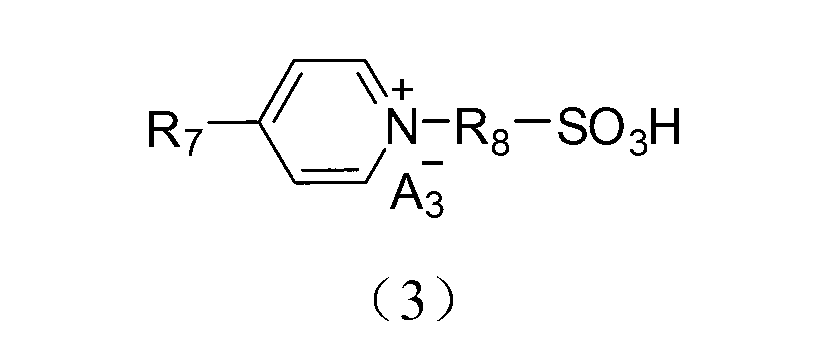
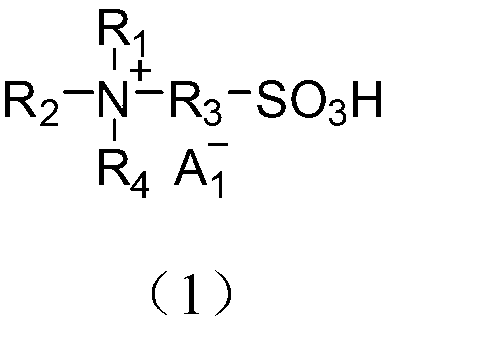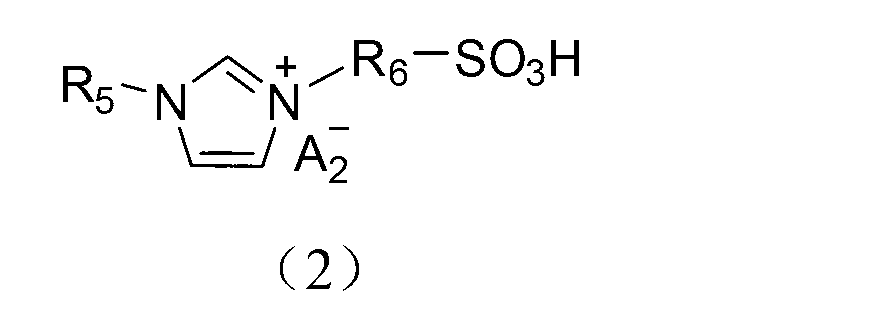Application of acidic ionic liquid in catalyzing and synthesizing diphenolic acid and/or diphenolic acid ester
A technology of acidic ionic liquid and bisphenolic acid ester, applied in the field of application of acidic ionic liquid in catalytic synthesis of bisphenolic acid and/or bisphenolic acid ester
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] (1) Add 0.15mol biimidazole to a 250mL single-necked flask and then add 100mL acetonitrile to dissolve; dissolve 0.30mol 1,3-propane sultone in 20mL toluene and slowly add it dropwise to the flask under ice-bath stirring conditions. After the dropwise addition, the temperature was raised to 50°C to react for 2 hours. After the reaction, the product was filtered and precipitated, and the precipitate was washed three times with 50 mL of ether to obtain diimidazole propanesulfonic acid inner salt (DIM-SO 3 );
[0079] (2) Take 0.1mol diimidazole propanesulfonic acid inner salt (DIM-SO 3 ) in 100mL deionized water, add 0.2mol trifluoromethanesulfonic acid, heat up to 90°C and stir and heat for 5h. After the reaction is over, put the reaction product in a vacuum drying oven and dry at 100°C for 10h , the obtained final product is detected by nuclear magnetic resonance, and the data are as follows: 1 H NMR (400MHz,D 2 O): δ=1.85(s,2H),2.26(m,2H),2.84(t,2H),4.20(s,2H),4.31(...
Embodiment 2
[0082] (1) Add 0.15mol N,N,N′N′-tetramethyl-1,3-propanediamine to a 250mL single-necked flask, then add 100mL acetonitrile to dissolve, and dissolve 0.30mol 1,3-propanesulfonic acid Dissolve the ester in 20mL of toluene and slowly add it dropwise to the flask under the condition of stirring in an ice bath. After the dropwise addition, raise the temperature to 50°C and react for 2 hours. , to get N,N,N',N'-tetramethyl-1,3-propanediamine (DTA-SO 3 );
[0083] (2) Take 0.1mol N,N,N',N'-tetramethyl-1,3-propanediamine (DTA-SO 3 ) was dissolved in 100mL deionized water, added 0.2mol mercaptopropanesulfonic acid and heated to 90°C, stirred and heated for 5h. The final product of the reaction was detected by nuclear magnetic resonance, and the detection data were as follows: 1 H NMR (400MHz,D 2 O): δ=2.15(m,2H), 2.25(m,1H), 2.91(t,2H), 3.08(s,6H), 3.35(t,2H), 3.46(t,2H).
[0084] From the above data, the obtained product was deduced to be N1,N1,N2,N2-tetramethyl-N1,N2-bis(3-sulfo...
Embodiment 3
[0086] (1) Add 0.15mol 4,4′-bipyridine to a 250mL single-necked flask, then add 100mL acetonitrile to dissolve, dissolve 0.30mol 1,3-propane sultone in 20mL toluene, and slowly add it dropwise to the ice In the flask under the condition of bath stirring, after the dropwise addition, the temperature was raised to 50°C for 2 hours of reaction. After the reaction, the reaction product was filtered and precipitated, and the obtained precipitate was washed three times with 50 mL of ether to obtain the inner salt 4,4'-bipyridine propane Sulfonic acid inner salt (DPY-SO 3 );
[0087] (2) Take 0.1mol 4,4'-bipyridylpropanesulfonic acid inner salt (DPY-SO 3 ) was dissolved in 100mL deionized water, added 0.2mol mercaptopropanesulfonic acid and heated to 90°C, stirred and heated for 5h. The final product of the reaction was detected by nuclear magnetic resonance, and the detection data were as follows: 1 H NMR (400MHz,D 2 O): δ=2.45(m,1H), 2.95(t,1H), 4.81(t,1H), 8.48(d,1H), 9.08(d,1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com