Sertraline hydrochloride intermediates (+/-)-tetralone and chiral chromatographic splitting method thereof
A technology of sertraline hydrochloride and chiral chromatography, which is applied in the directions of material separation, analysis materials, measuring devices, etc., can solve the problems such as infeasible chemical separation methods, and achieve the effect of quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
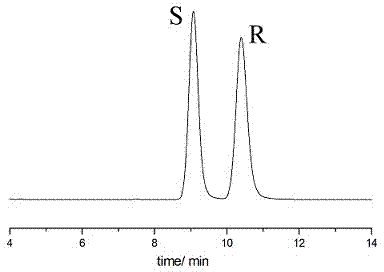
Embodiment 1
[0038] Instruments and Conditions
[0039] High performance liquid chromatography: Shimadzu LC-15C
[0040] Chromatographic column: Enantiopak CCDP column (150x4.6 mm)
[0041] Mobile phase: n-hexane:isopropanol=95:5 (v:v)
[0042] Flow rate: 1.0mL / min
[0043] Injection volume: 10μL
[0044] Detection wavelength: 214nm (maximum absorption wavelength)
[0045] Experimental steps:
[0046] Weigh 25 mg of sertraline intermediate (±)-Tetralone, place it in a 25.00 mL volumetric flask, add mobile phase to dilute to 25.00 mL. as a mixed sample solution.
[0047] Take 4 respectively S - Tetralone and 4 R - Tetralone each 25mg, respectively placed in 25.00mL volumetric flask, adding mobile phase to dilute to 25.00mL, as a qualitative control solution.
[0048] Take the control solution and the mixed sample solution respectively, carry out high-performance liquid chromatography separation and analysis according to the above conditions, and record the chromatograms. see attac...
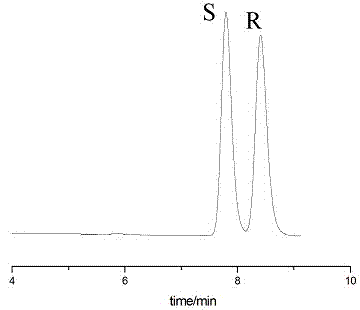
Embodiment 2
[0050] Instruments and Conditions
[0051] High performance liquid chromatography: Shimadzu LC-15C
[0052] Column: Enantiopak CADP column (150x4.6 mm)
[0053] Mobile phase: n-hexane: ethanol = 99:1 (v:v)
[0054] Flow rate: 1.0mL / min
[0055] Injection volume: 10μL
[0056] Detection wavelength: 214nm (maximum absorption wavelength)
[0057] Experimental steps:
[0058] Weigh 25mg of sertraline intermediate (±)-Tetralone, place it in a 25.00mL volumetric flask, add mobile phase to dilute to 25.00mL. as a mixed sample solution.
[0059] Take 4 respectively S - Tetralone and 4 R - Tetralone each 25mg, respectively placed in 25.00mL volumetric flask, adding mobile phase to dilute to 25.00mL, as a qualitative control solution.
[0060] Take the control solution and the mixed sample solution respectively, carry out high-performance liquid chromatography separation and analysis according to the above conditions, and record the chromatograms. see attached results figure...
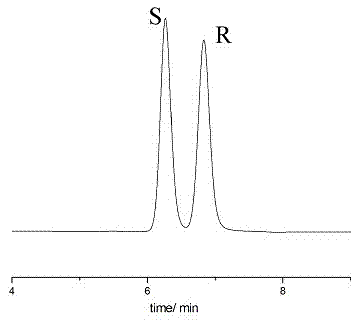
Embodiment 3
[0062] Instruments and Conditions
[0063] High performance liquid chromatography: Shimadzu LC-15C
[0064] Chromatographic column: Enantiopak CCDP column (150x4.6 mm)
[0065] Mobile phase: n-hexane: ethanol = 90:10 (v:v)
[0066] Flow rate: 1.0mL / min
[0067] Injection volume: 10μL
[0068] Detection wavelength: 214nm (maximum absorption wavelength)
[0069] Experimental steps:
[0070] Weigh 25 mg of sertraline intermediate (±)-Tetralone, place it in a 25.00 mL volumetric flask, add mobile phase to dilute to 25.00 mL. as a mixed sample solution.
[0071] Take 4 respectively S - Tetralone and 4 R - Tetralone each 25mg, respectively placed in 25.00mL volumetric flask, adding mobile phase to dilute to 25.00mL, as a qualitative control solution.
[0072] Take the control solution and the mixed sample solution respectively, carry out high-performance liquid chromatography separation and analysis according to the above conditions, and record the chromatograms. see attac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com