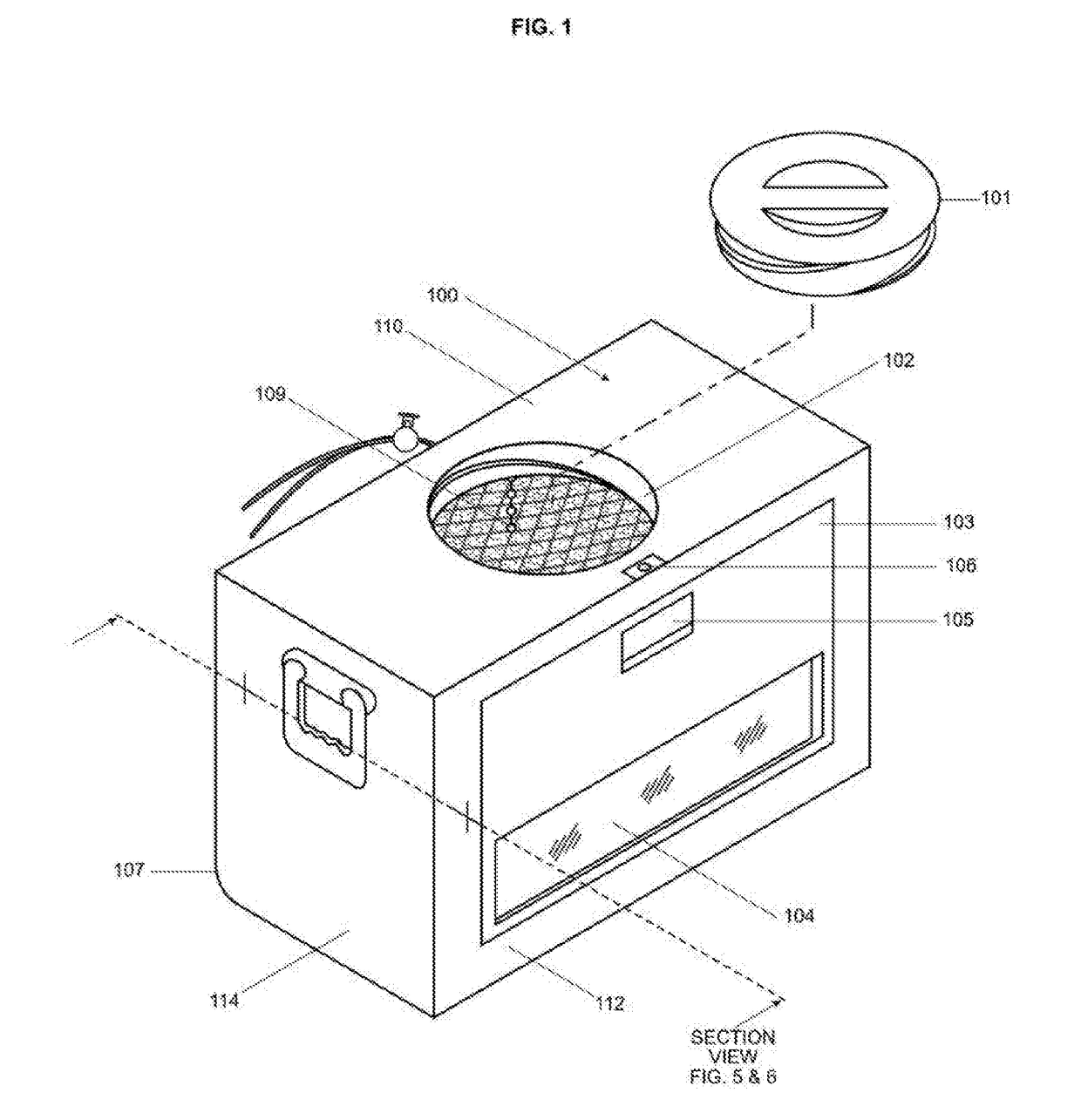
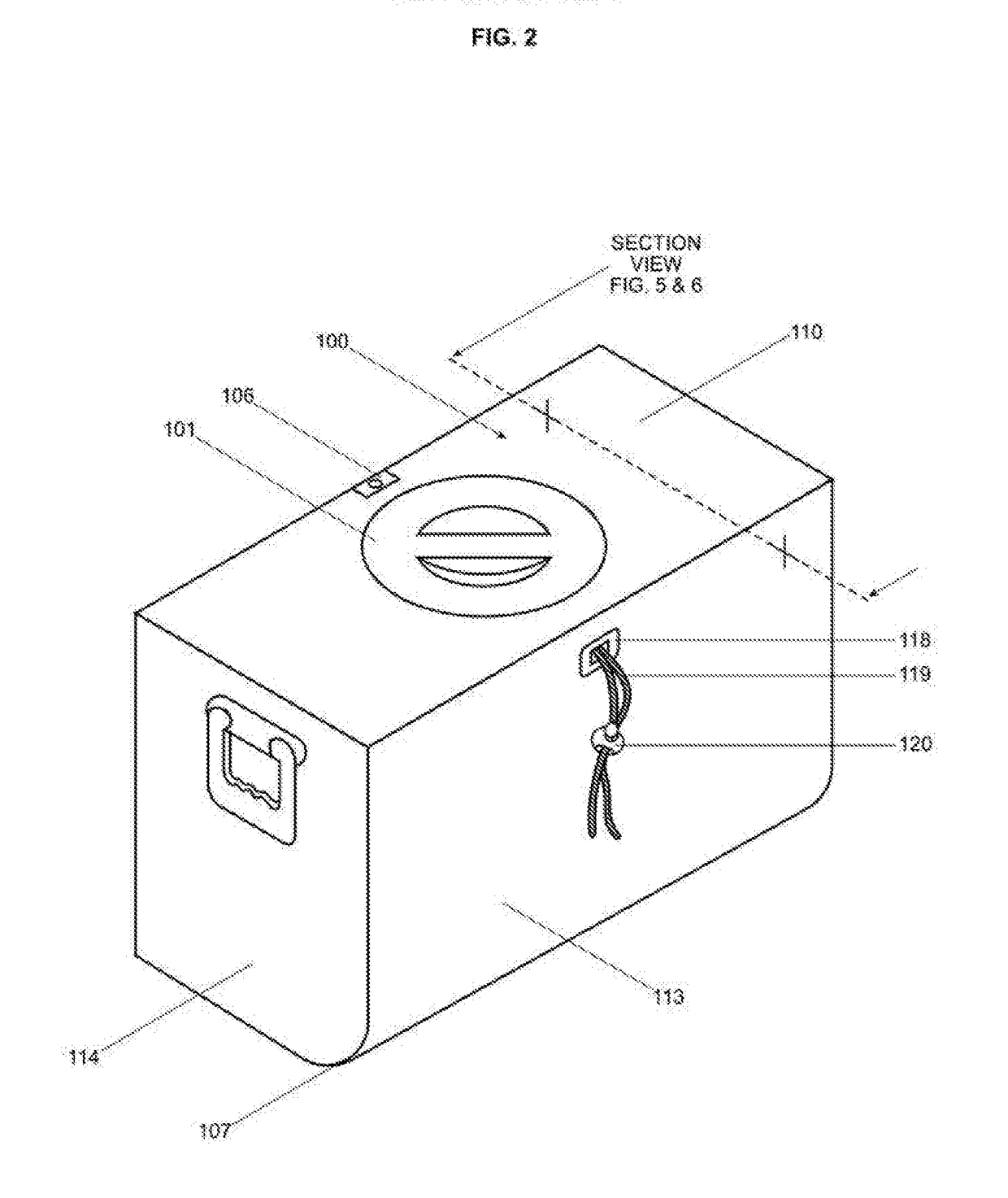
Retractable ice cooler
InactiveUS20170254578A1Improve cooling effectEasy to moveLighting and heating apparatusLighting arrangementMarine engineeringChiller
One embodiment of an improved portable ice cooler that eases access to the cooled contents by integrating in a single apparatus an adjustable ice containment device, a front door, a window, internal lighting and a specialized external contour. The benefits these features provide are a significant reduction in the discomfort and difficulty currently encountered when placing, locating, and removing contents from a current state of the art ice cooler that is accessed from the top or front. The ice containment device further offers the capability of holding a desired level of retraction to the ice within the cooler. The provision for retraction of the ice and thereby the ability to adjust both the proximity of the ice to the items in the cooler as well as the weight of the ice upon those items can be utilized to cool fragile objects.
Owner:KRIESEL ROGER MARK
Microfluidic Biological Extraction Chip
ActiveUS20100112667A1Increase reagent contactReduce bubble formationMicroorganism lysisSolid sorbent liquid separationElectric fieldPlanar electrode
A microfluidic cartridge for isolating biological molecules having a capture chamber containing functionalized solid supports maintained in a fluidized state provides reduced pressure drops and bubble formation during microfluidic extraction. The cartridge may include an electric field lysis chamber and / or a chemical lysis chamber. The electric-field lysis chamber may comprise an electrically insulating structure arranged between two opposing planar electrodes.
Owner:CFD RES COPORATION
Bone block assemblies and their use in assembled bone-tendon-bone grafts
ActiveUS7763071B2Add featureHigh tensile strengthSuture equipmentsSkin implantsBone CortexBone tendon bone
The present invention has multiple aspects. In its simplest aspect, the present invention is directed to an intermediate bone block comprising a machined segment of cortical bone, cancellous bone or both, the intermediate having a face comprising one to ten compression surfaces and one to ten cavities, the compression surfaces suitable for compressing soft tissue, the cavities for receiving and holding overflow soft tissue. The cavities are preferably channels, and more preferably channels having an omega cross-sectional profile. The invention is also directed to bone block assemblies suitable for binding to a soft tissue to form an implantable graft, and to such implantable grafts. A particularly preferred graft is a bone-tendon-bone graft.
Owner:RTI BIOLOGICS INC
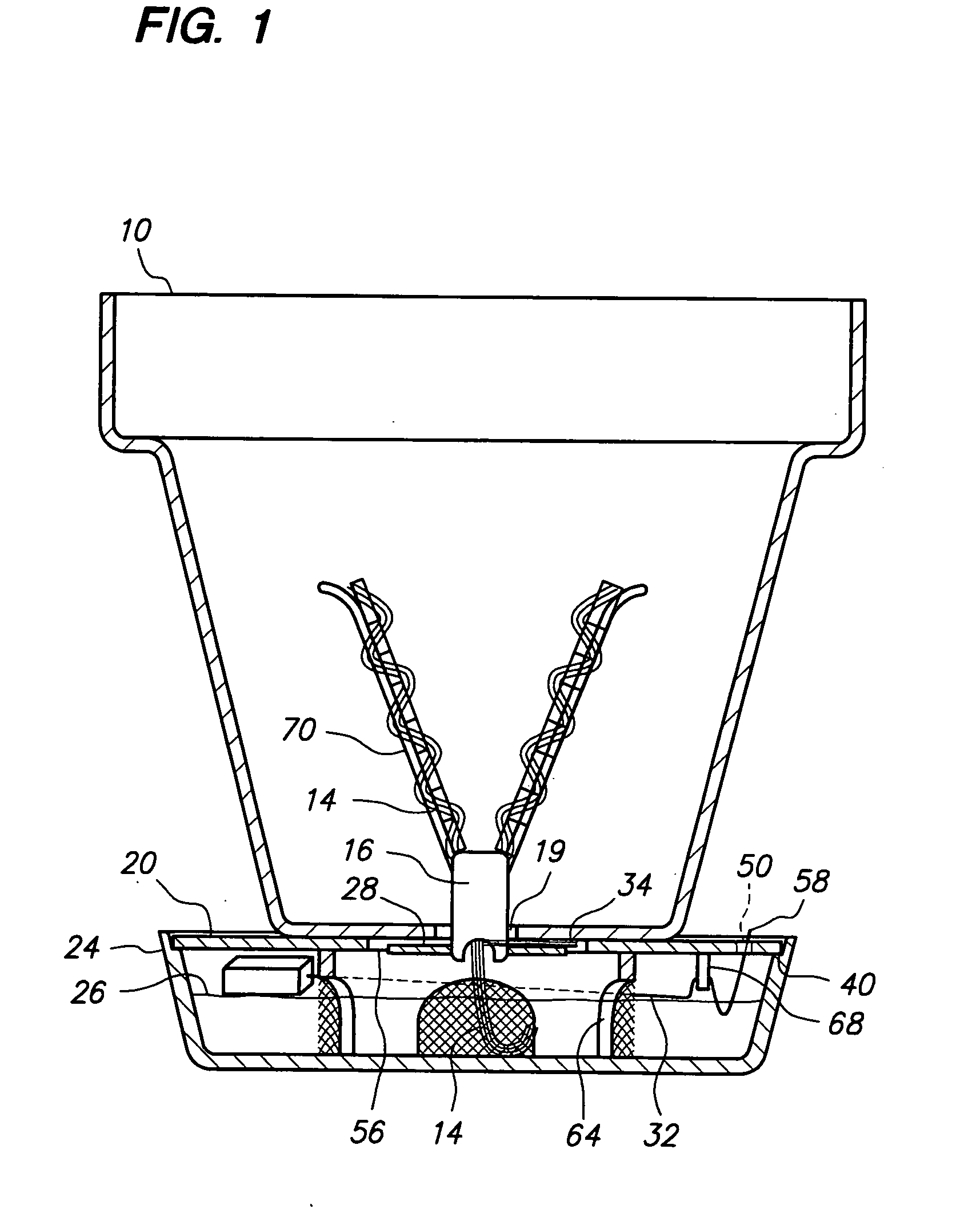
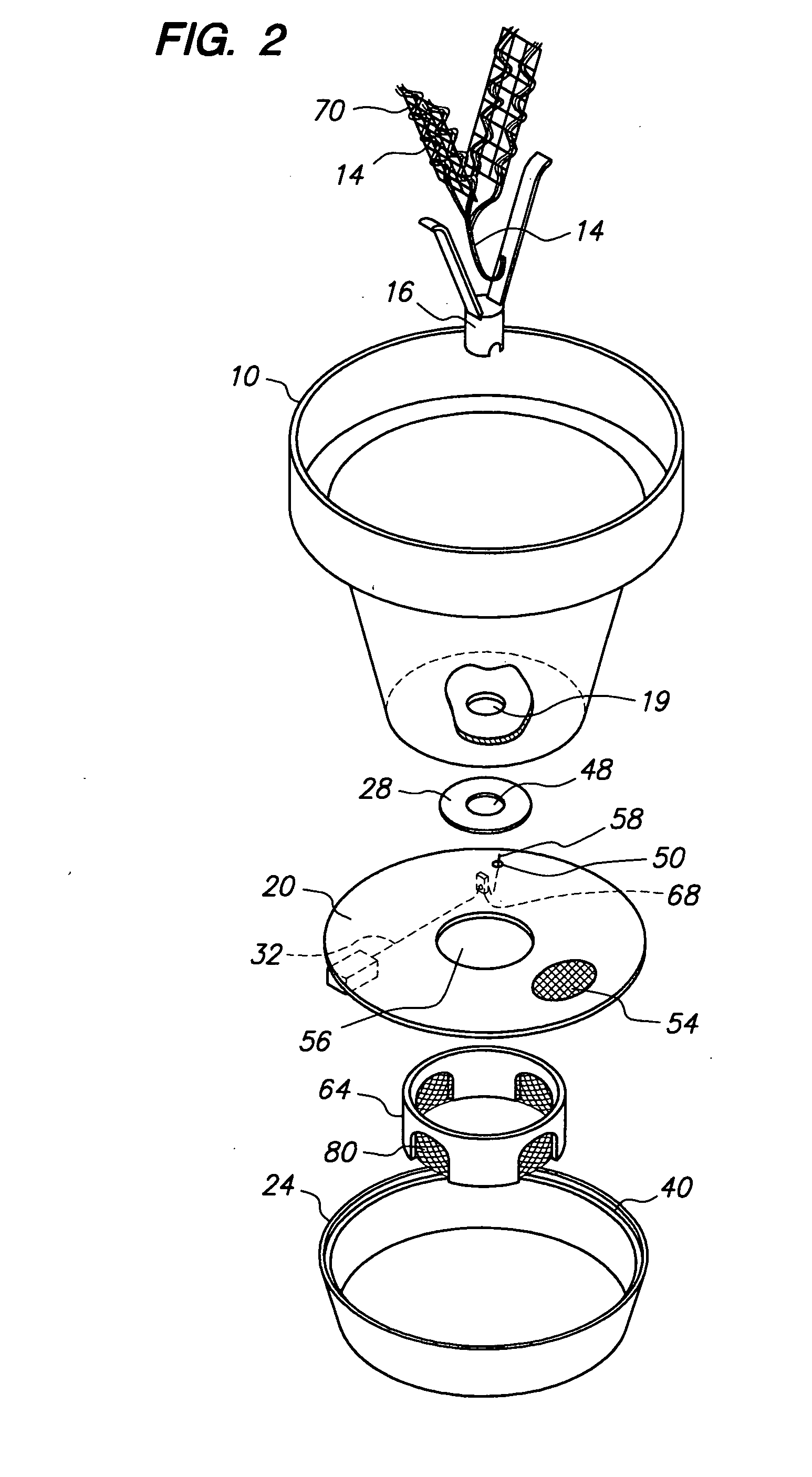
Potted plant watering system
InactiveUS20090064576A1Easily and consistently controlMinimizes water level indicator damage and confusion in water levelSelf-acting watering devicesWatering devicesWater flowConfusion
A potted plant watering system that uses the capillary action of a wick to draw water up from a water reservoir and into the soil of a potted plant via a plurality of fingers of a wick support member that secure the wick in predetermined patterns and maximizes soil contact during wet and dry conditions and distributes water evenly throughout the plant pot. A water flow control device attaches to the wick support member and by separating the wick's strands from contact with the water, controls the flow of water through the wick. A float mechanism provides for a reservoir level indication with minimum rise and arc of the float rod above the surface of the water reservoir and therefore minimizes risk of rod damage and observer confusion. The wick, flow control, and water level indication systems can be used with any existing plant pot equipped with a drainage hole.
Owner:SUGAREK STEVEN LEE SUGAREK
Hydroponic plant container with highly oxygenated nutrient solution using continuous air injection and continuous coriolis effect mixing
InactiveUS8667734B2Increase dissolved oxygen levelsMaximize contactSelf-acting watering devicesAgriculture gas emission reductionPlant rootsNutrient solution
A hydroponic plant container with a reservoir of “highly oxygenated” nutrient solution that is the result of continuous direct air injection into the bottom of the reservoir, at a specific range of trajectories, along with continuous Coriolis effect mixing. The highly oxygenated nutrient solution is used to provide a deep water culture for the lower section of the plant roots, to saturate the middle section of the plant roots with fine droplets of the highly oxygenated nutrient solution, and to saturate the growing media and the upper section of the plants roots with a continuous drip of the highly oxygenated nutrient solution.
Owner:JOHNSON DAN +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com