Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
39results about How to "Improve the overall yield of synthesis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
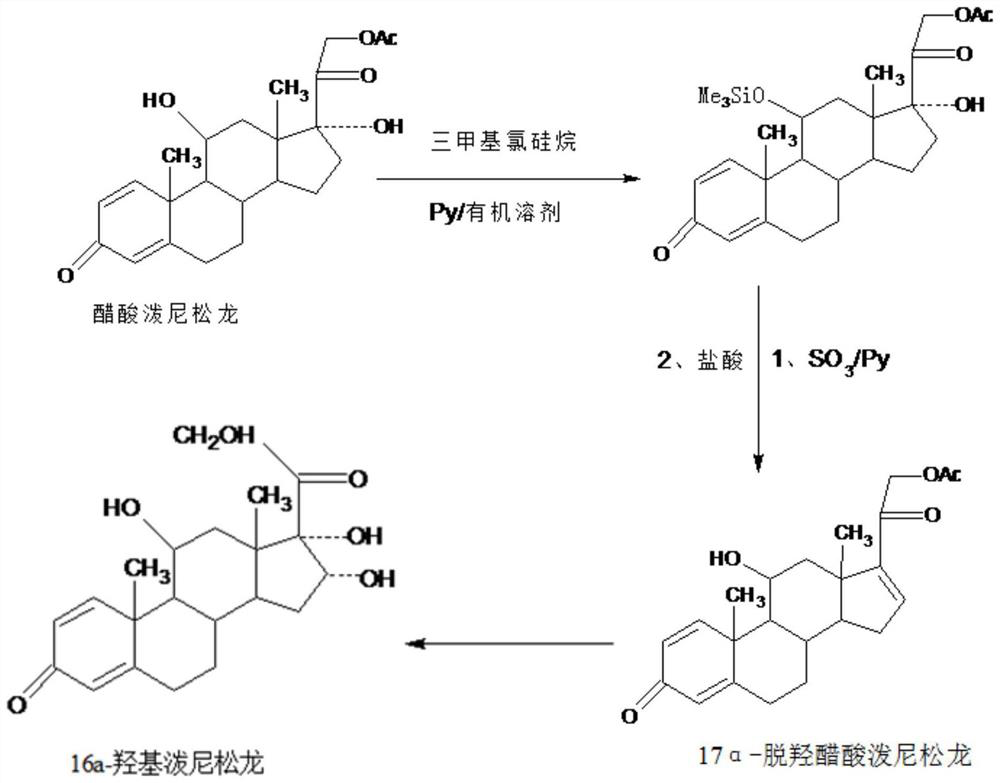
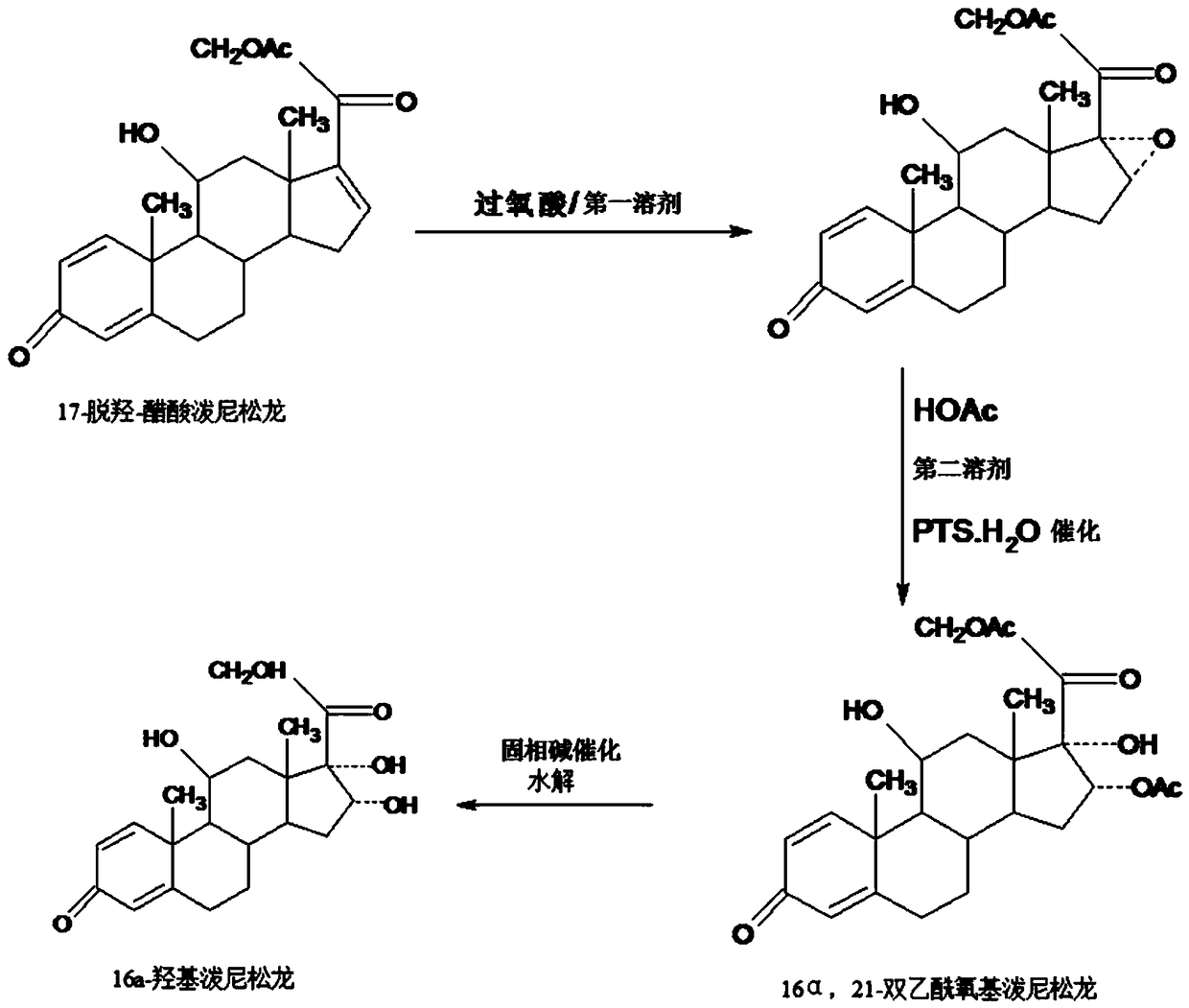
Preparation method of 16a-hydroxy prednisolone
ActiveCN107488203AInhibition of rearrangement and ring expansion side reactionsReduce generationPhysical/chemical process catalystsOrganic chemistry methodsOrganic solventAlcohol
The invention discloses a preparation method of 16a-hydroxy prednisolone. The preparation method comprises the following steps: dissolving 16a-hydroxy prednisolone acetate into an organic solvent, adding an inert solid carrier adsorbed with strong base as a hydrolysis reaction solid-phase base catalyst, and hydrolyzing 21-acetate to obtain a 16a-hydroxy prednisolone crude product; and carrying out lower alcohol recrystallization on the crude product under the condition of below C4 to obtain a 16a-hydroxy prednisolone competitive product, wherein the refined weight yield is 85% to 90%, and the preparation weight total yield is 75% to 80%. The solid carrier is selected from aluminum oxide, silica gel or calcium carbonate; the base catalyst is selected from sodium carbonate; and the organic solvent is selected from methylbenzene or chloroform. Compared with a traditional method, the method disclosed by the invention is simple and convenient in production operation, impurities generated in traditional production can be greatly reduced, and the total yield for synthesis is greatly improved; compared with the traditional method, the production cost is reduced by 10% to 15%; and a synthetic reaction solvent can be recycled, and industrial production is facilitated.
Owner:HUNAN KEREY BIOTECH
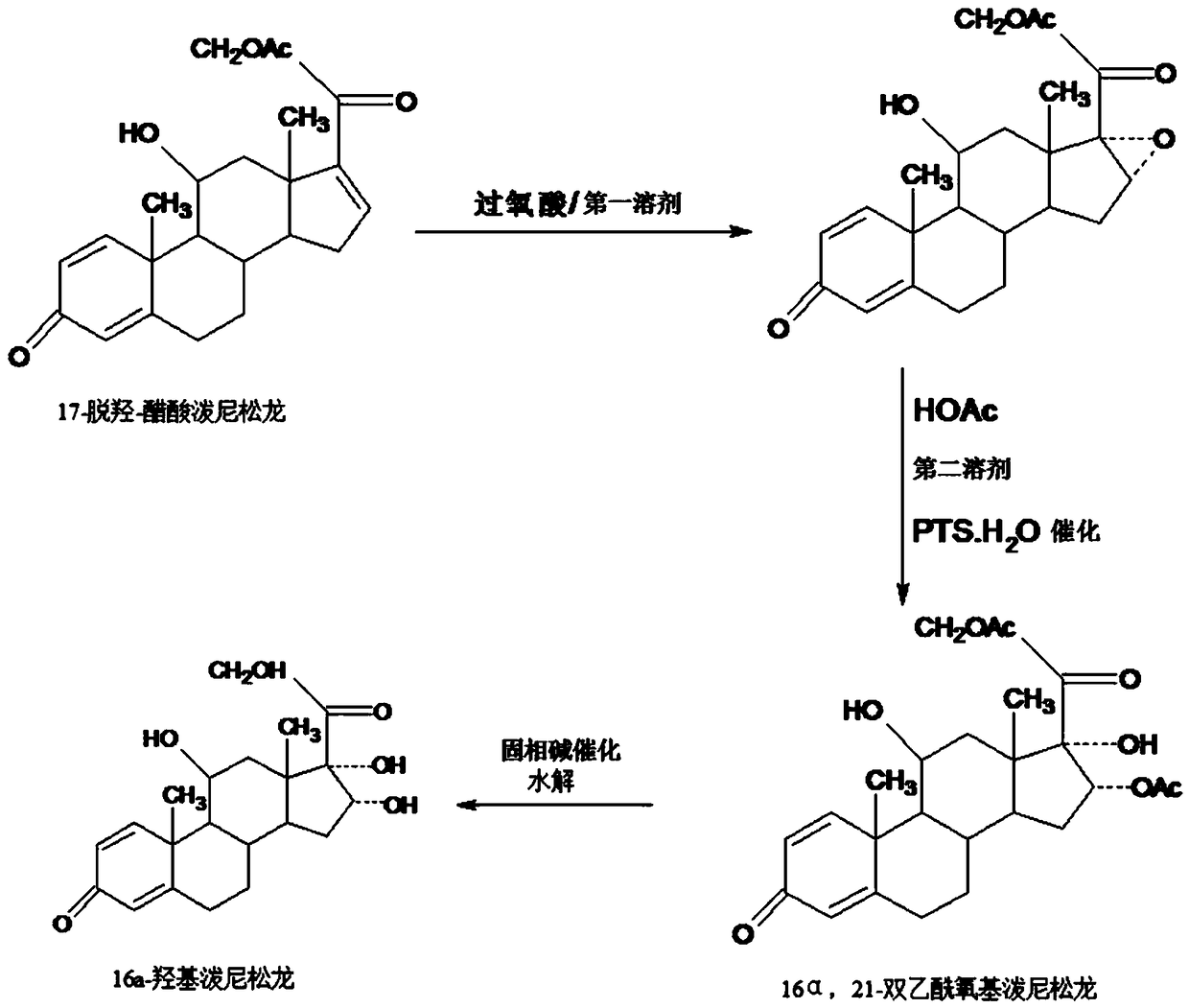
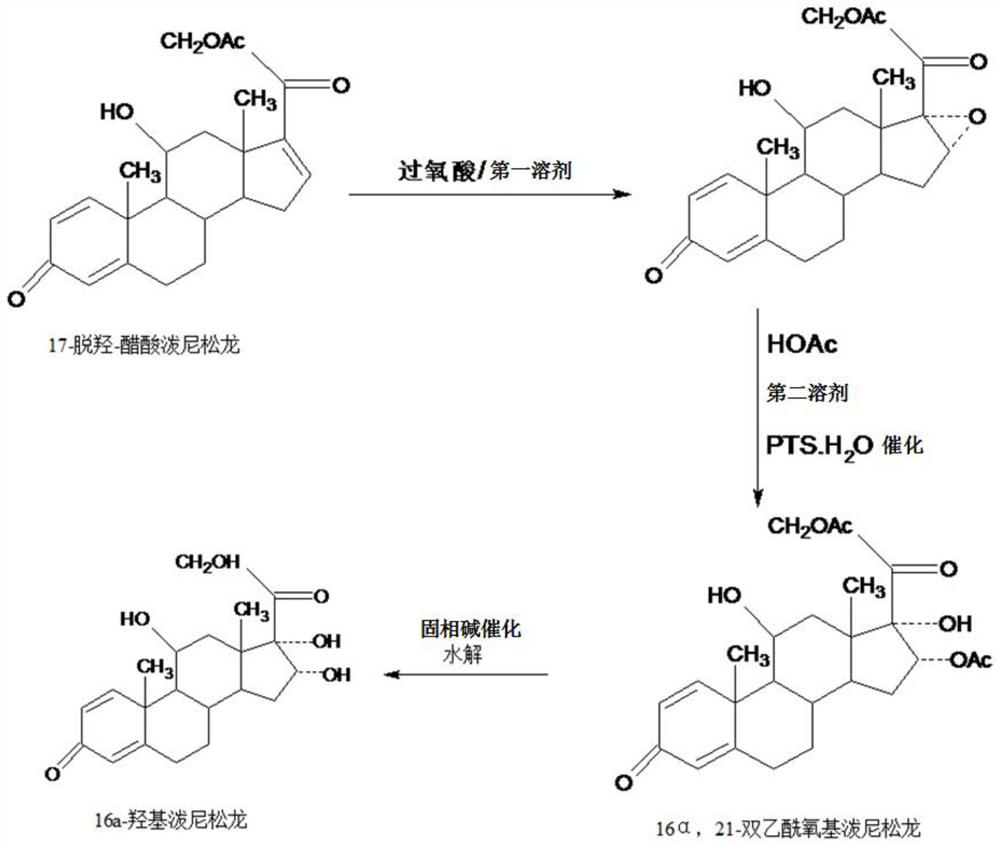
Method for preparing 16a-hydroxyl prednisolone product
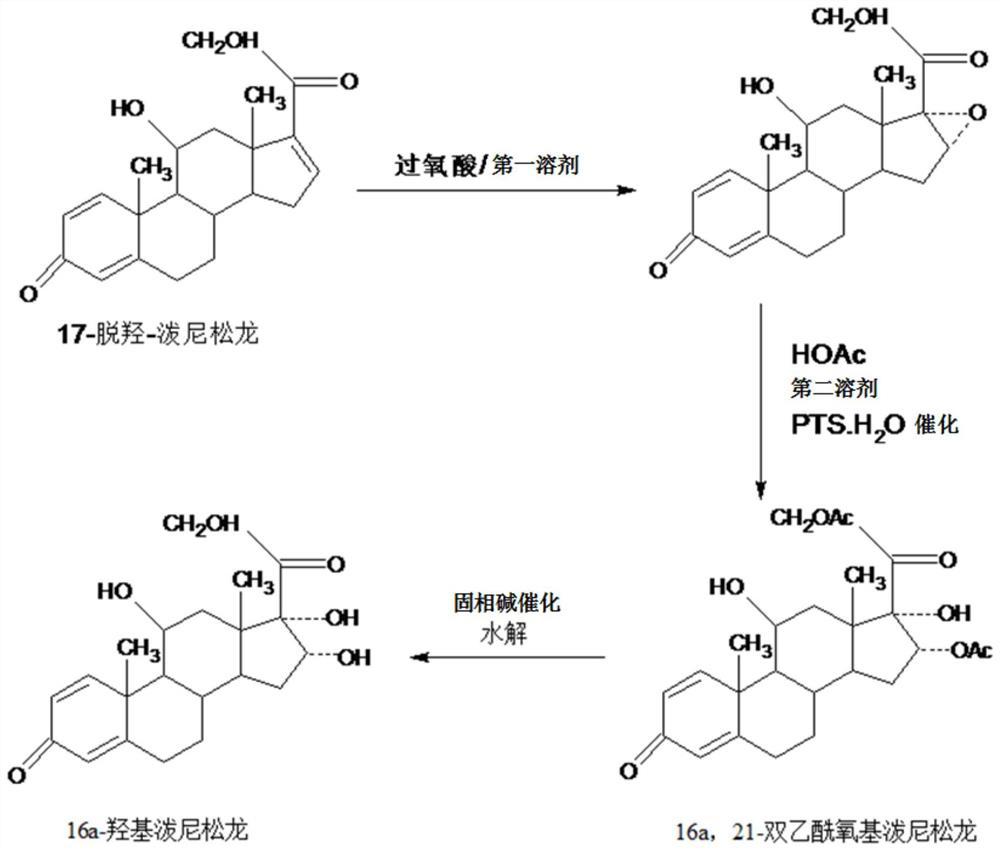
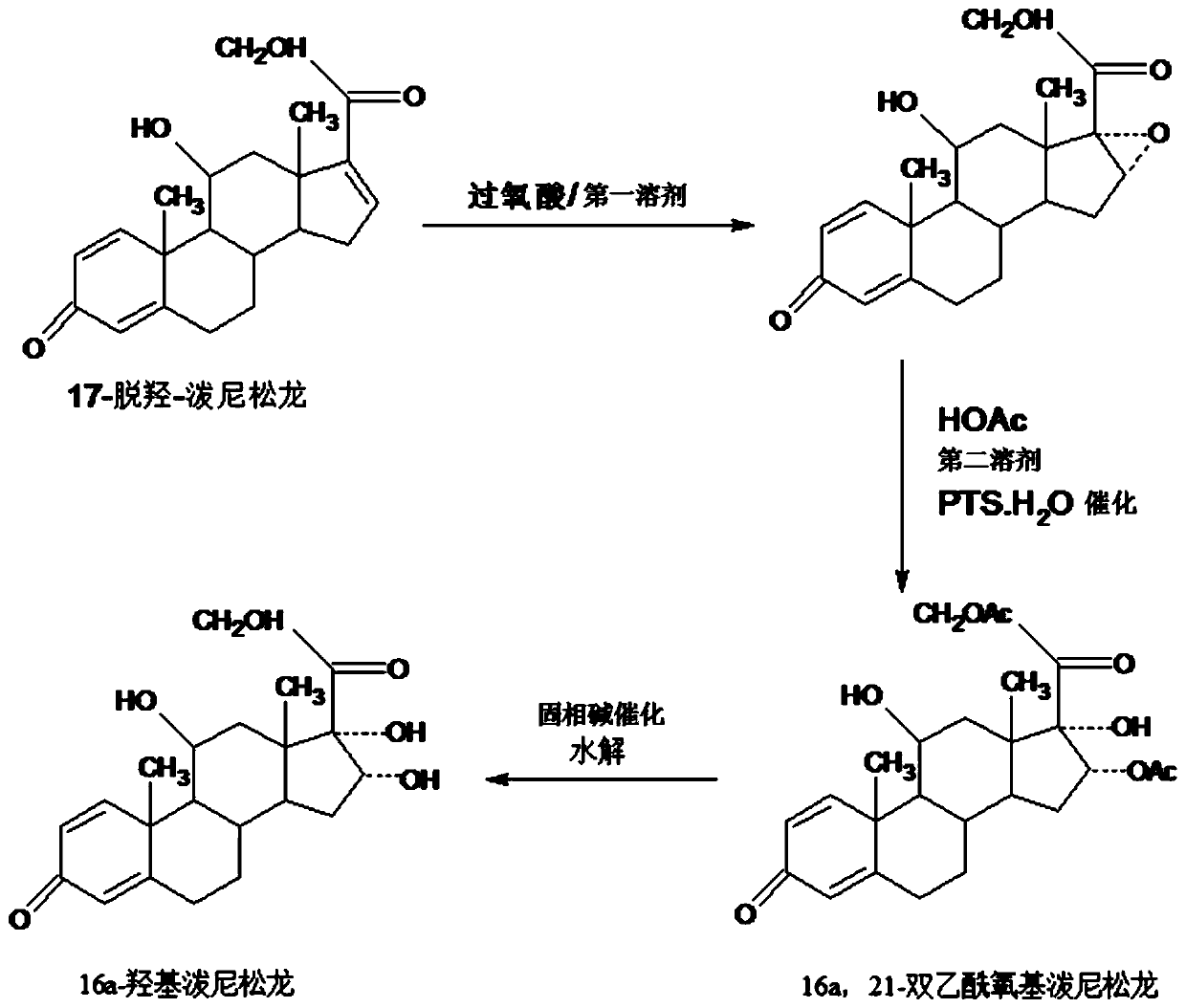
InactiveCN109232696AAvoid many difficulties such as difficult purificationEasy to operateSteroidsAcetic acidOrganic solvent
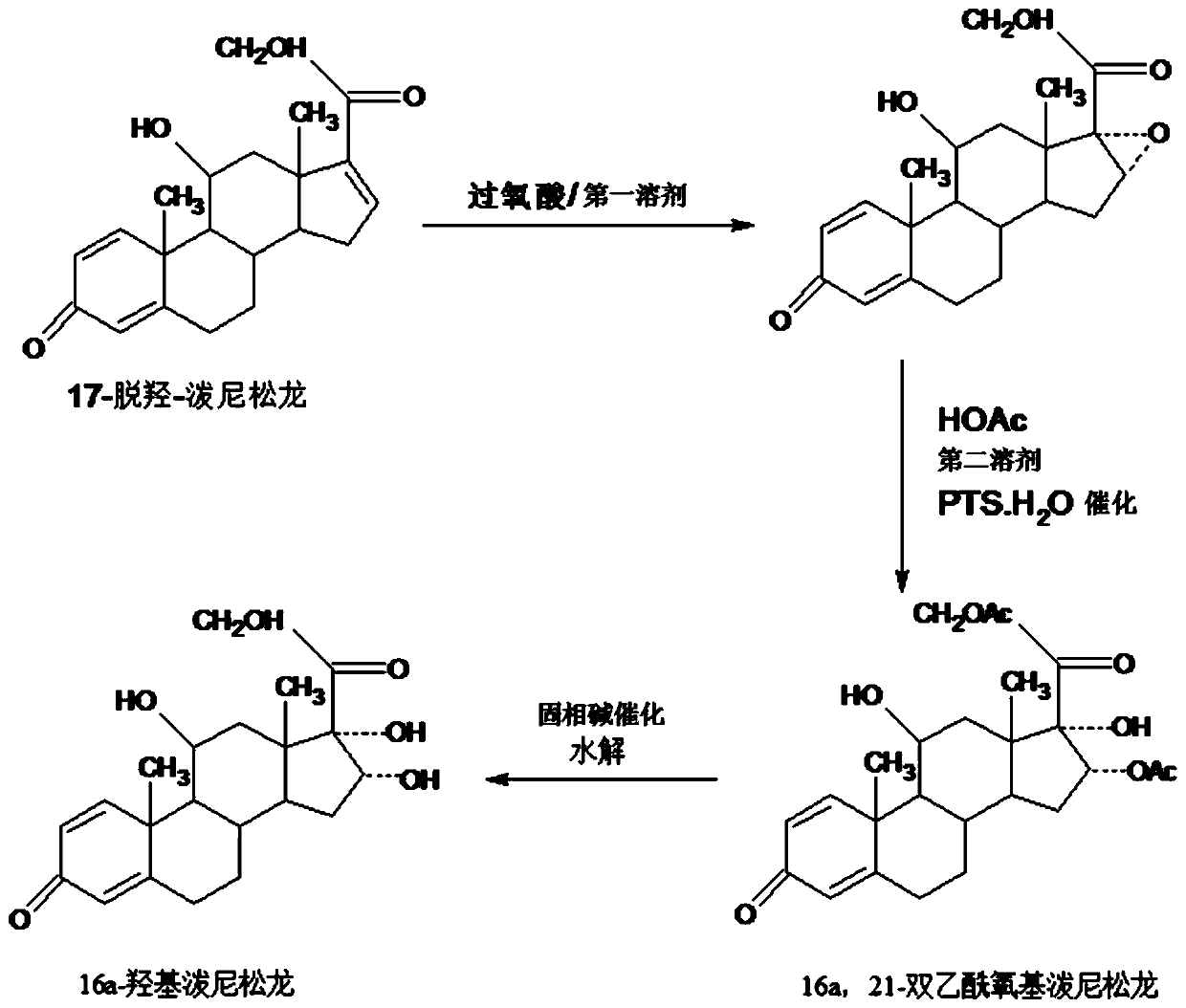
The invention provides a method for preparing a 16a-hydroxyl prednisolone product. The method comprises the steps: firstly, subjecting 17a-deshydroxy prednisolone acetate, which serves as a starting raw material, and organic peroxy acid to an epoxidation reaction at 16,17 sites in a first organic solvent, so as to prepare epoxide; subjecting the epoxide and glacial acetic acid to a ring-opening reaction under the catalysis of an acid catalyst in a second organic solvent, so as to prepare 16a,21-diacetoxyl prednisolone; then, dissolving the 16a,21-diacetoxyl prednisolone in a third organic solvent, and hydrolyzing acetate of two positions under the catalysis of a solid-phase alkali catalyst, so as to prepare 16a-hydroxyl prednisolone; finally, subjecting the crude 16a-hydroxyl prednisoloneobtained through solid-phase alkali-catalyzed hydrolysis to heated refluxing, decoloring and recrystallization by low carbon alcohols of C4 or less, thereby obtaining the 16a-hydroxyl prednisolone product. The 16a-hydroxyl prednisolone is prepared by the efficient, environment-friendly and cheap method.
Owner:HUNAN KEREY BIOTECH
Preparation method of 16alpha-hydroxy prednisolone
InactiveCN109081861AAvoid many difficulties such as difficult purificationEasy to operateSteroidsSodium carbonatePrednisolone acetate
The invention provides a preparation method of 16alpha-hydroxy prednisolone, comprising: subjecting 17alpha-deshydroxy prednisolone acetate as an initial material to 16,17-epoxidation with an organicperoxy acid in a first organic solvent to obtain an epoxide; subjecting the epoxide, in a second organic solvent, to ring-opening reaction with glacial acetic acid under the catalysis of an acid catalyst to obtain 16alpha,21-diacetoxy prednisolone; dissolving 16alpha,21-diacetoxy prednisolone in a third organic solvent, and hydrolyzing acetates in two positions under the catalysis of a solid alkaline catalyst, wherein the solid alkaline catalyst is made by adsorbing sodium carbon or sodium hydroxide to aluminum oxide, silicone or calcium carbonate as a support. The 16alpha-hydroxy prednisoloneis prepared via the method which is efficient, environmentally and fair in cost.
Owner:HUNAN KEREY BIOTECH
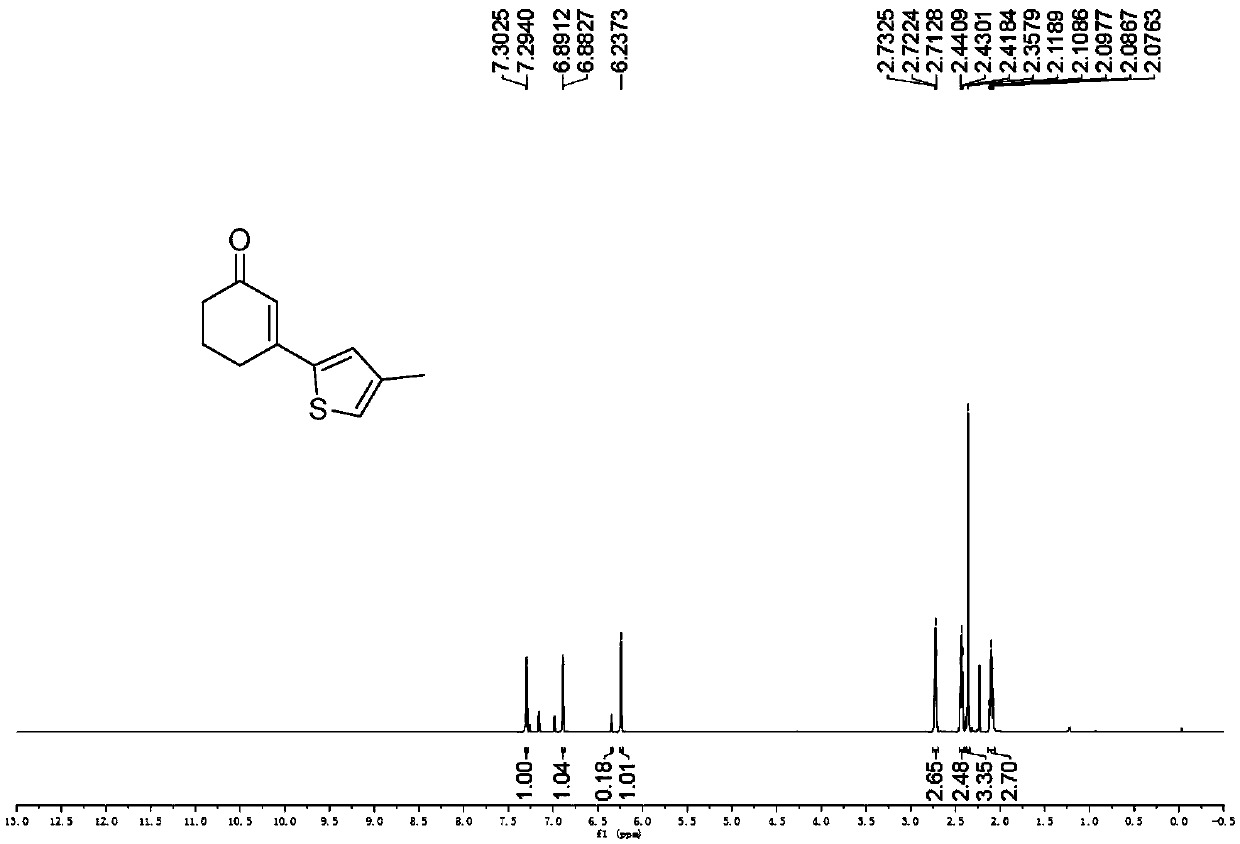
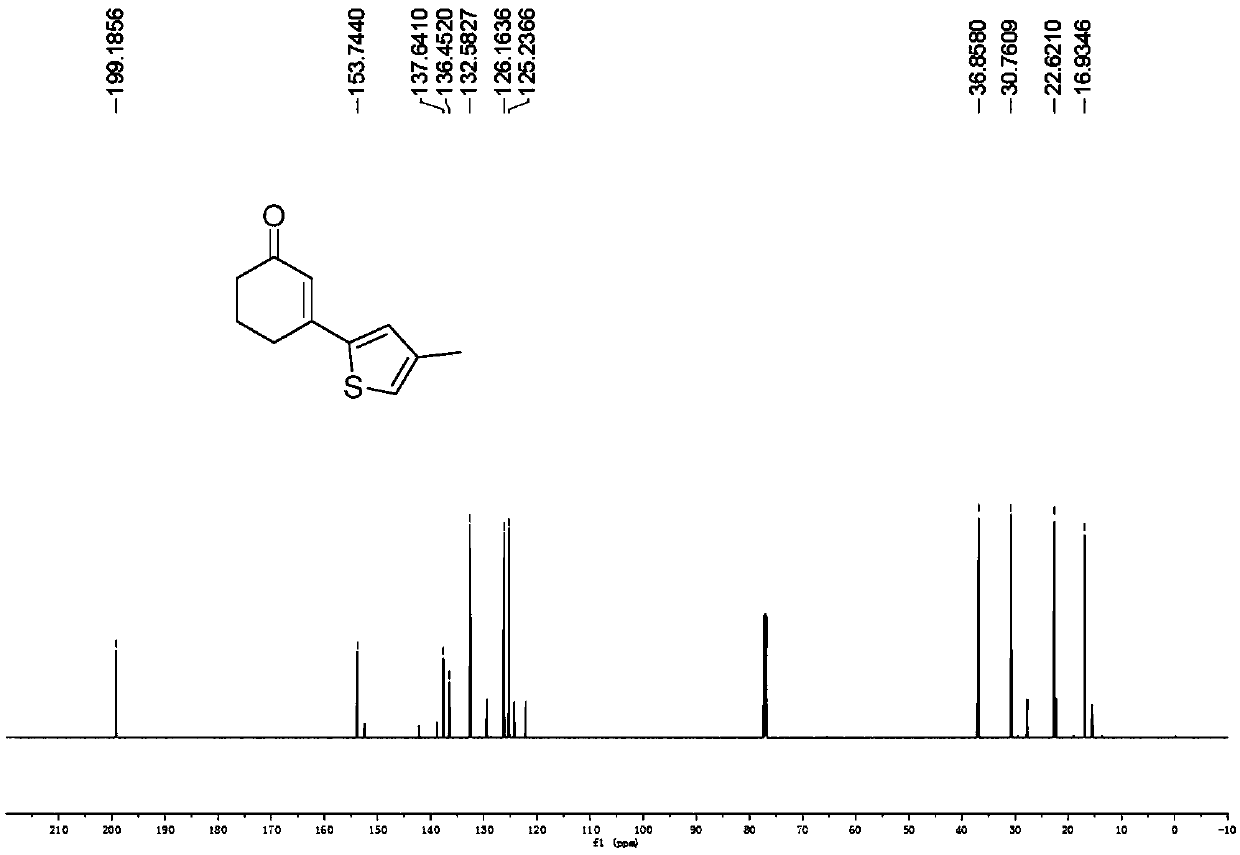
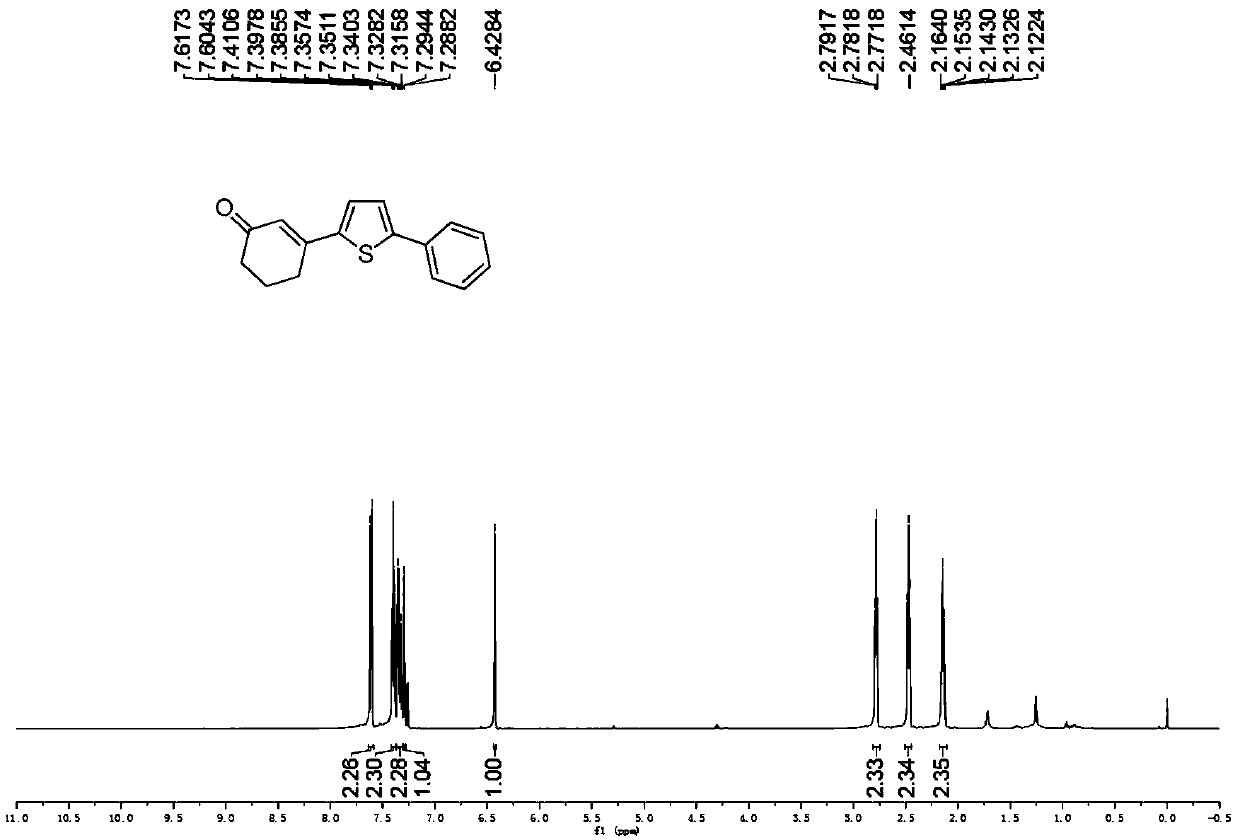
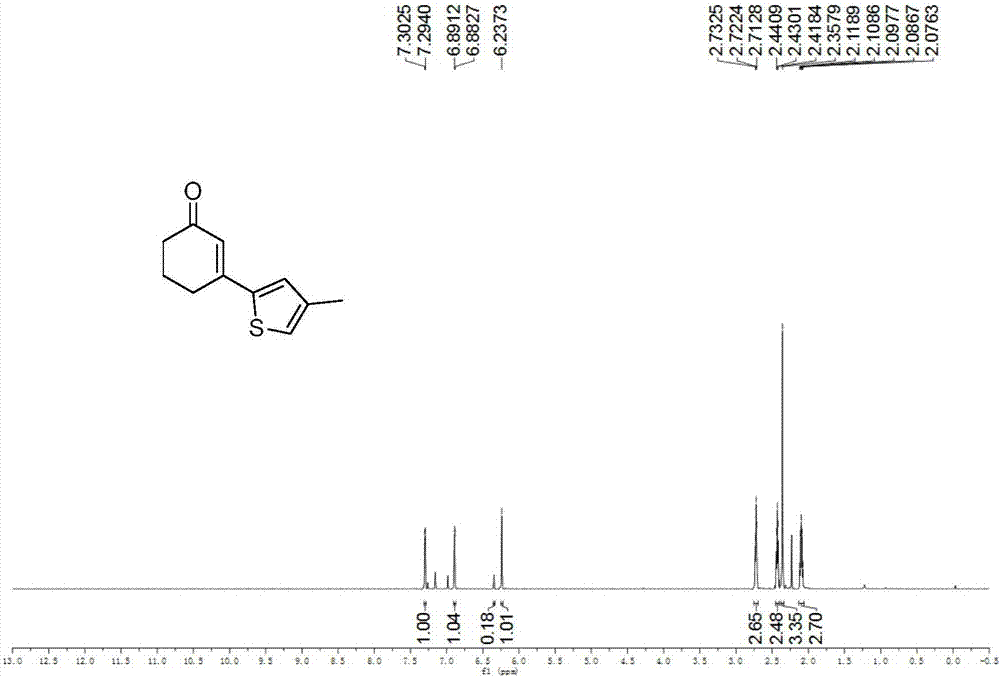
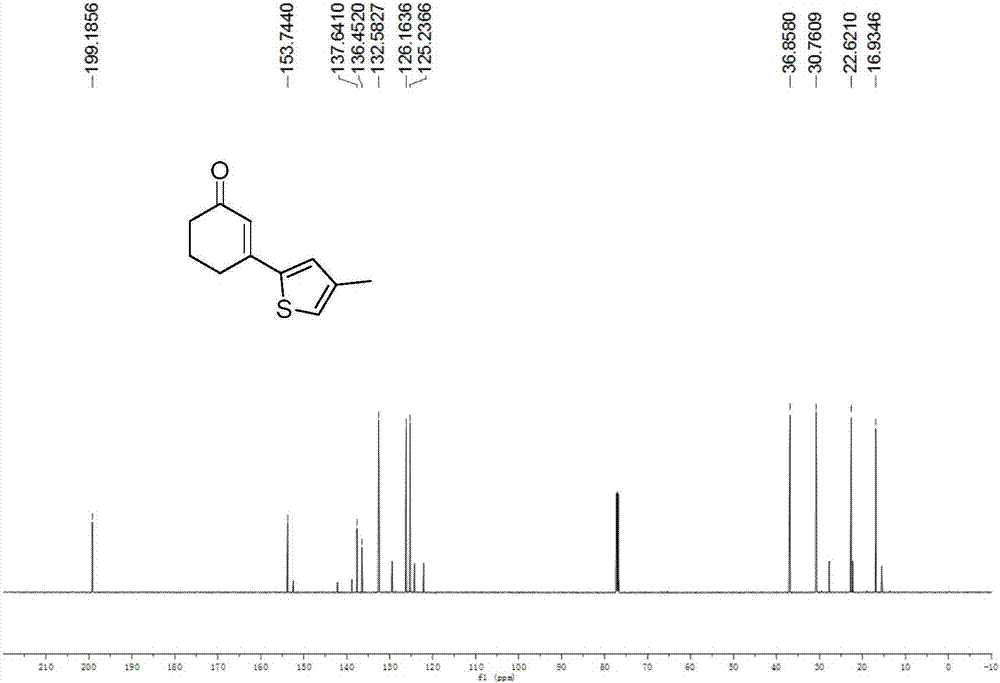
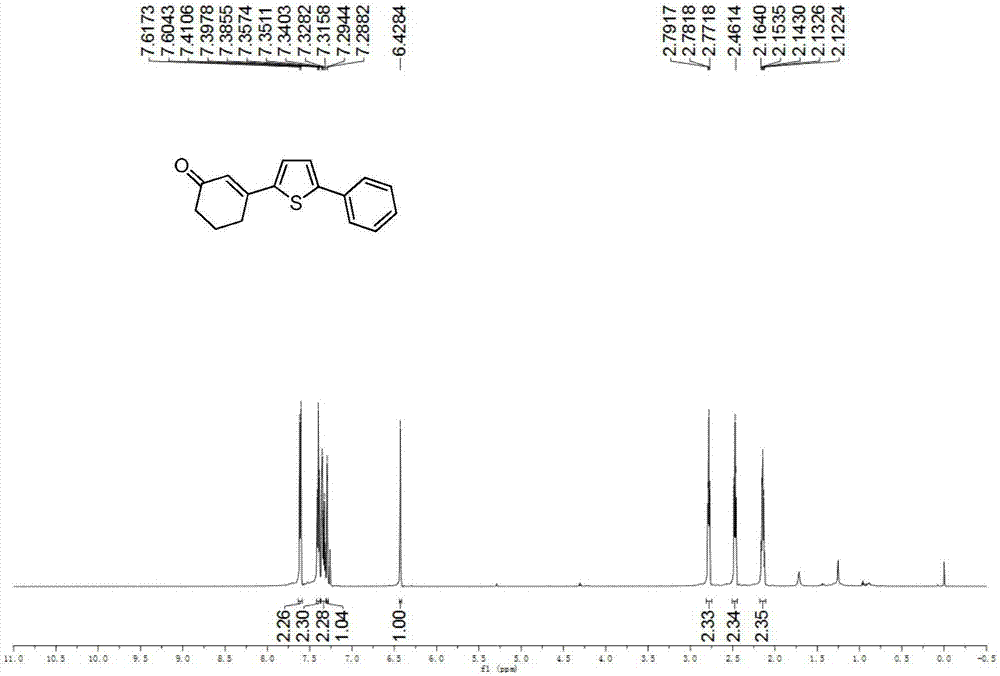
Preparation method of 3-(thiophene-2-yl)hexanaphthene-2-ketene derivatives
ActiveCN107141279AThe reaction steps are simpleImprove the overall yield of synthesisOrganic chemistryThiophene derivativesChemistry
The invention relates to the technical field of a preparation method of thiophene derivatives, in particular to a preparation method of 3-(thiophene-2-yl)hexanaphthene-2-ketene derivatives. The preparation method of the 3-(thiophene-2-yl)hexanaphthene-2-ketene derivatives comprises the following steps: (1) adding cyclohexenone, thiophene or substituted thiophene, palladium chloride, silver carbonate, N-acetoglycocoll and silver hexafluoroantimonate into a reactor sequentially according to the molar ratio of 3:1:0.1:2.5:0.1:0.2, adding a solvent to dissolve the reactants, mixing uniformly at a room temperature, and reacting at 60 to 120 DEG C for 5 to 48 hours; (2) after the reaction, cooling the reactor to a room temperature, dissolving with ethyl acetate, washing with a saturated ammonium chloride aqueous solution and a saturated sodium chloride aqueous solution sequentially, drying the organic phase by using anhydrous sodium sulfate, filtering and rotating on a rotary evaporator to remove the solvent; (3) separating and purifying the residues through silica-gel column chromatography after the solvent is removed through rotation, collecting a target product, performing rotary evaporation to remove the solvent, and pumping and drying by an oil pump.
Owner:SHANXI UNIV
Method for extracting nitration product from N-ethyl carbazole nitration solvent distillation residue
ActiveCN103254117ASimple extraction processImprove efficiencyOrganic chemistryEthyl phosphateActive carbon
The invention discloses a method for extracting a nitration product from N-ethyl carbazole nitration solvent distillation residue. The method is characterized by comprising the following steps of: putting the N-ethyl carbazole nitration solvent distillation residue and an organic solvent in a container, heating while stirring to a reflux state, stirring for 0.5-6 hours while preserving heat, adding a flocculant and continuing stirring for 0.5-6 hours while preserving heat, and then filtering while the solution is hot or taking out the supernatant liquid from the container after standing, thereby obtaining 3-nitro-N-ethyl carbazole solution; and in order to improve the quality of the 3-nitro-N-ethyl carbazole solution, further adding active carbon to the solution for absorption bleaching, and after stirring for 0.5-6 hours while preserving heat, filtering while the solution is hot, thereby obtaining the solution of 3-nitro-N-ethyl carbazole. The method for extracting the nitration product from N-ethyl carbazole nitration solvent distillation residue has the advantages that the extraction process is simple and high in efficiency, the yield of permanent violet nitration is greatly improved and further the total yield of permanent violet synthesis is improved, the production cost of permanent violet is reduced, the waste residue is reduced and the environmental protection effect is good.
Owner:南通龙晨新材料科技有限公司
Preparation method of N-[4-(triethyl aminomethyl) benzoyl] caprolactam bromide
The invention discloses a synthesis method of N-[4-(triethyl aminomethyl) benzoyl]caprolactam bromide. The method comprises the following four steps: chloridizing methylbenzoic acid and then carrying out reaction on chloridized methylbenzoic acid and caprolactam; and carrying out benzyl position-halogenation reaction and triethylamine salifying reaction so as to obtain the white solid N-[4-(triethyl aminomethyl) benzoyl] caprolactam bromide finally. The synthesis method is simple in synthesis process and convenient for operation; the N-[4-(triethyl aminomethyl) benzoyl]caprolactam bromide prepared by the synthesis method has the advantages of wide raw material source, cheap price, low production cost cleanliness and no pollution and is environment-friendly; and yield of the product is high, and the yield of the final product is 55-65%.
Owner:SHANGHAI INST OF TECH
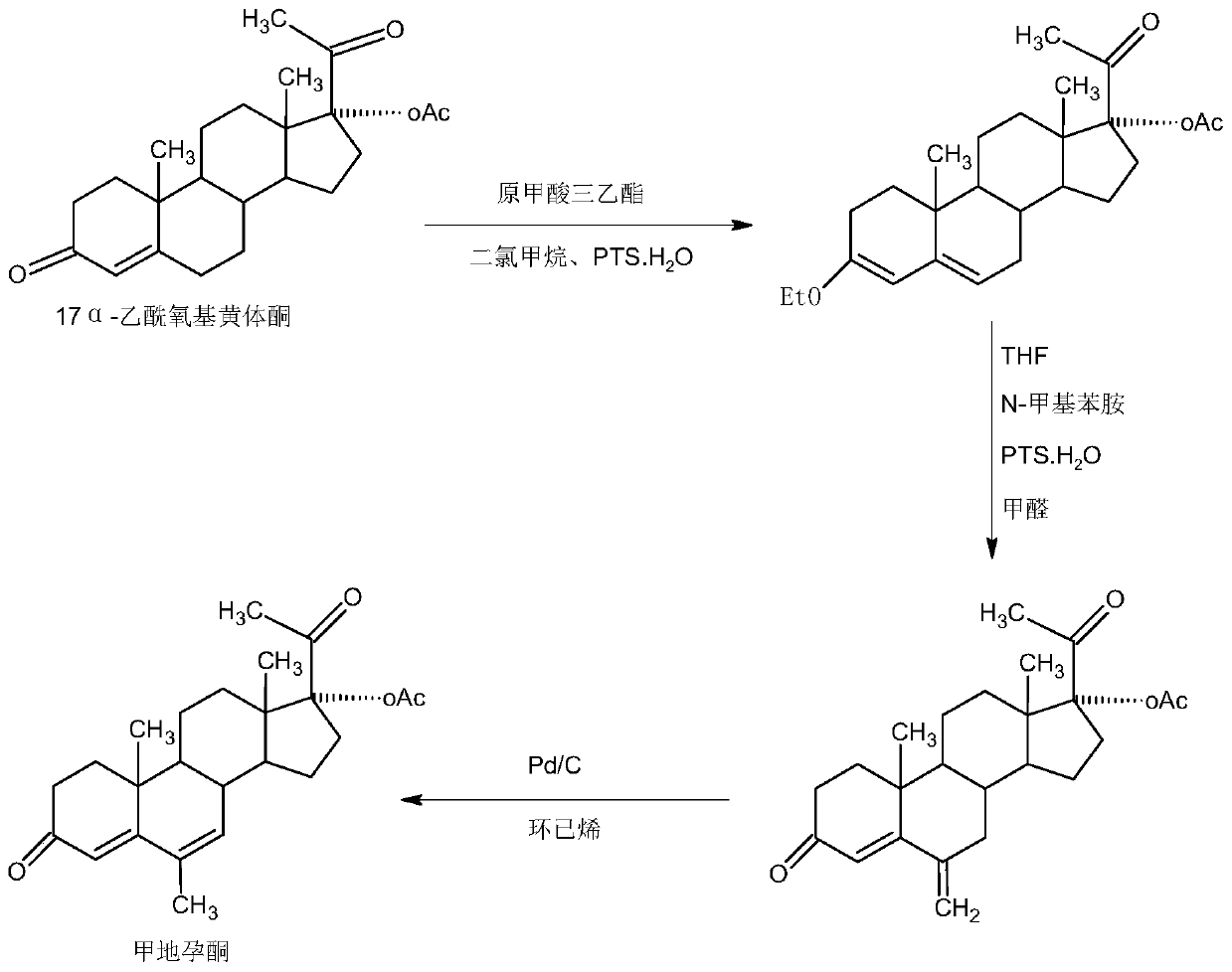
Preparation method of fluoromethyl ketone peptide series compounds
InactiveCN106317161AConvenient sequential couplingImprove the overall yield of synthesisPeptide preparation methodsBulk chemical productionCarboxyl radicalSide chain
The invention relates to a preparation method of fluoromethyl ketone peptide series compounds. The preparation method comprises the following steps: (1) preparing Fmoc-Asp(OR0)-FMK from Fmoc-Asp(OR0)-OH; (2) removing the side chain protective group (R0) from Fmoc-Asp(OR0)-FMK to obtain Fmoc-Asp-FMK with a naked side chain carboxyl group; (3) taking 2-CTC resin as the solid phase carrier to synthesize Fmoc-Asp-FMK-CTC amino acid resin; (4) using Fmoc-Asp-FMK-CTC to sequentially couple the protected amino acids (An) to obtain R1-An-Asp-FMK-CTC; (5) using a TFE / DCM reagent (20%) to cut R1-An-Asp-FMK-CTC peptide resin to obtain totally protected peptide R1-An-Asp-FMK; step (6) modifying and protecting the side chain carboxyl group of carbon terminal Asp in R1-An-Asp-FMK to obtain totally protected peptide R1-An-Asp(OR2)-FMK; and (7) if in the peptide sequence, amino acids other than the carbon terminal Asp have a side chain protective group, carrying out cracking so as to remove the protective groups. The provided method has the advantages of mild synthesis conditions and simple and stable technology.
Owner:HYBIO PHARMA
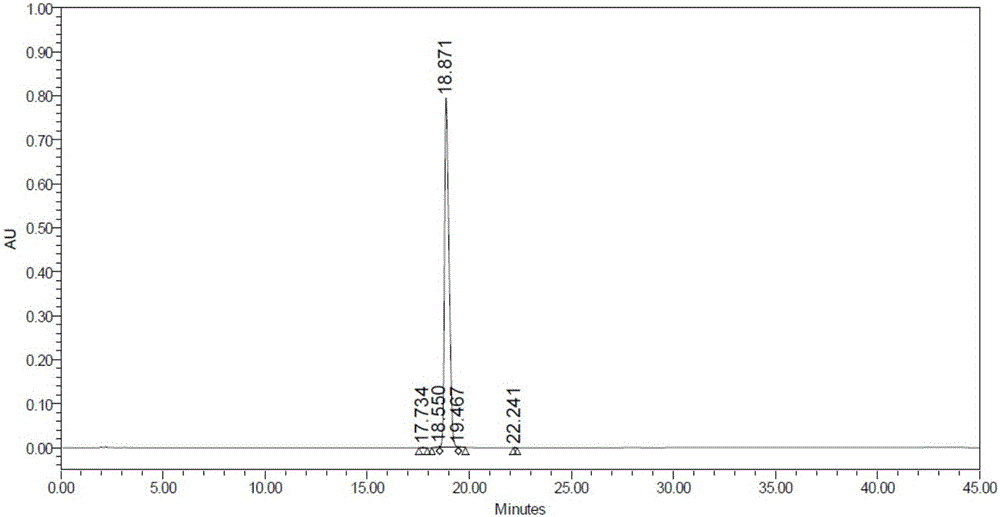
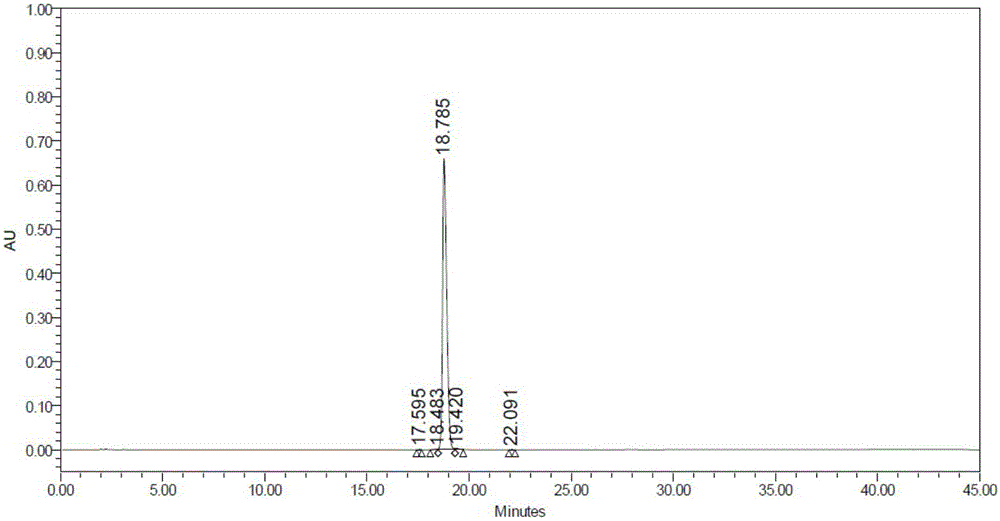
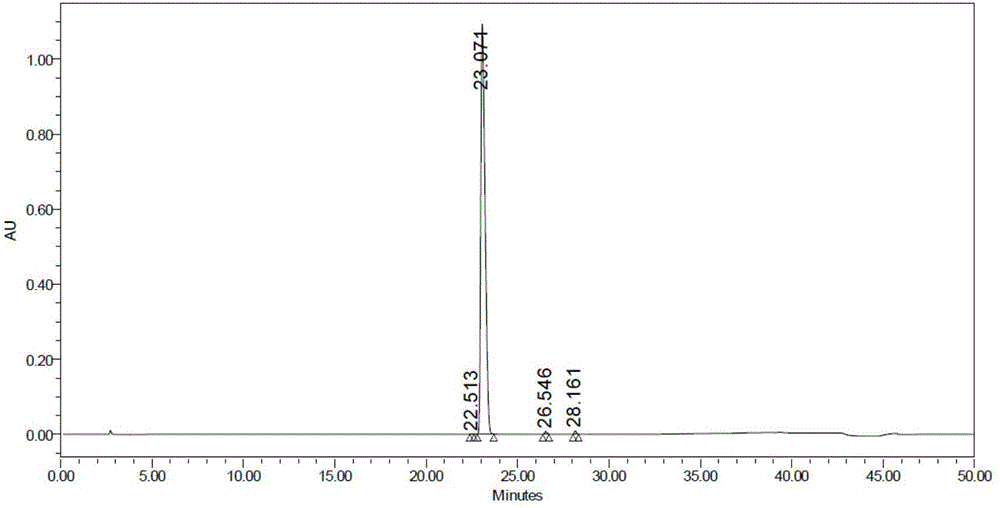
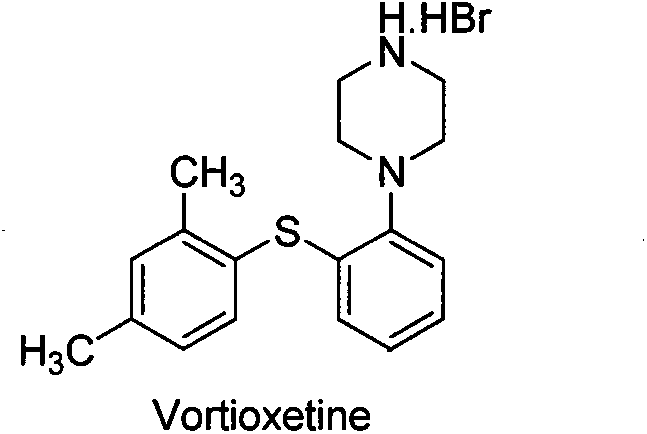
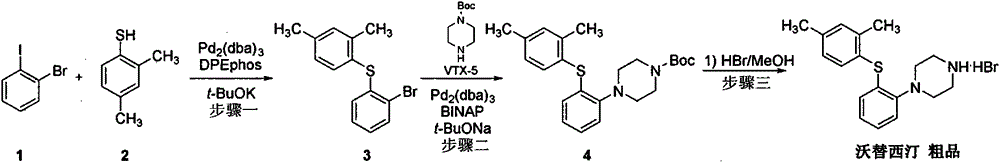
Beta type efficient vortioxetine hydrobromide crystal transformation method
ActiveCN106316985ASimplify refining operationsImprove the overall yield of synthesisOrganic chemistry methodsSolventChemical purity
The invention provides an efficient and concise method for refining and crystal transformation of vortioxetine hydrobromide, and belongs to the technical field of chemical drug synthesis. A vortioxetine synthesis precursor tert-butyl-4-(2-(2,4-dimethyl thiophenol)phenyl)piperazine-1-carbonate has an N-Boc protection group removed by adopting an isopropanol-hydrobromic acid mixed solution, a reaction system is cooled and crystallized, a vortioxetine hydrobromide isopropanol solvate is obtained and is subjected to water azeotropic distillation for one time, and the beta type vortioxetine hydrobromide can be safely and efficiently obtained. The method reduces multi-step tedious refining operation, and also can effectively solve the problems that toluene solvent residues, inorganic salt residues and metal palladium residues in the prior art; and the obtained beta type vortioxetine hydrobromide meets medical needed chemical purity and crystal form purity, and is suitable for industrial production.
Owner:BEIJING SHENLANHAI BIO PHARM TECH
Preparation method of finished 16alpha,21-diacetoxy prednisolone
ActiveCN109081860AAvoid many difficulties such as difficult purificationEasy to operateSteroidsAcetic acidAlcohol
The invention provides a preparation method of finished 16alpha,21-diacetoxy prednisolone product, comprising: subjecting 17alpha-deshydroxy prednisolone acetate as an initial material to 16,17-epoxidation with an organic peroxy acid in a first organic solvent to obtain an epoxide; subjecting the epoxide, in a second organic solvent, to ring-opening reaction with glacial acetic acid under the catalysis of an acid catalyst to obtain the target product, 16alpha,21-diacetoxy prednisolone; subjecting the crude 16alpha,21-diacetoxy prednisolone to heating reflux discoloration and crystallization with a lower carbon alcohol of C4 and below so as to obtain the finished 16alpha,21-diacetoxy prednisolone. The intermediate, 16alpha,21-diacetoxy prednisolone, to 16alpha-hydroxy prednisolone is prepared herein via the method that is efficient, environmentally friendly and fair in cost.
Owner:HUNAN KEREY BIOTECH
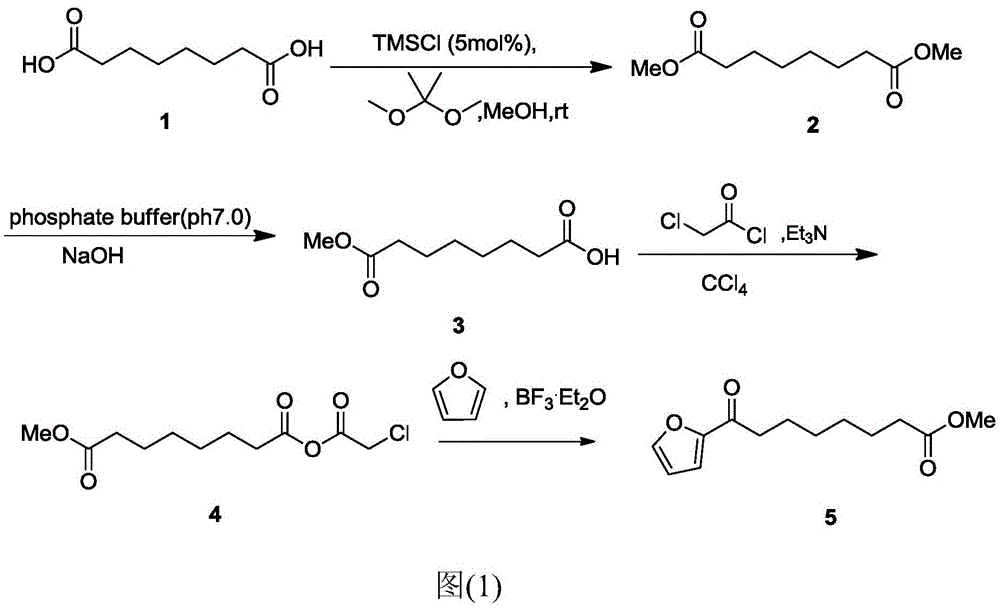
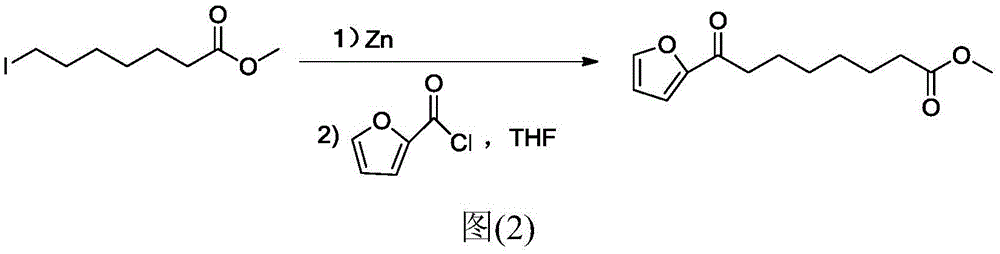
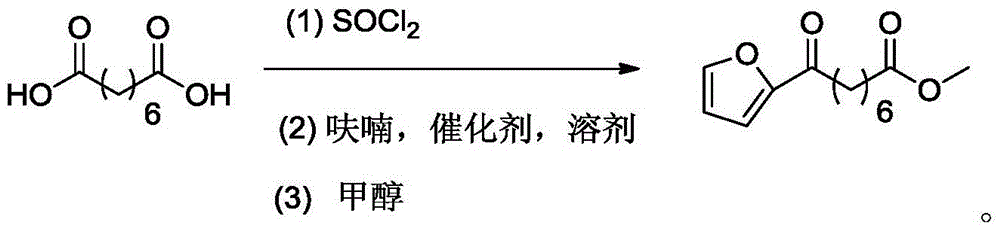
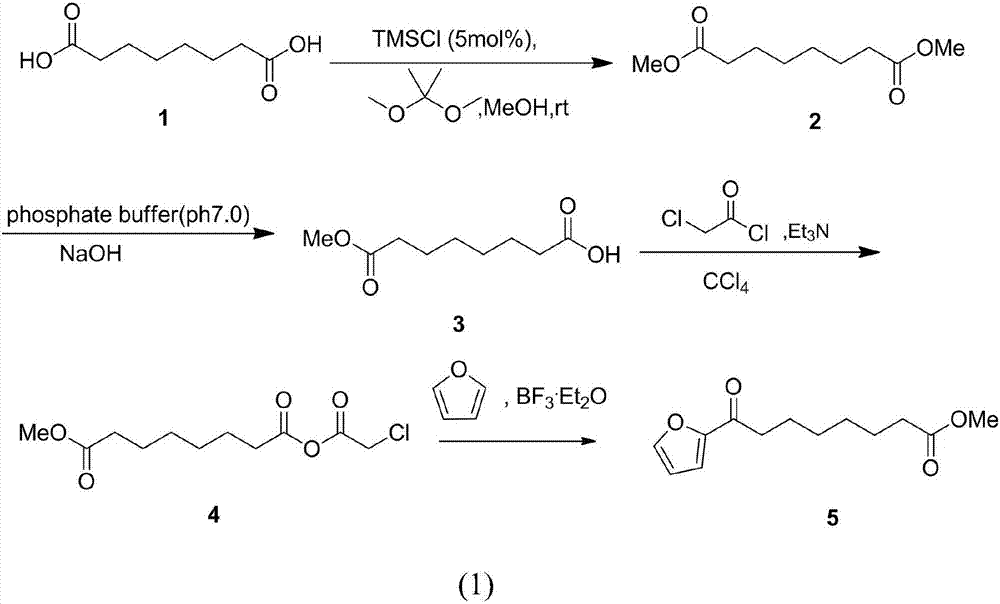
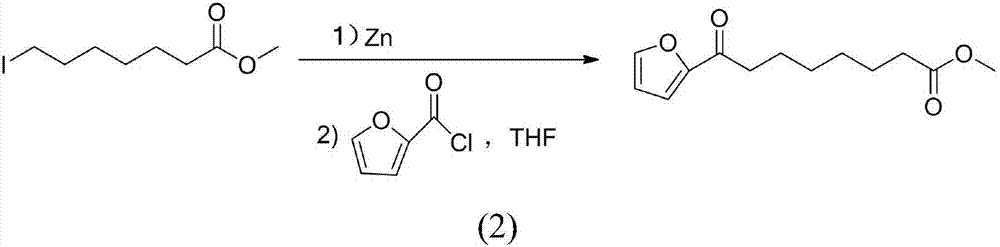
Chemical synthetic method of 8-furan-8-oxomethyl caprylate
ActiveCN105646403ASolve hard-to-get puzzlesThe synthetic route is simpleOrganic chemistryFuranChemical synthesis
The invention relates to a chemical synthetic method of 8-furan-8-oxomethyl caprylate. The method comprises the following steps of adding octanedioic acid and sulfoxide chloride into a reaction bulb, agitating an obtained mixture at a room temperature until a reaction is complete, and distilling to remove the sulfoxide chloride under normal pressure; afterwards, adding a solvent and a catalyst into the reaction bulb to carry out another reaction, dropwise adding methanol into the reaction bulb for quenching, decompressing the reaction bulb and distilling to remove the solvent, dissolving a target product by using an extracting solution to obtain a solution, and distilling the solution under reduced pressure to obtain a yellowish oily 8-furan-8-oxomethyl caprylate pure product. According to the chemical synthetic method of the 8-furan-8-oxomethyl caprylate, a synthetic strategy of a one-pot method is adopted; by proceeding from the low-cost octanedioic acid, the octanedioyl chloride is prepared with a high yield; afterwards, the octanedioyl chloride and furan are subjected to a regioselectivity Friedel-Crafts acylation reaction; finally, acyl chloride at the other end is quenched by using the methanol to directly generate methyl ester to obtain the product; by using the method, the synthetic route of the target product is simplified; the reaction time is greatly shortened; the synthetic total yield is improved; therefore, the production cost is decreased; the chemical synthetic method has the advantages of being low in cost, higher in yield, simple to operate and suitable for industrial production, and the like.
Owner:ZHEJIANG UNIV OF TECH
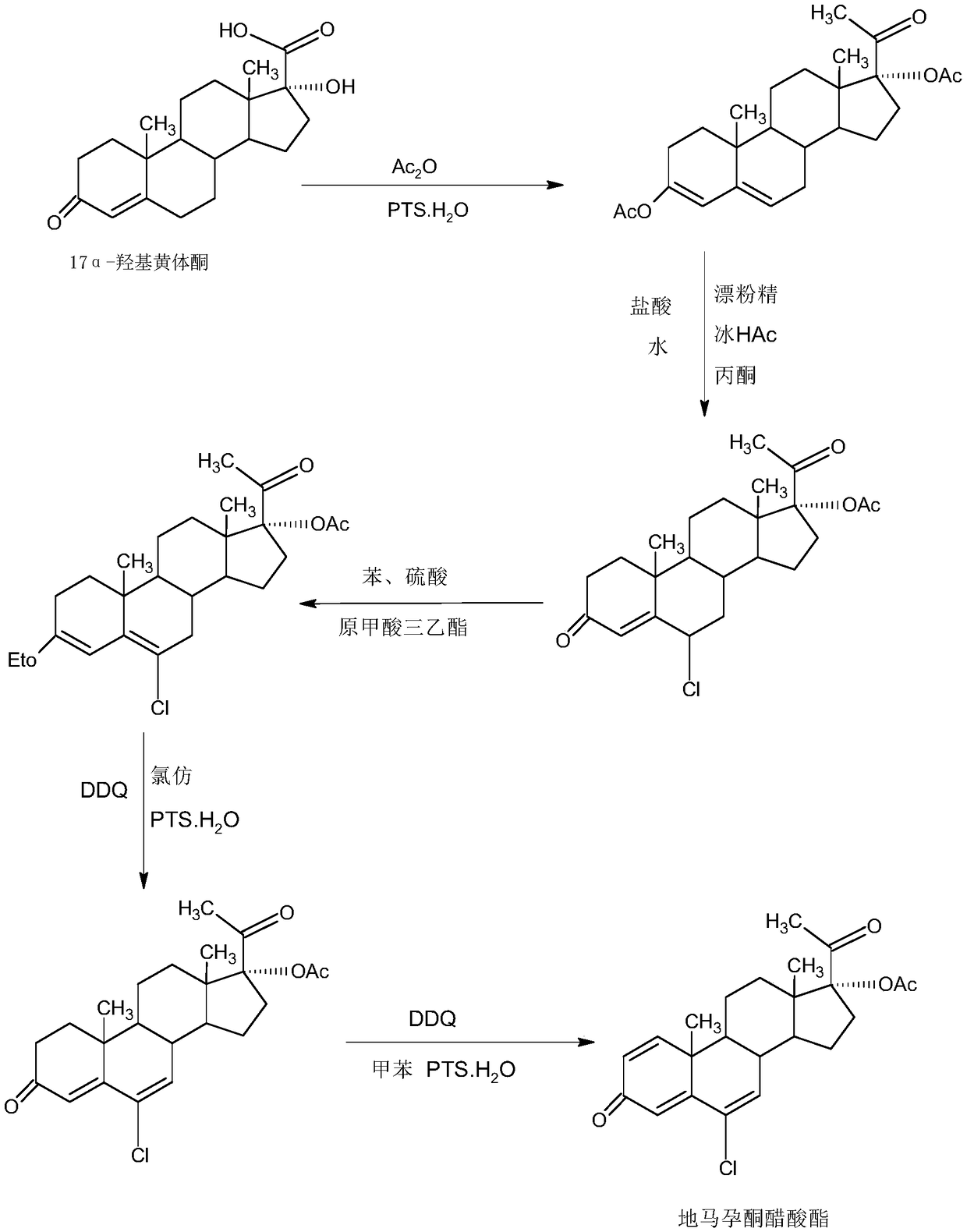
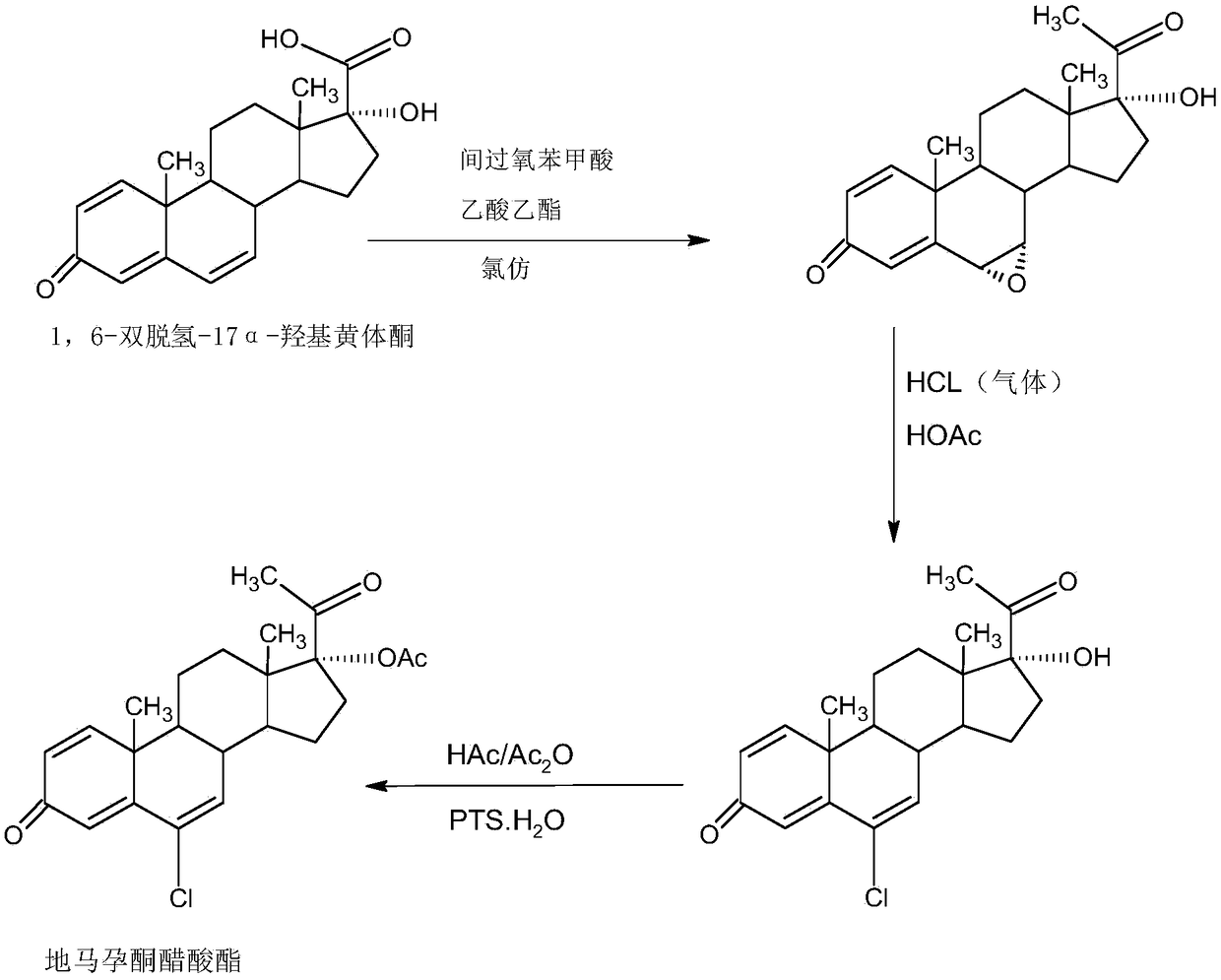
Method for preparing delmadinone acetate
InactiveCN109456382AEasy to operateProduction is economical and environmentally friendlyMicroorganism based processesSteroidsActivated carbonAlcohol
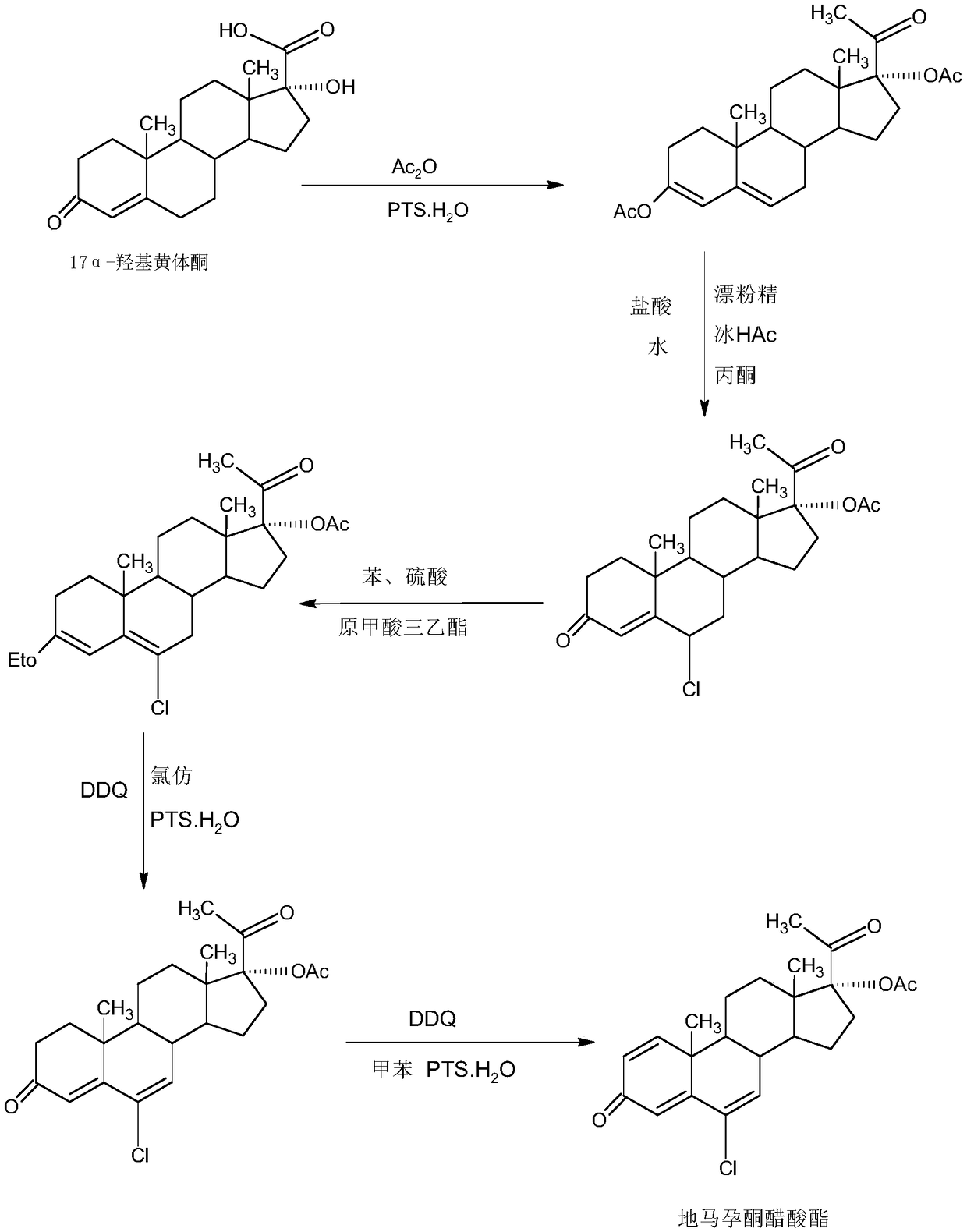
The invention provides a method for preparing delmadinone acetate. The method comprises the following steps that 1,6-didehydro-17a-hydroxyprogesterone is adopted as a raw material to first synthesizea 6-position epoxide, then the epoxide is heated, refluxed, discolored and re-crystallized by using active carbon in lower alcohol of C4 or less to obtain an epoxide product, then the epoxide productis used as a raw material to synthesize a 6-position chloride to obtain delmadinone, and finally by virtue of 17-position esterification, the delmadinone acetate can be obtained. Compared with a traditional synthetic method of the delmadinone acetate, the method of the invention has the advantages of simple process operation, economical and environment-friendly production, high synthetic total yield, good product quality, low production cost and the like.
Owner:HUNAN KEREY BIOTECH
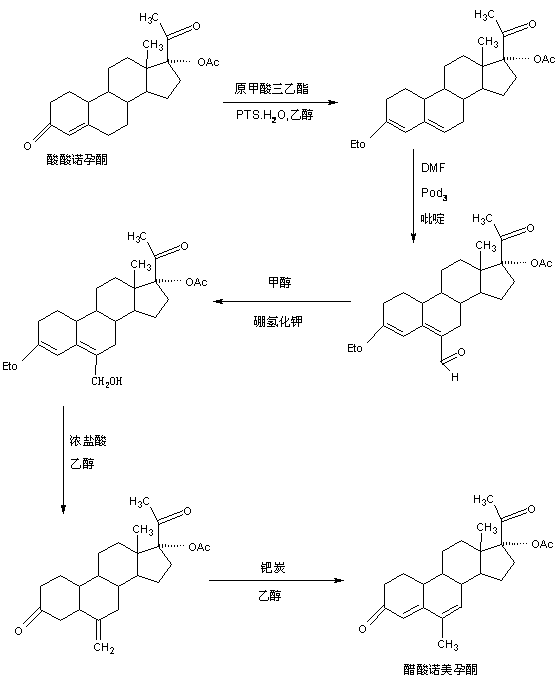
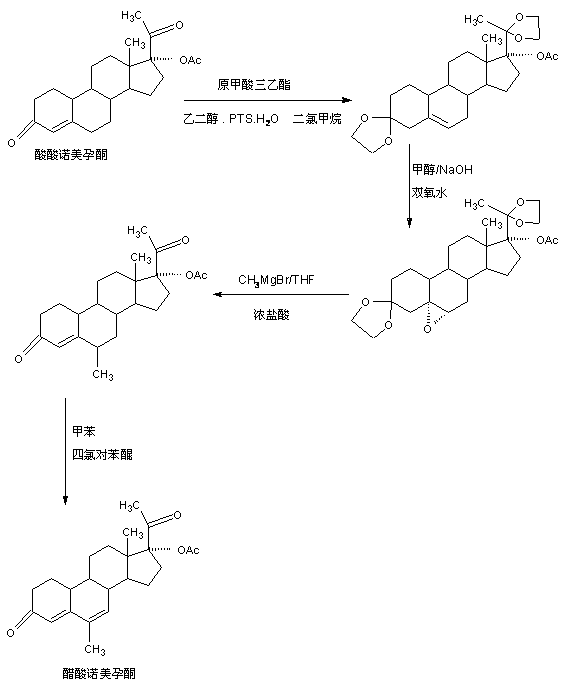
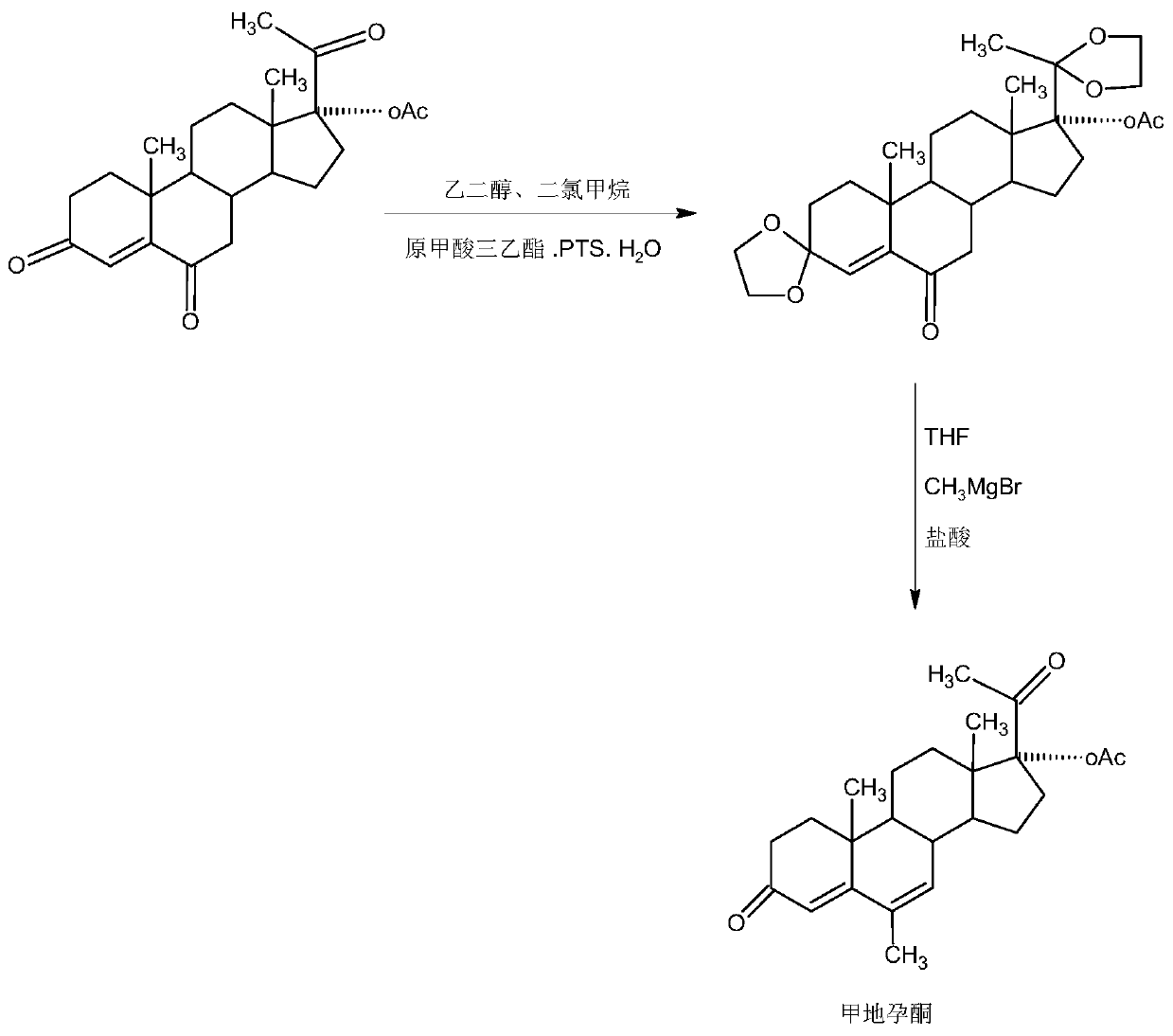
The preparation method of nomegestrol acetate
InactiveCN107629102BShort synthetic routeEasy to operateSteroidsCarboxylic acid salt preparationEpoxyDehydrogenation
The invention discloses a preparation method of nomegestrol acetate. The method comprises that gestonorone acetate as a raw material is dissolved in an organic solvent and then undergoes a reaction with ethylene glycol under acid catalysis in the presence of triethyl orthoformate to produce diketal, the diketal is dissolved in an organic solvent and undergoes a reaction with hydrogen peroxide under alkali catalysis to produce an epoxy compound, the epoxy compound is dissolved in an organic solvent and undergoes a Grignard addition reaction with methylmagnesium halide, the reaction product is hydrolyzed in a strong acid solution and is subjected to dehydration deprotection so that a methyl compound is obtained, and the methyl compound is dissolved in an organic solvent and undergoes a dehydrogenation reaction with tetrachloro-p-benzoquinone to produce nomegestrol acetate. The nomegestrol acetate has HPLC content of 99.0-99. 5% and a four-step synthesis total yield of 60-62%. Compared with the traditional method, the method has the advantages of simple and convenient operation, economy, environmental friendliness, high total synthesis yield and good product quality and reduces a costby 35-40%. The solvent used by the method can be recovered and recycled and is conducive to industrial production.
Owner:HUNAN KEREY BIOTECH
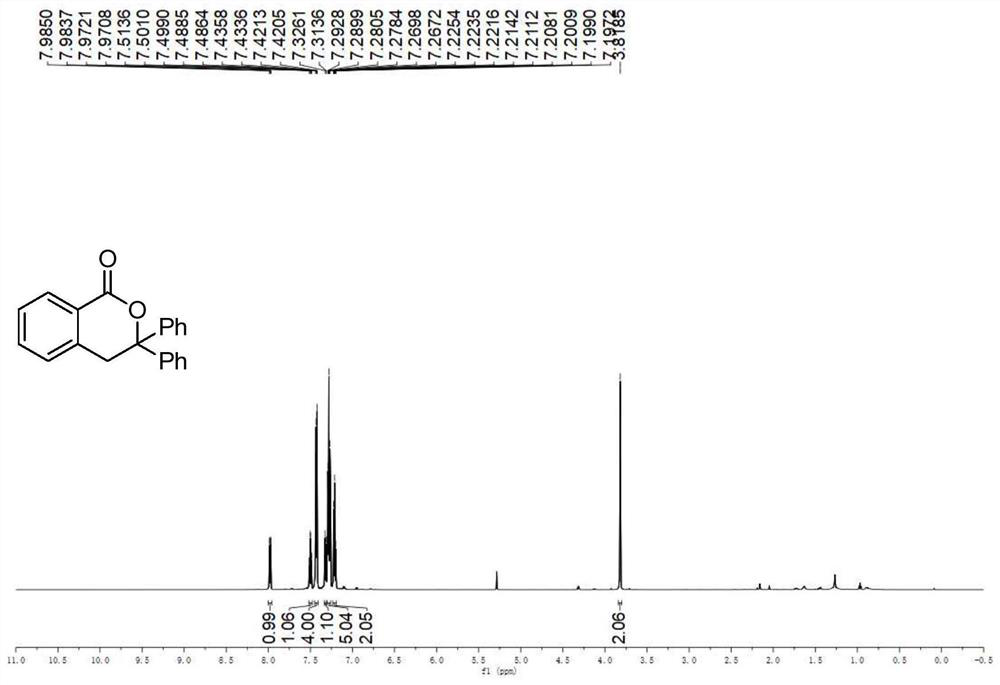
A kind of preparation method of the compound containing isochroman-1-one skeleton
ActiveCN108774206BImprove toleranceThe reaction steps are simpleOrganic chemistryBenzoic acidNatural product
The invention provides a method for preparing a compound containing isochroman-1-one skeleton, specifically methyl o-iodobenzoate substituted with substituents and 1, The 1-disubstituted olefin is used as a raw material, and a compound containing an isochroman-1-one skeleton is prepared through a cross-coupling reaction. The method does not use any directing group, and no additional steps are required to introduce or remove the directing group; the method has a wide range of functional groups and the tolerance of the functional group; the method has the advantages of cheap and easy-to-obtain raw materials, simple steps, and high overall yield of synthesis , The advantages of low total cost of synthesis. The prepared isochroman-1-one skeleton-containing compound has broad application prospects in the synthesis of pesticides, pharmaceutical intermediates, complex natural products, and the like.
Owner:SHANXI UNIV
A kind of preparation method of 3-(thiophen-2-yl)cyclohex-2-enone derivative
ActiveCN107141279BThe reaction steps are simpleImprove the overall yield of synthesisOrganic chemistryCyclohexenoneRotary evaporator
The invention relates to the technical field of a preparation method of thiophene derivatives, in particular to a preparation method of 3-(thiophene-2-yl)hexanaphthene-2-ketene derivatives. The preparation method of the 3-(thiophene-2-yl)hexanaphthene-2-ketene derivatives comprises the following steps: (1) adding cyclohexenone, thiophene or substituted thiophene, palladium chloride, silver carbonate, N-acetoglycocoll and silver hexafluoroantimonate into a reactor sequentially according to the molar ratio of 3:1:0.1:2.5:0.1:0.2, adding a solvent to dissolve the reactants, mixing uniformly at a room temperature, and reacting at 60 to 120 DEG C for 5 to 48 hours; (2) after the reaction, cooling the reactor to a room temperature, dissolving with ethyl acetate, washing with a saturated ammonium chloride aqueous solution and a saturated sodium chloride aqueous solution sequentially, drying the organic phase by using anhydrous sodium sulfate, filtering and rotating on a rotary evaporator to remove the solvent; (3) separating and purifying the residues through silica-gel column chromatography after the solvent is removed through rotation, collecting a target product, performing rotary evaporation to remove the solvent, and pumping and drying by an oil pump.
Owner:SHANXI UNIV
A new method for preparing 16a-hydroxyprednisolone
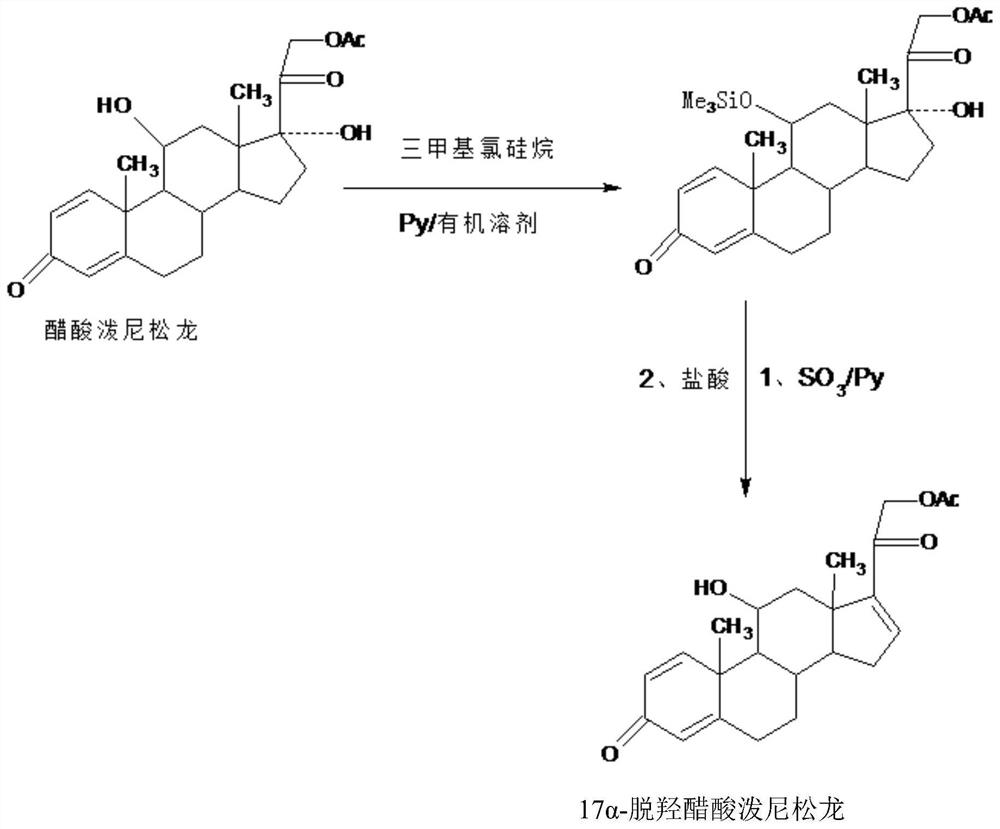
The invention provides a new method for preparing 16alpha-hydroxyprednisolone. The method comprises the following steps that A, a protector is prepared, wherein prednisolone acetate and trimethylchlorosilane are catalyzed by organic base to generate a 11th-site silicon etherification reaction, and then the protector is obtained; B, dehydration, hydrolysis deprotection and refining are conducted, wherein the protector and SO3 are catalyzed by organic base to generate a 17th-site dehydration reaction, after the reaction, acid is directly added to make the 11th site deprotected, and a crude 17alpha-dehydroxylated prednisolone acetate product is obtained; the crude product is refined to obtain a 17alpha-dehydroxylated prednisolone acetate product; C, the 17alpha-dehydroxylated prednisolone acetate product is used as a raw material to prepare the 16alpha-hydroxyprednisolone. The problems are solved that in a traditional production technology of the 17alpha-dehydroxylated prednisolone acetate, more side reactions and impurities are generated in the dehydration process, and the impurities are difficult to refine; the total yield of the synthesized 16alpha-hydroxyprednisolone is greatly improved, and the production cost is reduced.
Owner:HUNAN KEREY BIOTECH
A kind of method for preparing 16a-hydroxyprednisolone
ActiveCN109265507BEasy to operateThe production process is economical and environmentally friendlySteroidsPtru catalystOrganosolv
Owner:HUNAN KEREY BIOTECH
A kind of method for preparing 16a-hydroxyprednisolone product
ActiveCN109232697BEasy to operateThe production process is economical and environmentally friendlySteroidsPtru catalystOrganosolv
The invention provides a method for preparing a 16a-hydroxyprednisolone product. The method comprises the steps that 17a-dehydroprednisolone is used as a raw material, and firstly the starting material 17a-dehydroprednisolone performs an epoxidation reaction with organic peroxide acid at 16 and 17 sites in a first organic solvent to prepare an epoxy material; then the epoxy material reacts with glacial acetic acid under catalysis of an acid catalyst in a second solvent for loop opening, and thus 16a,21-diacetoxyprednisolone is prepared; 16a,21-diacetoxyprednisolone is dissolved in a third organic solvent, under the catalytic action of a solid-phase base catalyst, acetic ester on the two sites are hydrolyzed, and thus 16a-hydroxyprednisolone is prepared; and finally the 16a-hydroxyprednisolone crude product obtained by solid-phase base-catalyzed hydrolysis is heated and refluxed, decolorized, and recrystallized through low-carbon alcohol below C4, and thus the 16a-hydroxyprednisolone product is obtained. According to the method, 16a-hydroxyprednisolone is prepared in an efficient, environment-friendly and low-cost mode.
Owner:HUNAN KEREY BIOTECH
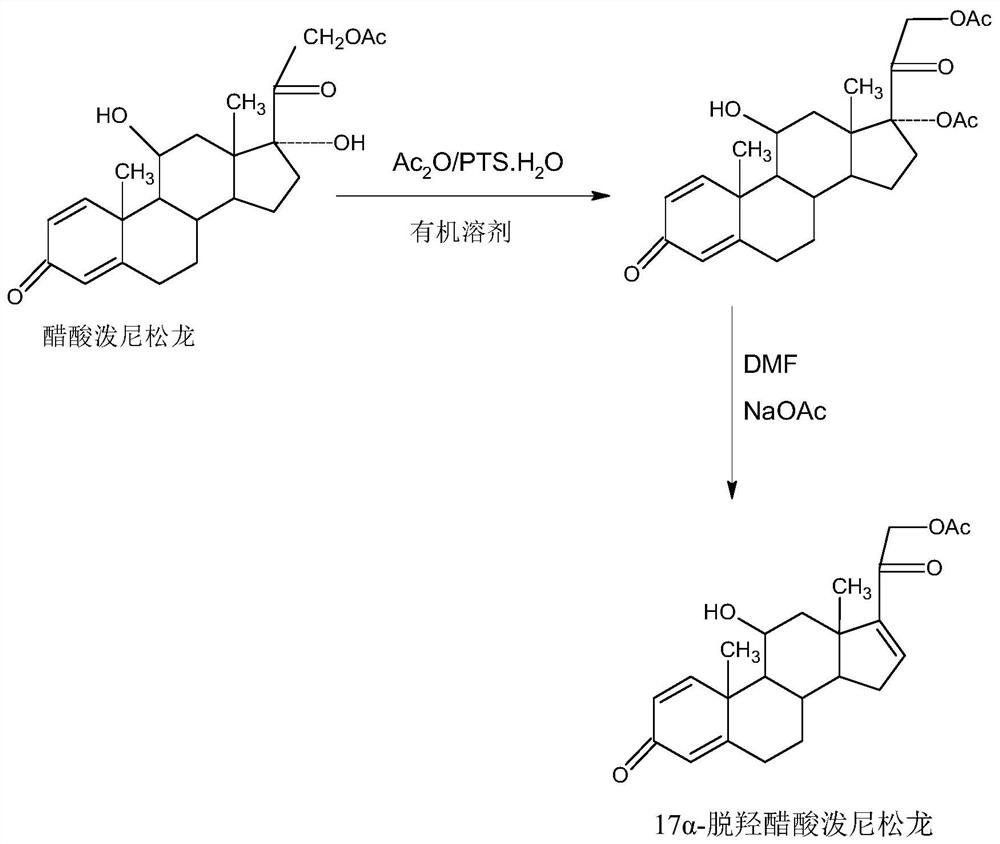
A kind of preparation method of 17a-prednisolone dehydroxyacetate product
The invention provides a preparation method of a 17a-dehydroxy prednisolone acetate product. The method includes the following steps: step A, dissolving prednisolone acetate in a first organic solvent, and subjecting the solvent and trimethylchlorosilane to 11th-position silicon etherification reaction under the catalysis of organic base to obtain a protector; step B, in a second organic solvent,subjecting the protector and SO3 to 17th-position dehydration reaction under the catalysis of the organic base, directly adding acid for treatment after the reaction to deprotect the 11th position, and obtaining a crude 17a-dehydroxy prednisolone acetate product; subjecting the crude product to decolorization and recrystallization in the presence of C4-below low carbon alcohol and activated carbonto obtain the 17a-dehydroxy prednisolone acetate product. The preparation method has the advantages that the problems such as many side reactions and impurities and difficulty in impurity refining inthe dehydration reaction in a traditional production process of 17a-dehydroxy prednisolone acetate are solved, the total synthesis yield is remarkably increased, and the production cost is reduced.
Owner:HUNAN KEREY BIOTECH
A kind of preparation 16a, the method for 21-diacetyloxy prednisolone
ActiveCN109251230BEasy to operateThe production process is economical and environmentally friendlySteroidsAcetic acidPtru catalyst
The invention provides a method for preparing 16a,21-diacetyloxy prednisolone. The method comprises the following steps: adopting 17a-deshydroxy prednisolone as a raw material, firstly, enabling the starting raw material 17a-deshydroxy prednisolone to generate epoxidation reaction with organic peroxy acid on 16 and 17 sites in a first organic solvent to prepare an epoxy product; enabling the epoxyproduct to react with glacial acetic acid under the catalysis of an acid catalyst in a second organic solvent to be subjected to ring opening, to prepare a target product, i.e., 16a,21-diacetyloxy prednisolone. According to the efficient, environment-friendly and fair-price method, the intermediate 16a,21-diacetyloxy prednisolone of 16 alpha-hydroxyprednisolone is prepared.
Owner:HUNAN KEREY BIOTECH
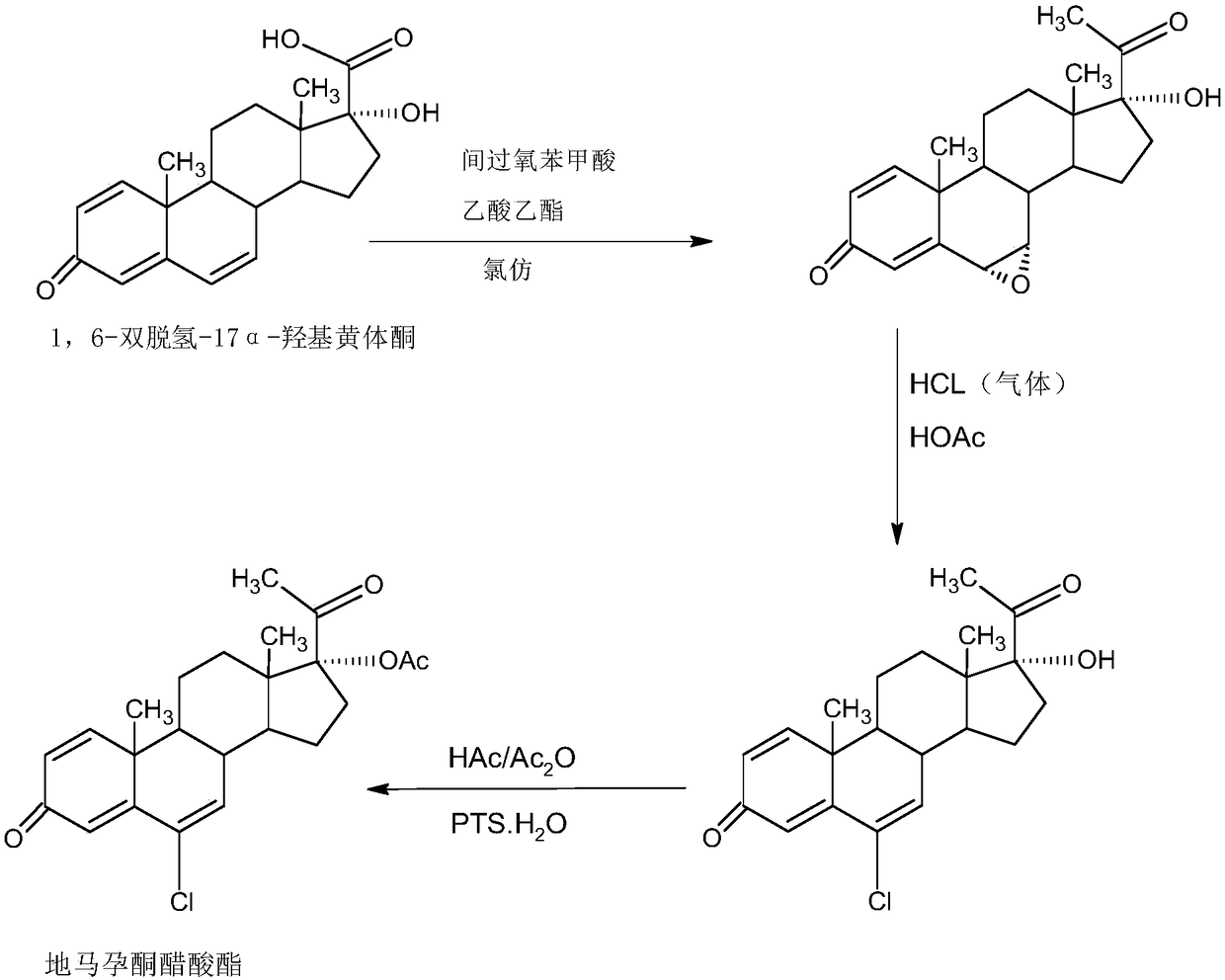
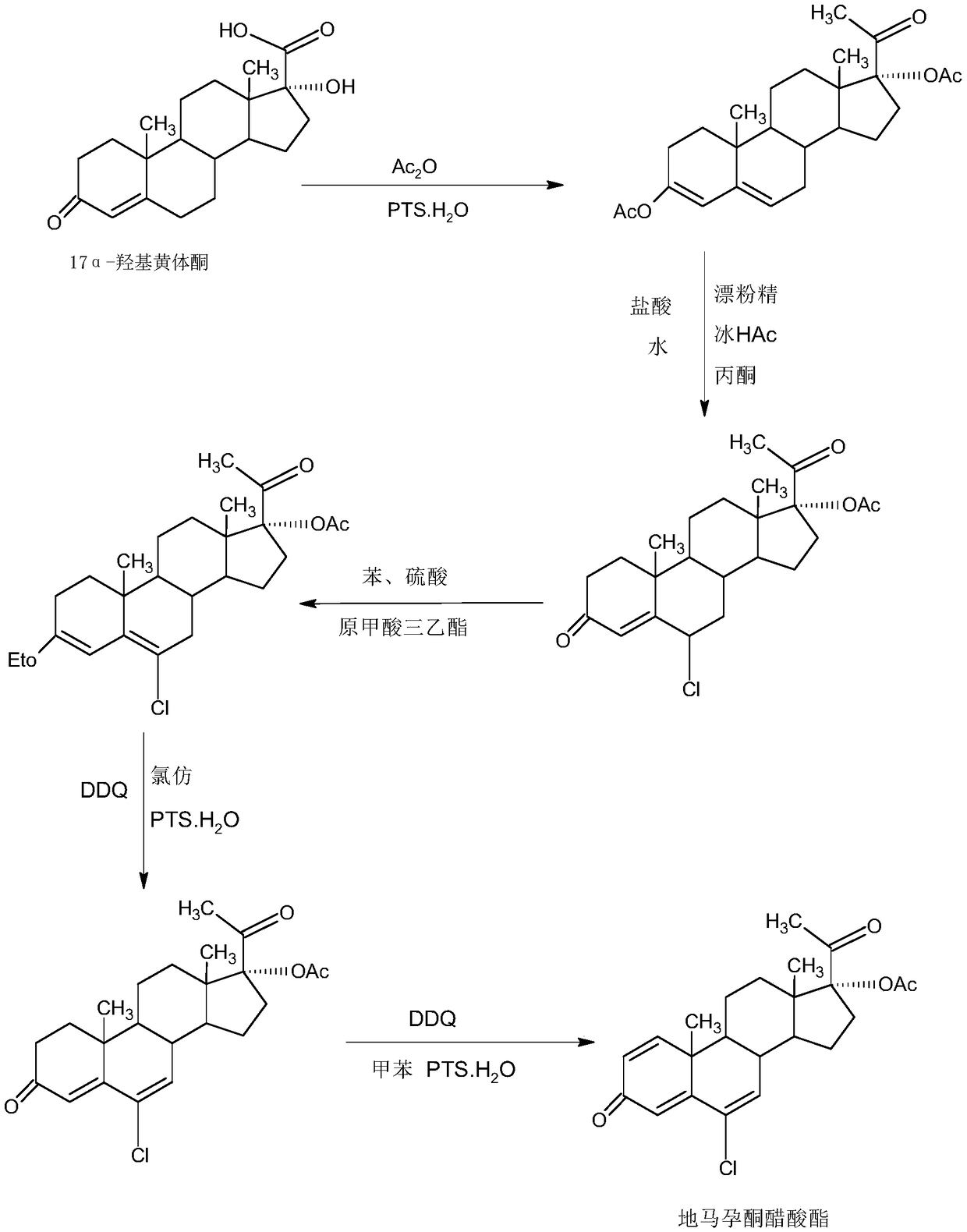
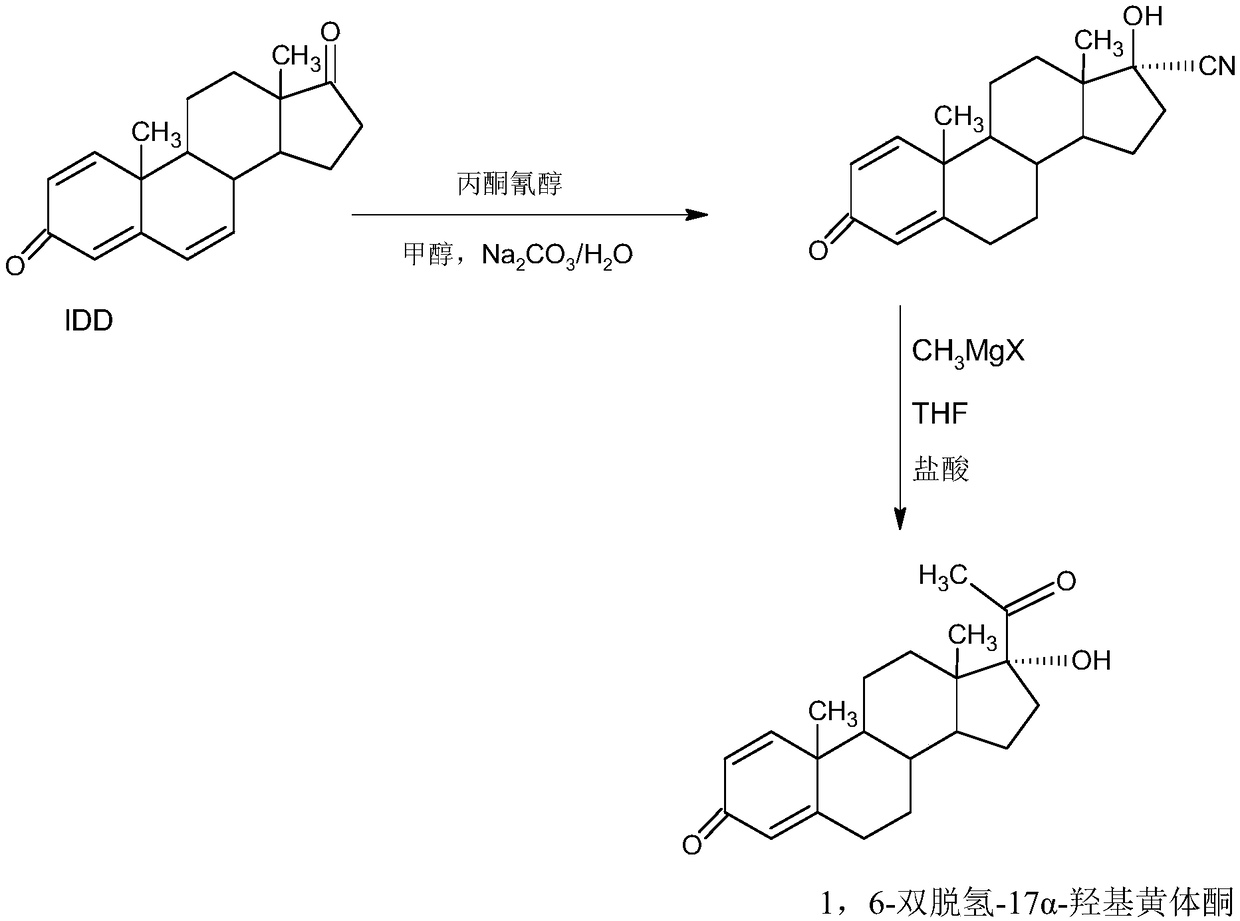
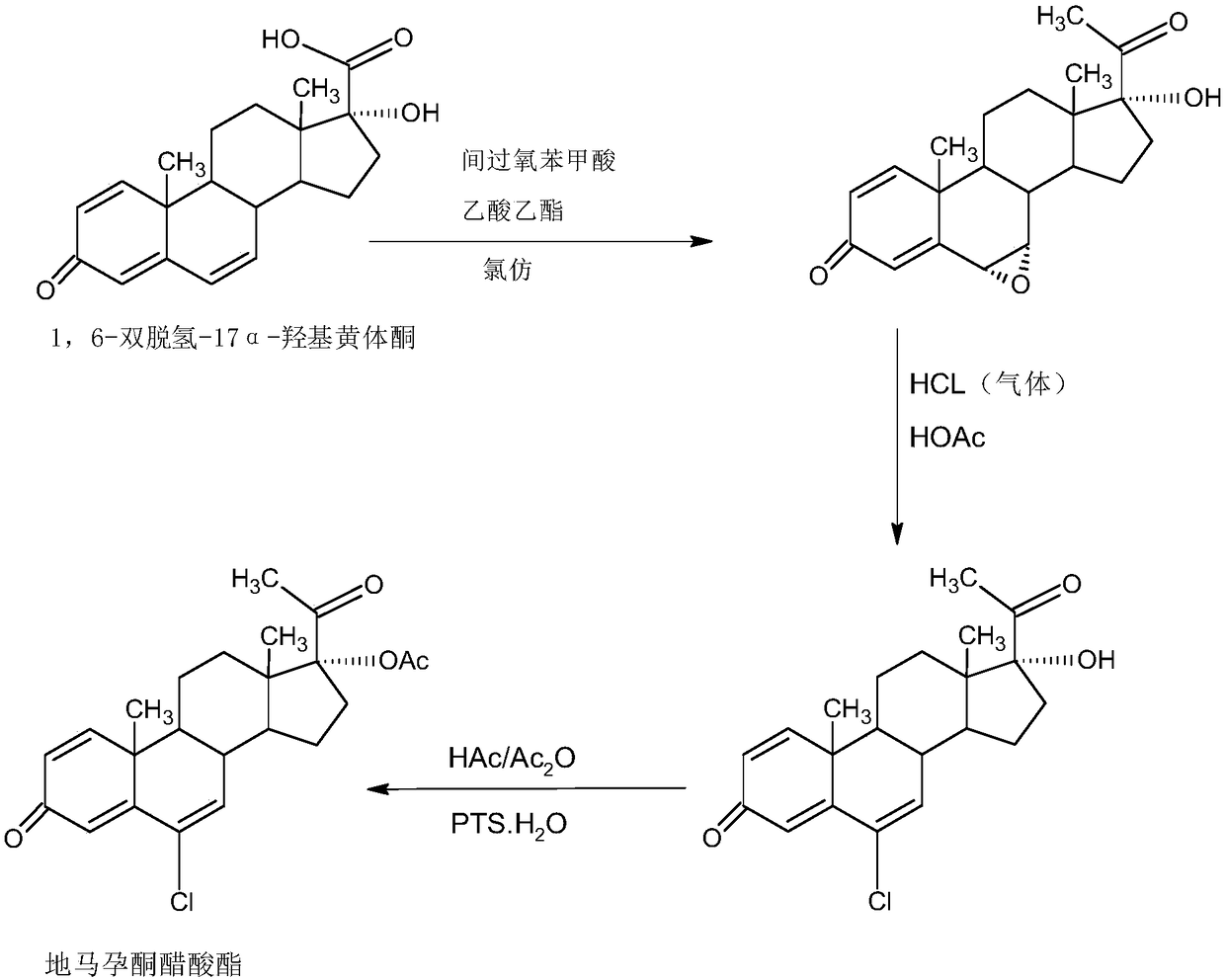
Delmadinone acetate preparation method
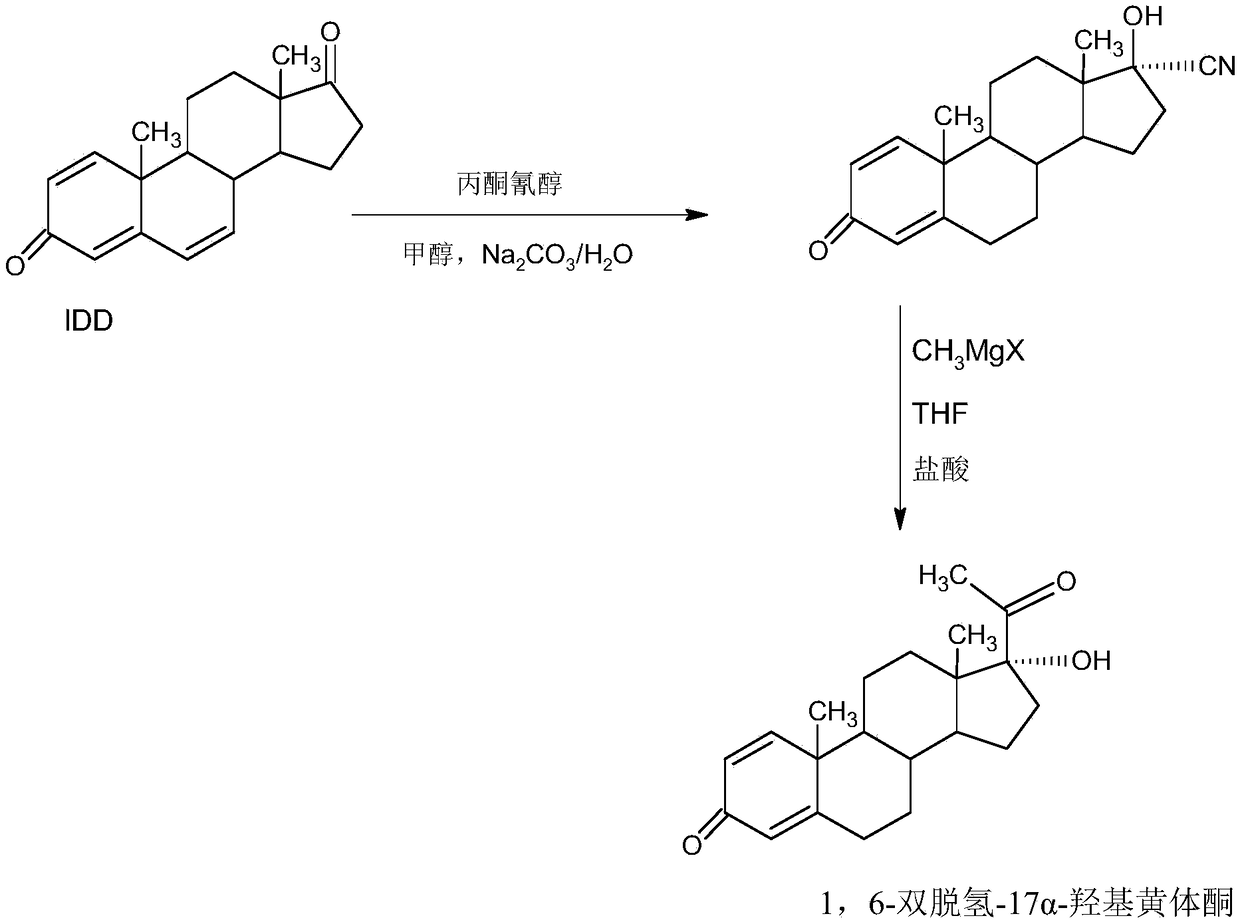
InactiveCN109369764AEasy to operateProduction is economical and environmentally friendlyOrganic chemistry methodsSteroidsEpoxySynthesis methods
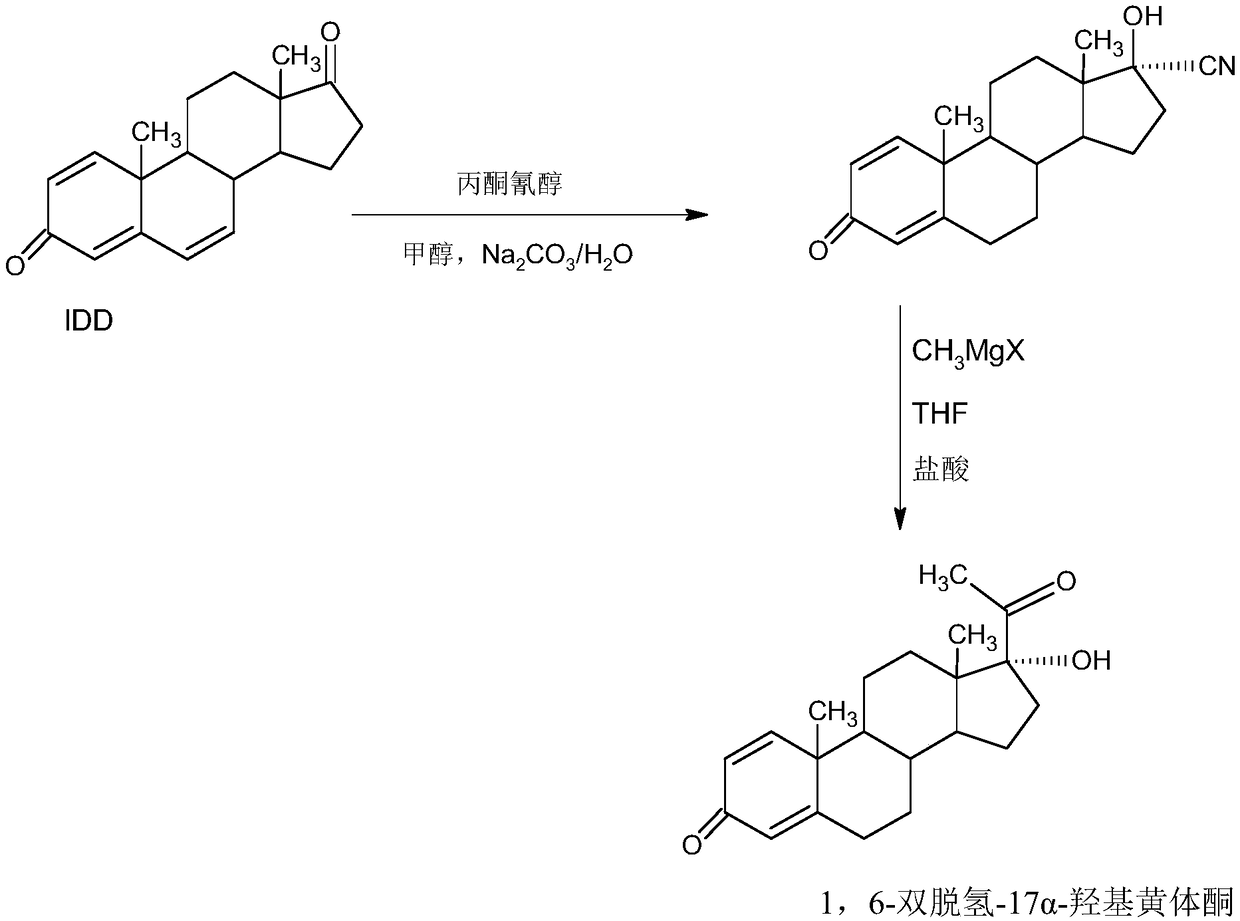
The invention provides a delmadinone acetate preparation method which comprises the following steps: by taking 1,4-androstadienedione, that is, IDD, as a raw material, firstly, enabling 17-site ketonein IDD molecules to react with acetone cyanohydrins in a first organic solvent under catalysis of an alkali, and introducing beta-hydroxyl and alpha-cyan into the 17-site so as to obtain hydroxyl cyanogens; preparing 1,6-bidehydrogenation-17a-hydroxyl progesterone from the hydroxyl cyanogens in the presence of methyl magnesium halide, a second organic solvent and an acid; and further synthesizinga 6-site epoxy substance, further synthesizing 6-site chloride so as to obtain delmadinone, and finally carrying out 17-site esterification, thereby obtaining delmadinone acetate. Compared with a conventional synthesis method, the delmadinone acetate preparation method provided by the invention has multiple advantages of being simple and convenient in process operation, economic and environmental-friendly in production, high in total synthesis yield, high in product quality, low in production cost, and the like.
Owner:HUNAN KEREY BIOTECH
A kind of preparation method of 16a-hydroxyprednisolone
ActiveCN107488203BInhibition of rearrangement and ring expansion side reactionsReduce generationPhysical/chemical process catalystsSteroidsOrganic solventAlcohol
Owner:HUNAN KEREY BIOTECH
A kind of 16a, the preparation method of 21-diacetyloxyprednisolone product
ActiveCN109081860BAvoid many difficulties such as difficult purificationEasy to operateSteroidsAcetic acidPtru catalyst
The invention provides a preparation method of finished 16alpha,21-diacetoxy prednisolone product, comprising: subjecting 17alpha-deshydroxy prednisolone acetate as an initial material to 16,17-epoxidation with an organic peroxy acid in a first organic solvent to obtain an epoxide; subjecting the epoxide, in a second organic solvent, to ring-opening reaction with glacial acetic acid under the catalysis of an acid catalyst to obtain the target product, 16alpha,21-diacetoxy prednisolone; subjecting the crude 16alpha,21-diacetoxy prednisolone to heating reflux discoloration and crystallization with a lower carbon alcohol of C4 and below so as to obtain the finished 16alpha,21-diacetoxy prednisolone. The intermediate, 16alpha,21-diacetoxy prednisolone, to 16alpha-hydroxy prednisolone is prepared herein via the method that is efficient, environmentally friendly and fair in cost.
Owner:HUNAN KEREY BIOTECH
Method for preparing delmadinone acetate product
InactiveCN109369763AEasy to operateProduction is economical and environmentally friendlyOrganic chemistry methodsSteroidsActivated carbonKetone
The invention provides a method for preparing a delmadinone acetate product. The method comprises the following steps: by taking IDD (1,4-Androstadienedione) as a raw material, firstly, enabling 17-site ketone in IDD molecules to react with acetone cyanohydrins in a first organic solvent under catalysis of an alkali, and introducing beta-hydroxyl and alpha-cyan into the 17-site so as to obtain hydroxyl cyanogens; preparing 1,6-bidehydrogenation-17a-hydroxyl progesterone from the hydroxyl cyanogens in the presence of methyl magnesium halide, a second organic solvent and an acid; further synthesizing a 6-site epoxy substance, further synthesizing 6-site chloride so as to obtain delmadinone, and finally carrying out 17-site esterification so as to obtain delmadinone acetate; and further carrying out heating backflow decoloring and recrystalization on the obtained delmadinone acetate with activated carbon in lower-carbon alcohol with the carbon number of smaller than 4, thereby obtaining the delmadinone acetate product. Compared with a conventional synthesis method, the method provided by the invention has multiple advantages of being simple and convenient in process operation, economic and environmental-friendly in production, high in total synthesis yield, high in product quality, low in production cost, and the like.
Owner:HUNAN KEREY BIOTECH
Preparation method of delmadinone
InactiveCN109456381AEasy to operateProduction is economical and environmentally friendlySteroidsEpoxySynthesis methods
The invention provides a preparation method of delmadinone. The preparation method comprises the steps: 1,4-androstadienedione, namely IDD is adopted as a raw material; firstly, 17-position ketone inIDD molecules reacts with acetone cyanohydrin in a first organic solvent under alkali catalysis, and beta-hydroxyl and alpha-cyan are introduced in the 17 position to obtain hydroxyl cyanide; then thehydroxyl cyanide is prepared under presence of methylmagnesium halide, a second organic solvent and acid to obtain 1,6-didehydro-17a-hydroxyprogesterone; and then 6-position epoxy is synthesized, andthen 6-position chloride is synthesized to obtain the delmadinone. Compared with a traditional synthesis method of delmadinone and delmadinone acetate, the preparation method has the multiple advantages that process operation is easy and convenient, production is economical and environmentally friendly, the total yield of synthesis is high, the product quality is good, and the production cost islow.
Owner:HUNAN KEREY BIOTECH
The preparation method of megestrol
The invention provides a megestrol acetate preparation method. The method comprises the steps that 6-keto-17a-acetoxy progesterone serves as the raw material, the raw material is dissolved into an organic solvent, in the presence of triethyl orthoformate, a catalytic reaction is conducted with glycolic acid, and double ketals are obtained; the double ketals are dissolved into an organic solvent, a Grignard reaction is conducted with a Grignard reagent, after the reaction is completed, under the strong acid effect, Grignard hydrolysis is conducted, deprotection and dehydration are conducted simultaneously, and a crude megestrol acetate product is synthesized through a two-step reaction; the crude product is subjected to decoloration and recrystallization through activated carbon, and a megestrol acetate product is obtained, wherein the HPLC content ranges from 99.0% to 99.5%, the melting point ranges from 213 DEG C to 220 DEG C, and the two-step synthetic weight total yield ranges from 80% to 85%. Accordingly, compared with a traditional method, the synthetic route is short, the technological operation is easy and convenient, production is economical and environmentally friendly, the synthetic total yield is increased by 30% or above compared with that of the traditional method, and the production cost is lowered by 30%-35%; the solvents used in the technology can be recycled and applied mechanically, economy and environmental friendliness are achieved, and the method is very beneficial to industrial production.
Owner:HUNAN KEREY BIOTECH
A kind of chemical synthesis method of 8-furan-8-oxooctanoic acid methyl ester
ActiveCN105646403BSolve hard-to-get puzzlesThe synthetic route is simpleOrganic chemistryFuranChemical synthesis
The invention relates to a chemical synthetic method of 8-furan-8-oxomethyl caprylate. The method comprises the following steps of adding octanedioic acid and sulfoxide chloride into a reaction bulb, agitating an obtained mixture at a room temperature until a reaction is complete, and distilling to remove the sulfoxide chloride under normal pressure; afterwards, adding a solvent and a catalyst into the reaction bulb to carry out another reaction, dropwise adding methanol into the reaction bulb for quenching, decompressing the reaction bulb and distilling to remove the solvent, dissolving a target product by using an extracting solution to obtain a solution, and distilling the solution under reduced pressure to obtain a yellowish oily 8-furan-8-oxomethyl caprylate pure product. According to the chemical synthetic method of the 8-furan-8-oxomethyl caprylate, a synthetic strategy of a one-pot method is adopted; by proceeding from the low-cost octanedioic acid, the octanedioyl chloride is prepared with a high yield; afterwards, the octanedioyl chloride and furan are subjected to a regioselectivity Friedel-Crafts acylation reaction; finally, acyl chloride at the other end is quenched by using the methanol to directly generate methyl ester to obtain the product; by using the method, the synthetic route of the target product is simplified; the reaction time is greatly shortened; the synthetic total yield is improved; therefore, the production cost is decreased; the chemical synthetic method has the advantages of being low in cost, higher in yield, simple to operate and suitable for industrial production, and the like.
Owner:ZHEJIANG UNIV OF TECH
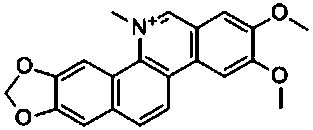
Preparation method of nitidine chloride
InactiveCN109942590AHigh yieldImprove the overall yield of synthesisOrganic chemistryPhenylboronic acidNitidine chloride
The invention discloses a preparation method of nitidine chloride. The method comprises the following steps of 1) catalyzing 4,5-dimethoxy-2-(methoxycarbonyl) phenylboronic acid to react with azobenzene bornene to obtain an intermediate 1 by using a metal palladium / ligand; 2) dissolving the intermediate 1 with an organic solvent, then adding trifluoroacetic acid, and conducting a room temperaturereaction to obtain an intermediate 2; 3) methylating the intermediate 2, oxidizing, reducing, and treating with hydrochloric acid to obtain the nitidine chloride. The synthetic route of the method issimpler, and the total synthesis yield of the nitidine chloride is as high as 72% or more.
Owner:YUNNAN MINZU UNIV
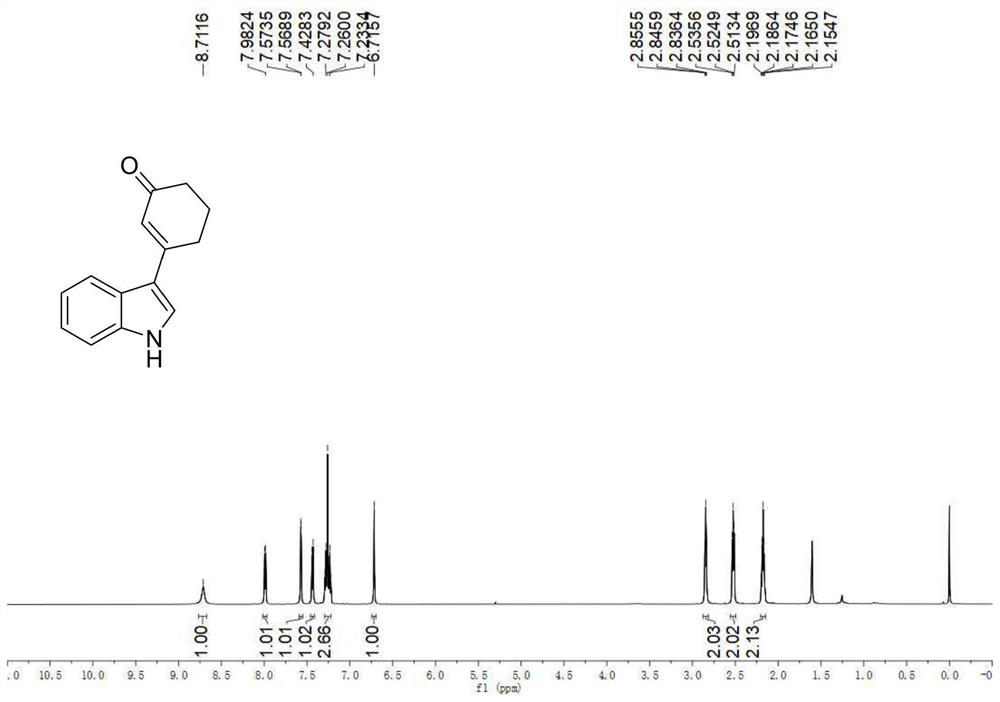
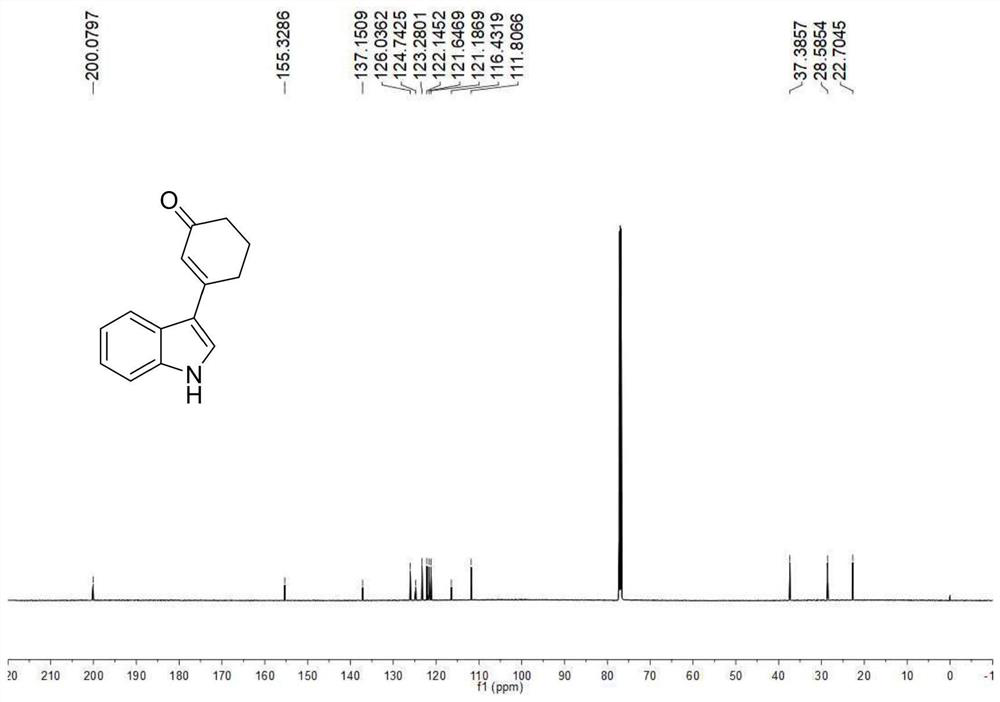
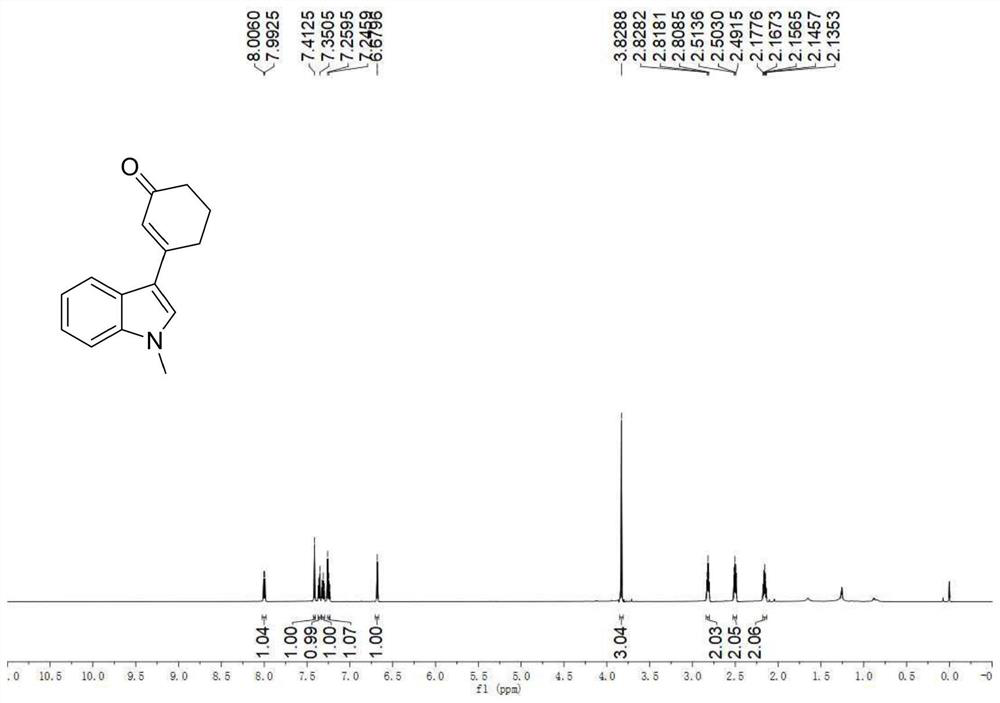
A kind of preparation method of 3-indolated cyclohexenone compound
ActiveCN110117246BImprove compatibilityMild reaction conditionsOrganic chemistryQuinoneCyclohexenone
The invention belongs to the technical field of preparation methods of indolated cyclohexenone derivatives, and specifically relates to a preparation method of 3-position indolated cyclohexenone compounds. The steps are as follows: molar ratio 1:4:0.1:0.2 :1.5 Indole or substituted indole, cyclohexenone, palladium trifluoroacetate, 2,5-dimethyl-8-trifluoromethyl-3,4-dihydro-2H-pyrano[2 ,3‑b] Add quinoline and tert-butanol peroxide into the reactor, add solvent to dissolve and mix well, react at 50°C for 24 to 41 hours; after the reaction is completed, cool to room temperature, dilute the reactant and wash with saturated aqueous sodium chloride solution , separating the organic phase from the aqueous phase, extracting the aqueous layer with ethyl acetate, combining the organic phases, filtering, and spinning off the solvent; separating and purifying the residue after spinning off the solvent, spinning off the solvent, and pumping dry to obtain the target product. The preparation method has the advantages of cheap and easy-to-obtain raw materials, simple reaction steps, high atom utilization rate and the like.
Owner:SHANXI UNIV
A method for extracting nitration products from n-ethylcarbazole nitration solvent distillation residue
ActiveCN103254117BImprove nitrification yieldImprove the overall yield of synthesisOrganic chemistryRefluxOrganic solvent
The invention discloses a method for extracting a nitration product from N-ethyl carbazole nitration solvent distillation residue. The method is characterized by comprising the following steps of: putting the N-ethyl carbazole nitration solvent distillation residue and an organic solvent in a container, heating while stirring to a reflux state, stirring for 0.5-6 hours while preserving heat, adding a flocculant and continuing stirring for 0.5-6 hours while preserving heat, and then filtering while the solution is hot or taking out the supernatant liquid from the container after standing, thereby obtaining 3-nitro-N-ethyl carbazole solution; and in order to improve the quality of the 3-nitro-N-ethyl carbazole solution, further adding active carbon to the solution for absorption bleaching, and after stirring for 0.5-6 hours while preserving heat, filtering while the solution is hot, thereby obtaining the solution of 3-nitro-N-ethyl carbazole. The method for extracting the nitration product from N-ethyl carbazole nitration solvent distillation residue has the advantages that the extraction process is simple and high in efficiency, the yield of permanent violet nitration is greatly improved and further the total yield of permanent violet synthesis is improved, the production cost of permanent violet is reduced, the waste residue is reduced and the environmental protection effect is good.
Owner:南通龙晨新材料科技有限公司
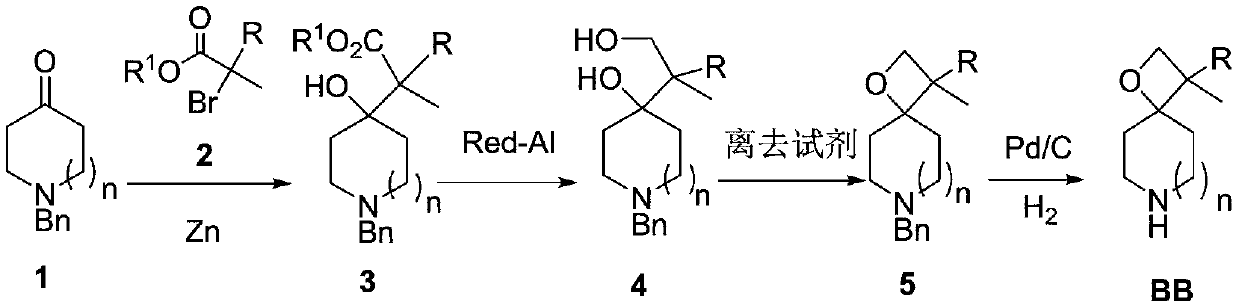
Preparation method of piperidine spiro derivative
InactiveCN111039949AImprove the overall yield of synthesisThe synthetic route is simpleOrganic chemistryCyclic ketoneCarboxylic ester
The invention discloses a preparation method of a piperidine spiro derivative, and belongs to the field of synthesis of medical intermediates. The method comprises the following steps: 1, enabling N-benzyl substituted cyclic ketone 1 to react with bromo-carboxylic ester 2 in the presence of zinc powder to obtain an addition product 3, reducing with sodium bis(2-methoxyethoxy)aluminiumhydride to obtain a diol product 4, performing cyclization in the presence of a leaving reagent to obtain bicyclo-spirooxetane 5, and finally performing palladium-carbon catalytic hydrogenation debenzylation to obtain a BB piperidine spiro compound. The synthesis route is simple, the total synthesis yield is high, the method is suitable for various different rings, a series of compounds are obtained, and a newdiversified structural fragment is provided for new drug development.
Owner:CHEMVON BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Preparation method of N-[4-(triethyl aminomethyl) benzoyl] caprolactam bromide Preparation method of N-[4-(triethyl aminomethyl) benzoyl] caprolactam bromide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dc67888e-fb2f-4fe7-ba13-86179e0858f0/101210122024.PNG)
![Preparation method of N-[4-(triethyl aminomethyl) benzoyl] caprolactam bromide Preparation method of N-[4-(triethyl aminomethyl) benzoyl] caprolactam bromide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dc67888e-fb2f-4fe7-ba13-86179e0858f0/101210122014.PNG)
![Preparation method of N-[4-(triethyl aminomethyl) benzoyl] caprolactam bromide Preparation method of N-[4-(triethyl aminomethyl) benzoyl] caprolactam bromide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dc67888e-fb2f-4fe7-ba13-86179e0858f0/2010105819641100002DEST_PATH_IMAGE004.PNG)