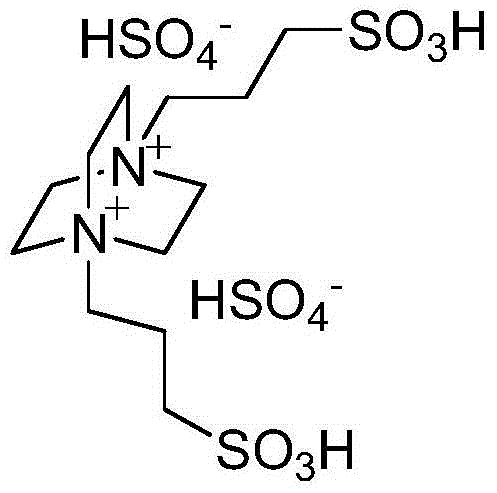
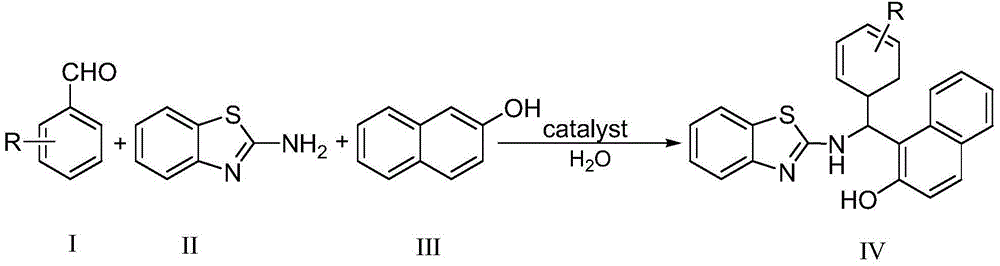
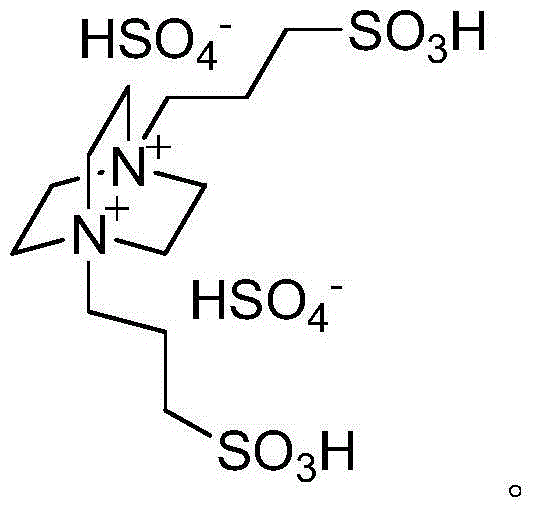
Green method for catalytically synthesizing 2'-aminobenzothiazolyl-arylmethyl-2-naphthol
An aminobenzene and arylmethyl technology, applied in the field of organic chemical synthesis, can solve problems such as large loss, and achieve the effects of high acid density, good catalytic activity, and low environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 1mmol of benzaldehyde, 1mmol of 2-aminobenzothiazole, 1mmol of β-naphthol, 3ml of water and 0.05mmol of bissulfonate acidic ionic liquid into a 25ml single-necked flask with a stirring bar and a reflux condenser. Stir vigorously at 80°C for 5 minutes, TLC (thin plate chromatography) detection, the raw material point disappears, cool to room temperature, and filter with suction, the obtained filter residue is recrystallized with 95% ethanol aqueous solution, and after vacuum drying, the pure product 2′-aminobenzothiazole is obtained -Phenylmethyl-2-naphthol, the yield is 94%. Add benzaldehyde, 2-aminobenzothiazole and β-naphthol directly to the filtrate for repeated use.
[0021] 2′-Aminobenzothiazole-phenylmethyl-2-naphthol: 1 H NMR (500MHz, DMSO-d 6 ): δ=6.97~7.86(m, 16H, ArH / CH), 8.80(s, 1H, -NH-), 10.13(s, 1H, -OH); IR(KBr): ν=3379, 1623, 1595 , 1544, 1518, 1445, 1332, 1270, 814, 750cm -1
Embodiment 2
[0023] Add 1mmol of 2-chlorobenzaldehyde, 1mmol of 2-aminobenzothiazole, 1mmol of β-naphthol, 5ml of water and 0.05mmol of bissulfonate acidic ionic liquid into a 25ml single-necked flask with a stirring bar and a reflux condenser. Vigorously stirred and reacted at 80°C for 4 minutes, detected by TLC (thin plate chromatography), the raw material point disappeared, cooled to room temperature, and filtered with suction, the obtained filter residue was recrystallized with 95% ethanol aqueous solution, and the pure product 2′-aminobenzothiazole was obtained after vacuum drying -(2-Chlorophenyl)methyl-2-naphthol, the yield is 95%. Directly add 2-chlorobenzaldehyde, 2-aminobenzothiazole and β-naphthol to the filtrate for repeated use.
[0024] 2'-Aminobenzothiazole-(2-chlorophenyl)methyl-2-naphthol: 1 H NMR (500MHz, DMSO-d 6 ): δ=6.96~8.04(m, 15H, ArH / CH), 8.82(s, 1H, -NH-), 9.91(s, 1H, -OH); IR(KBr): ν=3384, 1628, 1597 , 1543, 1440, 1319, 1271, 815, 754cm -1
Embodiment 3
[0026] Add 1mmol of 4-nitrobenzaldehyde, 1mmol of 2-aminobenzothiazole, 1mmol of β-naphthol, 5ml of water and 0.05mmol of bissulfonate acidic ionic liquid into a 25ml single-necked flask with a stirring bar and a reflux condenser. Vigorously stirred and reacted at 85°C for 3.5 minutes, detected by TLC (thin plate chromatography), the raw material point disappeared, cooled to room temperature, and filtered with suction, the obtained filter residue was recrystallized with 95% ethanol aqueous solution, and after vacuum drying, the pure product 2′-aminobenzo Thiazole-(4-nitrophenyl)methyl-2-naphthol, yield 96%. Directly add 4-nitrobenzaldehyde, 2-aminobenzothiazole and β-naphthol to the filtrate for repeated use.
[0027] 2'-Aminobenzothiazole-(4-nitrophenyl)methyl-2-naphthol: 1 H NMR (500MHz, DMSO-d 6 ): δ=7.06~8.14(m, 15H, ArH / CH), 8.92(s, 1H, -NH-), 10.21(s, 1H, -OH); IR(KBr): ν=3393, 1626, 1599 , 1534, 1443, 1349, 1270, 813, 753cm -1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com