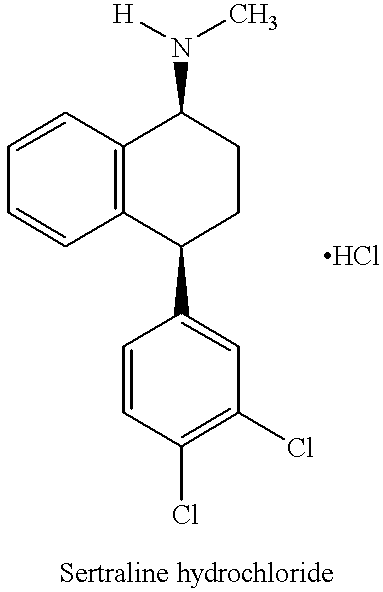
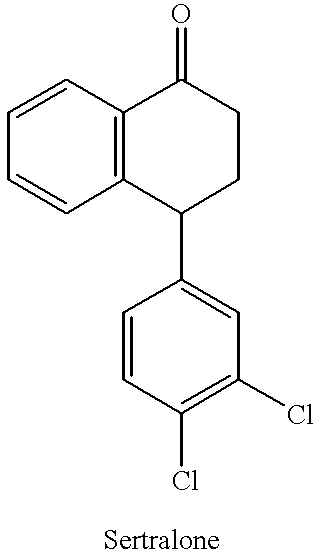
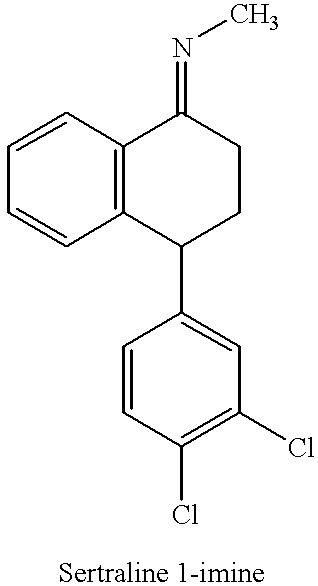
Novel process for preparing (+)-cis-sertraline
a technology of cis/trans-sertraline and process, which is applied in the field of new process for preparing (+)cissertraline, can solve the problems of cis/trans-sertraline as described, the cost of multiple recrystallizations, and the complexity of 518 patents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0028] Step 1: Preparation of Sertraline-1-imine (Schiff Base):
[0029] Sertralone (100 g) was dissolved in toluene (1400 mL) and the solution so obtained was cooled to 0-5.degree. C. Methyl amine gas (38.7 g) was bubbled through the solution while maintaining the temperature between 0-5.degree. C. To the above solution, TiCl.sub.4 (20 mL) was added dropwise while maintaining the temperature below 10.degree. C. The reaction mixture was allowed to warm to room temperature and then was stirred at room temperature for 3 hours. Upon completion of the reaction, TiO.sub.2 was removed by filtration and the filtrate was evaporated to dryness. The solid obtained after evaporation was sertraline-1-imine (101.17 g; yield 100%).
[0030] Step 2: Preparation of (.+-.)-cis / trans-sertraline (Sertraline Racemate) Free Base:
[0031] A slurry of sertraline-1-imine (Schiff base) (10 g) in t-butyl-methyl-ether (MTBE) (270 mL) was hydrogenated in the presence of Pd / C (10% loading) at 40.degree. C., at 1 atm H....
example 2
[0040] (.+-.)-Sertraline hydrochloride (5 g) was dissolved in ethanol (20 mL) and KOH powder (85%) was added to the solution. The slurry was stirred at room temperature for 2.5 hrs. After stirring the solids were removed by filtration and the solution was treated with D-(-)-mandelic acid (2.66 g). Precipitation occurred and the stirring was continued for 24 hours. (+)-Sertraline-mandelate was isolated by filtration and washed with ethanol and then dried to yield 2.70 g of (+)-sertraline-mandelate.
[0041] Optical purity of (+)-sertraline-mandelate was established by chiral HPLC methods. Table 3 provides additional data and reaction conditions concerning the optical resolution of sertraline. In Table 3, the % Enantiomer RR is the percent area of the RR-enantiomer as determined by chiral HPLC; Chiracel OD-H, 250.times.4.6 nm, 5.mu., column temperature 5.degree. C. In Table 3, the Yield % is the yield of optical resolution, based on the % SS-enantiomer of sertraline hyd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com