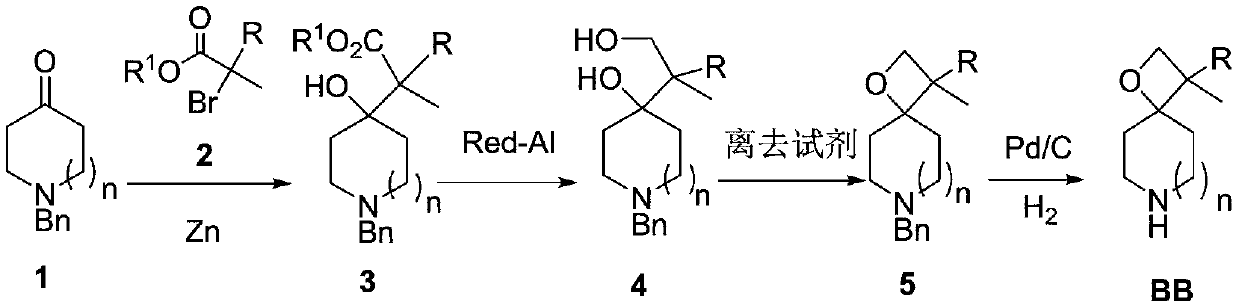
Preparation method of piperidine spiro derivative
A technology of derivatives and spiro rings, applied in the field of synthesis of pharmaceutical intermediates, can solve problems such as inappropriateness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] Step 1: In a 10L three-necked flask equipped with mechanical stirring, add 189g of N-benzyl-4-piperidone 1a [1.0mol], 71.5g of activated zinc powder [1.1mol] and 2.0L of anhydrous tetrahydrofuran in sequence. Under protection, turn on mechanical stirring. Then the temperature was raised to 70-80°C, and 2.0g of ethyl bromide 1,1-dimethylcarboxylate 2 was added dropwise. After stirring and initiation (about 5 minutes), 210g [1.1mol] of bromide was added dropwise to the reaction system. A mixture of ethyl 1,1-dimethylcarboxylate 2 dissolved in 500 mL of anhydrous tetrahydrofuran. The rate of addition was such that the temperature of the system did not exceed 80°C. After the addition was complete, the reaction was continued for 2 hours while the temperature was maintained. TLC detected that the raw materials disappeared, and the reaction was stopped. After cooling to room temperature, the above reactants were separated into layers, the aqueous phase was extract...
Embodiment 2
[0039]
[0040] Step 1: In a 10L three-necked flask equipped with a mechanical stirrer, add 175g [1.0mol] N-benzyl-3-pyrrolone 1b, 71.5g activated zinc powder [1.1mol] and 2.0L anhydrous tetrahydrofuran, under nitrogen protection , turn on the mechanical stirring. Then the temperature was raised to 70-80°C, 2.0g of 2a was added dropwise, and after the reaction was initiated with stirring, a mixture of 210g of ethyl bromocarboxylate 2a [1.1mol] dissolved in 500mL of anhydrous tetrahydrofuran was continued to be added dropwise to the reaction system. It is advisable to control the temperature of the system not to exceed 80°C for the rate of addition, and continue stirring for 2 hours after addition. TLC detects that the starting material disappears, and the reaction is stopped. After the post-treatment in Example 1, 251 g of light yellow oily liquid 3b was obtained, M / z=292 [M+1], and the yield was 86.2%.
[0041]Step 2: In a 5.0L three-necked flask equipped with a mechanic...
Embodiment 3
[0045]
[0046] Step 1: In a 10.0L three-necked flask equipped with mechanical stirring, add 203g of N-benzyl-4-homopiperidone 1c [1.0mol], 71.5g of activated zinc powder [1.1mol] and 2.0L of anhydrous 2-formazol Based tetrahydrofuran, under the protection of nitrogen, start the mechanical stirring. Then the temperature was raised to 70-80°C, 2.0g of ethyl bromocarboxylate 2a was added dropwise, and after stirring (about 10 minutes), 200g of ethyl bromocarboxylate 2a [1.1mol] dissolved in 500mL of anhydrous 2-methyltetrahydrofuran mixed solution. During the dropwise addition process, the temperature of the system should not exceed 80°C. After the addition, the reaction was continued for 2 hours, and the reaction was stopped after TLC detected that the raw materials disappeared. After cooling to room temperature, the layers were separated, the aqueous phase was extracted with 3.0L ethyl acetate, and the organic phases were combined. Wash with saturated brine and concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com