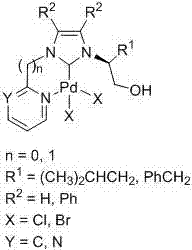
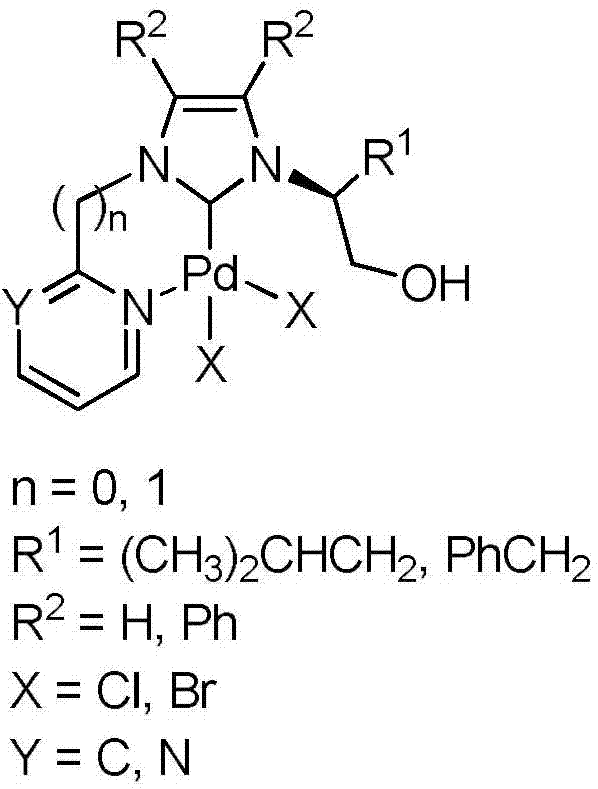
Heteroaryl-containing chelating nitrogen heterocyclic carbene palladium compound and preparation method thereof
A technology for nitrogen-heterocyclic carbene and palladium compounds, which is applied in the field of organic compound synthesis, can solve problems such as hindering the further application of nitrogen-heterocyclic carbene palladium compounds, difficult separation and recovery of homogeneous catalysts, and no active groups, etc., and achieves a simple and easy synthesis method Line, easy to separate, simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) In a 250mL three-neck flask, dissolve L-leucinol (60mmol, 7.03g) and ammonium chloride (60mmol, 3.21g) in 120mL of methanol, and then add the mass percentage dropwise with a constant pressure funnel under ice-water bath cooling. 36% formaldehyde solution (60mmol, 4.6mL) and 40% mass percent glyoxal solution (60mmol, 7.6mL). After the dropwise addition, the temperature was raised to 60° C. for 5 h, and the reaction solution was spin-dried to obtain a reddish-brown viscous liquid. The above reddish-brown viscous liquid was dissolved in 150mL NaOH solution (2M), extracted with dichloromethane (20mL×3), the organic phases were combined, and washed with anhydrous NaOH 2 SO 4 Dry, filter, remove the solvent under reduced pressure, separate and purify by column chromatography to obtain pure (S)-2-(1-imidazolyl)-4-methylpentanol (7.57 g, yield 75%).
[0029] (2) Add (S)-2-(1-imidazolyl)-4-methylpentanol (5.0mmol, 0.84g), 2-bromopyridine (3mL) and 2mL toluene into a 50mL r...
Embodiment 2
[0033] Step (1) is the same as embodiment 1
[0034] (2) Add (S)-2-(1-imidazolyl)-4-methylpentanol (5.0mmol, 0.84g), 2-chloropyrimidine (6mmol, 0.69g) and 15mL toluene into a 50mL round bottom flask , heated to 110°C for 72h, cooled to room temperature, collected viscous yellow solid in the system, separated and purified by column chromatography to obtain N-(1-hydroxy-4-methyl-2-pentyl)-N'- Pure (2-pyrimidinyl)-imidazolium chloride salt (1.12 g, yield 79%). 1 H NMR(400MHz,DMSO):δ10.34(s,1H,CH in imidazole),9.08(d,J=4.9Hz,2H,CH in pyrimidine),8.55(t,J=1.8Hz,1H,CH in imidazole), 8.22(t, J=1.7Hz, 1H, CH in imidazole), 7.79(t, J=4.9Hz, 1H, CH in pyrimidine), 5.47(t, J=5.4Hz, 1H, CH 2 OH),4.75-4.71(m,1H,NCH),3.68-3.77(m,2H,CH 2 OH),1.99-1.92(m,1H,CH 2 CH(CH 3 ) 2 ),1.67-1.60(m,1H,CH 2 CH(CH 3 ) 2 ),1.43-1.36(m,1H,CH 2 CH(CH 3 ) 2 ), 0.93 (d, J=6.5Hz, 3H, CH 2 CH(CH 3 ) 2 ),0.88(d,J=6.6Hz,3H,CH 2 CH(CH 3 ) 2 ))ppm. 13 C NMR (100MHz, DMSO): δ160.5, 152.7, 136.3, 12...
Embodiment 3
[0038] (1) In a 250mL three-necked flask, dissolve L-phenylalaninol (60mmol, 9.07g) and ammonium chloride (60mmol, 3.21g) in 120mL of methanol, and drop the mass of A 36% formaldehyde solution (60mmol, 4.6mL) and a 40% mass percent glyoxal solution (60mmol, 7.6mL). After the dropwise addition, the temperature was raised to 60° C. for 5 h, and the reaction solution was spin-dried to obtain a reddish-brown viscous liquid. The above yellow oily viscous substance was dissolved in 150mL NaOH solution (2M), extracted with dichloromethane (20mL×3), the organic phases were combined, and washed with anhydrous NaOH 2 SO 4 Dry, filter, remove the solvent under reduced pressure, and separate and purify by column chromatography to obtain pure (S)-2-(1-imidazolyl)-3-phenylalaninol (9.71 g, yield 80%).
[0039] (2) Add (S)-2-(1-imidazolyl)-3-phenylalaninol (5.0mmol, 1.01g), 2-bromopyridine (3mL) and 2mL toluene into a 50mL round bottom flask and heat to After reacting at 110°C for 72 hour...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com