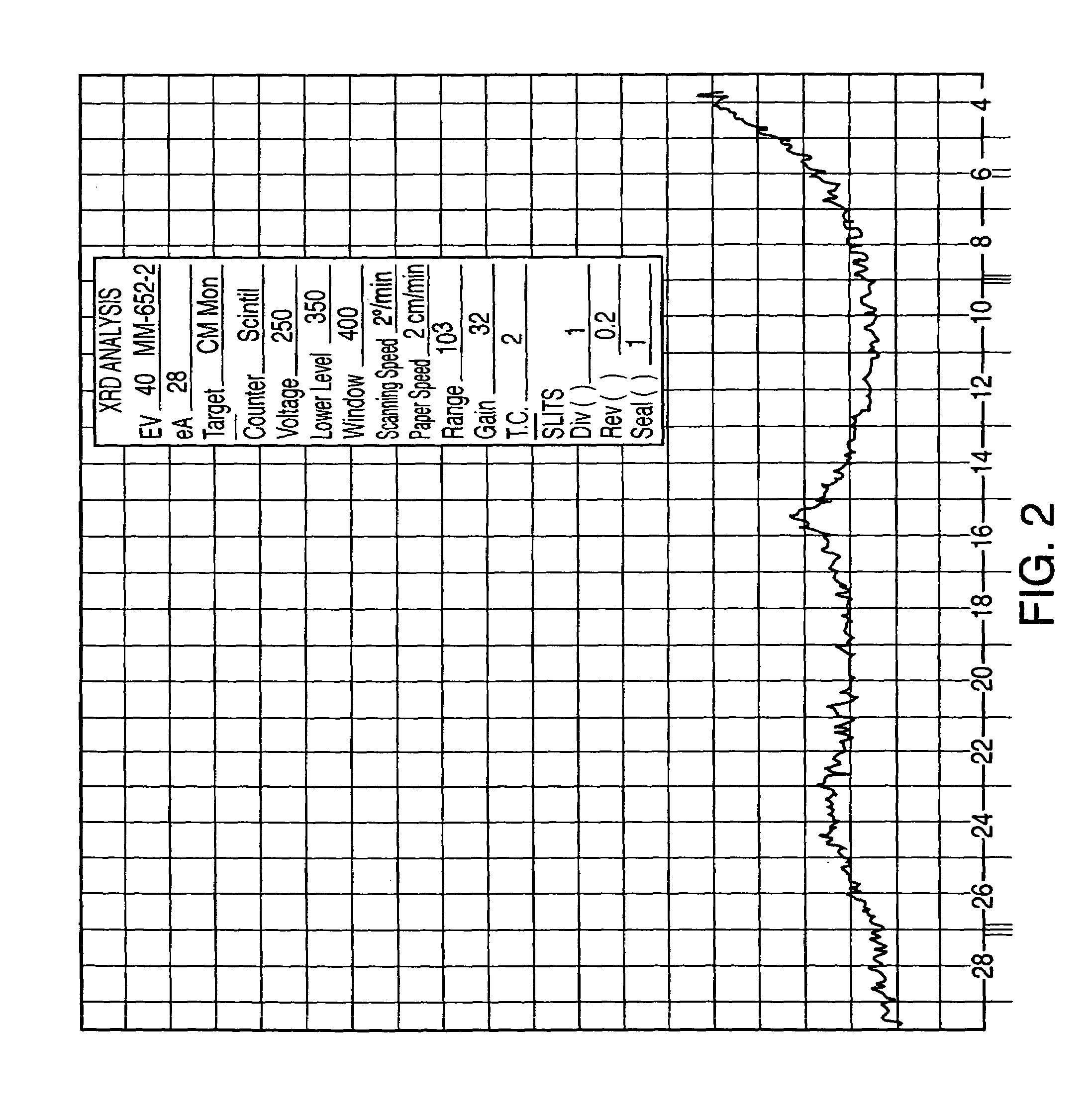
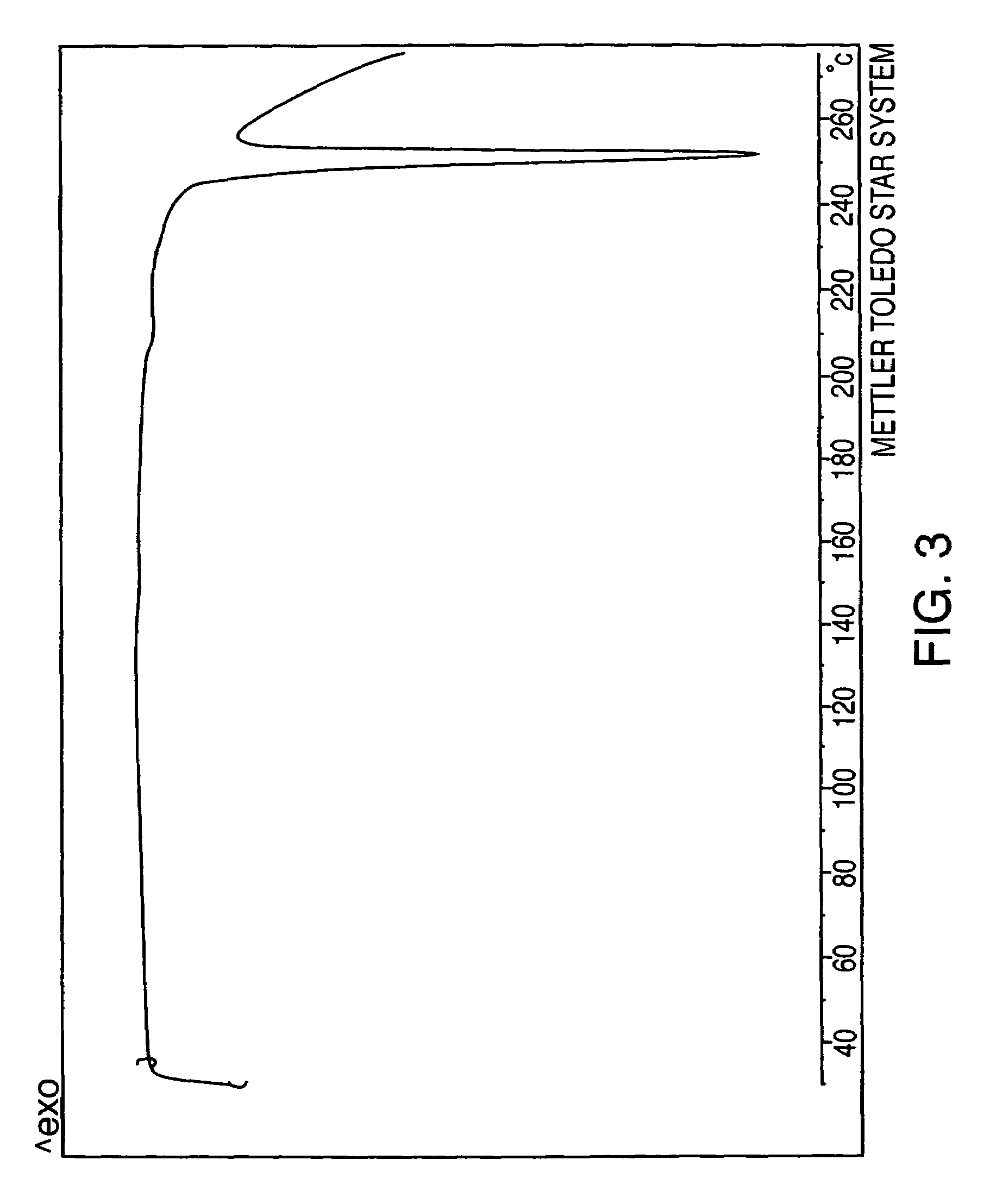
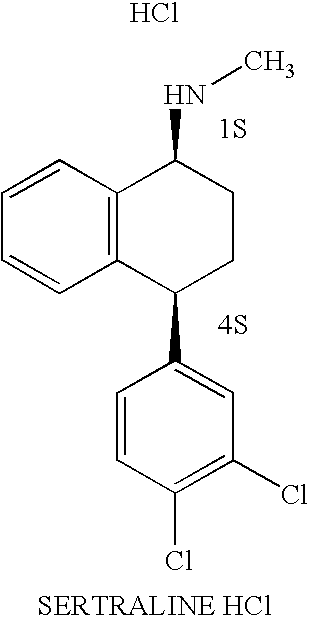
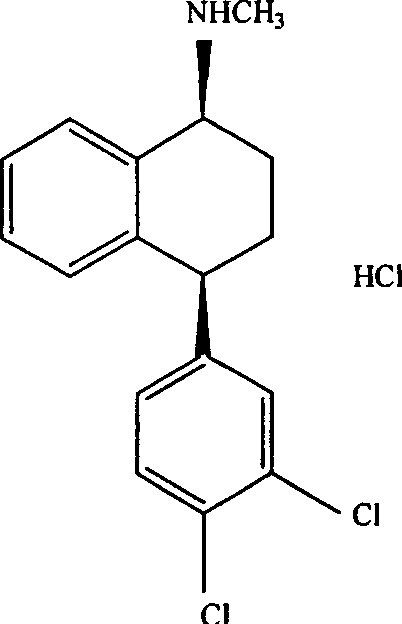
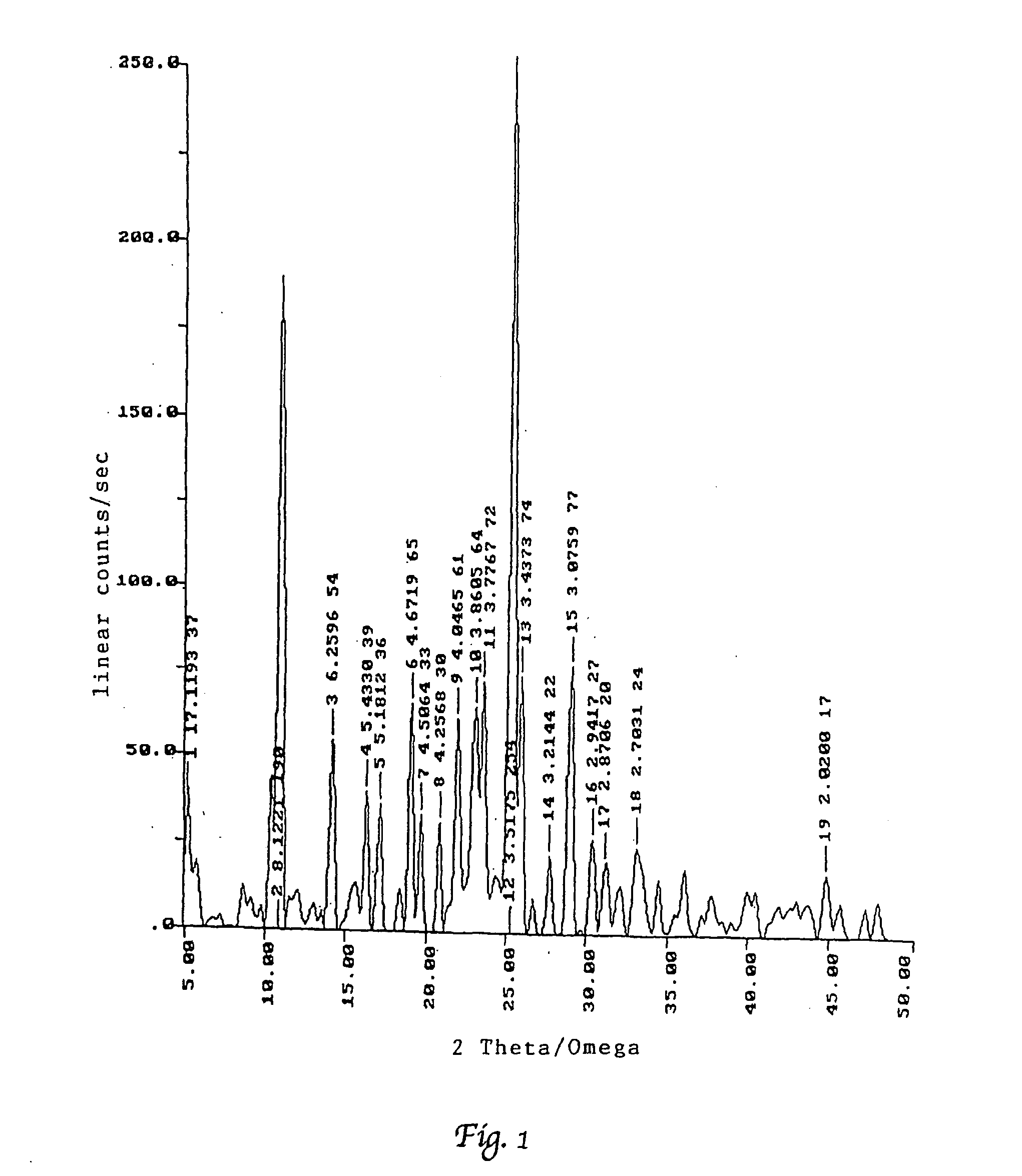
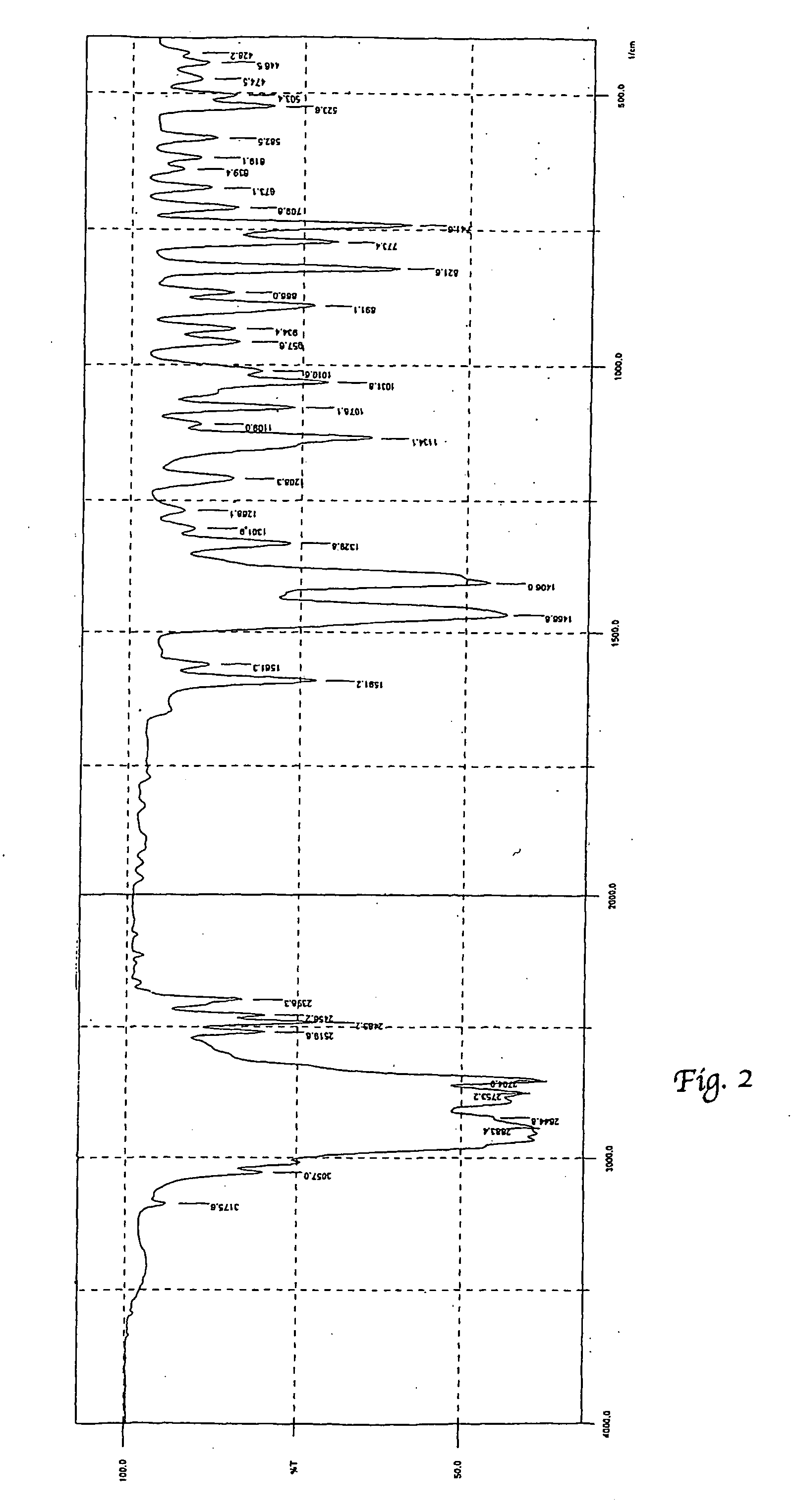
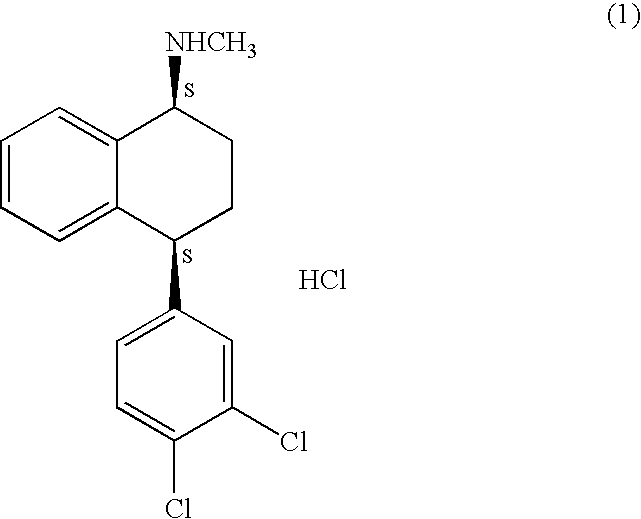
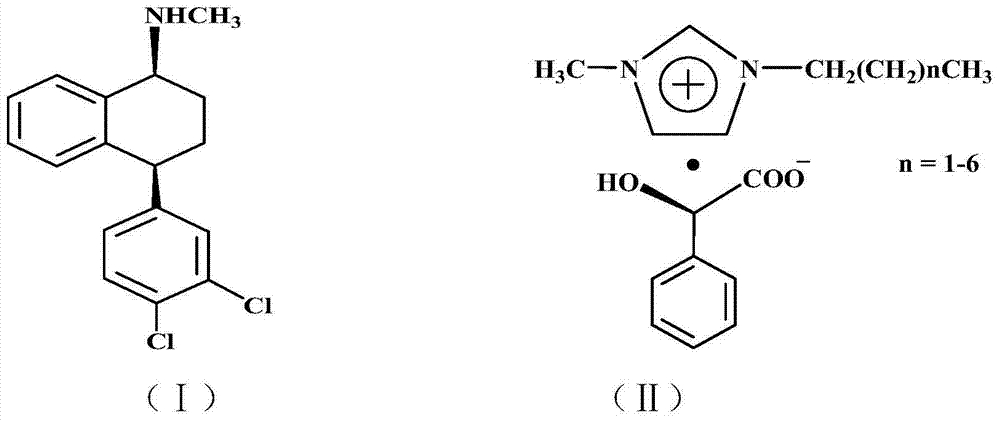
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40 results about "Sertraline Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
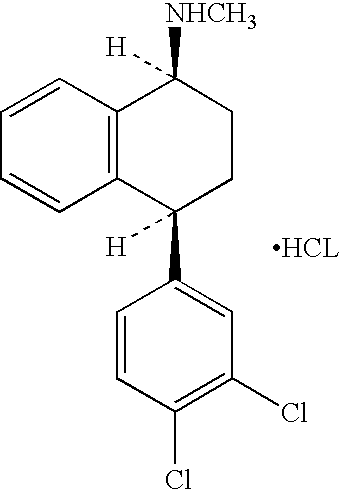
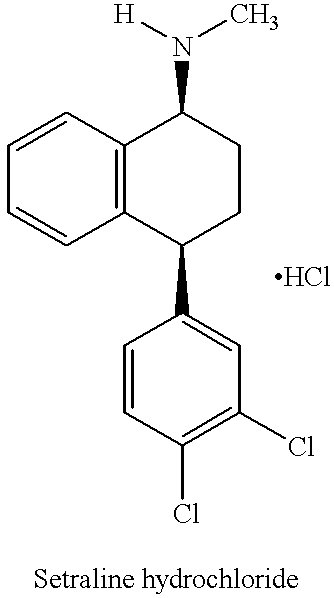
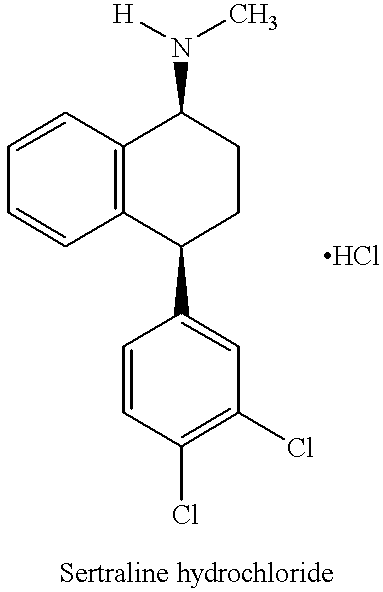
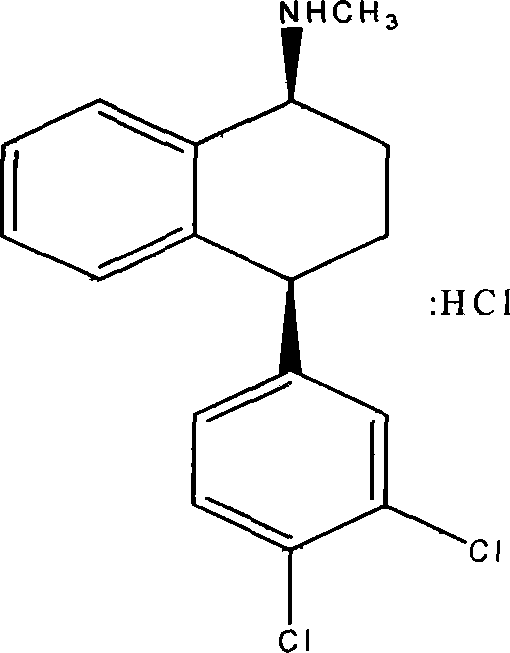
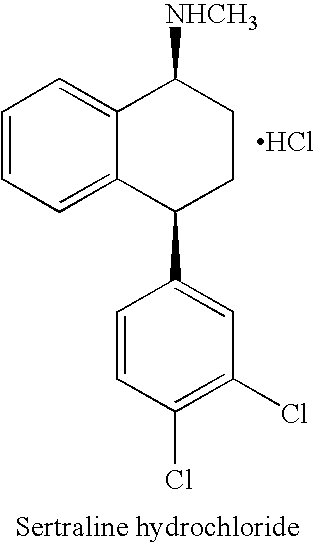
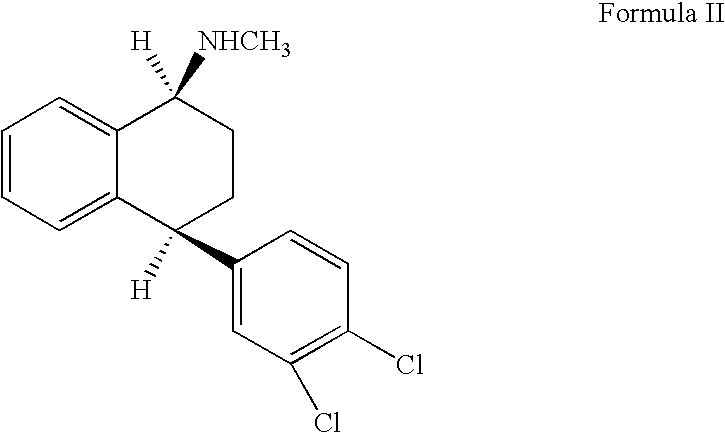
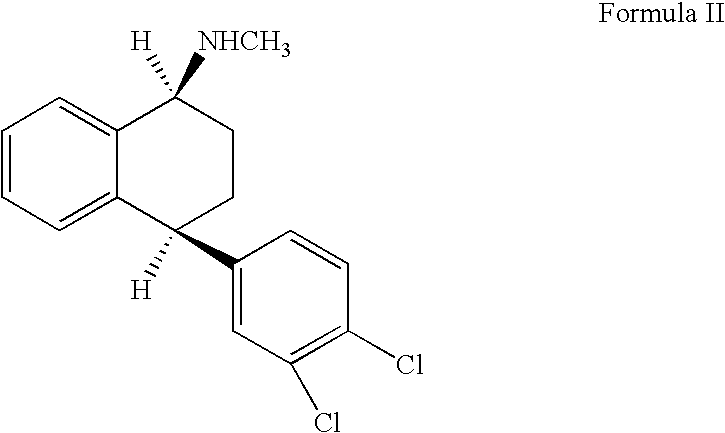
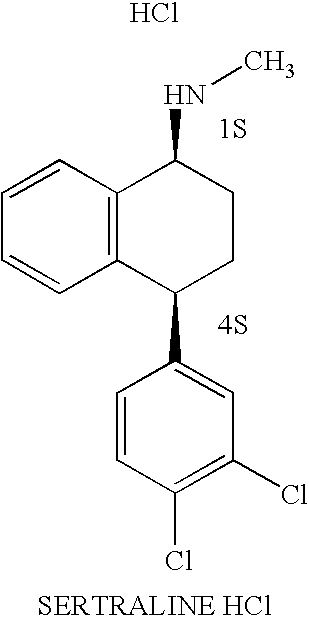
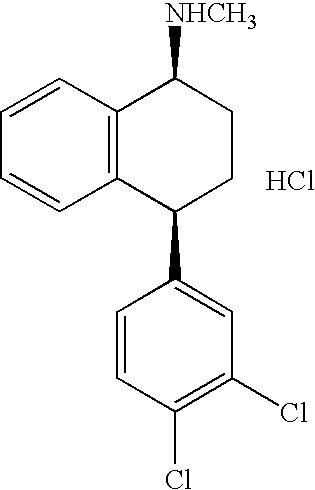
The hydrochloride salt of sertraline, a synthetic derivative of naphthalenamine with anti-serotoninergic and anti-depressant properties. Sertraline appears to selectively inhibit the neuronal uptake of serotonin, raising serotonin levels in the CNS.
Serotonin reuptake inhibitor formulations
A process for preparing amorphous paroxetine hydrochloride or sertraline hydrochloride is provided, which comprises preparing a solution in which paroxetine hydrochloride or sertraline hydrochloride and a water-soluble polymer are dissolved in a co-solvent of a volatile organic solvent and water. Said solution is dried to obtain a composition comprising amorphous paroxetine hydrochloride or sertraline hydrochloride and the water-soluble matrix.
Owner:ANDRX PHARMA INC
Serotonin reuptake inhibitor formulations
InactiveUS20020156066A1Easy to operateBiocidePowder deliveryOrganic solventSerotonin receptor inhibitors
A process for preparing amorphous paroxetine hydrochloride or sertraline hydrochloride is provided, which comprises preparing a solution in which paroxetine hydrochloride or sertraline hydrochloride and a water-soluble polymer are dissolved in a co-solvent of a volatile organic solvent and water. Said solution is dried to obtain a composition comprising amorphous paroxetine hydrochloride or sertraline hydrochloride and the water-soluble matrix.
Owner:ANDRX PHARMA INC
Stable Sertraline Hydrochloride Formulation and Method
InactiveUS20060257475A1Good storage stabilityTreatment and amelioration of numerous physical maladiesBiocideOrganic active ingredientsDosage formDrug
Pharmaceutically stable solid pharmaceutical dosage forms of sertraline hydrochloride Form II and Form V polymorphs formed by direct compression.
Owner:BOEHRINGER INGELHEIM INT GMBH
Process for preparing (+)-cis-sertraline
InactiveUS6552227B2Improved and efficient and cost-effective purificationQuick and efficientAmino compound purification/separationOrganic compound preparationChemistrySertraline Hydrochloride
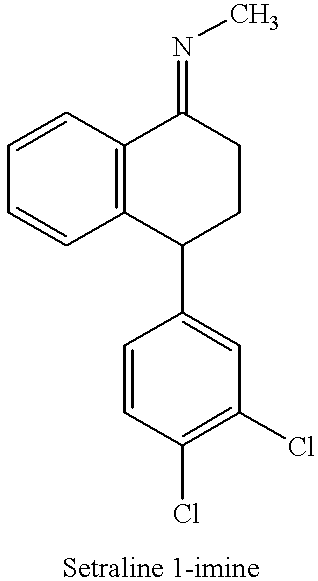
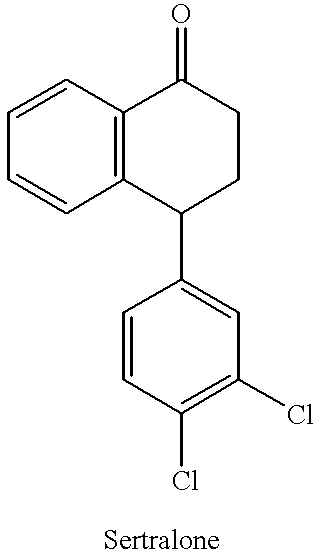
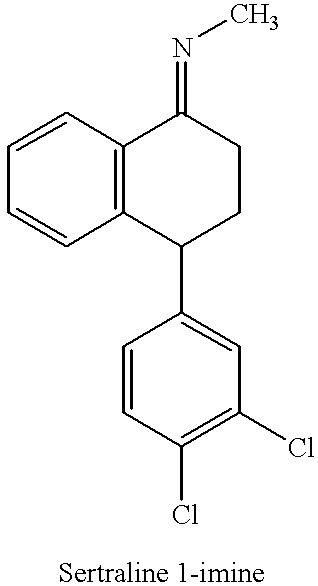
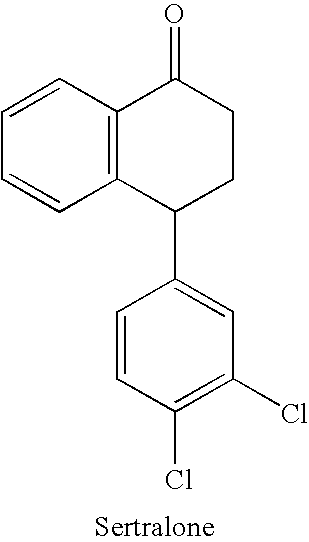
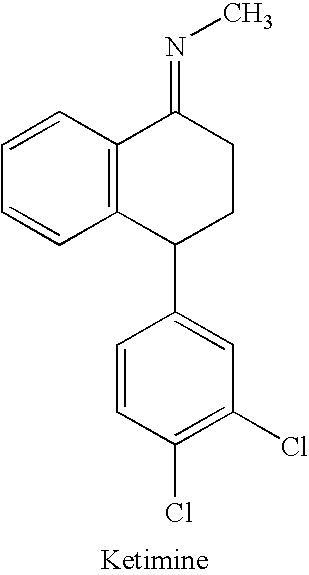
The present invention is directed to (+)-cis-sertraline hydrochloride and methods of preparation. The present invention also includes processes for making sertraline having a cis / trans ratio greater than 3:1, greater than or equal to 8:1, or between about 8:1 and about 12:1, from the Schiff base of sertralone, sertraline-1-imine.
Owner:TEVA PHARM USA INC
Sertraline hydrochloride form II and methods for the preparation thereof
InactiveUS20050032906A1Yield lossHigh yieldBiocideOrganic active ingredientsMethyl groupPhenyl group
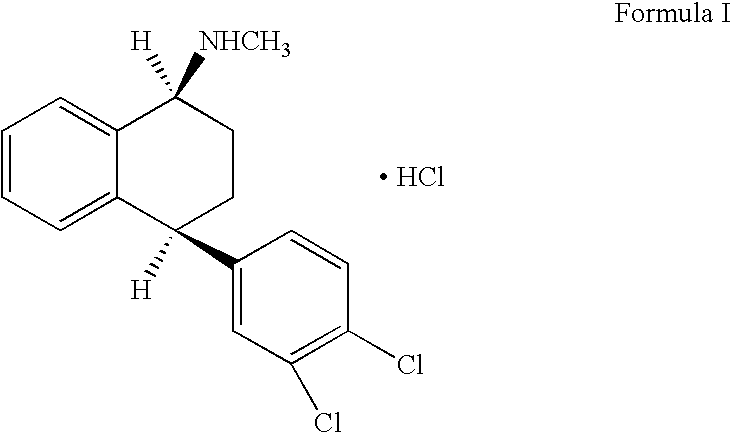
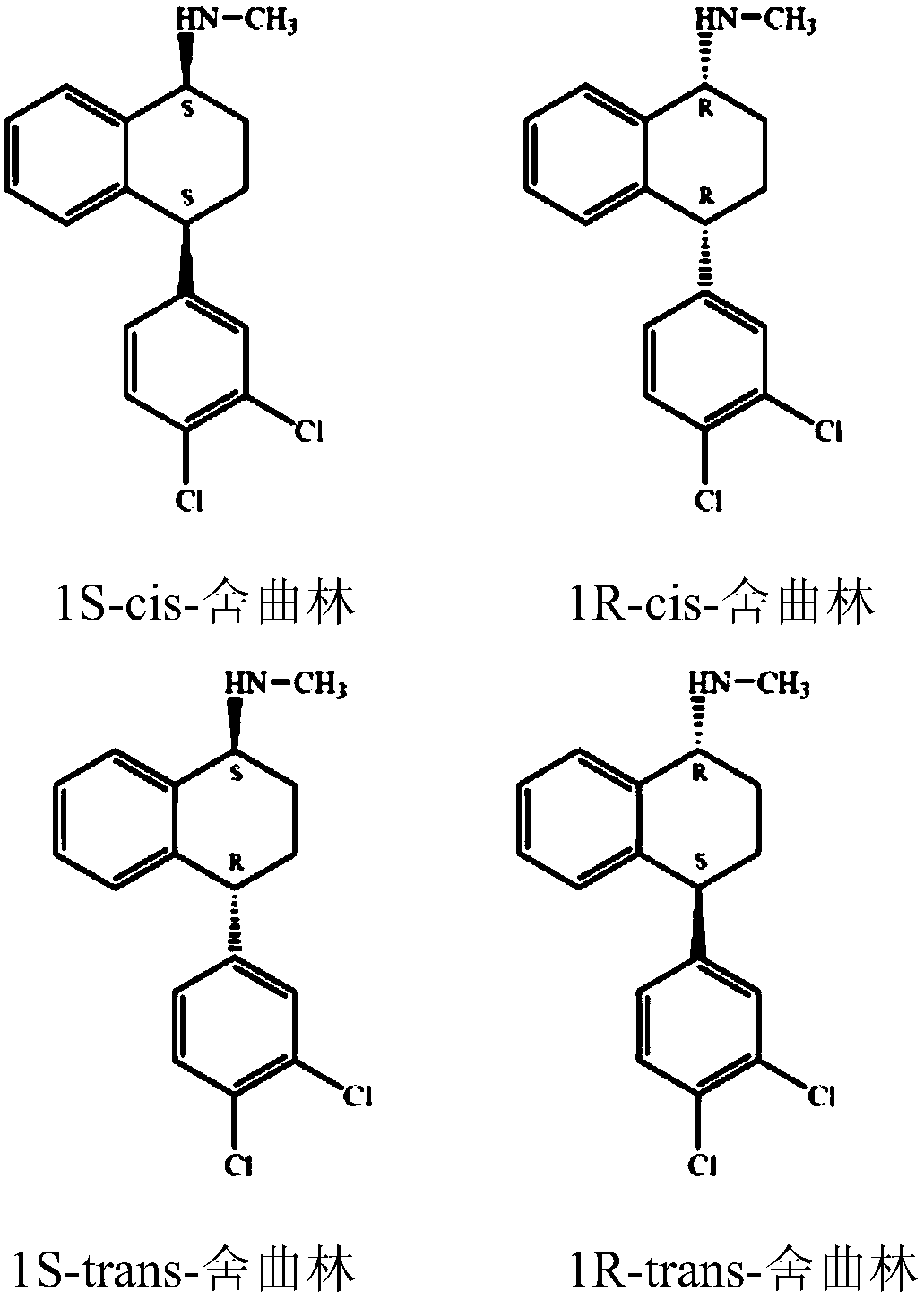
This invention relates to a method for the preparation of sertraline hydrochloride, (1S-cis)-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-1-naphtalenamine hydrochloride, in its crystalline form II.
Owner:RECORDATI IND CHEM E FARM SPA
Novel process for preparing (+)-cis-sertraline
InactiveUS20020019570A1Improved and efficient and cost-effective purificationQuick and efficientAmino compound purification/separationOrganic compound preparationChemistryImine
The present invention is directed to (+)-cis-sertraline hydrochloride and methods of preparation. The present invention also includes processes for making sertraline having a cis / trans ratio greater than 3:1, greater than or equal to 8:1, or between about 8:1 and about 12:1, from the Schiff base of sertralone, sertraline-1-imine.
Owner:TEVA PHARM USA INC
Sertraline hydrochloride oral liquid and preparation method thereof
InactiveCN101623252AImprove solubilityGuaranteed curative effectOrganic active ingredientsNervous disorderChemistrySertraline Hydrochloride
The invention discloses a sertraline hydrochloride oral liquid and a preparation method thereof. The sertraline hydrochloride oral liquid has the advantages of convenient administration, steady storage at low temperature or normal temperature, quick absorption, good mouthfeel, more suitability for senile patients, simple process and material conservation.
Owner:BEIJING D VENTUREPHARM TECH DEV
Orally disintegrating tablet containing sertraline hydrochloride and preparation method thereof
InactiveCN105616367ASimple processGood taste masking effectOrganic active ingredientsNervous disorderOrally disintegrating tabletPOLACRILIN POTASSIUM
The invention discloses a preparation method of an orally disintegrating tablet containing sertraline hydrochloride. The orally disintegrating tablet contains a sertraline hydrochloride-polacrilin potassium compound and other pharmaceutically acceptable auxiliary materials. An ion exchange technology is adopted to mask taste, and the orally disintegrating tablet is prepared by wet granulation and tabletting and can be disintegrated in the oral cavity without needing water, thereby effectively masking the bitter, pungent and spicy taste of the main active ingredient, namely sertraline hydrochloride. The preparation method is simple and convenient in process and suitable for industrial production.
Owner:AVENTIS PHARMA HAINAN
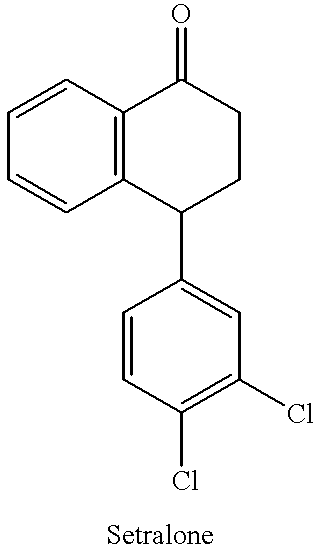
Sertraline hydrochloride intermediates (+/-)-tetralone and chiral chromatographic splitting method thereof
InactiveCN104122342AEfficient analysisEfficient separationComponent separationChromatographic separationStationary phase
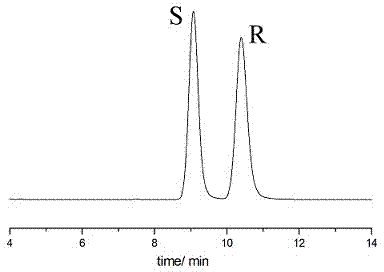
The invention relates to a liquid chromatographic separation and analysis method, and particularly relates to sertraline hydrochloride intermediates (+ / -)-tetralone and a chiral chromatographic splitting method thereof. The sertraline hydrochloride intermediates (+ / -)-tetralone and the chiral chromatographic splitting method thereof are provided, wherein the method comprises the following steps: taking the sertraline hydrochloride intermediates (+ / -)-tetralone, and dissolving in an aliphatic hydrocarbon-alcohol mixed liquid; and providing a polysaccharide chiral stationary phase, taking the solution for sample introduction, and carrying out chiral chromatographic separation under a condition with the aliphatic hydrocarbon-alcohol mixed liquid as a mobile phase. A coating type polysaccharide chiral stationary phase material is adopted for effective separation and analysis on the sertraline hydrochloride intermediates and enantiomers thereof, impurities brought by preparation of sertraline hydrochloride with a new process are effectively controlled, and the quality of raw material drugs and preparations can be further controlled.
Owner:RAFFLES PHAMRMATECH CO LTD +1
Preparation method of chiral sertraline hydrochloride
ActiveCN103570555ASimple and fast operationImprove efficiencyAmino compound purification/separationAlcoholMandelic acid
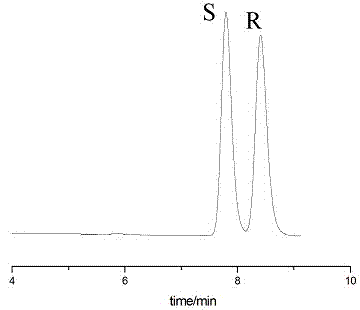
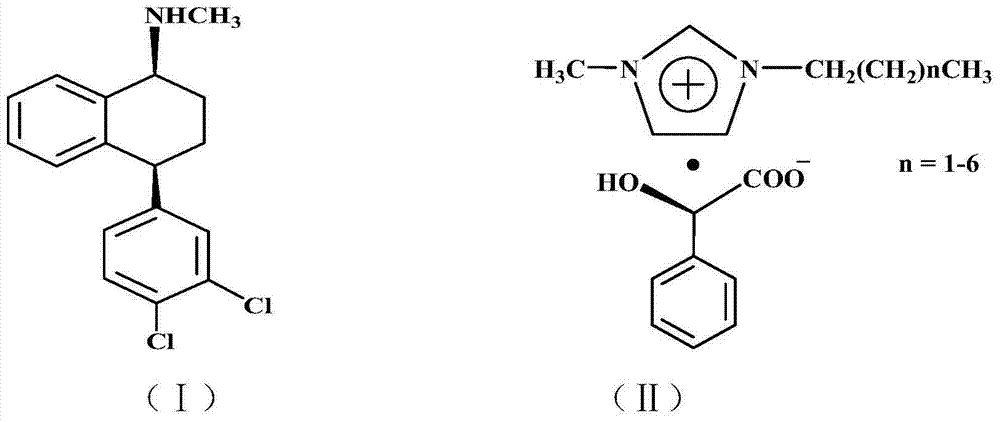
The invention discloses a preparation method of chiral sertraline hydrochloride. The method comprises the following steps: mixing racemic sertraline and a chiral ionic liquid of D-mandelic acid; slowly stirring at 10-100 DEG C for full reaction; after reaction, adding absolute ethyl alcohol into the reaction mixed liquid for continuously stirring for 1 hour; filtering; hydrolyzing the filter cakes; acidizing by hydrochloric acid to obtain Cis-(1S, 4S)-sertraline hydrochloride; and recovering the filtrate to obtain the chiral ionic liquid which is recycled. According to the invention, the chiral ionic liquid is taken as a solvent and a resolving agent, so that the yield is greater than or equal to 80%, the optical purity is greater than or equal to 98.0%, and the content is greater than or equal to 99.0%. The method is simple and convenient to operate, high in efficiency, less in three wastes and convenient in post-treatment, and the ionic liquid can be repeatedly used, so that the preparation method is an economical and practical green and environment-friendly technology.
Owner:上海衡山药业有限公司
Sertraline hydrochloride polymorphs
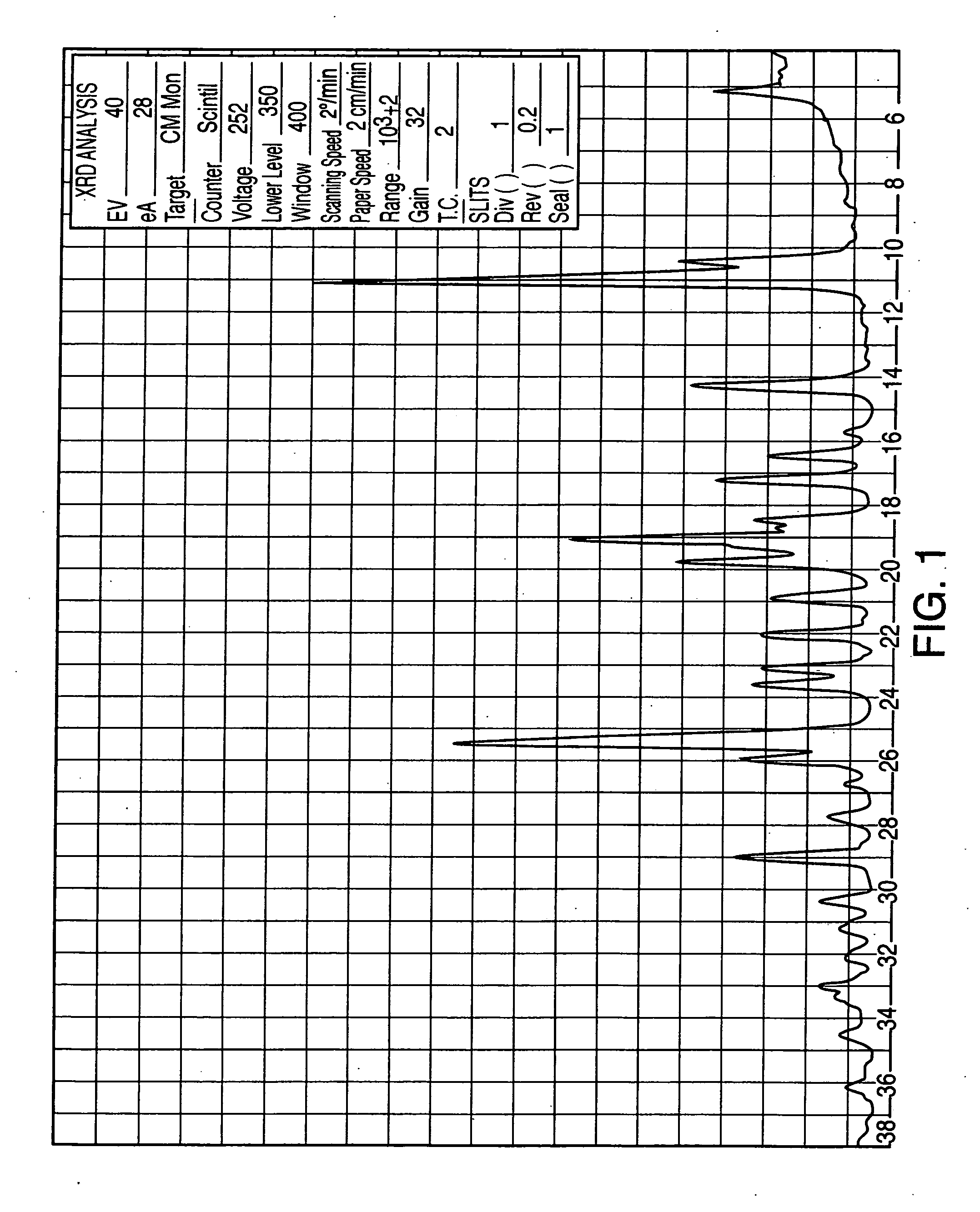
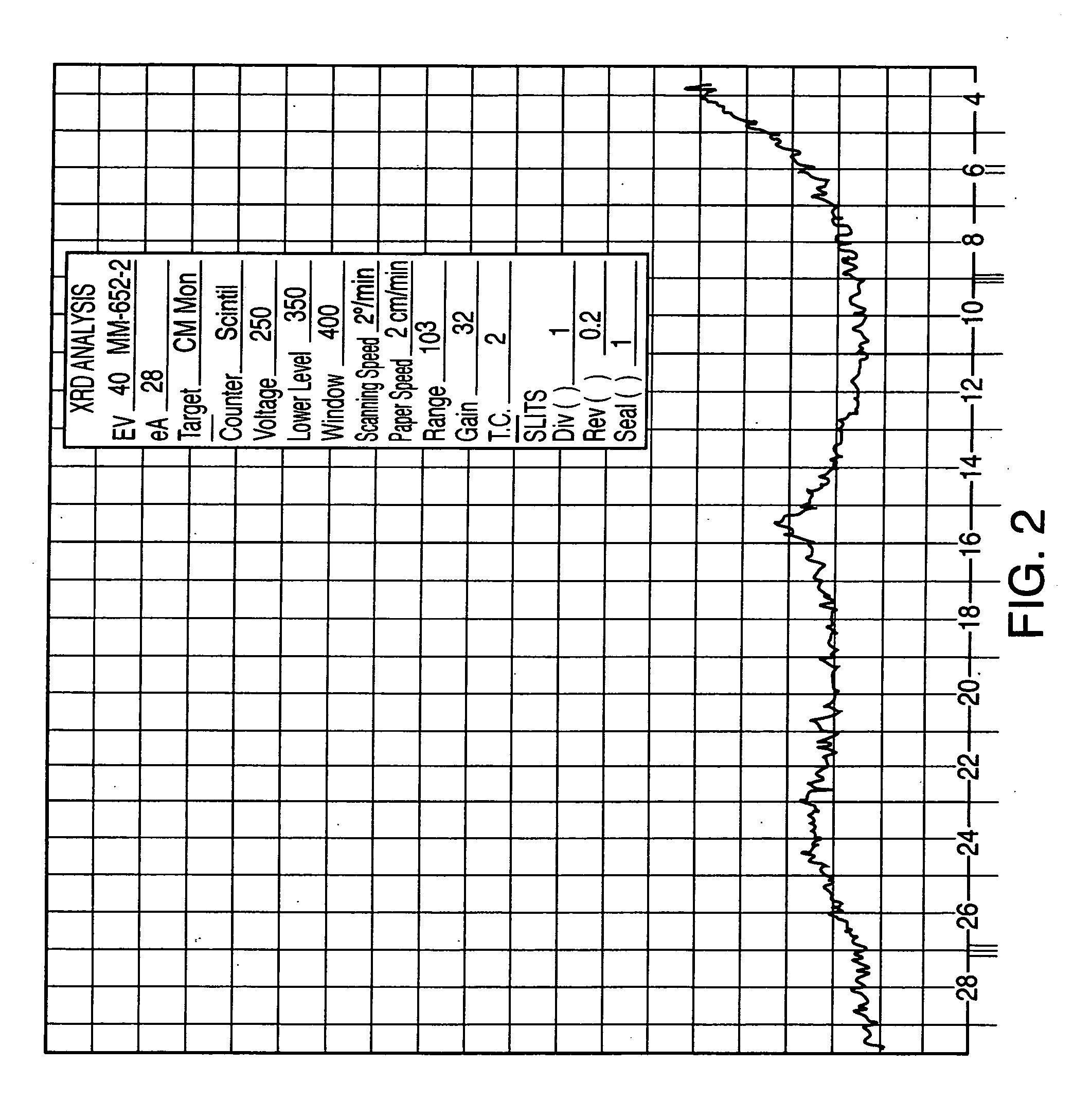
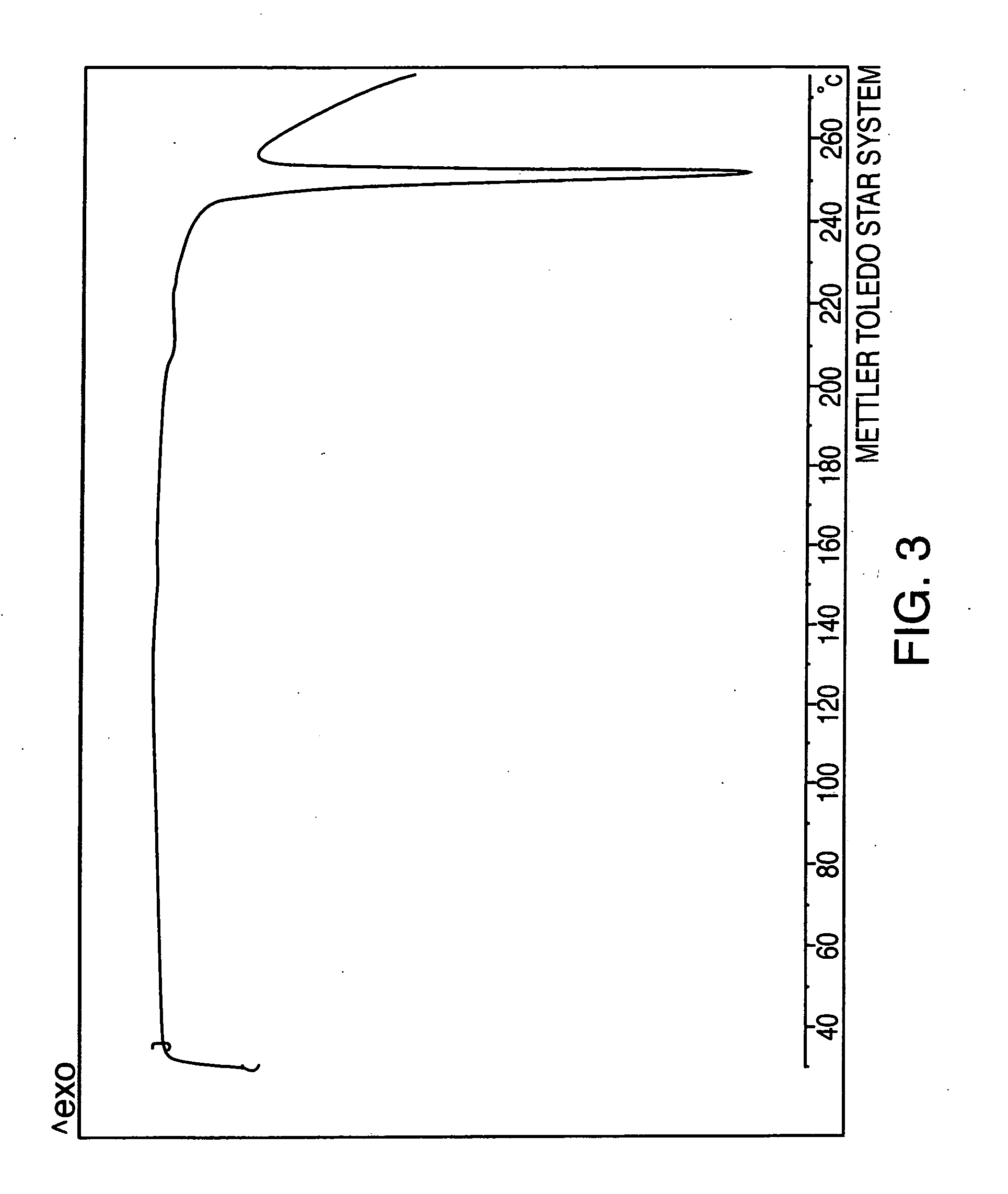
The present invention is directed to forms II, III, V, VI, VII, VIII, IX and X of sertraline hydrochloride and novel methods for their preparation. According to the present invention, sertraline hydrochloride polymorph II may be produced by slurrying sertraline hydrochloride polymorph VI in aprotic organic solvent. Sertraline hydrochloride polymorphic form III may be produced by heating sertraline hydrochloride polymorphs V and VI. Sertraline hydrochloride forms V and VI may be produced from either sertraline hydrochloride or sertraline base by crystallization. Sertraline hydrochloride Form VII may be produced by suspending sertraline chloride polymorph V in water, followed by filtration. Sertraline hydrochloride Forms VIII and IX may be produced by suspending sertraline base in water followed by acidification and filtration. Sertraline hydrochloride Form X may be produced by suspending sertraline hydrochloride in benzyl alcohol with heating, followed by filtration.
Owner:TEVA PHARMA IND LTD
Methods for preparing sertraline hydrochloride polymorphs
InactiveUS20050032907A1Increase productionOrganic active ingredientsBiocideHydrochlorideCrystallization
This invention relates to crystalline polymorphs of sertraline hydrocloride, i.e. (1S-cis)-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-1-naphtalenamine hydrochloride, denominated form I, II and V, and methods for their preparation.
Owner:RECORDATI CHEM & PHARMA
Sertraline hydrochloride form II and methods for the preparation thereof
Owner:RECORDATI CHEM & PHARMA
Process for the preparation of polymorphs of selective serotonin reuptake inhibitor
InactiveCN1902157AOrganic active ingredientsAmino compound purification/separationSerotonin receptor inhibitorsSerotonin reuptake inhibitor
Owner:CIPLA LTD
Sertraline hydrochloride polymorphs
The present invention is directed to forms II, III, V, VI, VII, VIII, IX and X of sertraline hydrochloride and novel methods for their preparation. According to the present invention, sertraline hydrochloride polymorph II may be produced by slurrying sertraline hydrochloride polymorph VI in aprotic organic solvent. Sertraline hydrochloride polymorphic form III may be produced by heating sertraline hydrochloride polymorphs V and VI. Sertraline hydrochloride forms V and VI may be produced from either sertraline hydrochloride or sertraline base by crystallization. Sertraline hydrochloride Form VII may be produced by suspending sertraline chloride polymorph V in water, followed by filtration. Sertraline hydrochloride Forms VIII and IX may be produced by suspending sertraline base in water followed by acidification and filtration. Sertraline hydrochloride Form X may be produced by suspending sertraline hydrochloride in benzyl alcohol with heating, followed by filtration.
Owner:TEVA PHARMA IND LTD
Sertraline hydrochloride dripping pills and the prepn
InactiveCN1526382ADisintegration and dissolution fastHigh dissolution rateOrganic active ingredientsNervous disorderMedicineMaterial consumption
The present invention is Sertraline hydrochloride dripping pill prepared through superfine crushing and dripping pill producing process. The Sertraline hydrochloride dripping pill has raised disintegrating and dissolving speed, high digestion rate and digestion degree, fast acting, high stability, reduced supplementary material consumption, low production cost and easy carrying about and taking. It may be sucked in mouth or swallowed, has excellent conformity and is especially suitable for children, the aged and other patients with dysphagia.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Method for separating non-geometric proportion cis-sertraline hydrochloride mixture
InactiveCN101100431AMake full use of resourcesAmino compound purification/separationPropanol1-Propanol
A method for separating cis-Schtrilin hydrochloride mixture is carried out by keeping temperature for mixture of cis-(+) Schtrilin hydrochloride and cis-(-) Schtrilin hydrochloride in solution of methanol, ethanol, 2-propanol, 1-propanol or their mixtures at a range of 40 deg. C to solvent reflux temperature, agitating for 1-16 hrs, filtering, separating to obtain cis-racemic Schtrilin hydrochloride, decreasing temperature and crystallizing to obtain final product. It's simple and safe and has full use of resources.
Owner:ZHEJIANG LEPU PHARMA CO LTD
Processes for preparing sertraline
InactiveUS20070010694A1Organic compound preparationAmino compound preparationCombinatorial chemistrySertraline Hydrochloride
Owner:TEVA PHARM USA INC
Process for converting trans-sertraline to cis-sertraline
A process for preparing sertraline hydrochloride includes reacting trans-N-methyl-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-1-napthaleneamine with a base in an aqueous basic organic solvent.
Owner:DR REDDYS LAB LTD +1
Taste-masking orally disintegrating tablets containing sertraline hydrochloride and preparation method thereof
InactiveCN106109432ASimple processGood taste masking effectOrganic active ingredientsNervous disorderFlavorOrally disintegrating tablet
The invention discloses a method for preparing taste-masking orally disintegrating tablets containing sertraline hydrochloride. The orally disintegrating tablets contain sertraline hydrochloride and other pharmaceutically acceptable excipients. The taste can be masked by adopting a granule coating technology, the prepared orally disintegrating tablets can be disintegrated in oral cavity without using water, bitterness and spicy and pungent flavor of sertraline hydrochloride, the main active ingredient of the orally disintegrating tablets, can be effectively masked, and the preparation method is simple and convenient in process and suitable for industrial production.
Owner:BEIJING VENTUREPHARM BIOTECH
Drug compound for resisting against anxiety and improving sleep and application thereof
InactiveCN107595849AOvercome the defect of prone to drug dependenceOvercome curative effectOrganic active ingredientsNervous disorderBULK ACTIVE INGREDIENTInsomnia
The invention relates to a drug compound for resisting against anxiety and improving sleep and an application thereof. The drug compound comprises the following active ingredients: tandospirone citrate, fluoxetine and sertraline hydrochloride. According to the invention, the tandospirone citrate, fluoxetine and sertraline hydrochloride are jointly used for treating anxiety disorder and insomnia, the defects of poor drug effect and easiness in generating drug dependence of the patient of single drug usage are overcome, the joint use of three drugs has the synergic effects of resisting against anxiety and improving sleep and the function of reducing toxicity can be achieved in the manner of reducing the clinical dosage of one or two drugs.
Owner:GUILIN HAOXIN TECH SERVICE
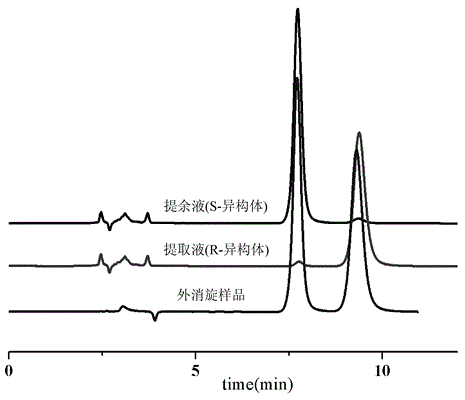
High-speed countercurrent chromatographic separation method for sertraline-hydrochloride cis-trans isomer
ActiveCN109651170AEasy to recycleMild conditionsAmino compound purification/separationOrganic chemistry methodsChromatographic separationHydrogen phosphate
The invention discloses a high-speed countercurrent chromatographic separation method for sertraline-hydrochloride cis-trans isomer. The method includes the specific steps that an organic solvent anda citric acid-sodium hydrogen phosphate buffer solution containing hydroxypropyl-beta-cyclodextrin are mixed according to the volume ratio of 1:(0.5-10) to form a solvent system, standing layering isconducted, an obtained organic phase serves as a stationary phase, and a water phase serves as a mobile phase; separation is conducted with the countercurrent chromatography, and cis-form sertraline hydrochloride and trans-form sertraline hydrochloride are obtained. In the separation and purification process, the condition is mild, the separation process is efficient and convenient and fast, the separation and preparation quantity is large, the separation degree is high, and both the purity of the cis-form sertraline hydrochloride obtained after purification and the purity of the trans-form sertraline hydrochloride obtained after purification are larger than 98%.
Owner:ZHEJIANG UNIV OF TECH
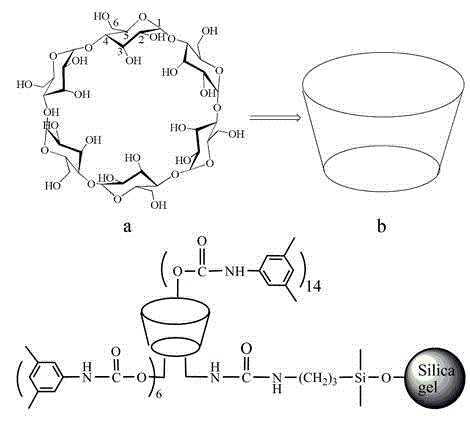
Application of bonded 3,5-dimethylcarbaniloylated beta-cyclodextrin chiral stationary phase in chiral analysis and/or separation of sertraline hydrochloride intermediate (+/-)-Tetralone
InactiveCN104826619AEasy to separateSimple post-processingComponent separationOther chemical processesChromatographic separationTetralone
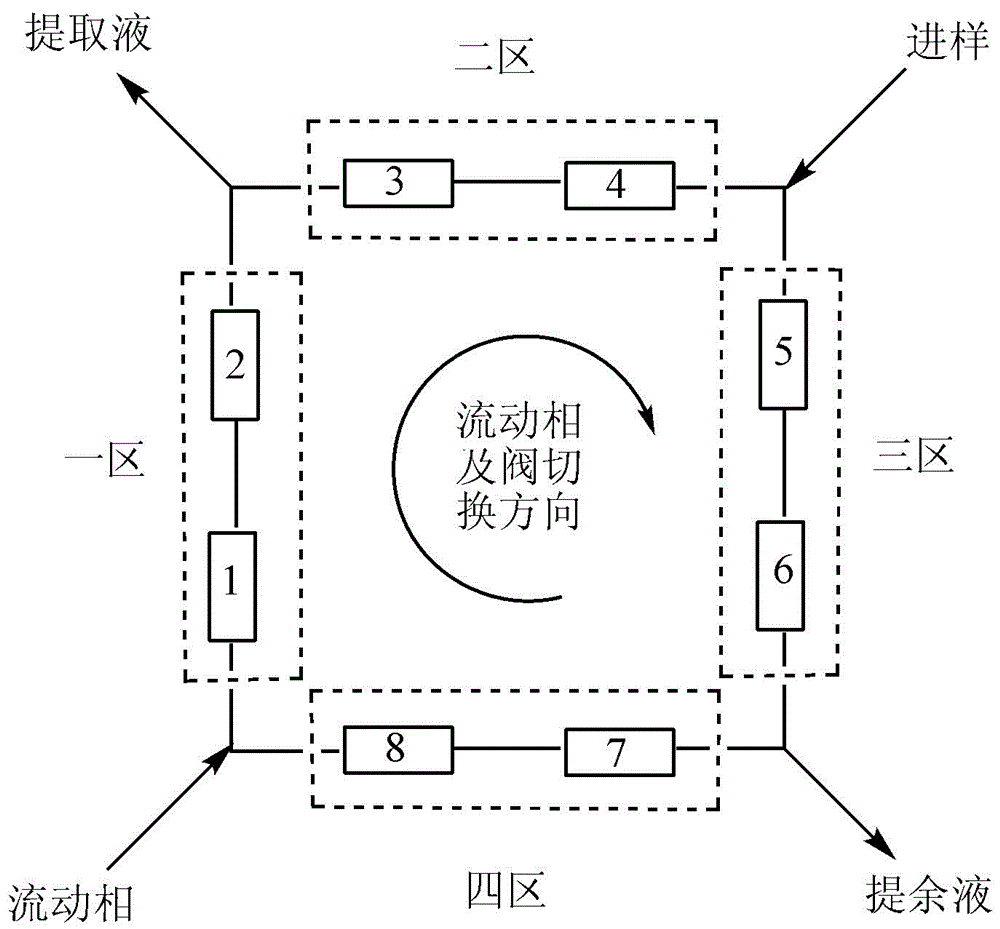
The invention discloses application of a bonded 3,5-dimethylcarbaniloylated beta-cyclodextrin chiral stationary phase in chiral analysis and / or separation of a sertraline hydrochloride intermediate (+ / -)-Tetralone. The invention also discloses application of the bonded 3,5-dimethylcarbaniloylated beta-cyclodextrin chiral stationary phase in chiral separation of a sertraline hydrochloride intermediate (+ / -)-Tetralone by simulated moving bed chromatography. The cyclodextrin chiral stationary phase can have better separating effects than the existing chiral stationary phases under identical conditions. The separation method, which adopts a four-region simulated moving bed system and uses a bonded 3,5-dimethylcarbaniloylated beta-cyclodextrin chiral stationary phase as a chiral column filler and an n-hexane-fatty alcohol mixed solution as a mobile phase, has the advantages of continuous sampling, high automation degree, high product purity and low mobile phase consumption, is suitable for industrialized chromatographic separation on (+ / -)-Tetralone to obtain the optically Tetralone isomer product, and widens the application range of the cyclodextrin chiral stationary phase in the preparative chromatography system.
Owner:广州研创生物技术发展有限公司
Methods for preparing sertraline hydrochloride polymorphs
This invention relates to crystalline polymorphs of sertraline hydrocloride, i.e. (1S-cis)-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-1-naphtalenamine hydrochloride, denominated form I, II and V, and methods for their preparation.
Owner:RECORDATI CHEM & PHARMA
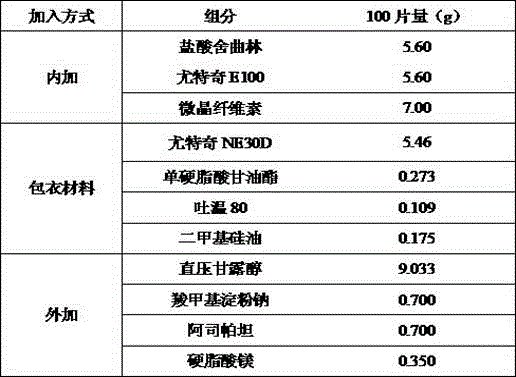
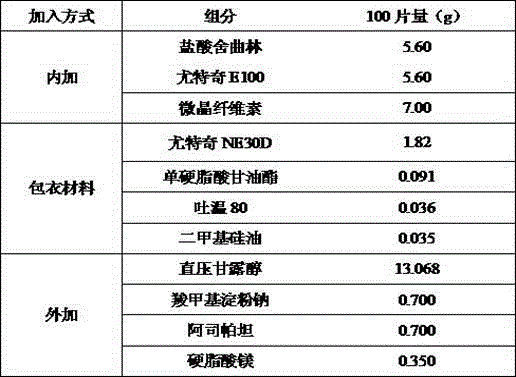
High-stability sertraline hydrochloride capsule and preparation method thereof
ActiveCN106309404ALong-term stabilityImprove stabilityOrganic active ingredientsNervous disorderSoft materialsLactose
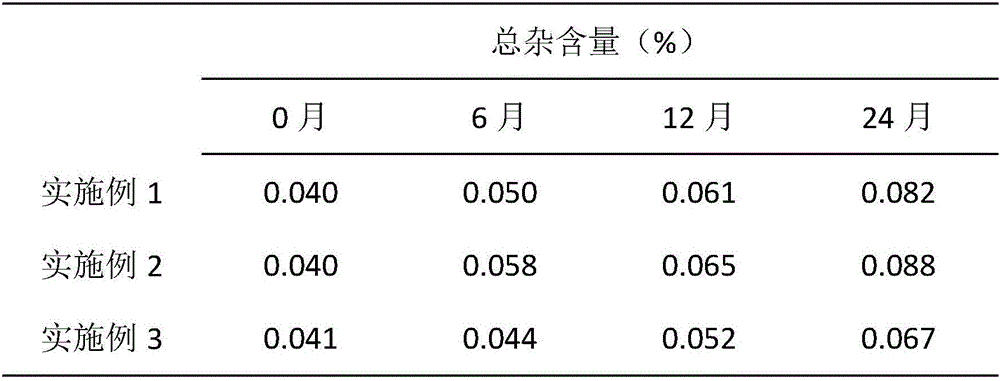
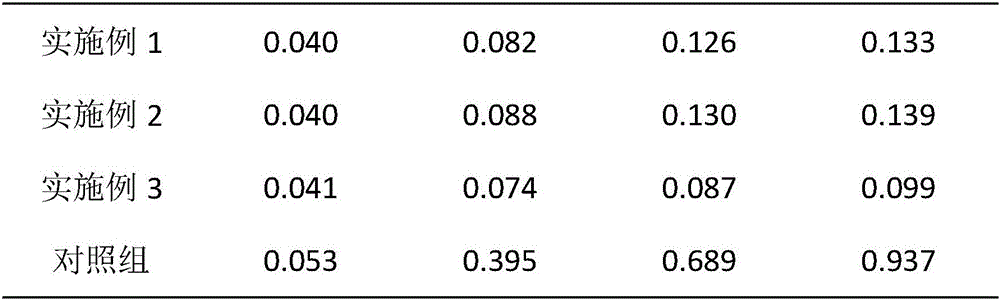
The invention provides a preparation method of a high-stability sertraline hydrochloride capsule. The method comprises the following steps: (1) mixing the following components in parts by weight: 20-25 parts of sertraline hydrochloride, 30-38 parts of lactose, 25-30 parts of microcrystalline cellulose, 20-22 parts of low-substituted hydroxypropyl cellulose and 5-10 parts of mannitol; (2) adding ethanol into the product obtained in the step (1) to prepare a soft material, screening, and granulating; (3) drying the product obtained in the step (2), and then granulating; and (4) filling the product obtained in the step (3) into a hard gelatin capsule shell. The capsule prepared by the invention has long-time stability; the capsule can be preserved for 24 months under the conditions of 30 DEG C and 70% RH (relative humidity), and the total impurity content does not exceed 0.09%; and the capsule can be preserved for 60 days under the conditions of 60 DEG C and 92.5% RH, and the total impurity content does not exceed 0.14%.
Owner:四川省百草生物药业有限公司
Preparation method of chiral sertraline hydrochloride
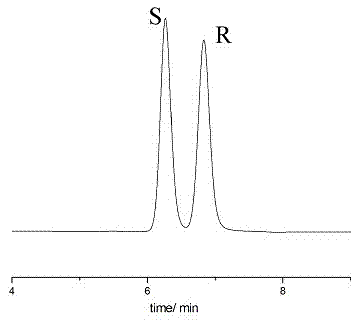
ActiveCN109627174AHigh recovery rateHigh yieldAmino compound purification/separationOrganic compound preparationInorganic saltsOrganic solvent
The invention discloses a preparation method of chiral sertraline hydrochloride. The method comprises the steps that cis-sertraline hydrochloride and alkali of alkali metal salt containing D-mandelicacid are mixed and stirred in a solvent, after dehydration, inorganic salt is filtered out, a filtrate is cooled and crystallized, and solid-liquid separation is carried out; coarse D-mandelic acidsertraline is recrystallized, and solid-liquid separation is carried out; refined D-mandelic acid sertraline reacts with inorganic alkali in a mixed solvent of water and the organic solvent, and dehydration, crystallization and solid-liquid separation are carried out; a filtrate and hydrogen chloride are mixed and stirred in an organic solvent, after salification is completed, solid-liquid separation, washing and drying are carried to obtain the chiral sertraline hydrochloride. The preparation method of the chiral sertraline hydrochloride has the advantages that the alkali metal salt of the D-mandelic acid is taken as a resolving agent, the yield of cis-(1S,4S)-hydrochloride sertraline is high, the single organic solvent can be adopted in the entire process, the recovery rate of the alkalimetal salt of the D-mandelic acid and solvent is high, the alkali metal salt and the solvent can be recycled, and the preparation method is simple in operation, safe, environmentally friendly and suitable for industrial production.
Owner:SHANGYU JINGXIN PHARMA +1
Process for preparation of polymorphic form of sertraline hydrochloride
InactiveUS20060167113A1Efficient and cost-effectiveEfficient and cost-effective processOrganic active ingredientsBiocideSolventBULK ACTIVE INGREDIENT
The invention discloses a process for preparation of sertraline salts particularly sertraline hydrochloride Form V by dissolving or suspending sertraline mandelate in a solvent, reducing the pH of the solution or the suspension and isolating salt of sertraline The invention also provides for a pharmaceutical composition comprising said sertraline salt as active ingredient
Owner:TORRENT PHARMA LTD
Preparation method of chiral sertraline hydrochloride
ActiveCN103570555BSimple and fast operationImprove efficiencyAmino compound purification/separationAlcoholMandelic acid
The invention discloses a preparation method of chiral sertraline hydrochloride. The method comprises the following steps: mixing racemic sertraline and a chiral ionic liquid of D-mandelic acid; slowly stirring at 10-100 DEG C for full reaction; after reaction, adding absolute ethyl alcohol into the reaction mixed liquid for continuously stirring for 1 hour; filtering; hydrolyzing the filter cakes; acidizing by hydrochloric acid to obtain Cis-(1S, 4S)-sertraline hydrochloride; and recovering the filtrate to obtain the chiral ionic liquid which is recycled. According to the invention, the chiral ionic liquid is taken as a solvent and a resolving agent, so that the yield is greater than or equal to 80%, the optical purity is greater than or equal to 98.0%, and the content is greater than or equal to 99.0%. The method is simple and convenient to operate, high in efficiency, less in three wastes and convenient in post-treatment, and the ionic liquid can be repeatedly used, so that the preparation method is an economical and practical green and environment-friendly technology.
Owner:上海衡山药业有限公司
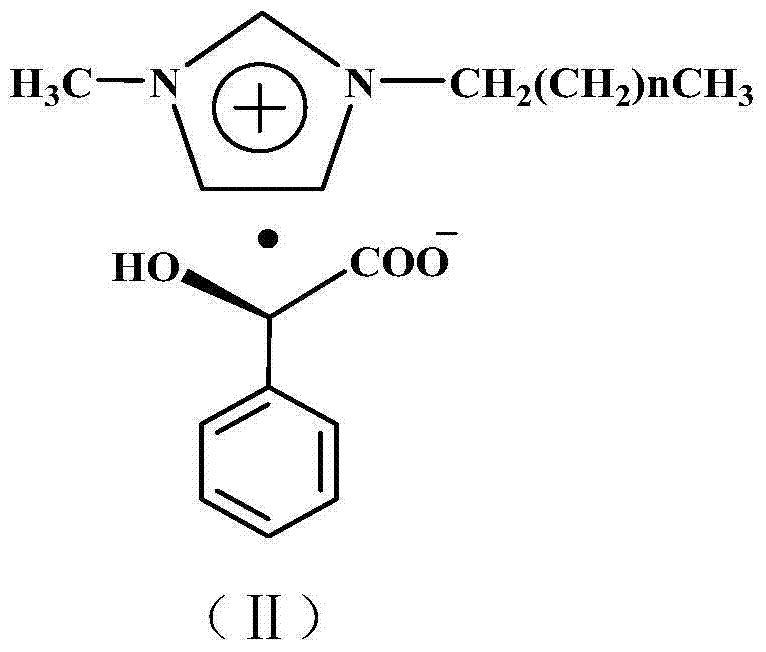
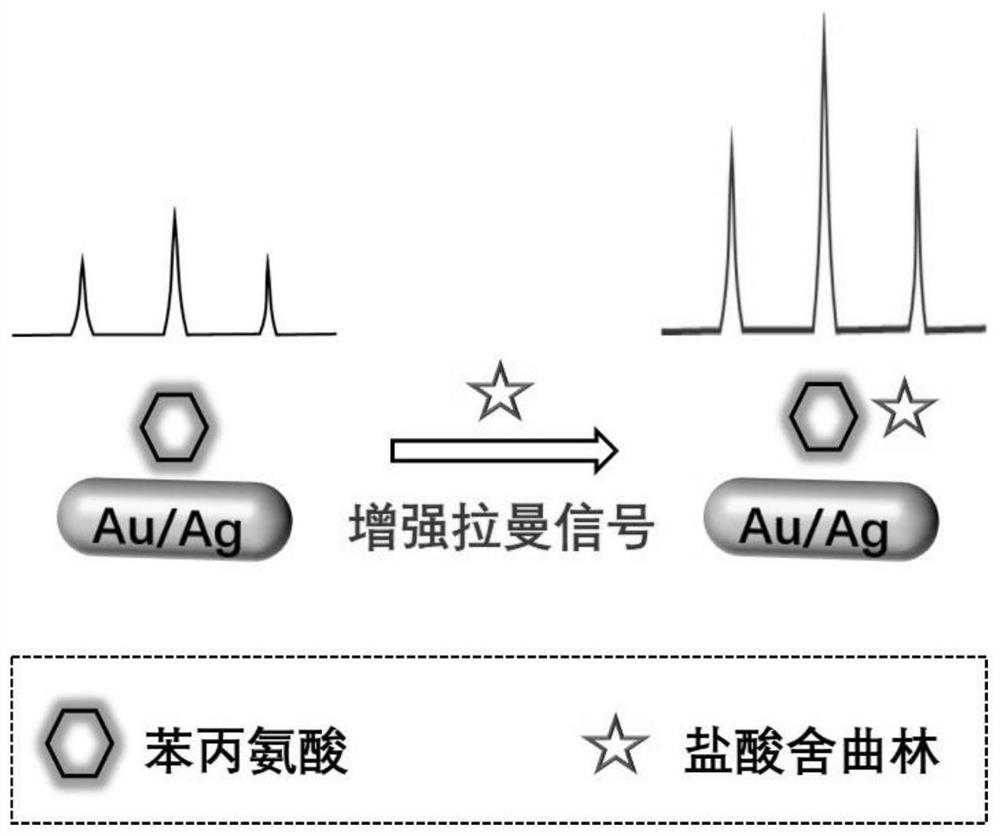
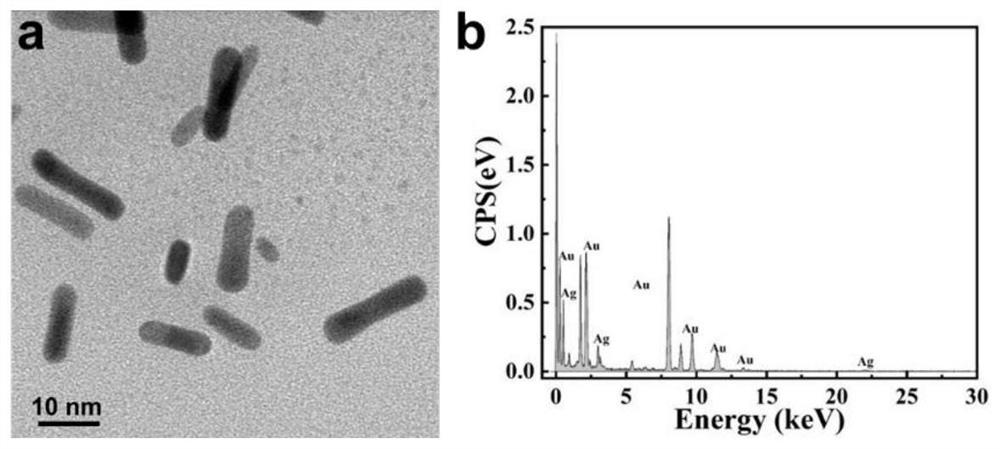
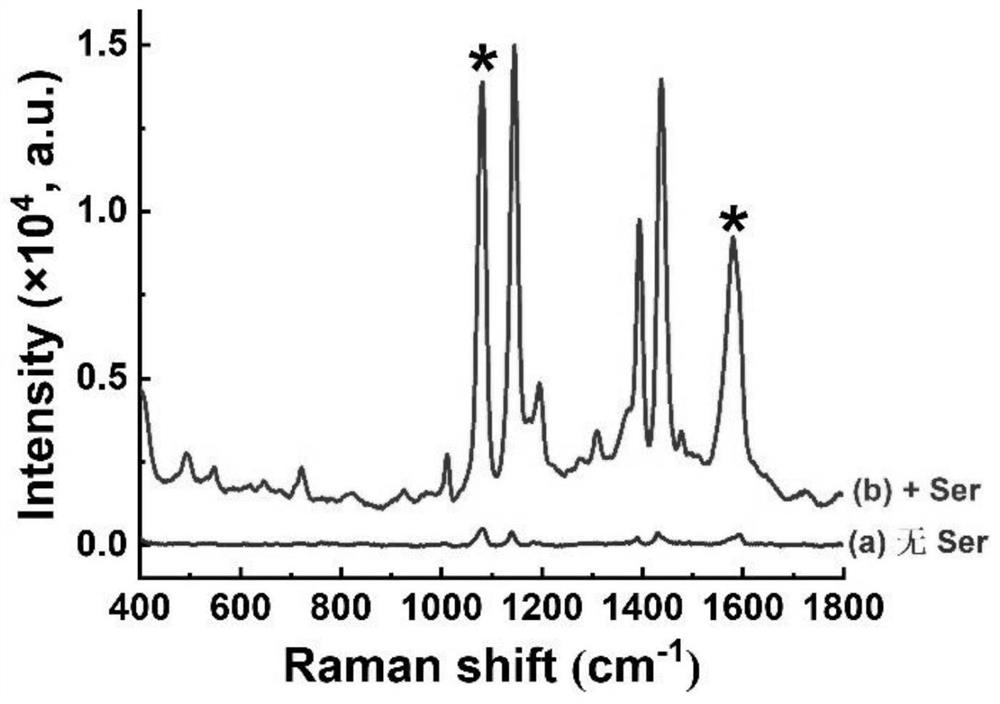
Raman spectrum detection method of phenylalanine enantiomer
PendingCN113588626ARealize detectionImprove accuracyRaman scatteringChiral selectivitySignal amplifier
The invention discloses a Raman spectrum detection method for phenylalanine enantiomers, and belongs to the field of spectral analysis and chiral recognition. The detection method comprises the following steps: taking a gold-silver nano composite material as a surface enhanced Raman scattering substrate, taking chiral sertraline hydrochloride as a chiral selective Raman signal amplifier, adsorbing phenylalanine on the surface of the substrate, and performing chiral selective interaction with the chiral sertraline hydrochloride on the surface of the gold-silver nano composite material, and then, measuring Raman scattering signals of phenylalanine, wherein the Raman scattering signals with the high enhancement multiple are D-phenylalanine, and otherwise, the Raman scattering signals are L-phenylalanine. The Raman scattering signal of phenylalanine is enhanced through interaction between chiral molecules, detection and distinguishing of phenylalanine enantiomers are achieved according to Raman spectrum differences, and the method is simple, sensitive and good in accuracy.
Owner:SHANGHAI NORMAL UNIVERSITY
Novel process for the preparation of sertraline hydrochloride form II
InactiveUS20090023955A1Organic compound preparationAmino compound preparationOrganic solventChemistry
The present invention relates to novel processes to produce sertraline hydrochloride Form II comprising the steps of forming a solution of sertraline hydrochloride in a polar organic solvent and adding this solution to a less polar organic solvent.
Owner:APOTEX PHARMACHEN INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com