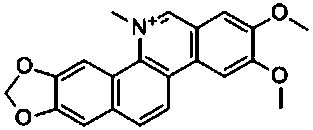
Preparation method of nitidine chloride
A kind of chlorinated edematine, reaction technology, applied in the field of medicine, can solve the problems of low total yield, low efficiency, poor water solubility, etc., and achieve the effect of simple synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0011] The preparation method of the chlorinated dimethicone of the present invention uses azabenzobornene and 4,5-dimethoxy-2-(methoxycarbonyl)phenylboronic acid as reaction raw materials, and undergoes a simple five-step reaction. The synthesis obtains chlorinated acetine, and its synthetic route is as follows:
[0012] .
[0013] The preparation method of described chlorinated acetine comprises the following steps:
[0014] 1) Azabenzobornene and 4,5-dimethoxy-2-(methoxycarbonyl)phenylboronic acid are used as raw materials for the reaction, and palladium acetate is used as the catalyst to obtain intermediate 1;
[0015] 2) Dissolving intermediate 1 in an organic solvent and adding trifluoroacetic acid or trifluoromethanesulfonic acid to react to obtain intermediate 2;
[0016] 3) Reaction of intermediate 2 with methyl iodide to obtain intermediate 3;
[0017] 4) Intermediate 4 is obtained by oxidation of intermediate 3;
[0018] 5) The intermediate 4 was reduced and d...
Embodiment 1
[0027] 1) Synthesis of Intermediate 1
[0028] Azabenzobornene (1 eq.) and 4,5- Dimethoxy-2-(methoxycarbonyl)phenylboronic acid (2-4 equivalents) was reacted at room temperature for 1 hour, and the reaction solution was detected by TLC. The solvent was evaporated to dryness under reduced pressure, and the residue was subjected to silica gel column chromatography to obtain Intermediate 1, a white solid, with a yield of 90%. 1 H NMR (400 MHz, CDCl 3 ) δ 7.22 (s, 1H), 7.13 (s, 1H), 6.72 (s, 1H), 6.65(s, 1H), 6.55 (t, J = 9.2 Hz, 1H), 6.02 (dd, J = 9.6, 4.8 Hz, 1H), 5.92 (d, J = 8.5 Hz, 2H), 5.18 (dd, J = 9.6, 7.6 Hz, 1H), 4.53 (d, J = 10.1 Hz, 1H),3.93 (s, 3H), 3.84 - 3.74 (m, 1H), 3.70 (s, 3H), 3.65 (s, 3H), 1.38 (s, 9H). 13 C NMR (CDCl 3 , 100 MHz): δ 168.1, 155.3, 147.3, 146.9, 137.8, 129.1, 128.5,128.4, 128.1, 127.4, 127.2, 107.1, 101.0, 79.4, 60.4, 56.1, 55.7, 52.4, 51.5,44.8, 28.4, 21.1, 14.2.
[0029] 2) Synthesis of Intermediate 2
[0030] Dissolve Intermed...
Embodiment 2
[0038] Repeat the method of embodiment 1, just make the following modifications:
[0039] 1 Change the chloroform in steps 3 and 4 to dichloromethane;
[0040] 2 Quench the reaction with wet tetrahydrofuran in step 5;
[0041] The product total yield that this implementation makes is 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com