Preparation method of chiral sertraline hydrochloride
A technology of sertraline hydrochloride and chirality, which is applied in the field of splitting of chiral sertraline hydrochloride, and achieves the effects of high efficiency, less three wastes and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
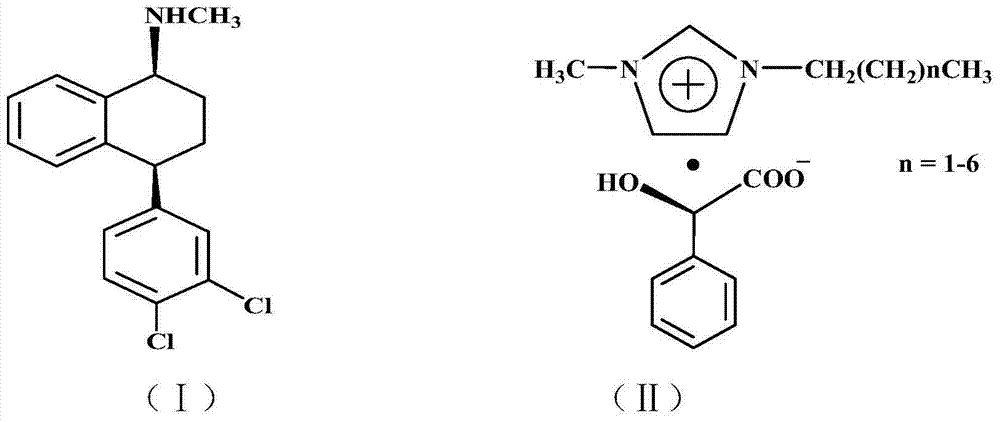
[0022] 306 g (1 mol) of racemic sertraline and 552 g (2 mol) of chiral D-1-methyl-3-propylimidazole mandelate ionic liquid were added to a 2L three-necked flask, and the mixture was heated at 50°C. After 5 hours of reaction, 306 ml of absolute ethanol was added and stirred for 1 hour, and filtered to obtain filter cake a and filtrate a. 50 ml of 10% aqueous sodium hydroxide solution was added to filter cake a, and stirred for 1 hour at room temperature for hydrolysis, and then Add 3×100 ml of dichloromethane to extract three times, combine the dichloromethane extracts and add 20% hydrochloric acid aqueous solution at room temperature under stirring until no new precipitates are formed, filter to obtain filter cake b and filtrate b, filter cake b is Cis-(1S,4S)-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-1-naphthylamine hydrochloride 137g, Yield 80%. Melting point: 248~250℃, optical purity ≥98.0%, content ≥99.0%.
Embodiment 2
[0024] 306 g (1 mol) of racemic sertraline and 828 g (3 mol) of chiral D-1-methyl-3-butylimidazolium mandelate ionic liquid were added to a 2L three-necked flask, and the mixture was heated at 80°C. After 3 hours of reaction, 1530 ml of absolute ethanol was added and stirred for 1 hour, and filtered to obtain filter cake a and filtrate a. In filter cake a, 50 ml of 10% aqueous sodium hydroxide solution was added and stirred for 1 hour at room temperature for hydrolysis, and then Add 3×100 ml of dichloromethane to extract three times, combine the dichloromethane extracts and add 20% hydrochloric acid aqueous solution at room temperature under stirring until no new precipitates are formed, filter to obtain filter cake b and filtrate b, filter cake b is Cis-(1S,4S)-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-1-naphthylamine hydrochloride 139 g, Yield 81.2%. Melting point: 248~250℃, optical purity ≥98.0%, content ≥99.0%.
Embodiment 3
[0026]306 g (1 mol) of racemic sertraline and 664 g (2 mol) of chiral D-1-methyl-3-hexylimidazolium mandelate ionic liquid were added to a 2L three-necked flask and reacted at 50 °C After 5 hours, 918 ml of anhydrous ethanol was added and stirred for 1 hour, filtered to obtain filter cake a and filtrate a, 50 ml of 10% aqueous sodium hydroxide solution was added to filter cake a, stirred for 1 hour at room temperature for hydrolysis, and then added Dichloromethane was extracted three times with 3 × 100 ml, the dichloromethane extracts were combined and 20% aqueous hydrochloric acid solution was added under stirring at room temperature until no new precipitates were formed, and filtered to obtain filter cake b and filtrate b, filter cake b That is Cis-(1S,4S)-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-1-naphthylamine hydrochloride 138g, received rate 81%. Melting point: 248~250℃, optical purity ≥98.0%, content ≥99.0%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com