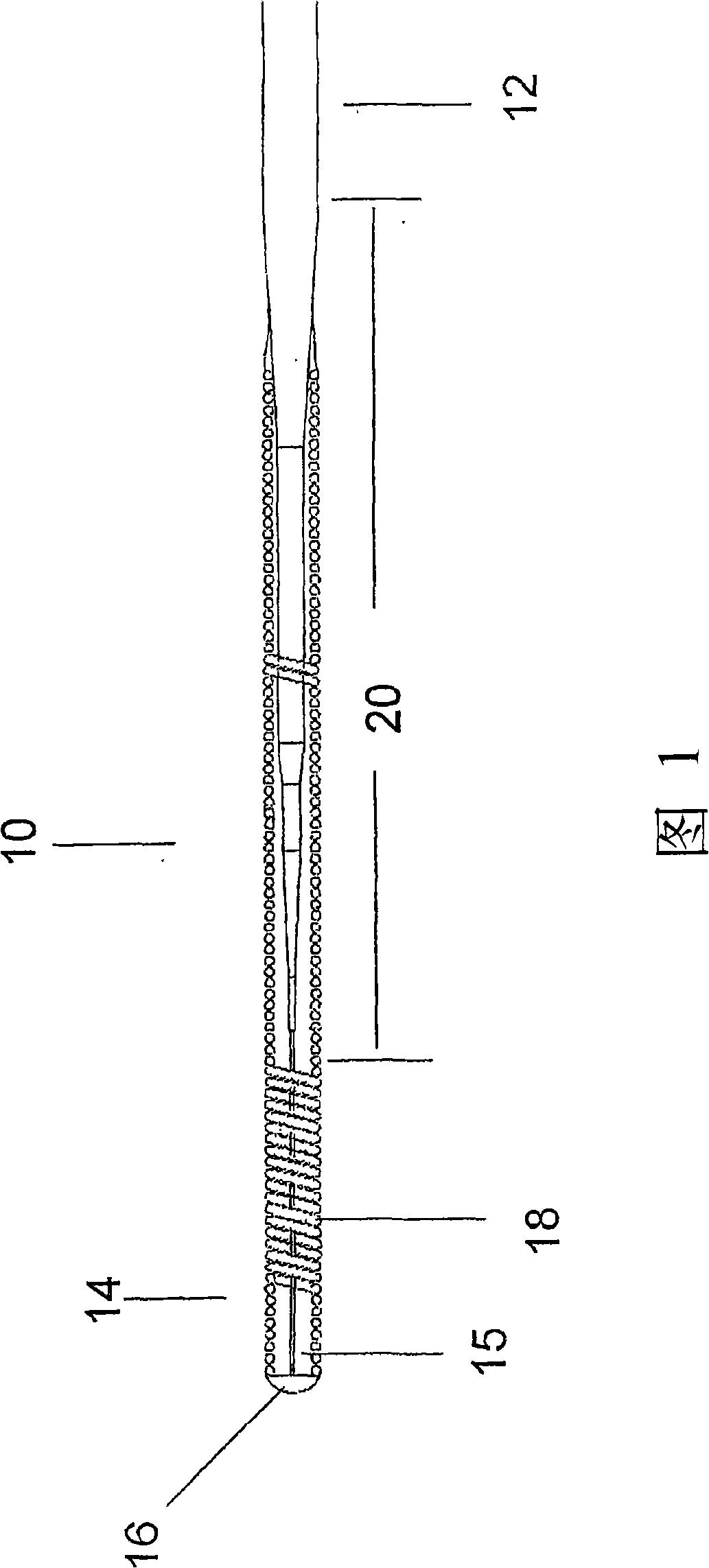
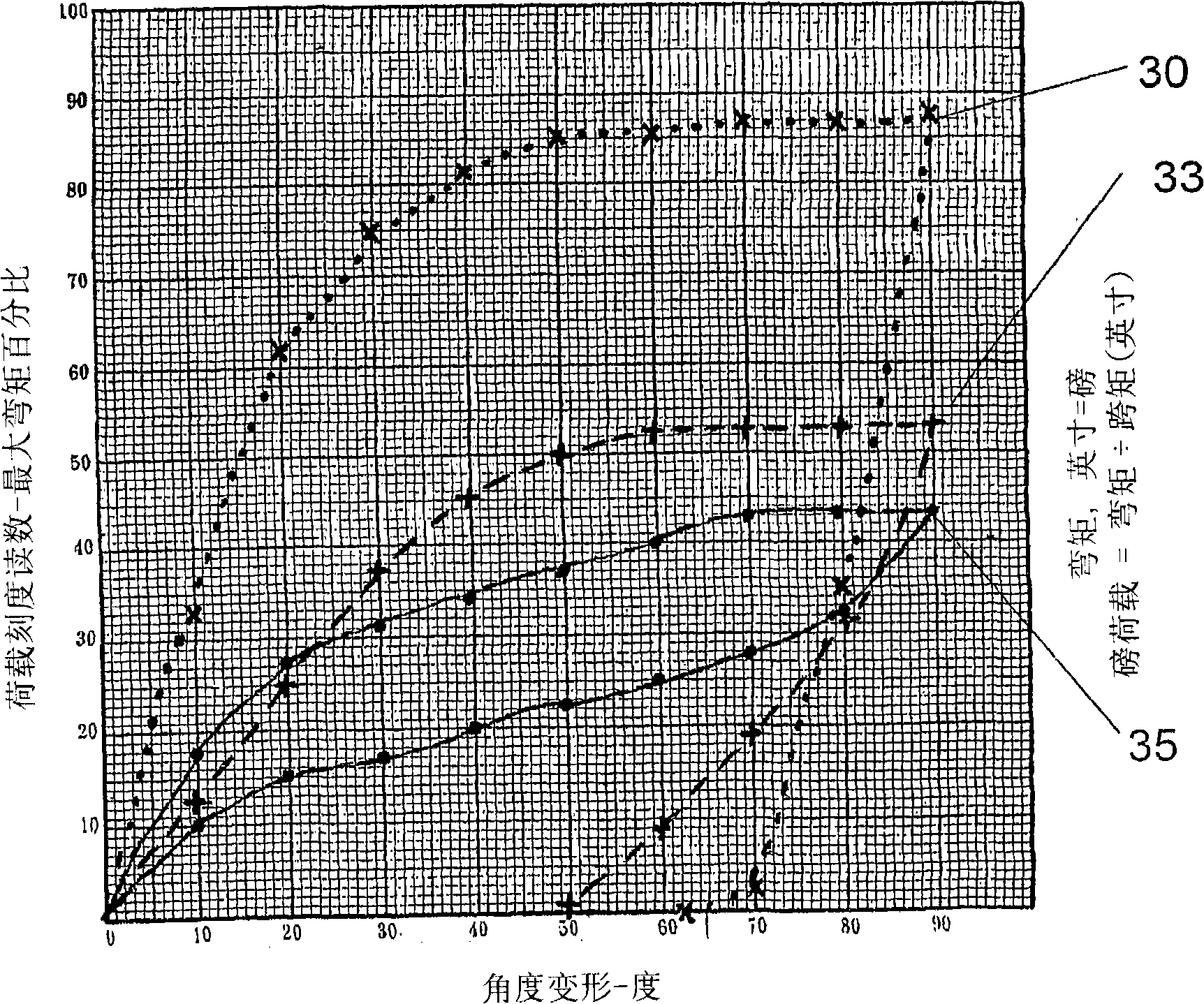
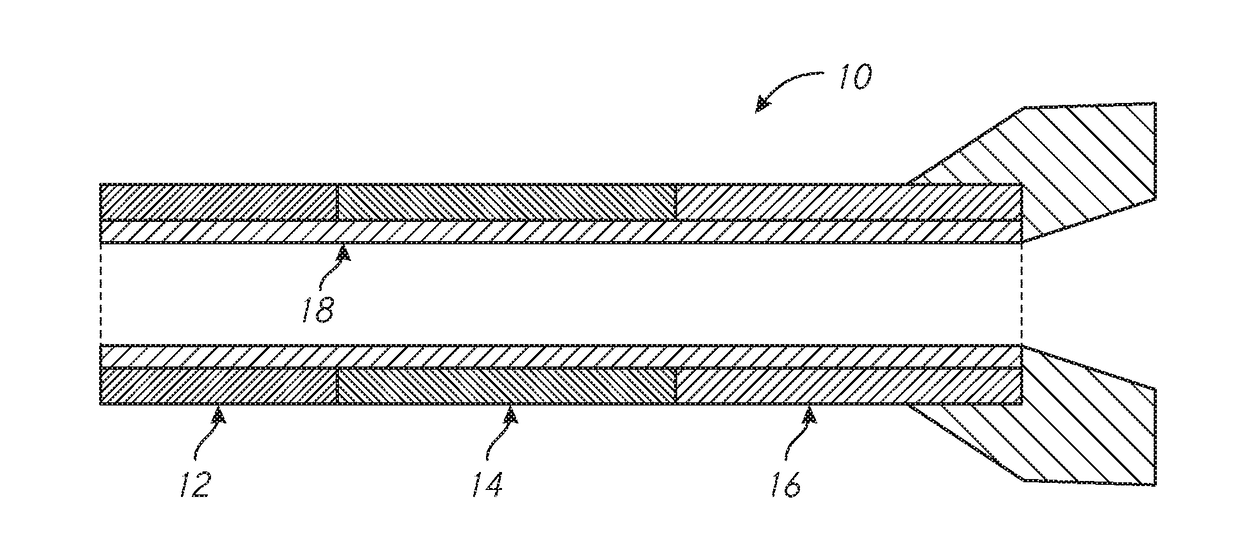
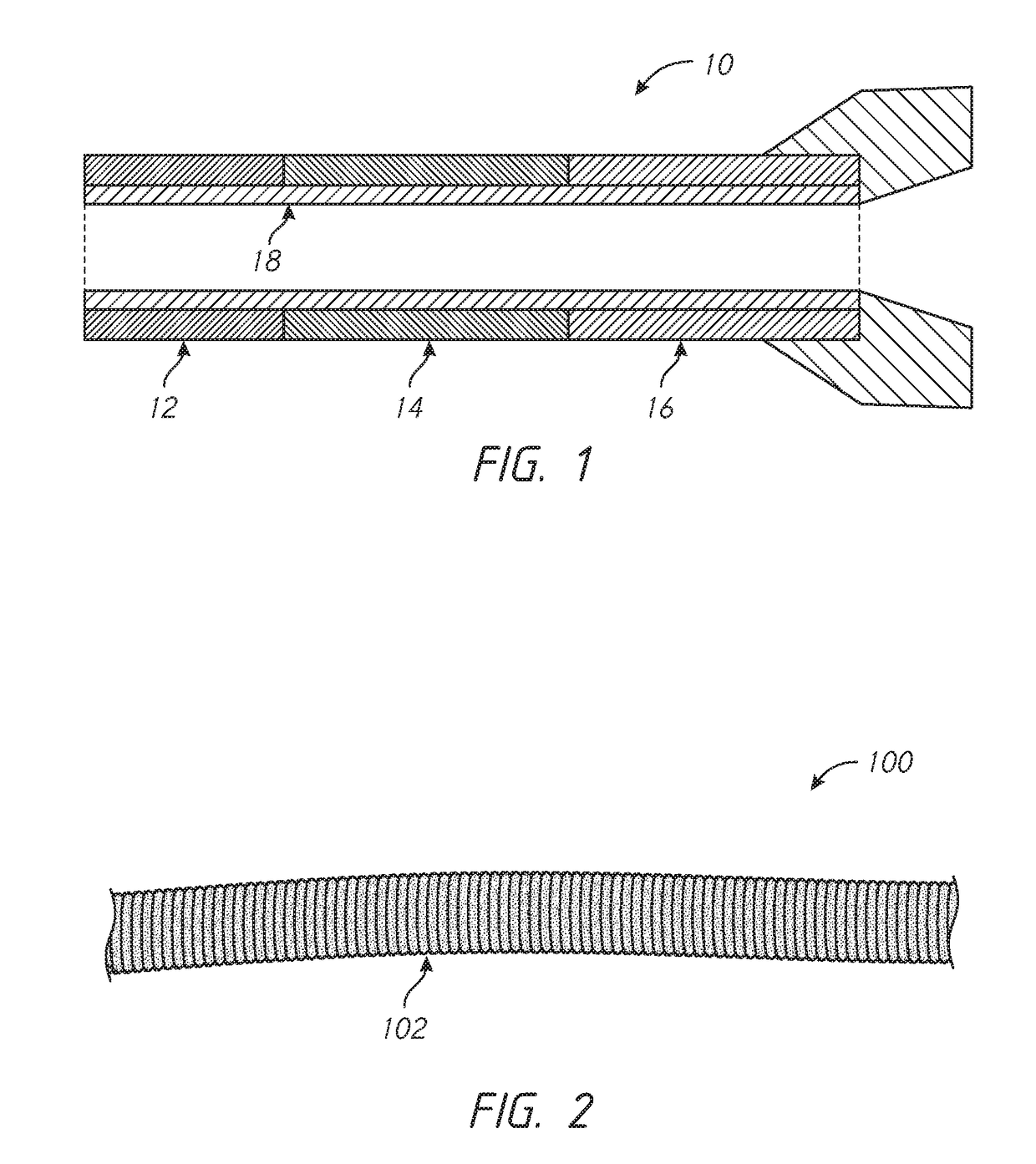
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
52results about How to "Improve kink resistance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
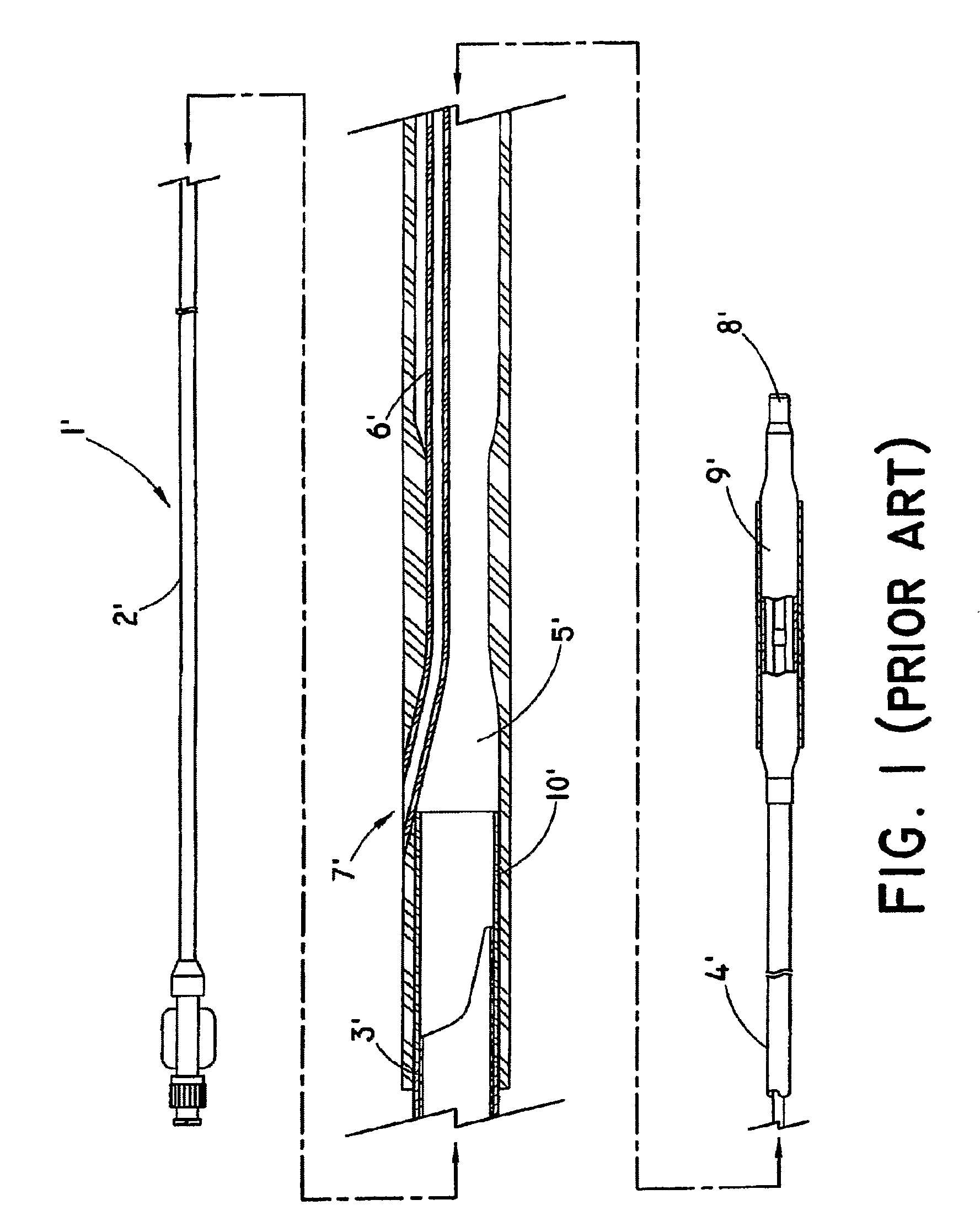
Advanced endovascular graft
This invention is a system for the treatment of body passageways; in particular, vessels with vascular disease. The system includes an endovascular graft with a low-profile delivery configuration and a deployed configuration in which it conforms to the morphology of the vessel or body passageway to be treated as well as various connector members and stents. The graft is made from an inflatable graft body section and may be bifurcated. One or more inflatable cuffs may be disposed at either end of the graft body section. At least one inflatable channel is disposed between and in fluid communication with the inflatable cuffs.
Owner:BOSTON SCI CORP
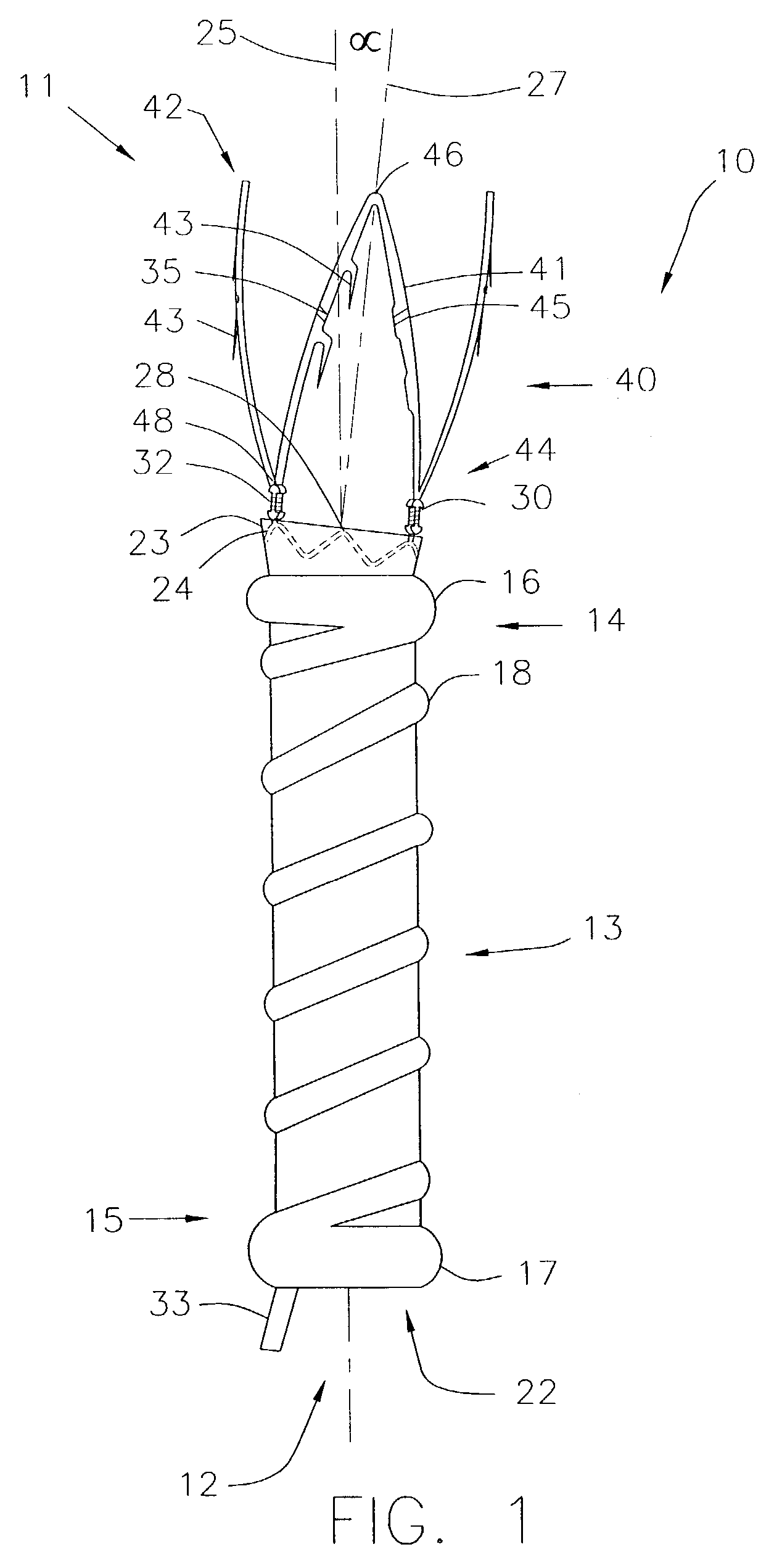
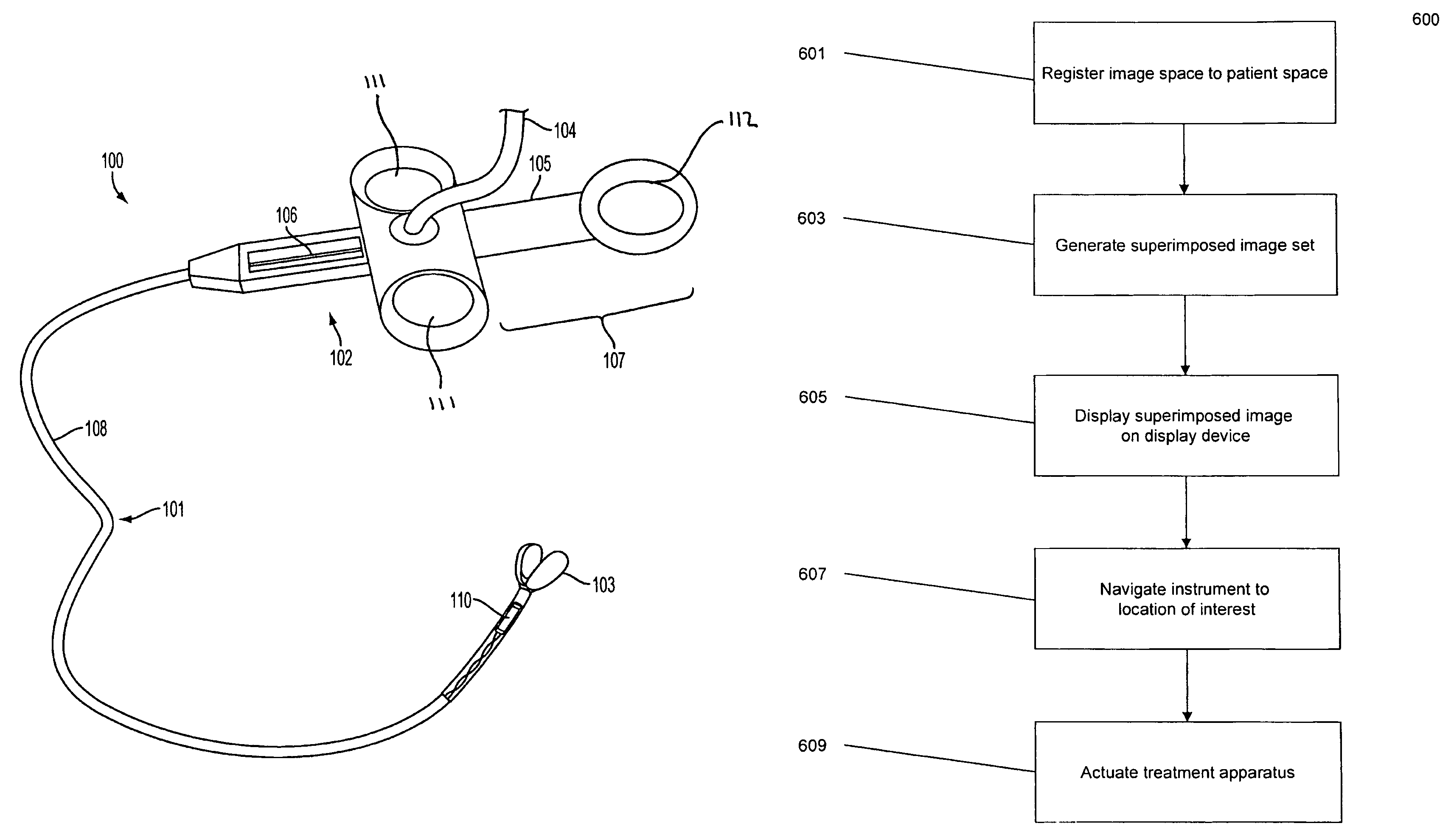
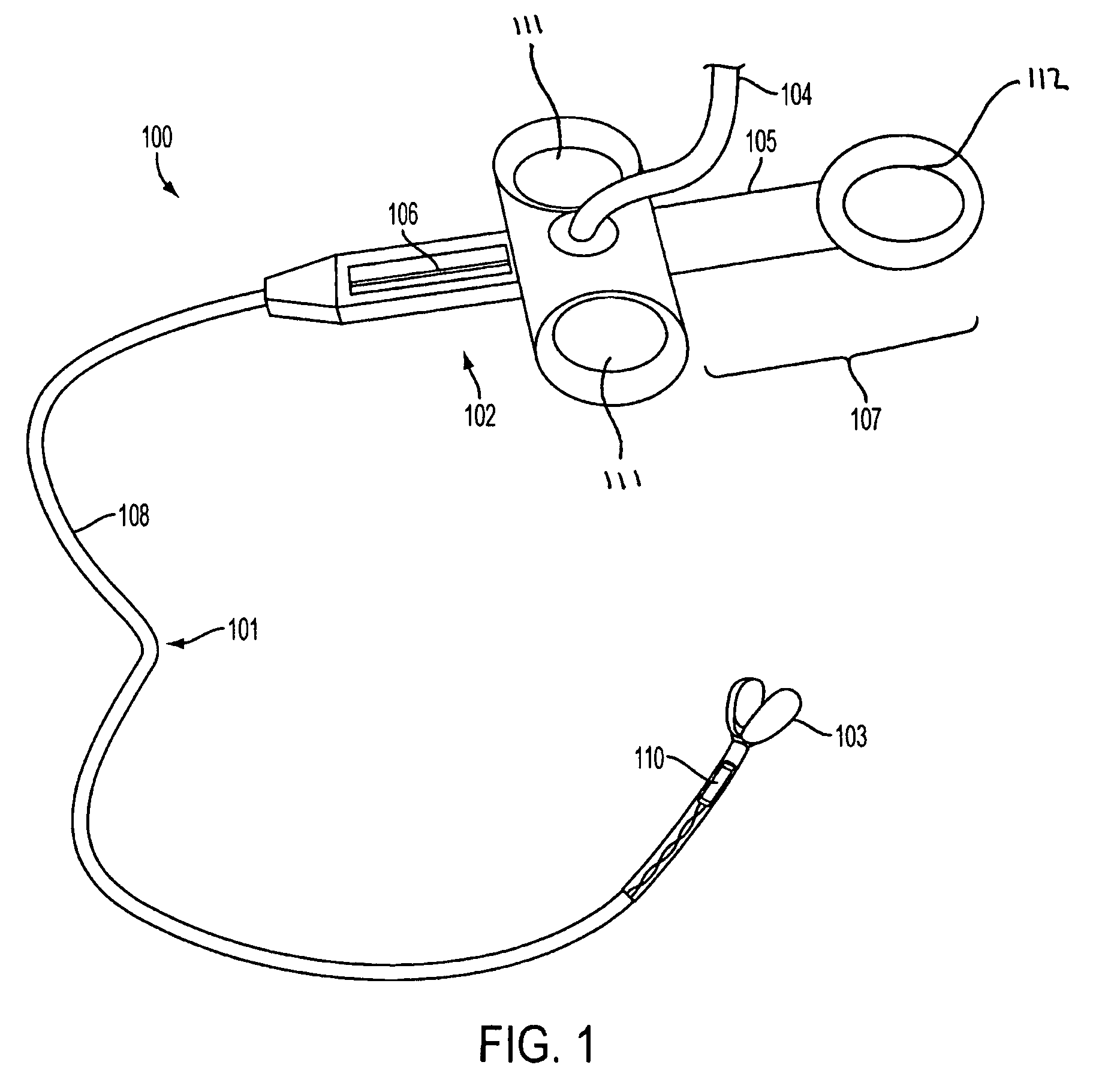
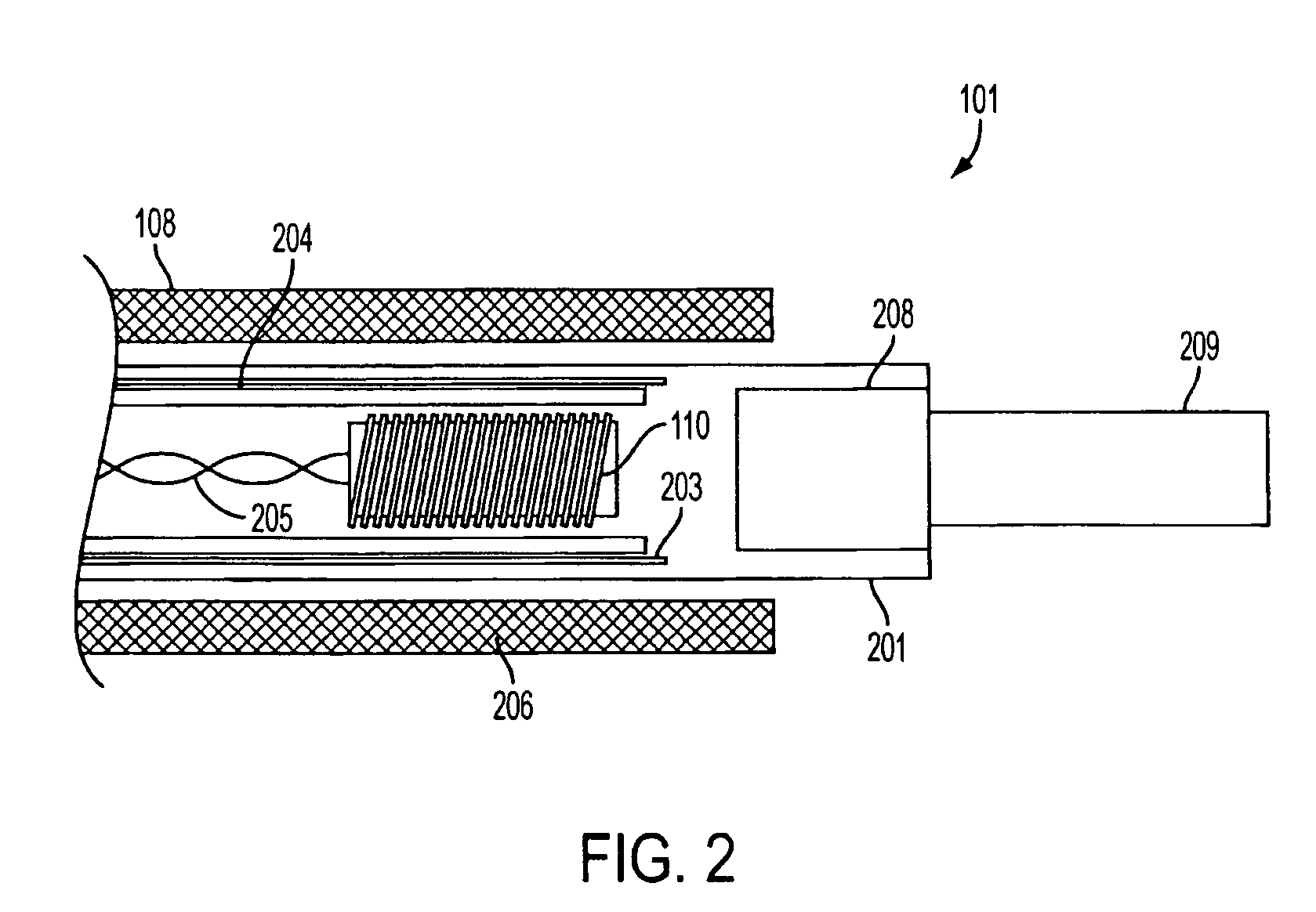
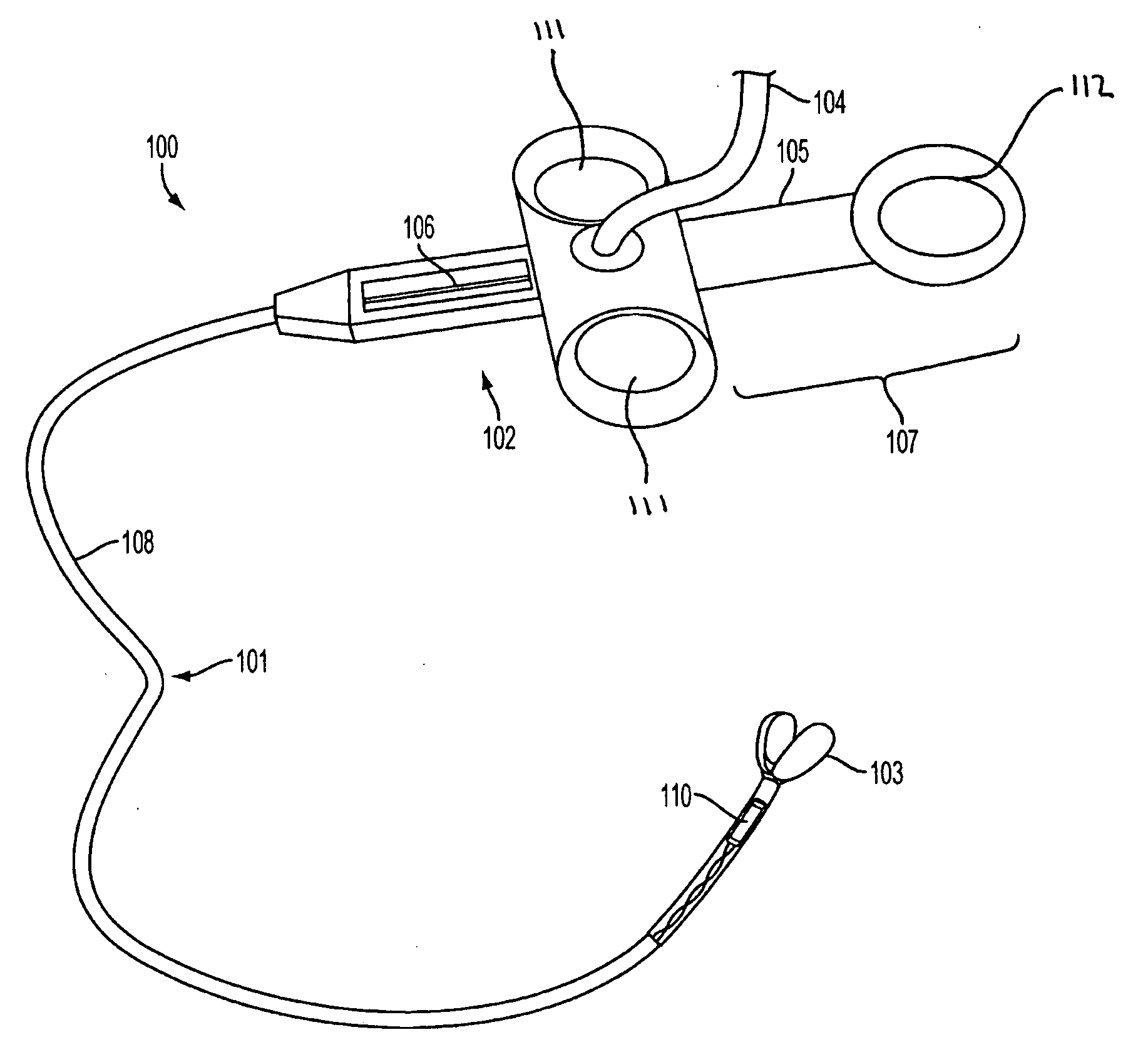
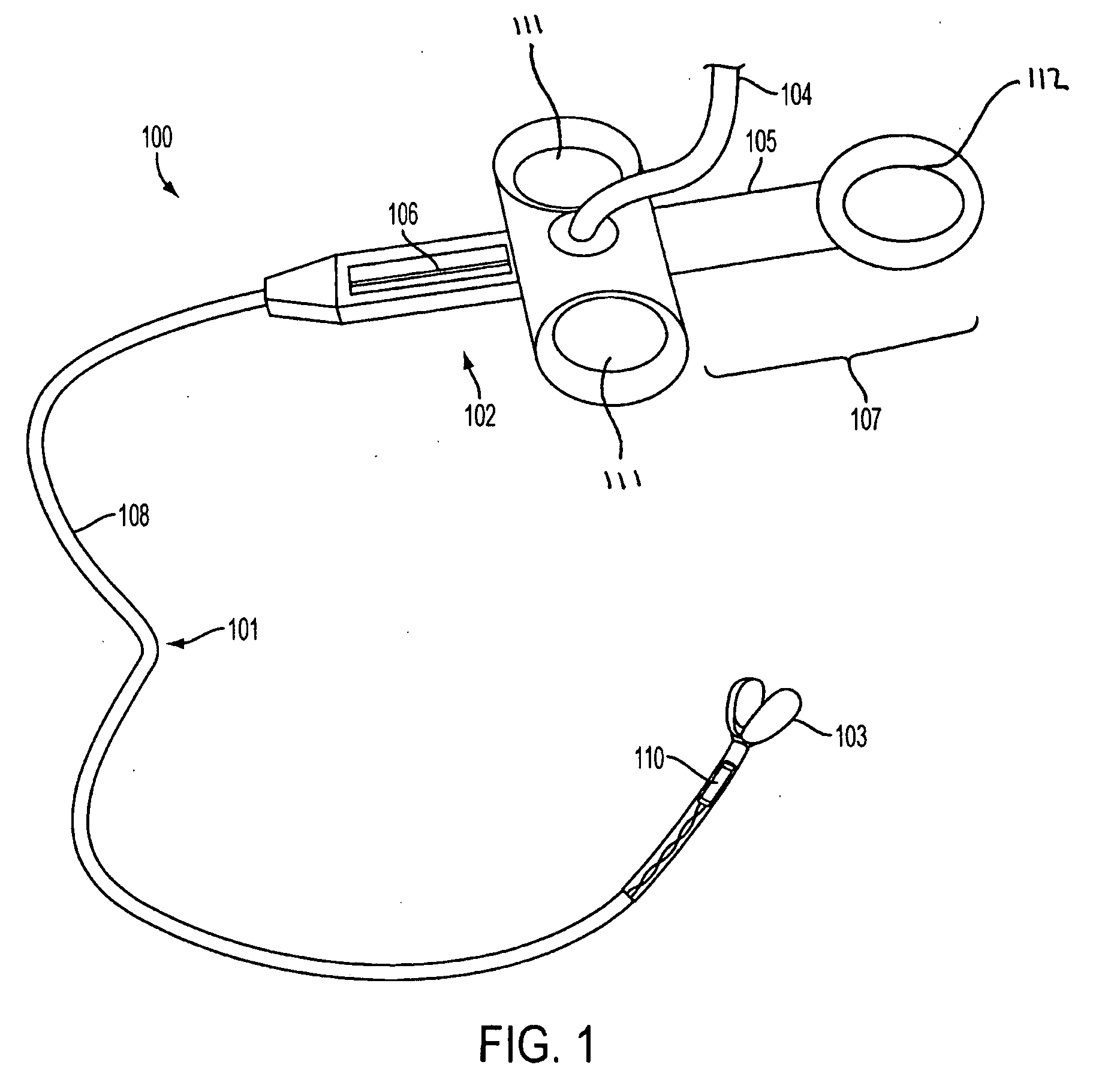
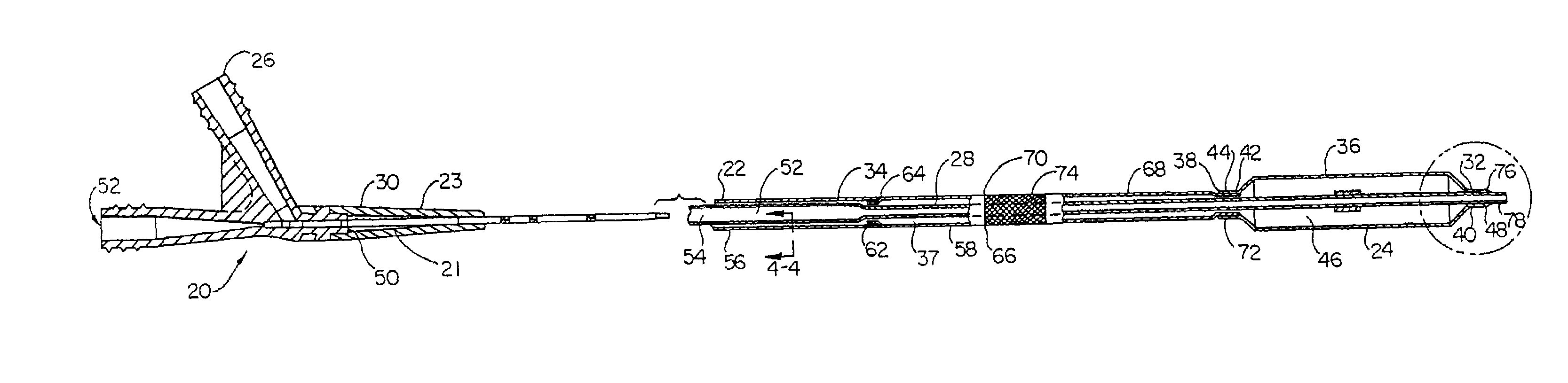
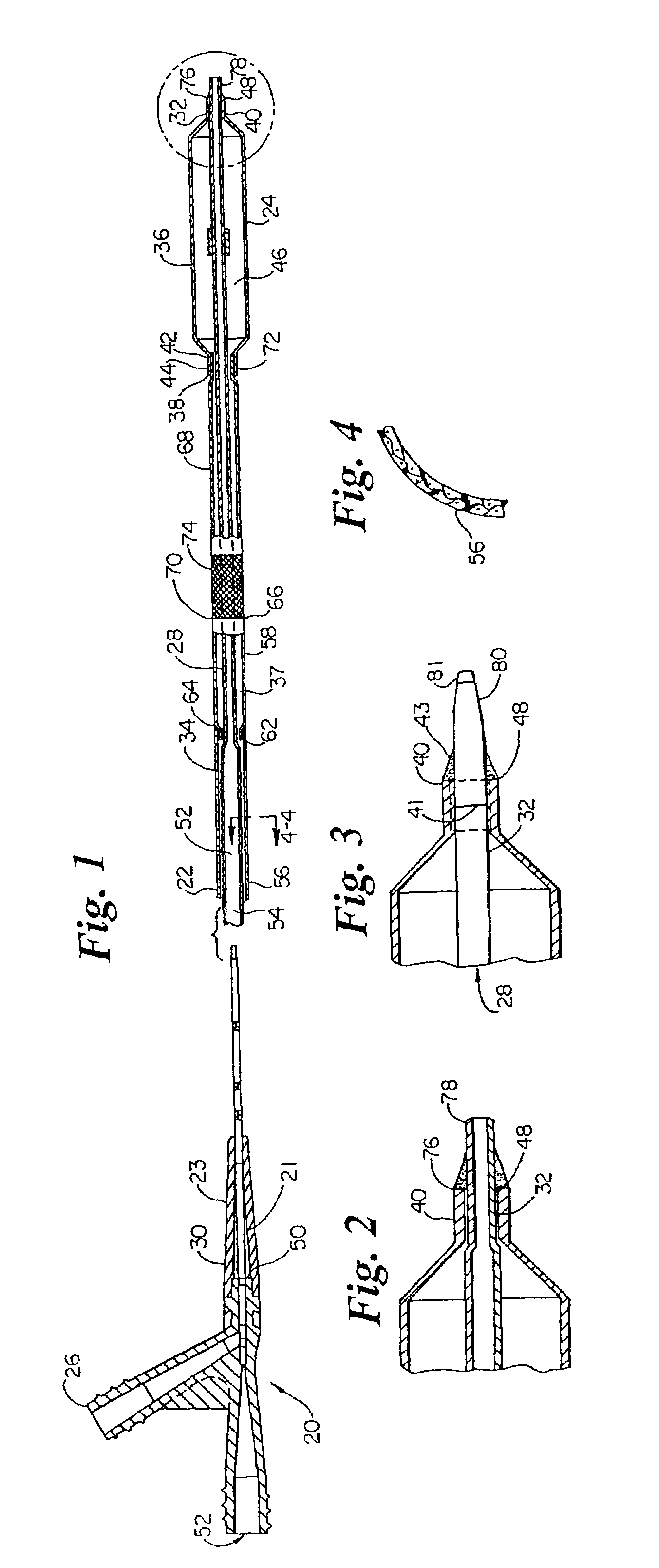
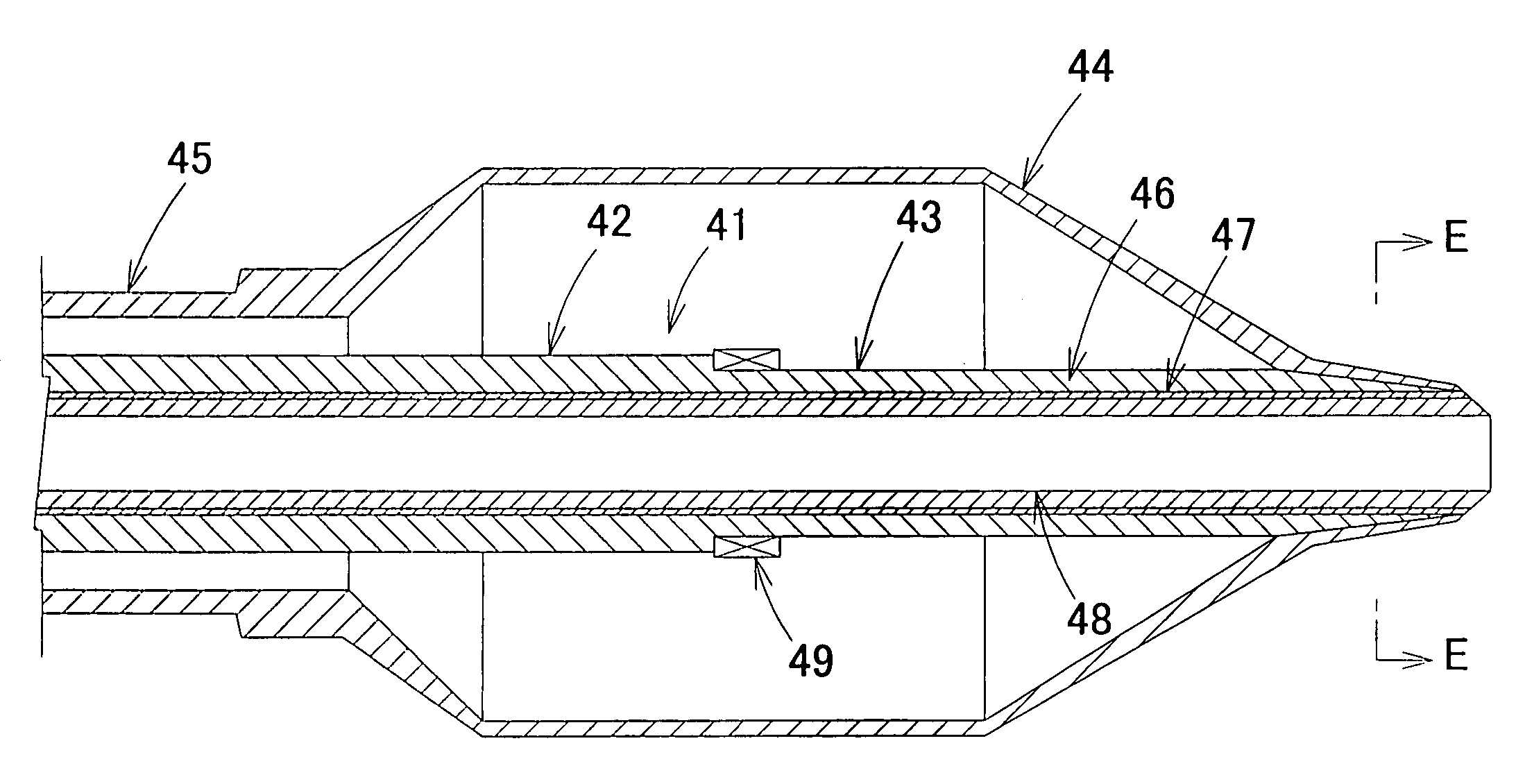
System, method and apparatus for navigated therapy and diagnosis
ActiveUS8632461B2Increase thrustHigh torque transmissionSurgical navigation systemsEndoscopesEngineeringMedical instruments
The invention provides an image-guided medical instrument that utilizes a tracking device to track the location of at least a portion of the instrument on at least one image of a patient's anatomy. The instrument may include a handle having an operating element, an elongated flexible body member connected to the handle, wherein the body member includes a lumen, a transmission element housed within the lumen of the body member, and a treatment apparatus connected to end of the transmission element, wherein actuation of the operating element actuates the treatment apparatus via the transmission element. The body member also includes at least one sensor element that is utilized by the tracking device to determine position information regarding the treatment apparatus that is used to display the location of the treatment apparatus on the at least one image of the patient's anatomy.
Owner:PHILIPS ELECTRONICS LTD
System, method and apparatus for navigated therapy and diagnosis
ActiveUS20070032723A1Increase thrustHigh torque transmissionSurgical navigation systemsEndoscopesTherapeutic DevicesEngineering
Owner:PHILIPS ELECTRONICS LTD
System and method for positioning implantable medical devices within coronary veins
An improved system and method for placing implantable medical devices (IMDs) such as leads within the coronary sinus and branch veins is disclosed. In one embodiment, a slittable delivery sheath and a method of using the sheath are provided. The sheath includes a slittable hub, and a substantially straight body defining an inner lumen. The body comprises a shaft section and a distal section that is distal to, and softer than, the shaft section. A slittable braid extends adjacent to at least a portion of one of the shaft section and the distal section. In one embodiment of the invention, the sheath further includes a transition section that is distal to the shaft section, and proximal to the distal section. The transition section is softer than the shaft section, but stiffer than the distal section.
Owner:MEDTRONIC INC
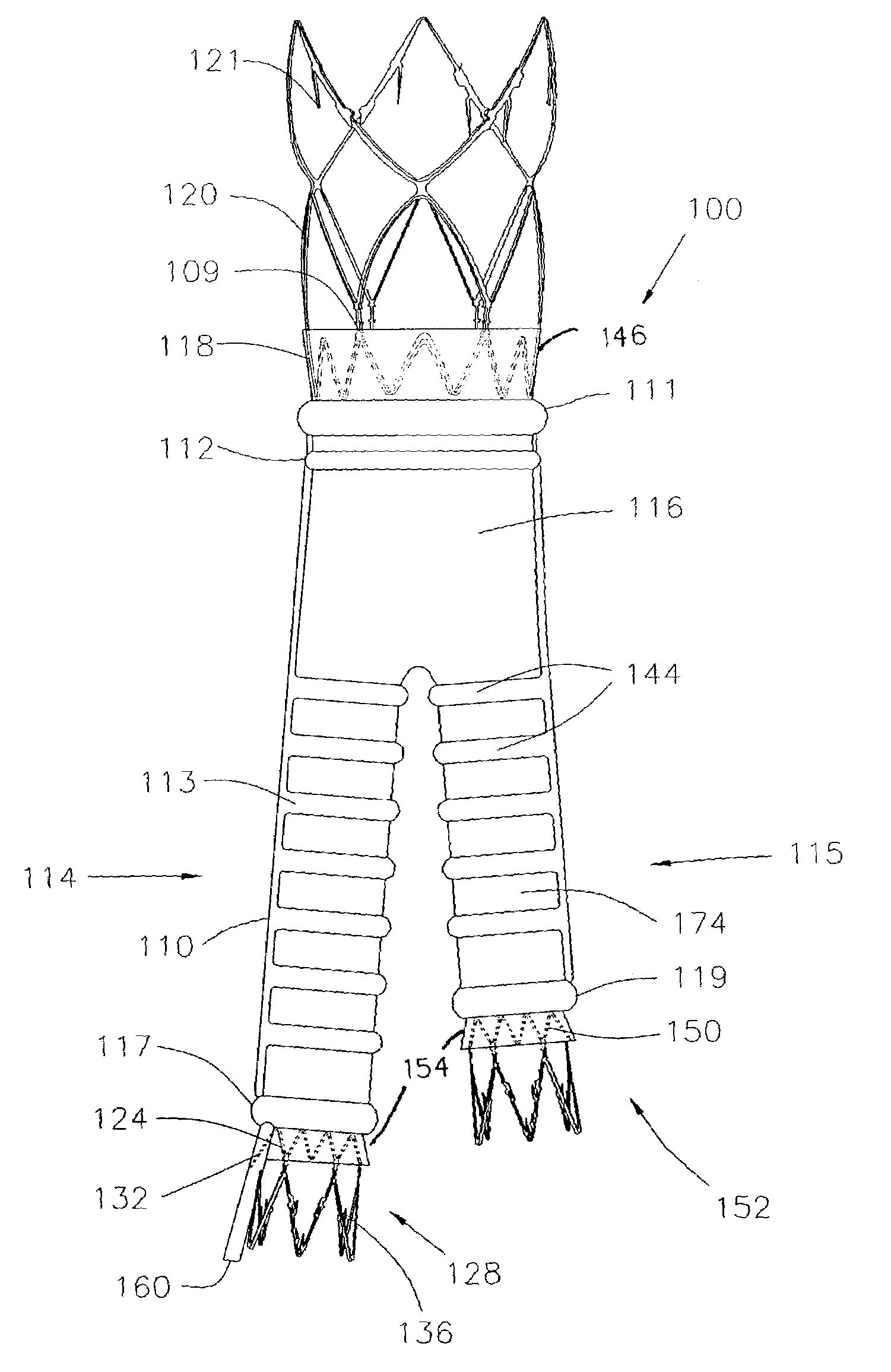
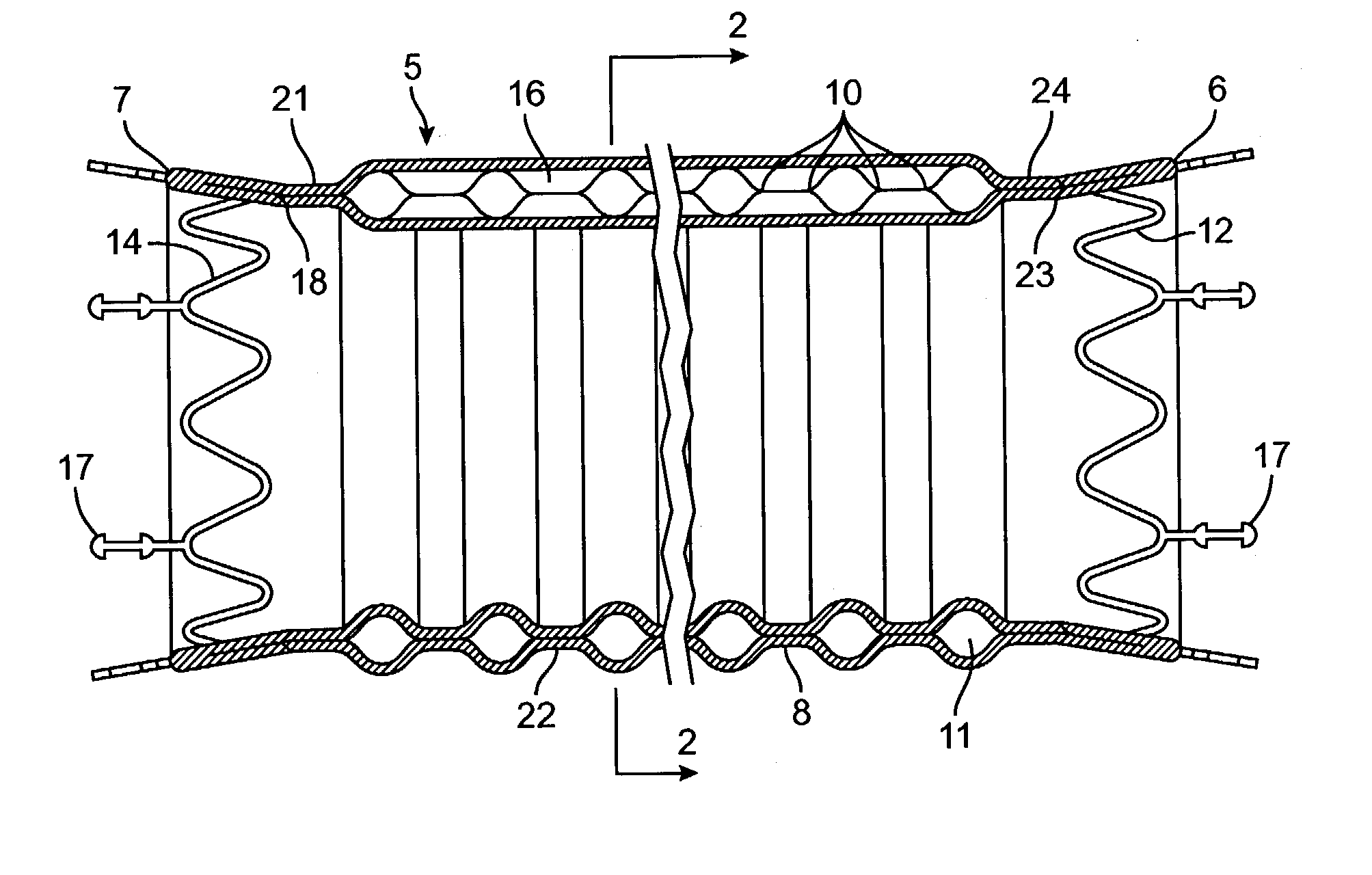
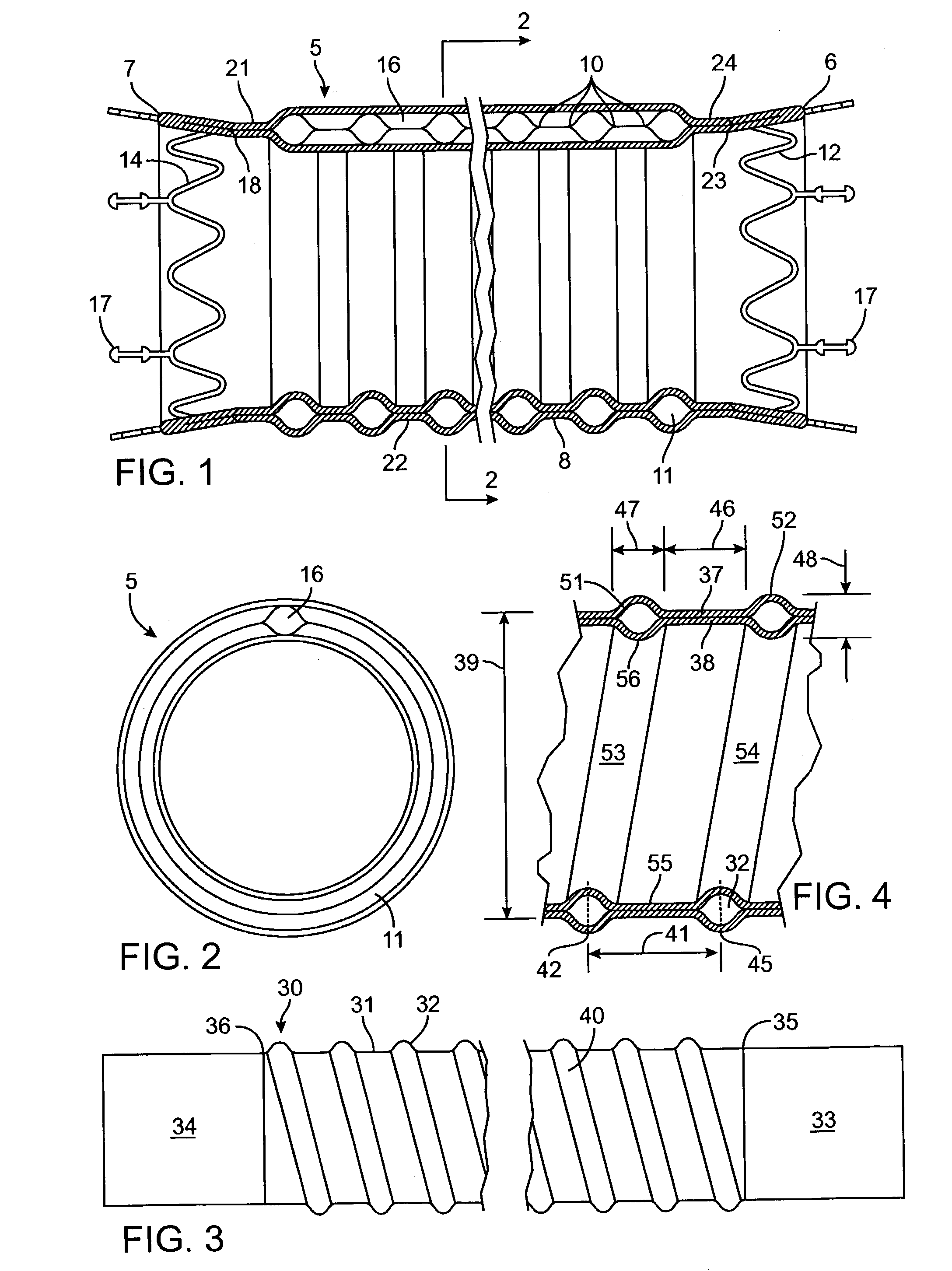
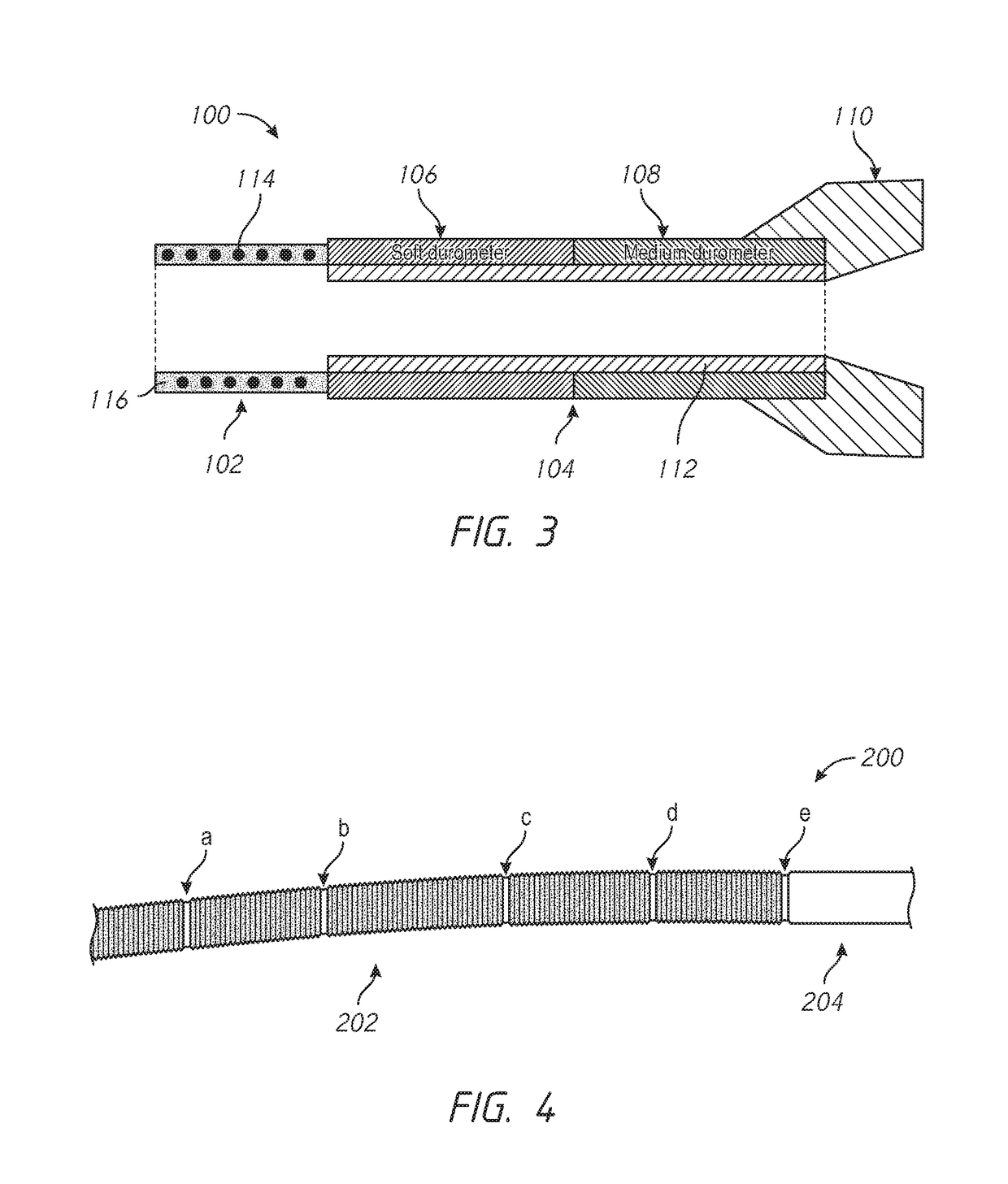
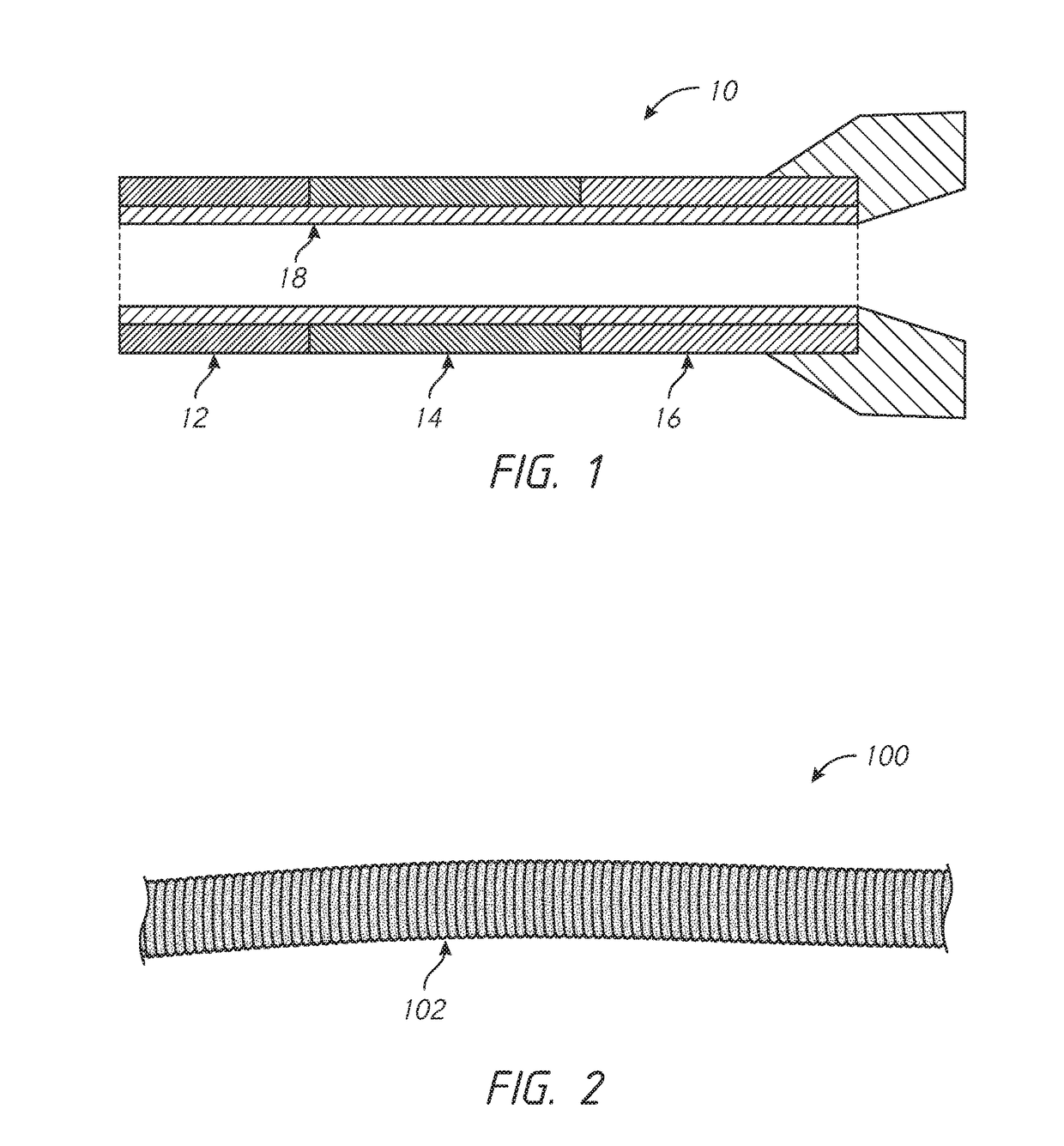
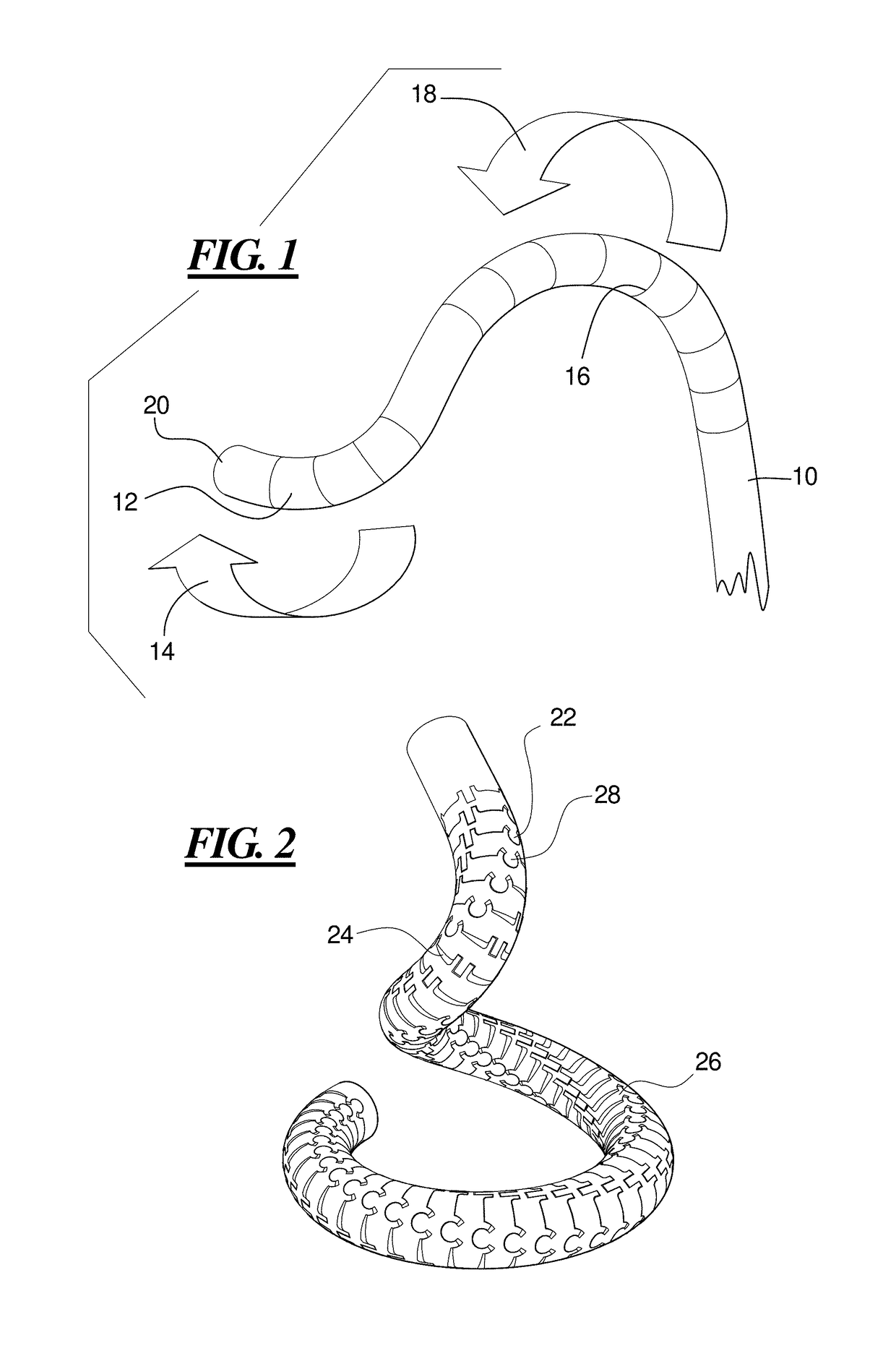
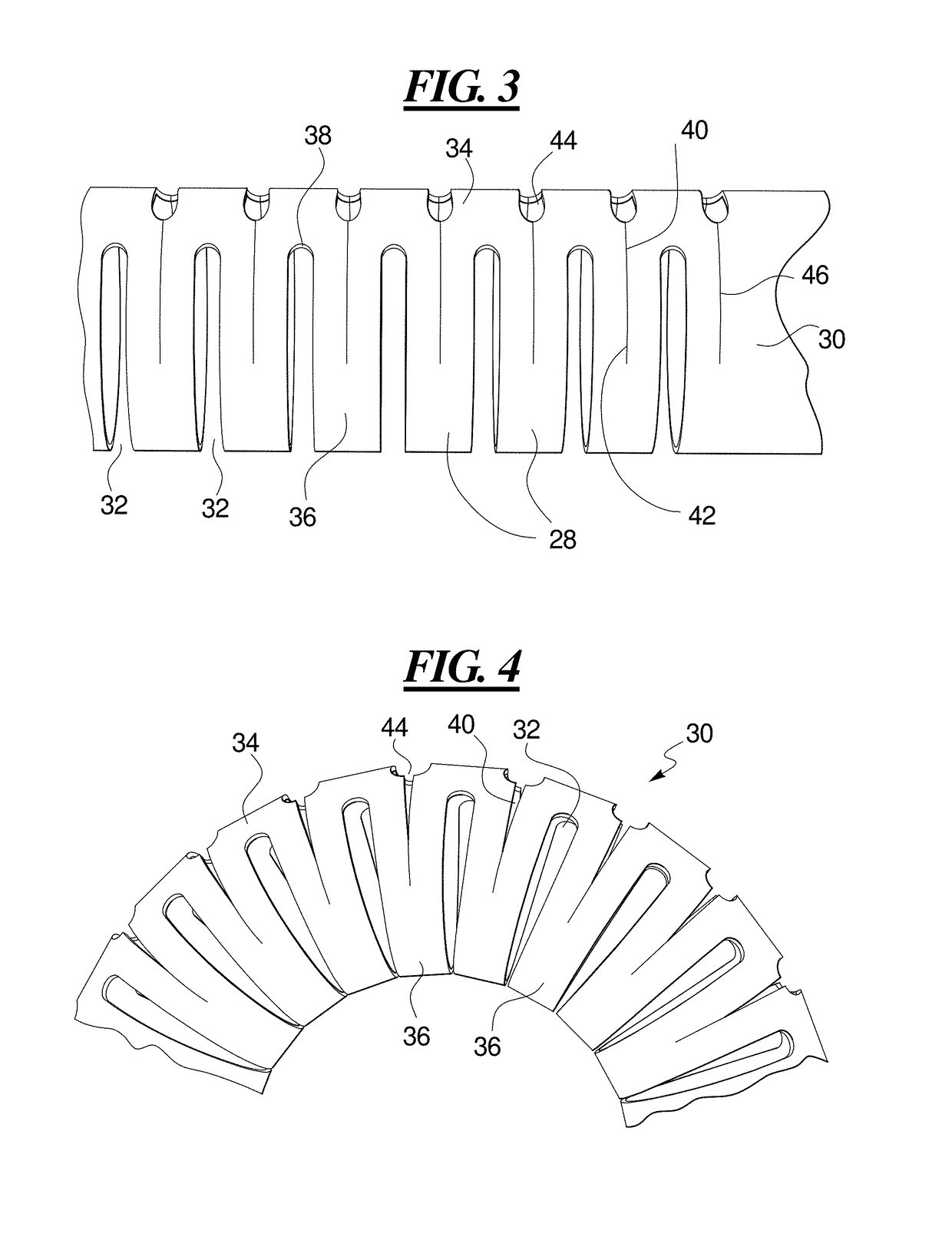
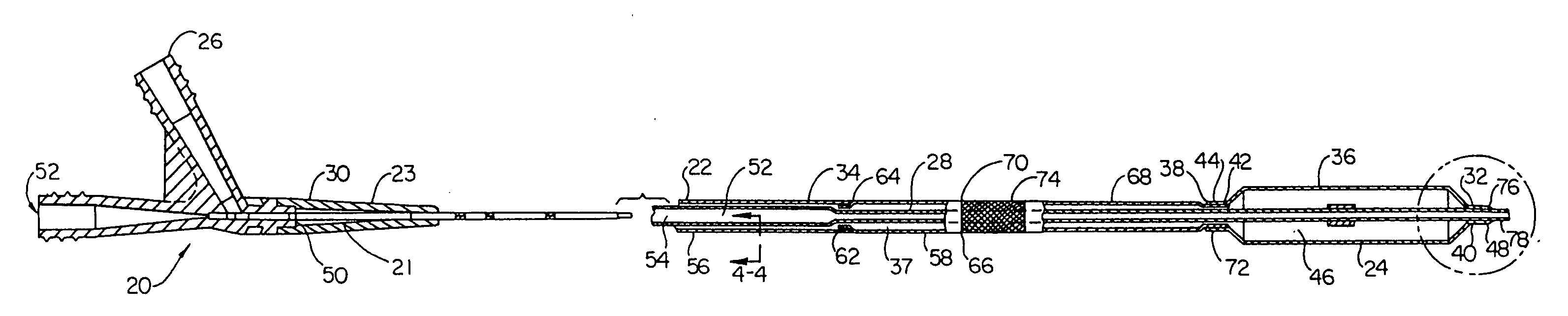
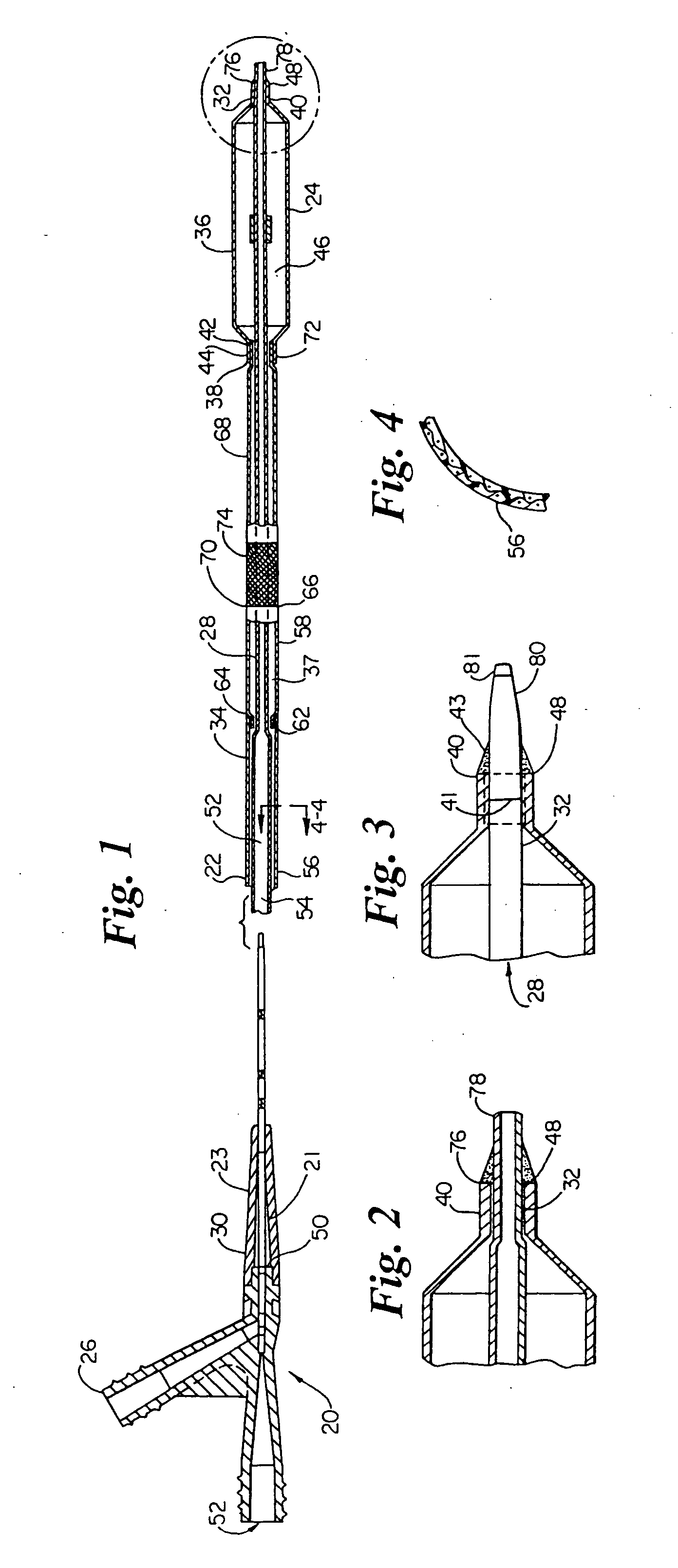
Kink resistant endovascular graft
InactiveUS7150758B2Maintain kink resistanceProvide complianceStentsBlood vesselsEngineeringBlood vessel
An intracorporeal device, such as an endovascular graft, having a tubular section with circumferential or helical radial support members. The radial support members may be inflatable channels which support the tubular structure of the graft and which are appropriately sized and longitudinally spaced to prevent or reduce kinking of the tubular structure upon bending of the tubular structure.
Owner:BOSTON SCI CORP
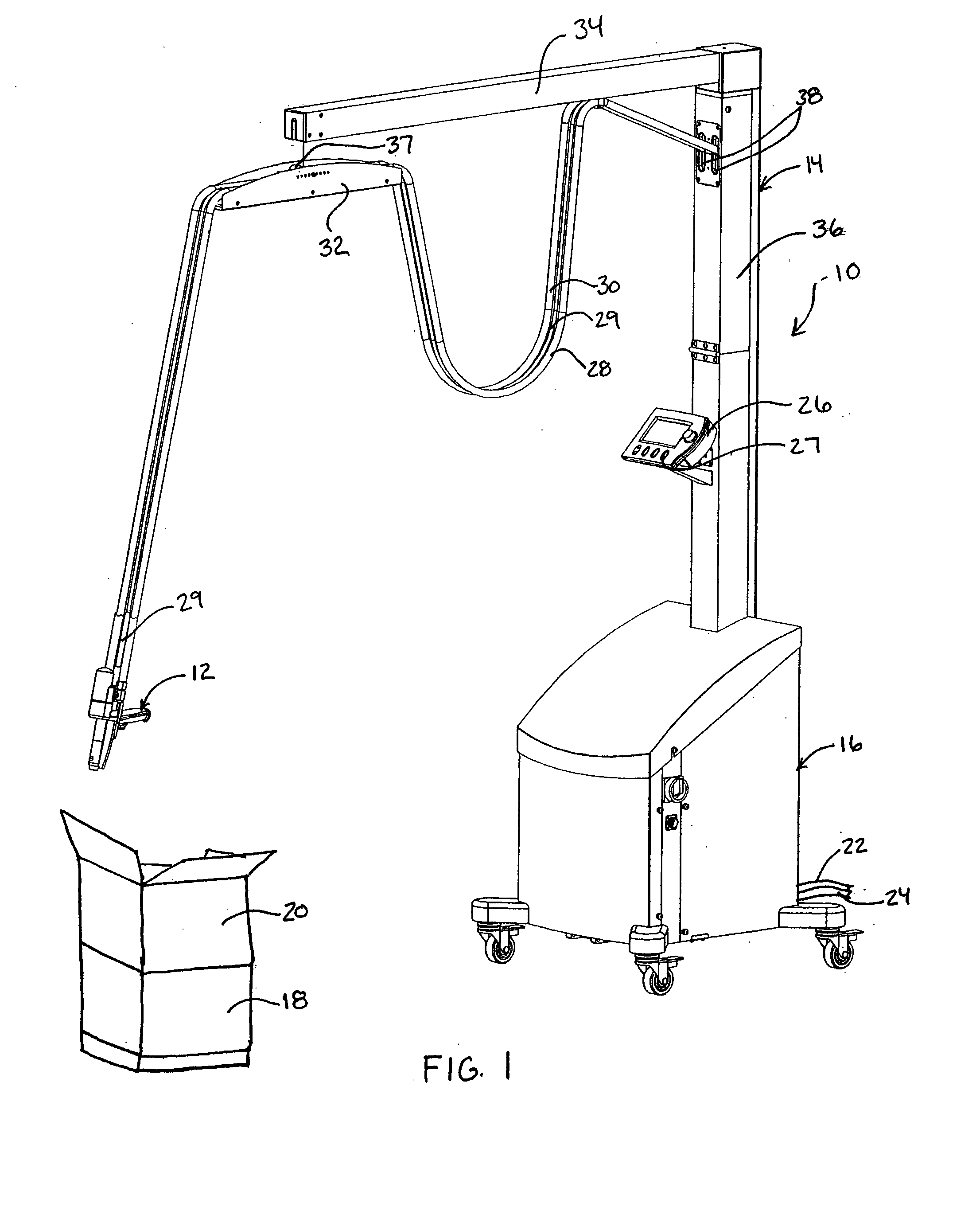
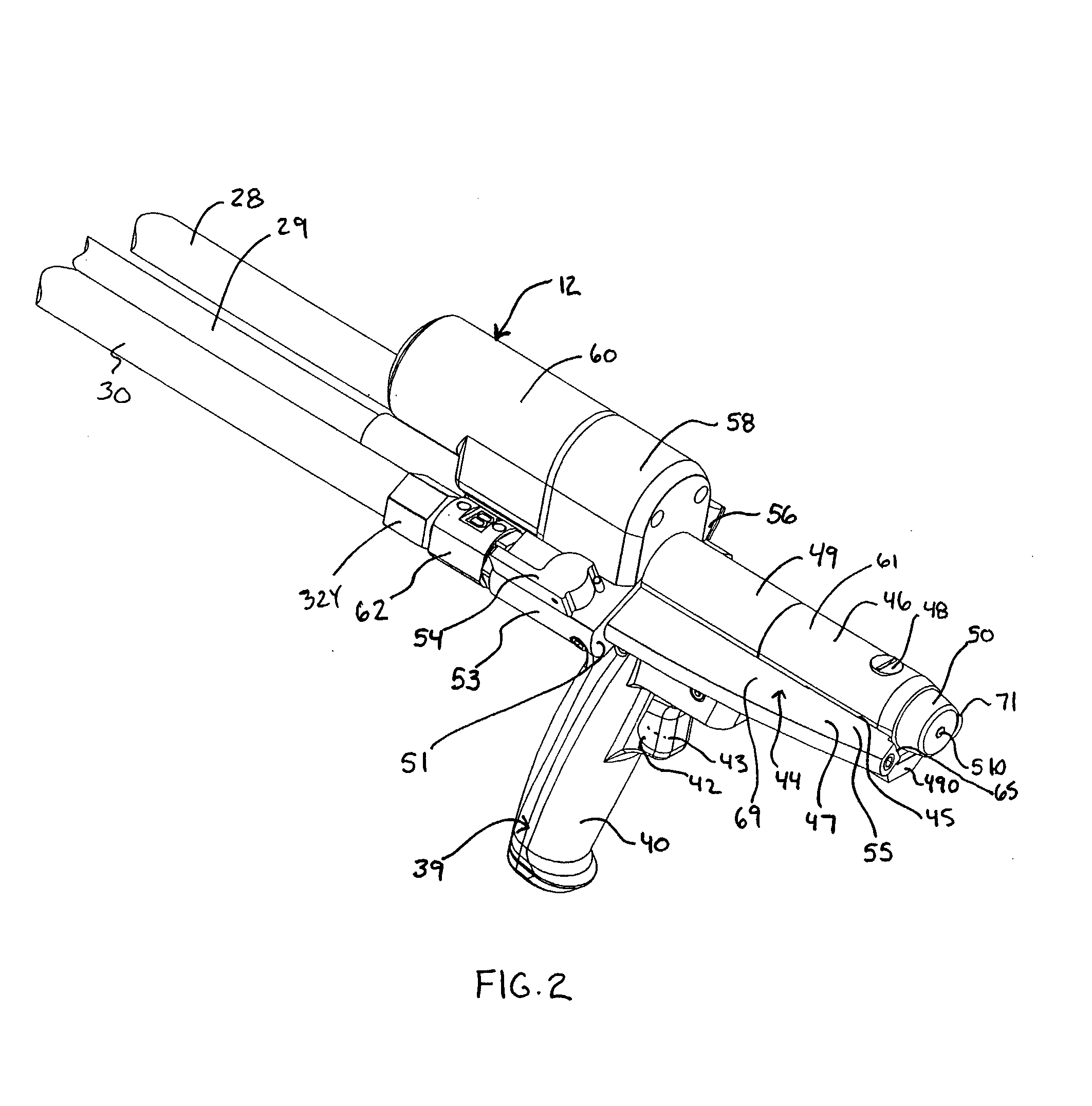
Hand Held Dispenser
ActiveUS20080135579A1Minimize downtimeEasy to disassembleLiquid flow controllersLarge containersTemperature controlHand held
A hand held dispenser system with associated dispensed material supply assembly as in separate source chemical foam precursor feeding into, for example, streamlined chemical passageways preferably each comprised of, in series, a castellated swivel hose filter, a valve assembly housing, a wing extensions of a manifold, which manifold supports a high efficiency drive system and is supported by a handle that provides for a compact assembly and receives a rugged trigger assembly. The manifold design provides for elongated filter and, temperature controlled cartridge heater insertion. There is further provided in a preferred embodiment a readily releasable electric source feed line plug connection at the butt end of the dispenser. The dispenser is well suited for the dispensing of methane foam as in a product packaging setting.
Owner:PREGIS INTELLIPACK CORP
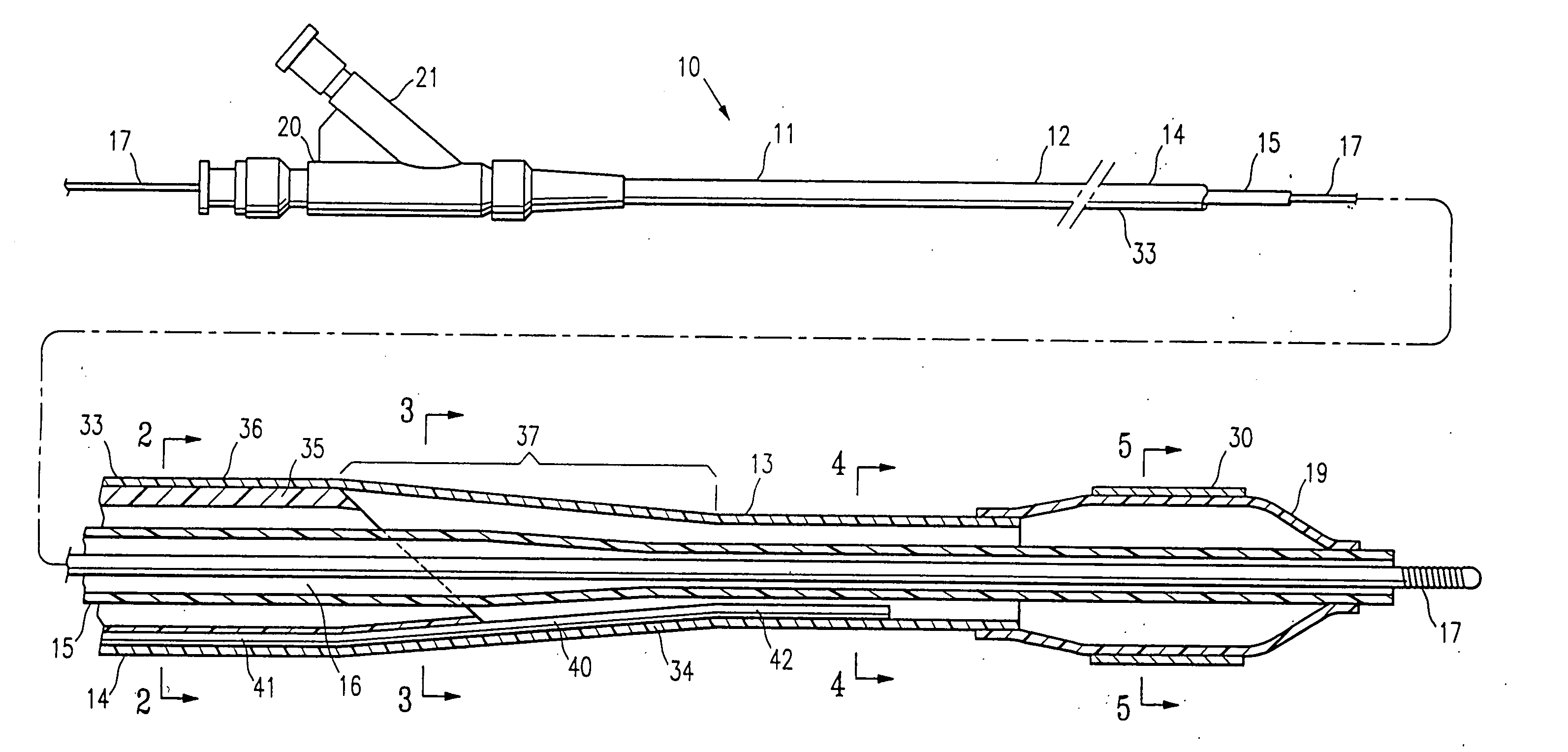
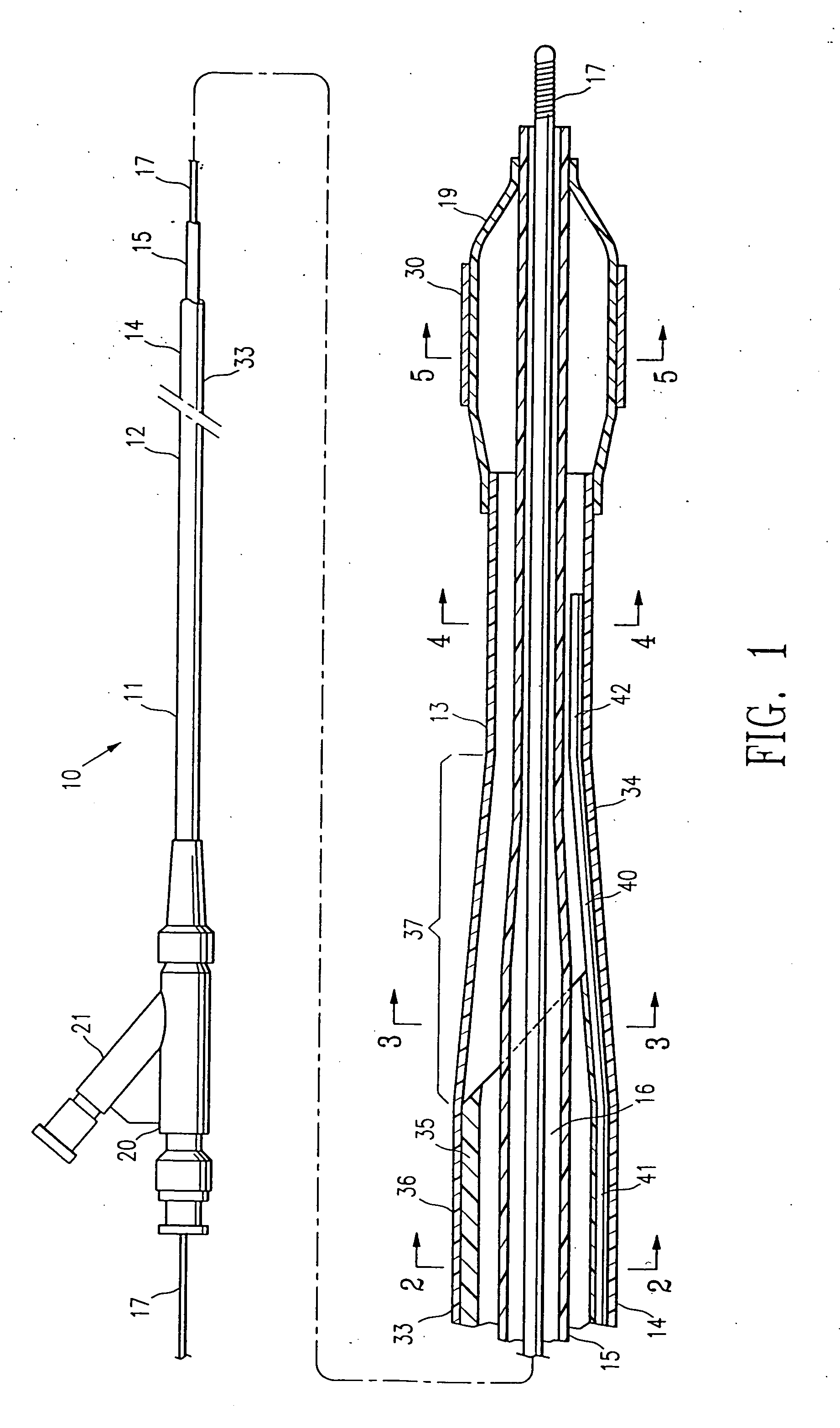
Catheter with spiral cut transition member
A spiral cut transition member is disclosed for controlling the transition in stiffness of a catheter from a stiffer more pushable proximal section to a more flexible and trackable distal section and increasing kink resistance. The transition member has a spiral cut provided therein to vary the flexibility of the transition member over its length. The pitch of the spiral cut can be varied to facilitate a gradual transition in flexibility along the catheter. The transition member may be used in conjunction with any type of catheter including single-operator-exchange type catheters, over-the wire type catheters, and / or fixed-wire type catheters.
Owner:BOSTON SCI SCIMED INC
System and method for positioning implantable medical devices within coronary veins
InactiveUS20090131873A1Easily advancedMinimizing chanceGuide needlesBalloon catheterVeinDistal portion
An improved system and method for placing implantable medical devices (IMDs) such as leads within the coronary sinus and branch veins is disclosed. In one embodiment, a slittable delivery sheath and a method of using the sheath are provided. The sheath includes a slittable hub, and a substantially straight body defining an inner lumen. The body comprises a shaft section and a distal section that is distal to, and softer than, the shaft section. A slittable braid extends adjacent to at least a portion of one of the shaft section and the distal section. In one embodiment of the invention, the sheath further includes a transition section that is distal to the shaft section, and proximal to the distal section. The transition section is softer than the shaft section, but stiffer than the distal section.
Owner:MEDTRONIC INC
Conductive filament of titanium molybdenum alloy
InactiveCN101360448AAppropriate rigidityThe process is simple and convenientGuide wiresSurgeryTitanium molybdenum alloyTitanium nickelide
A guidewire for medical use such as in vascular and nonvascular systems. The guidewire made from a titanium molybdenum alloy wire with a composition of approximately 78% titanium 11.5% molybdenum 6% zirconium and 4.5% tin by weight such that it is softer than stainless steel guidewires and stiffer than NiTi alloy guidewires. The distal end of the guidewire is of a smaller diameter and softer than the proximal end and fitted with a coil for springiness such that the distal end will bend when encountering curves in the body passageways. The distal tip may be heat treated for a gradient of softness with the distal tip being the softest. The distal end may also be tapered to provide an additional gradient of softness. A distal tip on the distal end of the guidewire protects the wall of the passageway from being punctured as the guidewire travels through the passageway. The resulting guidewire has properties between those of stainless steel guidewires and NiTi alloy guidewires for better torsion and stiffness characteristics.
Owner:MINNESOTA MEDICAL DEV
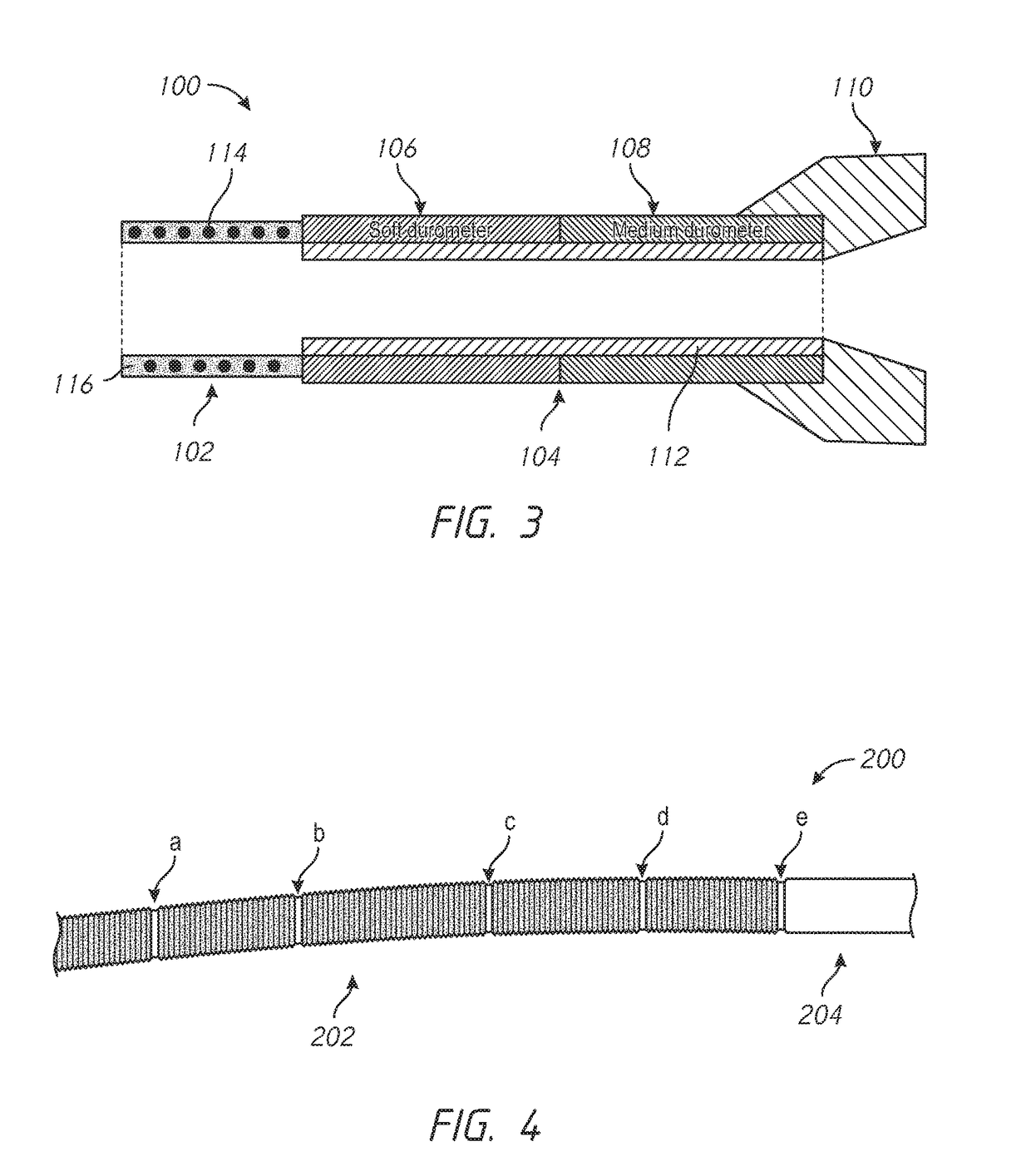
High flexibility, kink resistant catheter shaft
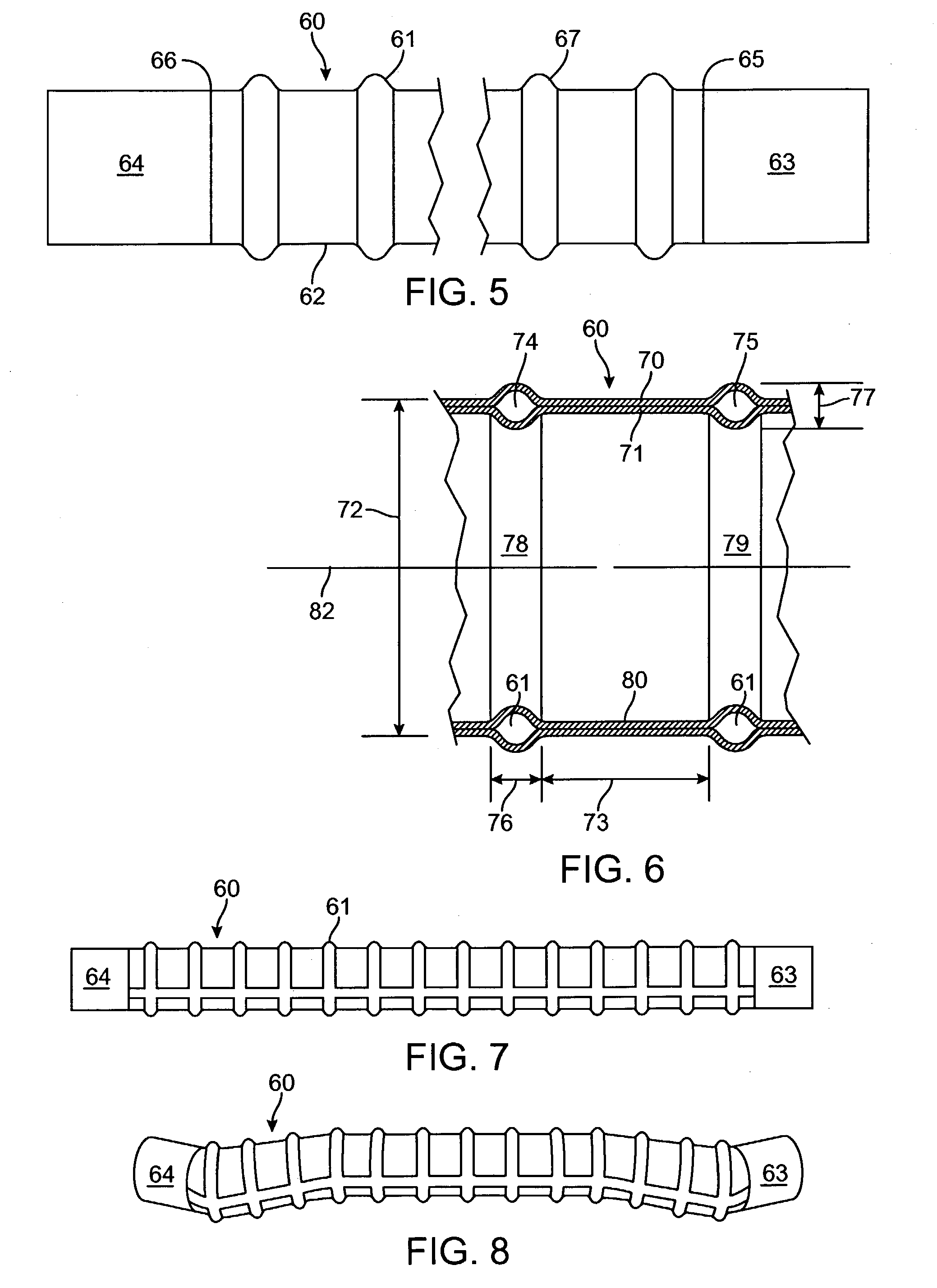
ActiveUS20180015254A1Improved resistance to compression or ovalization during bendingIncrease flexibilityStentsBalloon catheterMembrane configurationBiomedical engineering
An enhanced flexibility catheter shaft having an elongate flexible body with a proximal end, a distal end, and at least one lumen extending therethrough. A distal, flexible section on the body has a ribbed or corrugated tubular membrane having at least a first reinforcement structure, such as a first helical support, on a radially exterior or interior surface of the membrane and optionally a second reinforcement structure, such as a second helical support, on the other of the radially interior or exterior surface of the membrane
Owner:PERFUZE LTD
Medical balloon catheter
InactiveUS7247147B2Reduce discontinuityResolution problemSurgeryMedical devicesDistal portionBalloon catheter
The medical balloon catheter of the present invention comprises a catheter shaft composed of a distal end shaft and a proximal end shaft and a balloon on the distal end of the distal end shaft, wherein the proximal end shaft is composed of a single member and the distal end portion of the proximal end shaft is lower in rigidity than the other parts thereof. The present invention also provides a medical balloon catheter having a structure in which a tube for passing a guidewire inside thereof is arranged so as to pass inside the balloon and the balloon and tube are fused together in the vicinity of the distal end of the catheter, wherein the ratio of the outer diameter of the small-diameter portion on the distal end side of the tube to the outer diameter of the proximal end portion is no less than 0.85.
Owner:KANEKA CORP
High-modulus superelastic alloy wire for medical and dental purposes
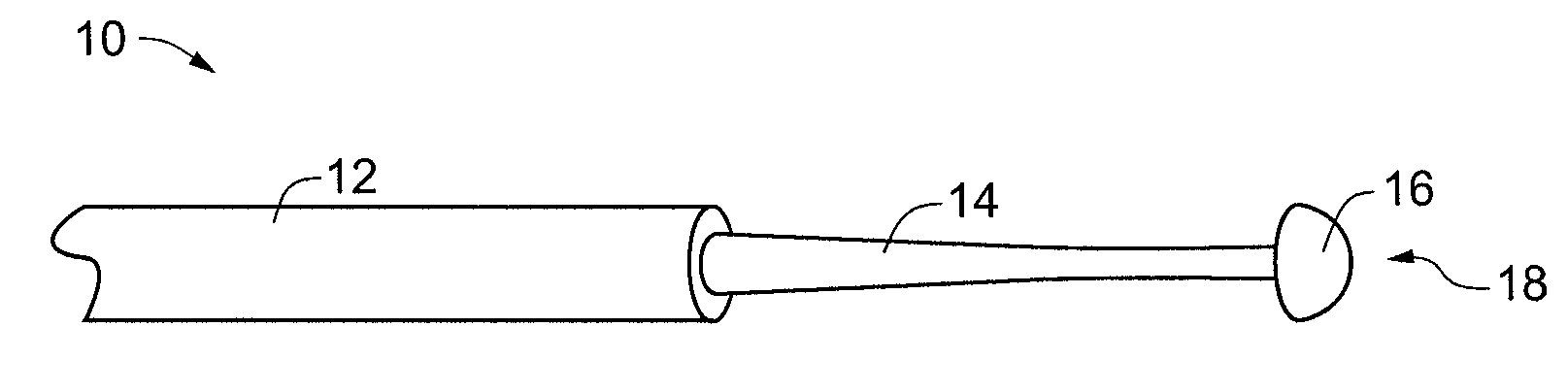
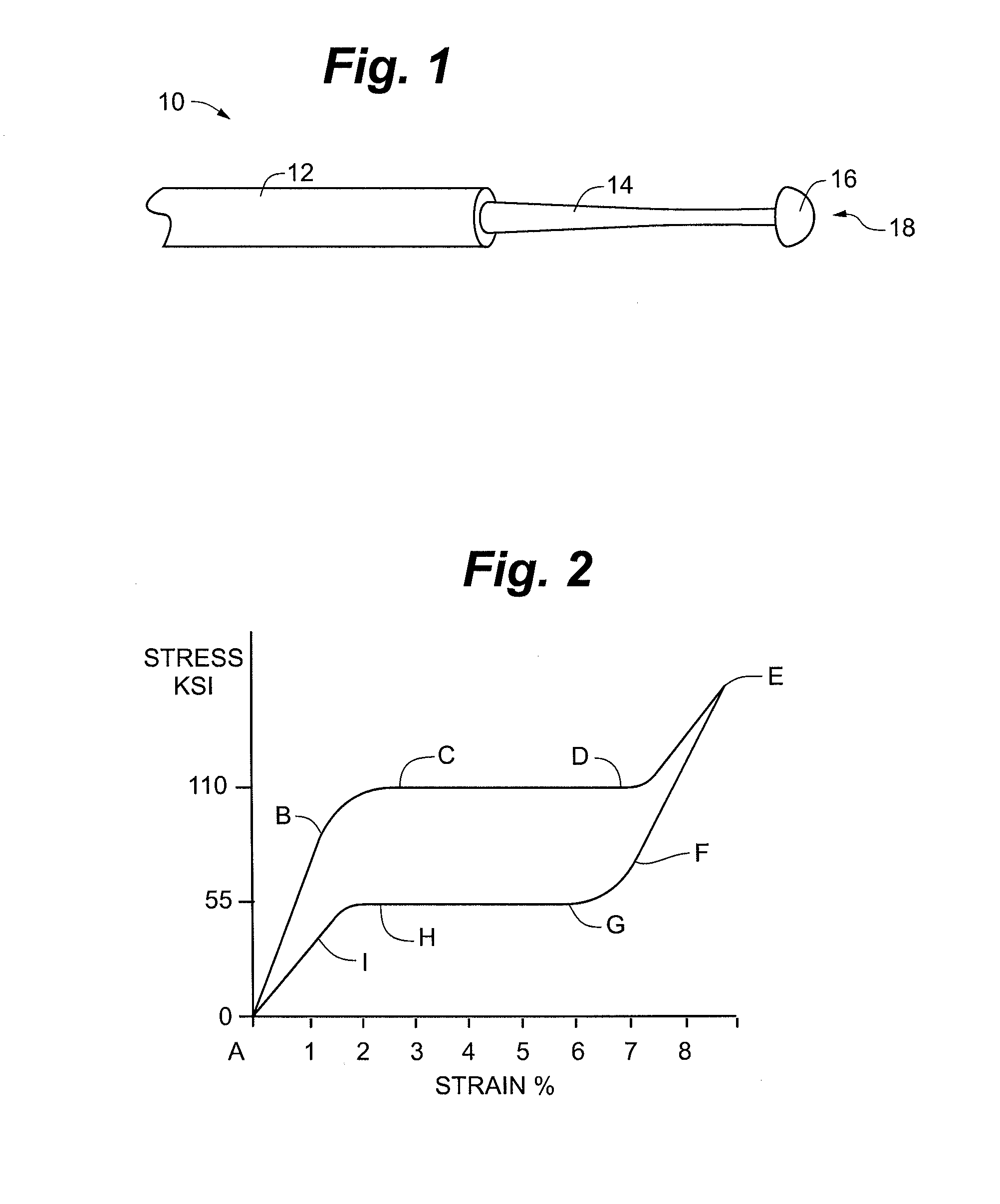
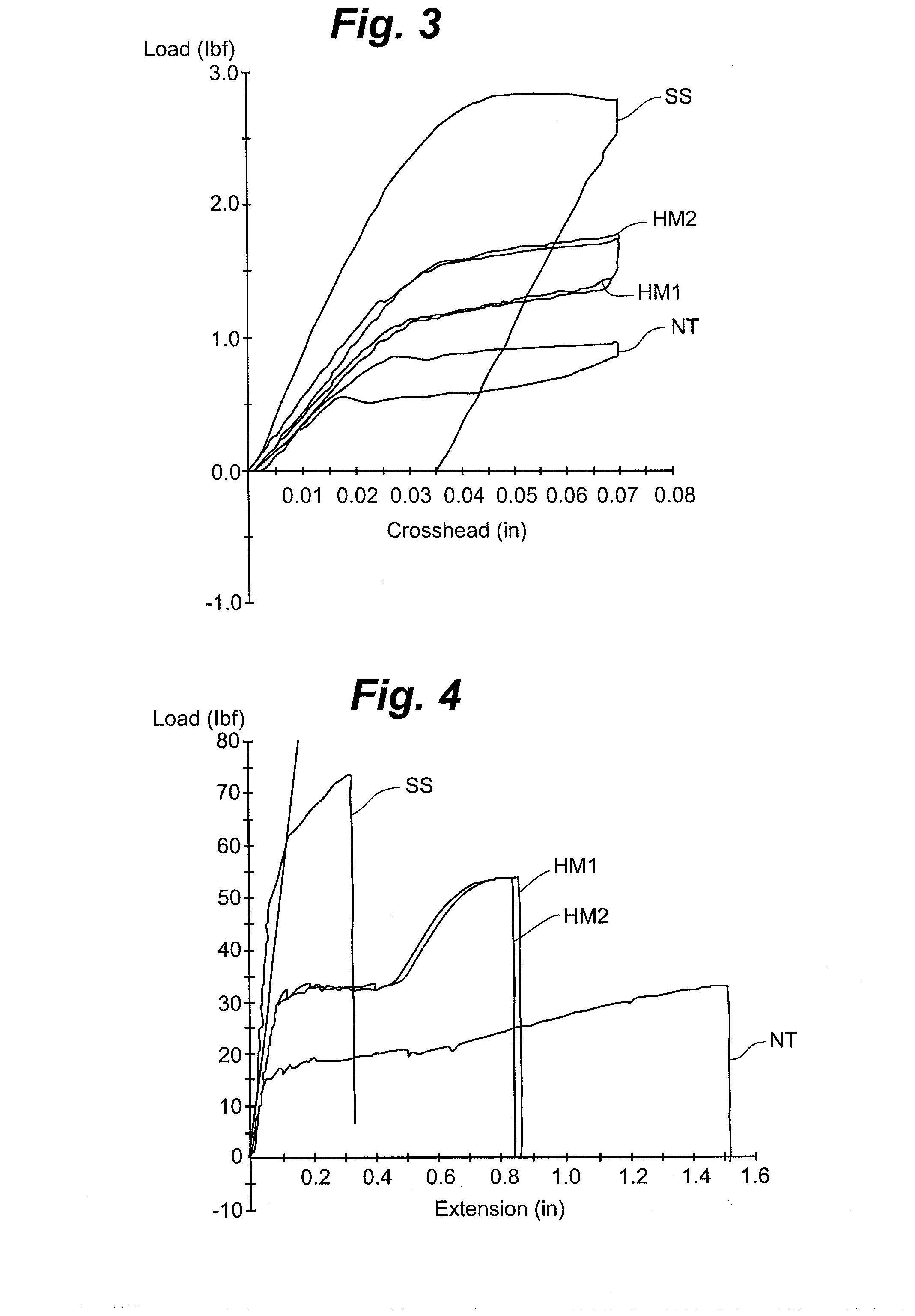
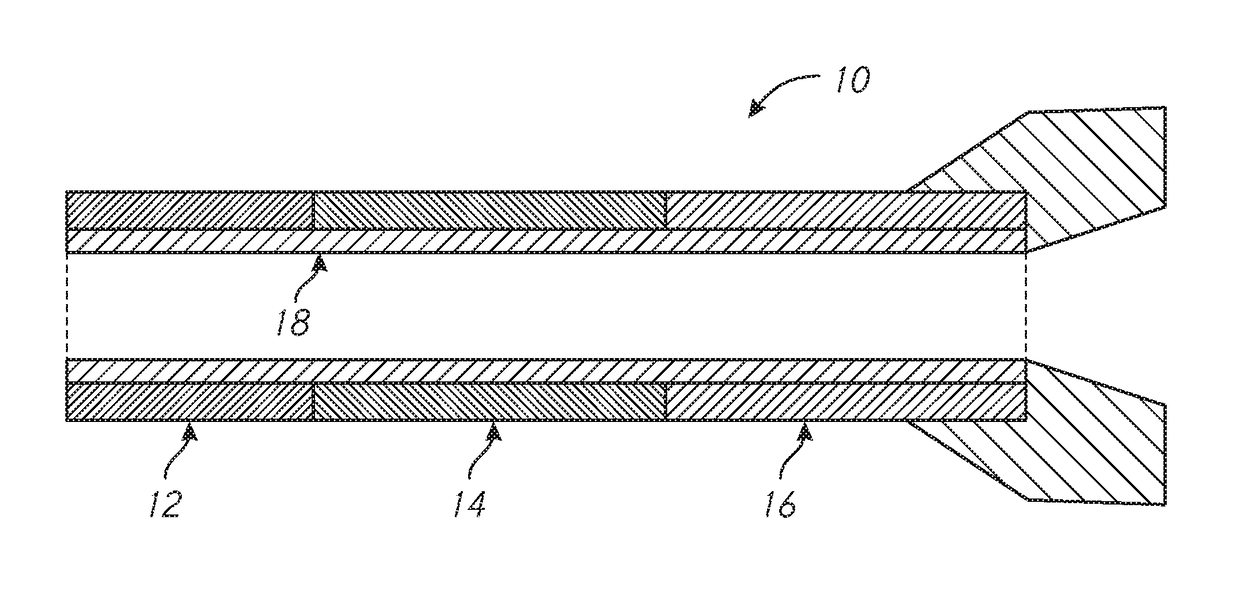
ActiveUS20110054351A1Improve kink resistanceIncrease stiffnessArch wiresGuide wiresArch wiresTitanium
A wire used in the medical field for guiding purposes, as well as in other fields, such as in the field of orthodontics for teeth aligning purposes. The wire, when prepared for use in such applications, exhibits an innovative blend of advantageous properties, including enhanced kink resistance over stainless steel wires and enhanced stiffness over Nitinol wires, which enhance its use as a medical guidewire or stylet, and further, as an arch wire in orthodontia applications.
Owner:HERAEUS MEDICAL COMPONENTS LLC
High flexibility, kink resistant catheter shaft
InactiveUS20180015248A1Trend downEasy to controlStentsBalloon catheterBiomedical engineeringCatheter
An enhanced flexibility catheter shaft having an elongate flexible body with a proximal end, a distal end, and at least one lumen extending therethrough. A distal, flexible section on the body has a ribbed or corrugated tubular membrane having at least a first reinforcement structure, such as a first helical support, on a radially exterior or interior surface of the membrane and optionally a second reinforcement structure, such as a second helical support, on the other of the radially interior or exterior surface of the membrane
Owner:PERFUZE LTD
Introducer sheath and introducer
ActiveUS20190021763A1Improve kink resistanceThin thicknessGuide needlesCannulasAliphatic hydrocarbonPolyamide
An introducer sheath includes a tubular body configured to be percutaneously inserted into a body lumen and a hub that is connected to a proximal portion of the tubular body, wherein the tubular body contains a polyamide resin having a structural unit derived from a cyclic aliphatic hydrocarbon, and a wall thickness / inner diameter ratio of the tubular body is 1 / 14 or less, and a kink radius / outer diameter ratio of the tubular body is less than 10.
Owner:TERUMO KK
Plastically deformable compositions and uses thereof
InactiveUS20080133001A1Enhance radial strengthEnhance anti kink resistanceStentsSurgeryChemistryFiber
A composition-of-matter, comprising one or more plastically deformable fiber is disclosed. The plastically deformable fiber(s) comprise a first and a second composition, where the first composition comprises at least one generally nondistensible polymer and the second composition comprises at least one agent capable of modulating distensibility of the generally nondistensible polymer(s).
Owner:NICAST LTD
High load steerable shaft and method for cardiac catheter
A tube or skeleton for a steerable catheter includes a cylindrical body structured to permit bending in at least one bending direction and to resist bending in directions transverse to the bending direction. The cylindrical body may include a laser cut metal tube, wires bent and connected to one another, or one or more coiled wires with axial support wires attached to the coil to define one or more bending directions to form bending segments. The skeleton includes axially stiff portions that resist compression when a pull wire is pulled to cause bending movement. The axially stiff portions may include a backbone, an alignment of pivot structures, connected axially extending portions of wire elements, or axially extending support wires or rods. Two or more bending portions may be provided, each with different bending directions. Complex bending shapes may be provided by arranging the segments in rotated positions along the skeleton.
Owner:CREGANNA UNLTD
Catheter having a multilayered shaft section with a reinforcing mandrel
InactiveUS20050090853A1Improve pushabilityIncrease resistanceStentsEnvelopes/bags making machineryMedicineCatheter device
A catheter having an elongated shaft having at least a section which is multilayered with a first layer and a second layer secured to the first layer, and a mandrel having at least a section between the first and second layers. In one presently preferred embodiment, the mandrel is in contact with an outer surface of the first layer and with an inner surface of the second layer.
Owner:DUCHAMP JACKY G
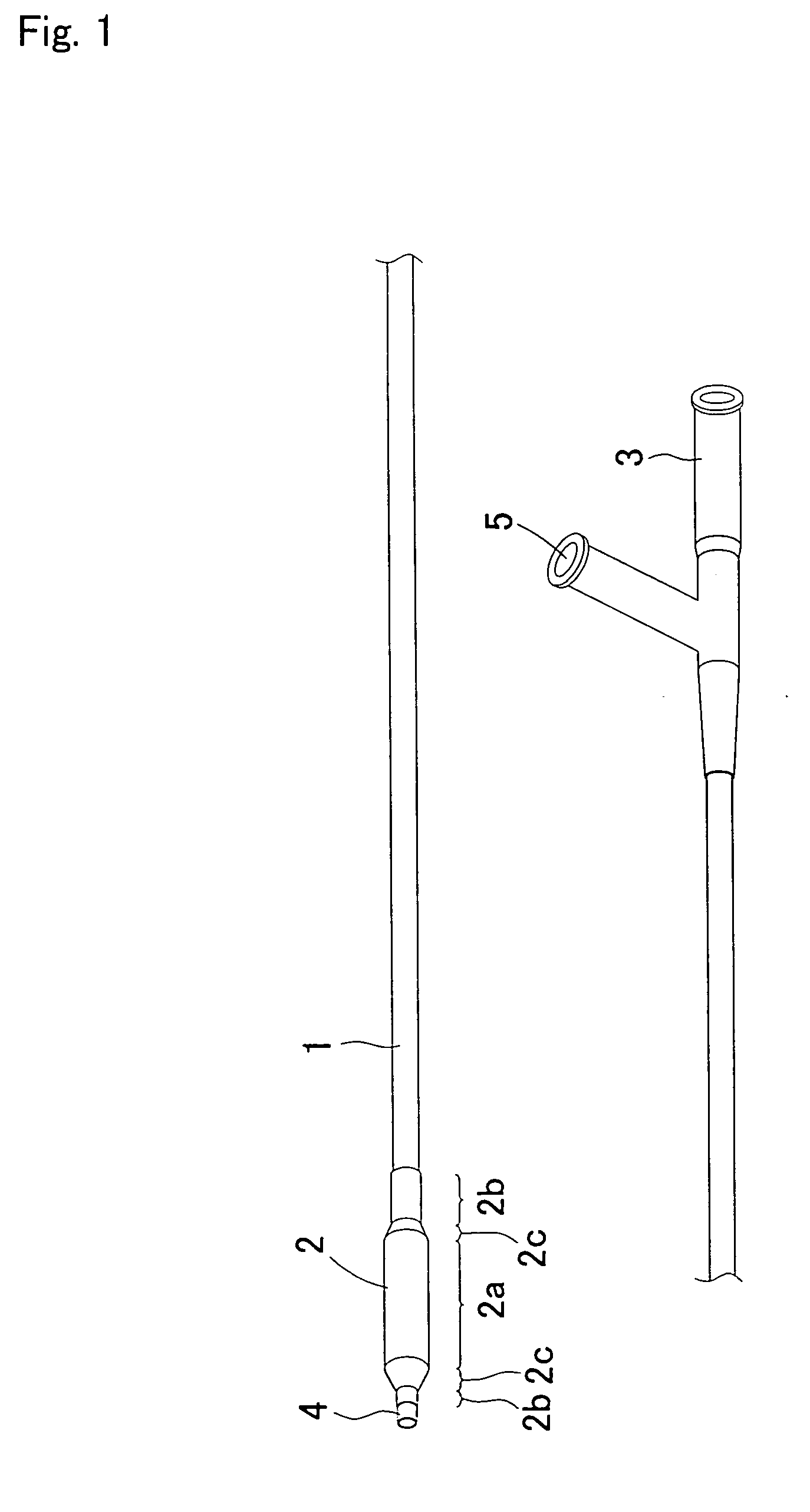
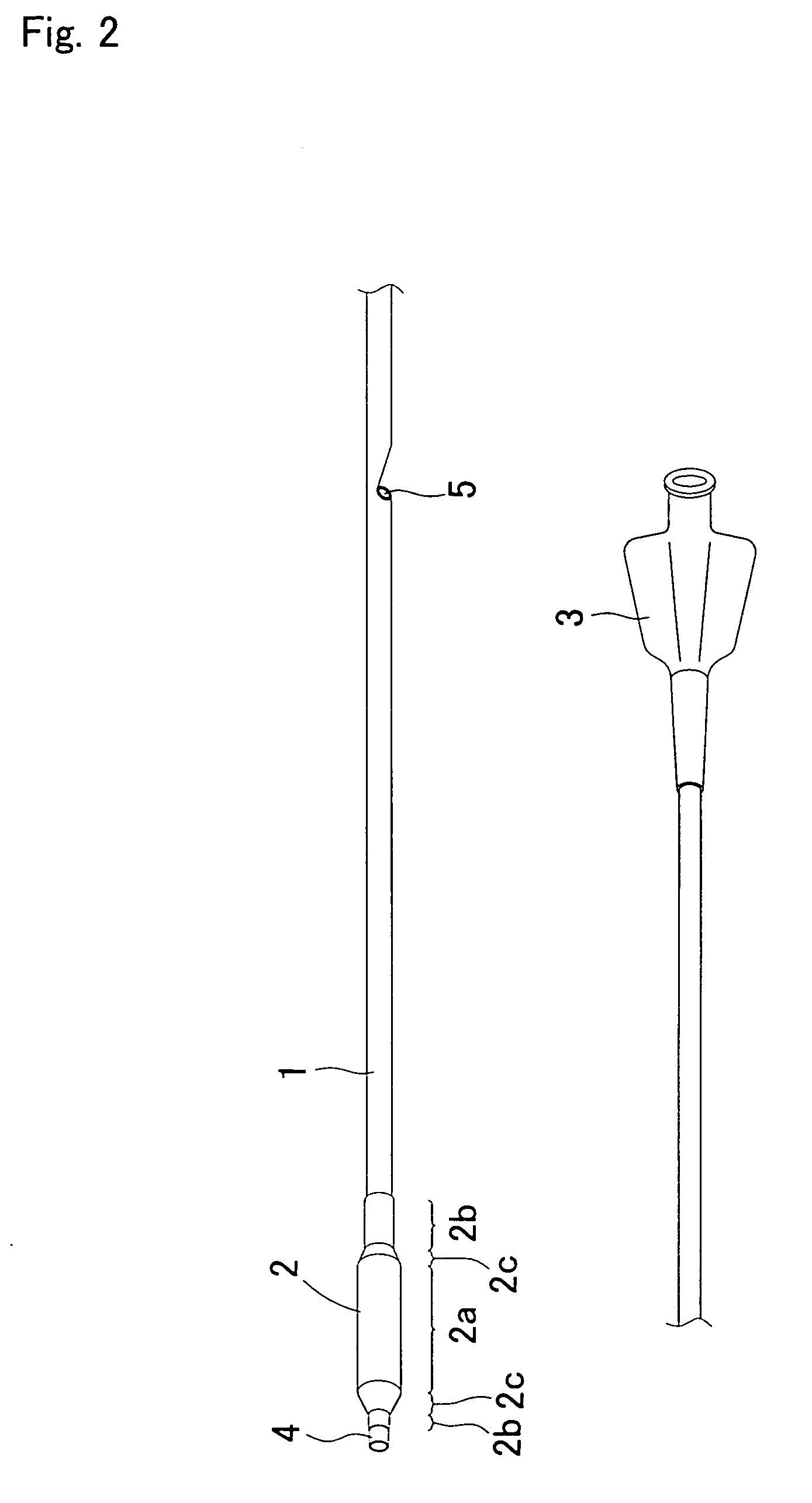
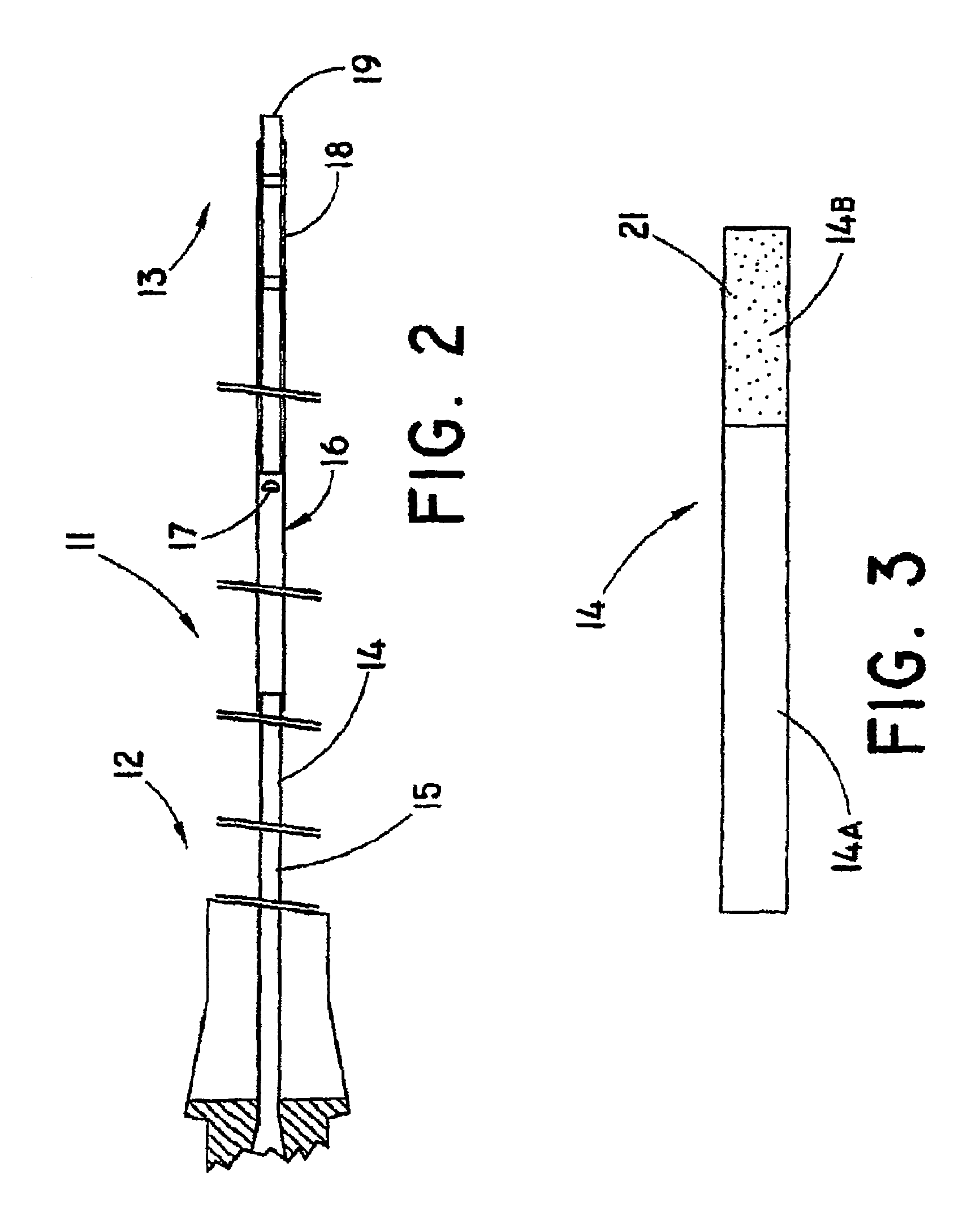
Guide wire for medical use
ActiveCN103354753AIncreased bending stiffnessImprove the pressing effectGuide wiresSurgeryCoil springEngineering
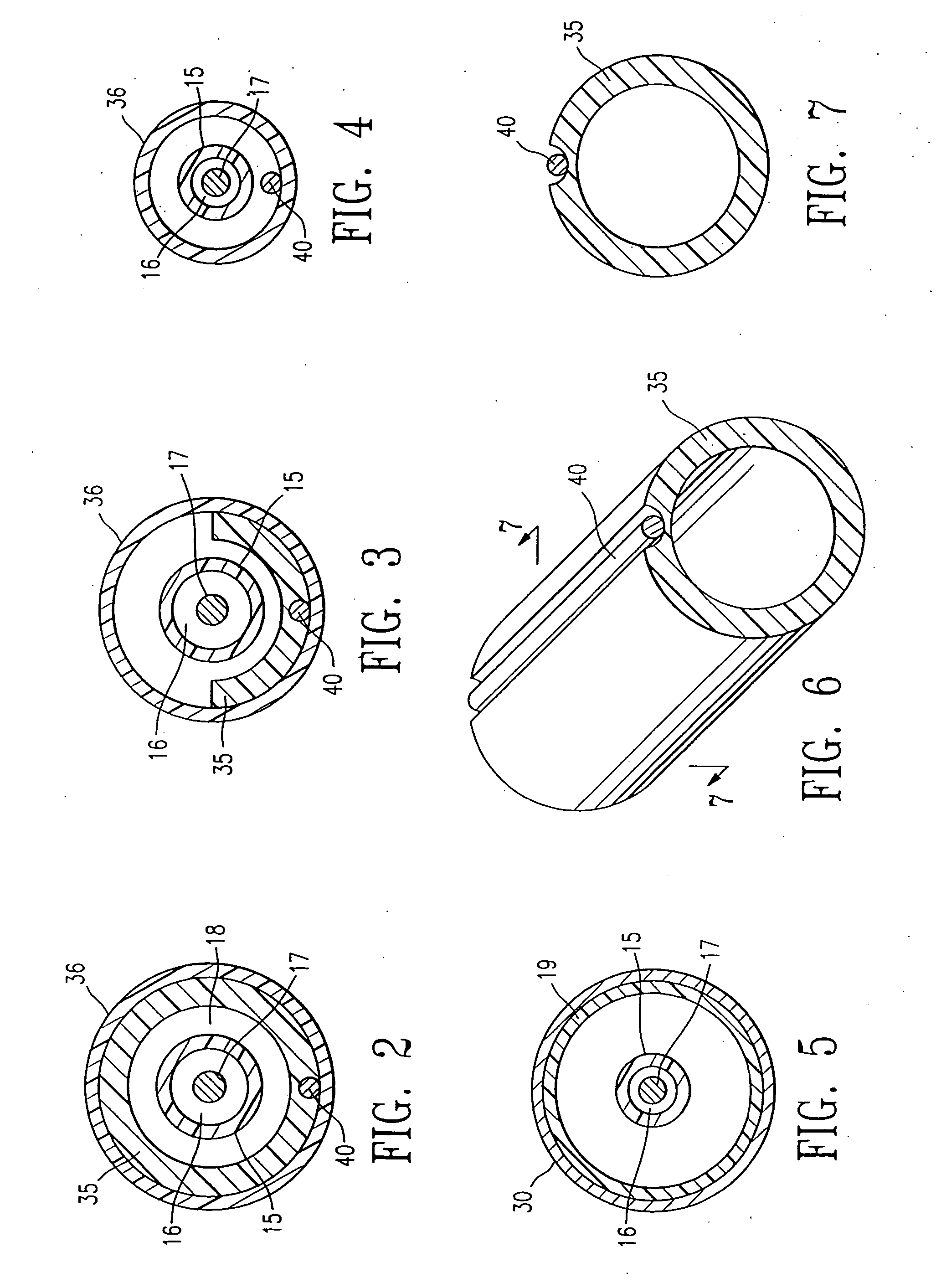
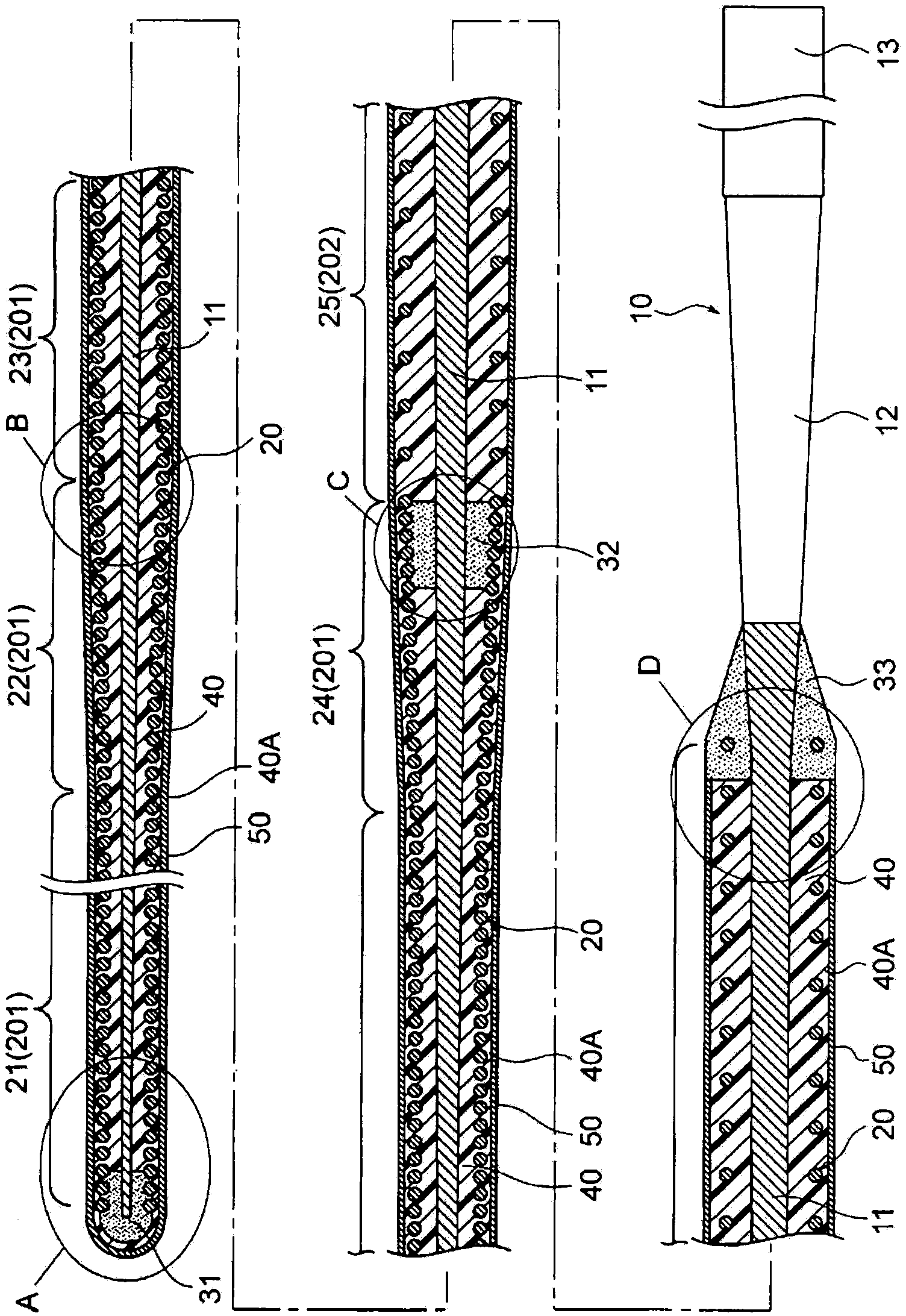
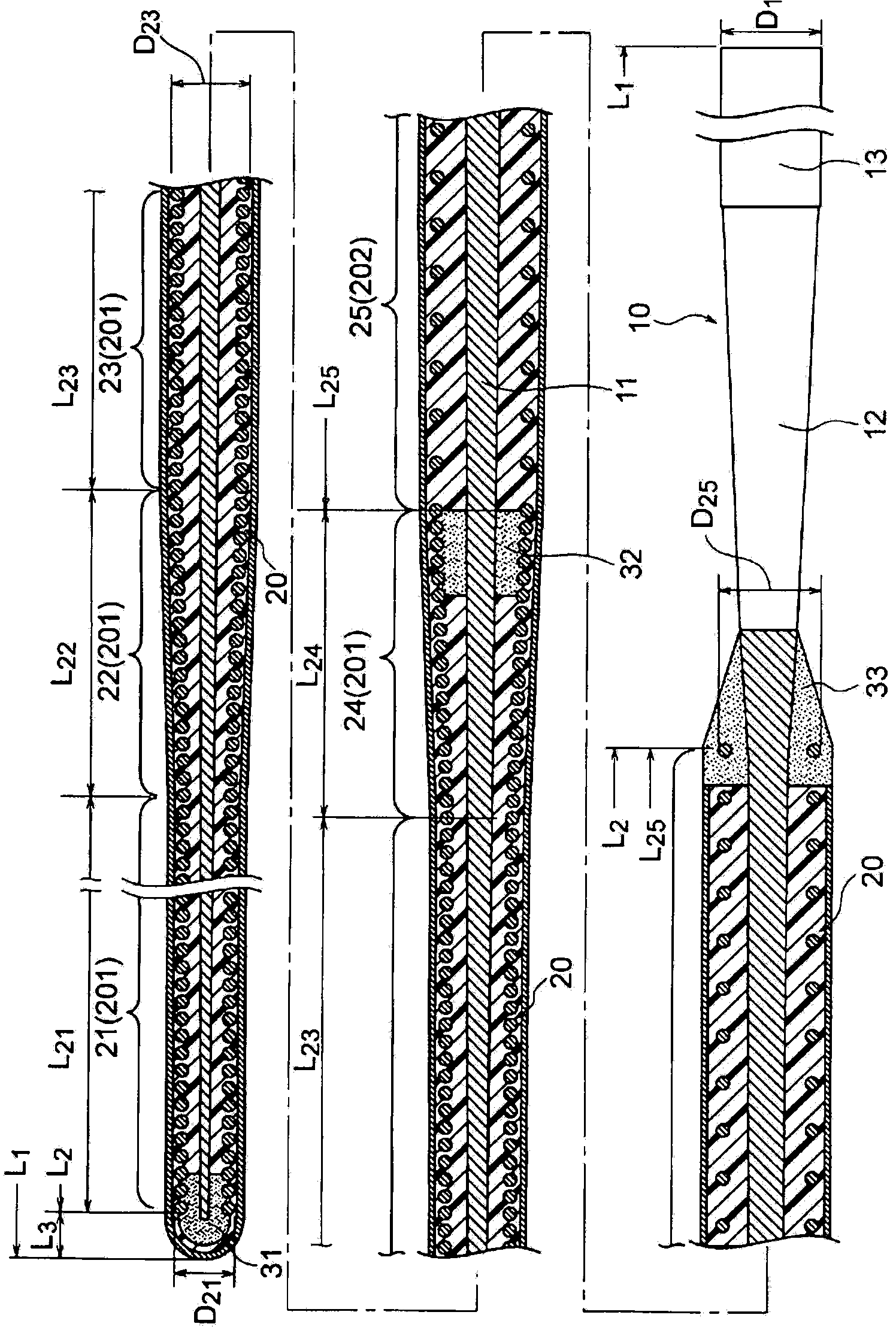
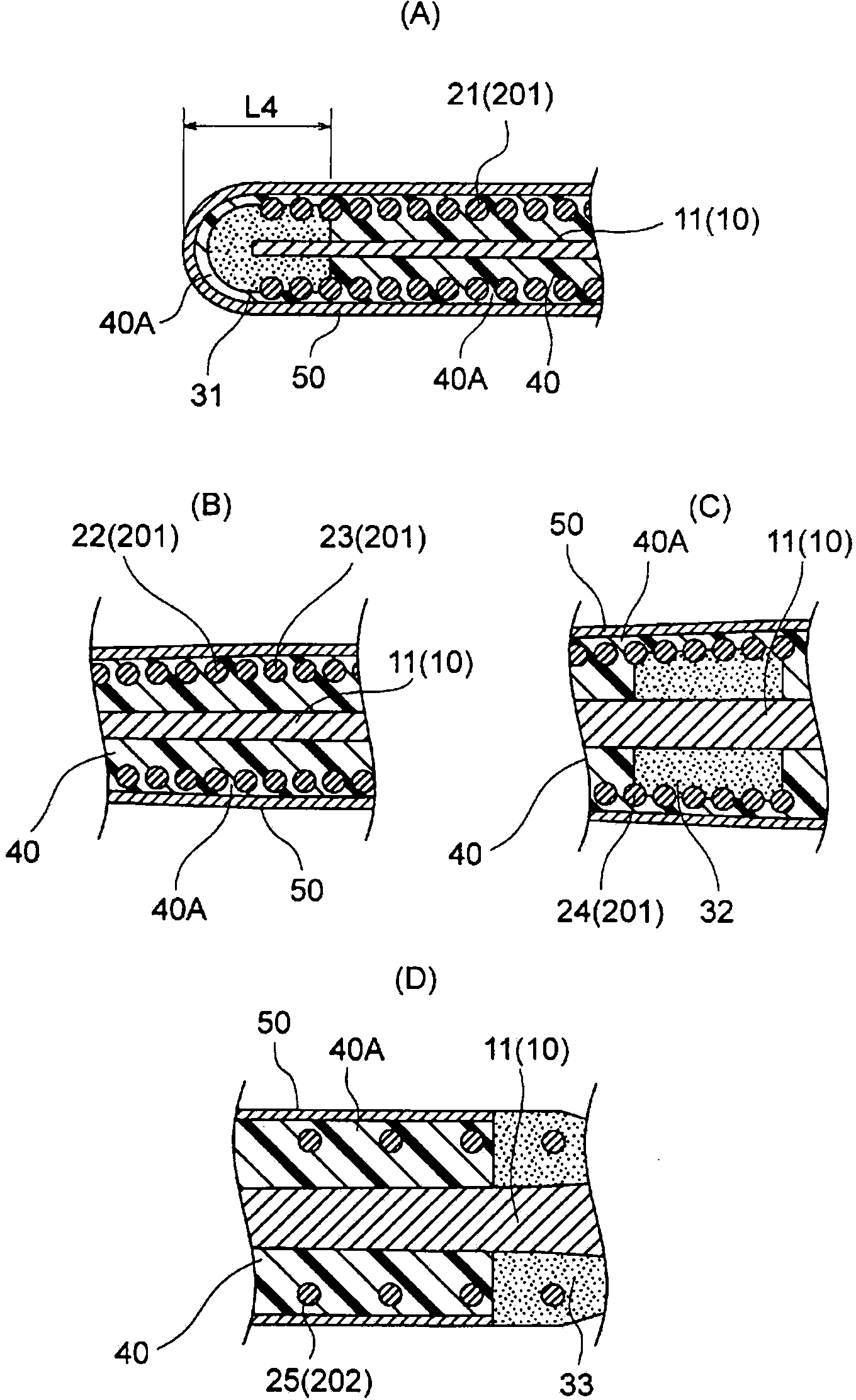
A guide wire for medical use is provided with a coil spring (20) mounted on a distal end side small diameter part (11) of a core wire (10) formed of austenitic stainless steel with a tensile strength of 2600 to 3000 MPa. The coil spring (20) has a tip end side small diameter part (21), a first taper part (22), a middle diameter part (23), a second taper part (24) and a back end side large diameter part (25). A closely coiled portion (201) is formed by the tip end side small diameter part (21), first taper part (22), middle diameter part (23) and second taper part (24), and a loosely coiled portion (202) is formed by the back end side large diameter part (25). The tip end portion of the tip end side small diameter part (21), the back end portion of the second taper part (24) and the back end portion of the back end side large diameter part (25) are affixed to the outer periphery of the distal end side small diameter part (11) of the core wire (10). This guide wire is capable of passing through even constricted areas, has high flexural rigidity, and also has exceptional push-in properties, torque conveyance properties and shape stability properties.
Owner:JAPAN LIFELINE CO LTD
Catheter with spiral cut transition member
Owner:BOSTON SCI SCIMED INC
Guidewire with internal pressure sensor
PendingUS20220175256A1Improve ductilityLower the heatGuide wiresMedical devicesGuide wiresStress sensors
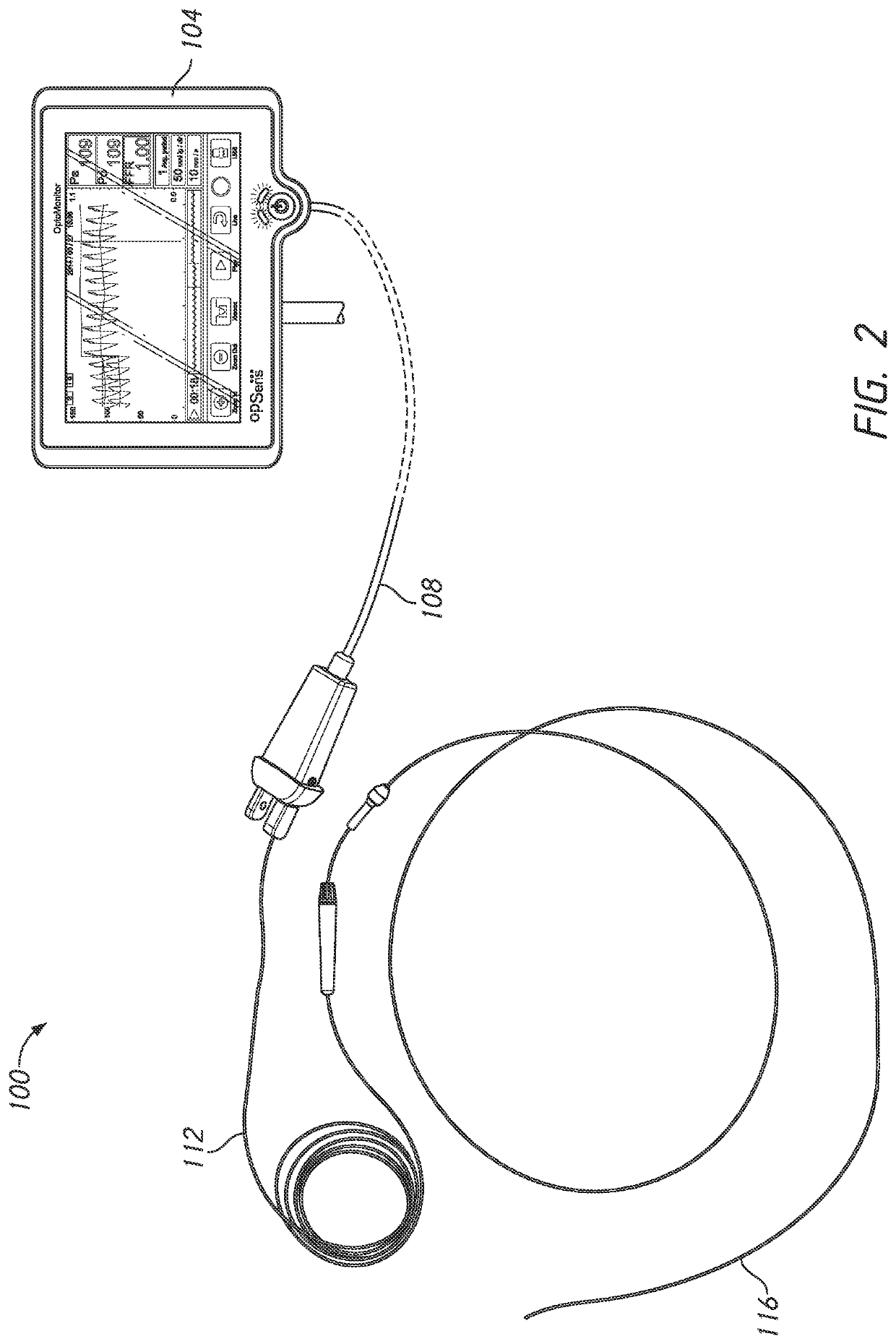
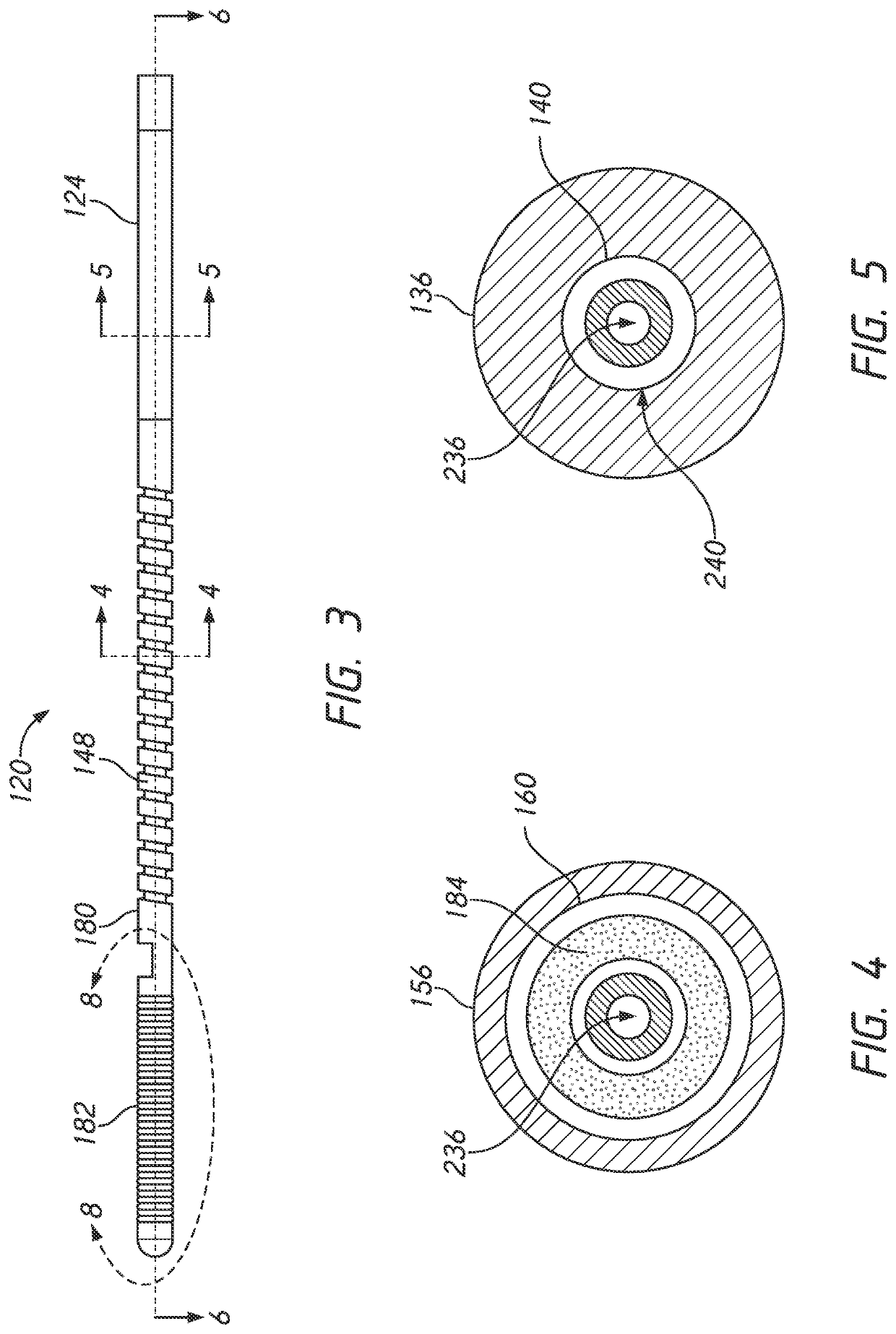
A pressure guidewire is provided that has a proximal end and a distal end. The pressure guidewire has a proximal section a sensor housing section, and an intermediate section. The proximal section extends from the proximal end of the pressure guidewire to a distal end of the proximal section. The sensor housing section is disposed adjacent to the distal end of the pressure guidewire. The intermediate section disposed between the proximal section and the sensor housing section. The intermediate section has a proximal end separate from the proximal section. The proximal end can be coupled to the distal end of the proximal section. The pressure guidewire has a tubular body positioned within the intermediate section. A pressure sensor is positioned in the sensor housing section
Owner:OPSENS
Reformable guidewire tip
ActiveUS20160038719A1Improve kink resistanceStable supportGuide wiresMedical devicesHigh speed rotational atherectomyMaterial Perforation
Devices and methods for providing an elastically deforming guidewire tip, capable of withstanding the extreme forces of high-speed rotational atherectomy, in particular orbital motion induced by an eccentric abrasive head, are disclosed. In certain embodiments, the reformable tip comprises an inner nitinol support coil, wherein the reformable tip may be attached to a larger proximal core for improved kink resistance and support for delivering adjunctive devices. In other embodiments, an inner nitinol support coil may be wrapped with a braided coil and / or a polymer sleeve. The resulting tip is more flexible with reduced risk of perforation than known guidewire tips.
Owner:CARDIOVASCULAR SYST INC
Embolism microcatheter
ActiveCN110538373AImprove overall performanceImprove passability and flexibilityMedical devicesCatheterUrologyEmbolism
The invention provides an embolism microcatheter, which comprises a catheter seat, a de-stressed tube and a catheter body. The near end of the catheter body is connected with the catheter seat, and the far end of the catheter body is provided with a mark ring; the de-stressed tube is arranged outside the catheter body in a sleeving mode, the length of the de-stressed tube is smaller than the length of the catheter body, and the near end of the de-stressed tube is connected with the catheter seat; and the catheter body is sequentially divided into an outer layer, a woven layer, a first combinedlayer, a second combined layer and an inner layer from outside to inside, and the outer layer comprises a first near section, a second near section, a transition section and a far section which are mutually connected in sequence in the direction of the far end of the catheter body. The two sets of combined layers are additionally arranged between the inner layer and the outer layer so that the inner layer and the outer layer can be combined better, the structure is more stable, and thus the pushing property, flexibility and the passing ability of the microcatheter are improved greatly.
Owner:SHANGHAI SHENQI MEDICAL TECH CO LTD
Peel-away sheath assembly
PendingCN112533661AImproved kink resistanceReduce the amount of forceGuide needlesIntravenous devicesPhysicsThermoplastic
Systems and methods for a multi-layered peel-away sheath assembly for insertion of a blood pump including a sheath hub and a sheath body having a proximal end that is connected to the sheath hub, anda distal end. The sheath body comprises multiple layers including a reinforcing layer. The reinforcing layer improves flexibility and kink resistance of the assembly The reinforcing layer can compriseLCP, PEBAX, stainless steel, Nitinol, or Kevlar. The reinforcing layer may be a laser-cut hypotube or a braided or coiled filament. The first layer material and the third layer material are thermoplastics, including PEBAX or TPU. The reinforcing layer has at least one discontinuity, which is aligned with peel-away lines in the sheath body to allow an operator to peel-away the assembly. The peel-away lines are formed of inner, outer notches, or both. The sheath hub also includes a discontinuity to allow the sheath hub to peel-away.
Owner:ABIOMED
Rapid exchange balloon catheter and method for making same
ActiveUS8043256B2Reduce stiffnessAvoid disadvantagesStentsSurgeryTransitional RegionBalloon catheter
A rapid exchange balloon catheter having a proximal end and a distal end, said catheter comprising: a tubular metal shaft body extending from the proximal end along a majority of the total length and having an inflation lumen arranged therein, a plastics distal end portion bonded to the metal body in extension thereof, said distal end portion being provided with an inflation lumen in communication with a balloon, and a guide wire lumen, said guide wire lumen extending from a proximal side port to a distal end opening. To reduce the resistance to kinking, the metal body comprises a transitional region having reduced stiffness at the position of bonding to the plastics distal end portion compared to a more proximal position along the metal body.
Owner:COOK MEDICAL TECH LLC
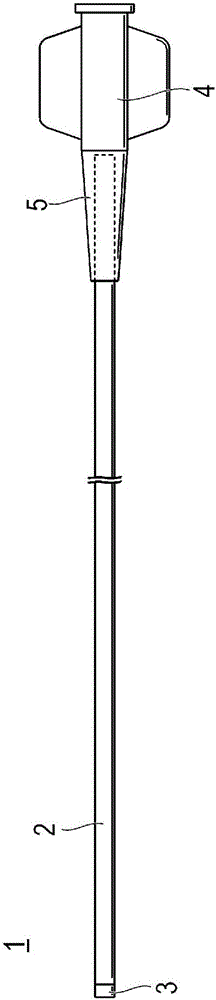
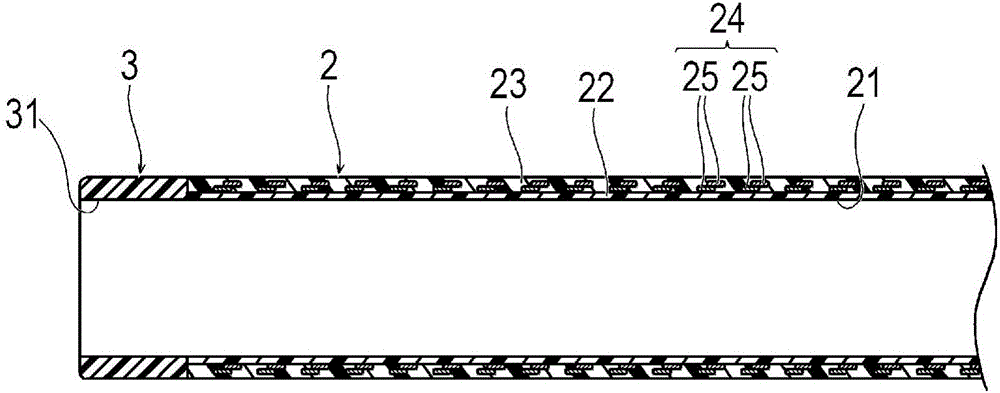
Catheter
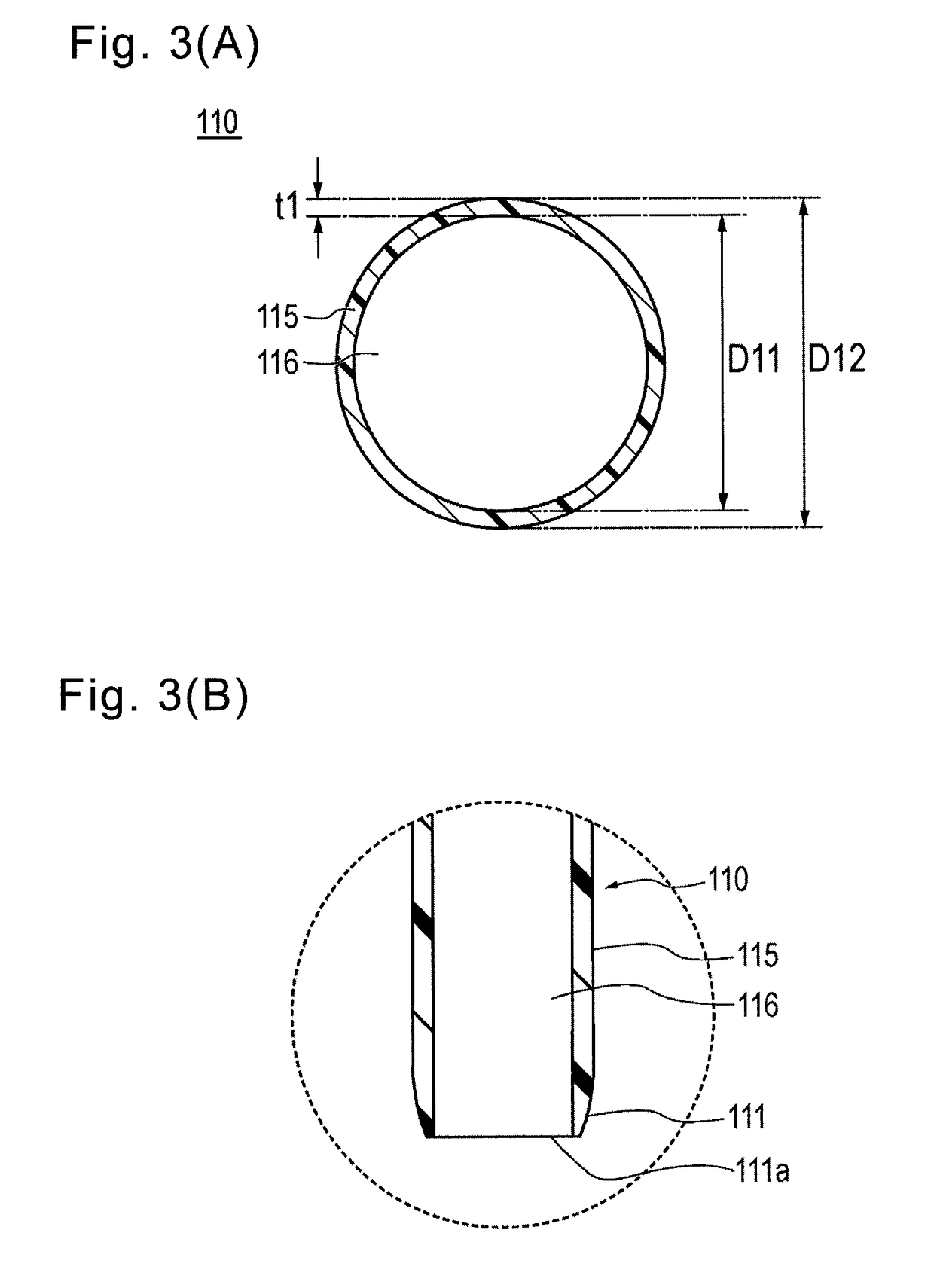
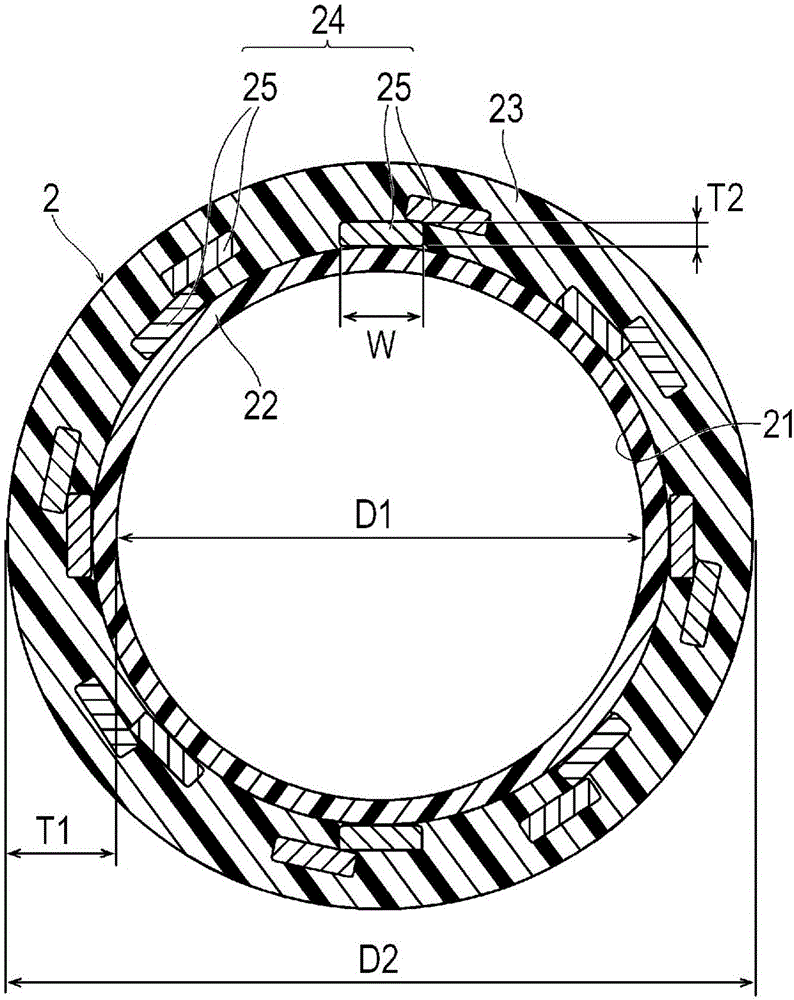
Provided is a catheter capable of favorably ensuring the passability of a medical instrument into the lumen of the catheter even when the inner diameter is large in comparison to the outer diameter, or when a kink occurs. A catheter (1) having an inner layer (22) for forming the inner surface of the catheter body (2), an outer layer (23) for forming the outer surface of the catheter body (2), and a plurality of reinforcing wires (25) embedded between the inner and outer surfaces, the catheter (1) being one which satisfies formula (1), formula (2) and formula (3), given that the inner diameter of the catheter body (2) is (D1), the outer diameter thereof is (D2), the wall thickness thereof is (T1), the wall thickness of the reinforcing wires (25) of the catheter body (2) is (T2), the effective width of the reinforcing wires (25) of the catheter body (2) is (W), and the number of reinforcing wires (25) is (N). 0.050mm<=T1<=0.100mm (formula (1)) T2 / T1>=0.25 (formula (2)) 60,000<=(D1*D2*W*N) / (T12*T2)<1,000,000 (formula (3))
Owner:TERUMO KK
High-compliance tissue engineering blood vessel preparation template and tissue engineering blood vessel
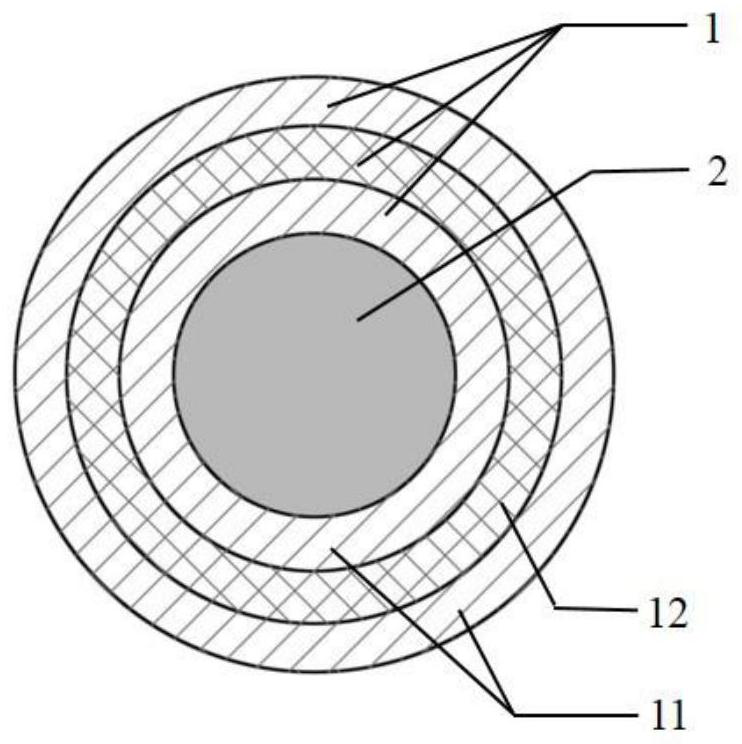
ActiveCN111714703AImprove complianceReduce the incidence of intimal hyperplasiaTissue regenerationBlood vesselsDecellularizationTunica intima
The invention belongs to the field of tissue engineering, and particularly relates to a high-compliance tissue engineering blood vessel preparation template. The high-compliance tissue engineering blood vessel preparation template comprises a framework main body (1) and an inner core (2), wherein the inner core (2) is arranged inside the framework main body (1); the outer diameter of the inner core (2) is matched with the inner diameter of the framework main body (1); the main body of the framework main body (1) comprises support layers (11) and degradation layers (12) in alternate arrangement; the inner surface and the outer surface of the framework main body are the support layers (11); and the degradation layers (12) are made of degradable materials. The high-compliance tissue engineering blood vessel preparation template is used for being implanted under the skin of an animal or being used for in-vitro tissue culture; then, the inner core (2) is pulled away; and after the decellularization treatment, the high-compliance tissue engineering blood vessel can be prepared. The high-compliance tissue engineering blood vessel preparation template has the beneficial effects that the tissue engineering blood vessel prepared by using the high-compliance tissue engineering blood vessel preparation template has good compliance; the intimal hyperplasia can be inhibited.
Owner:领博生物科技(杭州)有限公司
Vehicle fuel hose resistant to high pressure and low in permeability
A vehicle fuel hose resistant to high pressure and low in permeability is sequentially composed of a chloroprene rubber layer, a framework material layer, a chlorinated polyethylene rubber layer and a composite coating. The framework material layer is made of polyester fibers and steel wires. The composite coating is made of triisobutyl aluminum, vinyl silicone oil, polyimide, bentonite and silicon dioxide particles. The raw material of the composite coating is composed of, by weight, the triisobutyl aluminum of 5-10 parts, the vinyl silicone oil of 3-6 parts, the polyimide of 1-4 parts, the bentonite of 3-5 parts and the silicon dioxide particles of 8-15 parts. According to the hose, the framework material layer effectively improves the flexibility and the torsion resistance of the vehicle fuel hose, and the hose can be restored in shape after being subjected to high pressure; the granularity of the nano-meter silicon dioxide particles is basically uniform, so that the hose is low in permeability to liquid. Thus, the vehicle fuel hose can be widely applied to the vehicle field and has high practical value and economic value.
Owner:SHENYANG SAIYA RUBBER PRODS
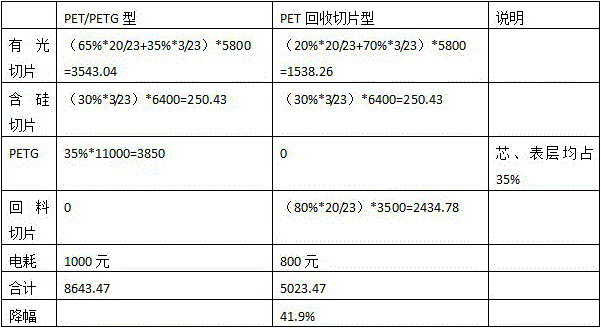
Low-cost eco-friendly polyester film for twist packaging and preparation method of polyester film
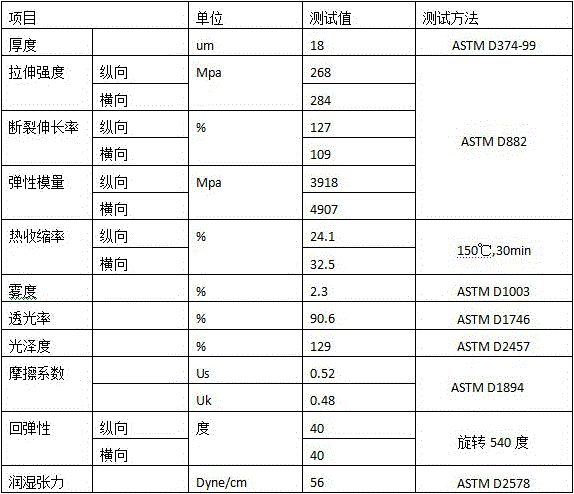
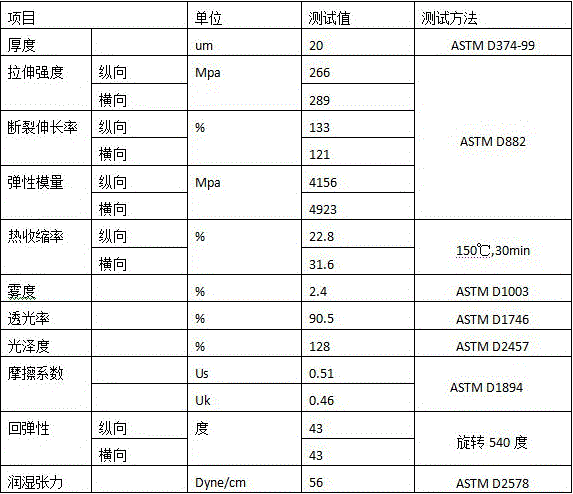
ActiveCN105966018ALow intrinsic viscosityImprove flexibilitySynthetic resin layered productsPolyesterSurface layer
The invention discloses a low-cost eco-friendly polyester film for twist packaging and a preparation method of the polyester film. The film comprises an upper and lower surface layers and a core layer. The core layer is composed of 20-30% of bright polyester chips and 70-80% of recovery chips. Each of the upper and lower surface layers is composed of 70% of bright polyester chips and 30% of silicon-contained chips. The core layer adopts PET recovery chips which are low in price and are clean. Meanwhile, longitudinal and transverse stretch technology is used for adjustment. Mainly, the longitudinal stretch ratio is reduced to 2.8-3.2, and the transverse stretch ratio is reduced to 3.5-3.7. A special low-temperature setting process is carried out at 80-120 DEG C to further reduce crystallization tendency of the film, so that the degree of crystallinity of the film can be controlled and the twist ability of the film is improved. A twist film having a resilience angle less than 45 degrees when rotated at 540 degrees, and the cost is reduced by about 40%.
Owner:SHANDONG FENGHUA PLASTIC TECH CO LTD
System and method for positioning implantable medical devices within coronary veins
An improved system and method for placing implantable medical devices (IMDs) such as leads within the coronary sinus and branch veins is disclosed. In one embodiment, a slittable delivery sheath and a method of using the sheath are provided. The sheath includes a slittable hub, and a substantially straight body defining an inner lumen. The body comprises a shaft section and a distal section that is distal to, and softer than, the shaft section. A slittable braid extends adjacent to at least a portion of one of the shaft section and the distal section. In one embodiment of the invention, the sheath further includes a transition section that is distal to the shaft section, and proximal to the distal section. The transition section is softer than the shaft section, but stiffer than the distal section.
Owner:MEDTRONIC INC
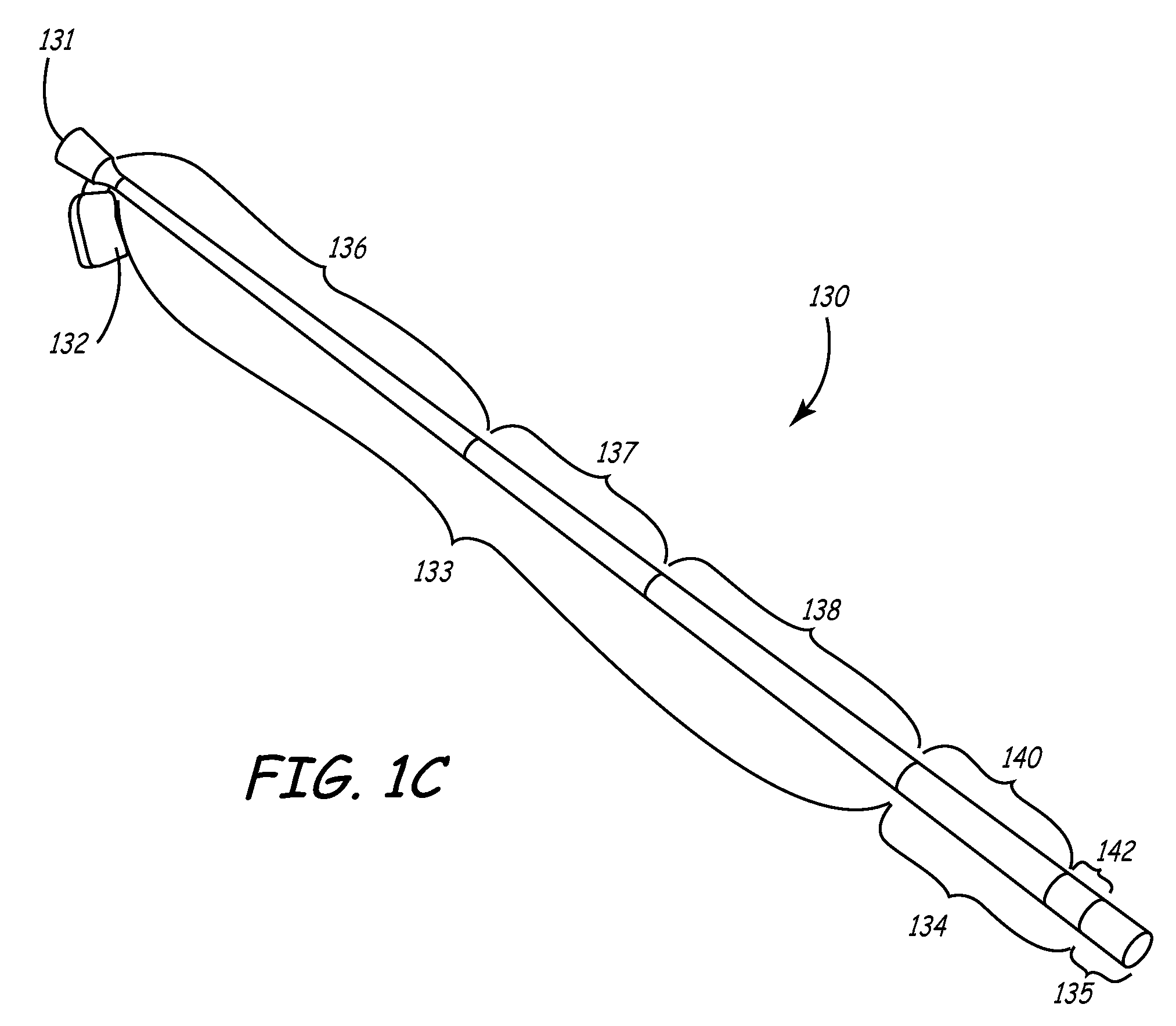
A self expanding flow diversion device with enhanced kink resistance and radial strength
PendingUS20210386566A1Reduce cross-sectional areaEnhance kink resistance and radial strengthStentsBlood vesselsFlow diversionBlood stream
A flow diverter device is used to redirect the blood flow inside the cerebral blood vessels and for the reduction of blood flow to the aneurysm, hence preventing the chance of aneurysm rupture as well as promoting the healing of the aneurysm. The novel design of the device, using a set of thicker wires, provides high kink resistance and radial strength. Two patterns of inter-braiding the thicker set of wires with the finer braid are disclosed, one having a checker-board and the other a ring structure. Both patterns are highly kink resistant with the checker-board design providing minimal loss in flexibility, whereas the ring design provides greater radial strength. The device could be made of super elastic materials like Nitinol wires with the thicker set being radio opaque. The device is highly kink resistant and sufficiently flexible for use in vasculature with complex bends.
Owner:SREE CHITRA TIRUNAL INST FOR MEDICAL SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com