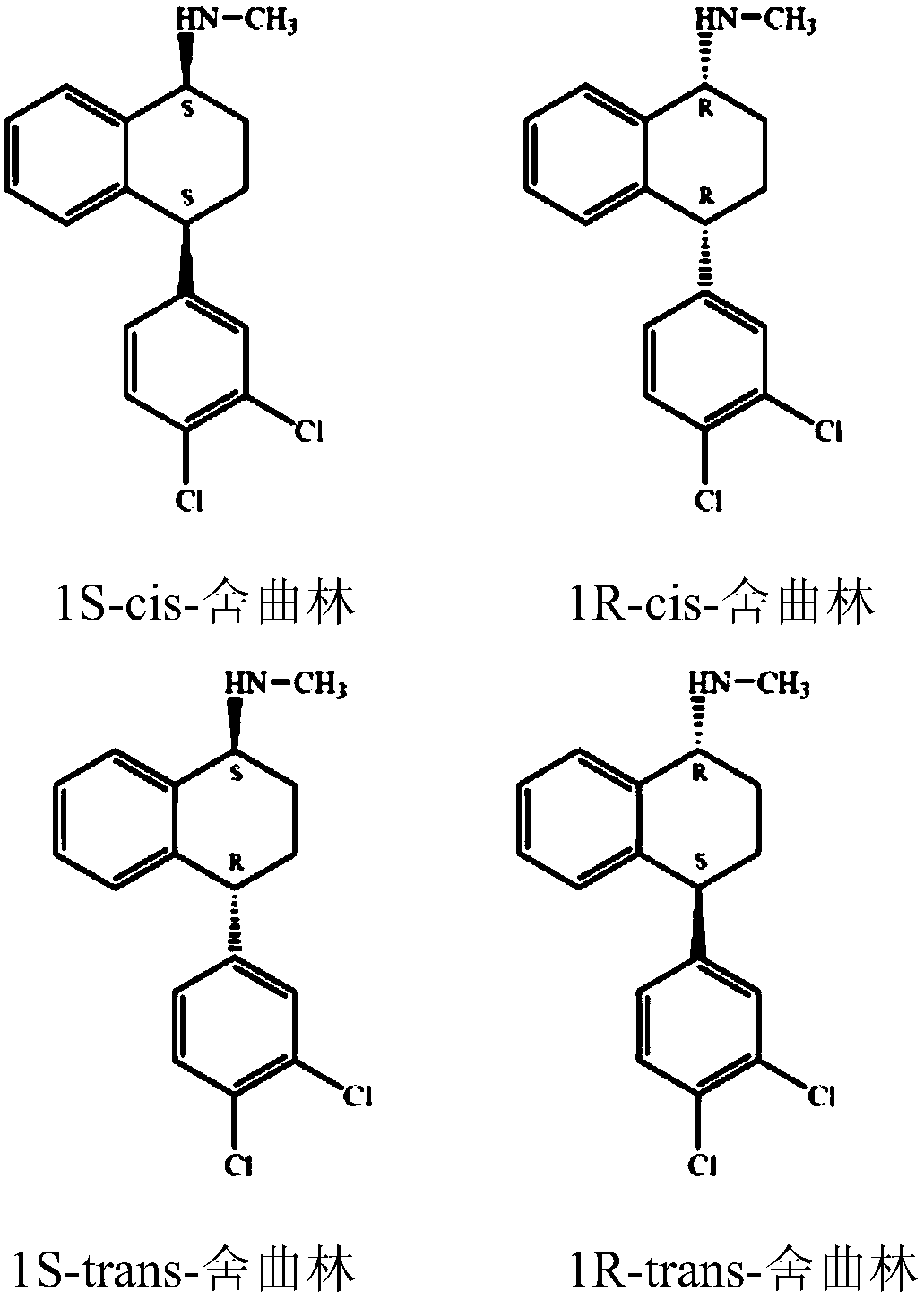
Preparation method of chiral sertraline hydrochloride
A technology of sertraline hydrochloride and sertraline hydrochloride, which is applied in the field of preparation of chiral sertraline hydrochloride, can solve the problems of low yield, unsafe use, low production efficiency, etc., achieve high yield, simple operation process, The effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] (1) 60 g of racemic cis-sertraline hydrochloride (the content of cis-(1S, 4S)-sertraline hydrochloride is 47%), 30.49 g of D-mandelic acid sodium salt, 130 g of n-butanol and 1.3 g of water g was added to a 500ml three-necked flask and reacted at 60°C for 3 hours, then heated up to distill off water, then heated to 118°C, filtered while hot to remove inorganic matter (mainly sodium chloride), rinsed with 10g of n-butanol, and combined to obtain filtrate 1 ;
[0048] (2) After the filtrate 1 was heated and completely dissolved, the temperature was lowered to 36.5° C., crystallized, centrifugally filtered, rinsed with 10 g of n-butanol to obtain the crude product of D-sertraline mandelate, and the combined liquids were obtained to obtain the filtrate 2;
[0049] (3) Mix the crude product of D-sertraline mandelate with 145g of n-butanol, heat up and dissolve completely, then cool down to 10°C, crystallize, centrifugally filter, rinse with 10g of n-butanol, and dry to obtai...
Embodiment 2
[0055] The preparation method of chiral sertraline hydrochloride is basically the same as in Example 1, except that the temperature in step (2) is lowered to 41°C.
[0056] Step (3), obtain 33.96g refined D-sertraline mandelic dry product;
[0057] In step (5), 23.18 g of cis-(1S,4S)-sertraline hydrochloride was obtained. The molar yield is 82.19%.
[0058] After testing, the purity of cis-(1S,4S)-sertraline hydrochloride was 99.51%, and the content was 99.87%.
[0059] In addition, 29.86 g of D-mandelic acid sodium salt was recovered according to the same method as in Example 1, and the molar recovery rate was 97.94%.
Embodiment 3
[0061] The preparation method of chiral sertraline hydrochloride is basically the same as in Example 1, except that the temperature in step (2) is lowered to 45°C.
[0062] Step (3), obtain 33.97g refined D-sertraline mandelic dry product;
[0063] In step (5), 23.15 g of cis-(1S,4S)-sertraline hydrochloride was obtained. The molar yield is 82.09%.
[0064] After testing, the purity of cis-(1S,4S)-sertraline hydrochloride was 99.49%, and the content was 99.61%.
[0065] In addition, 29.85 g of D-mandelic acid sodium salt was recovered according to the same method as in Example 1, and the molar recovery rate was 97.91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com