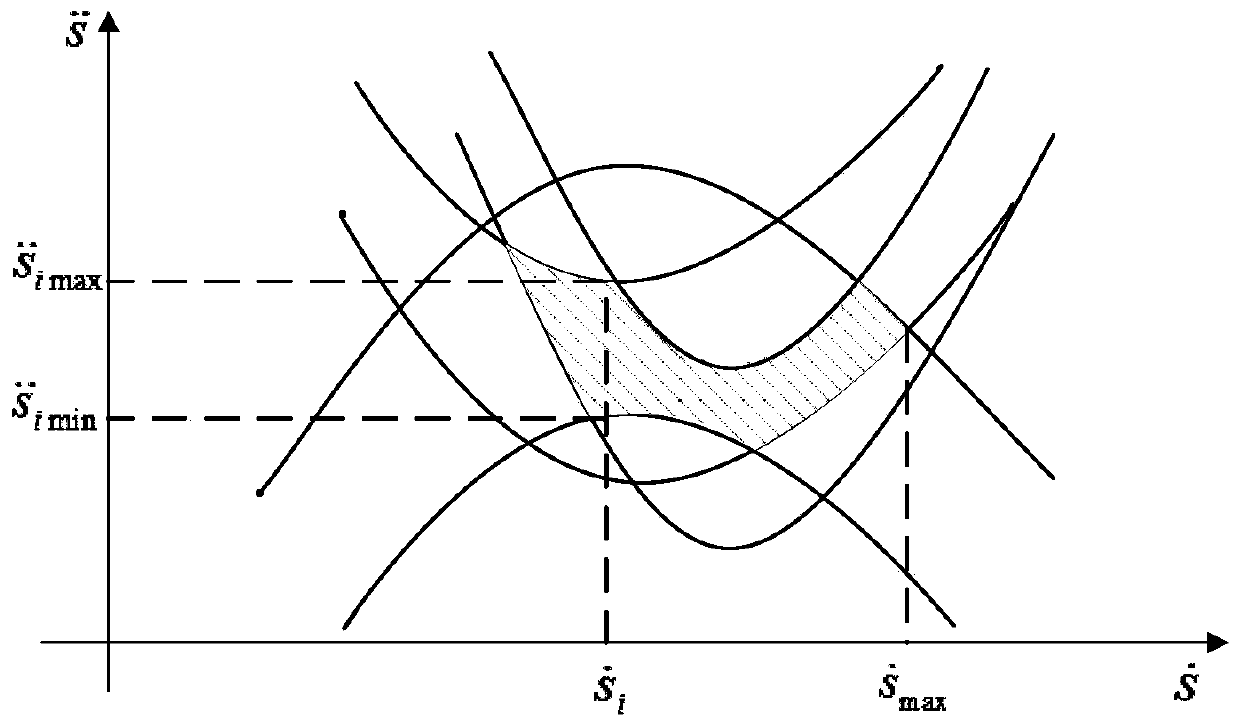
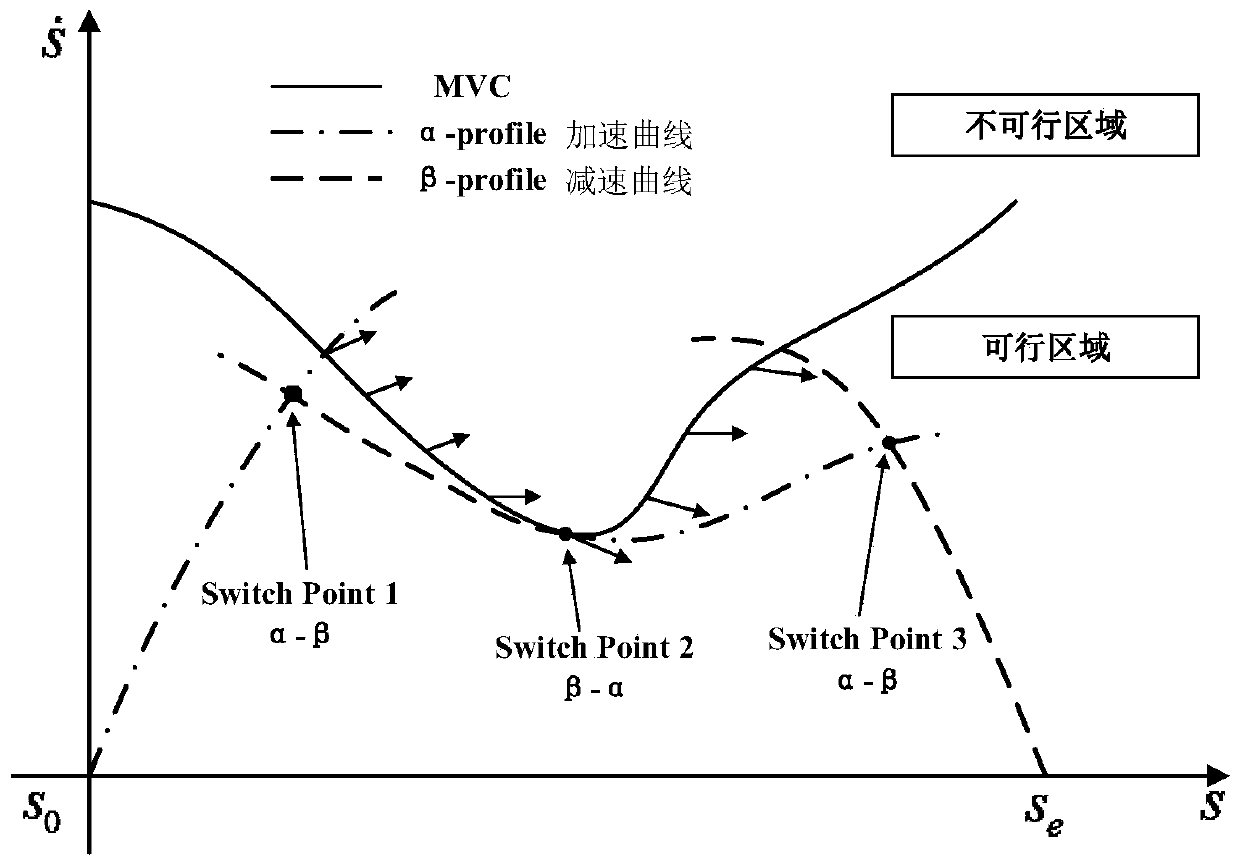
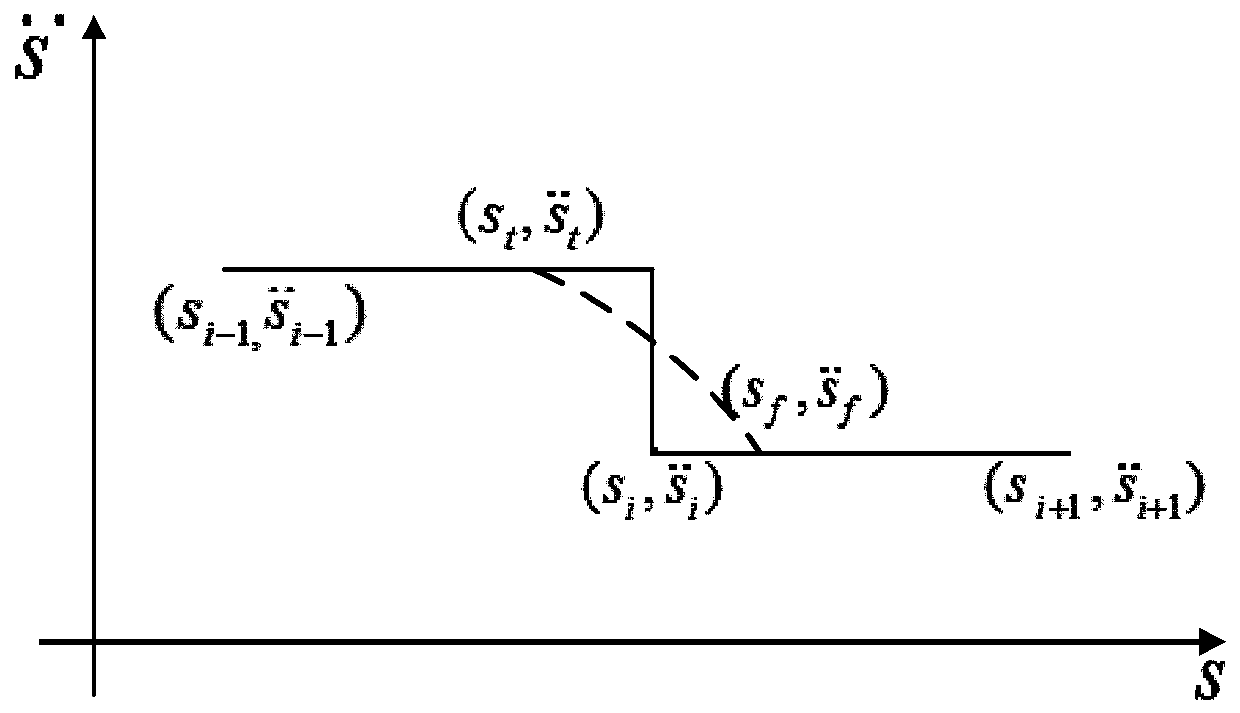
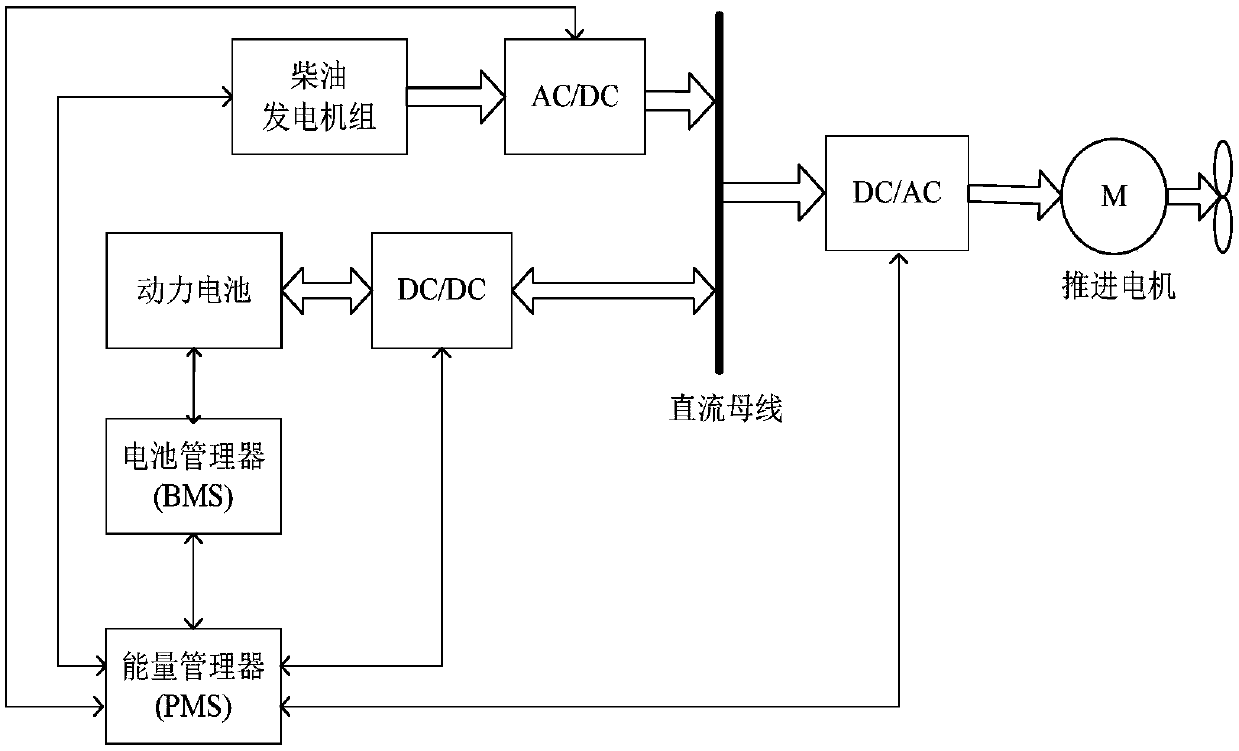
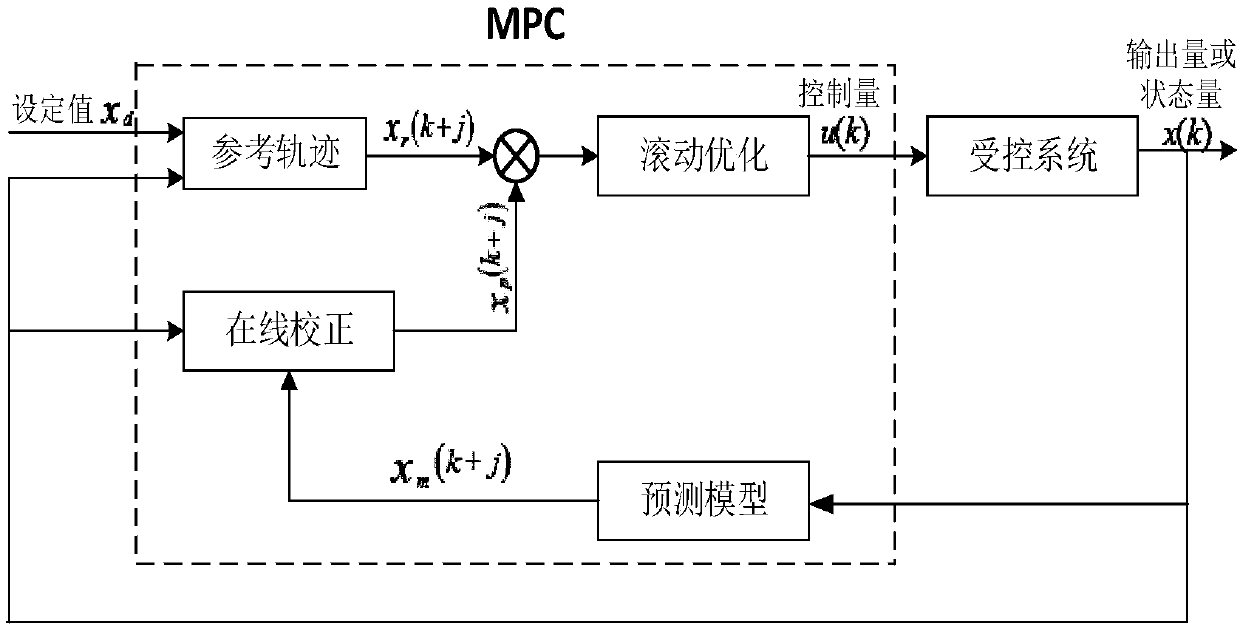
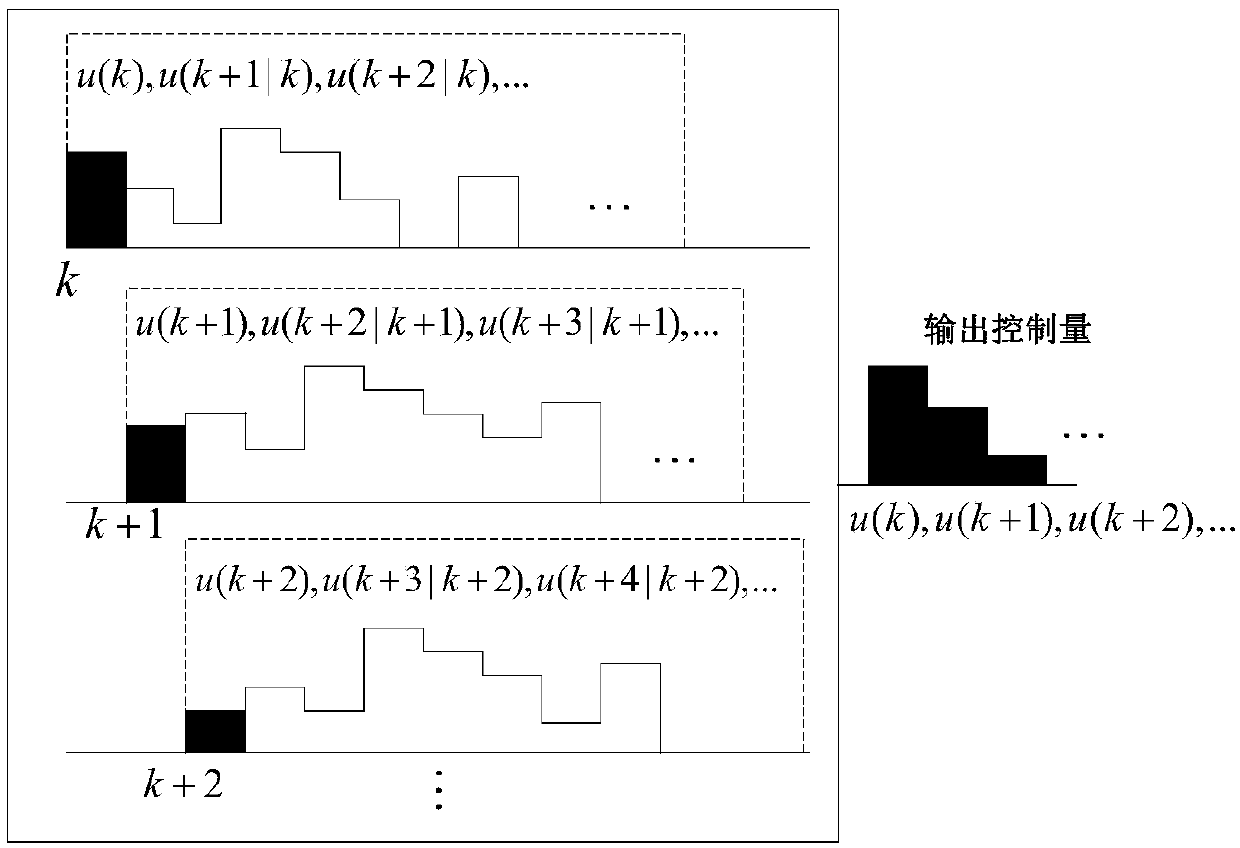
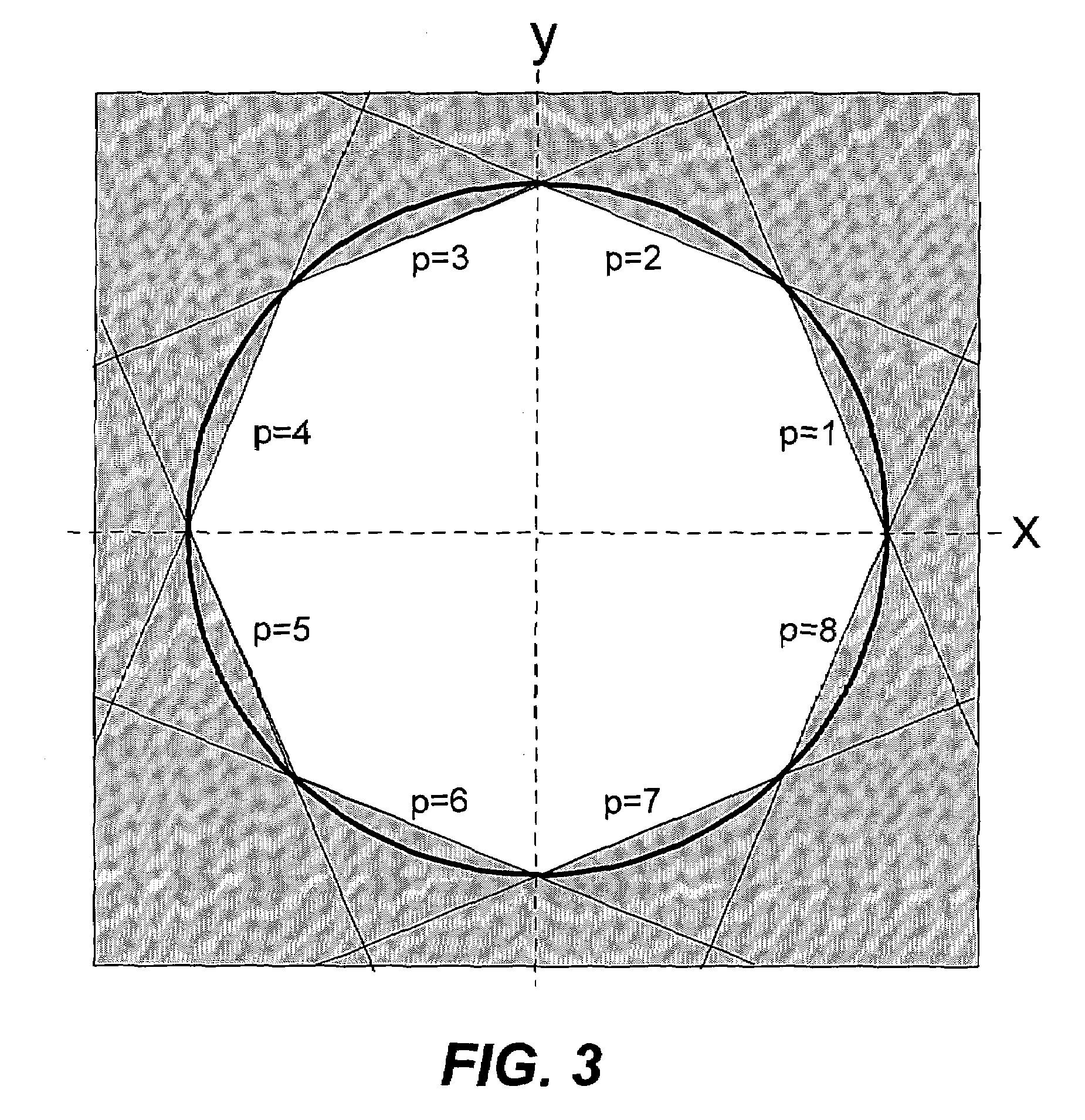
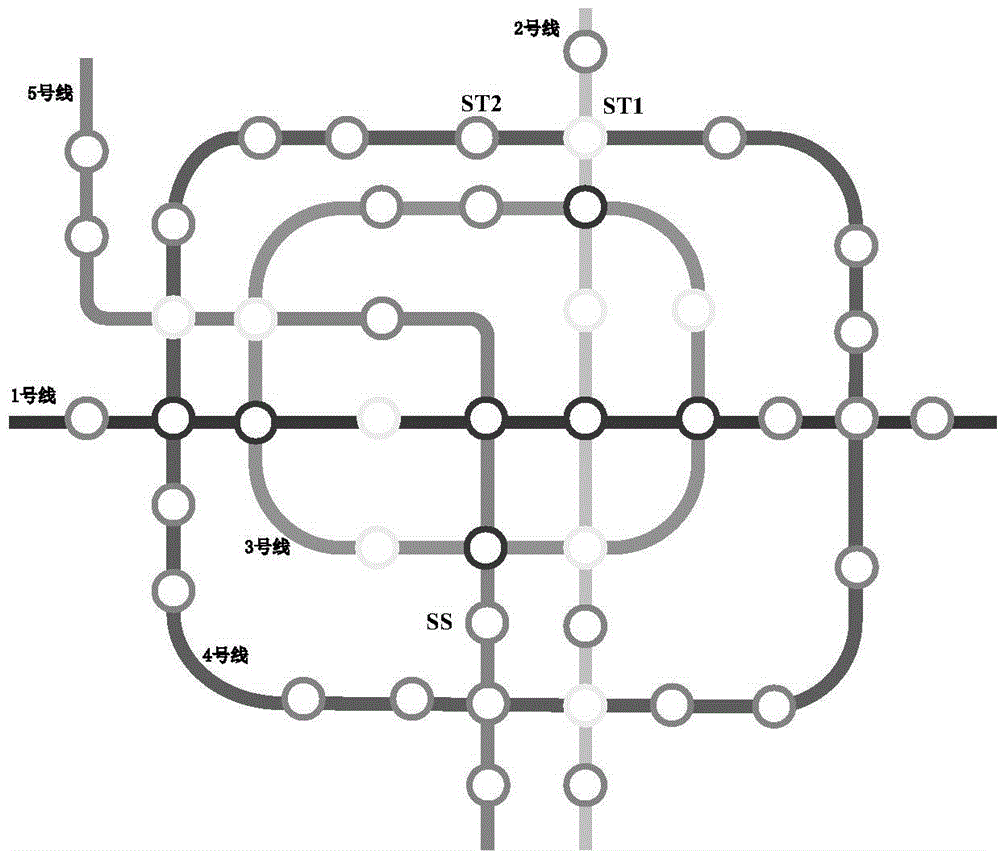
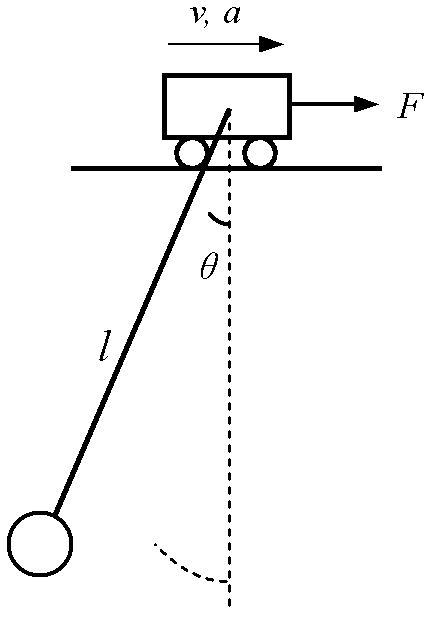
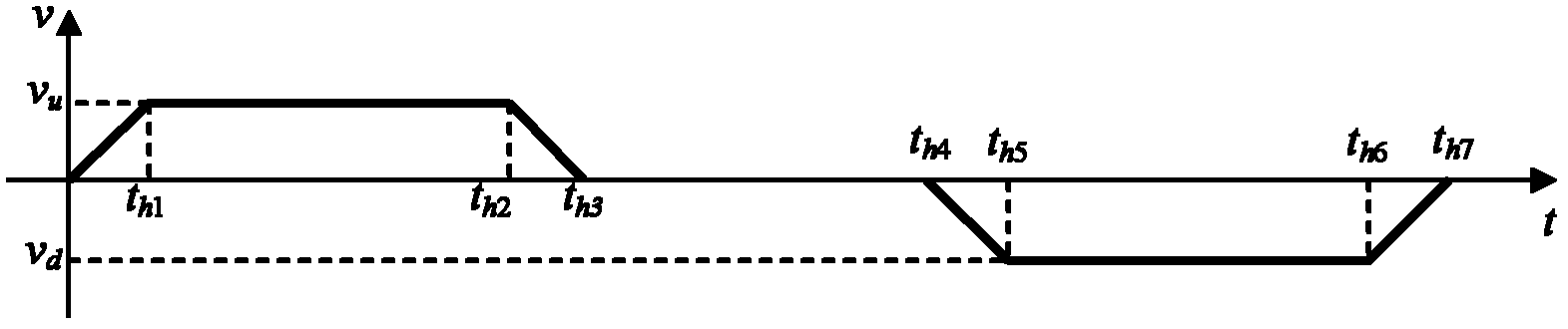
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
246 results about "Time optimal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
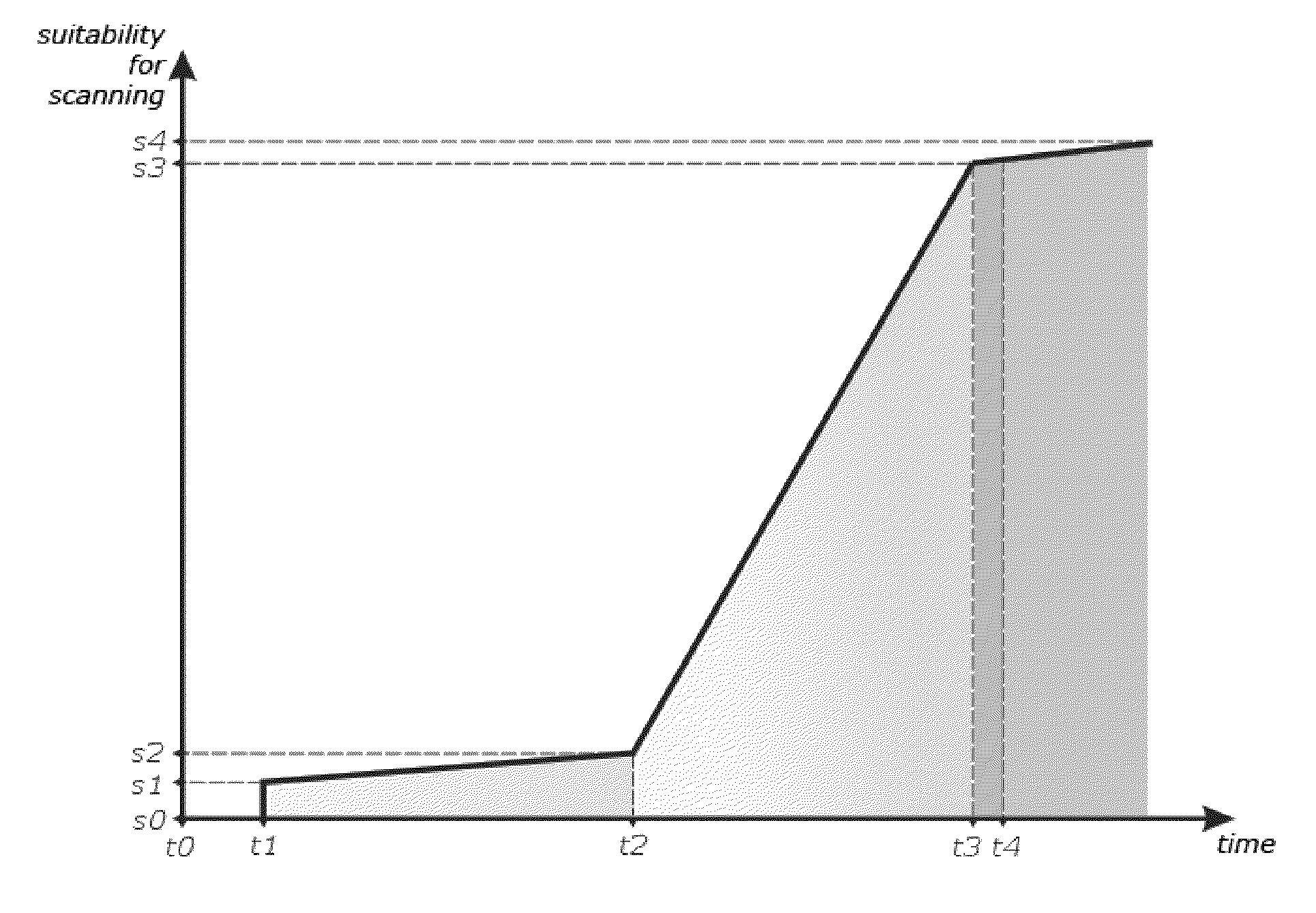
Optimal Time is a machine learning algorithm that looks at each user’s individual app engagement patterns and automatically sends messages when users are most likely to open.
Designs, interfaces, and policies for systems that enhance communication and minimize disruption by encoding preferences and situations
InactiveUS20050084082A1Enhance interpersonal communicationValue maximizationSpecial service for subscribersDigital computer detailsCost benefitStatus changed
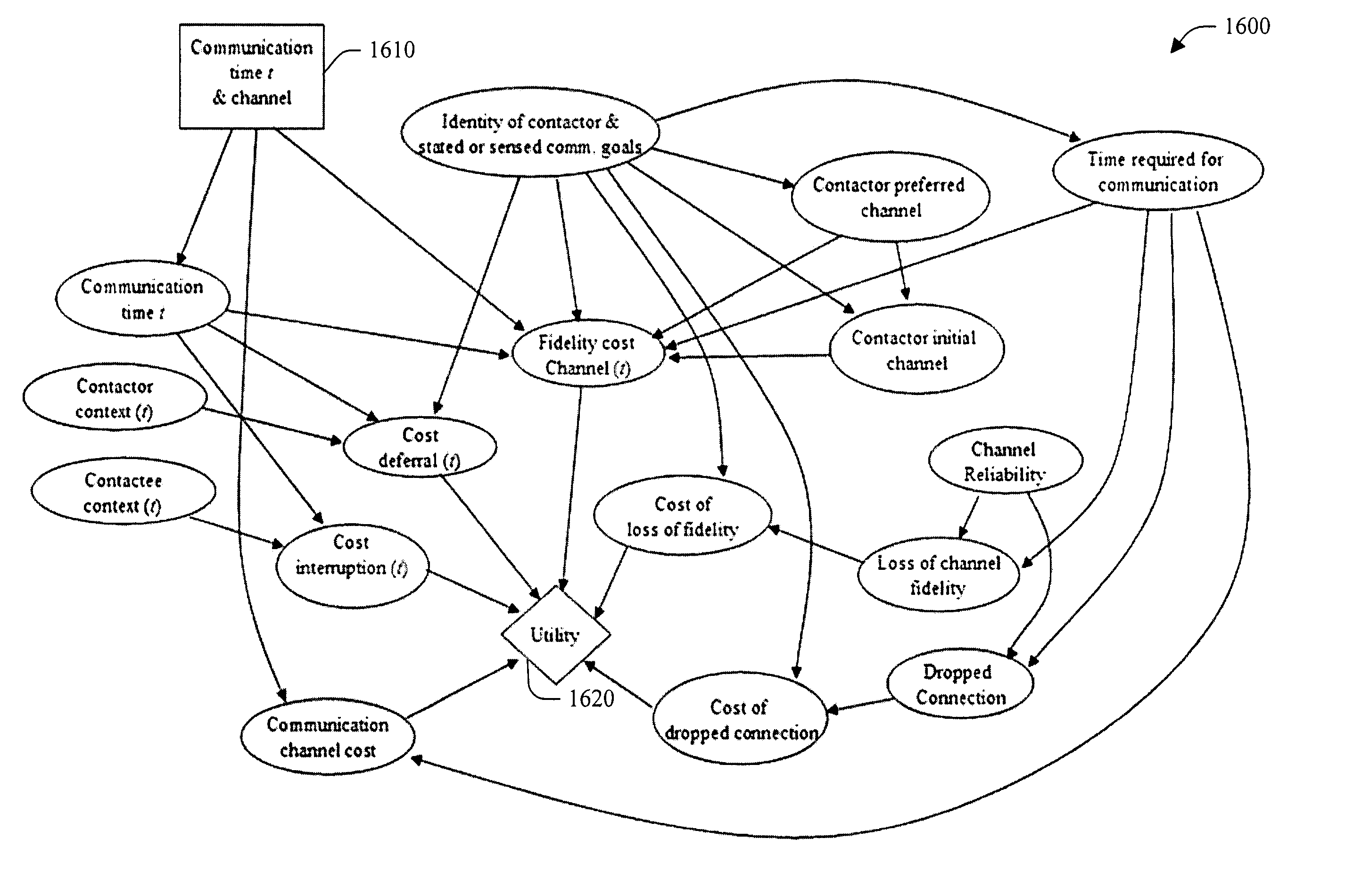
The present invention relates to utilizing identity and context-sensitive decision-making for handling communications, including, channel selection, routing, and rescheduling operations. The systems and methods provide a service that allows users to assess preferences regarding real-time call handling and performs dynamic decision-making about the best timing and channel for interpersonal communication. This service can be based on various cost-benefit analyses (e.g., basic and extended) that consider cost of interruption and preferences of contactors and contactees to guide communications, and / or on decision-making under uncertainty. Statistical models that are learned from data are joined with user preferences to generate expected costs of interruption for office activity and over time, based on a user's activities, locations, calendar information and preference assessments. In addition, statistical forecasting provides presence and availability predictions. The foregoing can provide an enhanced interpersonal communication system that can maximize the value and minimize the cost of communication among people.
Owner:MICROSOFT TECH LICENSING LLC
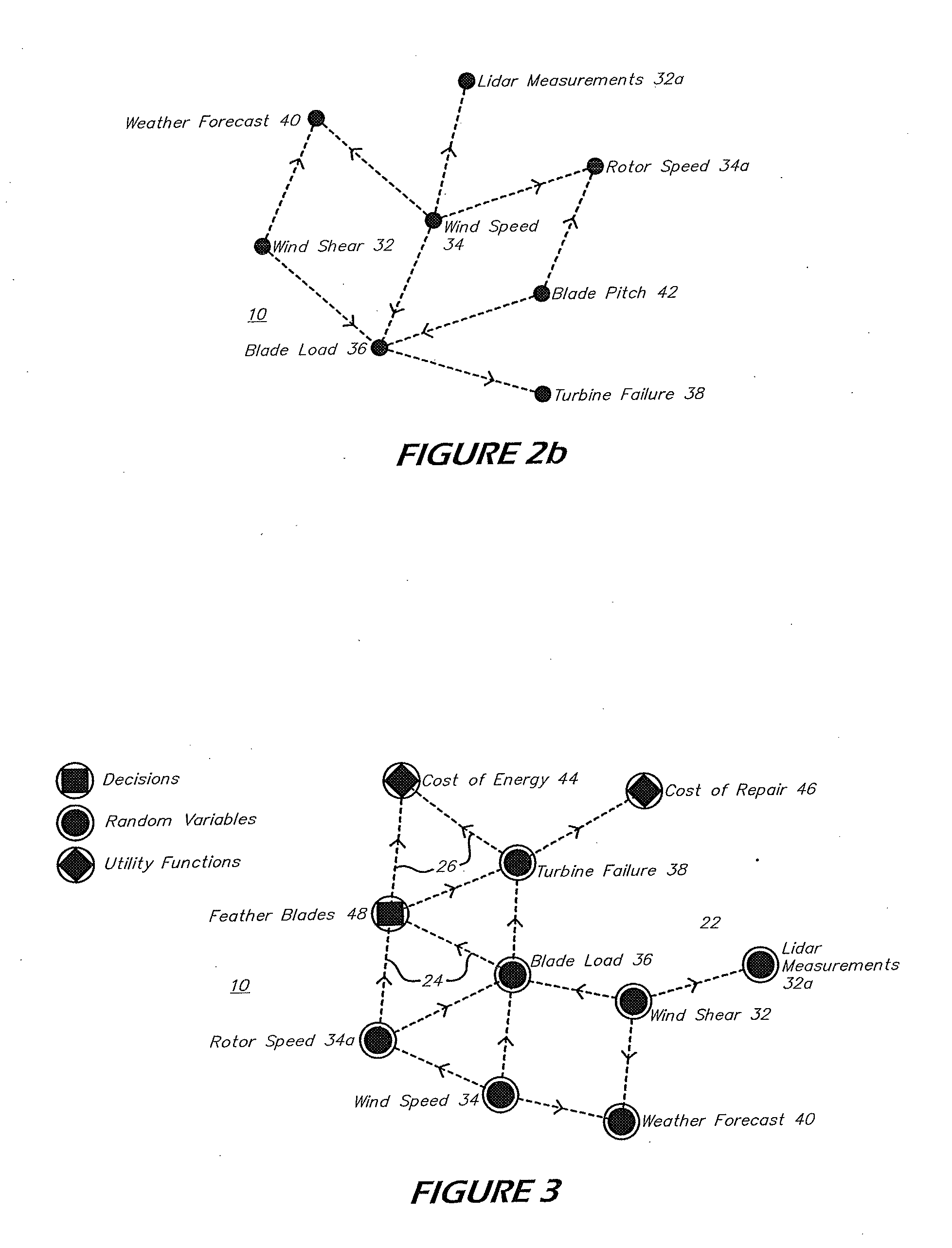
System and method for predicting power plant operational parameters utilizing artificial neural network deep learning methodologies
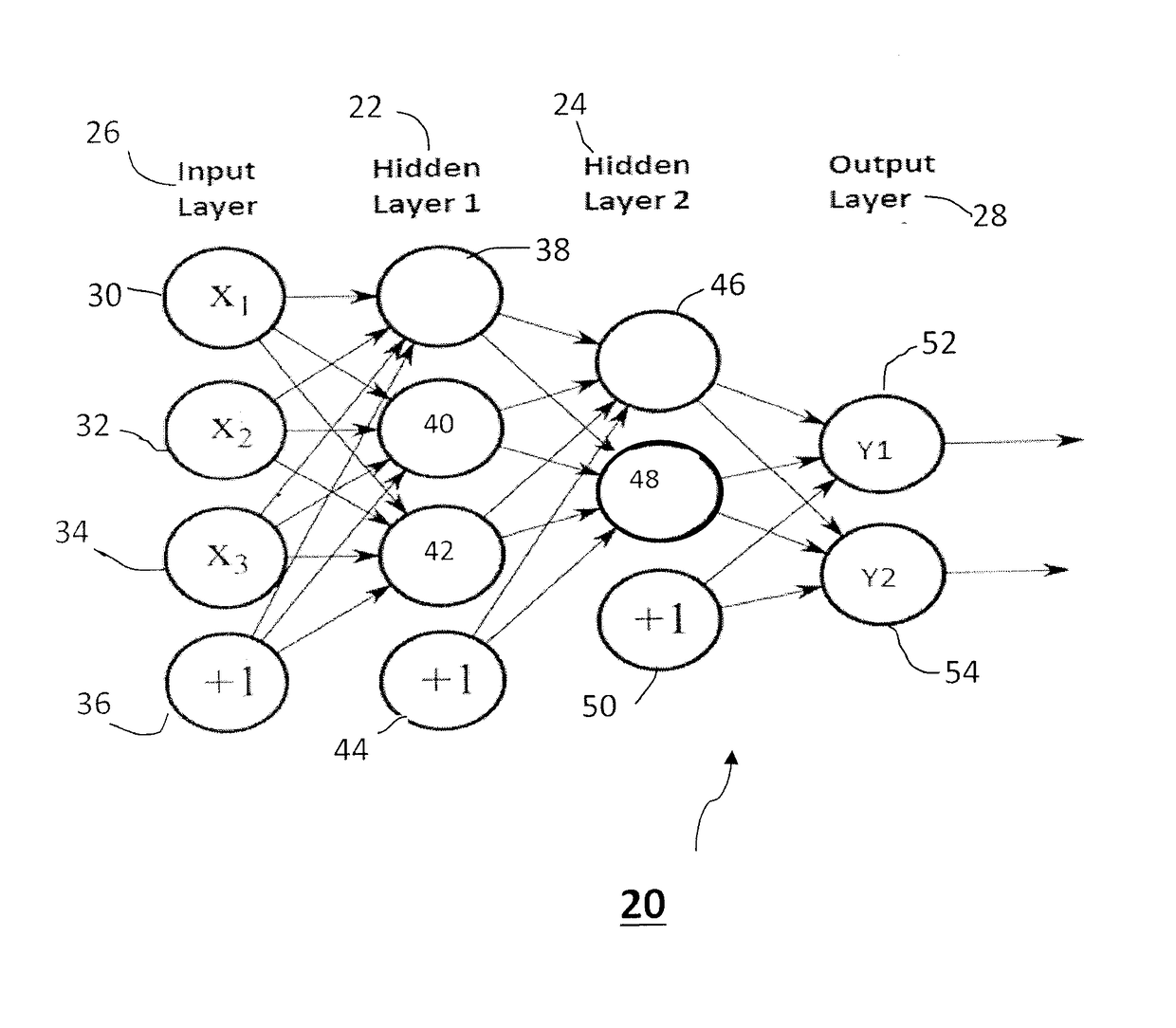
A system and method of predicting future power plant operations is based upon an artificial neural network model including one or more hidden layers. The artificial neural network is developed (and trained) to build a model that is able to predict future time series values of a specific power plant operation parameter based on prior values. By accurately predicting the future values of the time series, power plant personnel are able to schedule future events in a cost-efficient, timely manner. The scheduled events may include providing an inventory of replacement parts, determining a proper number of turbines required to meet a predicted demand, determining the best time to perform maintenance on a turbine, etc. The inclusion of one or more hidden layers in the neural network model creates a prediction that is able to follow trends in the time series data, without overfitting.
Owner:SIEMENS AG
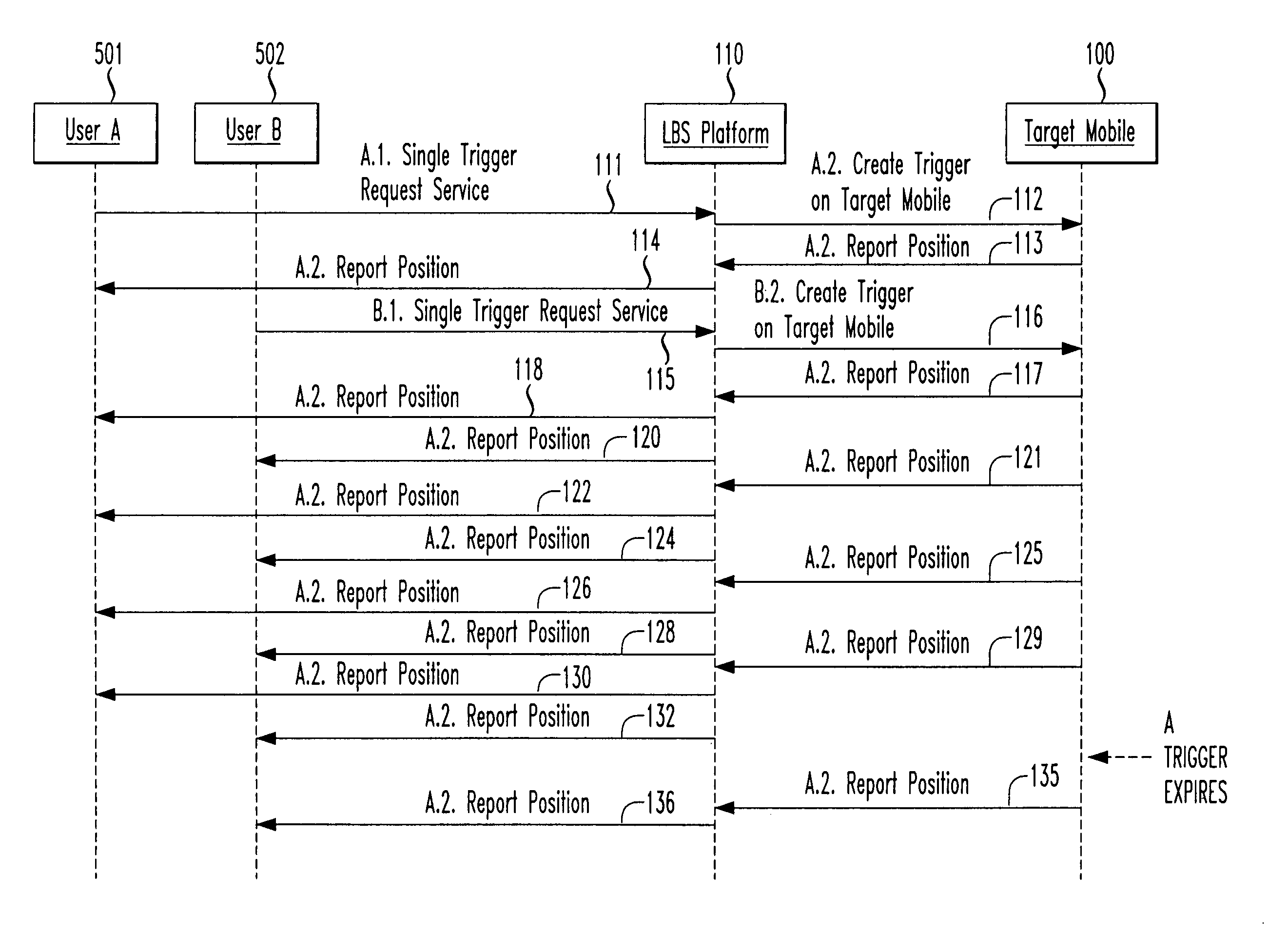
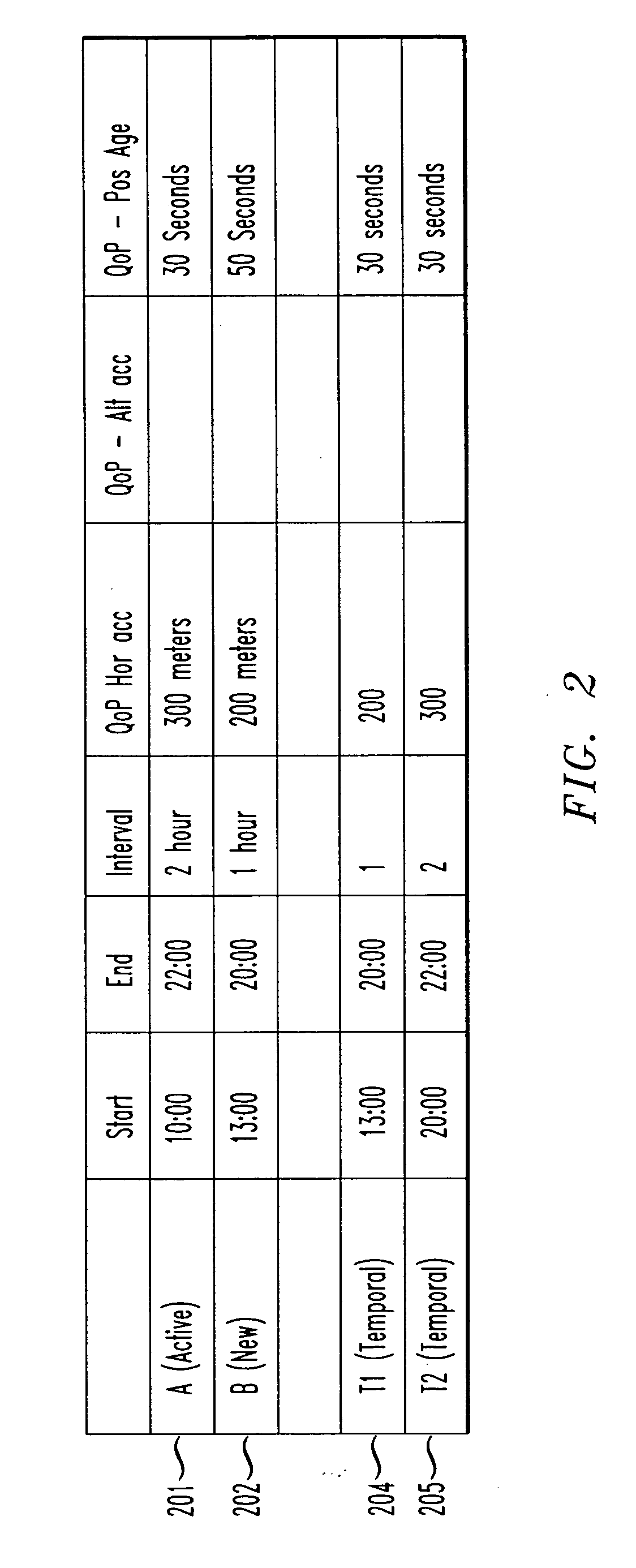
Creating optimum temporal location trigger for multiple requests
InactiveUS20070049288A1Network traffic/resource managementRadio/inductive link selection arrangementsMobile deviceTime optimal
Owner:TELECOMM SYST INC
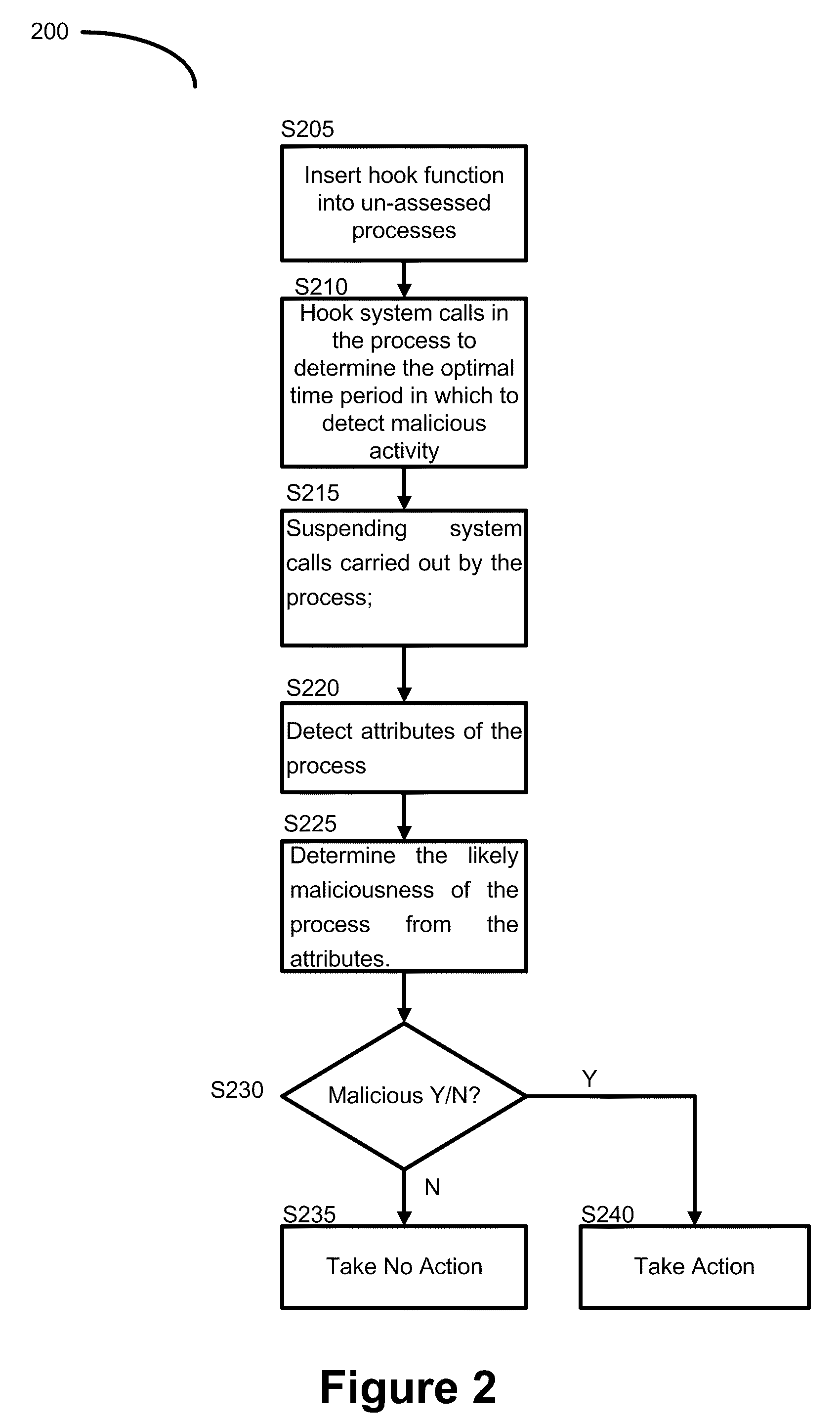
Method of detecting and blocking malicious activity
A method of detecting and blocking malicious activity of processes in computer memory during unpacking of a file after the code and data contained in the file are unpacked is described. The method includes inserting a hook function into one or more un-assessed processes running in the computer memory. A hook Is then placed on one or more system calls carried out by the one or more un-assessed processes; the one or more system calls determining an optimal time period in which to detect malicious activity in the un-assessed processes. During the optimal time period the one or more system calls carried out by the one or more un-assessed processes are suspended and attributes of the one or more un-assessed processes are detected and the likely maliciousness of the one or more un-assessed processes is determined from the attributes.
Owner:NORTONLIFELOCK INC
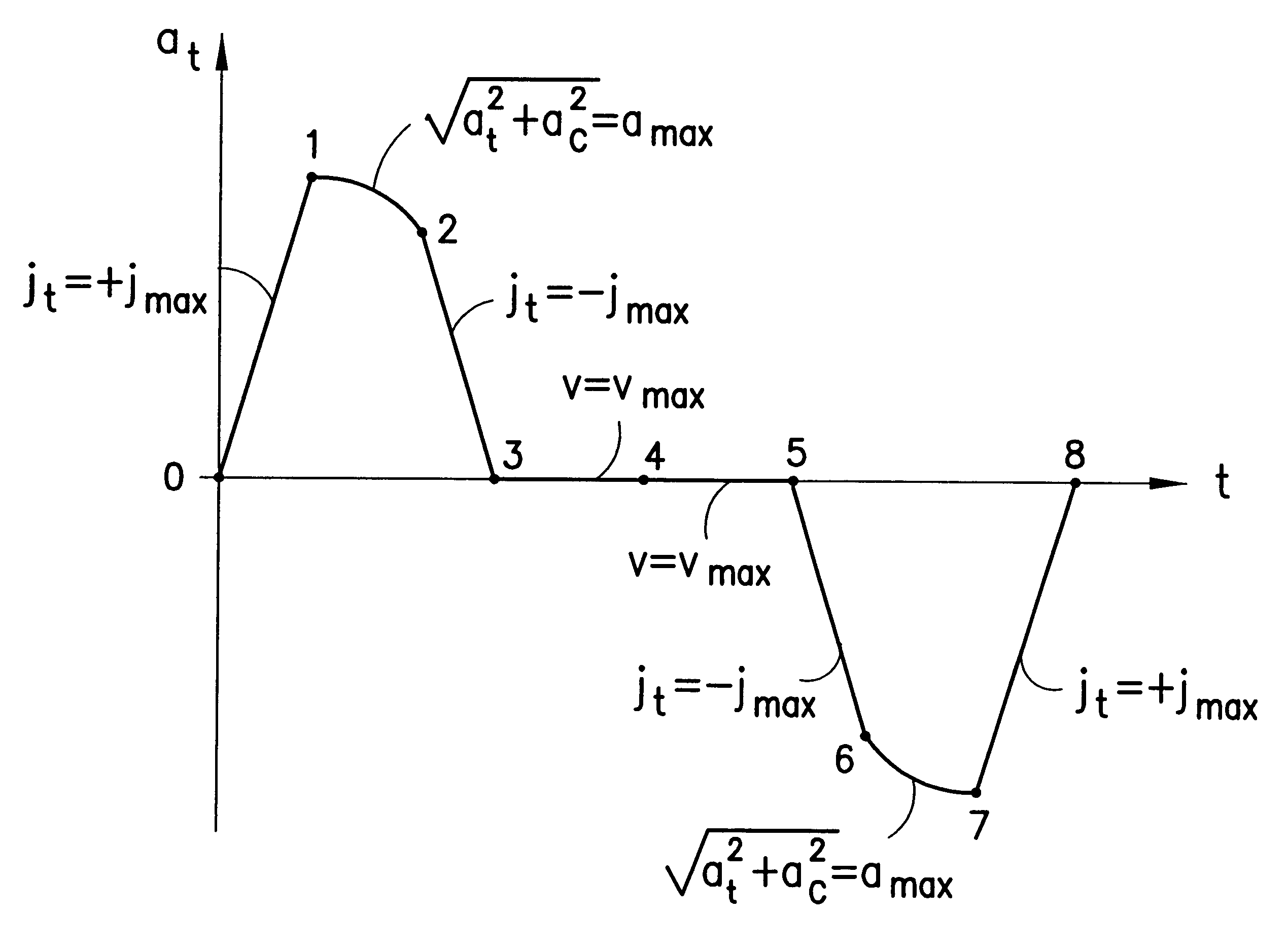
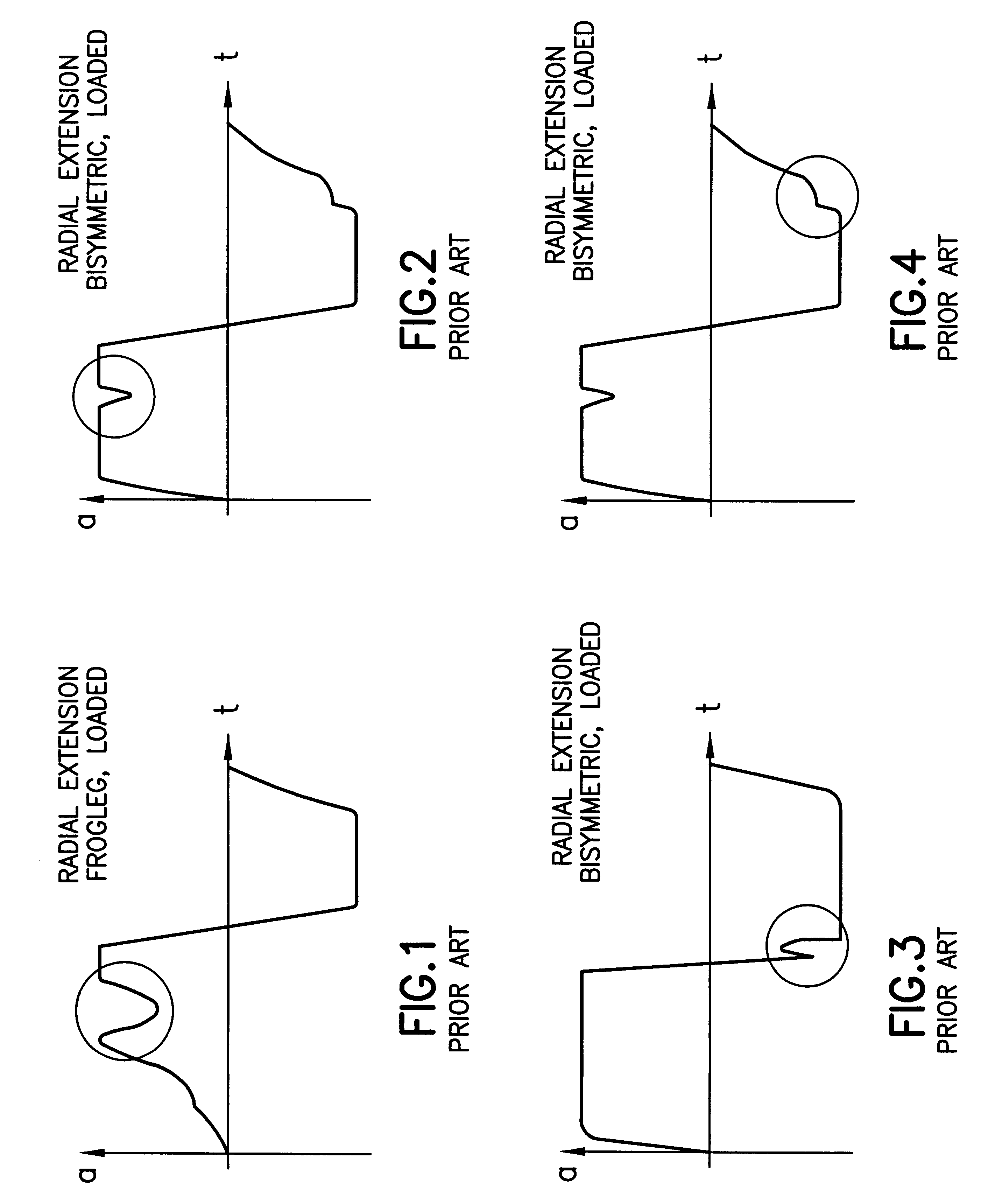
System of trajectory planning for robotic manipulators based on pre-defined time-optimum trajectory shapes
InactiveUS6216058B1Elimination of all typeShort travel timeProgramme-controlled manipulatorComputer controlEngineeringTrajectory planning
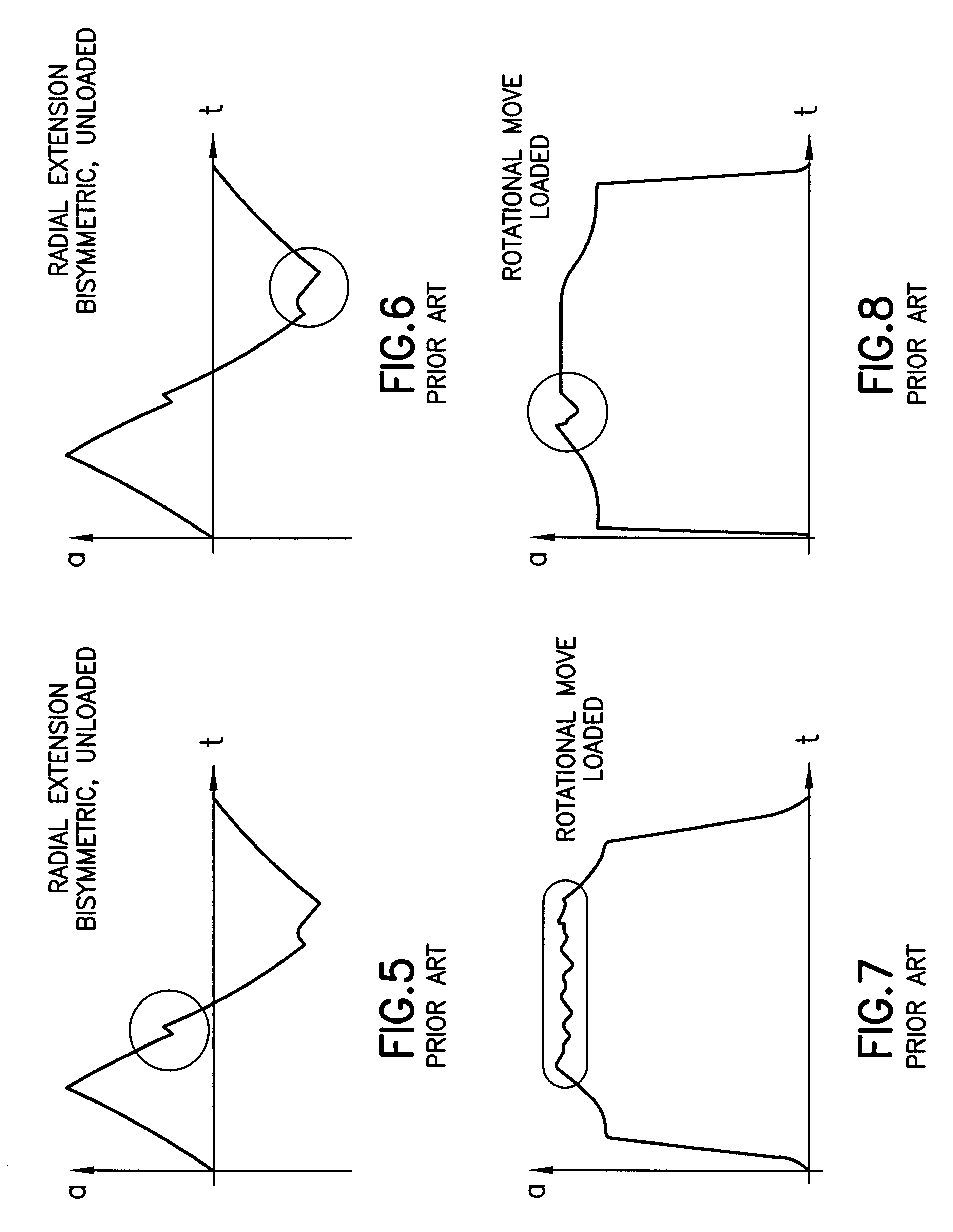
A system for providing the reliable and numerically efficient generation of time-optimum trajectories with easy-to-track or continuous acceleration profiles for simple and blended moves of single- and multi-arm robotic manipulators, such as an extension and retraction move along a straight line or a rotary move following a circular arc, with velocity, acceleration, jerk, and jerk rate constraints. A time-optimum trajectory is the set of the position, velocity, and acceleration profiles which describe the move of a selected end effector along a given path in the shortest time possible without violating given constraints, with a special case being an optimum abort trajectory, which brings the moving arm into complete rest in the shortest time. The invention involves firstly identifying the set of fundamental trajectory shapes which cover all possible combinations of constraints for a given category of moves, e.g., a move along a straight line or along a circular arc; next, decomposing the fundamental shapes into segments where a single constraint is active; and, then, determining the time optimum paths in the segments. As a result, a unique design of time-optimum trajectories is produced based on a set of pre-defined trajectory shapes. The invention also involves the blending of simple moves into a single trajectory by decomposing trajectories of the individual moves into their orthogonal components and overlapping them for a given time interval, which results in a non-stop move along a smooth transfer path.
Owner:BOOKS AUTOMATION US LLC
Four-dimensional (4D) image verification in respiratory gated radiation therapy
ActiveUS20080031404A1X-ray/infra-red processesMaterial analysis using wave/particle radiationFluoroscopic image4D Computed Tomography
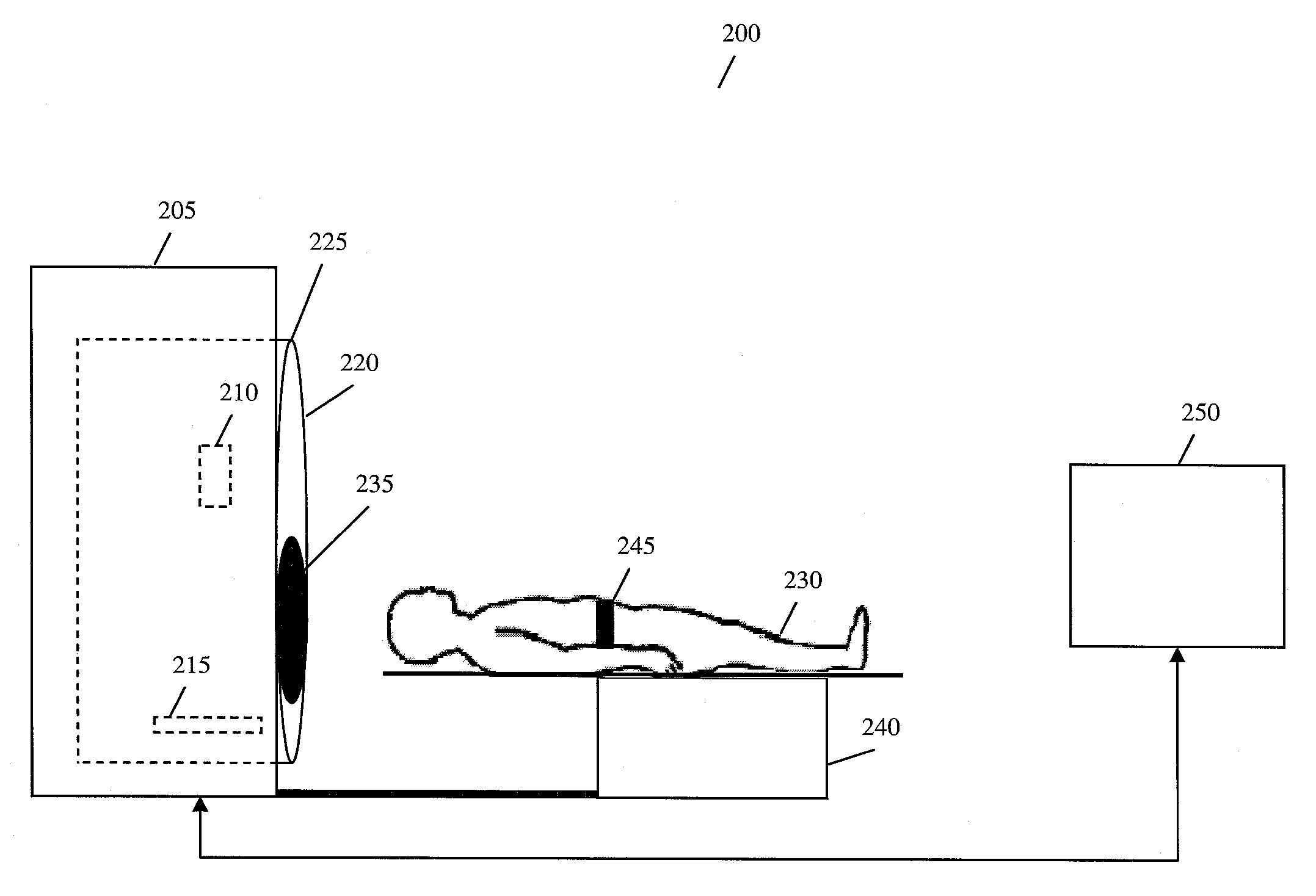
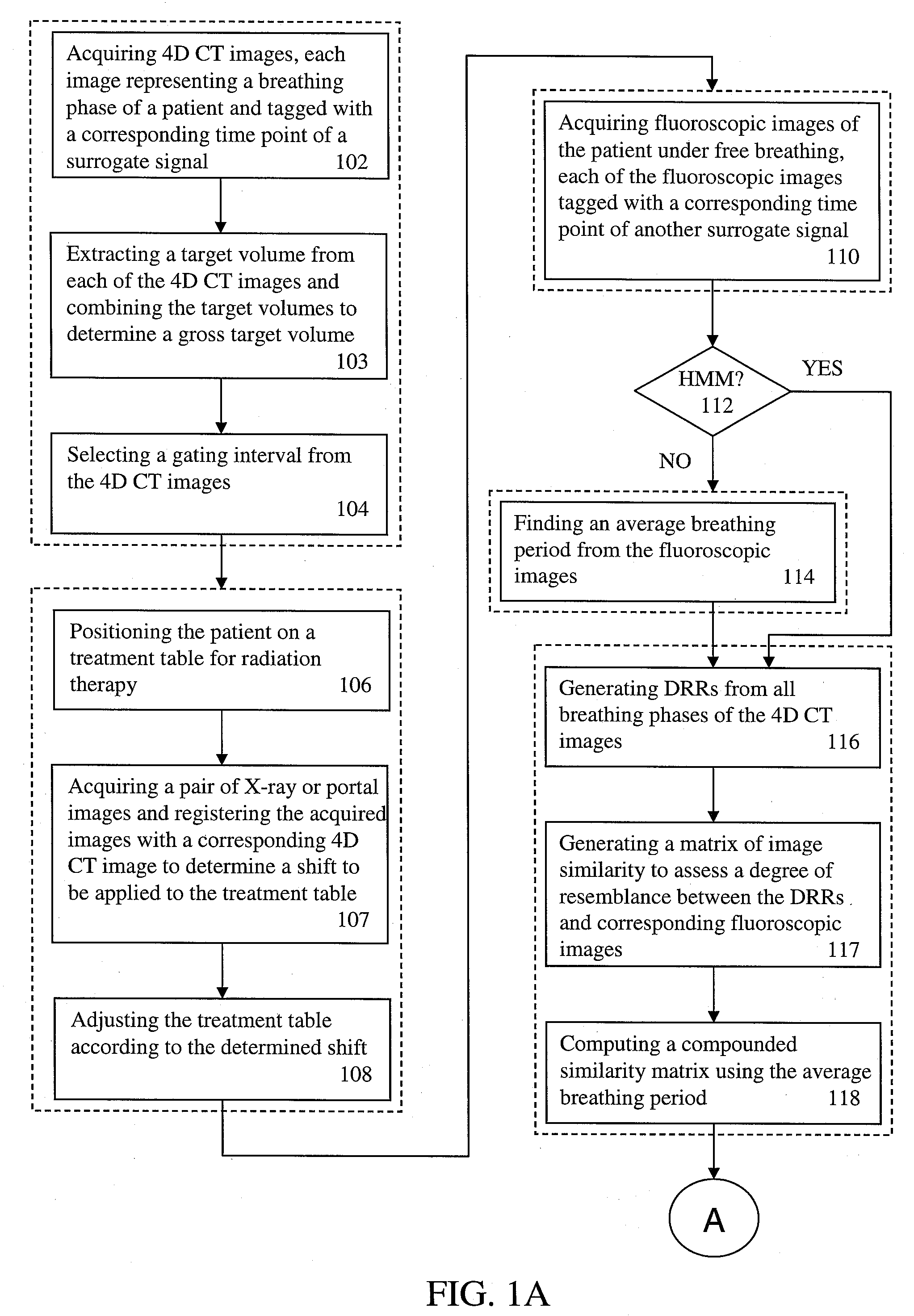
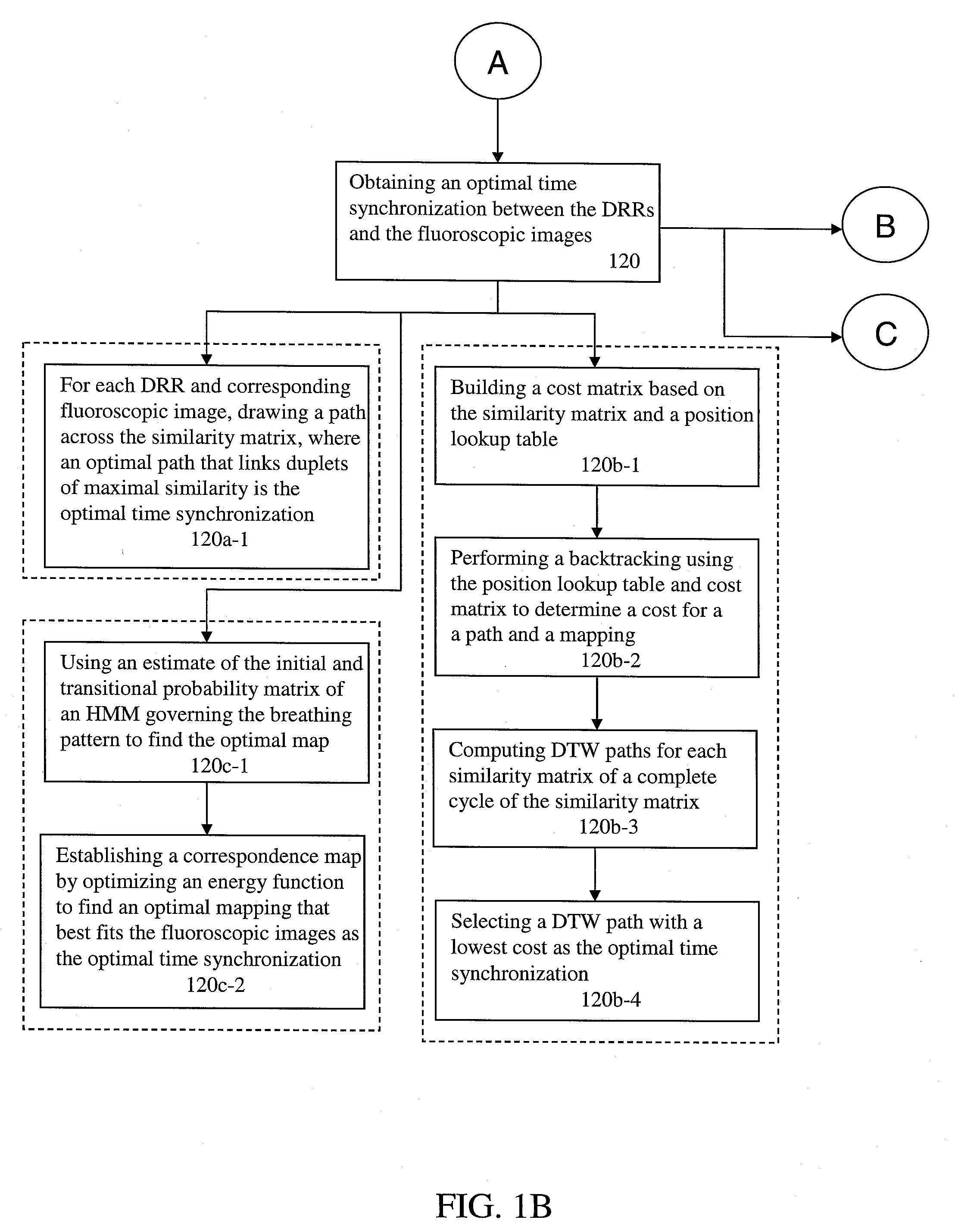
A method for four-dimensional (4D) image verification in respiratory gated radiation therapy, includes: acquiring 4D computed tomography (CT) images, each of the 4D CT images representing a breathing phase of a patient and tagged with a corresponding time point of a first surrogate signal; acquiring fluoroscopic images of the patient under free breathing, each of the fluoroscopic images tagged with a corresponding time point of a second surrogate signal; generating digitally reconstructed radiographs (DRRs) for each breathing phase represented by the 4D CT images; generating a similarity matrix to assess a degree of resemblance in a region of interest between the DRRs and the fluoroscopic images; computing a compounded similarity matrix by averaging values of the similarity matrix across different time points of the breathing phase during a breathing period of the patient; determining an optimal time point synchronization between the DRRs and the fluoroscopic images by using the compounded similarity matrix; and acquiring a third surrogate signal and turning a treatment beam on or off according to the optimal time point synchronization.
Owner:SIEMENS HEALTHCARE GMBH +1
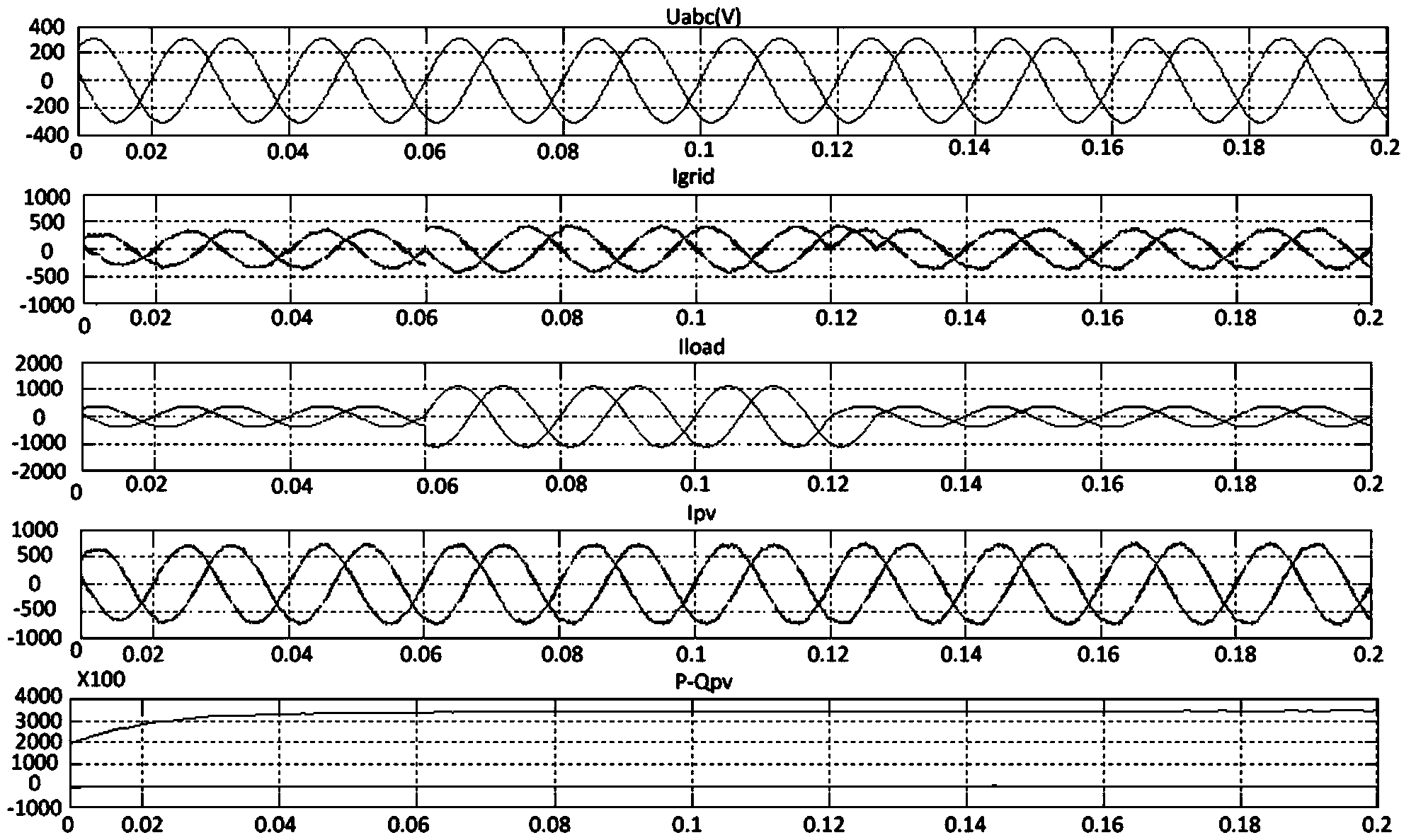
Real-time coordination and control method of photovoltaic micro-grid system
ActiveCN104242337AEfficient and economical operationAchieve Power Optimum MatchingSingle network parallel feeding arrangementsEnergy storageCapacitanceControl layer
The invention relates to the technical field of photovoltaic micro-grid control, and particularly discloses a real-time coordination and control method of a photovoltaic micro-grid system. A central real-time optimal control layer and a local control layer are involved in the real-time coordination and control method. The central real-time optimal control layer collects the voltage and frequency of an AC bus of the photovoltaic micro-grid system, the charge state of a storage battery pack, the output power of photovoltaic power generation micro-sources, the output power of the storage battery pack, the output power of a super capacitor and the real-time power of a load in real time, generates a real-time coordination and control strategy and a secondary frequency modulation and scheduling plan, and issues the strategy and the plan to all local controllers. The local control layer coordinates and controls all the micro-sources in real time, and particularly achieves the scheduling plan issued by the central real-time optimal control layer. According to the real-time coordination and control method, a micro-grid central optimizing controller can manage, coordinate and control all the local controllers in a unified mode, the micro-grid can efficiently and economically run, the output power of the photovoltaic power generation unit is utilized to the maximum extent, the output power fluctuation and the tracking load change of the photovoltaic power generation unit are restrained, the running method of the micro-grid is reasonably adjusted, and energy balance is achieved.
Owner:EAST GRP CO LTD
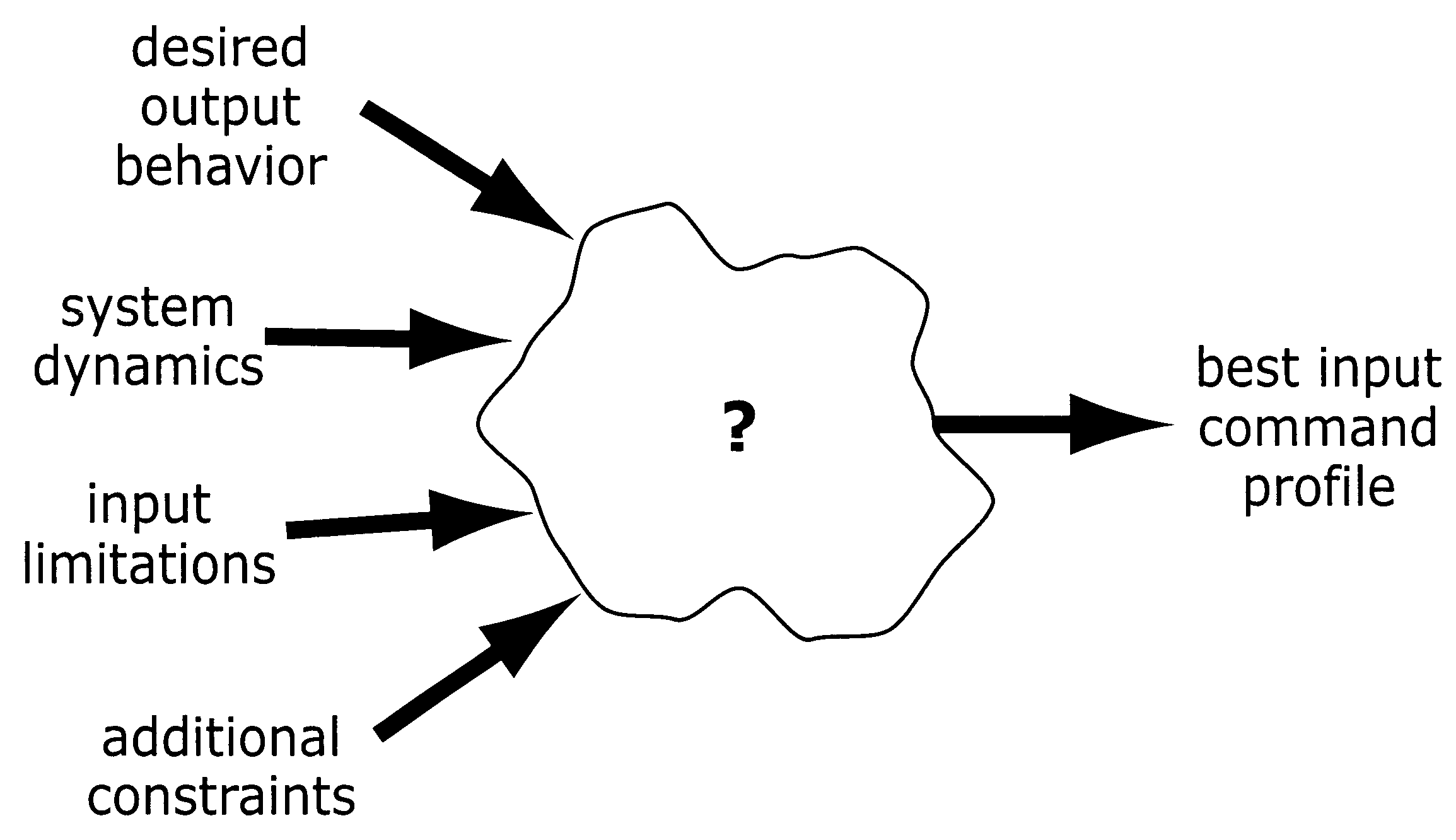
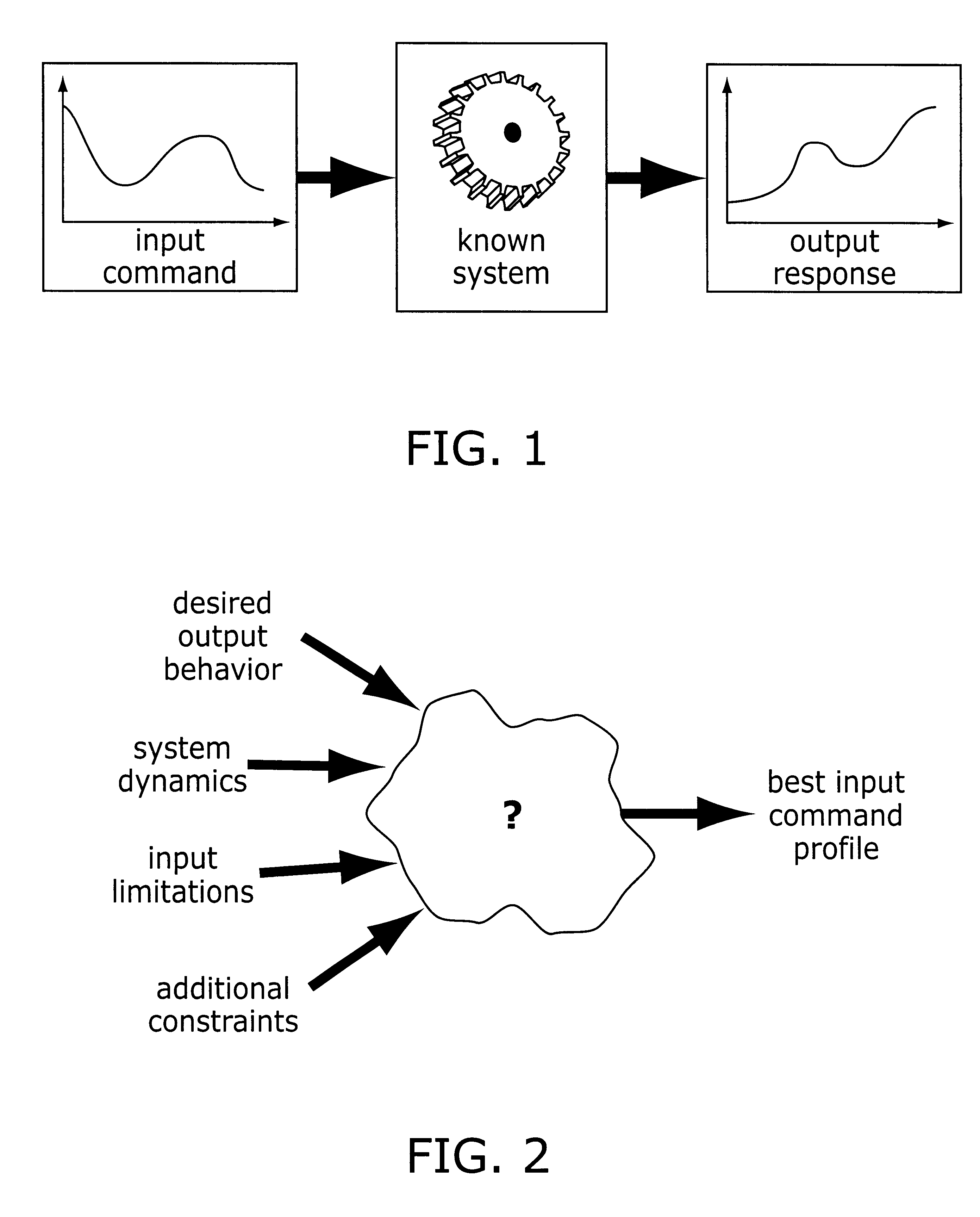
Method and apparatus for creating time-optimal commands for linear systems
The system described herein determines an input command profile for a dynamic system that can be modeled as a linear system, the input command profile for transitioning an output of the dynamic system from one point to another point. The system identifies characteristics of the dynamic system, and then selects a command profile which defines an input to the dynamic system based on the identified characteristics. The command profile comprises one or more pulses which rise and fall at switch times, and the command profile is useable with substantially any dynamic system that can be modeled as a linear system. The system then imposes a plurality of constraints on the dynamic system, at least one of the constraints being defined in terms of the switch times, and determines the switch times for the input to the dynamic system based on the command profile and the plurality of constraints.
Owner:MASSACHUSETTS INST OF TECH
System and method for predictive idle-time task initiation
InactiveUS20060190938A1Reduce the burden onAvoid distractionError detection/correctionMultiprogramming arrangementsIdle timeTime optimal
A system automatically determines probable idle times for a computing system and performs maintenance tasks, such as virus scanning, during these times. A prediction of probable idle times is based on an assessment of a user's past use or by an aggregate of information from several users if a company wishes to determine optimal times for running such tasks or pushing software patches to employees. A policy table set by the user or a company determines the priority of maintenance tasks to be run during the predicted idle time.
Owner:LENOVO (SINGAPORE) PTE LTD
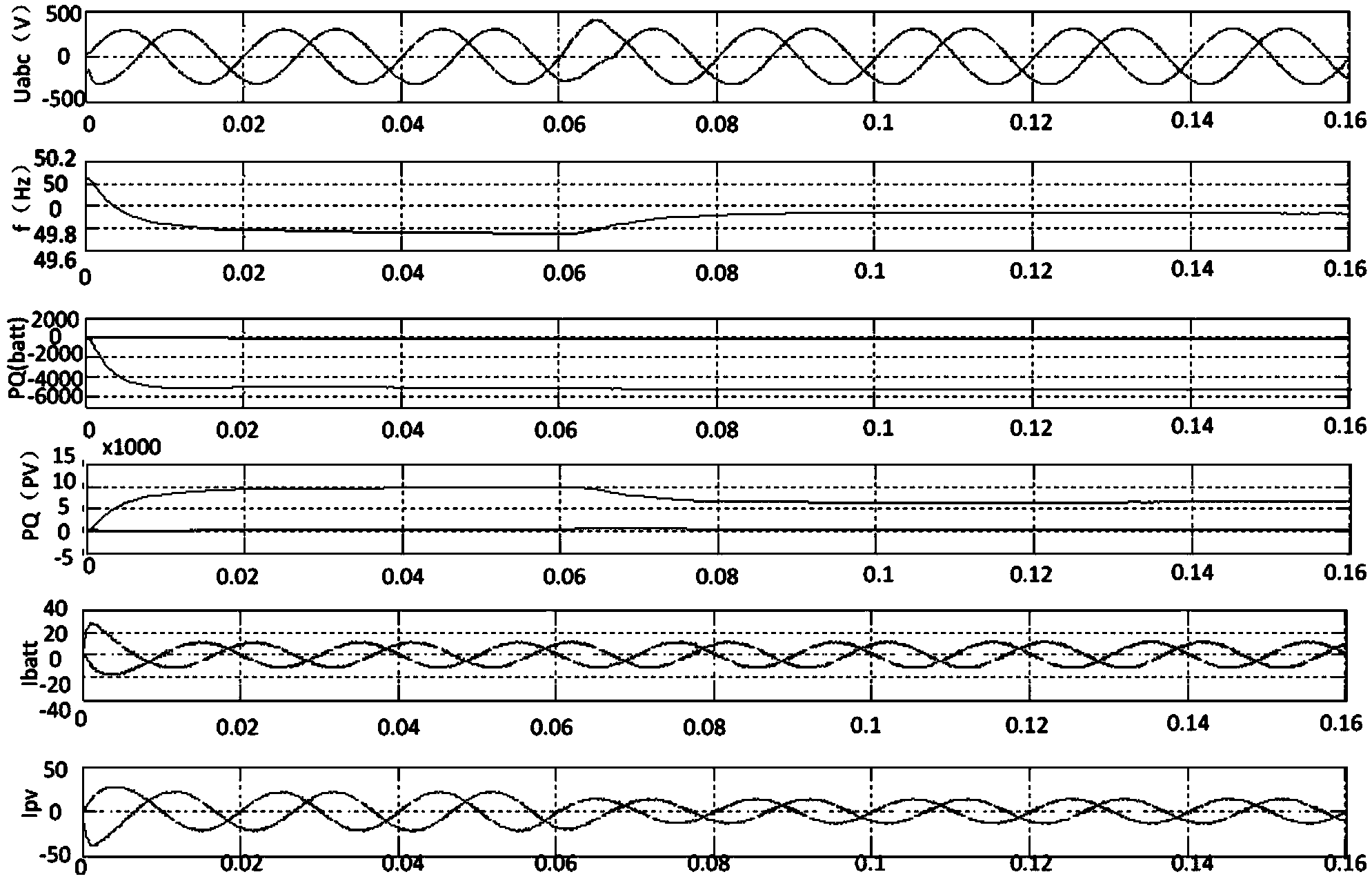
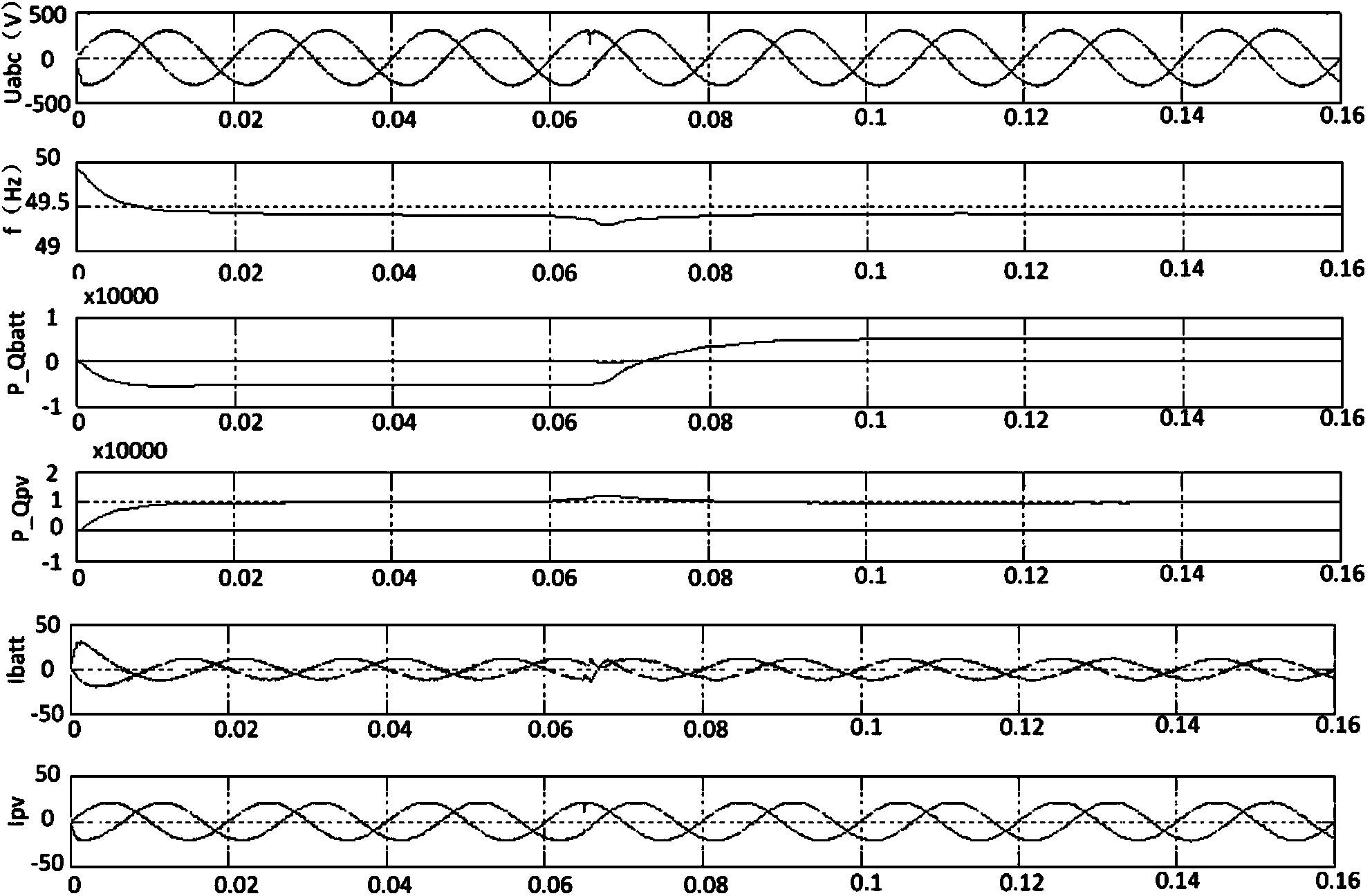
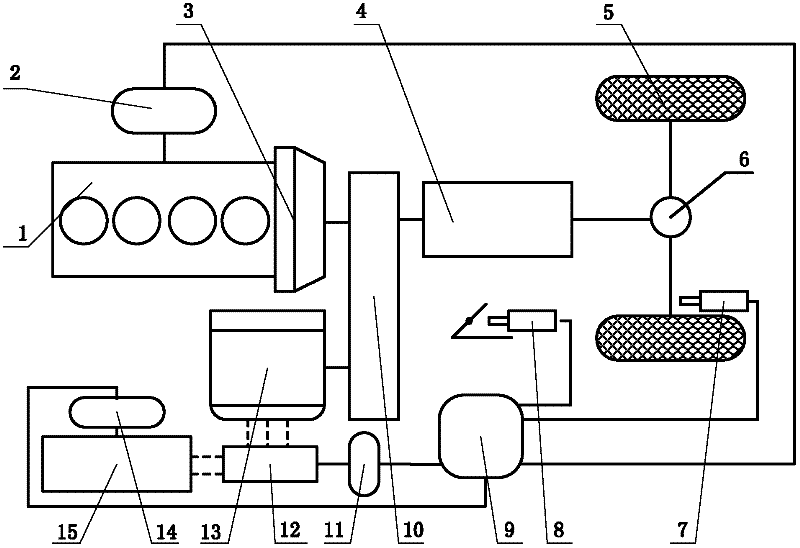
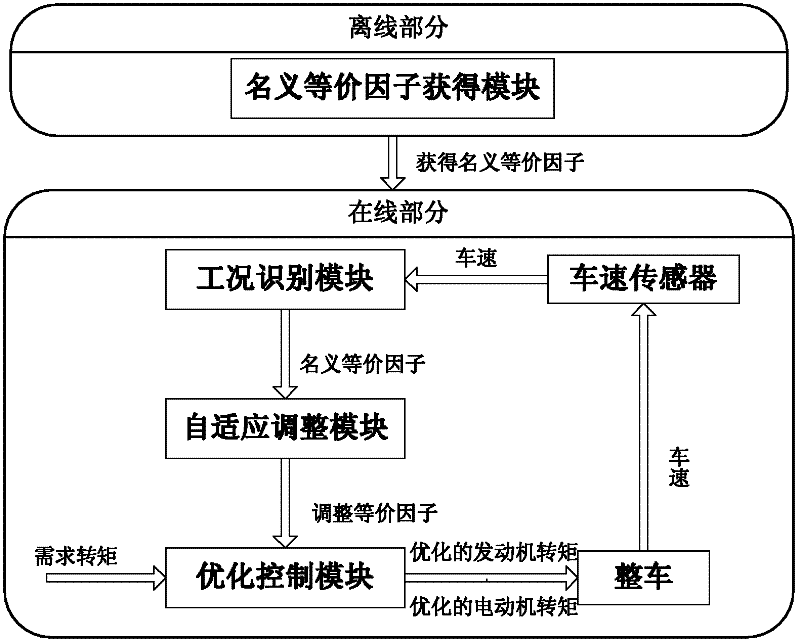
Minimum equivalent fuel consumption-based hybrid electrical vehicle control method
InactiveCN102416950AGuaranteed power balanceGuaranteed lifeHybrid vehiclesHybrid electrical vehicleTime control
The invention discloses a minimum equivalent fuel consumption-based hybrid electrical vehicle control method, which comprises the following steps of: acquiring a nominal equivalence factor offline; acquiring signals; identifying working conditions; performing adaptive regulation; and performing optimum control. Under the condition of meeting the requirement of dynamic property, the minimum equivalent fuel consumption-based hybrid electrical vehicle control method is adopted, adaptive regulation can be performed according to an actual working condition, and the electric quantity balance of a storage battery is ensured, so that the performance and service life of the storage battery are ensured. The nominal equivalence factor is acquired through simulation calculation in the offline state, and calculated quantity for real-time control of the whole vehicle is reduced. In addition, different from a global optimal control method in which a future vehicle running working condition is required to be known (the future vehicle running working condition is unpredictable actually), the method has high implementability; and by the method, the real-time optimal energy management decision can be provided, the fuel economy of the whole vehicle is further improved, and the emission is reduced.
Owner:DALIAN UNIV OF TECH
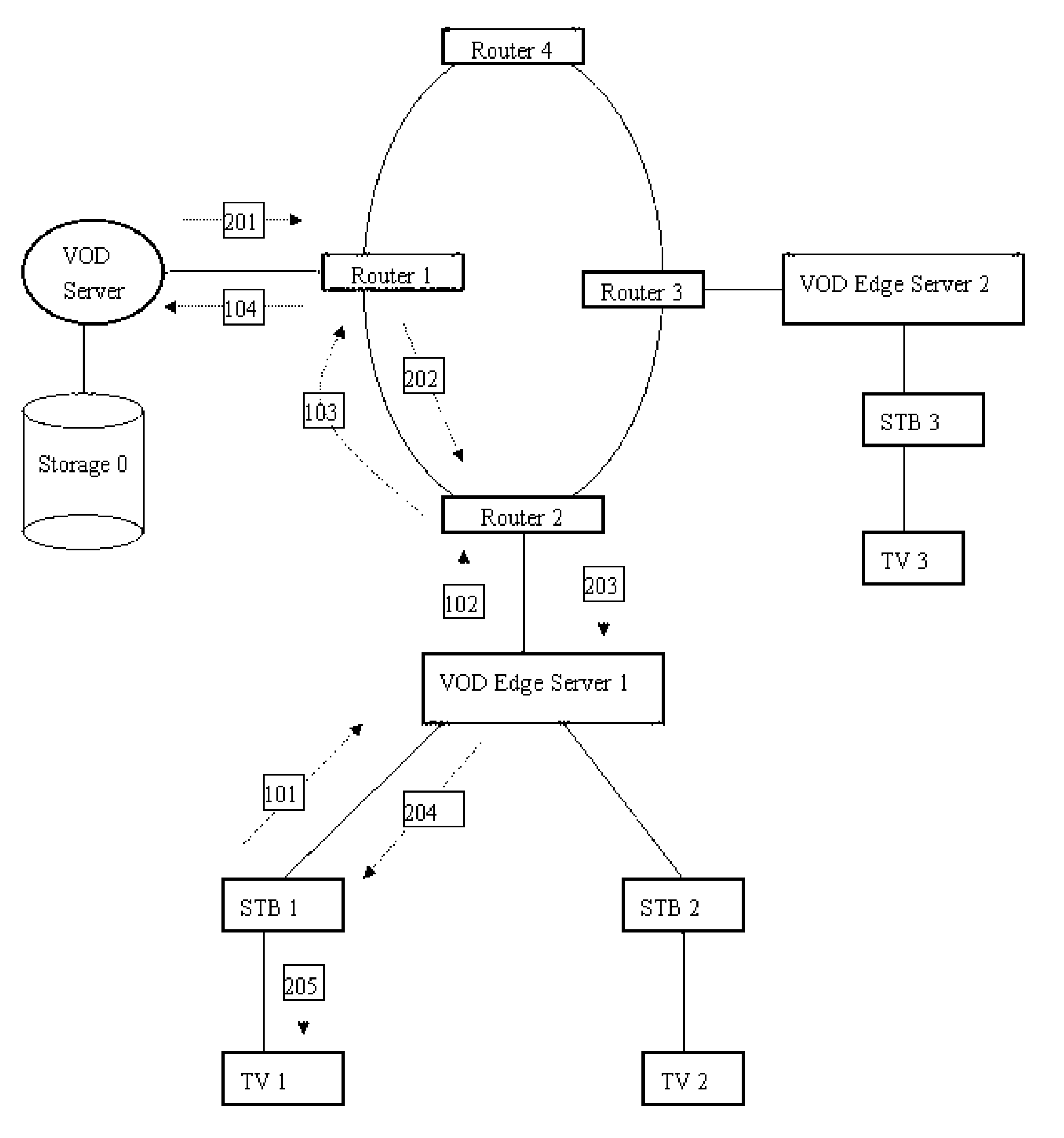
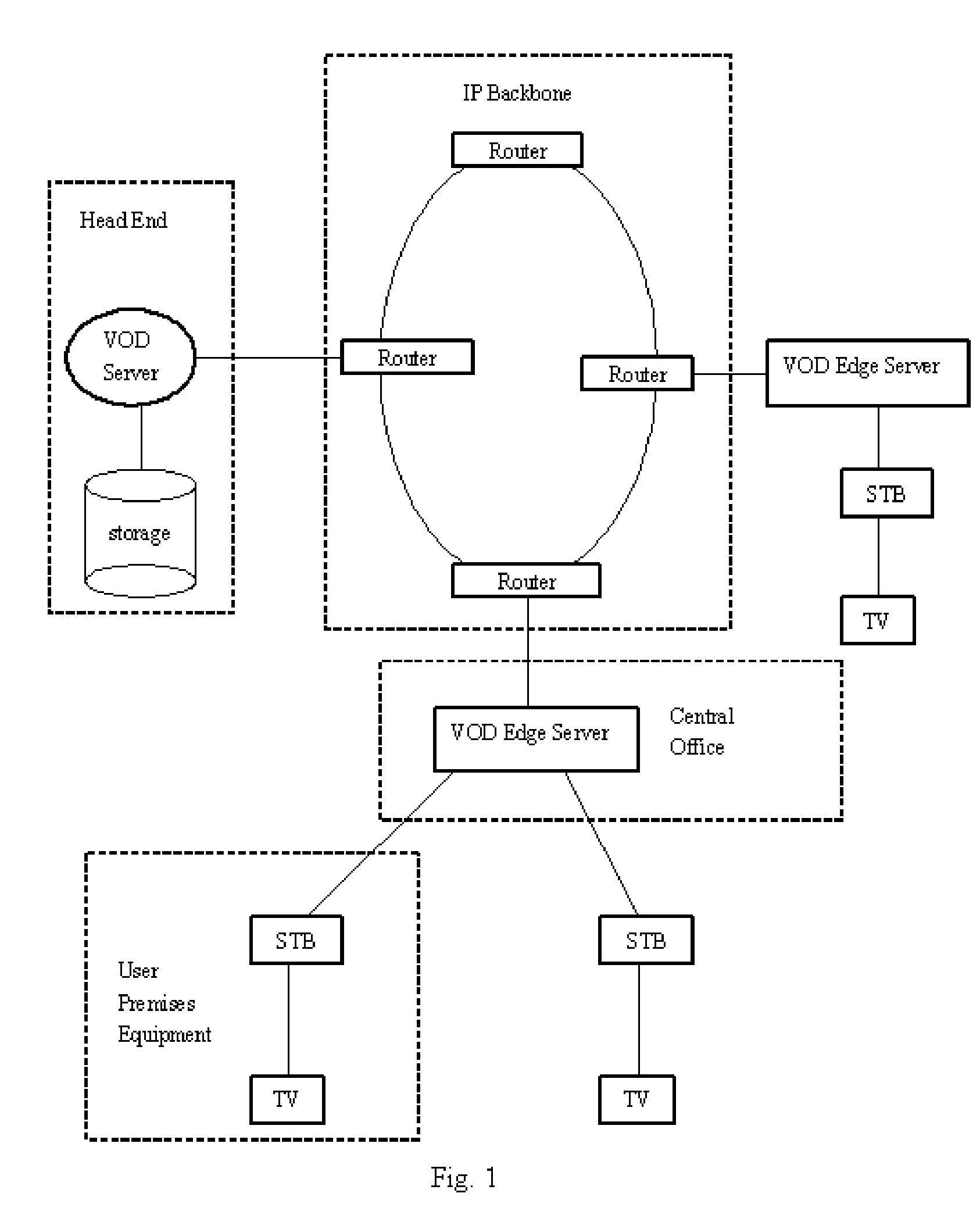
Method and system for video on demand (VOD) servers to cache content
A method and system of enabling VOD systems efficiently caching contents (video programs) on its edge servers or user premises equipment. A new user interface and a new VOD system component are introduced. The new user interface allows viewers to specify not only the name of the video programs, but also the time and date they want to start watching them. The new VOD system component is used to receive and store video requests from users and calculate when is the best time to start caching the video programs requested to edge servers or user premises equipment based on a set of criteria, such as user input from the new user interface, network traffic condition pattern during a day, etc.
Owner:XIE JIANHUA
Multi-robot distributed task assignment formation method facing dynamic task
ActiveCN106875090ADynamic task solvingSolve the problem of dynamic task assignmentForecastingResourcesSimulationTime distribution
The invention provides a multi-robot distributed task assignment formation method facing a dynamic task. The method comprises steps that the factors and difficulties that need to be considered in the task assignment are analyzed according to the state change of the task point in the environment map; when the task point occurs, the task assignment scheme is generated based on the multi-stage auction algorithm; and the robot performs the task according to the task assignment scheme. The method solves the problem of dynamic task assignment in the environment. The traditional auction algorithm adopts a one-time distribution to solve the given task, and there are great limitations in the face of dynamic tasks. The invention uses the resources of the robot to simulate the method on the VC ++ and Csharp platforms through a plurality of auctions and with the time optimal as the purpose. Through a large number of experimental simulation results, the improved auction algorithm is more effective than the traditional auction algorithm and can solve the dynamic task in the environment well, can meet the real-time demand through multiple assignments, and can give the approximately optimal solution.
Owner:CENT SOUTH UNIV
Resource optimization using environmental and condition-based monitoring
InactiveUS20130184838A1Increase in sizeIncreased susceptibilityWind motor controlMachines/enginesProbit modelAnalysis data
In a method for dynamically optimizing resource utilization in a system over time according to one or more objectives, data including information indicative of current environmental conditions, upcoming environmental conditions, a current state of a system configuration, and current system operating conditions is dynamically updated. Automatic analysis of the data using a probabilistic model based on conditional relationships is performed periodically. For each periodically generated set of possible system control actions, a probabilistic model is used to automatically analyze each possible system control action and an optimal system control action is selected based on a set of current utility functions. For each periodically generated set of possible system control actions, control of the system according to the optimal system control action selected from the possible system control actions. Resource optimization couples condition-based and environmental monitoring with automated reasoning and decision making technologies, to develop real time optimal control and decision strategies.
Owner:MICHIGAN AEROSPACE
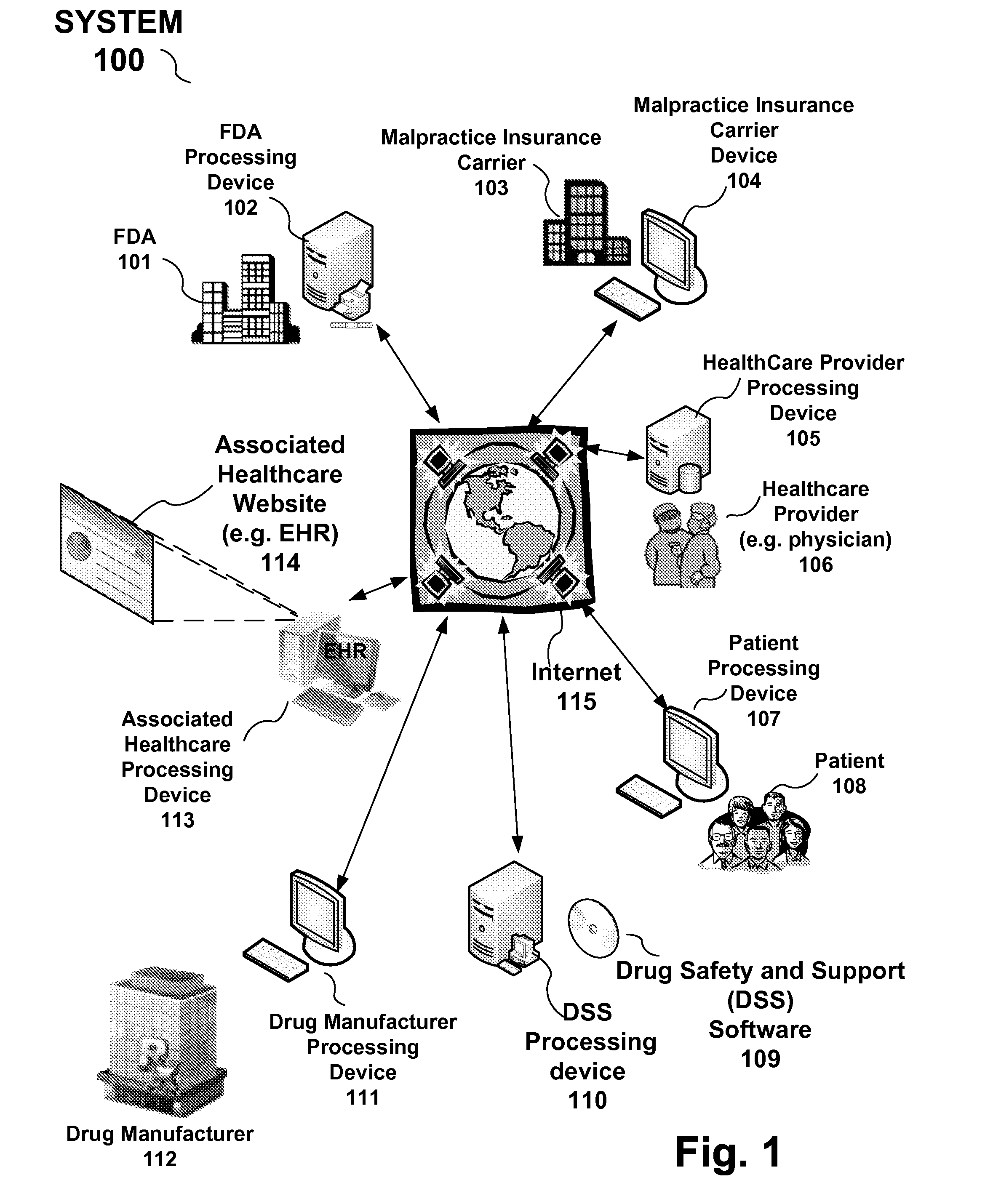
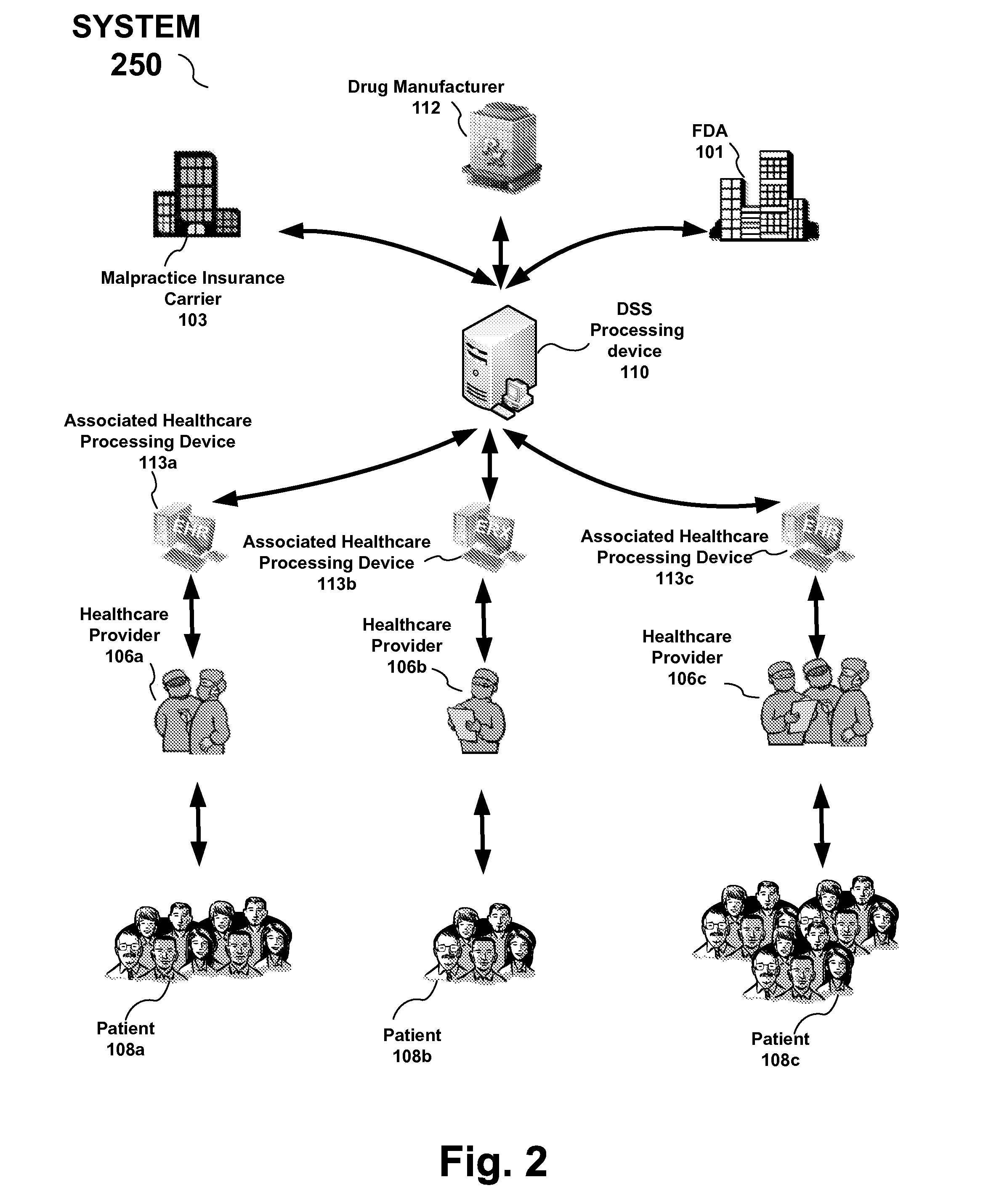
Drug and medical device safety and support information reporting system, processing device and method
A reporting system, including a processing device, and method provides drug and medical device safety and support information during a workflow of a healthcare provider. The system and method electronically acquires, maps, generates, compiles, verifies and transfers in real time critical information regarding particular drugs and medical devices required by healthcare providers, such as a prescriber, at the optimum time in which the healthcare provider needs the information. In an embodiment, the drug and medical device safety information is accessed from an associated healthcare website, such as an Electronic Health Record (EHR) web site, during a prescription stage in the healthcare providers workflow for a particular patient. The system and method also includes an adverse reporting system that allows for the drug and medical device safety information to be updated and accurately reported in an efficient and up to date manner. In an embodiment, a healthcare provider reports an adverse event or reaction to a drug by a patient during a workflow at a EHR web site. The adverse event information is forwarded to the Federal Drug Administration (FDA) and the particular drug manufacturer which may update the corresponding drug information. The updated drug information may then be reported by the system to insure that healthcare providers receive the most current, accurate and complete safety information during a workflow. In embodiments, the updated drug and medical information may be provided to malpractice insurance carriers to further ensure safety.
Owner:PDR NETWORK
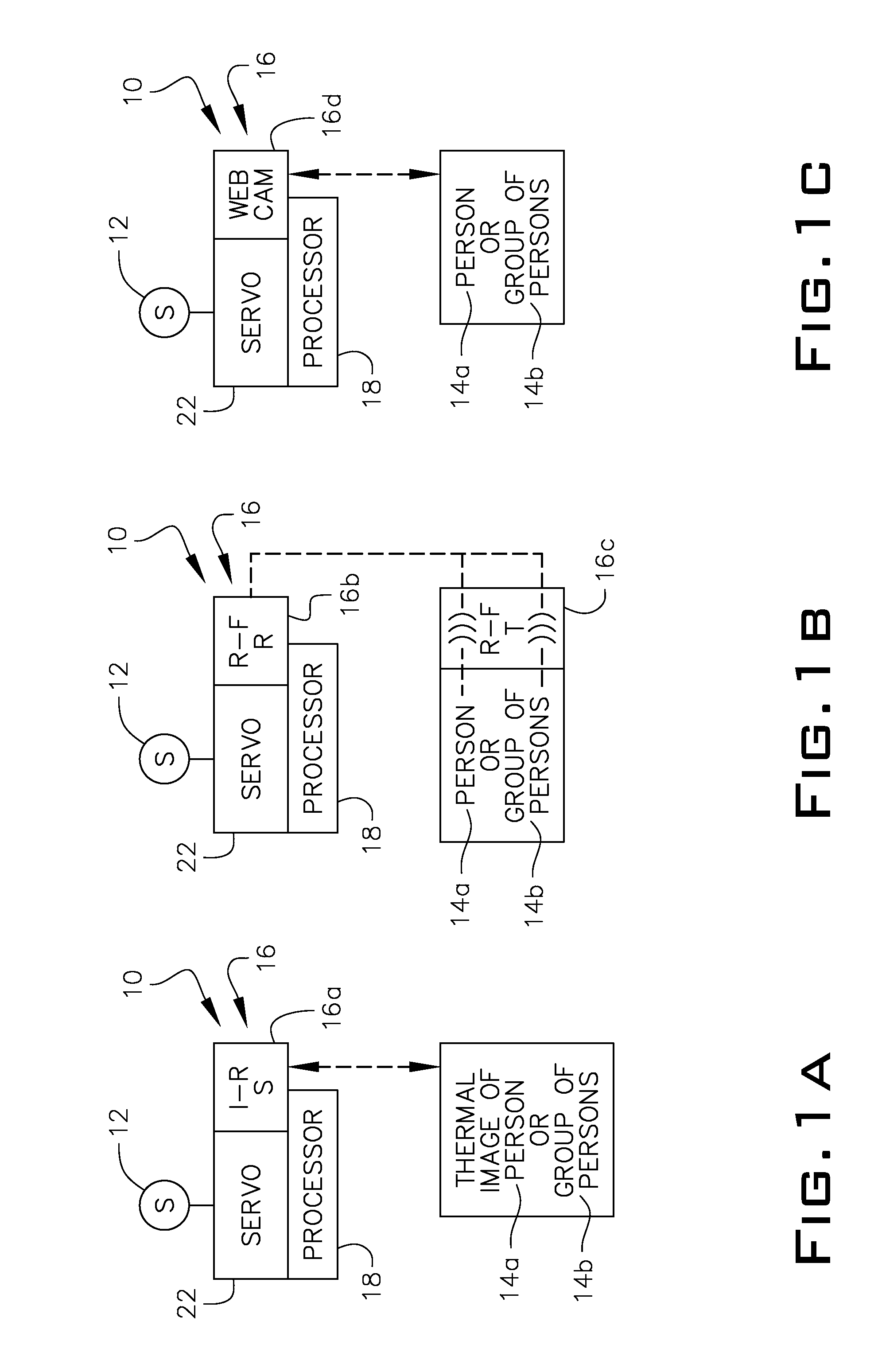
System and method for providing focused directional sound in an audio system
InactiveUS20120027226A1Rich soundManually-operated gain controlTelevision conference systemsEngineeringRadio frequency
A system and method for providing focused directional sound in an audio system where the system effectively uses regular speakers on servo stands with satellite speakers adapted to rotate on two axes. The system allows for the tracking of the speakers to focus the sound toward a person or persons as they move within the room, thereby providing for a real-time optimal sound in relation to the movement of the persons. Sensors for tracking and activating the servos can be done using infrared technology, facial recognition technology or radio frequency technology.
Owner:DESENBERG MILFORD
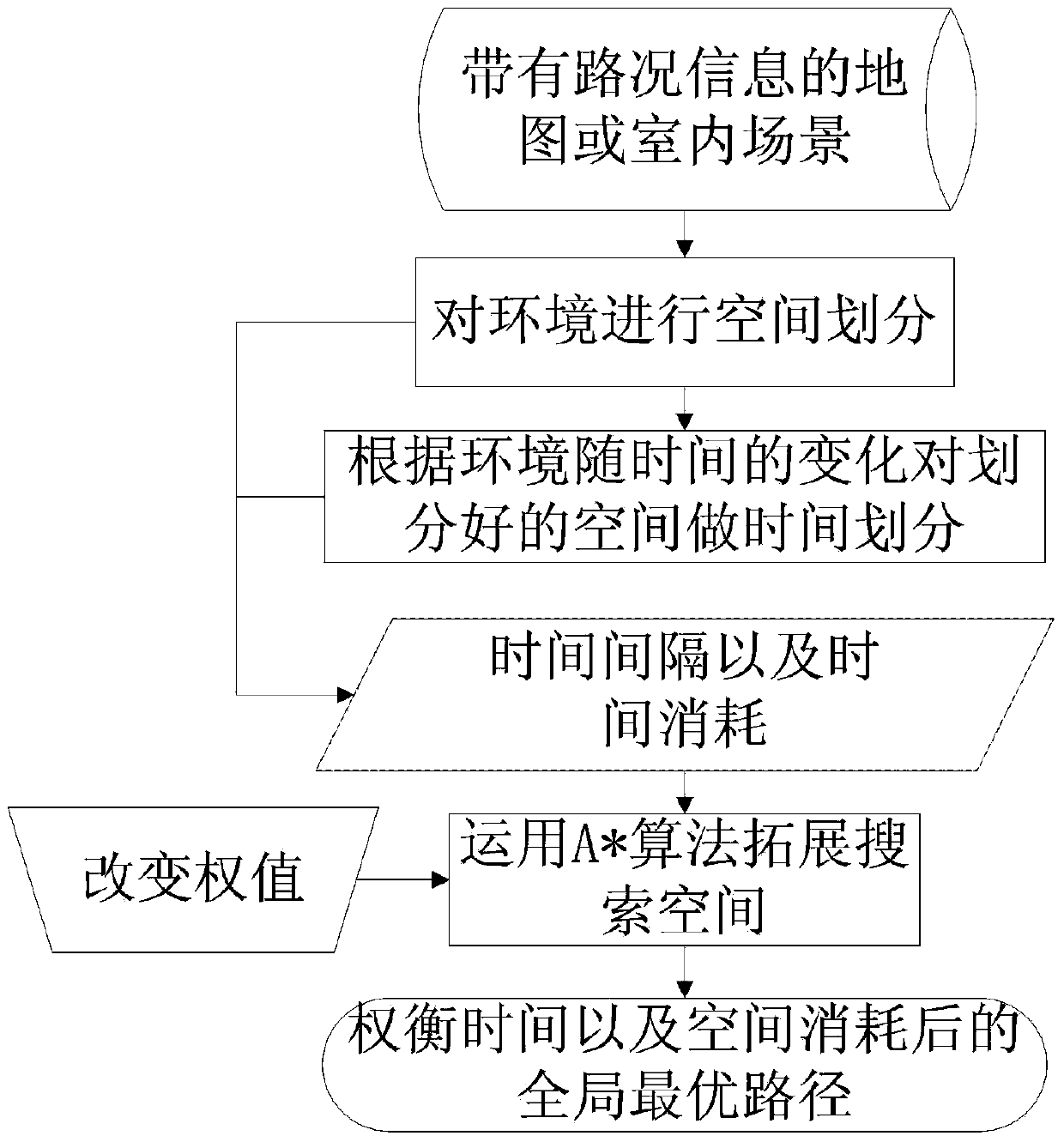
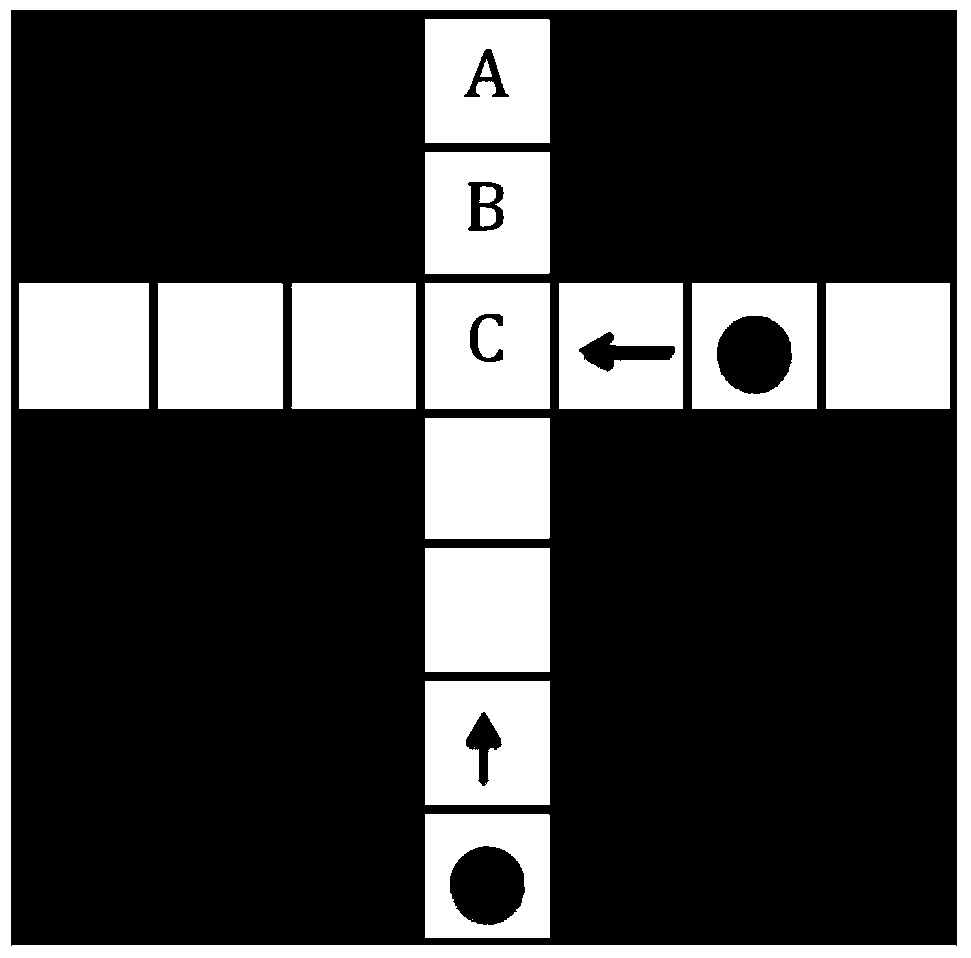
Method for seeking for overall situation time optimal path under dynamic time varying environment
ActiveCN103994768AMeets requirementsSolve the blocking problemInstruments for road network navigationNavigational calculation instrumentsOptimum routeTime cost
The invention discloses a method for seeking for an overall situation time optimal path under a dynamic time varying environment. The method comprises the following steps: extracting environment state information; molding the space of the environment; carrying out time division on the environment according to the environment state information, namely dividing a time axis into a plurality of time intervals and utilizing time consumption to represent an environment state of each time interval; utilizing an improved A* algorithm to search the environment state to obtain the overall situation time optimal path; introducing weight to realize a multi-scale path planning aim. The method can be used for realizing the aims of obstacle avoidance of indoor robots and path optimization of the complicated time varying environment of outdoor road traffic; the weighing can be carried out between time cost and distance cost according to different requirements of users to formulate the optimal path and accurately calculate the time spent for the route.
Owner:BEIJING JIAOTONG UNIV
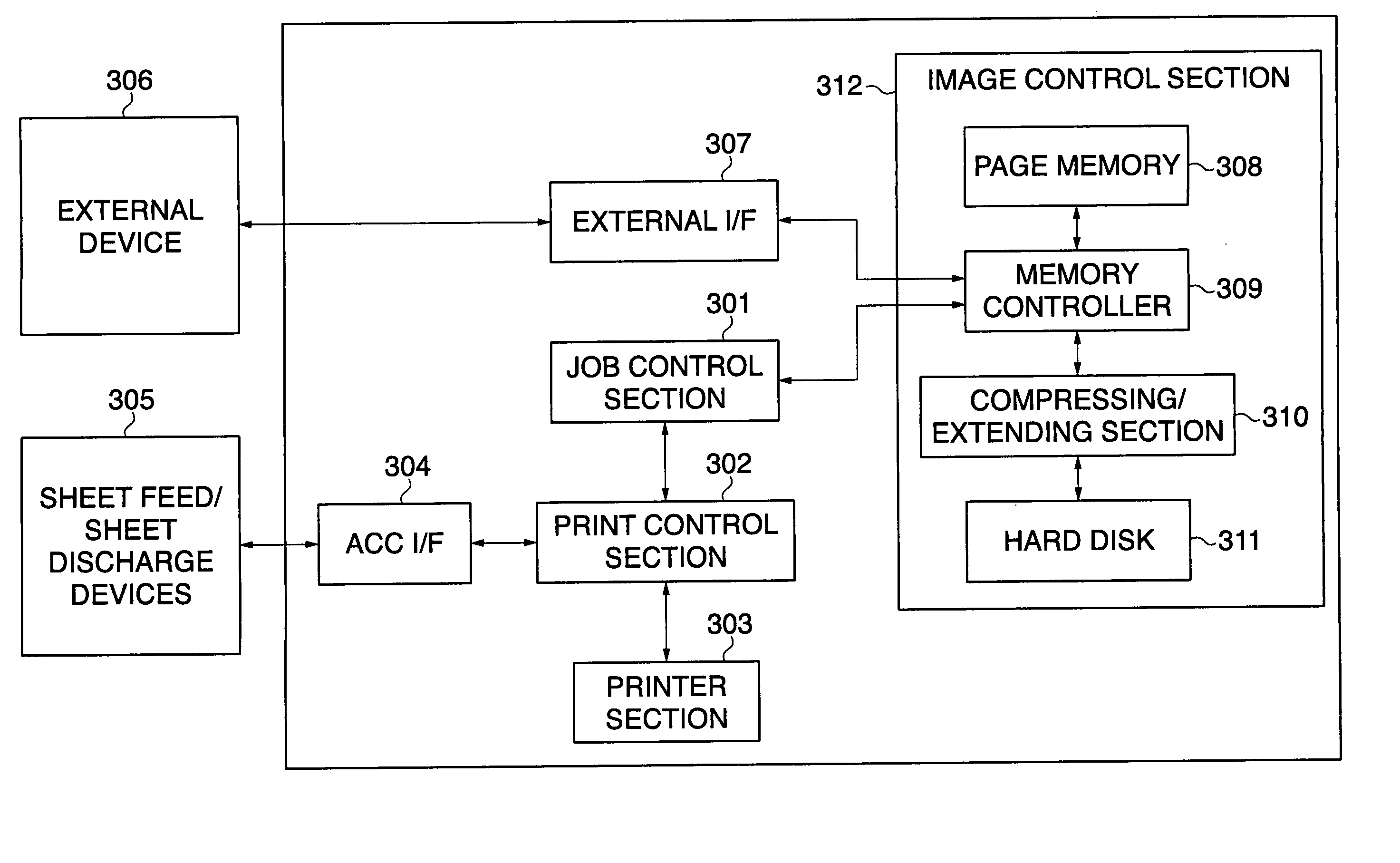
Image forming apparatus, control method therefor, and program for implementing the control method
InactiveUS20060023245A1Improve operating rateIncrease productivityEnergy efficient ICTDigital data processing detailsProduction rateImage formation
An image forming apparatus which is capable of bringing processing devices, such as sheet feed devices and post-processing devices connected respectively to the image forming apparatus, into operable states in timing optimal for carrying out an image forming job, thereby improving productivity and operation rate of the image forming apparatus as well as saving energy. A print job is analyzed to determine at least one sheet feed device and at least one post-processing device to be used in executing the print job, and states of the determined devices are checked to determine at least one of the sheet feed device and the post-processing device to be caused to start a preparatory operation in advance before the print job is executed. Power supply to the at least one of the sheet feed device and the post-processing device is turned on in timing such that the preparatory operation is completed in time for the start of execution of the print job.
Owner:CANON KK
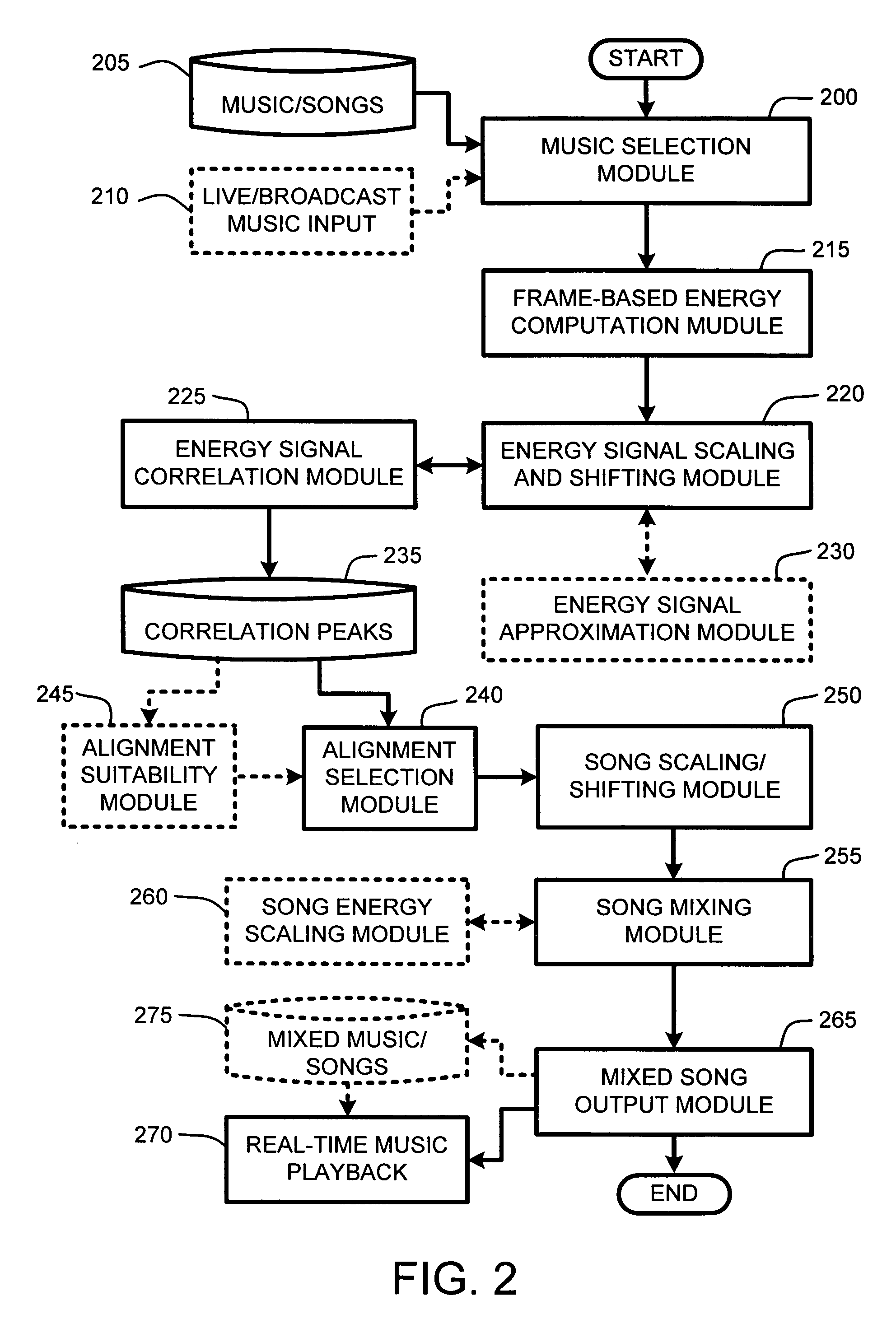
System and method for aligning and mixing songs of arbitrary genres
InactiveUS20060000344A1Reduce computational overheadIncrease rangeElectrophonic musical instrumentsFrame basedSound mixer
Owner:MICROSOFT TECH LICENSING LLC
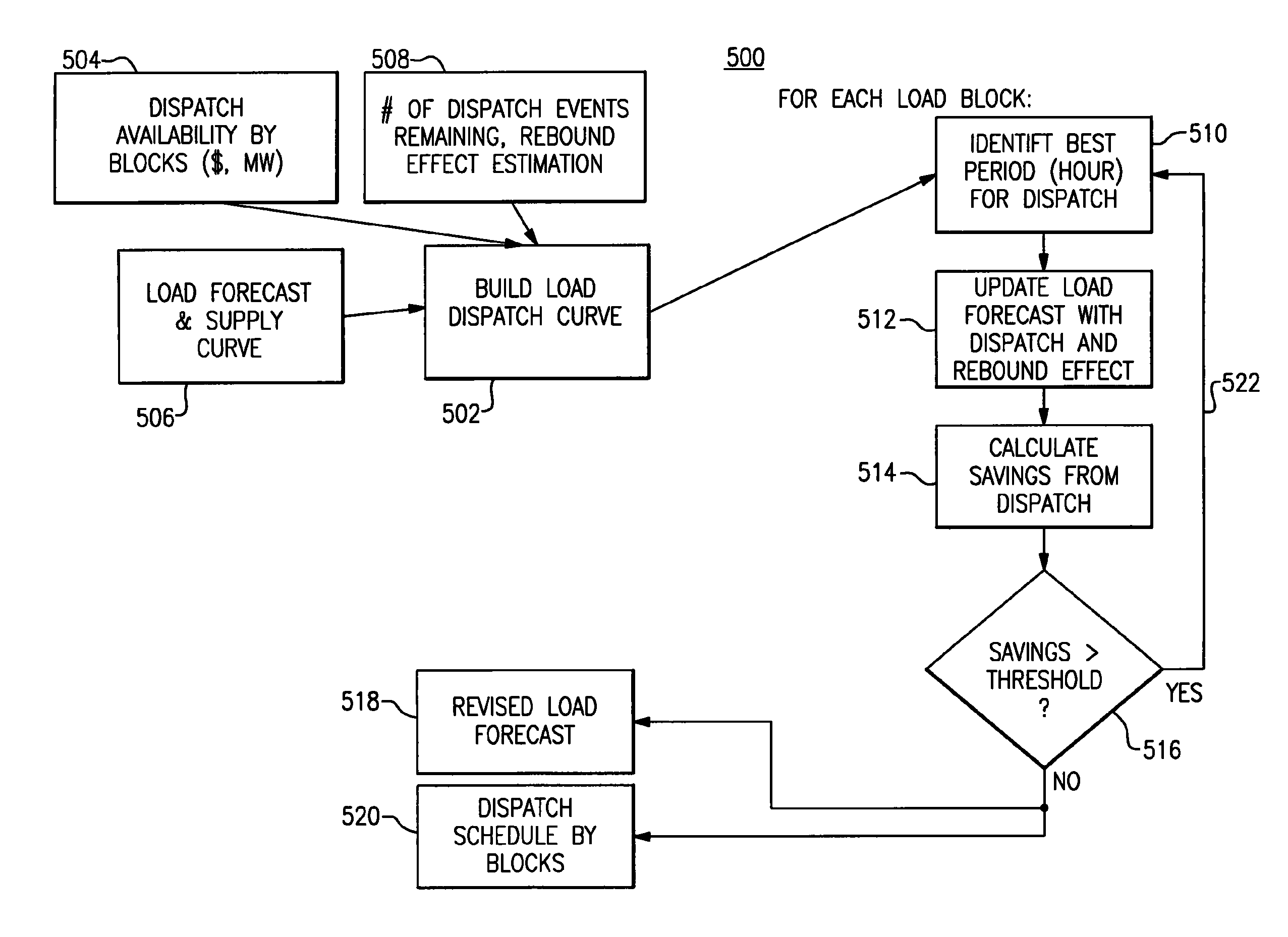
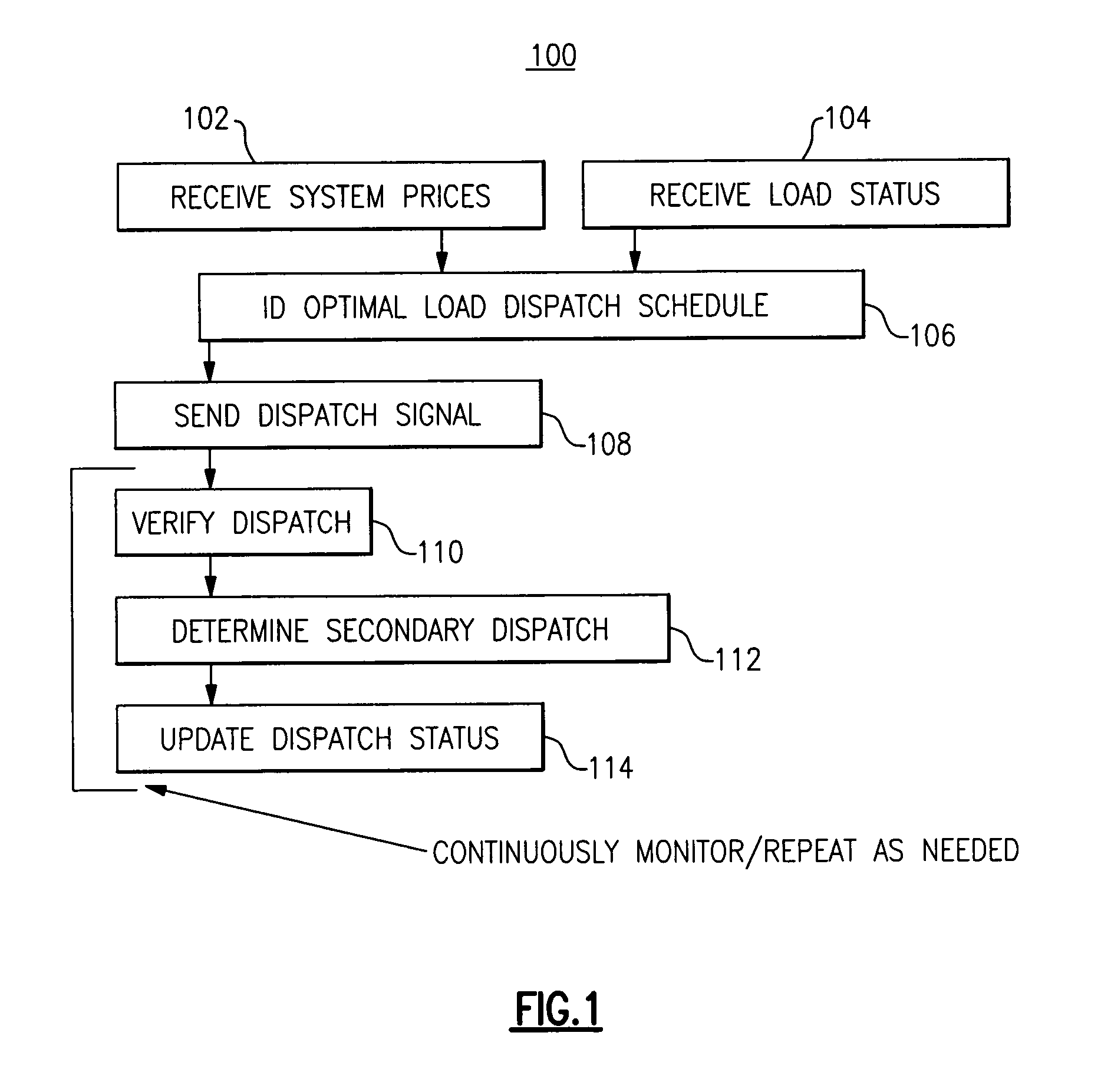
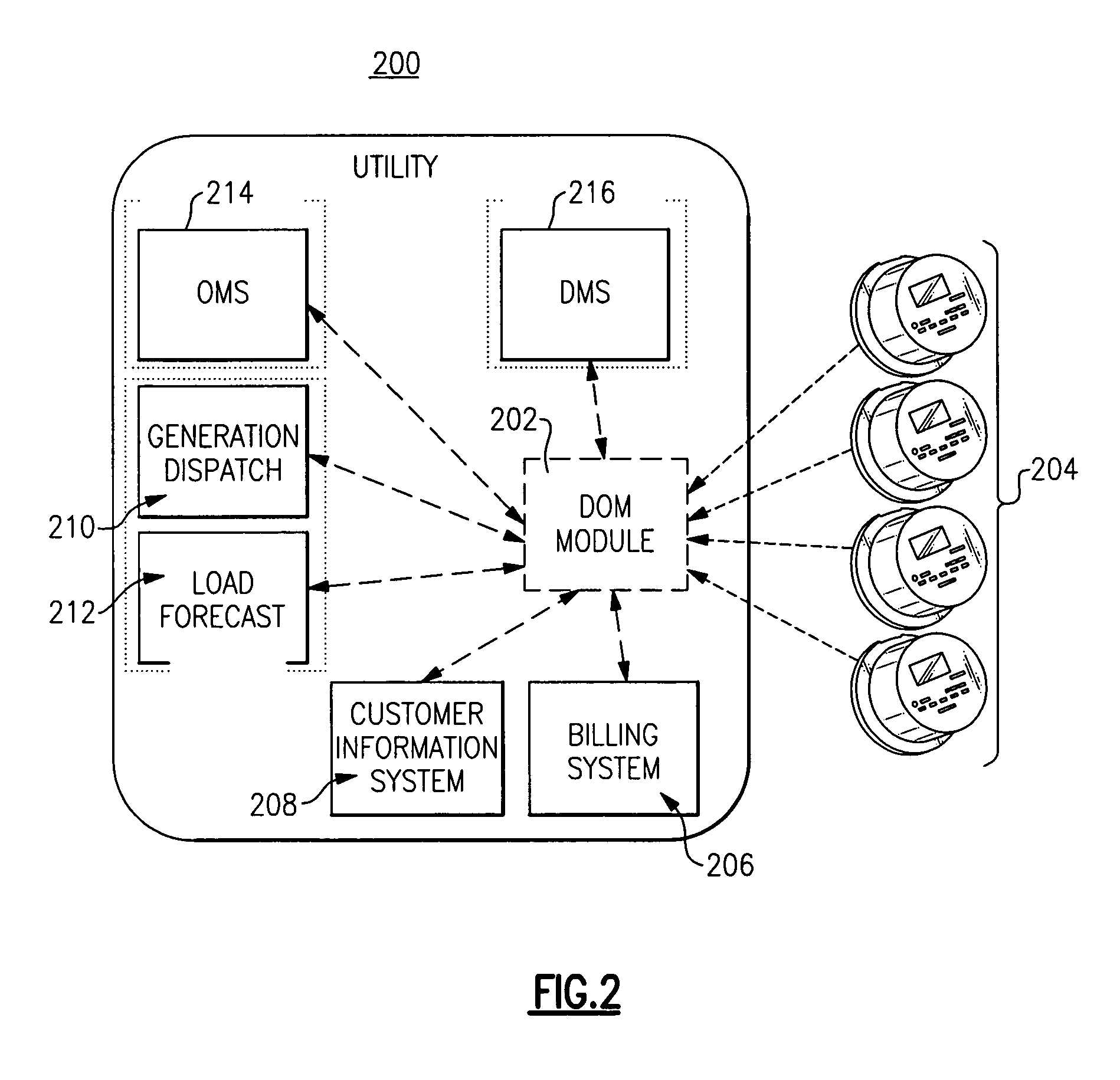
Optimal dispatch of demand side electricity resources
A method of load dispatch generates a load dispatch curve in response to both the opportunity cost of dispatching resources with a contractually limited number of dispatches in a given time period and estimated rebound effect data. The method identifies a best time period for dispatch based on the load dispatch curve and also supply curve data and generates a resultant load dispatch schedule. The resultant load dispatch schedule is transmitted to one or more smart home meters to dispatch loads in a manner that provides the greatest economic benefit.
Owner:GE DIGITAL HLDG LLC
Method and device for planning robot time optimal trajectory based on dynamic model
InactiveCN110209048AOptimize motion parametersRealize high-speed movementAdaptive controlDynamic modelsMotion parameter
The invention discloses a method and a device for planning a robot time optimal trajectory based on a dynamic model. The method for planning the robot time optimal trajectory comprises the following steps: converting joint motion constraint and geometric path constraint to a parameter space through a robot motion trajectory predetermined by dynamics modeling and parameterization; taking shortest time as an objective structural optimization problem, and solving through a numerical integration method to obtain an optimal motion parameter of the robot; and carrying out spline curve smoothing on acceleration for three times on a phase plane by considering the problem of joint vibration caused by sudden change of the acceleration. Compared with the conventional trapezoid acceleration trajectoryplanning method, the motion parameter of the robot can be optimized under the joint motion constraint and the geometric path constraint; the high-speed motion of the robot can be achieved; the performance of a joint motor is fully used; the motion speed of the robot can be further improved; pitch time for executing tasks is reduced, so that the operation efficiency of the robot is improved; and the method and the device have great significance to improvement of overall performance of the robot.
Owner:SOUTH CHINA UNIV OF TECH +1
Energy management method for hybrid ships based on model predictive control
InactiveCN107748498AImprove applicabilityImprove fuel efficiencyPropulsion based emission reductionPower plants using propulsion unit combinationsTime domainHorizon
In view of the problem that it is difficult to achieve real-time optimal control for the traditional hybrid ship control method, the invention presents an energy management method for hybrid ships based on model predictive control by adopting the idea of model predictive control. A Markov model is selected to predict the demanded power of a hybrid ship, and power distribution is optimized with theobjective of minimum fuel consumption in the prediction horizon by using a dynamic programming method based on the principle of model predictive control. The energy management method can improve thefuel economy of hybrid ships, and has good real-time performance.
Owner:SHANGHAI MARITIME UNIVERSITY
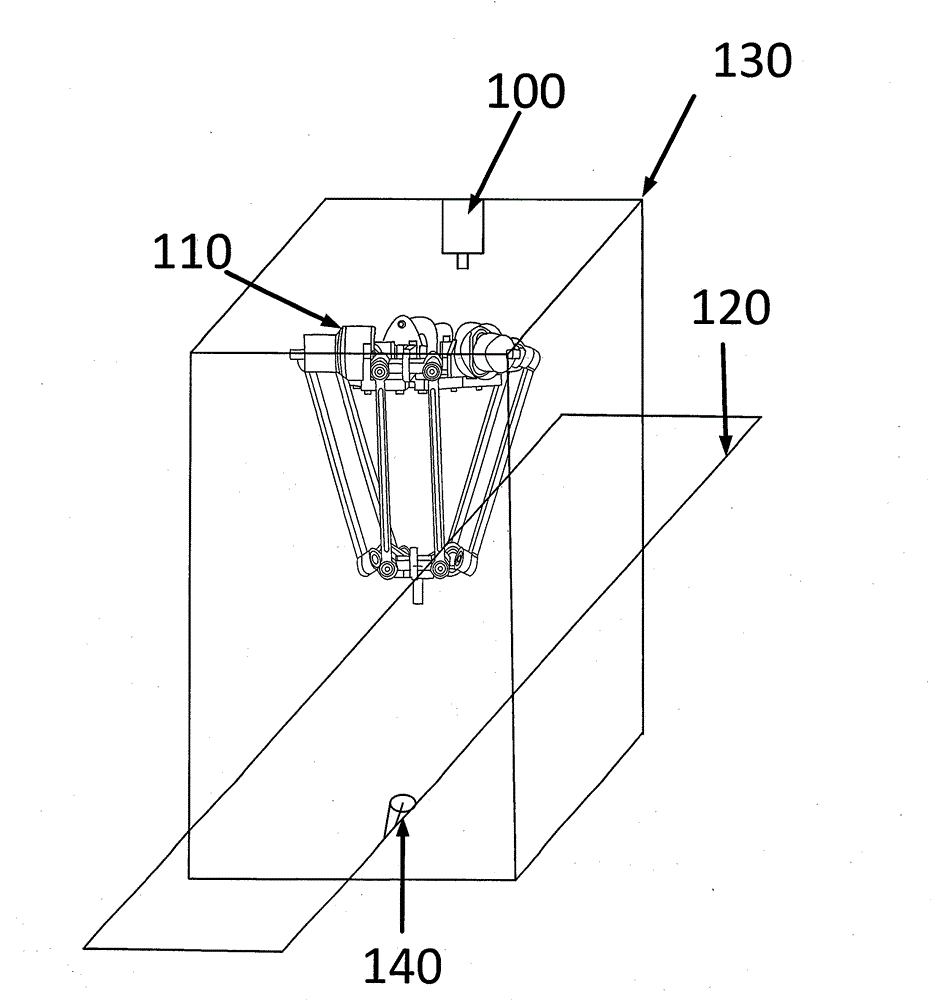
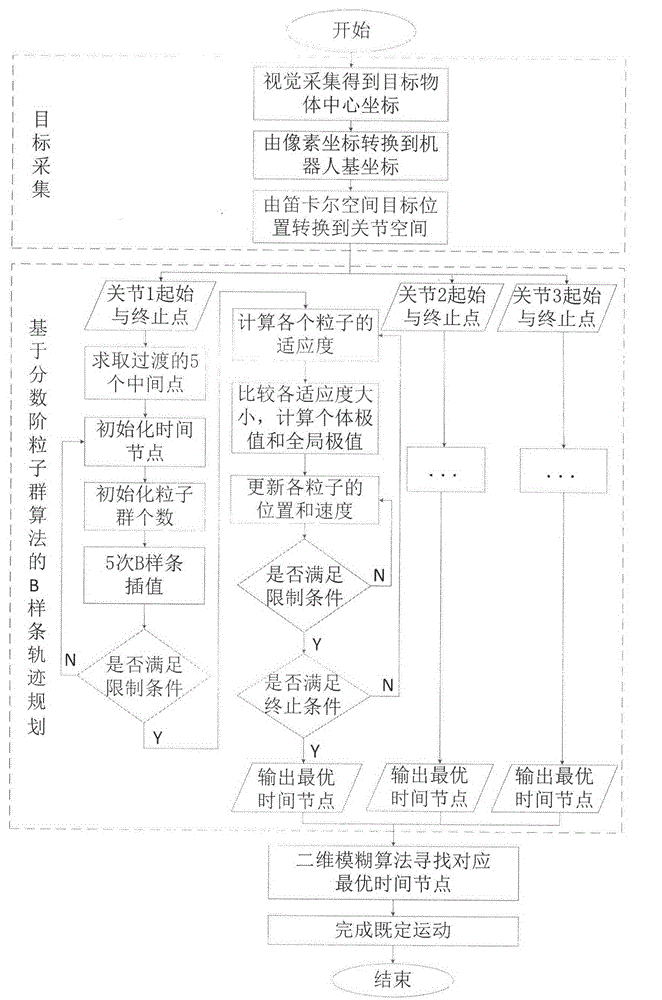
Delta robot time optimal trajectory planning method
ActiveCN104062902AImprove control efficiencyImprove control stabilityAdaptive controlNODALKinematics equations
The invention provides a vision-guided trajectory planning method on the basis of a Delta robot joint space. The method is applied to the optimal time motion of a Delta robot. The method comprises the following steps: solving a kinematic relation of the Delta robot; establishing an inverse kinematical equation for a motion from an end effector to each joint; acquiring a target position through an industrial intelligent camera; updating the target position on real time through an encoder; dividing a working area of the Delta robot into 9*13 subareas; planning the motion route of each joint at different areas through a B spline offline; maintaining smooth and continuous speed, acceleration and jerk; reducing the impact of a servo motor to a mechanical structure; improving the typical particle swarm optimization; speeding up to search time nodes with optimal solution by the fractional order particle swarm optimization in order to avoid local optimal solution; then performing the two-dimensional fuzzy method to select the optimal time node corresponding to the working area of the robot on line, and thus finishing control.
Owner:JIANGNAN UNIV +1
Variable-speed wind generating set maximum wind energy capturing method based on effective wind speed estimation
ActiveCN108334672AEasy to implementLow costWind motor controlNeural network algorithmsEconomic benefitsEngineering
The invention discloses a variable-speed wind generating set maximum wind energy capturing method based on effective wind speed estimation. The method comprises an effective wind speed estimating model and a maximum wind energy capturing controller. In order to acquire an effective wind speed estimated value, a training set of an SVR model is formed by normalized unit historical output data and historical wind speed measured values, penalty parameters and kernel function parameters are selected by a GA algorithm to obtain the trained SVR model, and the model gives out the wind speed estimatedvalue in an online manner; and when the maximum wind energy capturing controller is design, the real-time optimal wind wheel rotating speed estimated value is obtained according to effective wind speed which is given out by the effective wind speed estimating model, nonlinear characteristics and parameter uncertainty of the system are responded by robust factors and a neural network, and therefore, boundedness of rotating speed tracking errors and stability of a wind generating set system are realized. By the method, a mathematical model and parameters of the unit do not require to be used, adesign process is simple, an implementing cost is low, and the capacity of the unit and the economic benefit of a wind power plant can be improved.
Owner:ZHEJIANG UNIV
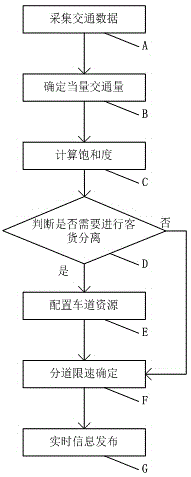
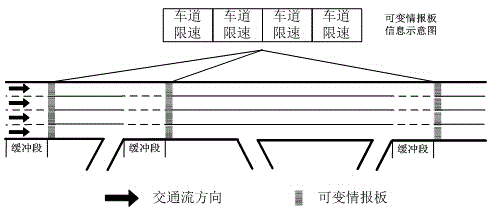
Real-time optimal configuration method for lane resources of multi-lane expressway
ActiveCN104794919AIncrease driving speedImprove traffic efficiencyArrangements for variable traffic instructionsTime informationMixed flow
The invention discloses a real-time optimal configuration method for lane resources of a multi-lane expressway. The real-time optimal configuration method for the lane resources of the multi-lane expressway comprises the following steps of traffic data acquisition, equivalent traffic volume confirmation, saturability calculation, passenger car and freight truck separation confirmation, lane resource configuration, lane speed limitation confirmation and real-time information issuing. Flow data of different vehicles running on the expressway are acquired and analyzed in real time, whether passenger cars and freight trucks on the expressway need to be separated from each other or not is determined, lane speed limitation measures under the condition of passenger car and freight truck separation and lane speed limitation measures under the condition of passenger car and freight truck combination are confirmed, and a real-time lane resource configuration scheme is formed. The lane resources of the expressway are distributed and controlled in real time, safety problems caused by mixed flow of the passenger cars and the freight trucks are reduced, speed advantages of the different vehicles can be played to a maximum extent, and the traffic efficiency of the expressway is improved.
Owner:LIAONING PROVINCIAL TRANSPORTATION PLANNING & DESIGN INST +2
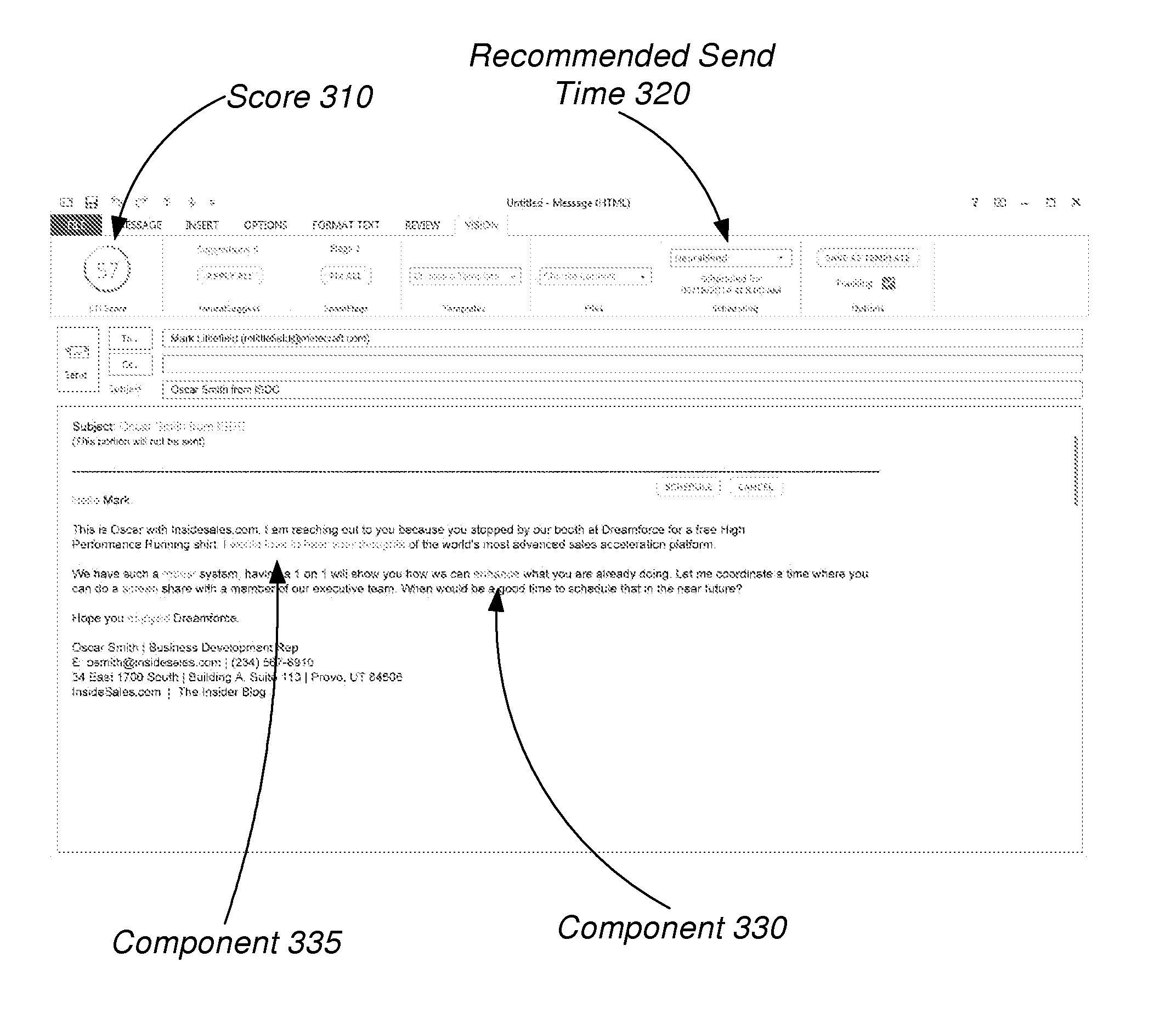
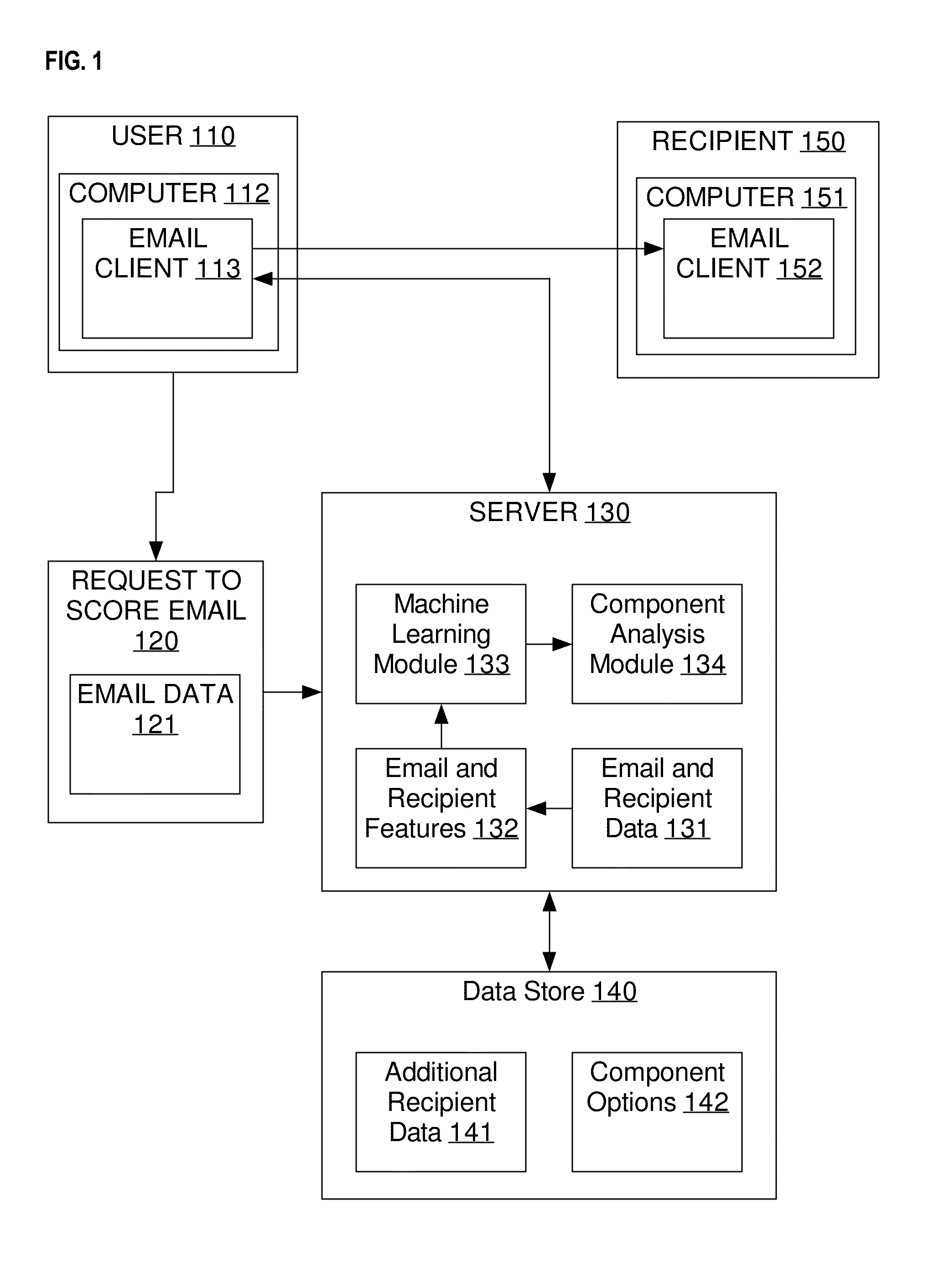
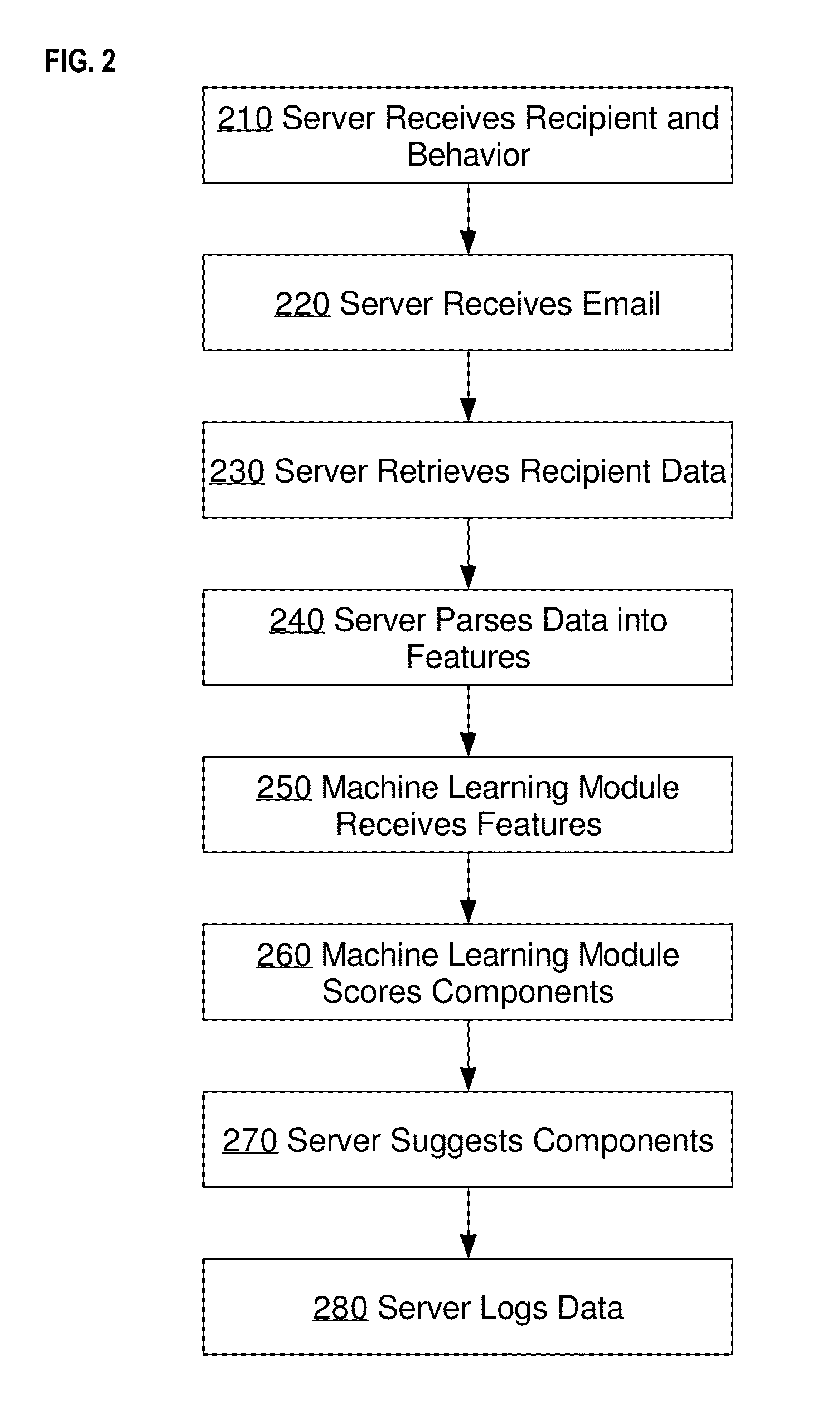
Email optimization for predicted recipient behavior: determining a likelihood that a particular receiver-side behavior will occur
Techniques are described herein for predicting one or more behaviors by an email recipient and, more specifically, to machine learning techniques for predicting one or more behaviors of an email recipient, changing one or more components in the email to increase the likelihood of a behavior, and determining and / or scheduling an optimal time to send the email. Some advantages of the embodiments disclosed herein may include, without limitation, the ability to predict the behavior of the email recipient and suggest the characteristics of an email which will increase the likelihood of a positive behavior, such as a reading or responding to the email, visiting a website, calling a sales representative, or opening an email attachment.
Owner:XANT INC
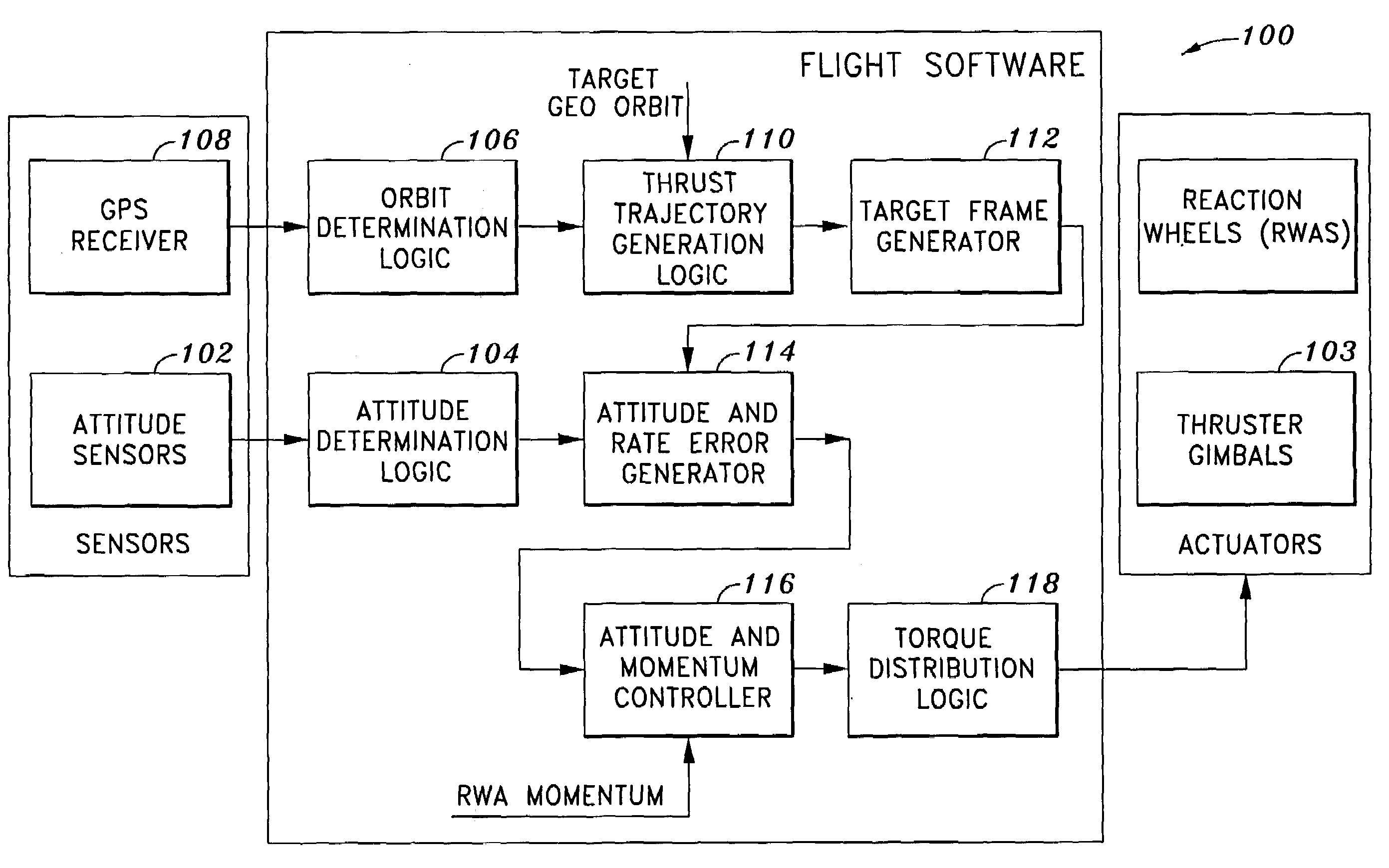
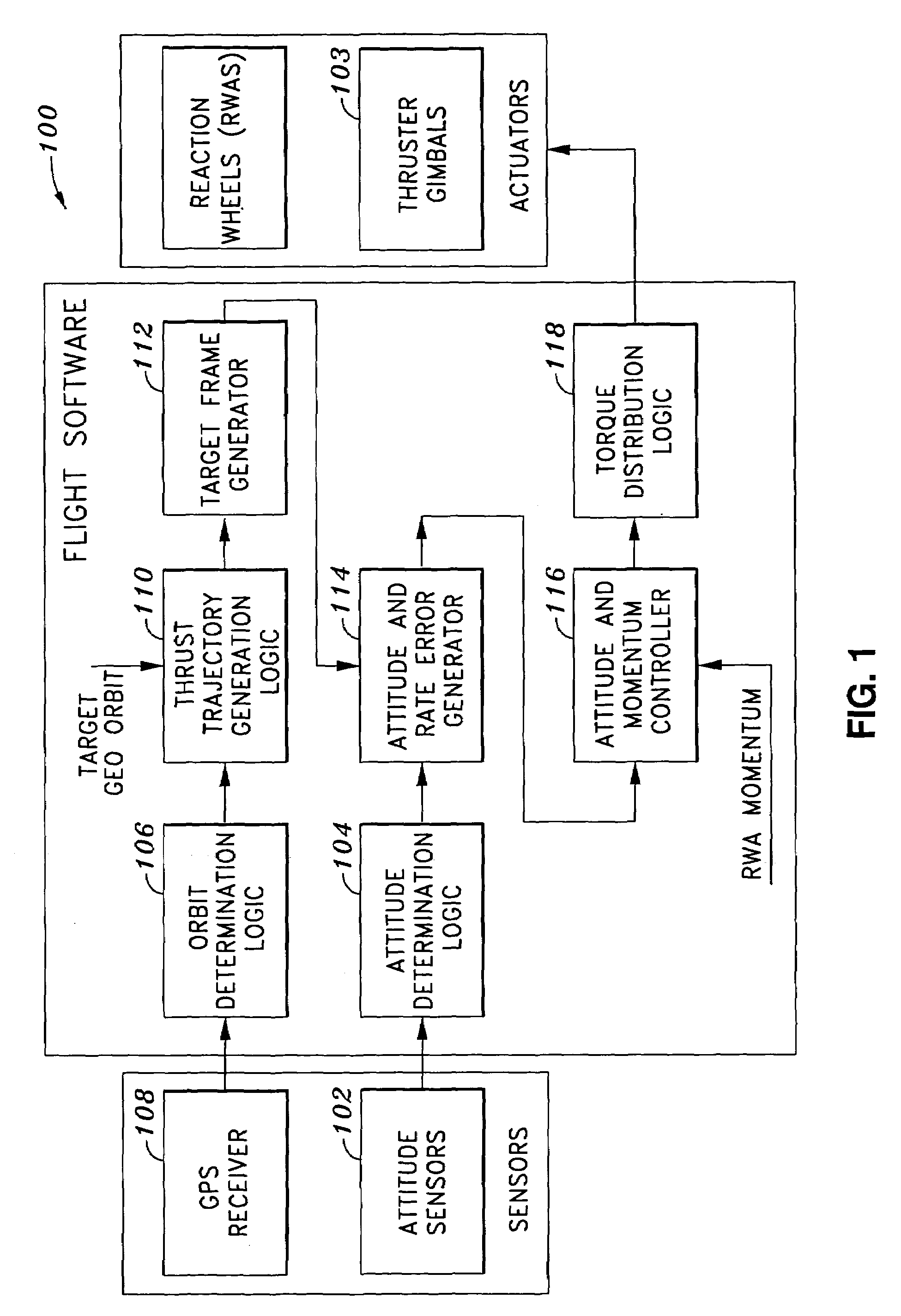
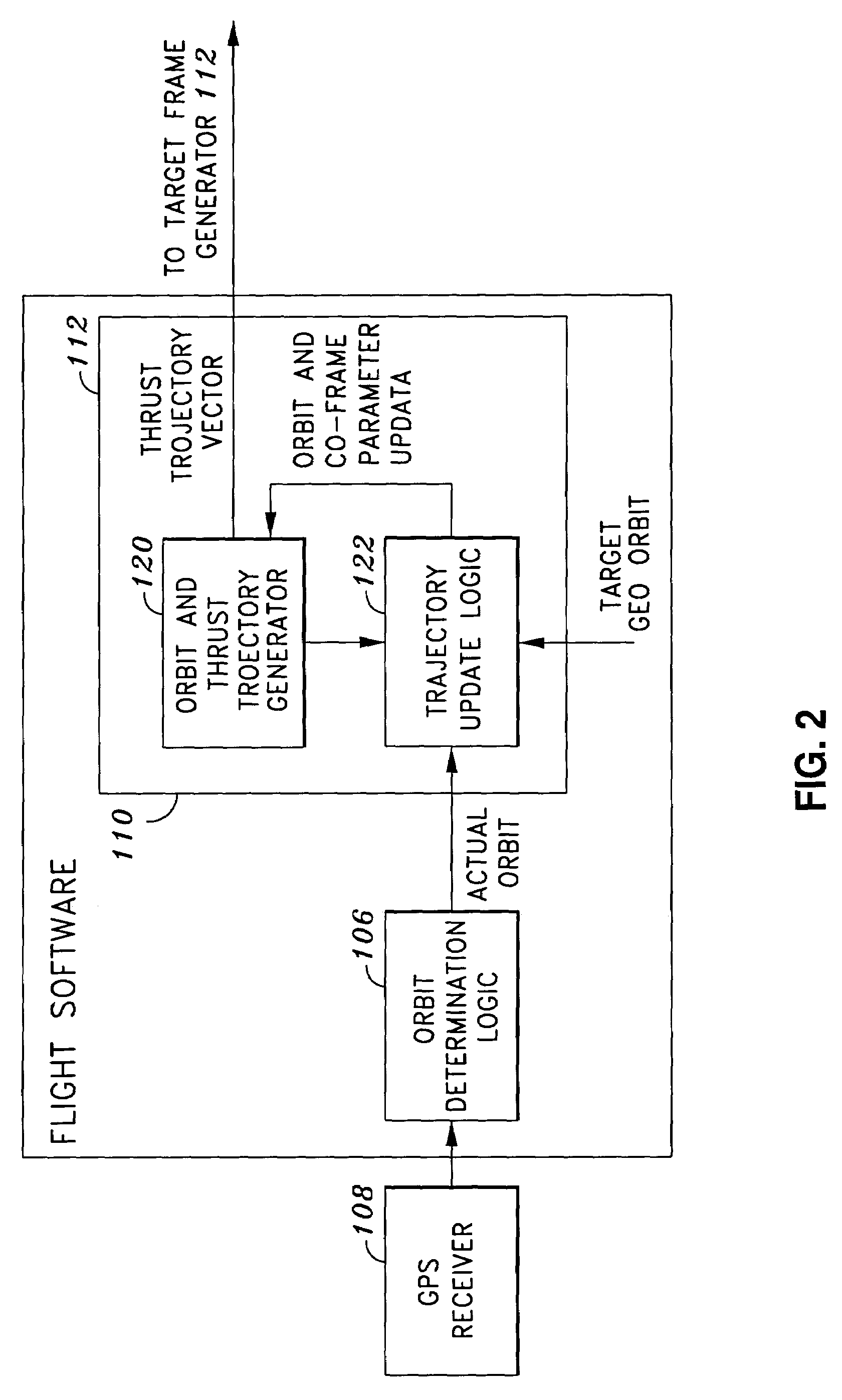
System and method of substantially autonomous geosynchronous time-optimal orbit transfer
InactiveUS7246775B1Reduce coverageImprove fuel efficiencyLaunch systemsVehicle position/course/altitude controlSynchronous orbitState parameter
Method of and system for on-board substantially autonomous control for transferring a spacecraft from an initial orbit to a final geosynchronous orbit, by a trajectory that minimizes remaining transfer time and orbit transfer fuel. The spacecraft determines its orbit using a GPS-based system to determine the spacecraft orbital elements. Based on the measured orbit error, corrected co-state parameters are calculated and used to generate an updated thrust trajectory. The corrections are calculated using an innovative numerical procedure, carried out repetitively at a fixed interval until the target geosynchronous orbit is achieved.
Owner:LOCKHEED MARTIN CORP
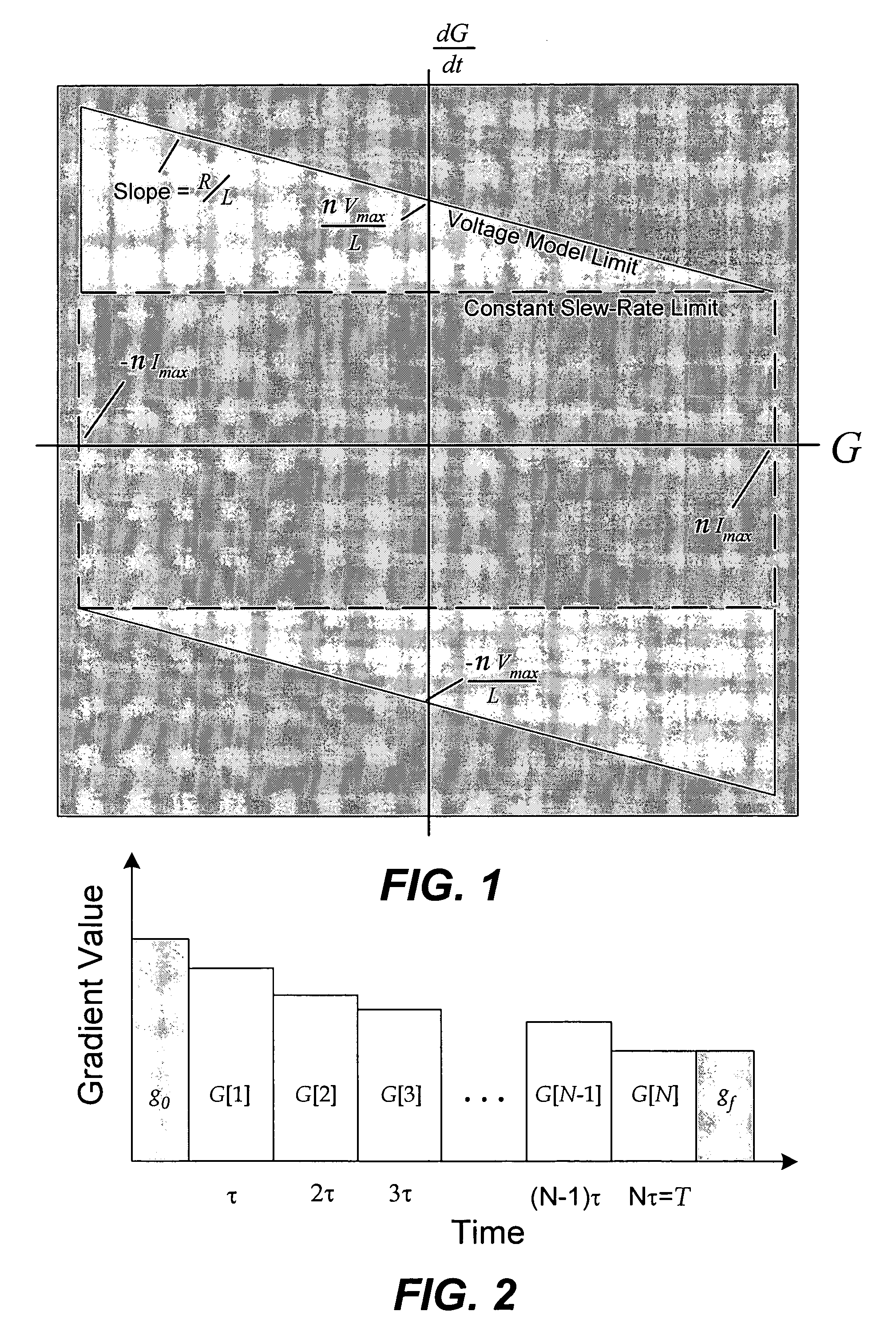
MRI gradient waveform design using convex optimization
A time-optimal MRI gradient design method utilizes constrained optimization to design minimum-time gradient waveforms that satisfy gradient amplitude and slew-rate limitations. Constraints are expressed as linear equations which are solved using linear programming, L1-norm formulation, or second-order cone programming (SOCP).
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Real-time information-based metro passenger service system
ActiveCN104408082ASolve uneven distributionAct as a guideForecastingGeographical information databasesTime informationMetro station
The invention relates to a real-time information-based metro passenger service system. Mobile terminal equipment automatically connects wireless sensors in a metro station and in metro compartments so as to perform data interaction with the servers to acquire relevant information in the metro station in real time, and the relevant information is used for passengers to view in real time; moreover, a heuristic search strategy based on the estimated arrival time of a metro, running time between stations, the current passenger flow information of the metro station and passenger flow information in each metro compartment is proposed, and therefore the optimal proposed ride scheme is obtained, and meanwhile, a time-optimal and distance-optimal scheme is obtained for the passengers to select; after the scheme is determined, according to the position conditions of the current passengers, a prompt of the next route is provided in real time; the passenger flow conditions of the compartments are determined according to the information of the wireless servers of the metro which is about to arrive, and the best waiting position proposal can be given by the system; after the passenger ride, prompt contents, including the time that the metro is about to arrive the next station, the get-off station and the get-off time of the passengers are given in real time, the exit scheme of the metro station and corresponding map information, can be given in real time.
Owner:大连海天兴业科技有限公司
Time optimal grab bucket operation method
The embodiment of invention discloses a time optimal grab bucket operation method. According to the method, an operation path of a grab bucket is a parabola compounded by hosting motion and trolley motion; the hoisting motion operates according to a trapezoidal velocity curve; both ascending motion and descending motion are accelerated to operate at full speed with the maximum acceleration and decelerated to stop with the maximum acceleration when a target location is reached, so that the grab bucket operates at high level at the earliest time and descends from the high level at the latest time; for the trolley motion, a two-step acceleration method is adopted at an acceleration stage, a two-step deceleration method is adopted at a deceleration stage, a two-step deceleration method or a bucket throwing deceleration method is adopted at the stage of approaching a hopper, and a two-step acceleration method or a bucket throwing acceleration method is adopted at the stage of leaving the hopper, wherein the hoisting motion is accelerated to the full speed, a trolley starts to move from a point B, and the grab bucket starts to move along the parabolic path; and when the grab bucket returns to a cabin of a ship, the grab bucket moves to the point H along the parabolic path, the trolley stops moving, and the hoisting starts to decelerate from the point H and stops at a point I.
Owner:上海港吉电气有限公司
Electric vehicle charging real-time optimization dispatching method
ActiveCN109523051AEnsure safe and economical operationSafe and economical operation meetsInternal combustion piston enginesForecastingTime informationDistribution power system
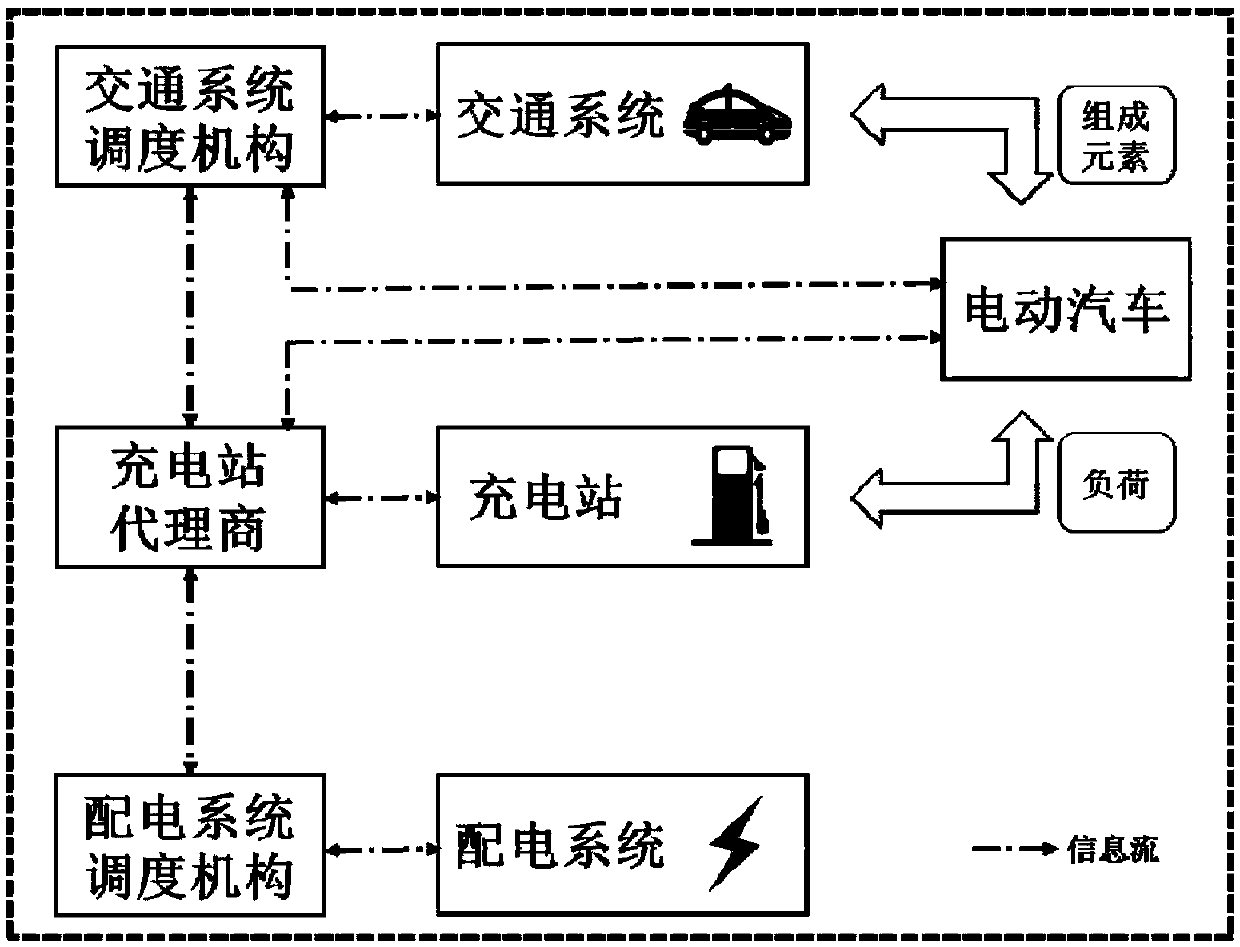
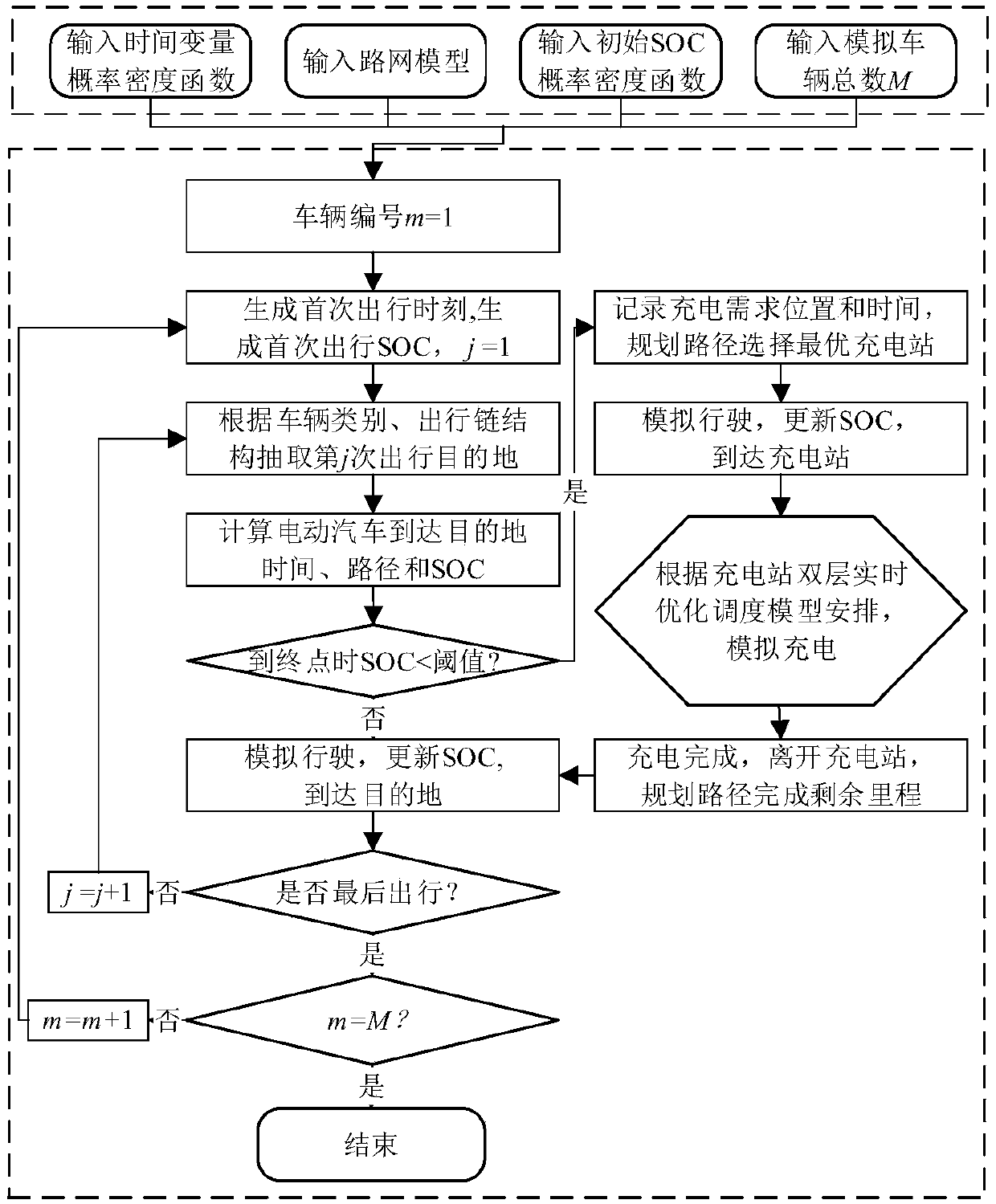
The invention discloses an electric vehicle charging real-time optimization dispatching method. The technical scheme adopted by the invention is as follows: Net-Electrified Transportation CooperativeSystem Architecture Based on Vehicle Interaction; A micro traffic assignment model based on travel chain tracking is established to track and simulate the real-time information of driving behavior, location distribution, state of charge and charging demand of electric vehicle users. Charging station selection and navigation strategy; A two-layer real-time optimal dispatching model is constructed to determine the specific charging scheme of electric vehicles in each charging station, which takes into account the safety of distribution system and the waiting time of users. YALMIP / CPLEX efficientcommercial solver is used to solve the upper and lower level problems iteratively. The invention can not only alleviate the traffic jam near the charging station; But also can make the load distribute evenly, reduce the network loss and ensure the safe and economical operation of the power network; Also can meet the EV charging demand, improve customer satisfaction, and ultimately achieve the overall optimization of electrified transportation system operation, taking into account the interests of many aspects.
Owner:STATE GRID ZHEJIANG ELECTRIC POWER +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com