Preparation method of 3-(thiophene-2-yl)hexanaphthene-2-ketene derivatives
A technology of cyclohexenone and derivatives, applied in the field of preparation of thiophene derivatives, to achieve the effects of reducing the total cost, increasing the total yield of synthesis, and simple reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
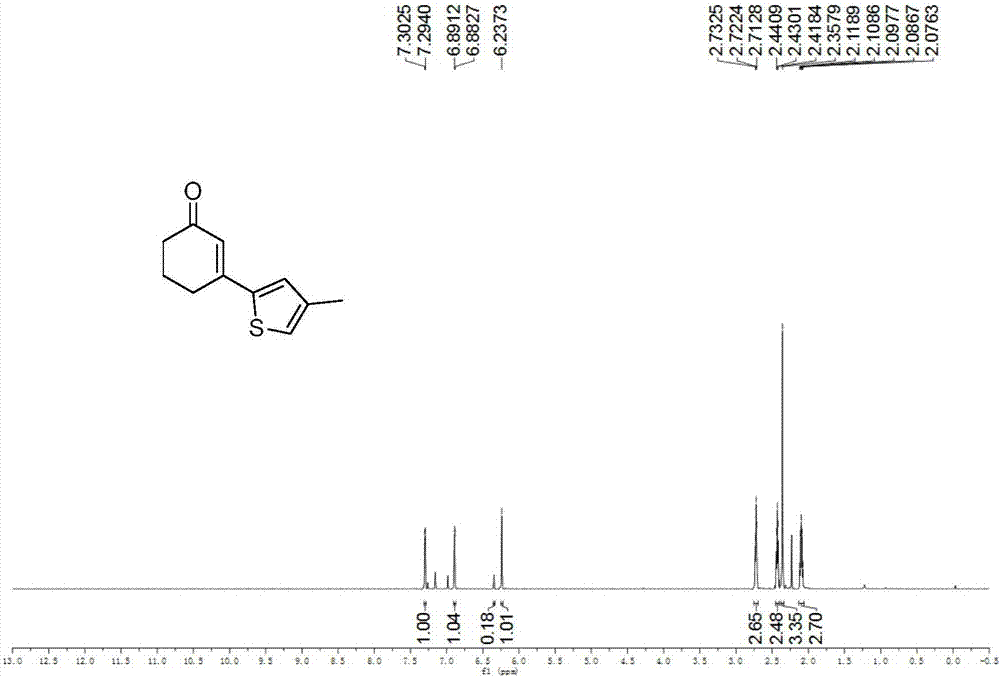
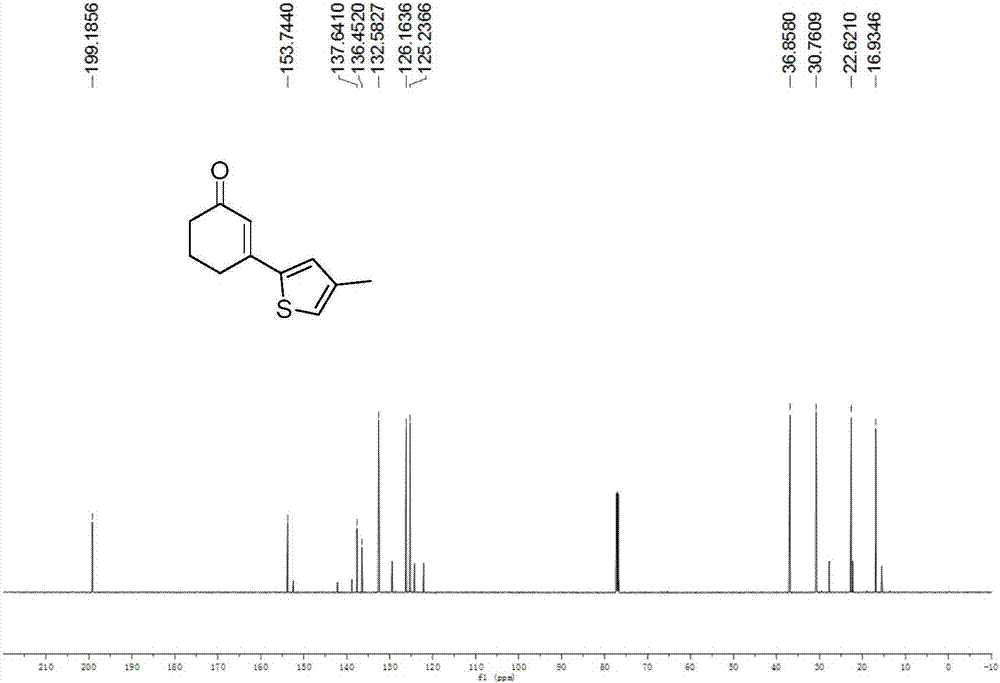
[0042] Example 1: Synthesis of 3-(3-methylthiophen-5-yl)cyclohex-2-enone
[0043] (1) 3-methylthiophene (0.024ml, 0.25mmol), cyclohexenone (0.072ml, 0.75mmol), palladium chloride (0.0044g, 0.0025mmol), N-acetylglycine (0.0029g, 0.0025mmol ), silver carbonate (0.1723g, 0.625mmol), silver hexafluoroantimonate (0.0172g, 0.05mmol), dimethylsulfoxide (0.05ml), 1,1,1,3,3,3-hexafluoro-2 -Propanol (0.05ml), 1,4-dioxane (1ml), stirred evenly in a clean and dry airtight reaction tube, heated to 60°C, and reacted for 24 hours.
[0044] (2) After the reaction is completed, cool the reaction tube to room temperature, add 40ml of ethyl acetate to dilute the reaction system and transfer it to a 100ml separatory funnel, add 20ml of saturated ammonium chloride aqueous solution, shake, let it stand, and remove the lower water phase Afterwards, add 20ml of saturated saline again, shake, let stand, remove the lower water phase, the organic phase is dried with anhydrous sodium sulfate, remove the...
Embodiment 2
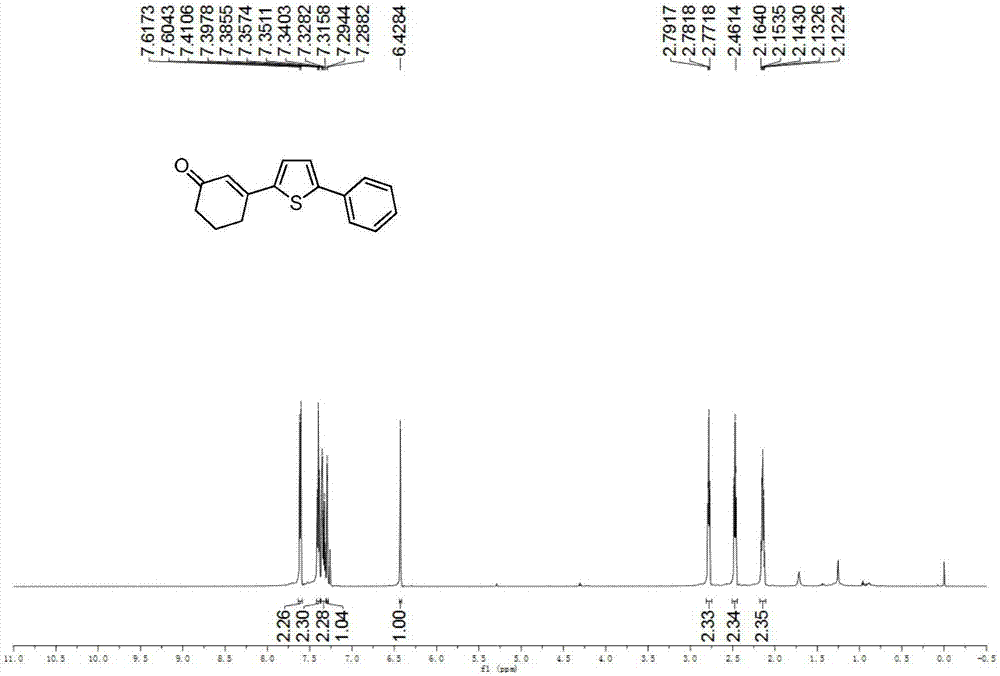
[0045] Example 2: Synthesis of 3-(5-phenylthiophen-2-yl)cyclohex-2-enone
[0046] (1) O-phenylthiophene (0.0401g, 0.25mmol), cyclohexenone (0.072ml, 0.75mmol), palladium chloride (0.0044g, 0.0025mmol), N-acetylglycine (0.0029g, 0.0025mmol), Silver carbonate (0.1723g, 0.625mmol), silver hexafluoroantimonate (0.0172g, 0.05mmol), dimethylsulfoxide (0.05ml), 1,1,1,3,3,3-hexafluoro-2-propane Alcohol (0.05ml), 1,4-dioxane (1ml), stirred evenly in a clean and dry airtight reaction tube, heated to 60°C, and reacted for 24 hours.
[0047] (2) After the reaction is completed, cool the reaction tube to room temperature, add 40ml of ethyl acetate to dilute the reaction system and transfer it to a 100ml separatory funnel, add 20ml of saturated ammonium chloride aqueous solution, shake, let it stand, and remove the lower water phase Afterwards, add 20ml of saturated saline again, shake, let stand, remove the lower water phase, the organic phase is dried with anhydrous sodium sulfate, remov...
Embodiment 3
[0048] Example 3: Synthesis of 3-(4-methoxythiophen-2-yl)cyclohex-2-enone
[0049] (1) 3-methoxythiophene (0.025ml, 0.25mmol), cyclohexenone (0.072ml, 0.75mmol), palladium chloride (0.0044g, 0.0025mmol), N-acetylglycine (0.0029g, 0.0025 mmol), silver carbonate (0.1723g, 0.625mmol), silver hexafluoroantimonate (0.0172g, 0.05mmol), dimethylsulfoxide (0.05ml), 1,4-dioxane (1ml), in clean dry Stir evenly in a sealed reaction tube, heat to 60°C, and react for 24 hours.
[0050] (2) After the reaction is completed, cool the reaction tube to room temperature, add 40ml of ethyl acetate to dilute the reaction system and transfer it to a 100ml separatory funnel, add 20ml of saturated ammonium chloride aqueous solution, shake, let it stand, and remove the lower water phase Afterwards, add 20ml of saturated saline again, shake, let stand, remove the lower water phase, the organic phase is dried with anhydrous sodium sulfate, remove the solvent under reduced pressure, and the residue is s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com