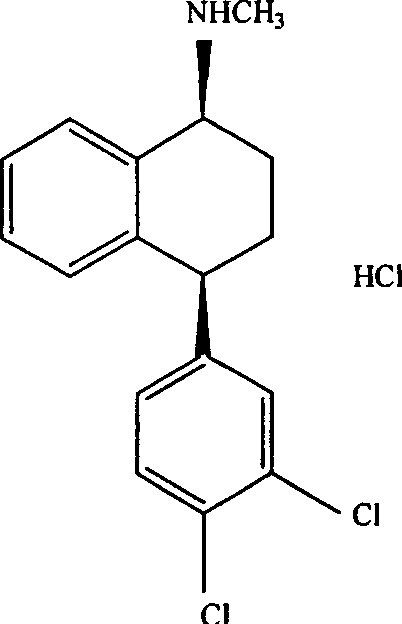
Process for the preparation of polymorphs of selective serotonin reuptake inhibitor
A technology of crystal form and solvent is applied in the field of preparation of selective serotonin reuptake inhibitor polymorph, and can solve the problems of undisclosed solid state properties of sertraline hydrochloride and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of Sertraline Hydrochloride Form II
[0041] 20 grams of sertraline acetate was suspended in a mixture of 100 ml of isopropanol and 4 ml of toluene. The mixture was heated to 50°C to obtain a clear solution, and dried hydrogen chloride gas was introduced to adjust the pH to 1-2. The reaction exothermed and the temperature rose to 60°C. The reaction was gradually cooled to room temperature. The precipitated solid was filtered and washed with isopropanol, and dried in a vacuum at 80° C. for 4 to 5 hours to obtain Sertraline Hydrochloride Form II.
Embodiment 2
[0043] Preparation of Sertraline Hydrochloride Form III
[0044] 20 grams of sertraline acetate was suspended in a mixture of 100 ml of isopropanol and 4 ml of toluene. The mixture was heated to 50°C to obtain a clear solution, and dried hydrogen chloride gas was passed through to adjust the pH to 1-2. The reaction exothermed and the temperature rose to 60°C. The reaction mixture was quickly cooled to 15-20°C in an ice bath within 15-20 minutes. The precipitated solid was filtered, washed with isopropanol, and dried in vacuum at 80° C. for 4 to 5 hours to obtain Sertraline Hydrochloride Form III.
Embodiment 3
[0046] Preparation of Sertraline Hydrochloride Form IV
[0047] Suspend 50 grams of sertraline acetate in 250 ml of isopropanol at room temperature. The mixture was heated to 50°C to obtain a clear solution, and dried hydrogen chloride gas was passed in to adjust the pH to 1-2. The reaction mixture was cooled to 15-20°C within 30 minutes with stirring. The precipitated solid is filtered and washed with isopropanol, and dried in a fluidized bed dryer at 60° C. for 4 to 5 hours to obtain Sertraline Hydrochloride Form IV.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com