Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
47 results about "Process validation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Process validation is the analysis of data gathered throughout the design and manufacturing of a product in order to confirm that the process can reliably output products of a determined standard. Regulatory authorities like EMA and FDA have published guidelines relating to process validation. The purpose of process validation is to ensure varied inputs lead to consistent and high quality outputs. Process validation is an ongoing process that must be frequently adapted as manufacturing feedback is gathered. End-to-end validation of production processes is essential in determining product quality because quality cannot always be determined by finished-product inspection. Process validation can be broken down into 3 steps: process design, process qualification, and continued process verification.
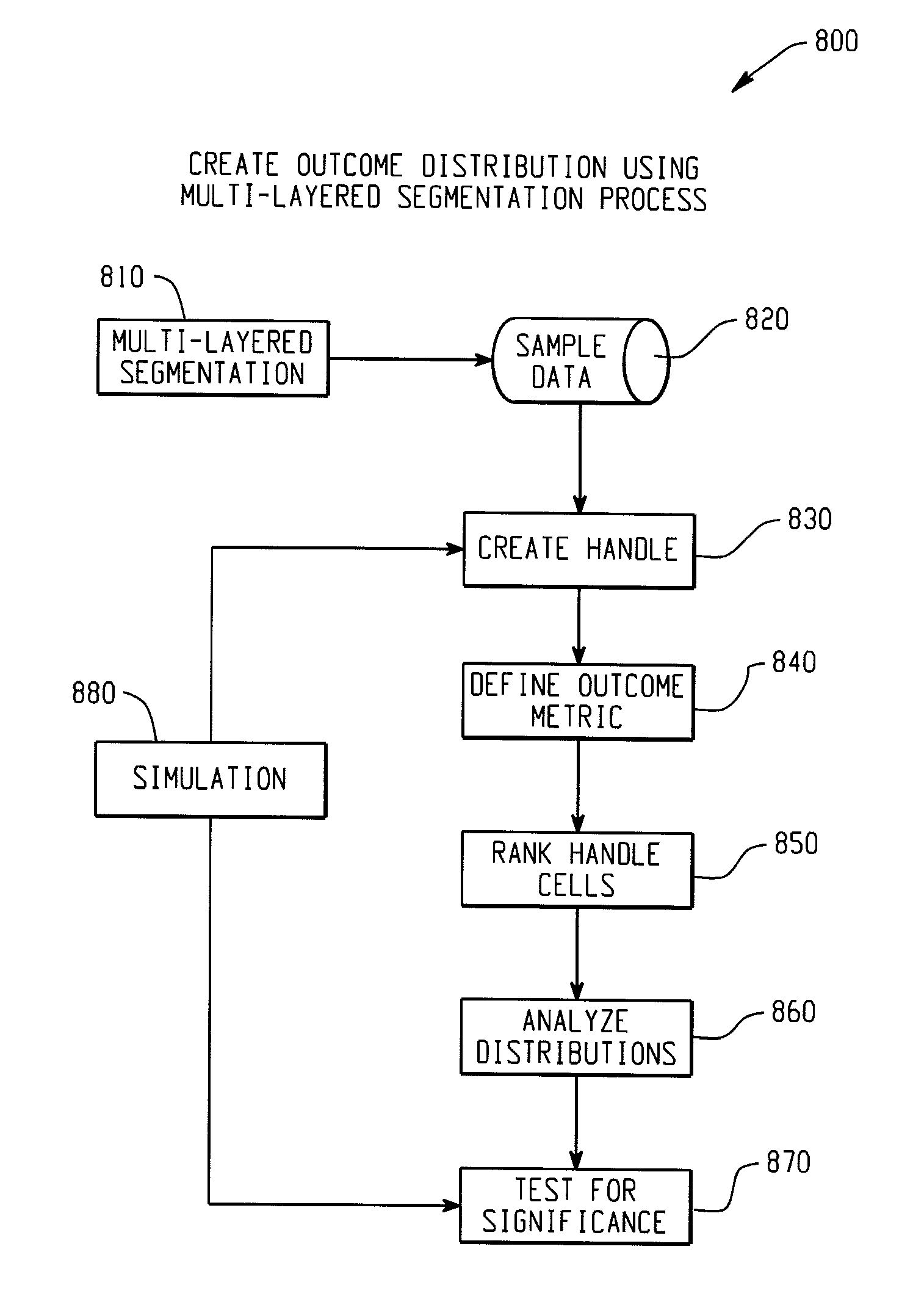
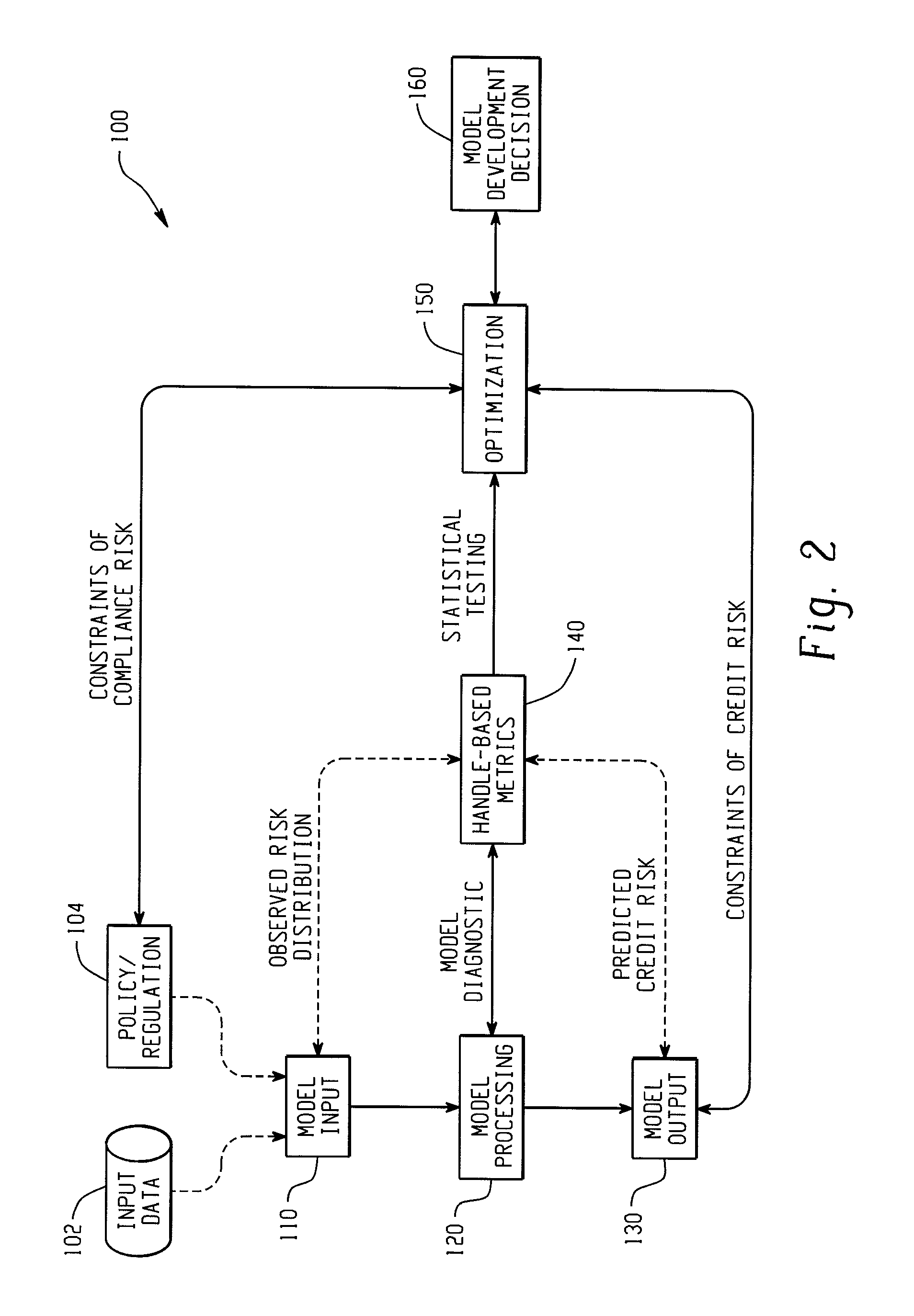
Computer-implemented systems and methods for integrated model validation for compliance and credit risk
ActiveUS8515862B2Accurate and explicit and testingMaximizing predictabilityFinanceFuzzy logic based systemsTheoretical computer scienceProcess validation
Computer-implemented systems and methods are provided for model validation of a model for compliance and credit risk. Model input, output, and processing validation areas are contained on a computer system. A handle data structure connects the model validation areas with handles that comprise a unified metric. A handle represents combinations of covariate patterns and describes the joint distribution of risk characteristics.
Owner:SAS INSTITUTE
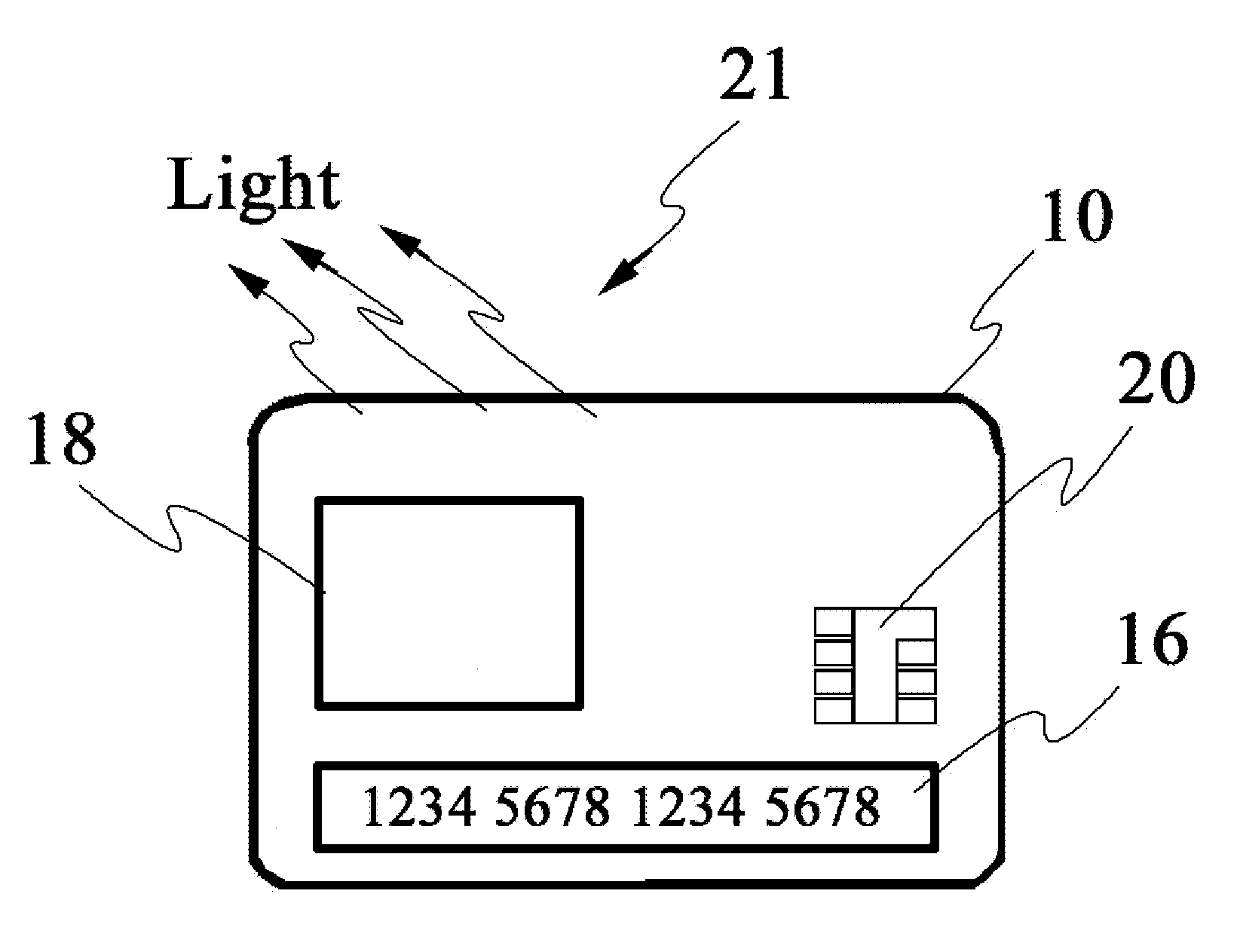
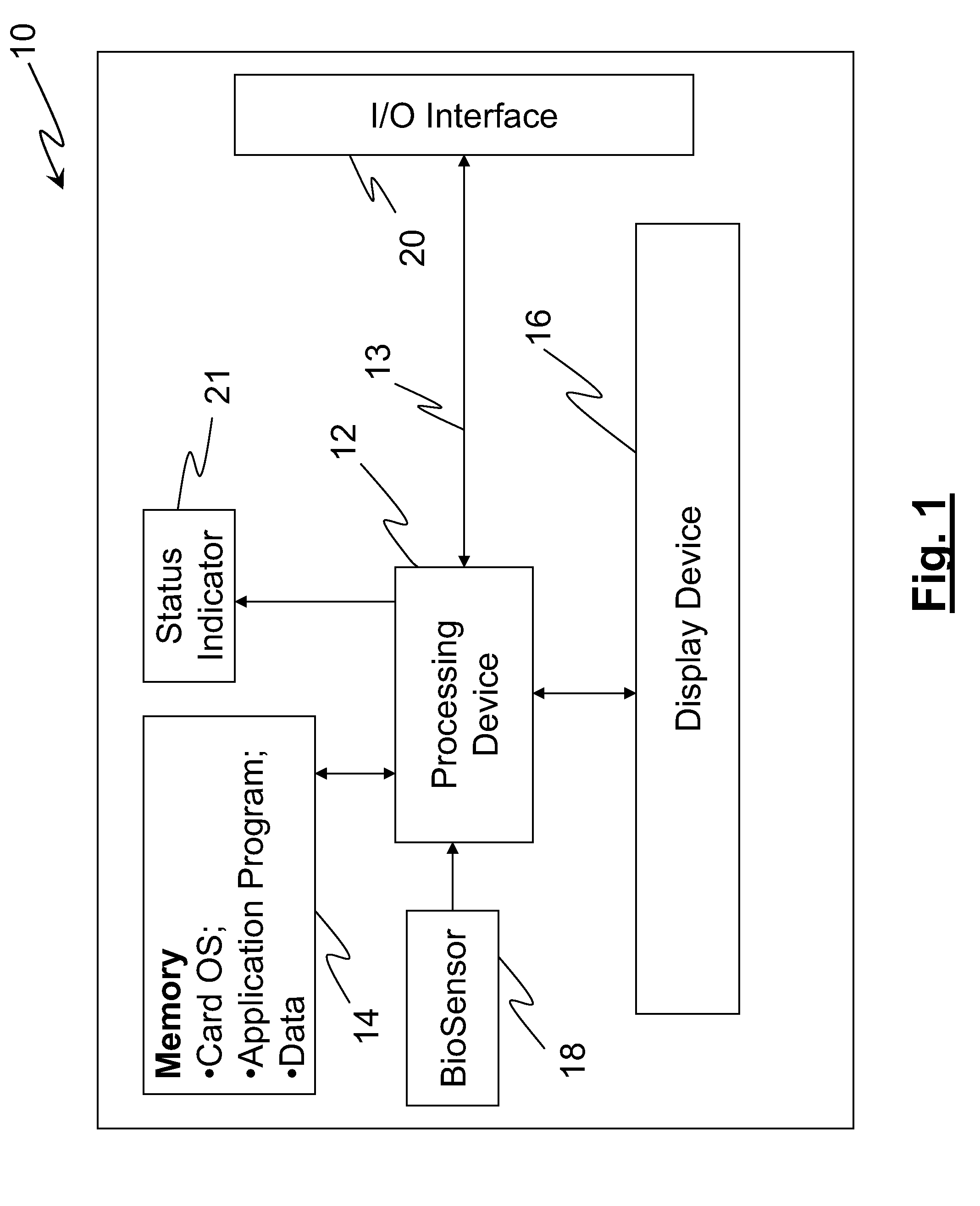
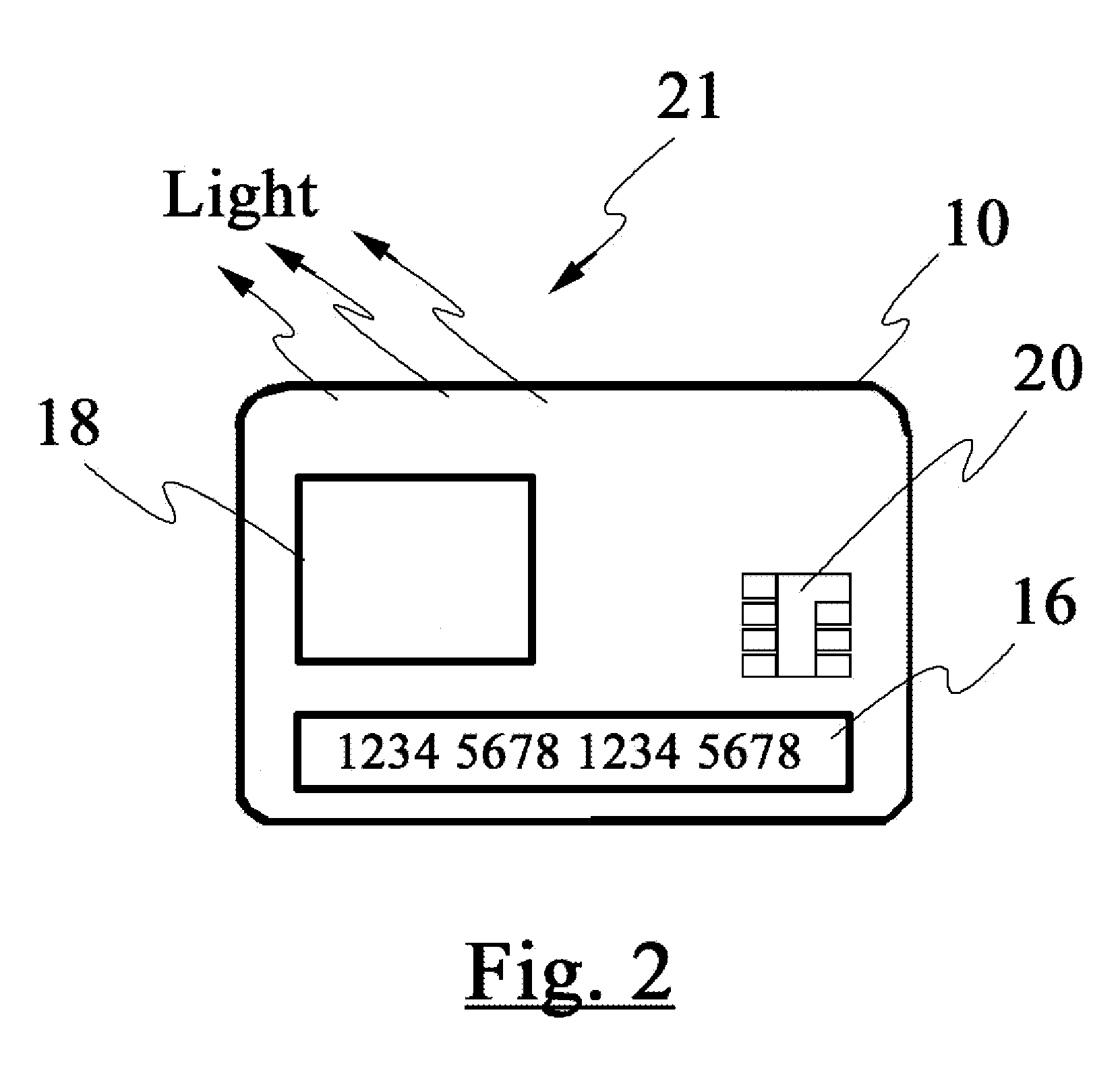
Smart apparatus for making secure transactions
InactiveUS20080223937A1Internal/peripheral component protectionDigital data authenticationDisplay deviceFinancial transaction
The disclosed invention relates to an apparatus and method for making secured transactions. Such an apparatus may include a smart card device configured with a display for display a static or dynamic number for making a secured transaction. The display is configured to display a valid card number after the user's identify has been verified using a biometric verification process. The device may further comprise a programmable magnetic strip that is programmed with user data after the user's identify has been verified. The apparatus may further include a cloning process to allow a second device to perform secure transactions. One such second device is a smart module associated with a vehicle.
Owner:PATENT ZERO
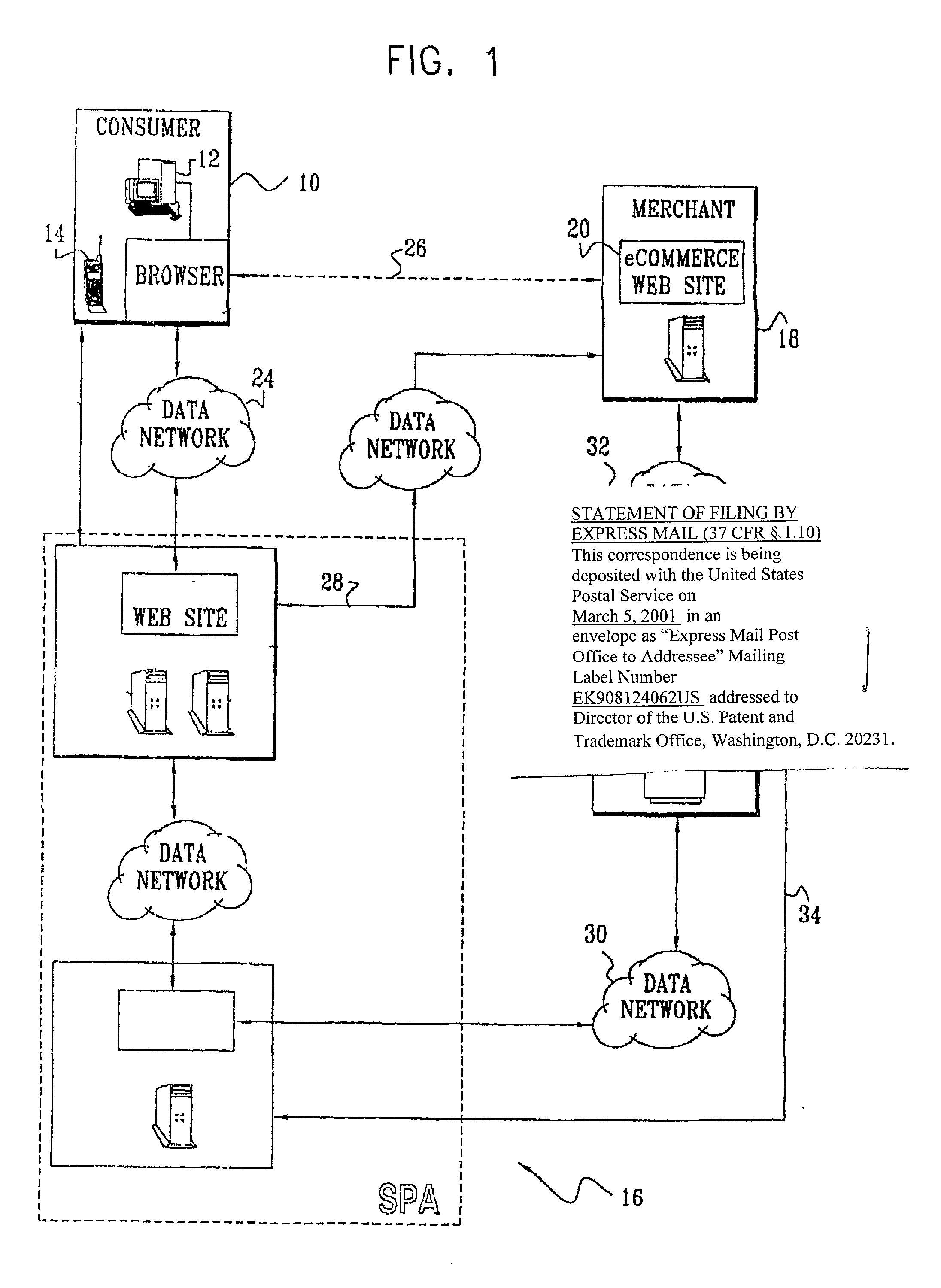
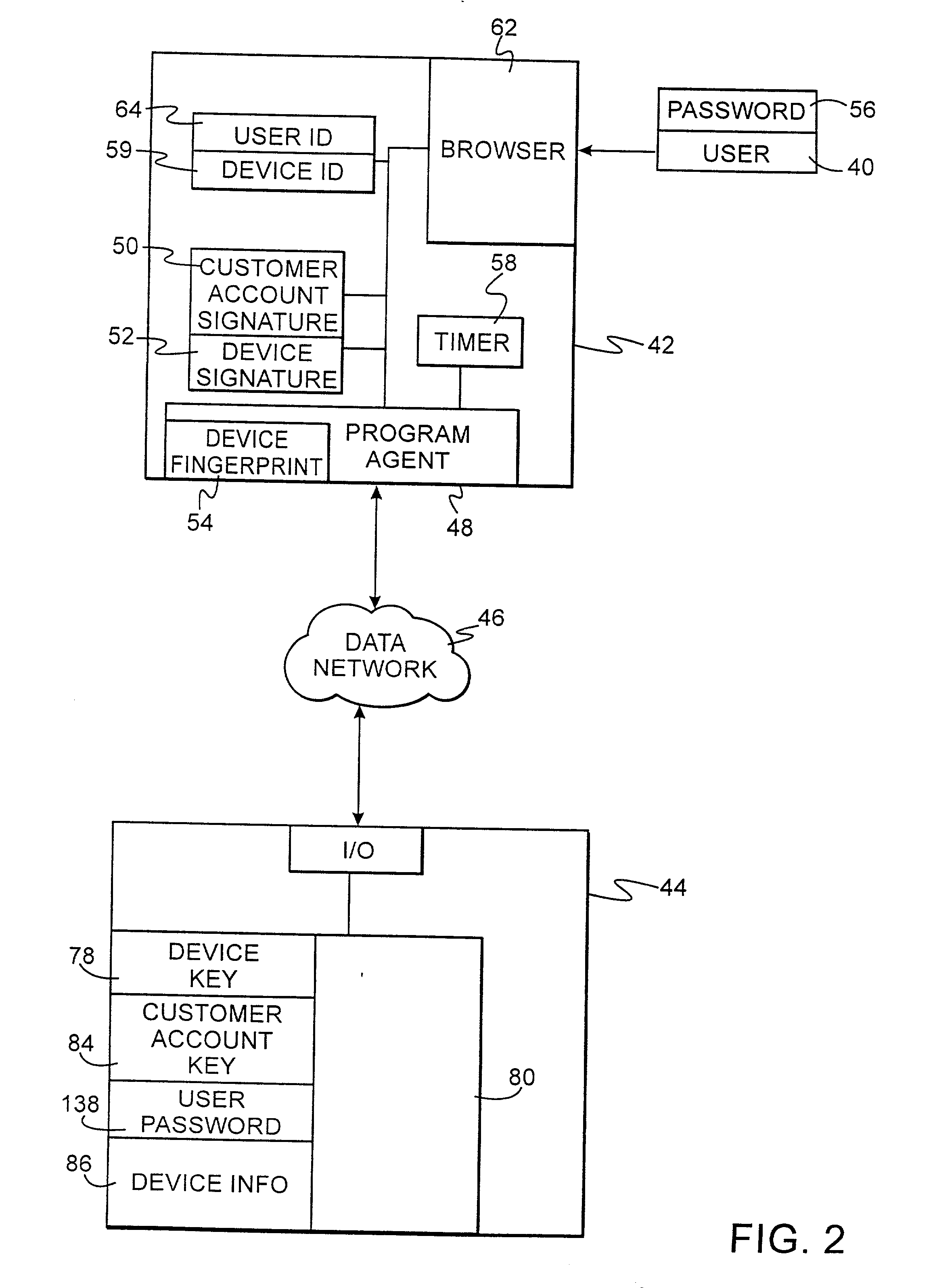
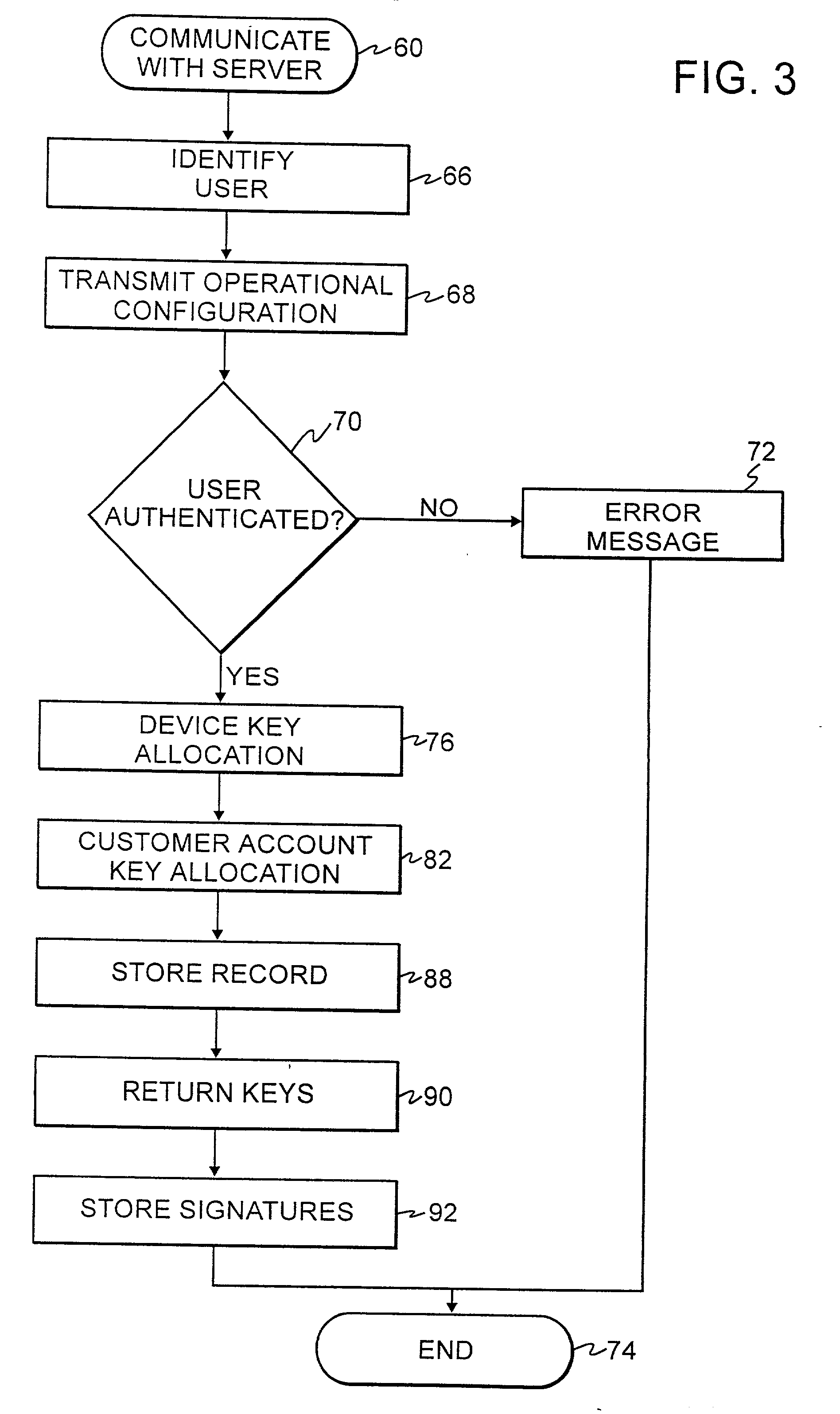
Authentication technique for electronic transactions
InactiveUS20010044896A1User identity/authority verificationDigital data authenticationPasswordRandom interval
A technique for authenticating a first party to a second party is applicable to electronic transactions. In addition to employing personal passwords, and a device operational parameter fingerprint, two signatures are employed, one being characteristic of the first party, and the other being associated with the computer or communications device of the first party. The signatures mutate at random intervals, responsive to mutation requests made by the device of first party to the device employed by the second party. The mutated signatures invalidate previous signatures, and are stored in the computing or communications devices of both parties. The mutation process authenticates the computer or communication device, and may also authenticate the password holder.
Owner:SCHWARTZ GIL +2
Computer-Implemented Systems And Methods For Integrated Model Validation For Compliance And Credit Risk
ActiveUS20090299896A1Improve efficiencyImprove fairnessFinanceFuzzy logic based systemsTheoretical computer scienceComputerized system
Computer-implemented systems and methods are provided for model validation of a model for compliance and credit risk. Model input, output, and processing validation areas are contained on a computer system. A handle data structure connects the model validation areas with handles that comprise a unified metric. A handle represents combinations of covariate patterns and describes the joint distribution of risk characteristics.
Owner:SAS INSTITUTE
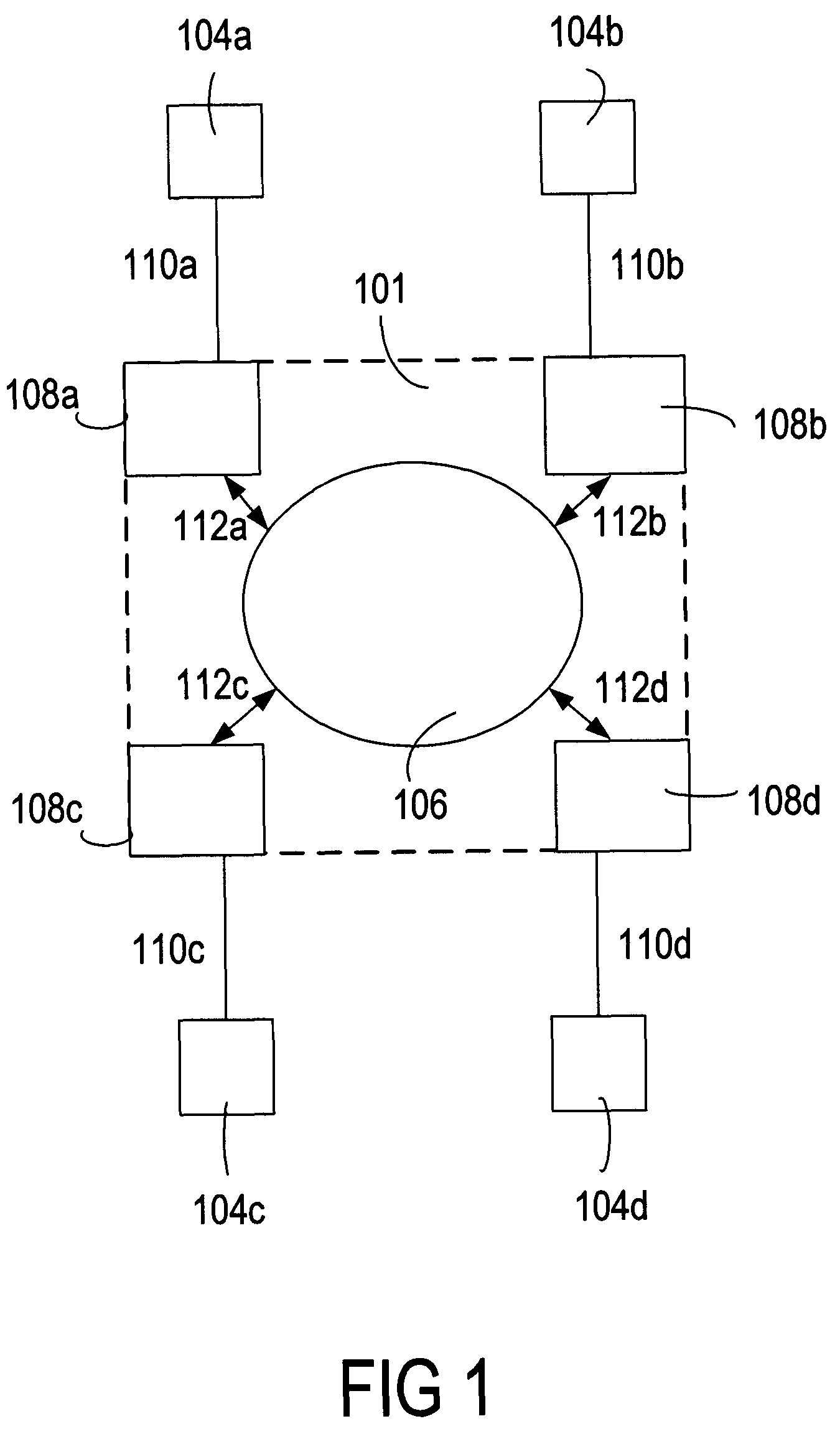
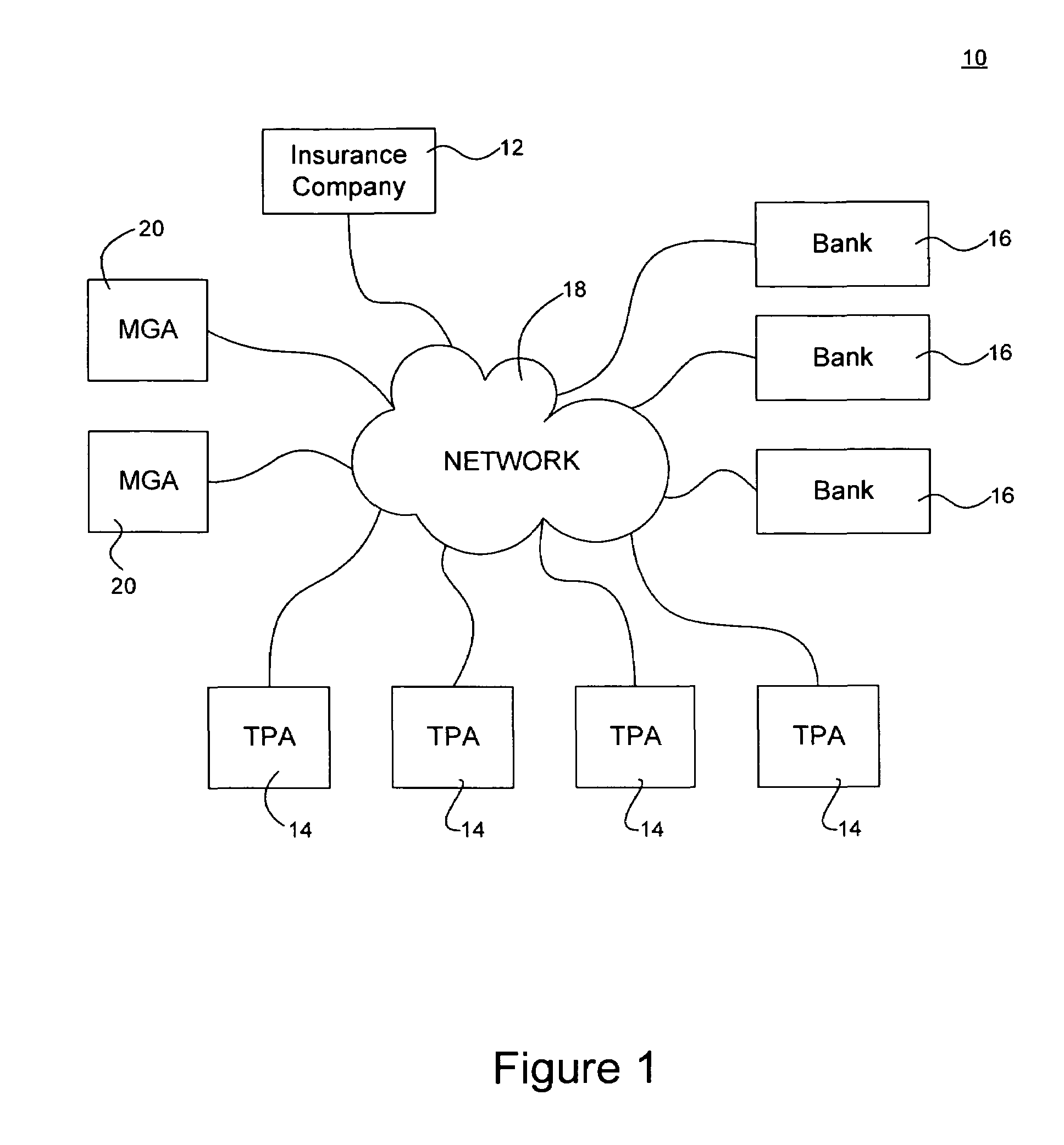
Virtual platform to facilitate automated production
InactiveUS20050137735A1Facilitate communicationSatisfies requirementData processing applicationsTotal factory controlTelecommunications linkCommunication link
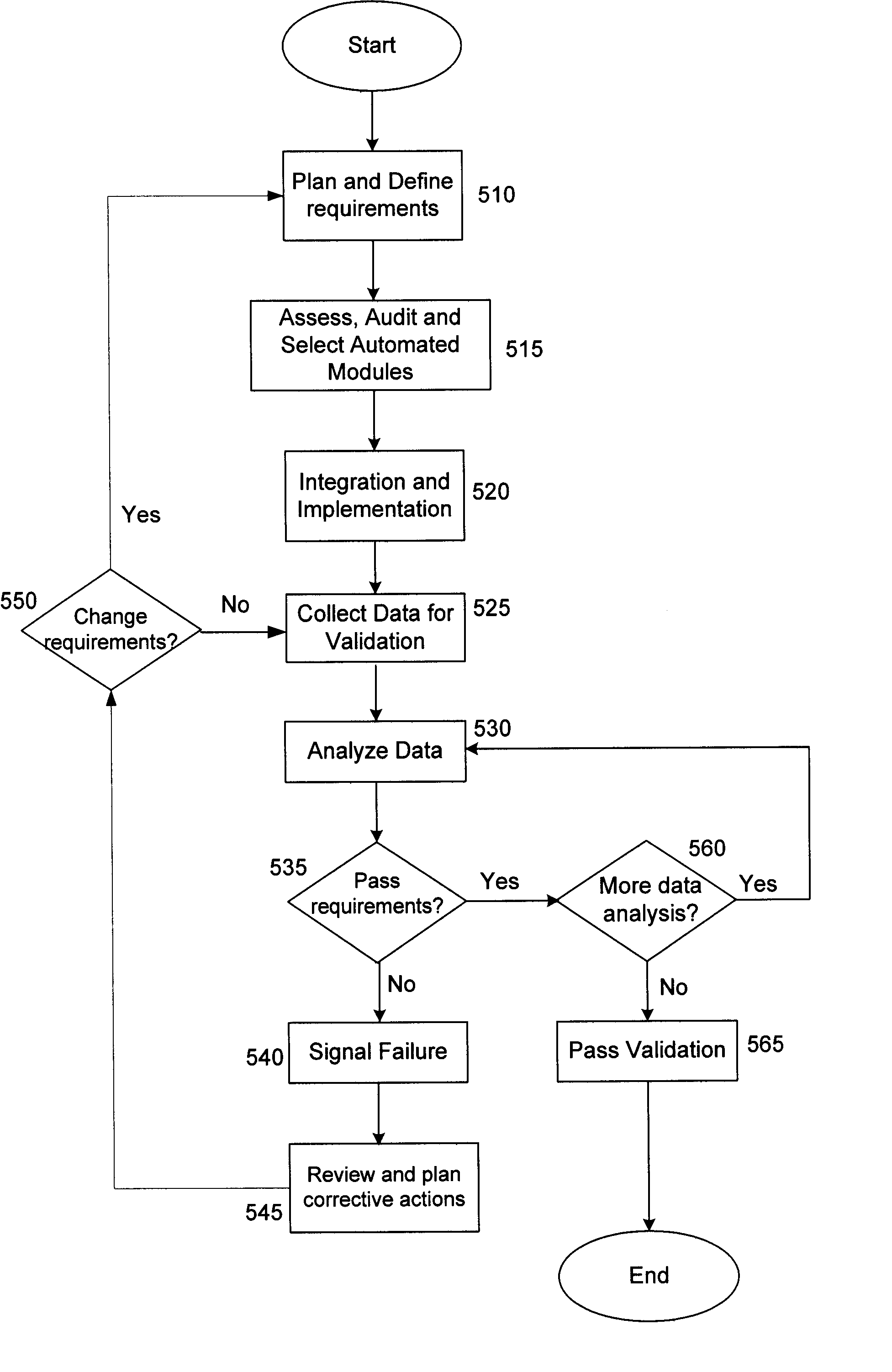
A method of automating validation in a manufacturing facility is disclosed. The method comprises defining requirements, selecting and integrating automated devices for manufacturing. A hub-box with communication links is used to integrate the automated devices. The hub-box controls and facilitates communication between automated devices. The hub-box further collects and analyzes processing data for validation of the process. By interconnecting the automated devices to a hub-box, processing data may be collected substantially real-time and accessed remotely, facilitating continuous process validation.
Owner:BEACONS PHARMA
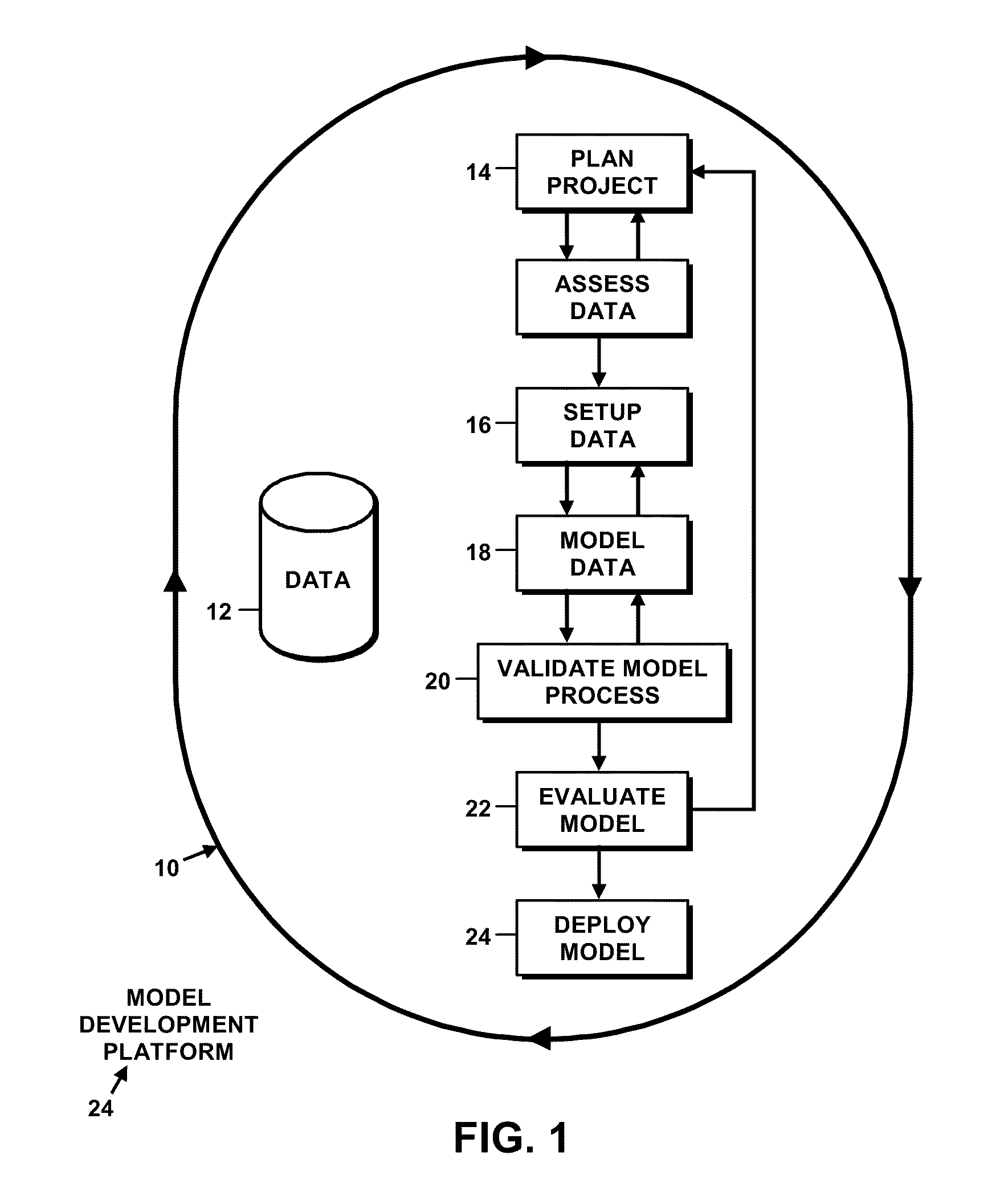
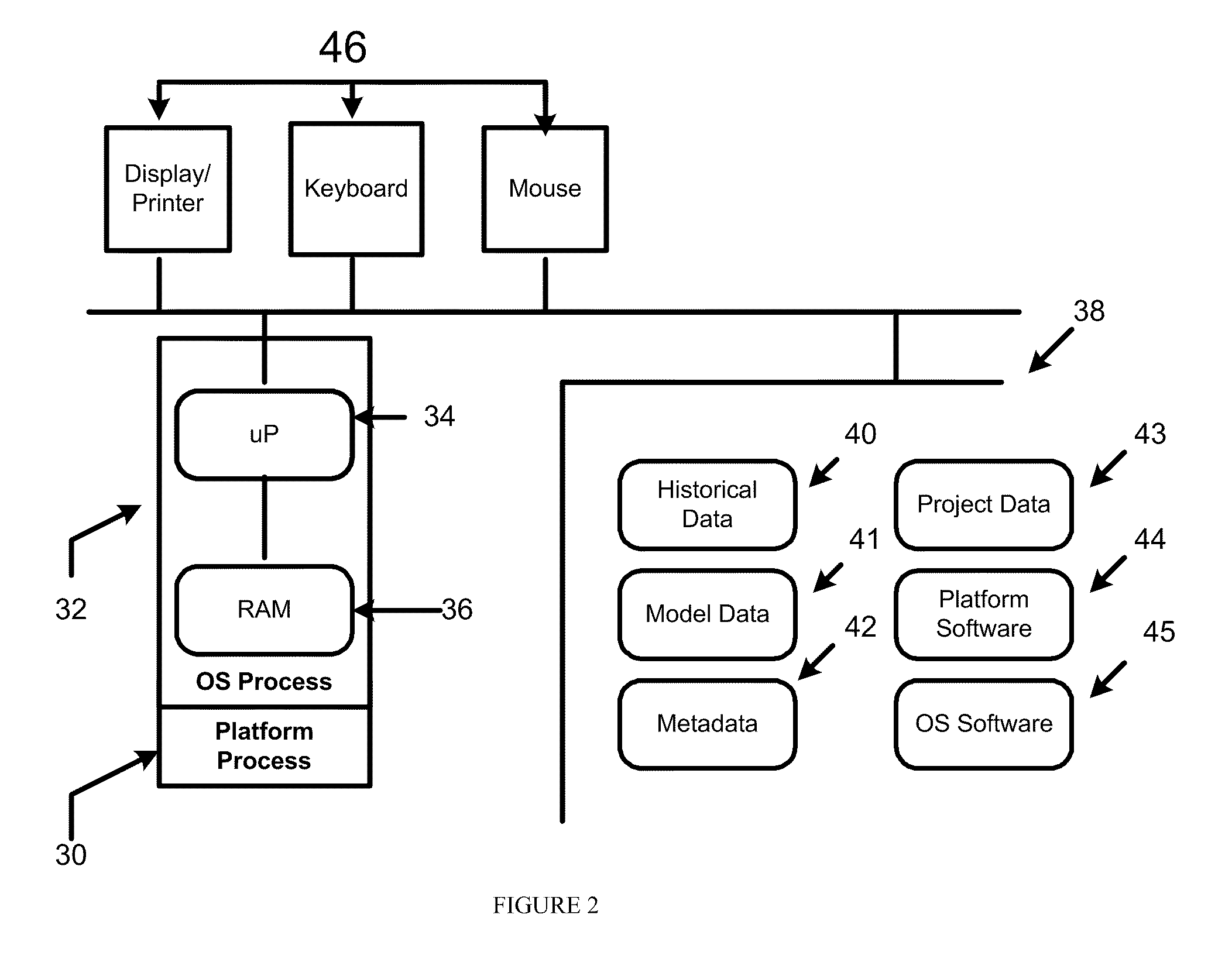
Predictive model development
InactiveUS20100010878A1Minimize development timeEasy to copyFinanceFuzzy logic based systemsGraphicsGraphical user interface
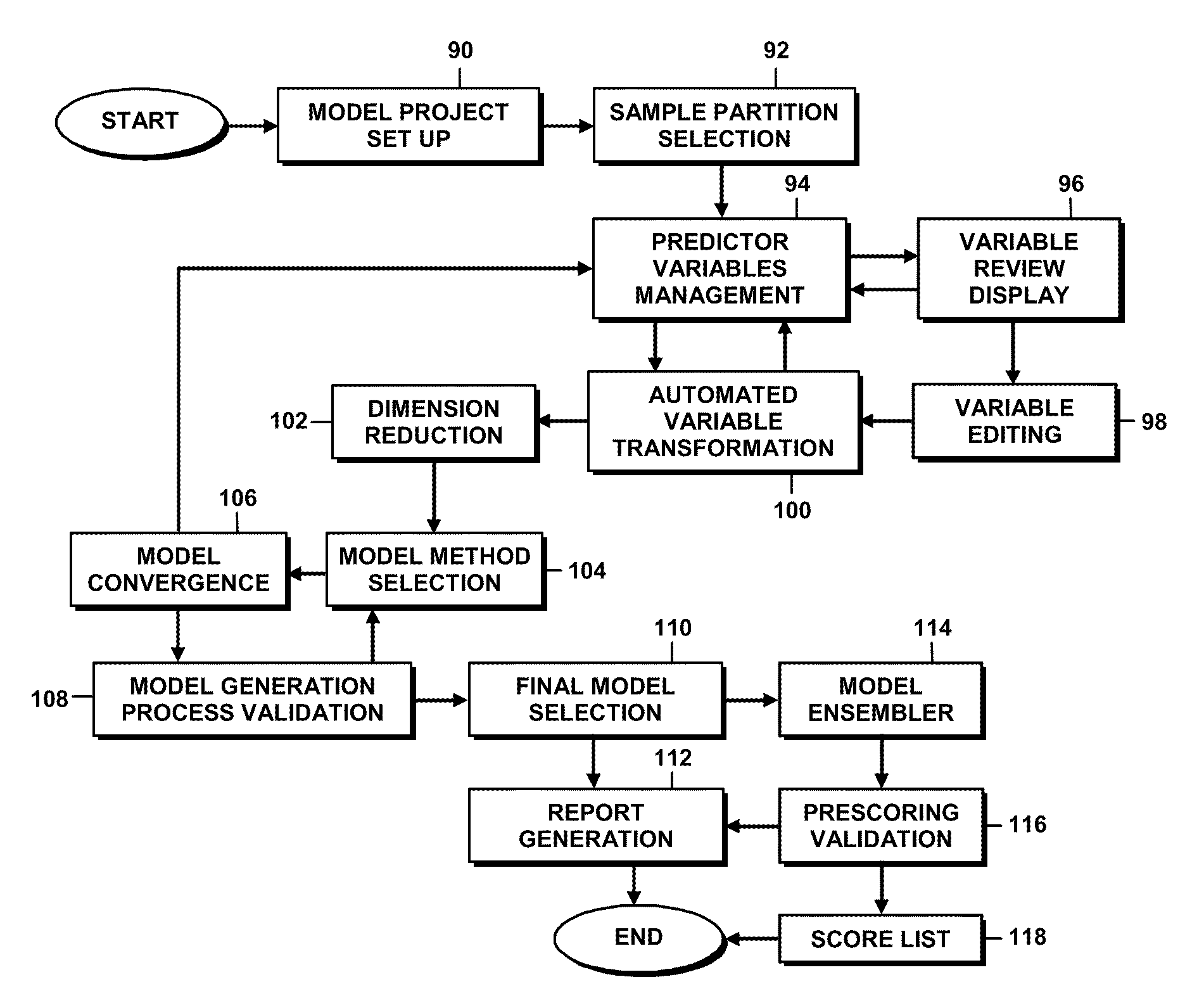
Models are generated using a variety of tools and features of a model generation platform. For example, in connection with a project in which a user generates a predictive model based on historical data about a system being modeled, the user is provided through a graphical user interface a structured sequence of model generation activities to be followed, the sequence including dimension reduction, model generation, model process validation, and model re-generation.In connection with a project in which a user generates a predictive model based on historical data about a system being modeled, and in which the project includes a series of user choice points and actions or parameter settings that govern the generation of the model based on rules, which direct the user to select and apply an optimal model.
Owner:BRINDLE DATA L L C
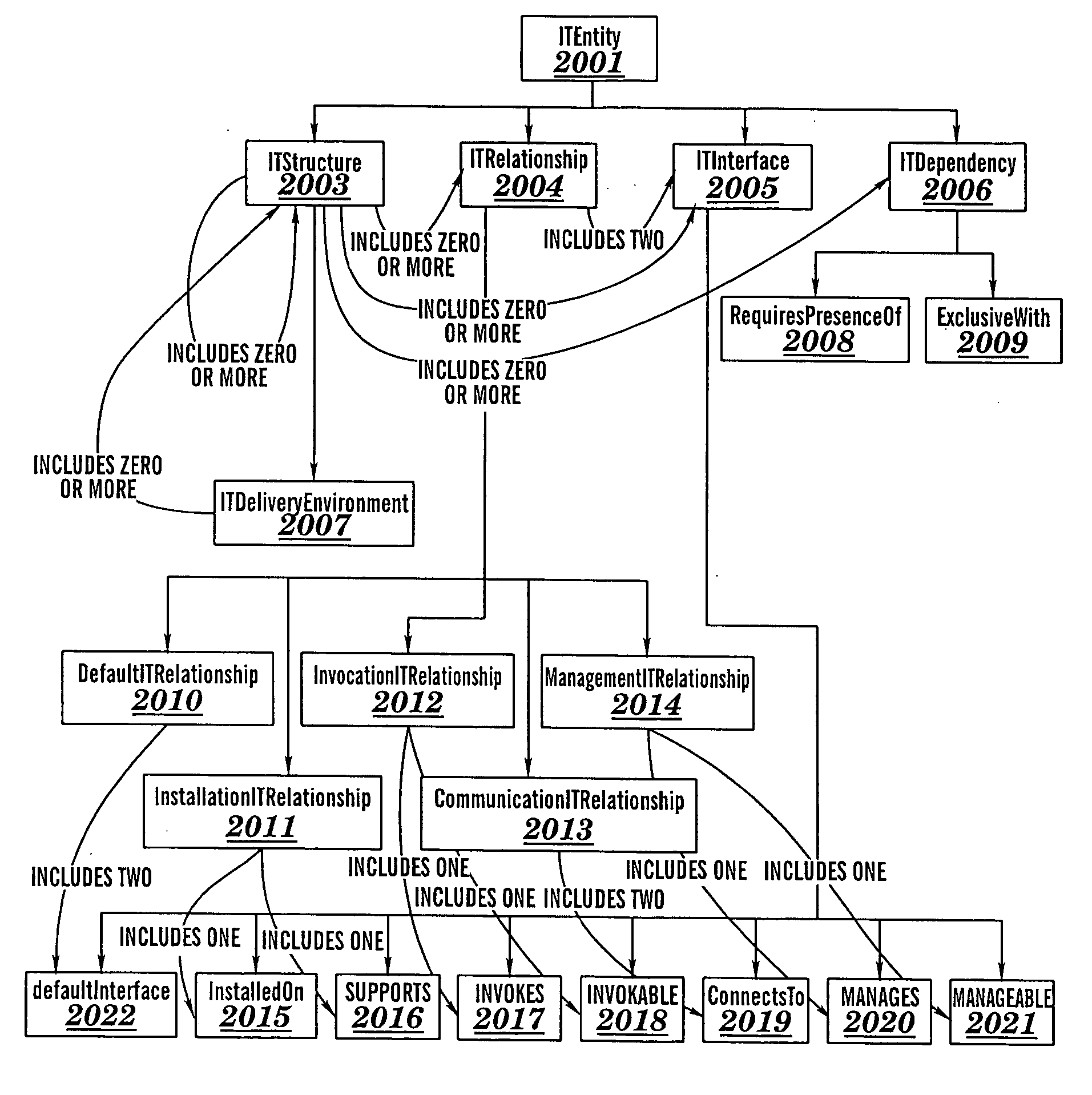
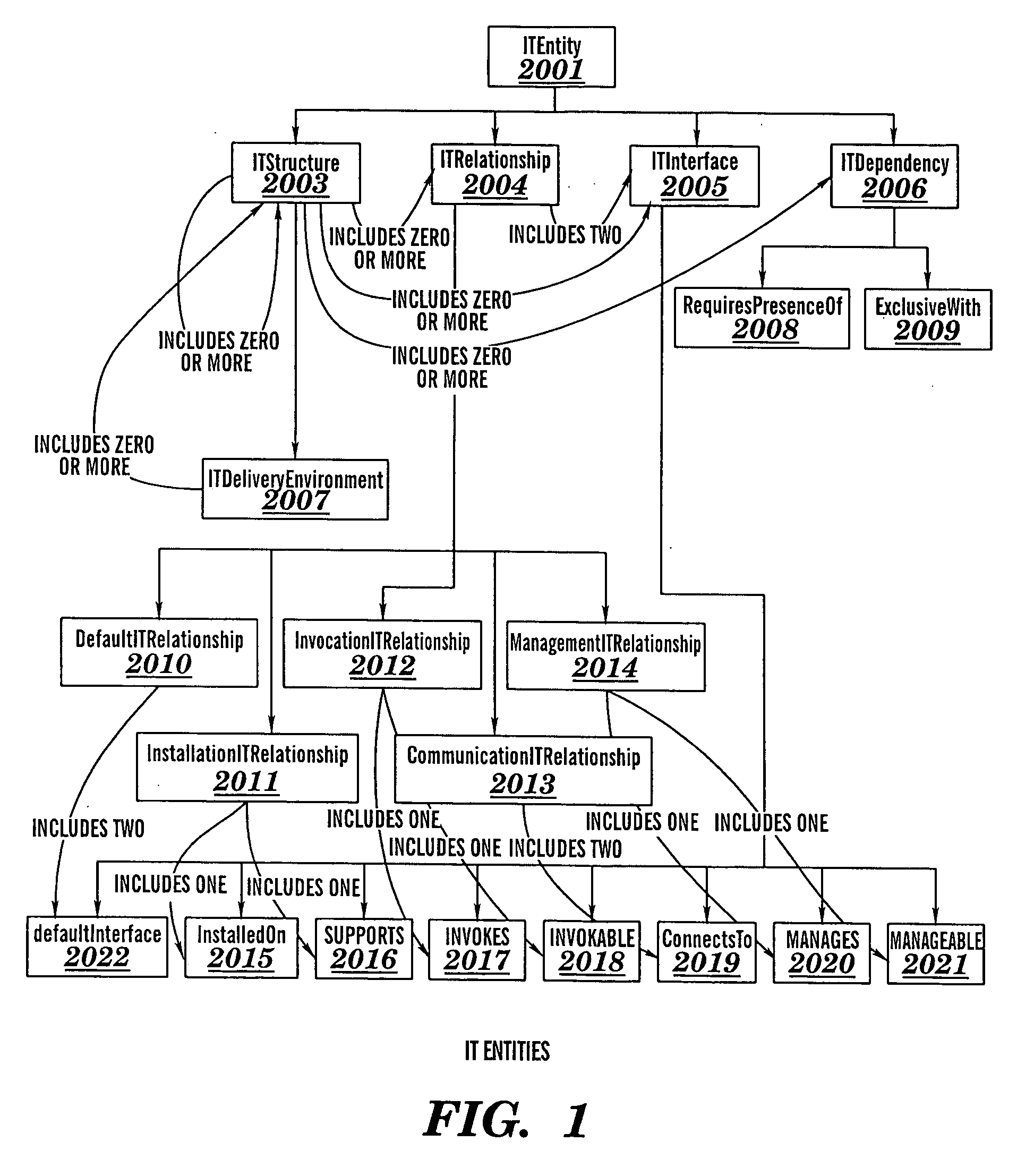
Automated verification of correctness of aspects of an information technology system
InactiveUS20060156274A1Reduce cost and user effortDigital data processing detailsUnauthorized memory use protectionInternet trafficProcess validation
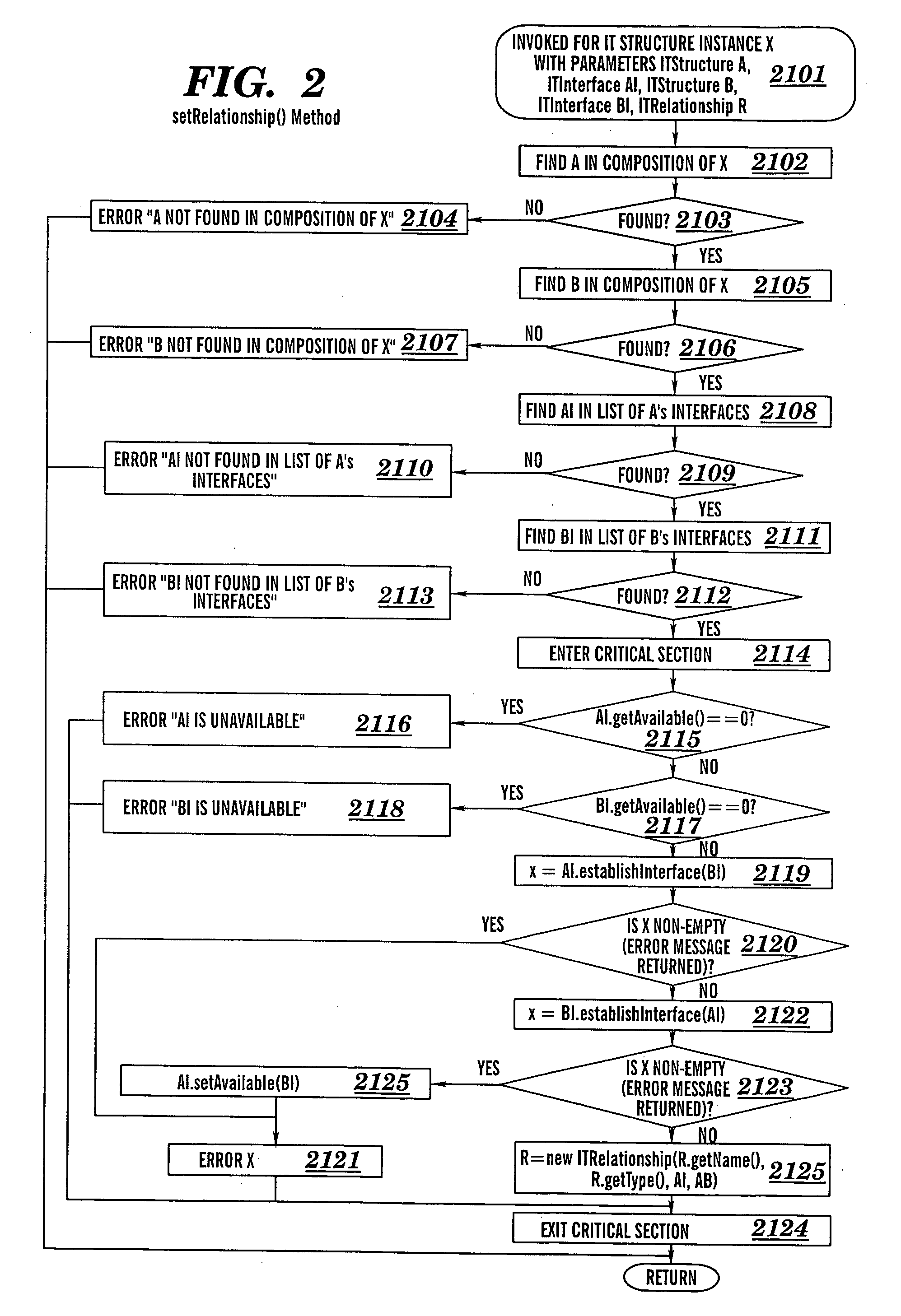
A method for verifying correctness of an Information Technology (IT) structure instance D of an IT structure R, a method for detecting an unauthorized change in an operating instance X of an IT structure R, a method for verifying conformance of an IT structure to an IT delivery environment, associated computer program products, and associated processes for integrating computing infrastructure. The method for verifying correctness of an IT structure instance D determines whether a reverse specification RD for D differs from R. The method for detecting an unauthorized change in an operating instance X of an IT structure R determines whether authorized changes in R have occurred. The method for verifying conformance of an IT structure to an IT delivery environment verifies compliance of the IT structure relating to: product standard compliance, compliance of software elements of the IT structure primitive composition, software application type compliance, and network traffic compliance.
Owner:KYNDRYL INC
Enhanced transfer framework for source or process verified products
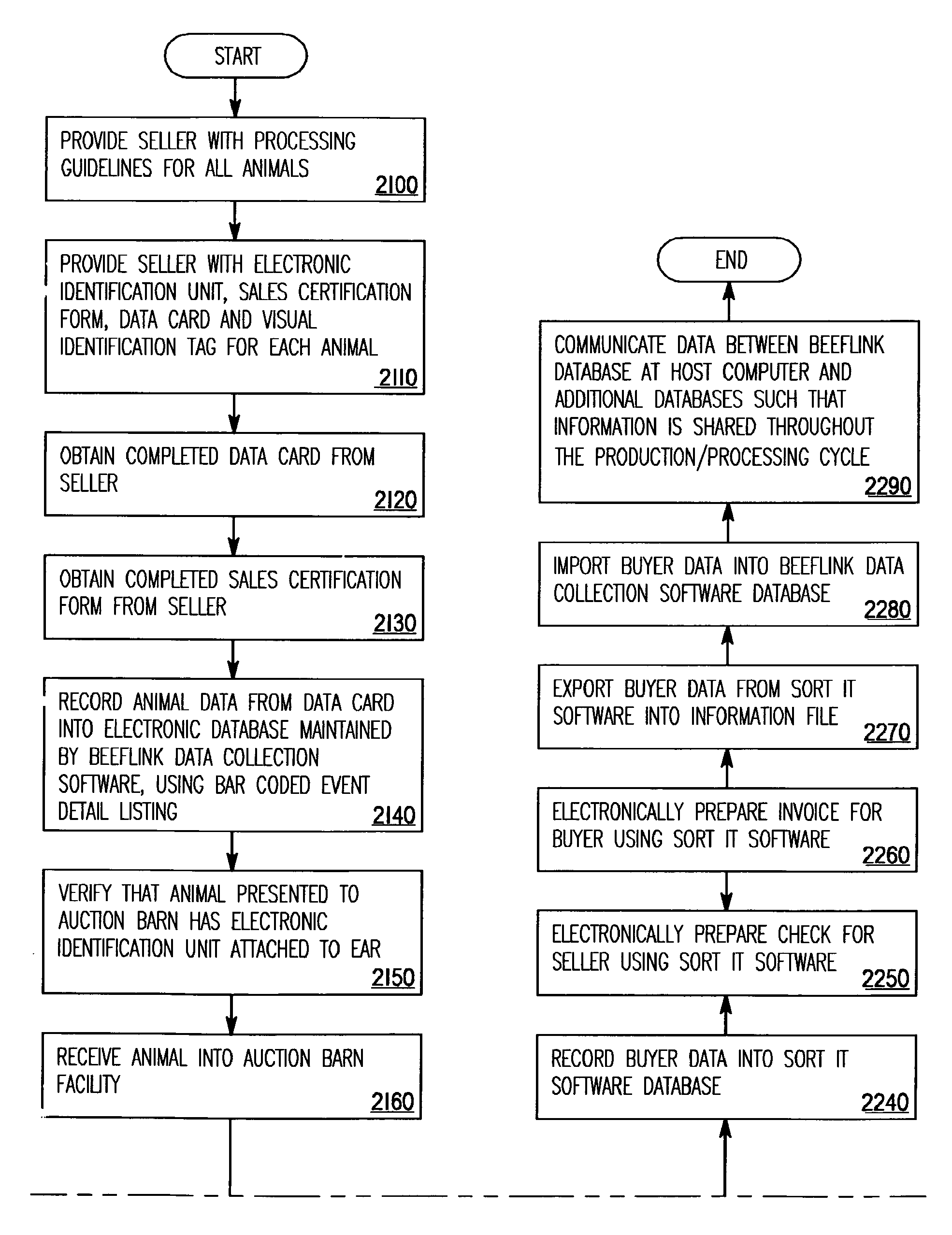
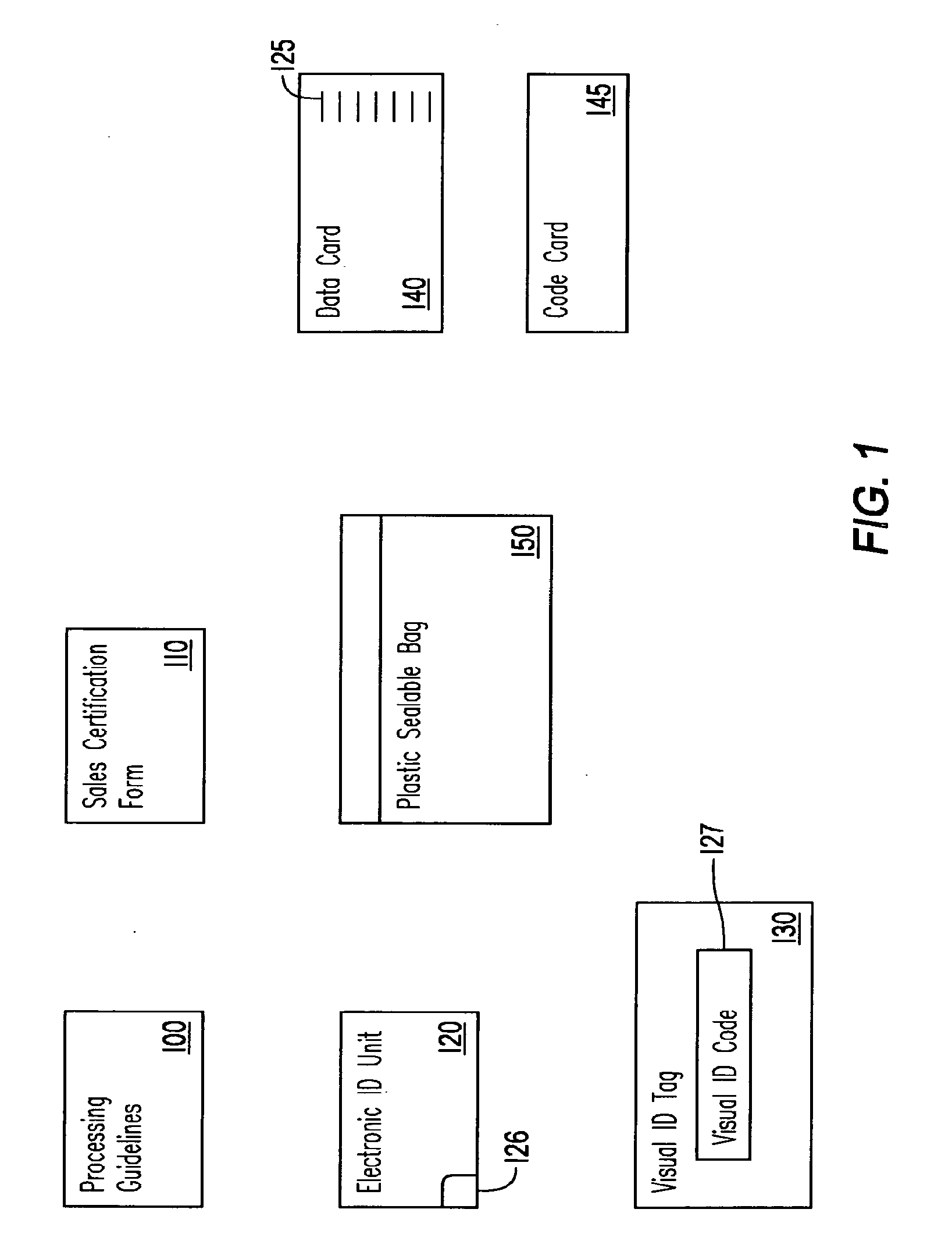
An enhanced transfer framework for source or process verified products, in which, from disparate selling entities, event data is received certifying that animals separately processed by the disparate entities each satisfy requirements associated with source or process verified animal products, and the animals are aggregated for sale as a group of certified source or process verified animals. As the group, the sale of the certified source or process verified animals is effected to a purchasing entity, the event data that identifies the disparate selling entities is filtered out, and the filtered event data is transferred to the purchasing entity.
Owner:AGINFOLINK HLDG INC BVI CORP
Method and system for ontology-enabled traceability in design and management applications
InactiveUS20120143570A1Enhanced Semantic RepresentationSpecial data processing applicationsNetwork management applicationDesign rule checking
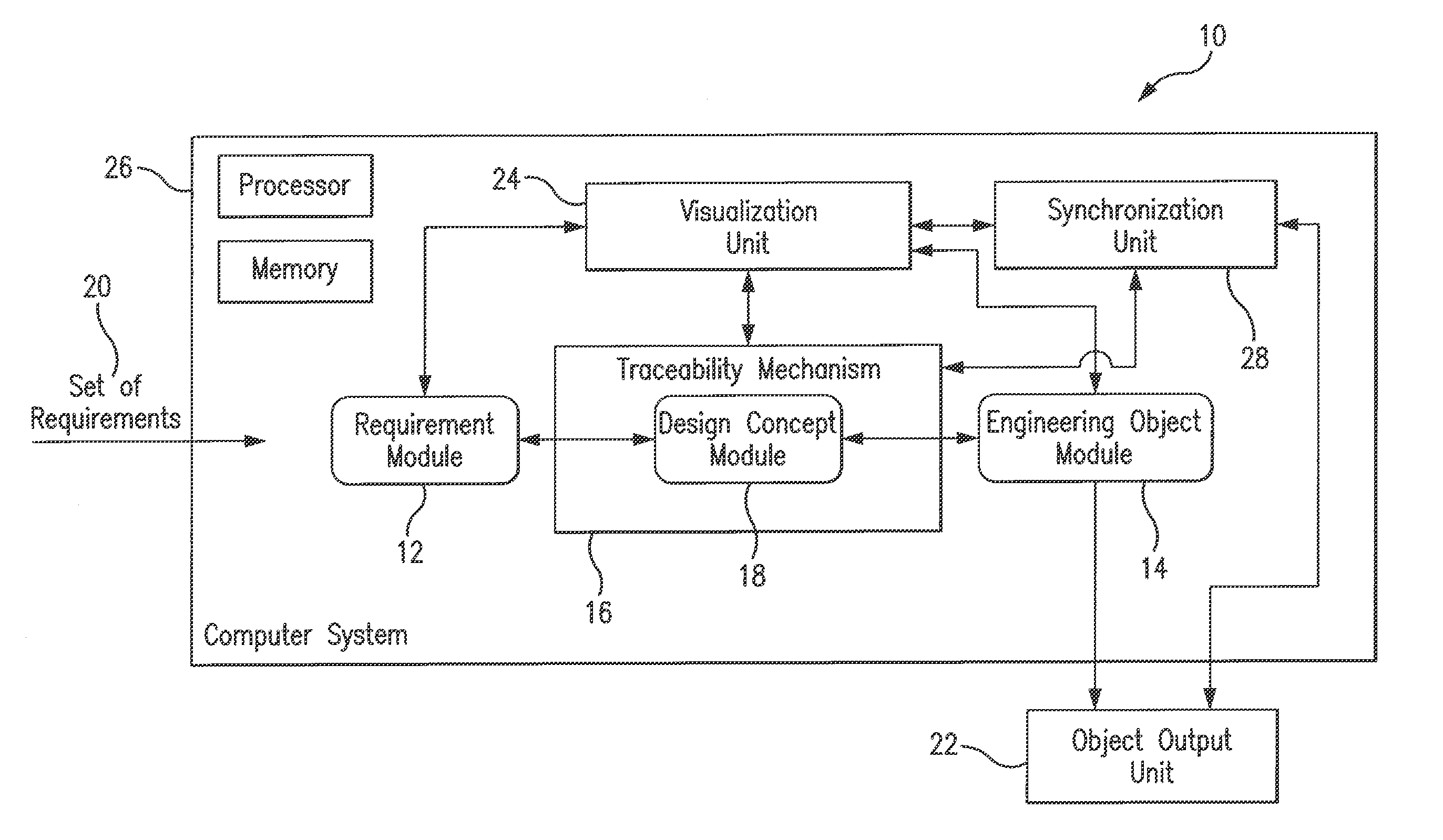
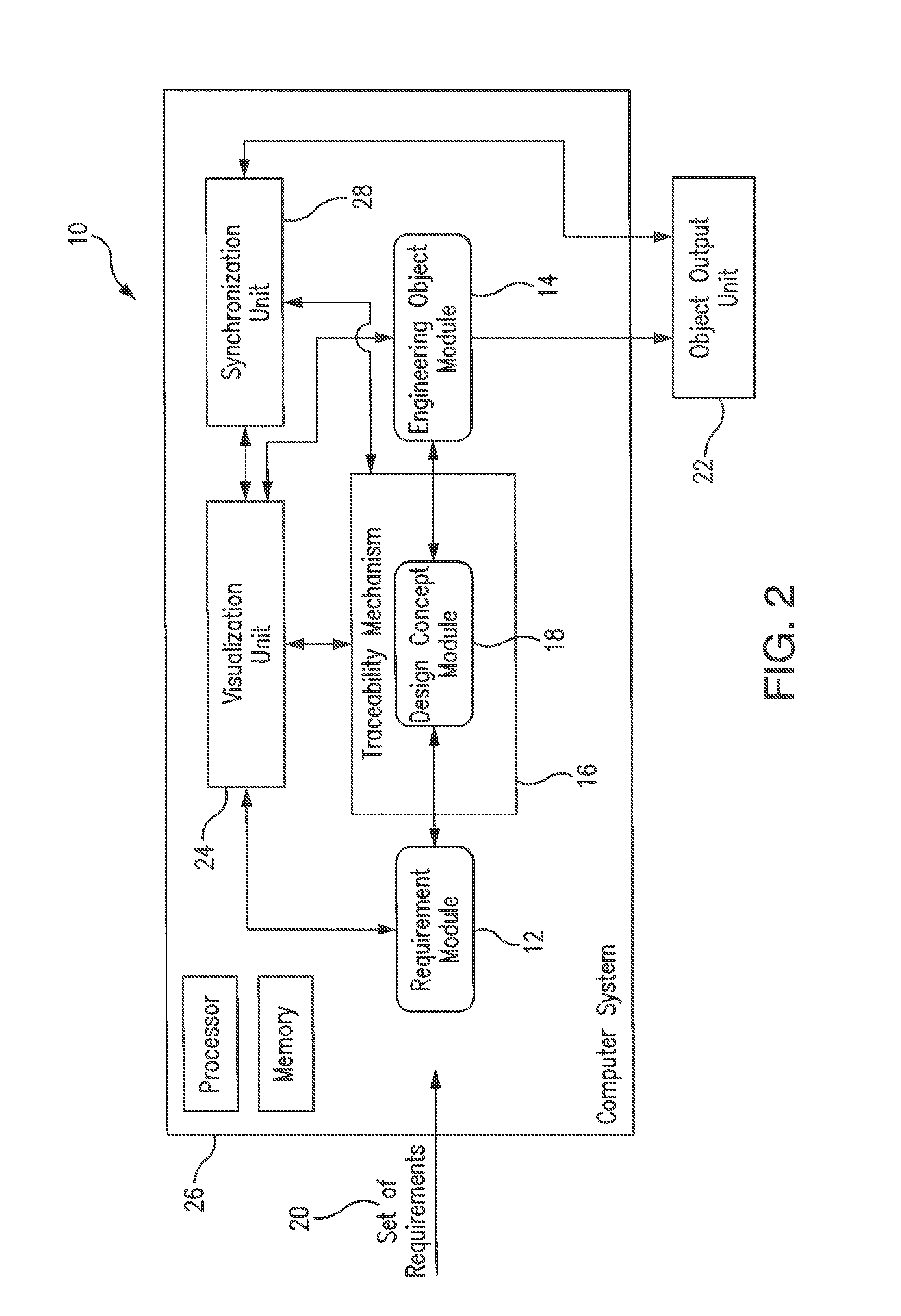
A system and method for ontology-enhanced traceability in design and management applications employ ontology nodes embedded in a processor executable traceability link (network) coupling processor executable requirement modules to processor executable engineering object modules to facilitate in all stages of engineering object development. The engineering object development occurs through multiple models of computation, control, and visualization platform networked together via ontology-enhanced traceability mechanism. Processor executable design rule checking module embedded in the design concept nodes creates a pathway for the development process validation and verification at early stages of the object lifecycle. Linking of ontologies / meta-models is performed for the purposes of supporting ontology-enabled traceability across multiple domains.
Owner:UNIV OF MARYLAND
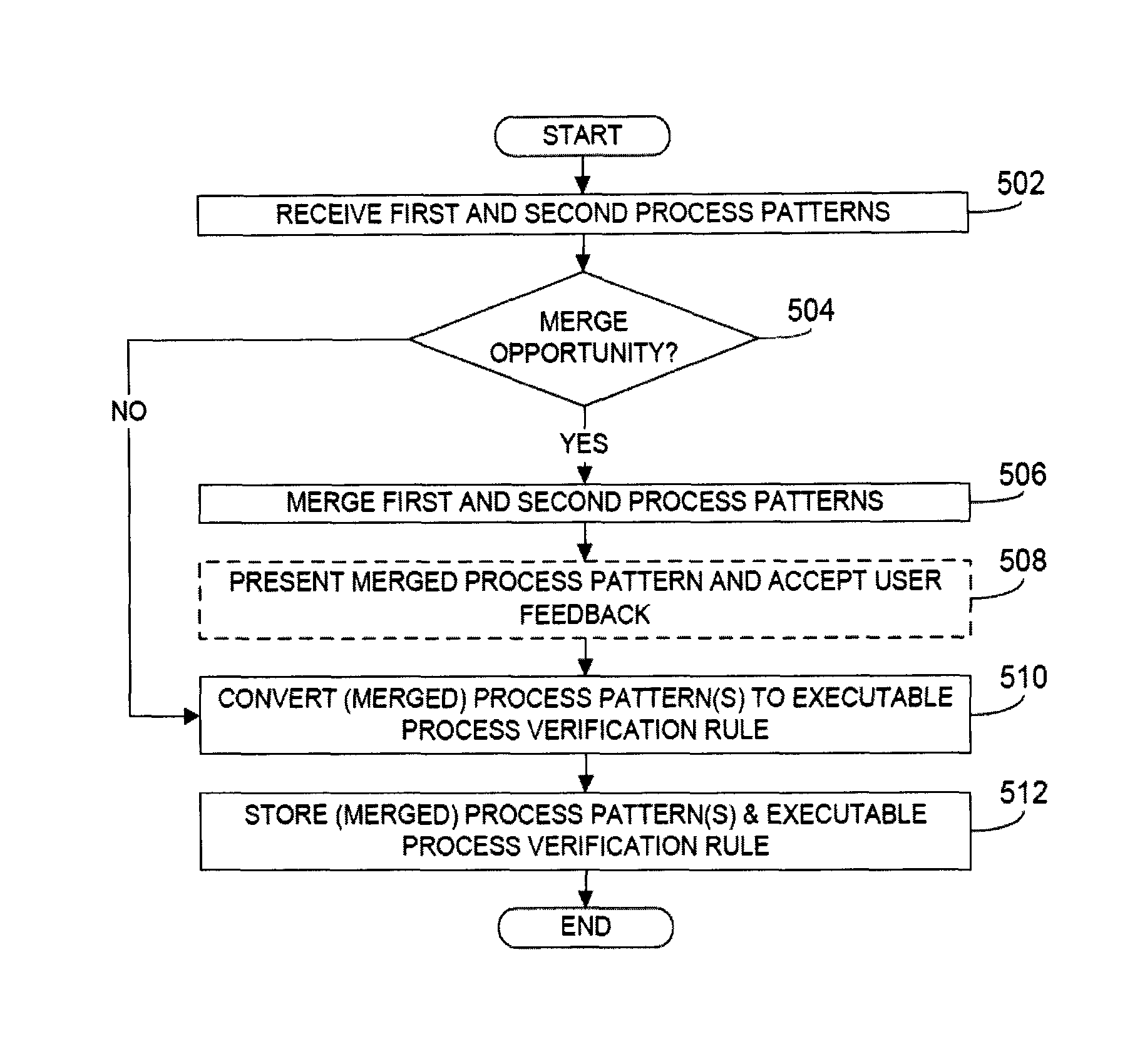
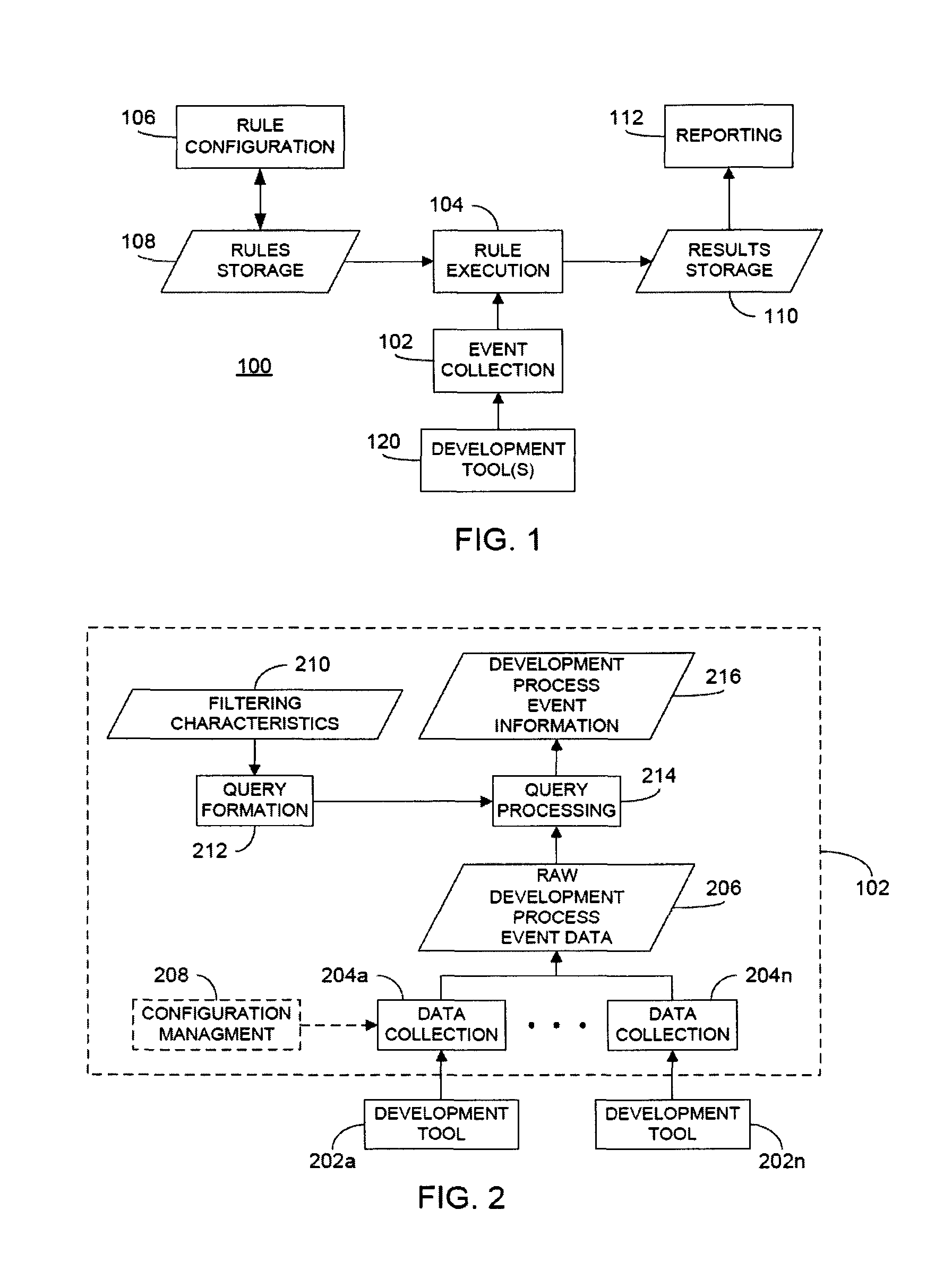
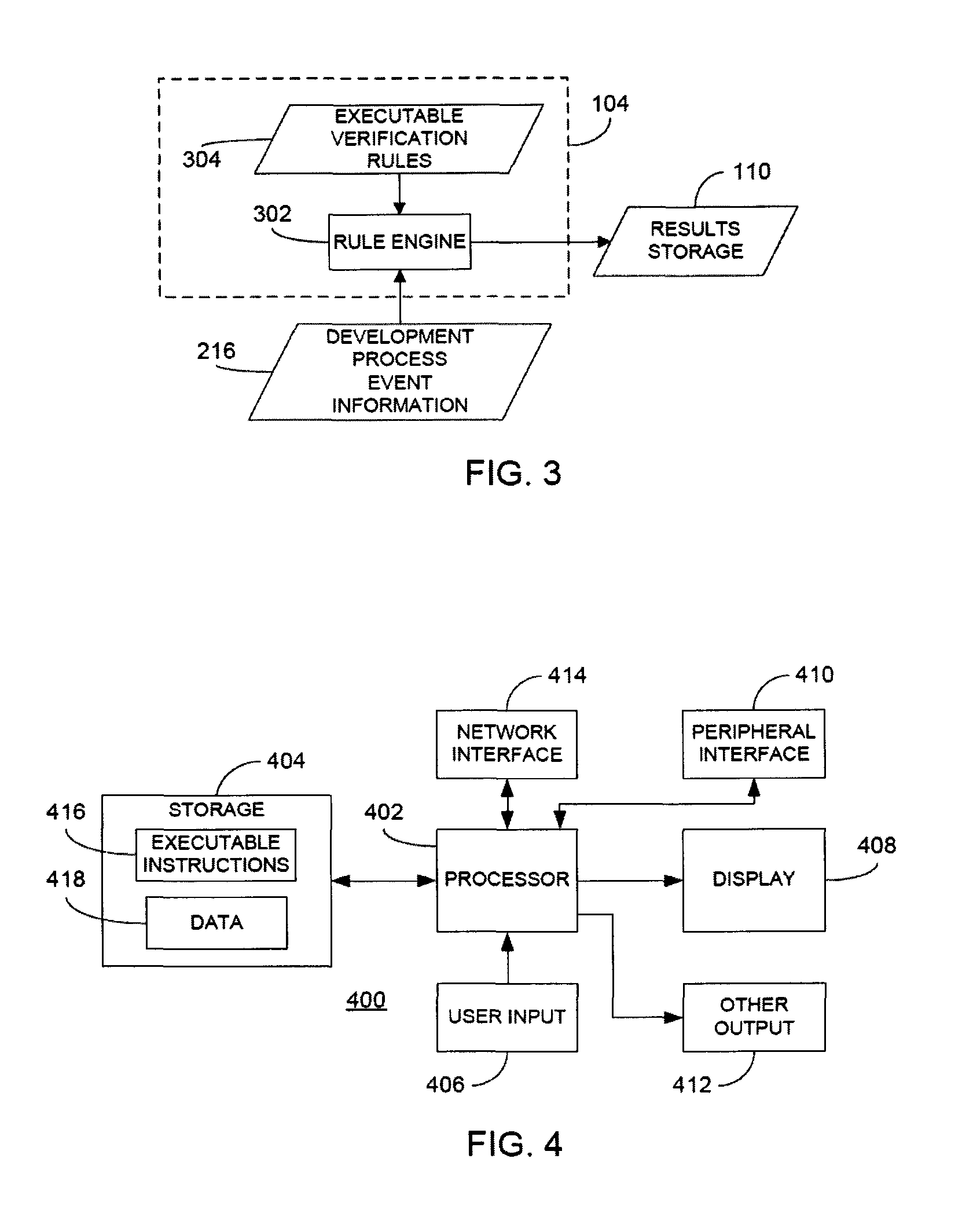
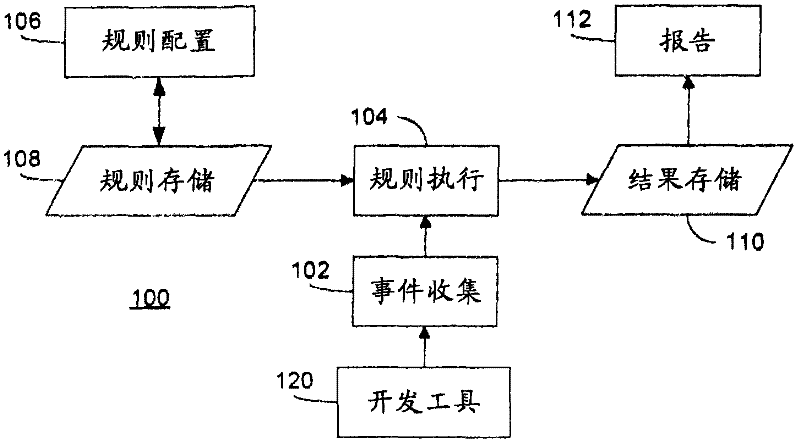
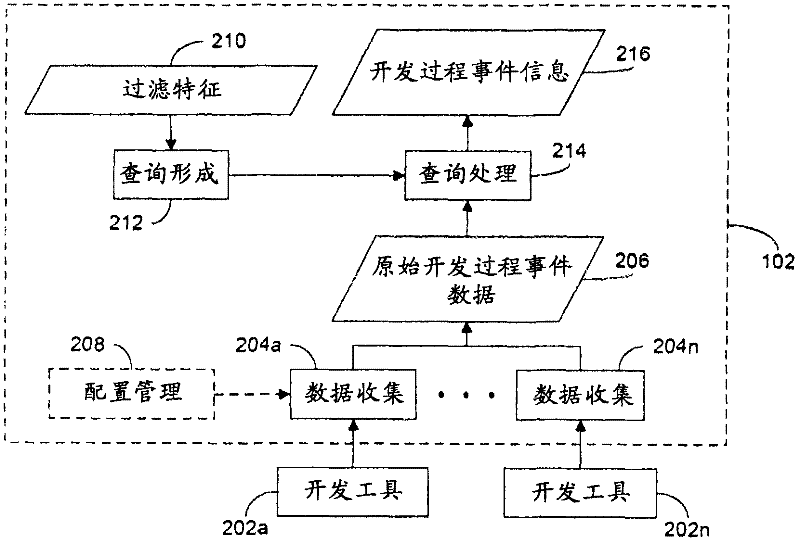
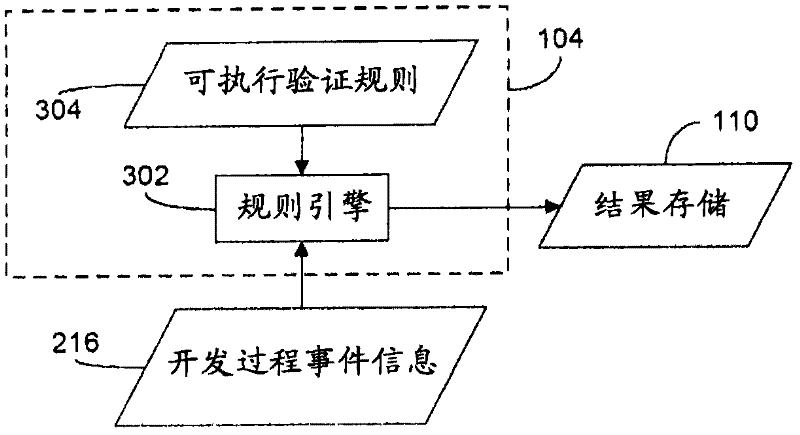
Rule merging in system for monitoring adherence by developers to a software code development process
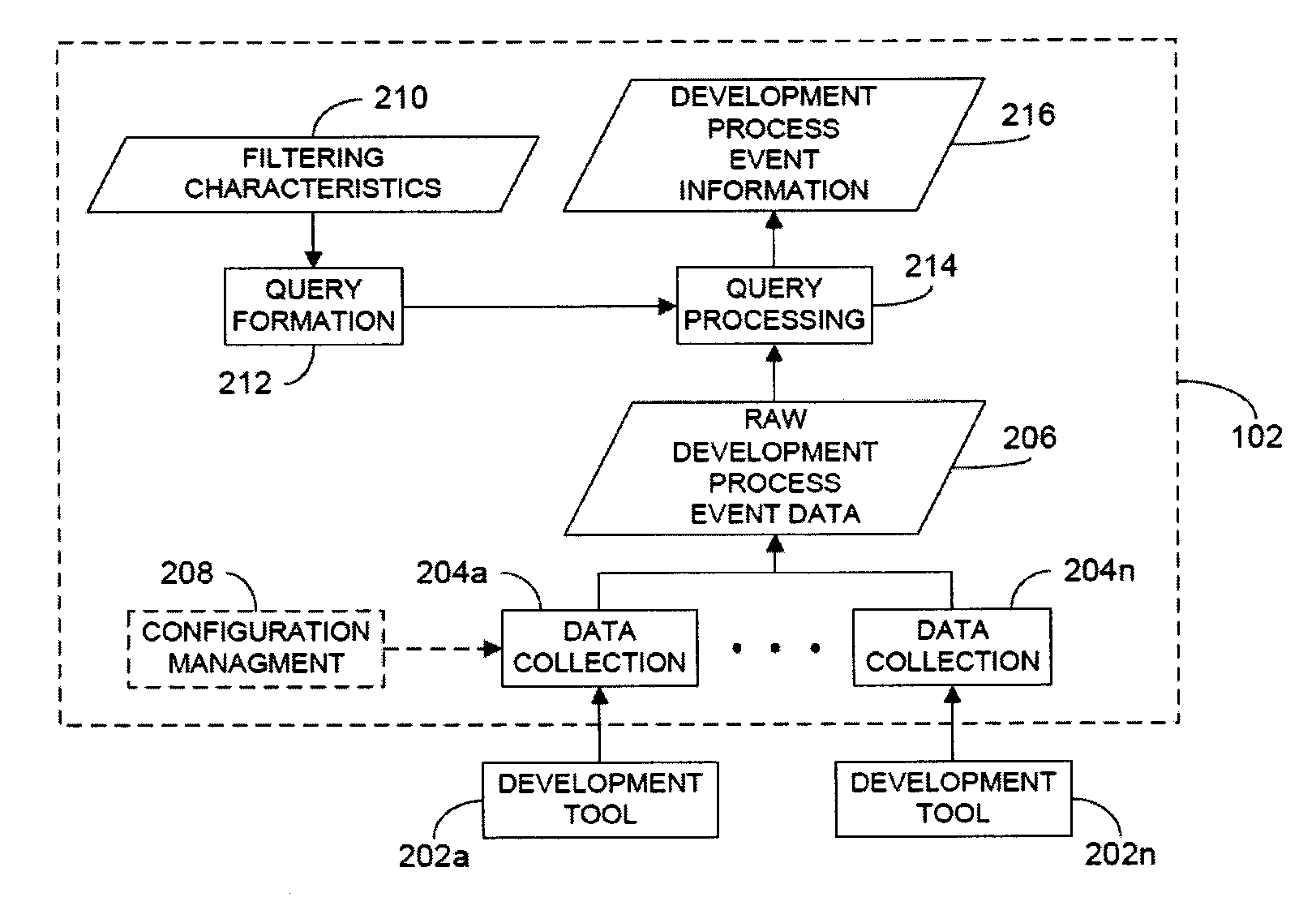
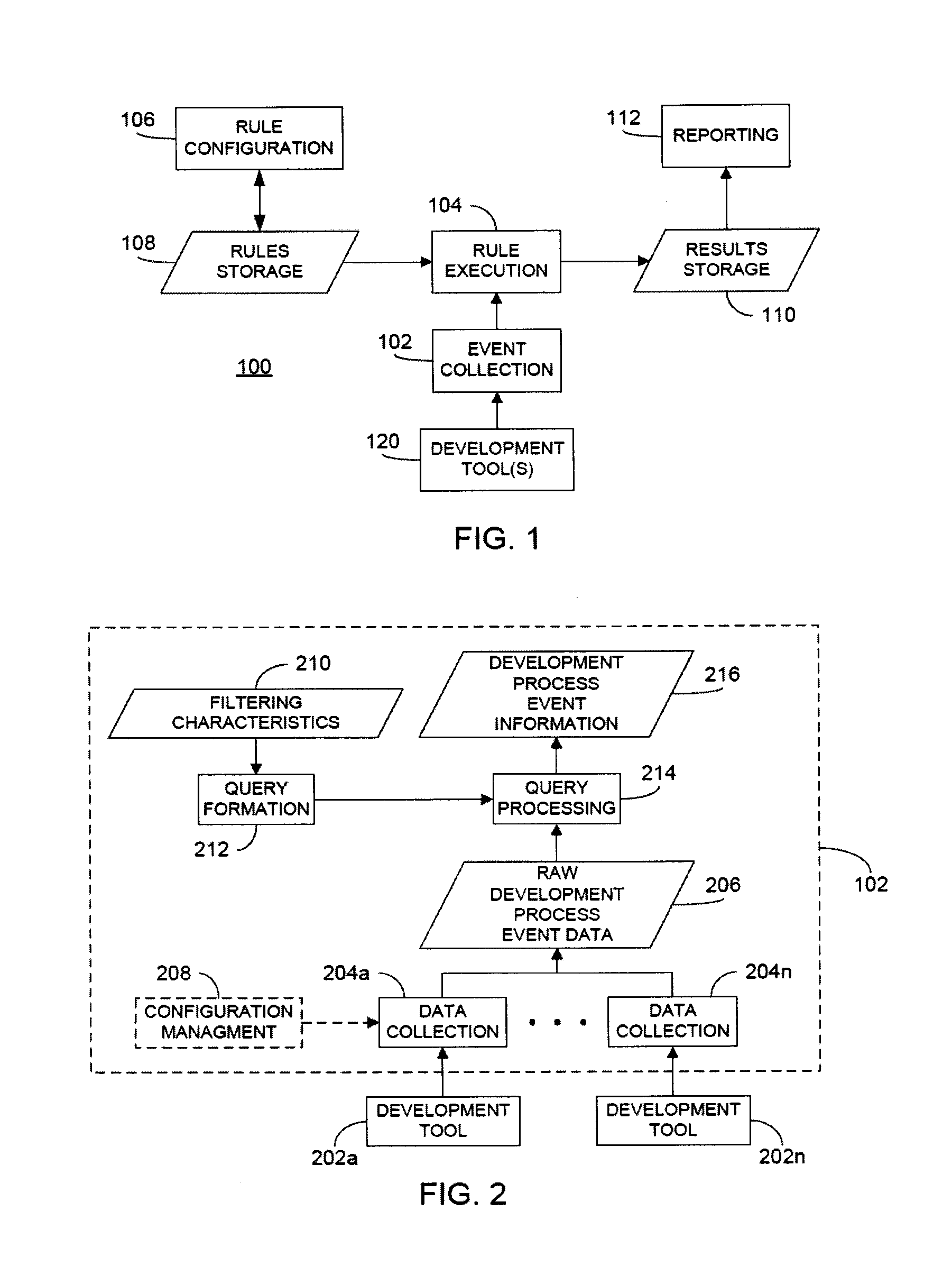
ActiveUS20120317541A1Improve efficiencyMagnifies processing burdenNuclear monitoringDigital computer detailsSoftware development processSubject-matter expert
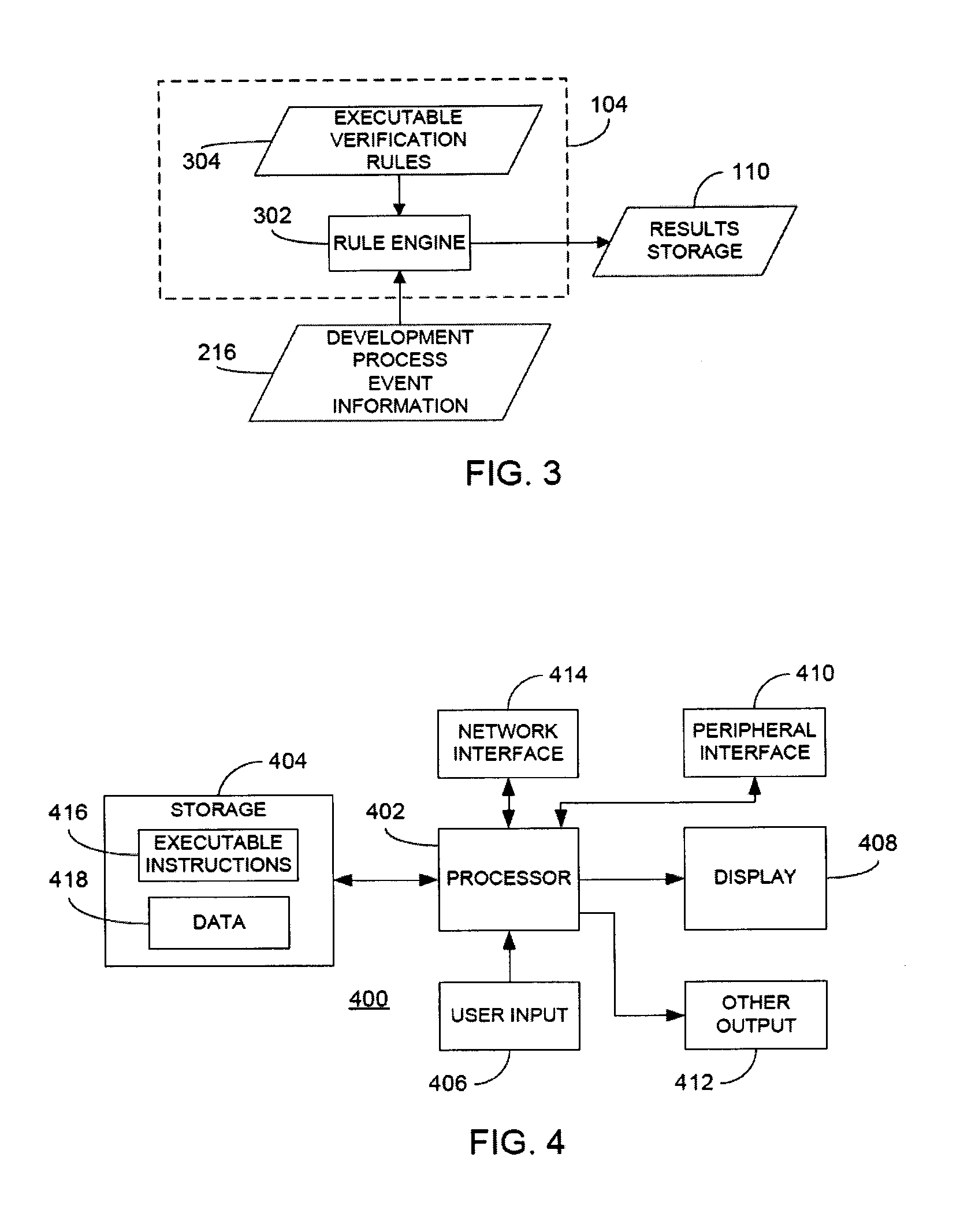
In a rule-based system for monitoring process adherence, first and second processing patterns are received and merged to provide a merged processing pattern. Each processing pattern, which may be expressed in a state graph representation, embodies at least a portion of a desired software code development process. Optionally, the merged processing pattern may be presented to a subject-matter expert to obtain feedback thereon. The merged processing pattern may then be converted into an executable process verification rule for use in monitoring process adherence. In an embodiment, development process event data is compared to the executable process verification rules. Violations of the rules result in the generation of failure indications that may be stored and subsequently reported as needed. In this manner, efficiency of automated process adherence monitoring systems may be improved when determining the level of compliance by developers with one or more software code development processes.
Owner:ACCENTURE GLOBAL SERVICES LTD
Intelligent data extraction
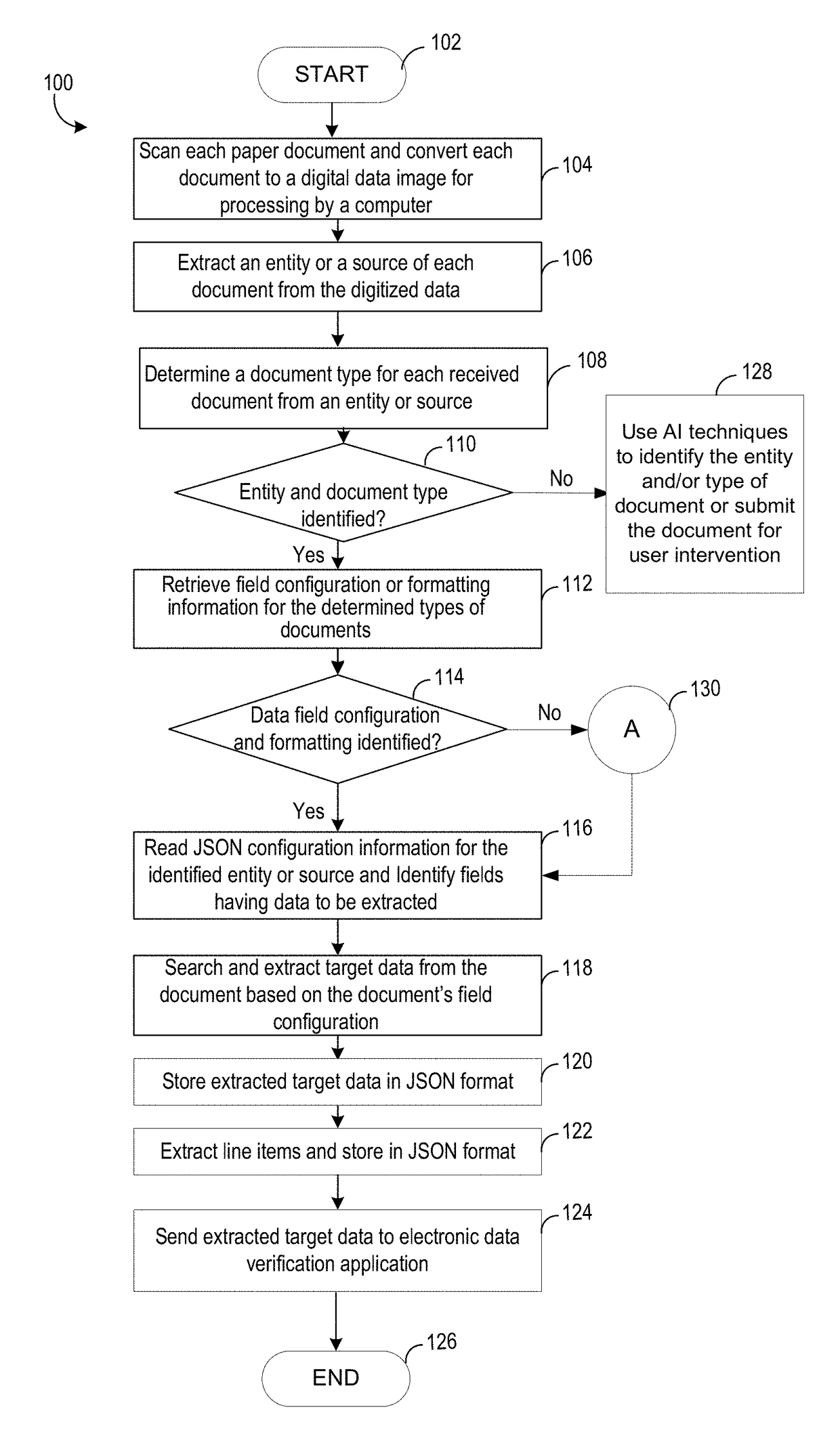
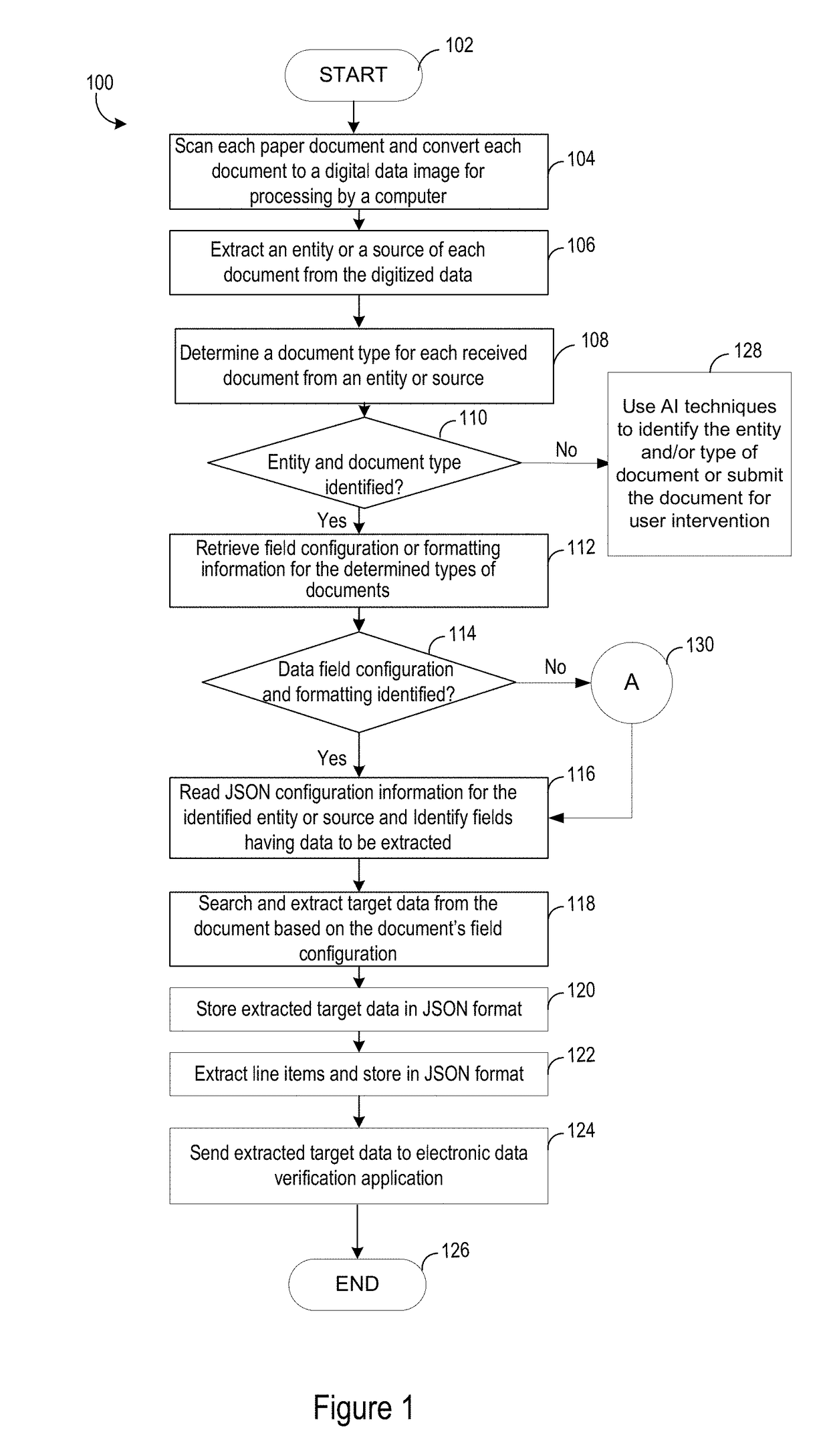
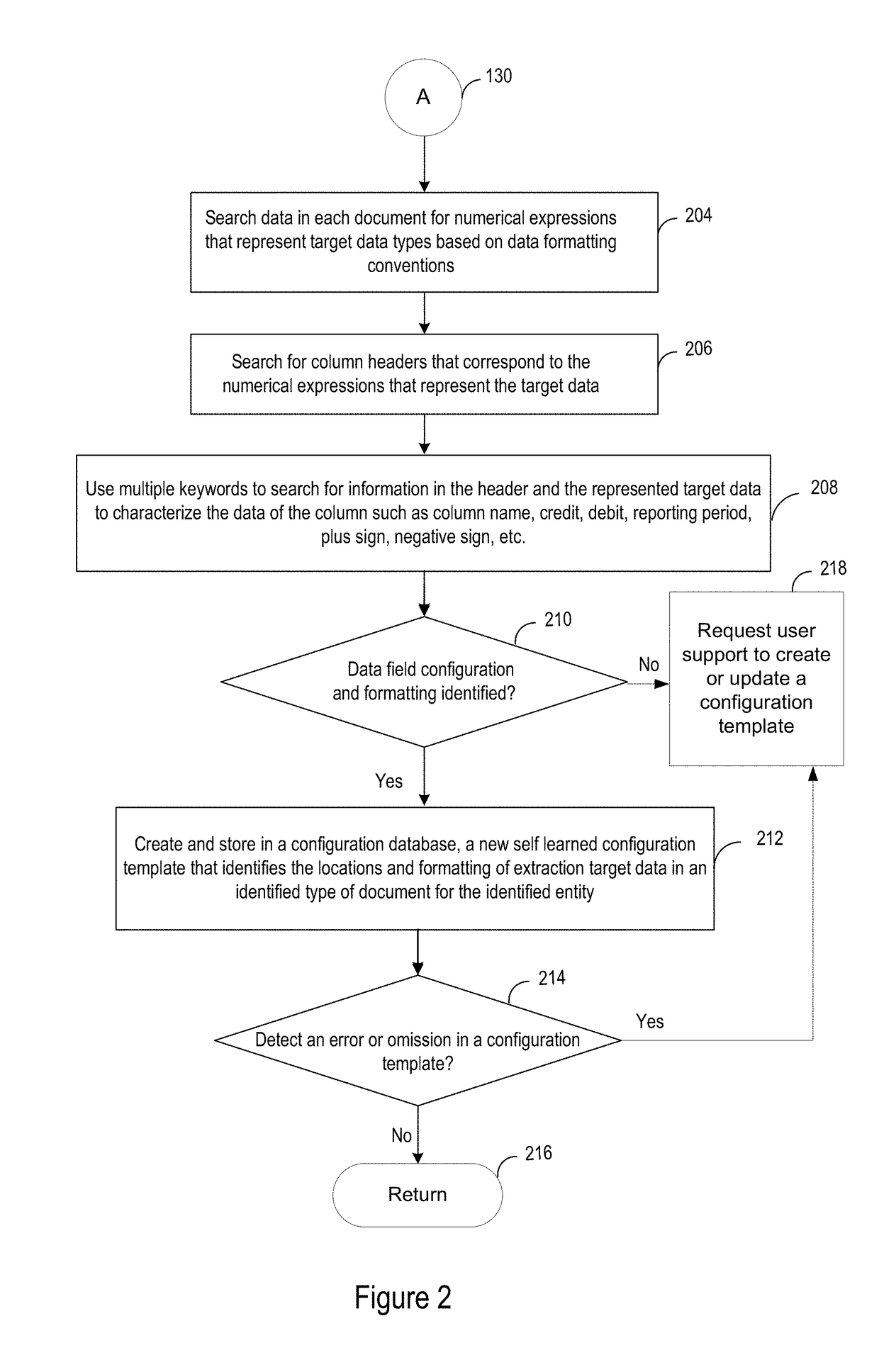
Electronically received data is validated based on a digital data image that is scanned from a paper document. Known paper document source entities, paper document types and associated paper document configuration information are stored in a database. The paper documents are converted to digital data images and optically processed to identify respective source entity and document type information represented within the digital data images. Appropriate document configuration information is retrieved based on association with the detected type of document. Validation target data is extracted from the digital data images based on the configuration information and processed. The electronically received data is validated based on the extracted and processed validation target data.
Owner:ACCENTURE GLOBAL SOLUTIONS LTD
Laser drilling system and method
InactiveUS7569794B2Reduce the amount requiredImprove processing efficiencyRecording apparatusPharmaceutical product form changeEngineeringHandling system
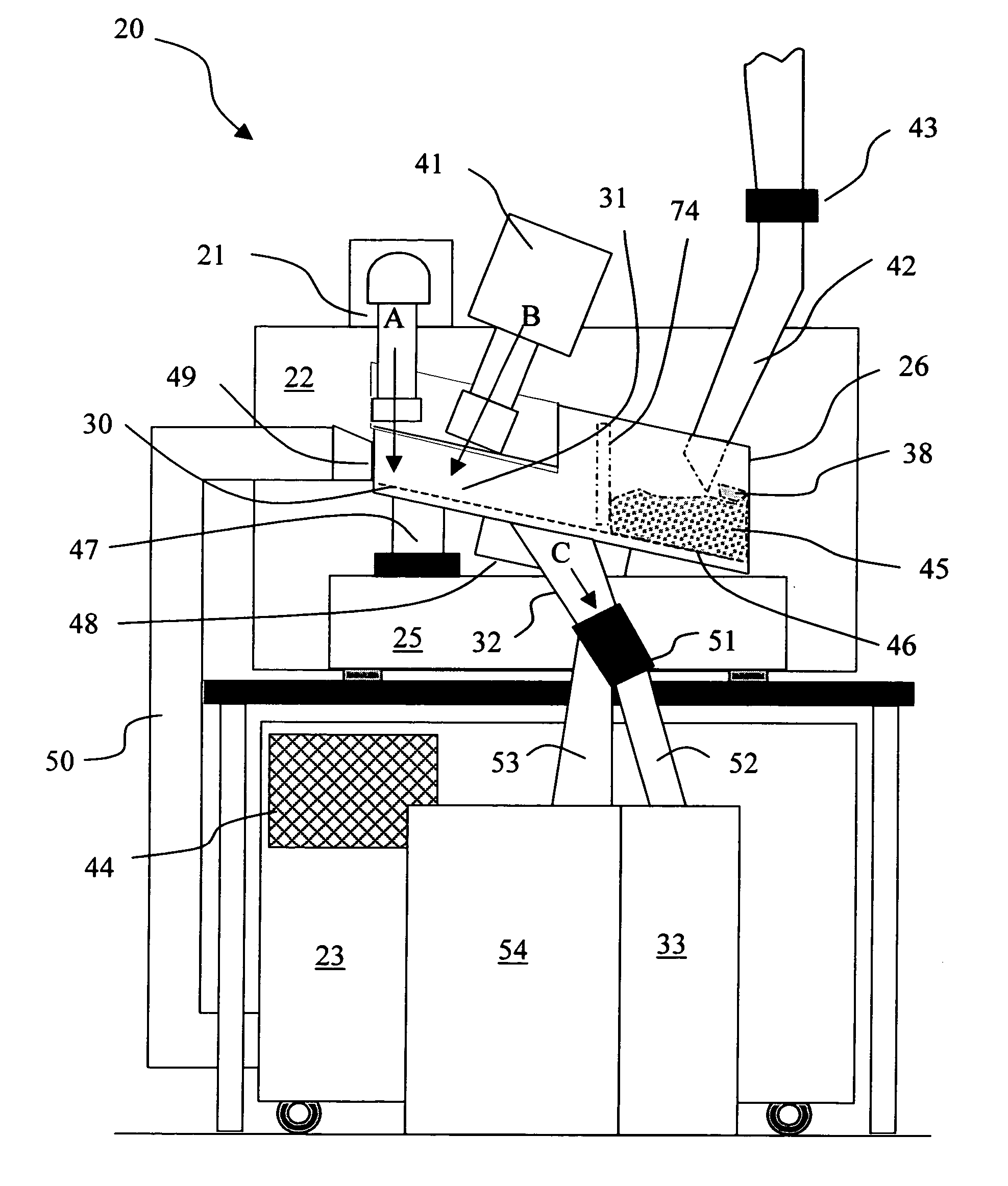
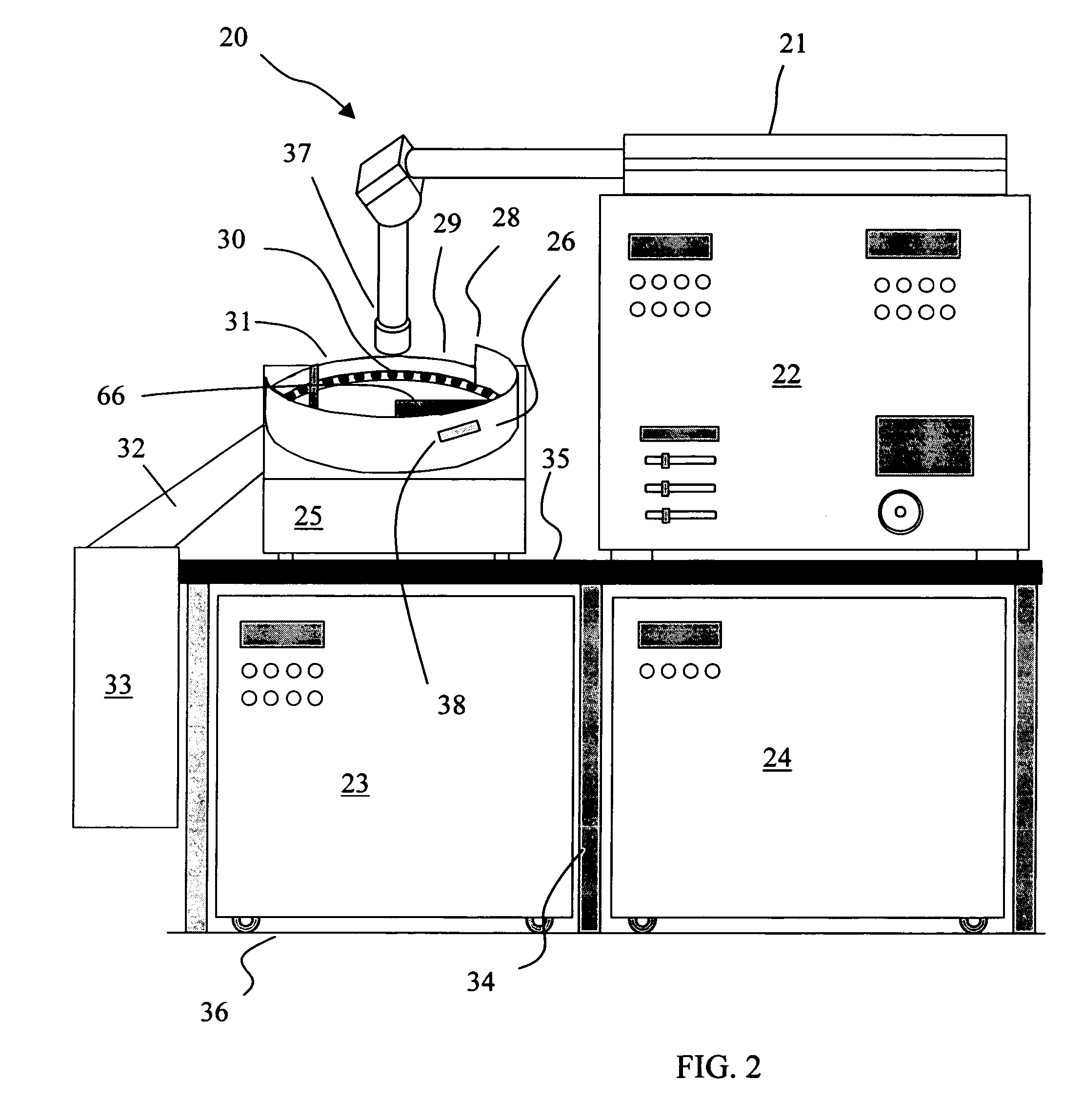
The present invention provides a laser drilling system for drilling holes or cavities in a solid, in particular a solid dosage form. The system includes a loading zone, firing zone, inspection zone and delivery zone. The system also includes optional components such as a process validation system, solids detector, color detector, solids rejection / repositioning means, accepted products receptacle, rejected products receptacle and / or solids inspection system. Operation of the laser device in the firing zone and of other optional equipment is synchronized with movement of a continuous solids indexer by way of an electronic synchronizer. A solids handling system includes an optional fill level detector that directly or indirectly controls solids loading means that fills a solids reservoir. The system can be run continuously, semicontinuously or batchwise. A solids rejection system in the system provides for reduced solids loss as compared to other laser drilling systems and solids recovery rates of 100% can be achieved.
Owner:ACELLA HLDG LLC +1
Laser drilling system and method
InactiveUS20050145605A1Reduce the amount requiredImprove processing efficiencyRecording apparatusPharmaceutical product form changeHandling systemProcess validation
The present invention provides a laser drilling system for drilling holes or cavities in a solid, in particular a solid dosage form. The system includes a loading zone, firing zone, inspection zone and delivery zone. The system also includes optional components such as a process validation system, solids detector, color detector, solids rejection / repositioning means, accepted products receptacle, rejected products receptacle and / or solids inspection system. Operation of the laser device in the firing zone and of other optional equipment is synchronized with movement of a continuous solids indexer by way of an electronic synchronizer. A solids handling system includes an optional fill level detector that directly or indirectly controls solids loading means that fills a solids reservoir. The system can be run continuously, semicontinuously or batchwise. A solids rejection system in the system provides for reduced solids loss as compared to other laser drilling systems and solids recovery rates of 100% can be achieved.
Owner:ACELLA HLDG LLC +1
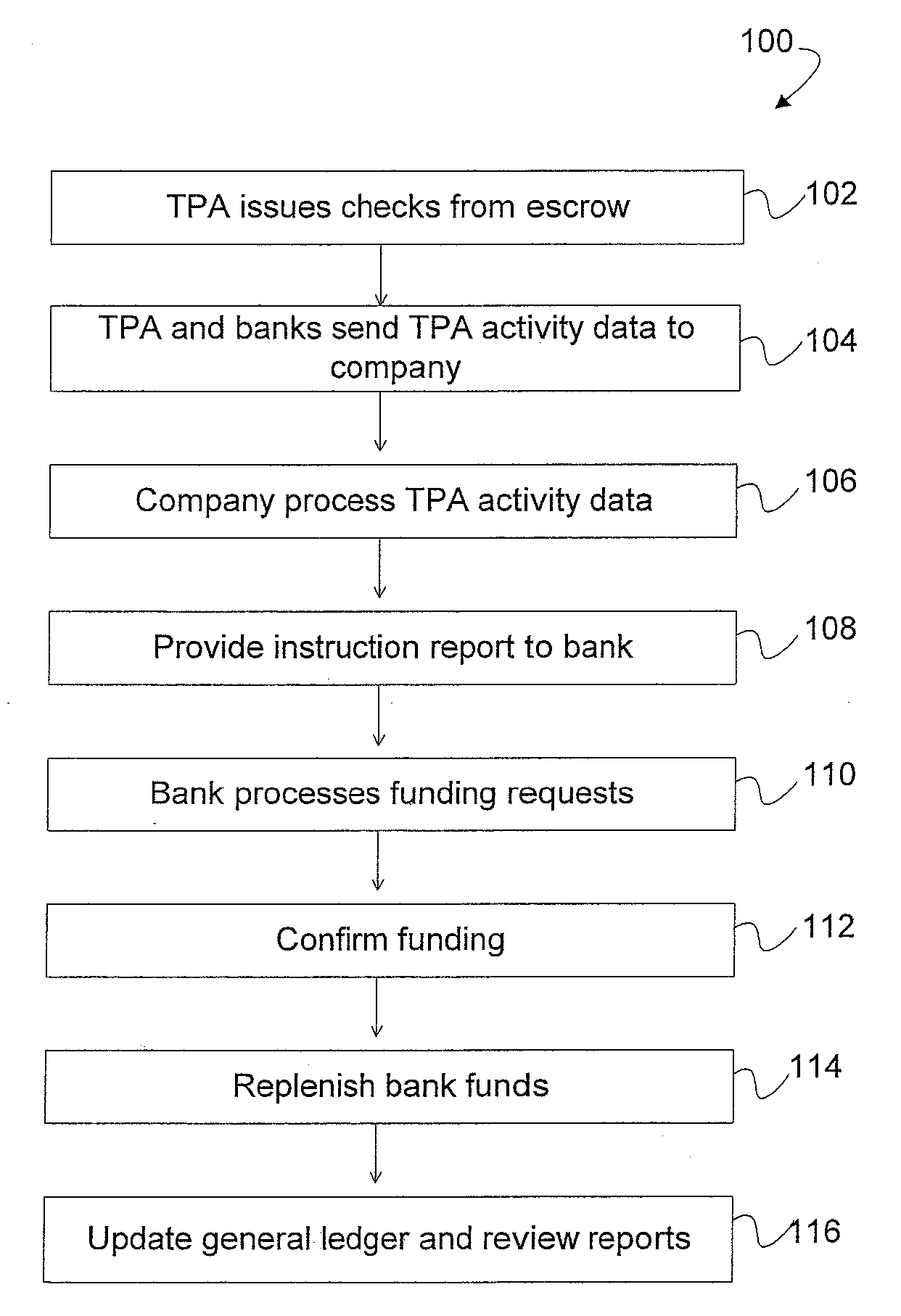
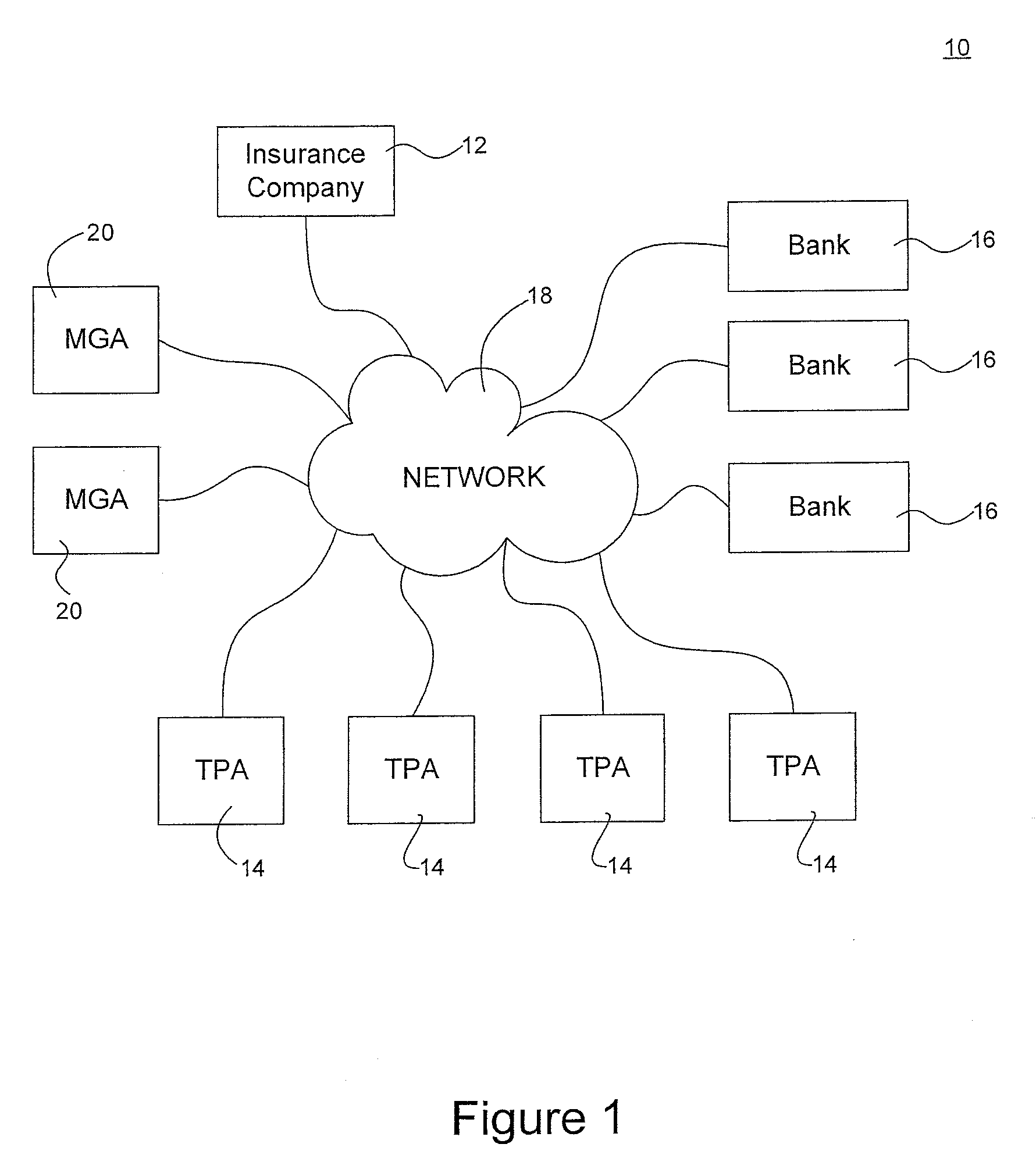
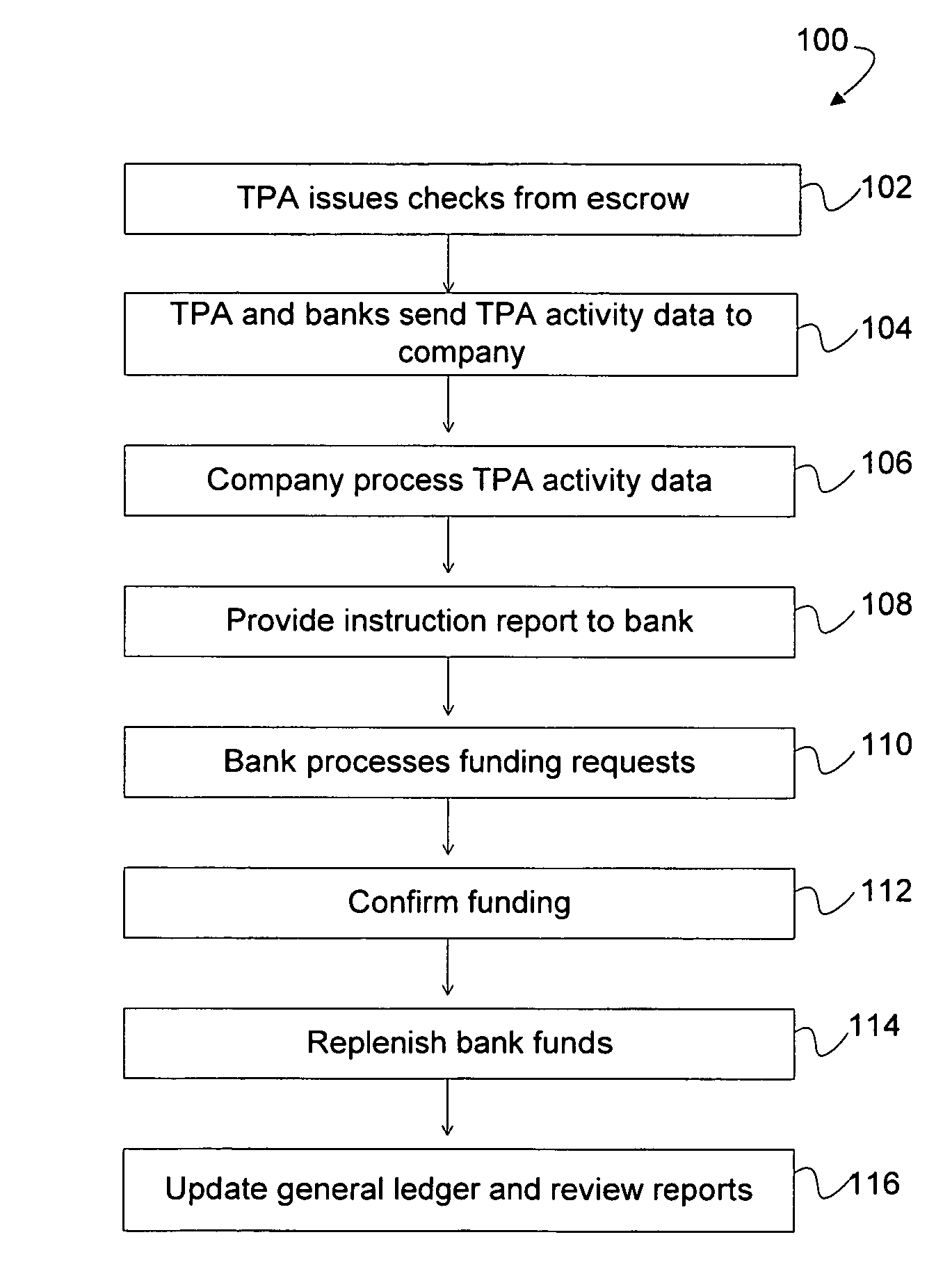
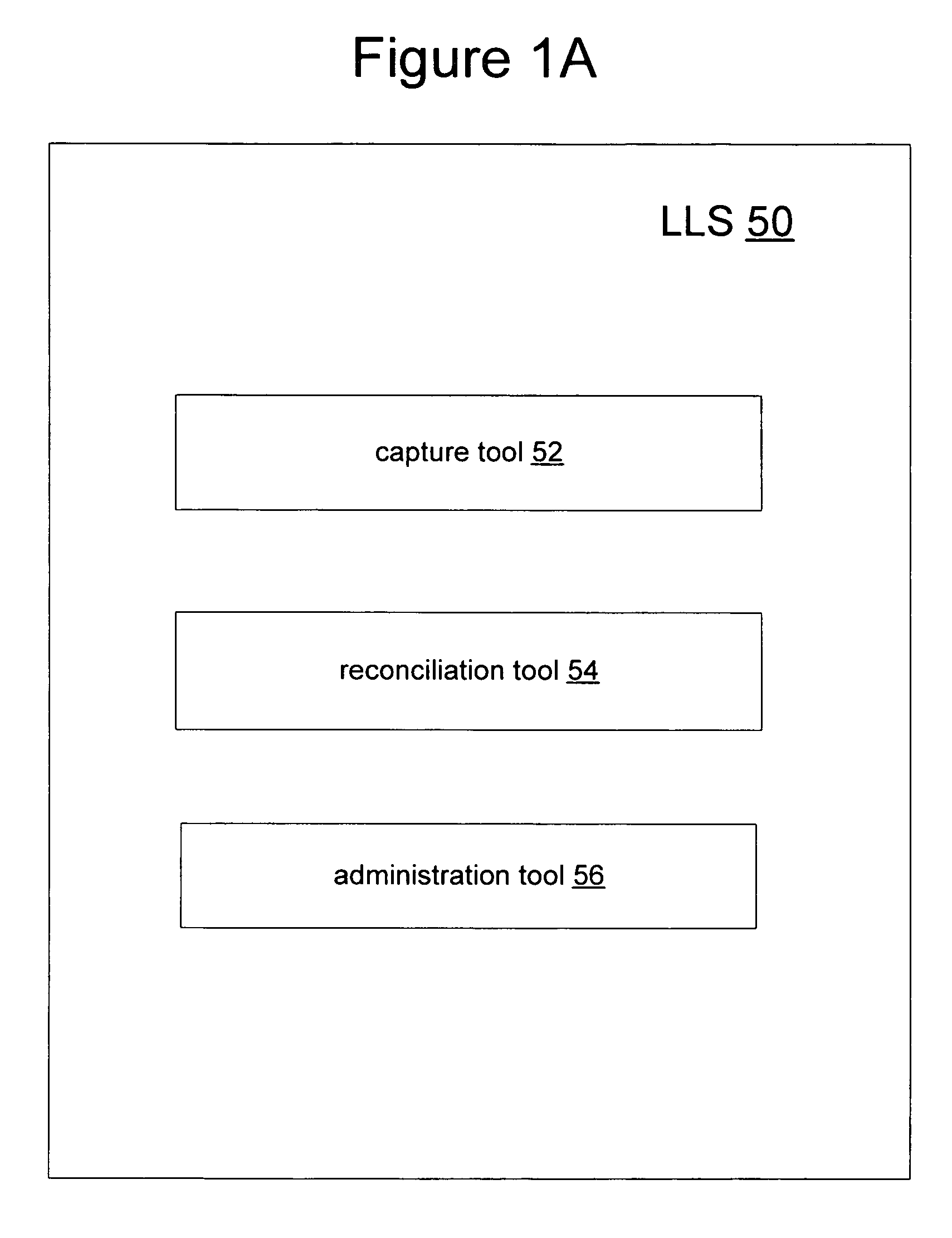
System for maintaining an escrow account for reimbursing administrators of payments
InactiveUS20090254381A1Accurate methodReducing escrow accountsFinancePayment architecturePaymentThird party
A loss ledger system to process third party fund requests on a daily basis, track historical requests, process validation on each request, maintain suspense / escrow and loss ledgers, and provide reconciliation assistance for accounting and claims processing departments. Additionally, the loss ledger system provides management reports, interfaces, security, audit and control and data conversion to facilitate minimizing escrowed resources, fraudulent activity and clerical errors.
Owner:ARCH CAPITAL GRP U S
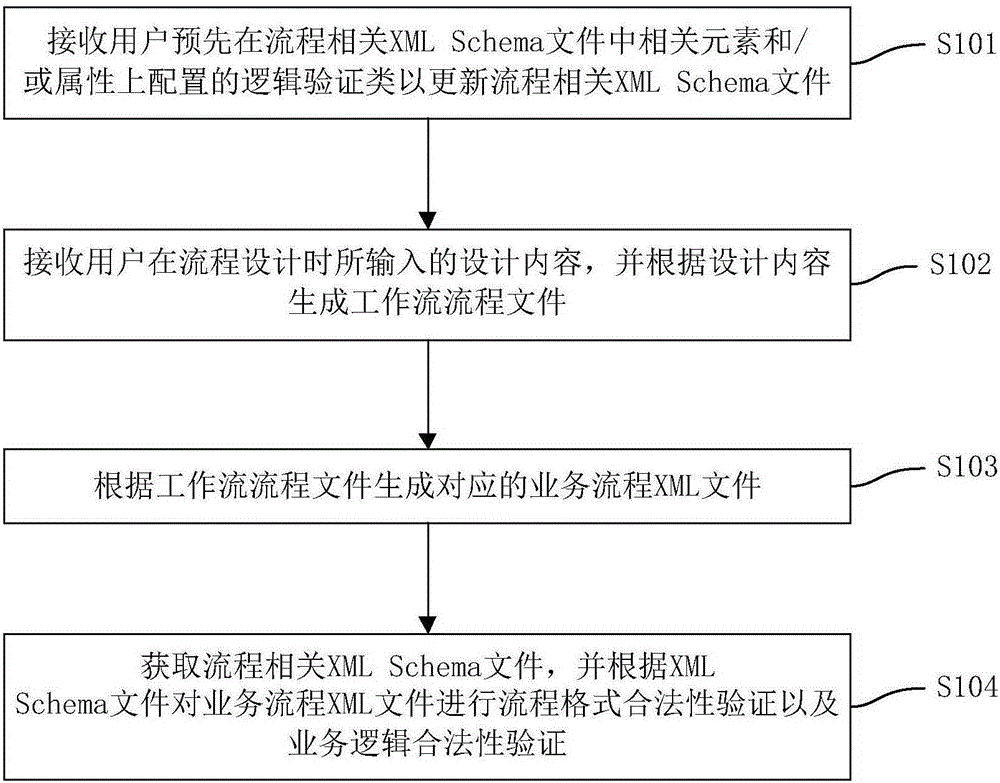
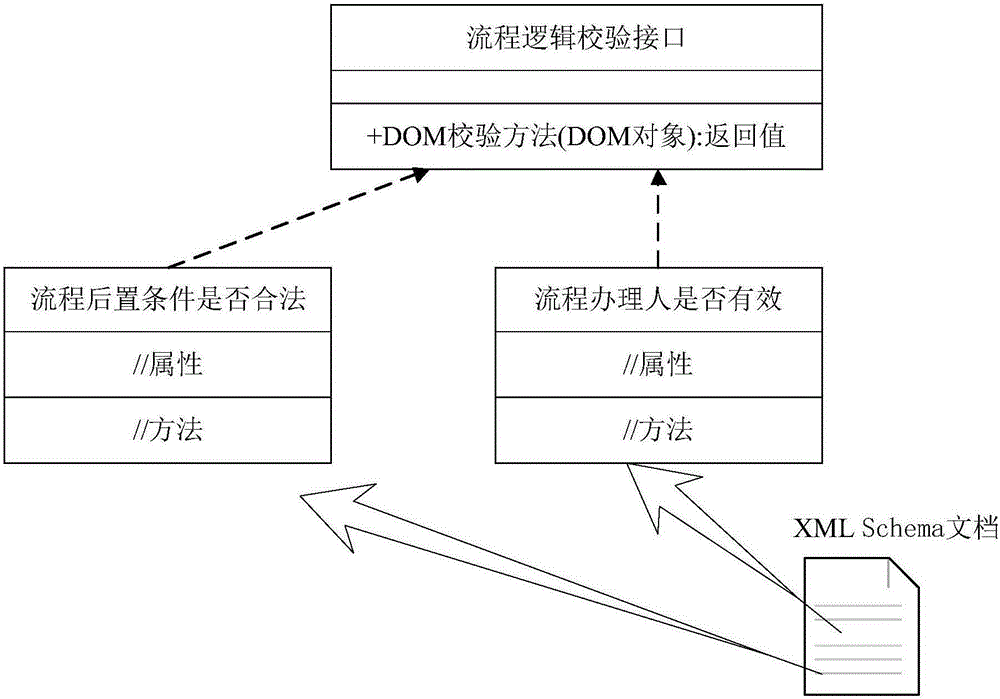
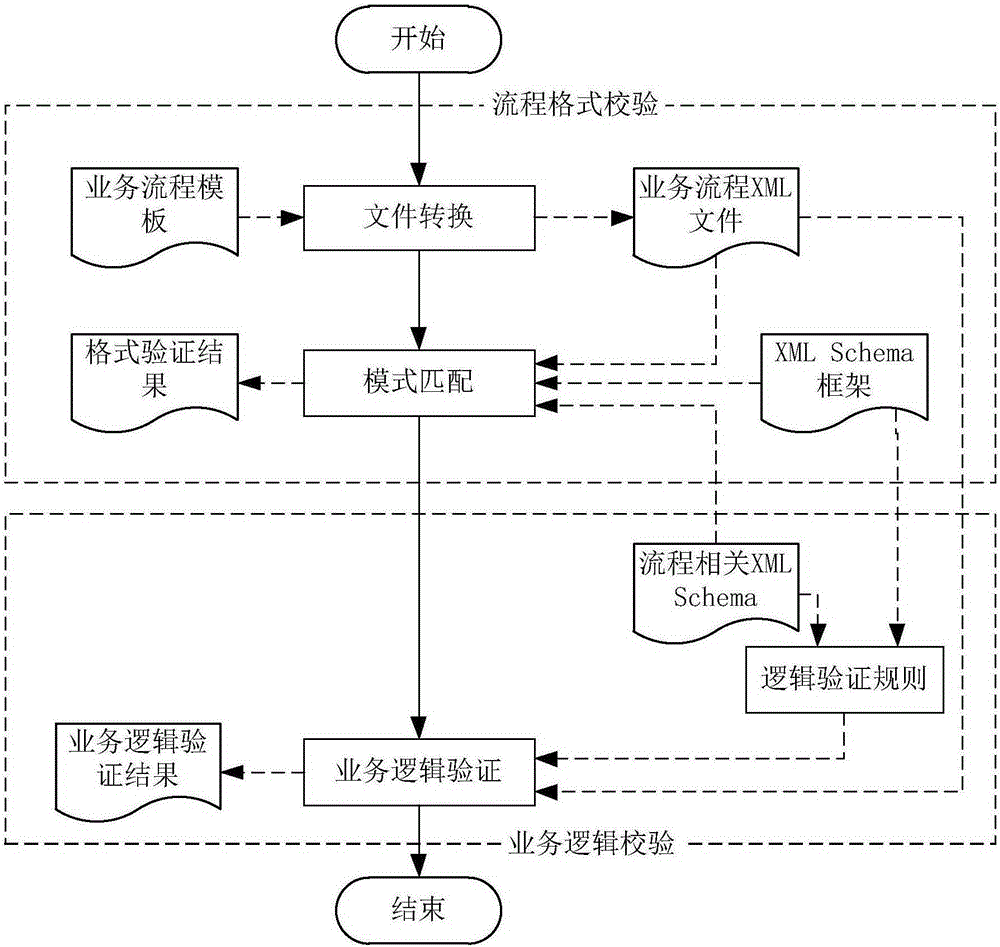
Validation method and validation device for workflow validity
ActiveCN105225066AImprove accuracyIncrease engagementOffice automationResourcesXML schemaBusiness Personnel
The invention discloses a validation method and a validation device for workflow validity. The method comprises the following steps: receiving logic validation classes which are configured on related elements and / or attributes in a process-related XML Schema file by a user in advance to update the process-related XML Schema file; receiving design content input by the user in a process design process and generating a workflow process file according to the design content; generating a corresponding business process XML file according to the workflow process file; and obtaining the process-related XML Schema file, and carrying out a process format validity validation and a business logic validity validation on the business process XML file according to the XML Schema file. The method supports the traditional process validation method and also supports the flow business logic validation; the accuracy of the validation result is improved; the participation degree of business personnel is strengthened; and the system implementation accuracy is improved.
Owner:NEUSOFT CORP
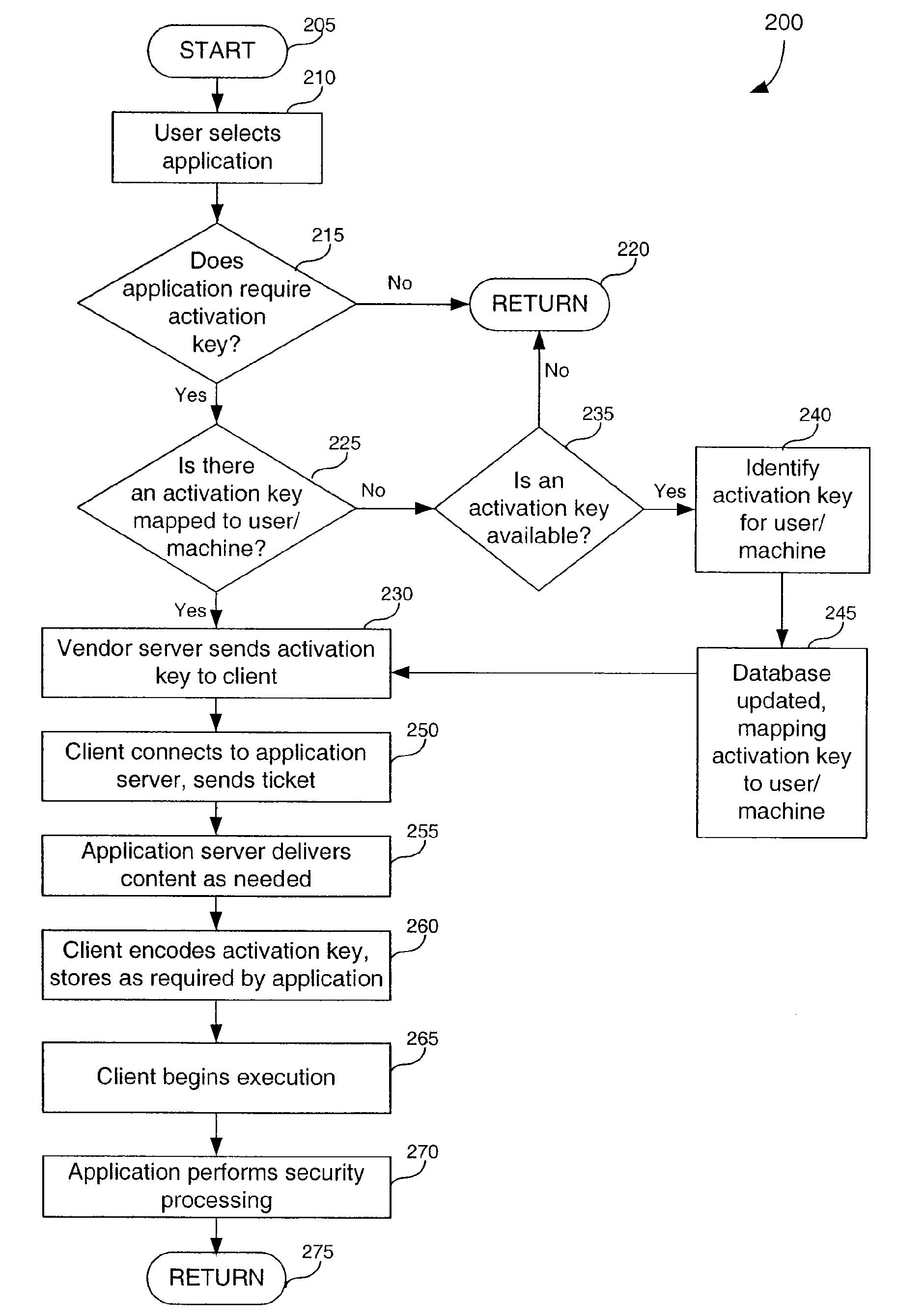
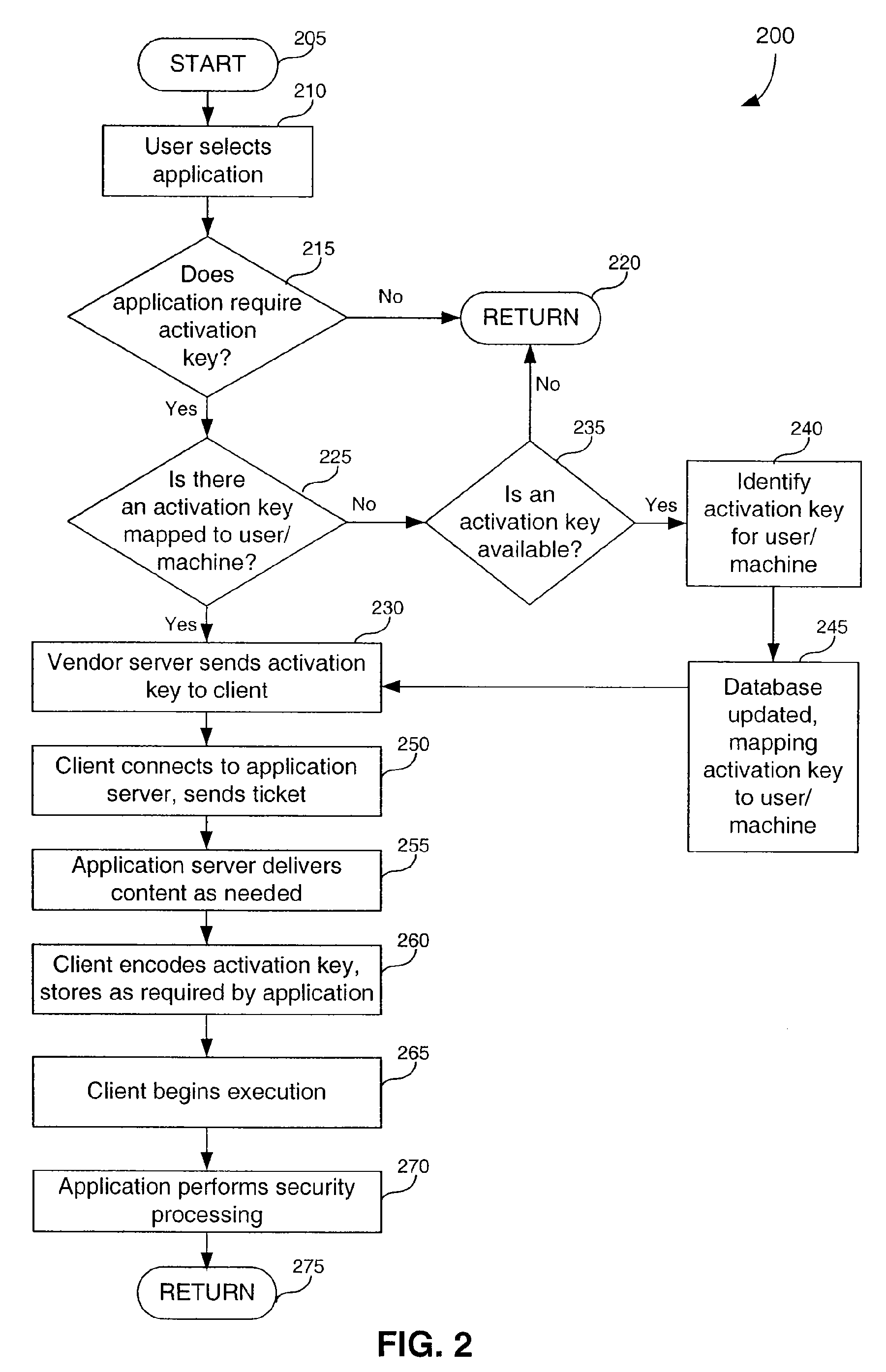
Useability features in on-line delivery of applications
InactiveUS20090237418A1Digital data processing detailsMemory adressing/allocation/relocationApplication softwareBiological activation
Systems, methods, and computer program products for enhancing useability of on-line delivered applications. Access control is provided by generating and delivering an activation key to a client whenever the client seeks access to an application. A security process, integral to the application, validates the key. With respect to displaying information, a client inserts itself between the application and the renderer. This allows the client to provide information to the renderer for display to the user.
Owner:EXENT TECH
Rule merging in system for monitoring adherence by developers to a software code development process
ActiveUS8621417B2Shorten the timeImprove efficiencyDigital computer detailsNuclear monitoringSoftware development processSubject-matter expert
In a rule-based system for monitoring process adherence, first and second processing patterns are received and merged to provide a merged processing pattern. Each processing pattern, which may be expressed in a state graph representation, embodies at least a portion of a desired software code development process. Optionally, the merged processing pattern may be presented to a subject-matter expert to obtain feedback thereon. The merged processing pattern may then be converted into an executable process verification rule for use in monitoring process adherence. In an embodiment, development process event data is compared to the executable process verification rules. Violations of the rules result in the generation of failure indications that may be stored and subsequently reported as needed. In this manner, efficiency of automated process adherence monitoring systems may be improved when determining the level of compliance by developers with one or more software code development processes.
Owner:ACCENTURE GLOBAL SERVICES LTD
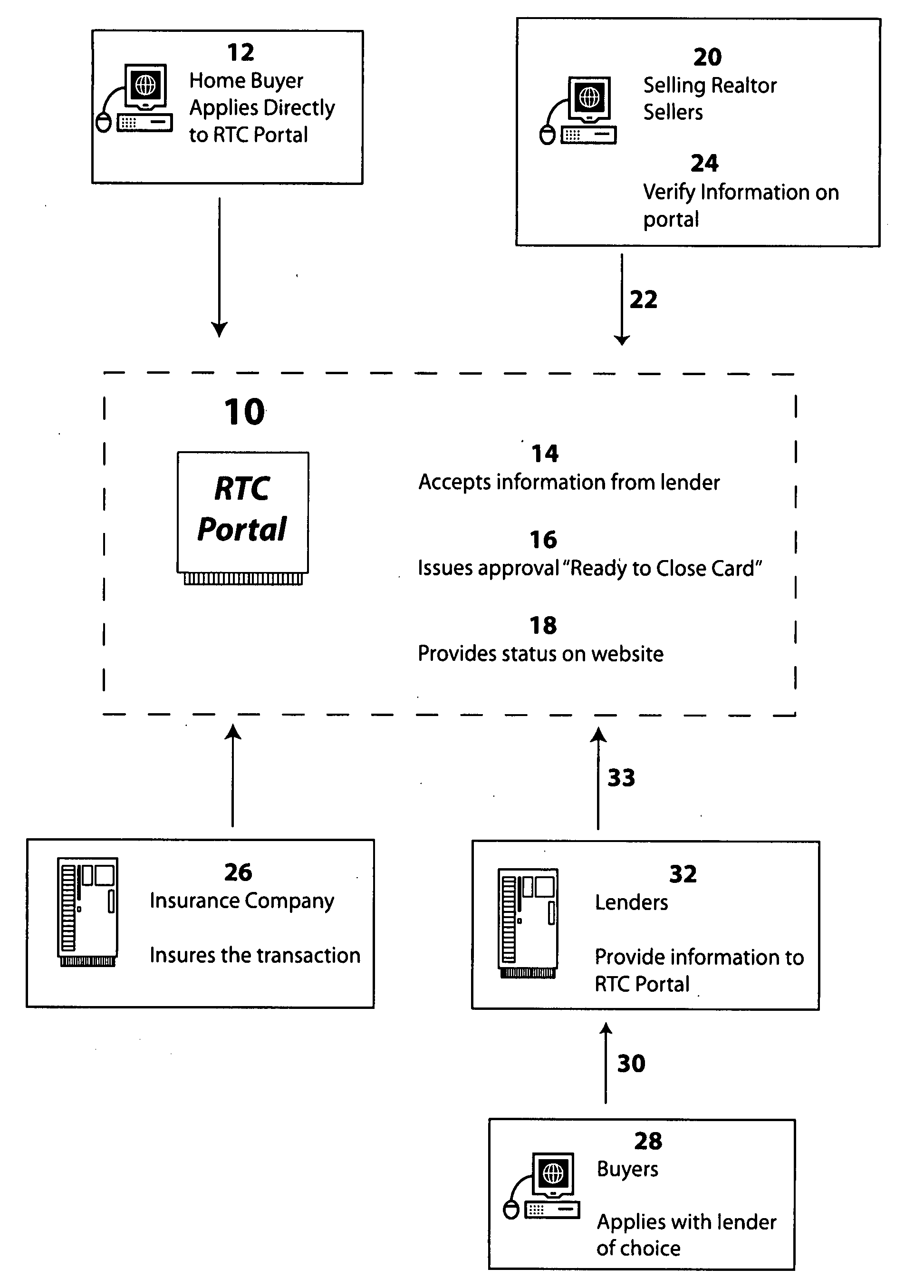
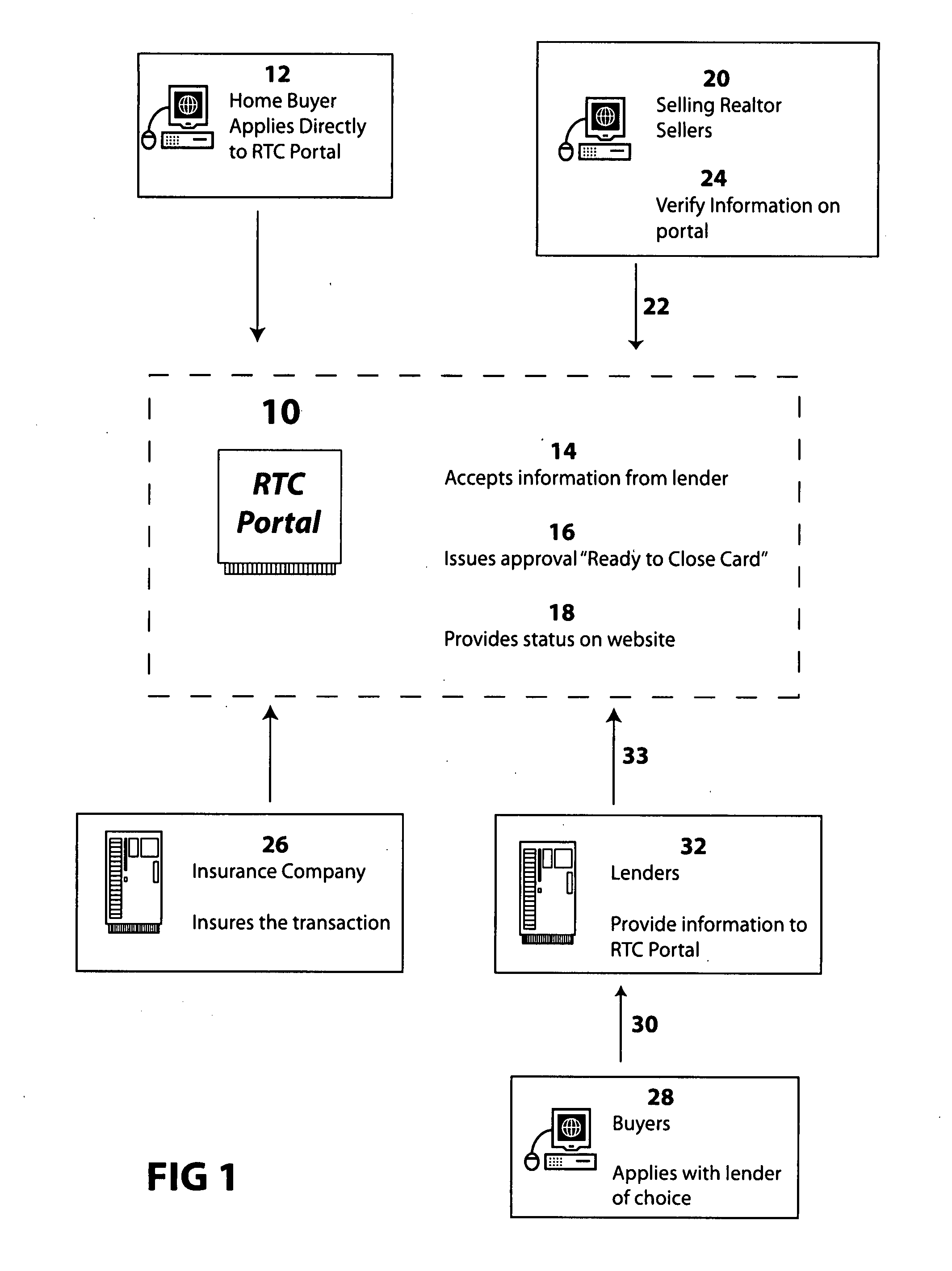
Method for home buyer loan approval process validation
InactiveUS20070208661A1FinanceBuying/selling/leasing transactionsValidation methodsProcess validation
Owner:MORAN WILLIAM
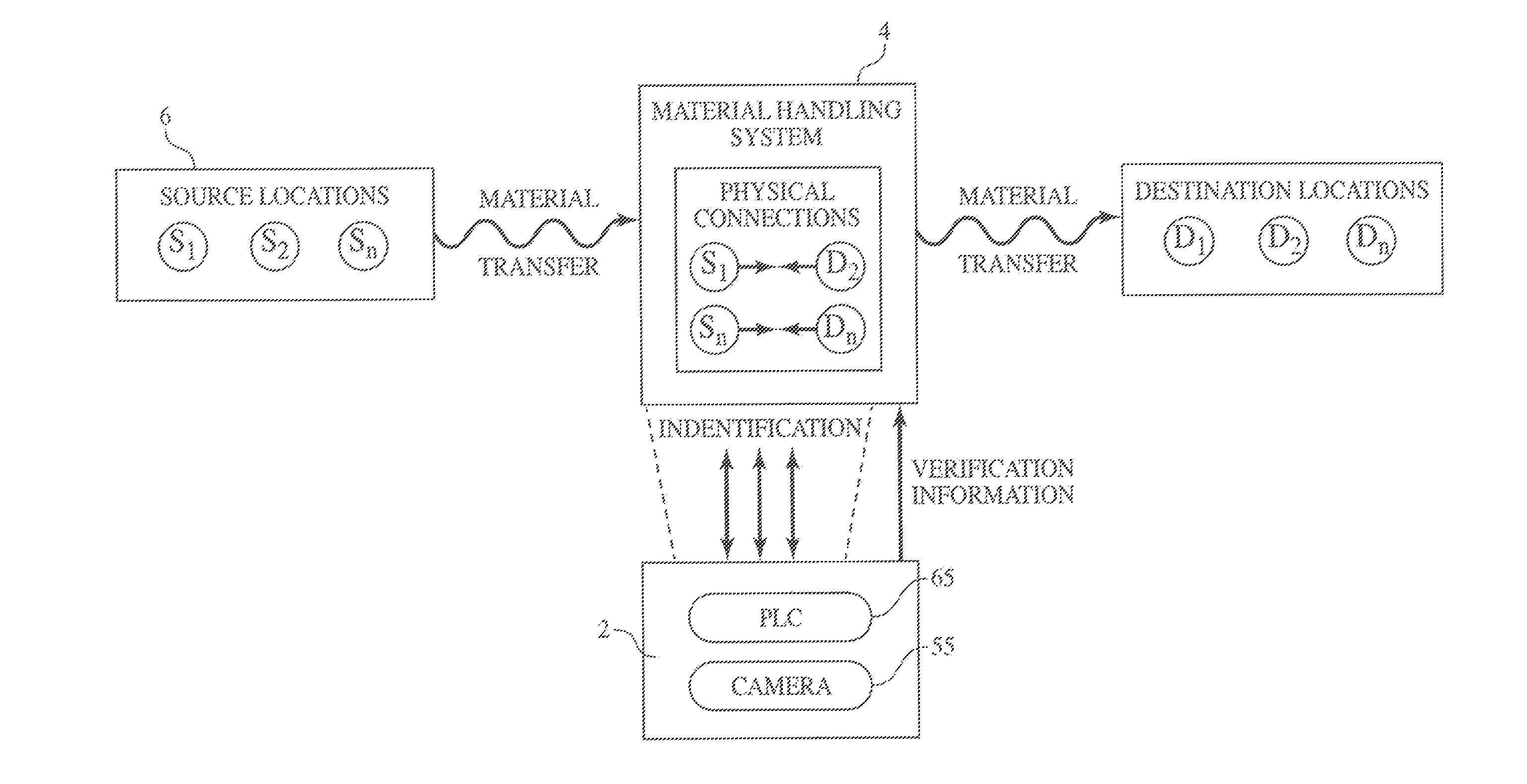
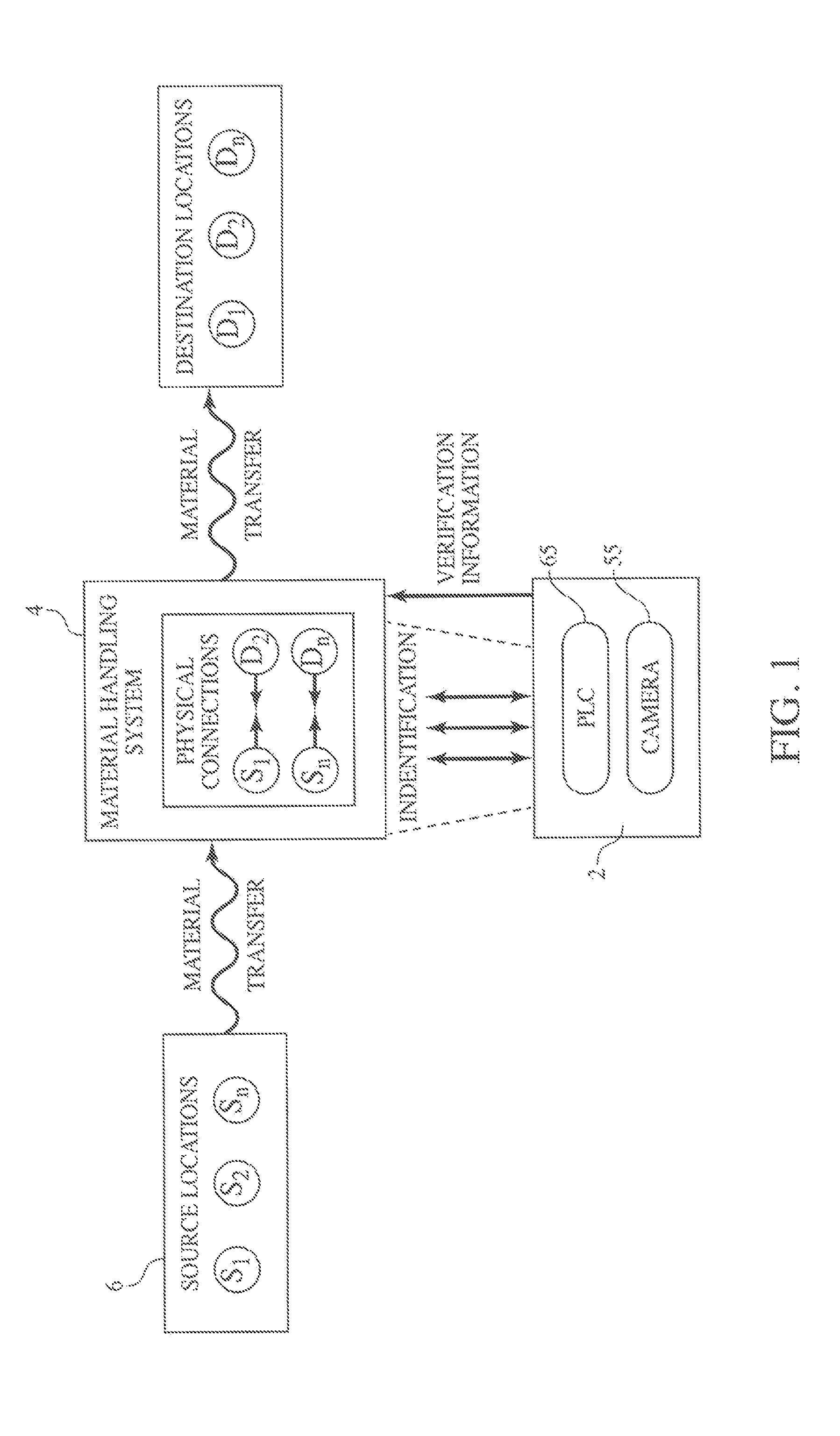
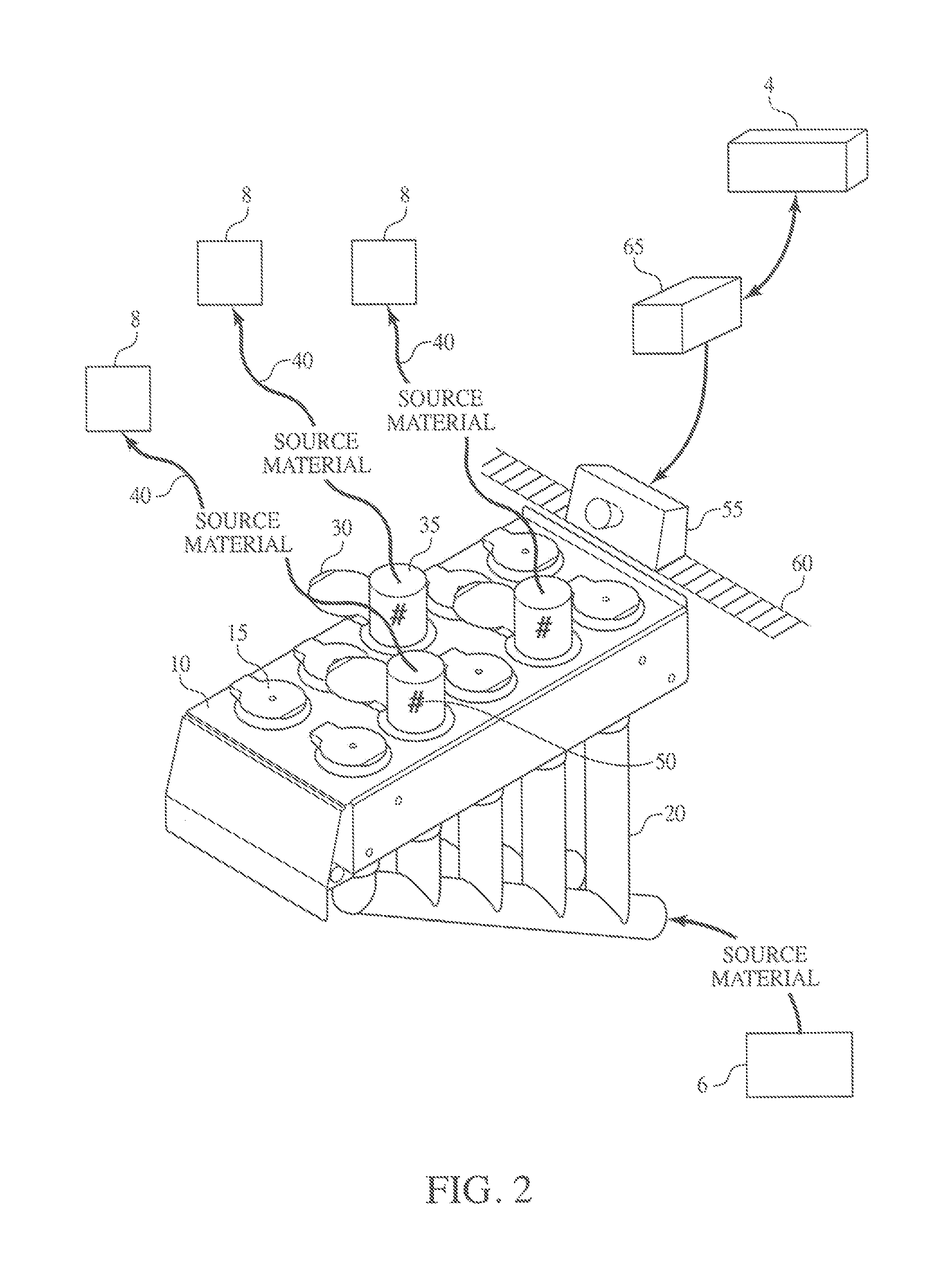
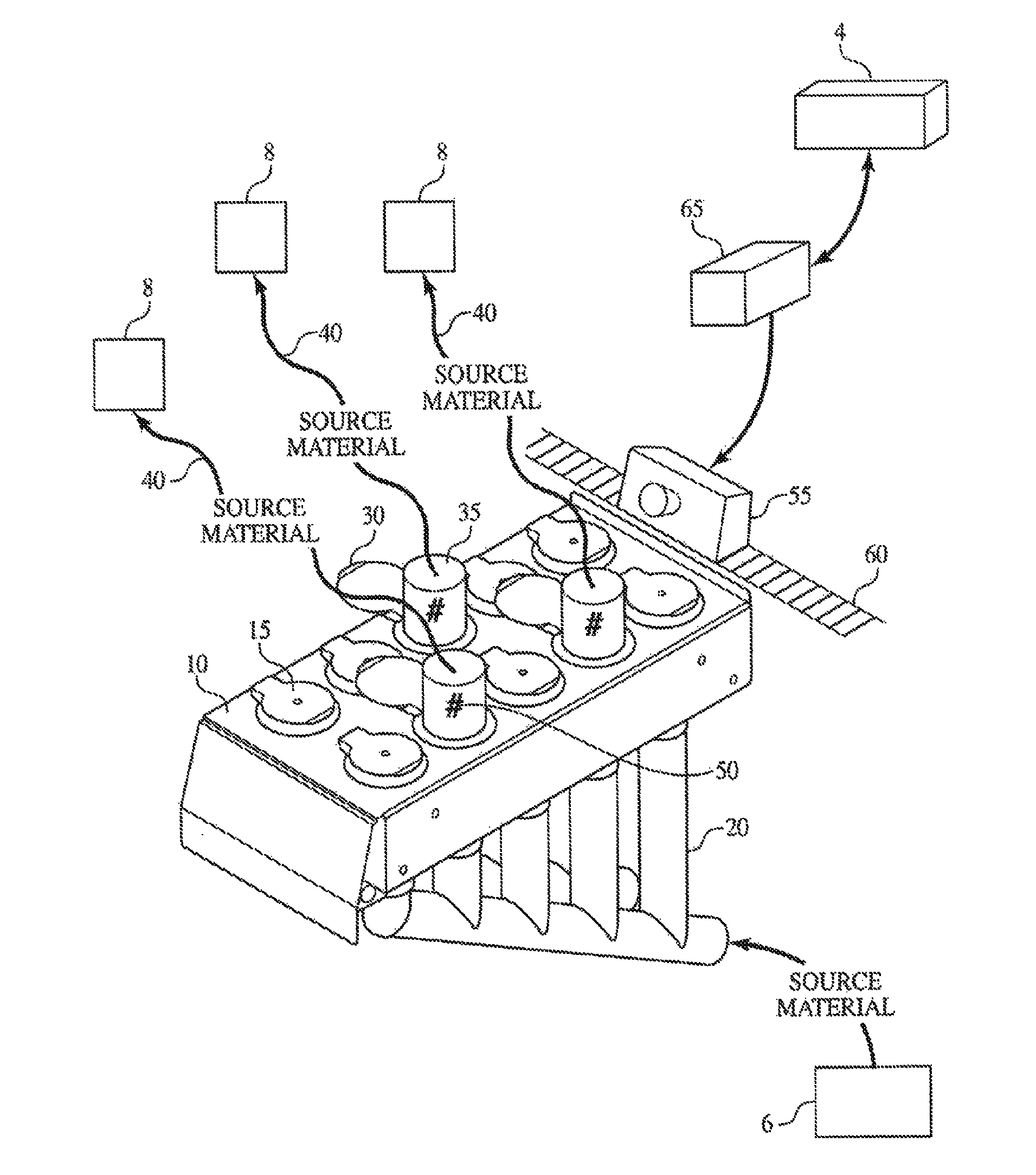
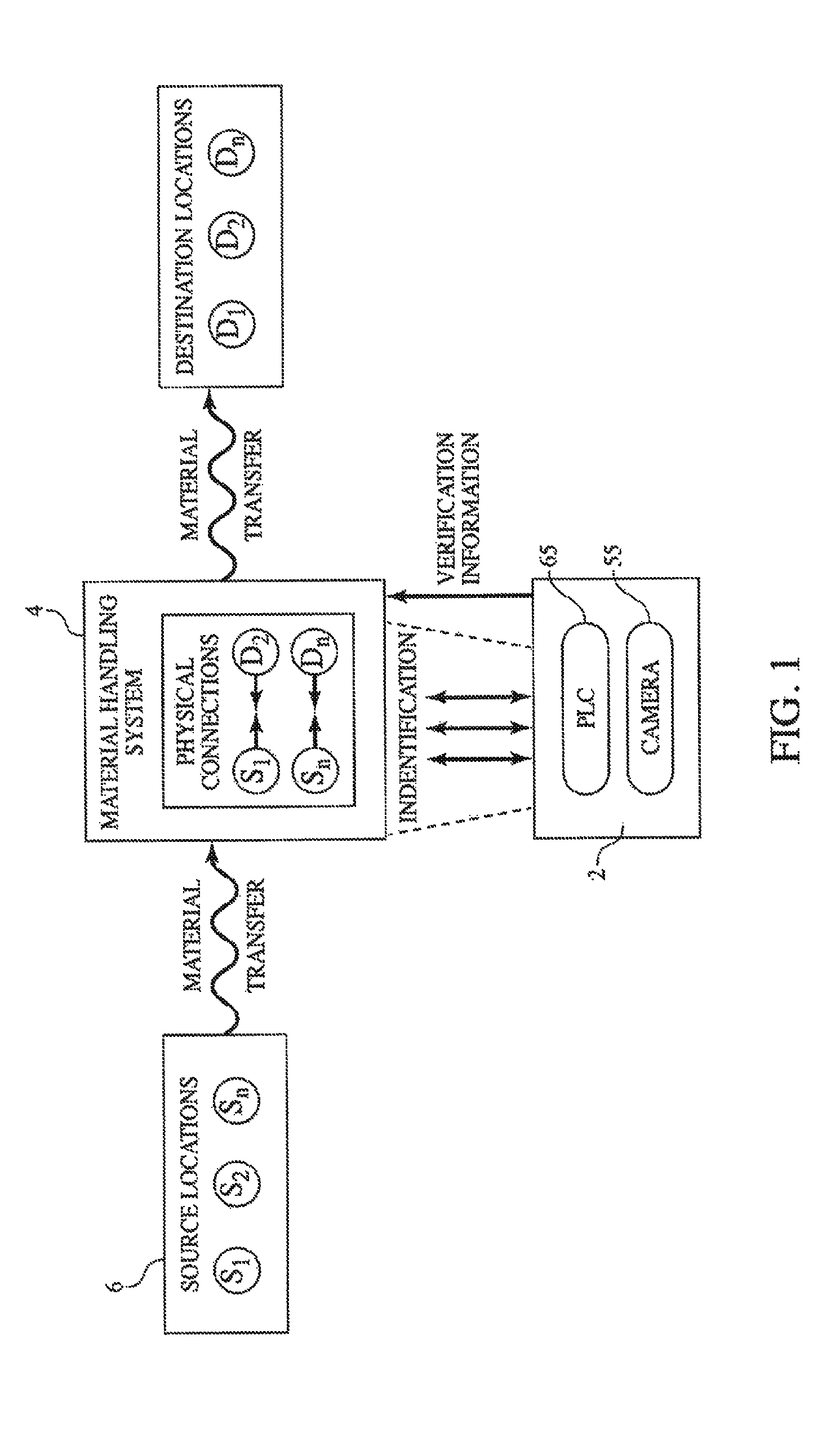
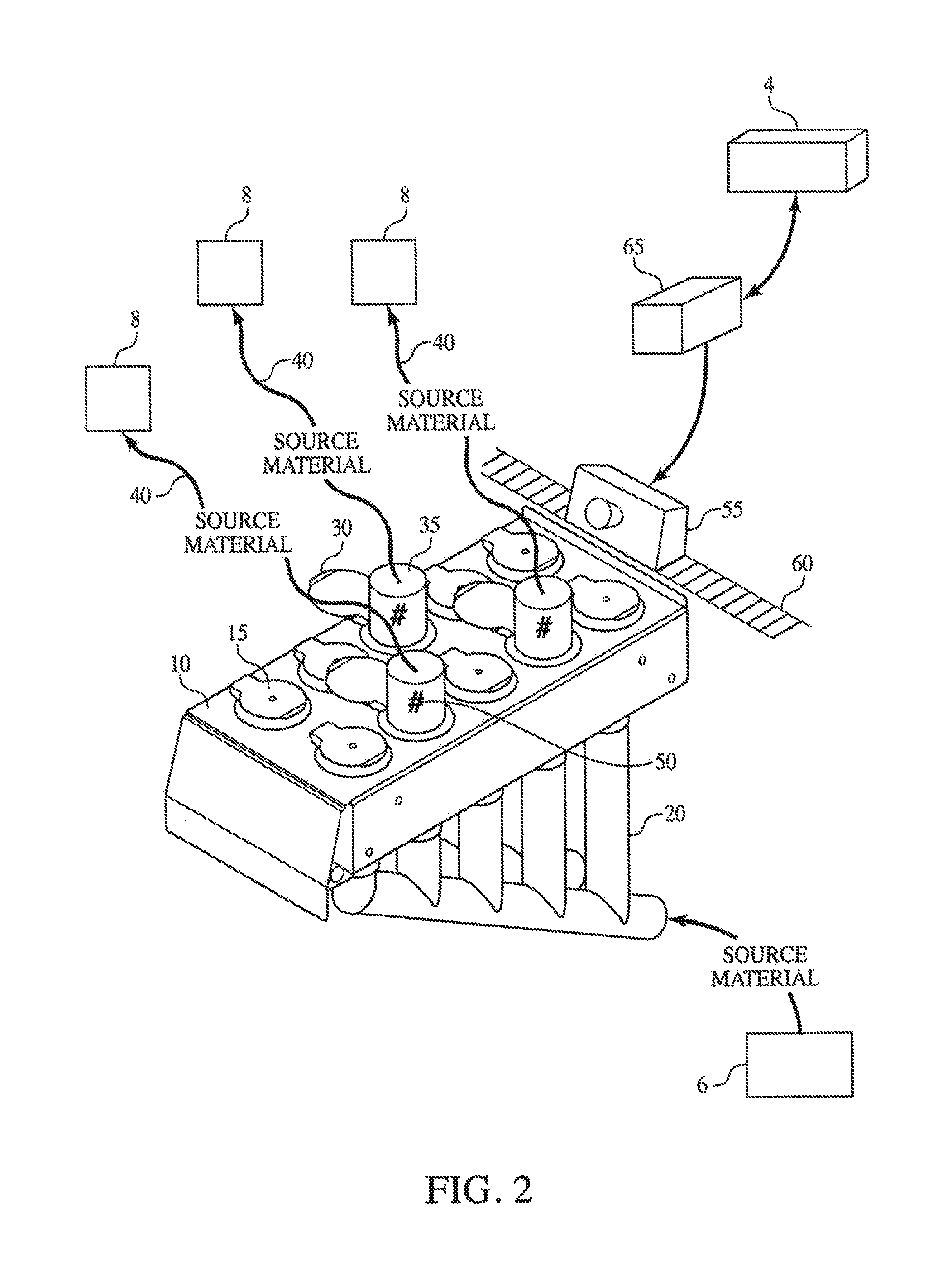
Method and Process of Verifying Physical Connections Within a Material Handling System
ActiveUS20130211572A1Reduce the possibilityLess susceptibleBulk conveyorsSpecial data processing applicationsHandling systemProcess validation
Manufacturing and industrial processes that involve the transfer of materials from source locations to destination location may utilize the presently described system and process to verify that the correct physical connections are established and maintained in a material handling system. The system collects information related to individual physical connections, including machine-identifiable indicia, and compares this information to data that defines the intended connections within the material handling system. In doing so, the system and process verify whether the proper physical connections are in place.
Owner:IPEG
Method and process of verifying physical connections within a material handling system
ActiveUS9304510B2Reduce the possibilityLess susceptibleBulk conveyorsSpecial data processing applicationsHandling systemProcess validation
Manufacturing and industrial processes that involve the transfer of materials from source locations to destination location may utilize the presently described system and process to verify that the correct physical connections are established and maintained in a material handling system. The system collects information related to individual physical connections, including machine-identifiable indicia, and compares this information to data that defines the intended connections within the material handling system. In doing so, the system and process verify whether the proper physical connections are in place.
Owner:IPEG
Virtual platform to facilitate automated production
InactiveUS7930053B2Data processing applicationsWithdrawing sample devicesTelecommunications linkCommunication link
Owner:BEACONS PHARMA
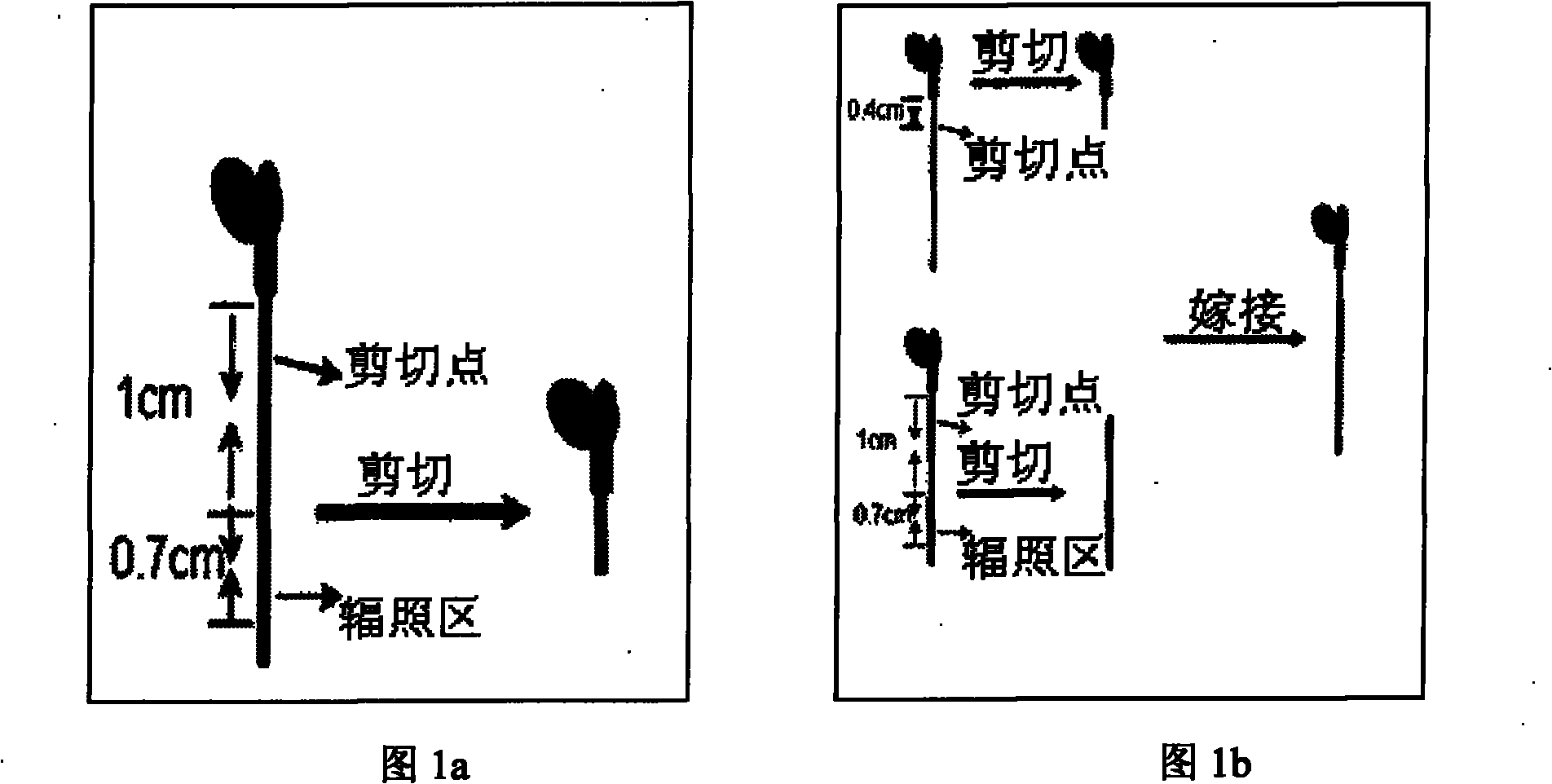
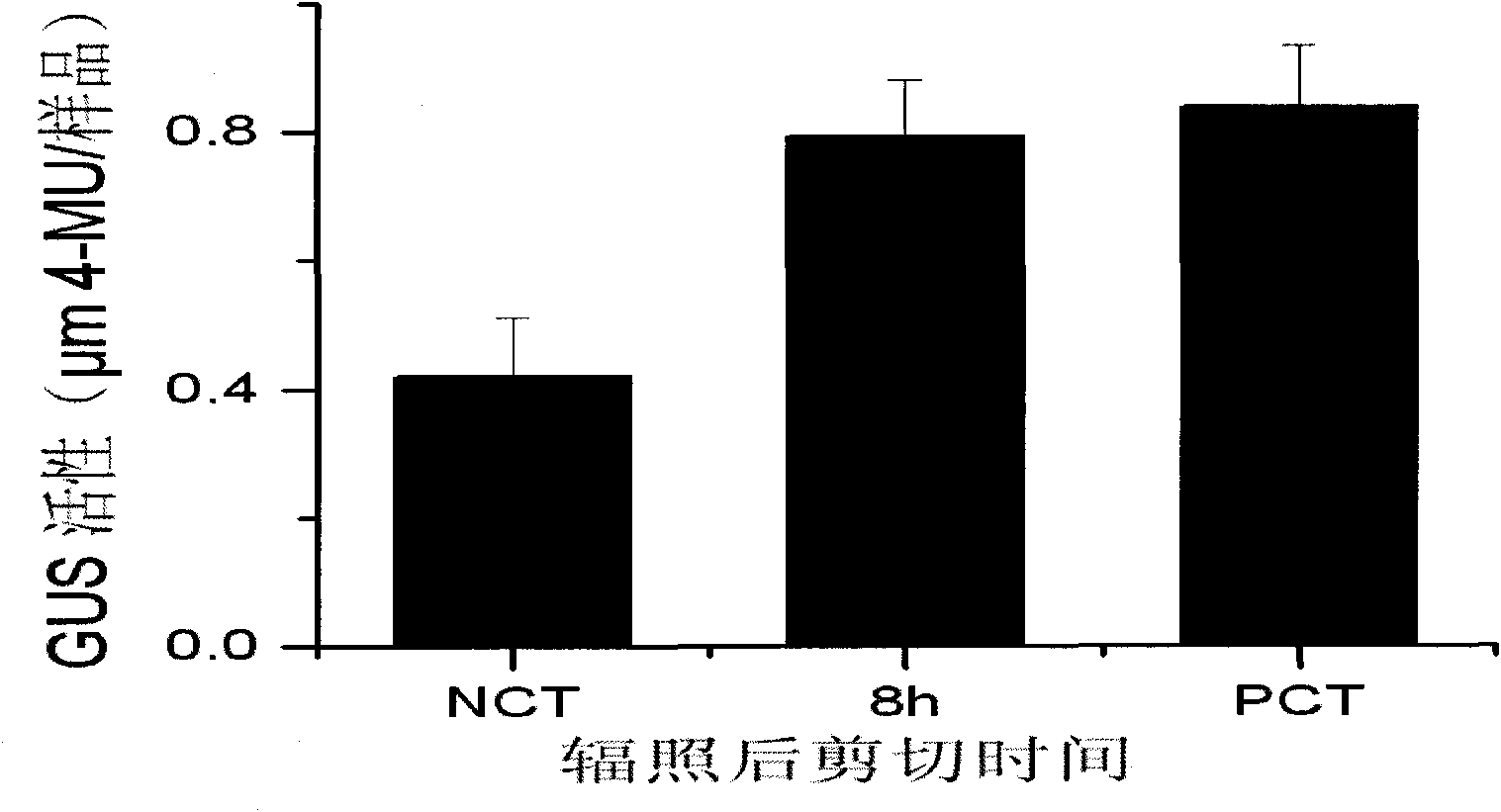
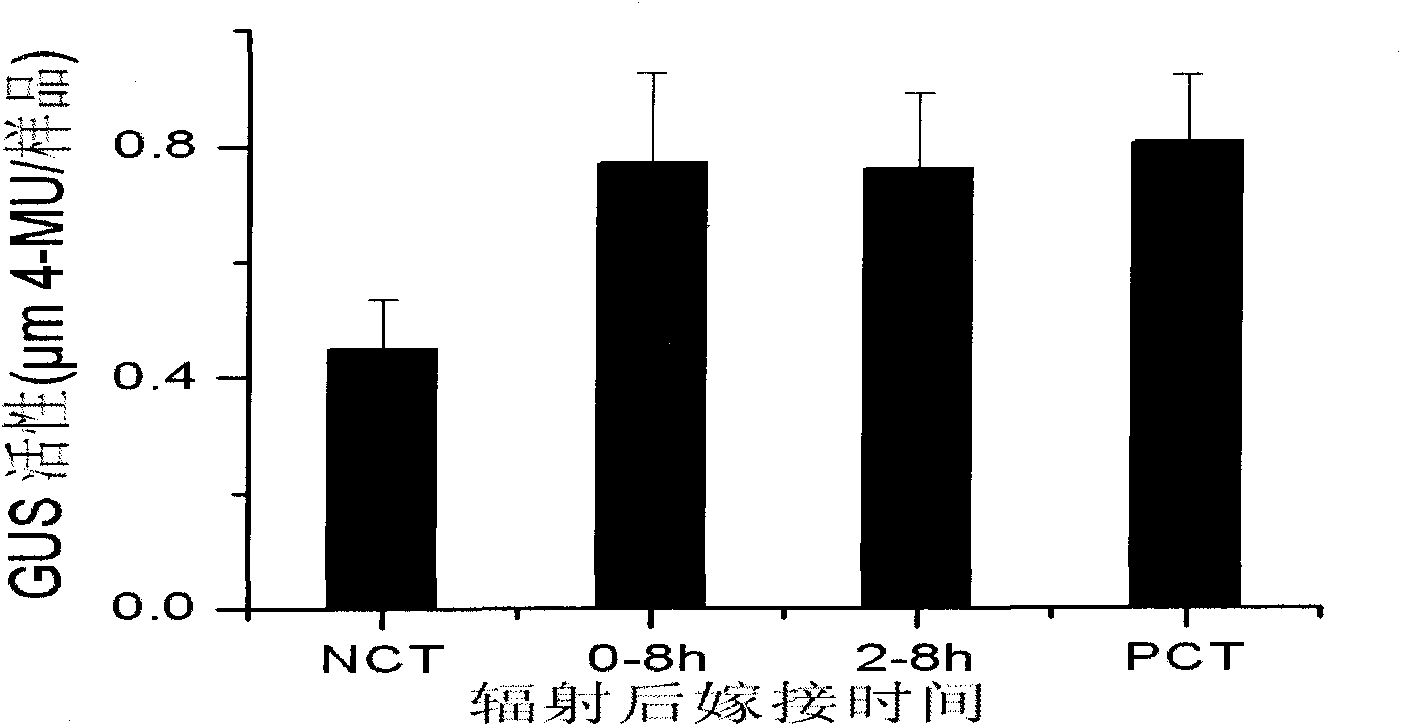
Experimental method for verifying early process of radiation bystander effect based on arabidopsis thaliana
The invention discloses an experimental method for verifying an early process of radiation bystander effect based on arabidopsis thaliana. The method comprises the following steps: adopting model plant arabidopsis thaliana as a material; carrying out ionizing radiation to the arabidopsis thaliana; then carrying out cutting and grafting treatment; extracting the AtRAD54 gene of new arabidopsis thaliana seedling which is grafted to the root tip of the radiated arabidopsis thaliana; and taking the expression level of the AtRAD54 gene as a detection end point. The innovation of the method lies in overcoming the limitation that the separation and connection of a radiated tissue (organ) and a non-radiated tissue (organ) can not be realized in animal experiments by adopting the characteristics of being capable of being cut and grafted which are caused by totipotency of a plant cell and providing a novel method system for studying the early process of remote radiation signals at the individual level.
Owner:INST OF PLASMA PHYSICS CHINESE ACAD OF SCI
Method for reimbursing administrators of payments
ActiveUS7567938B1Accurate methodReducing escrow accountsFinancePayment architecturePaymentThird party
A loss ledger system to process third party fund requests on a daily basis, track historical requests, process validation on each request, maintain suspense / escrow and loss ledgers, and provide reconciliation assistance for accounting and claims processing departments. Additionally, the loss ledger system provides management reports, interfaces, security, audit and control and data conversion to facilitate minimizing escrowed resources, fraudulent activity and clerical errors.
Owner:ARCH CAPITAL GRP U S
Method for risk-management over lifecycle of complex products and processes
InactiveUS20180075379A1Risk can be acceptedNone of methods is integratedResourcesKnowledge representationEngineeringCvd risk
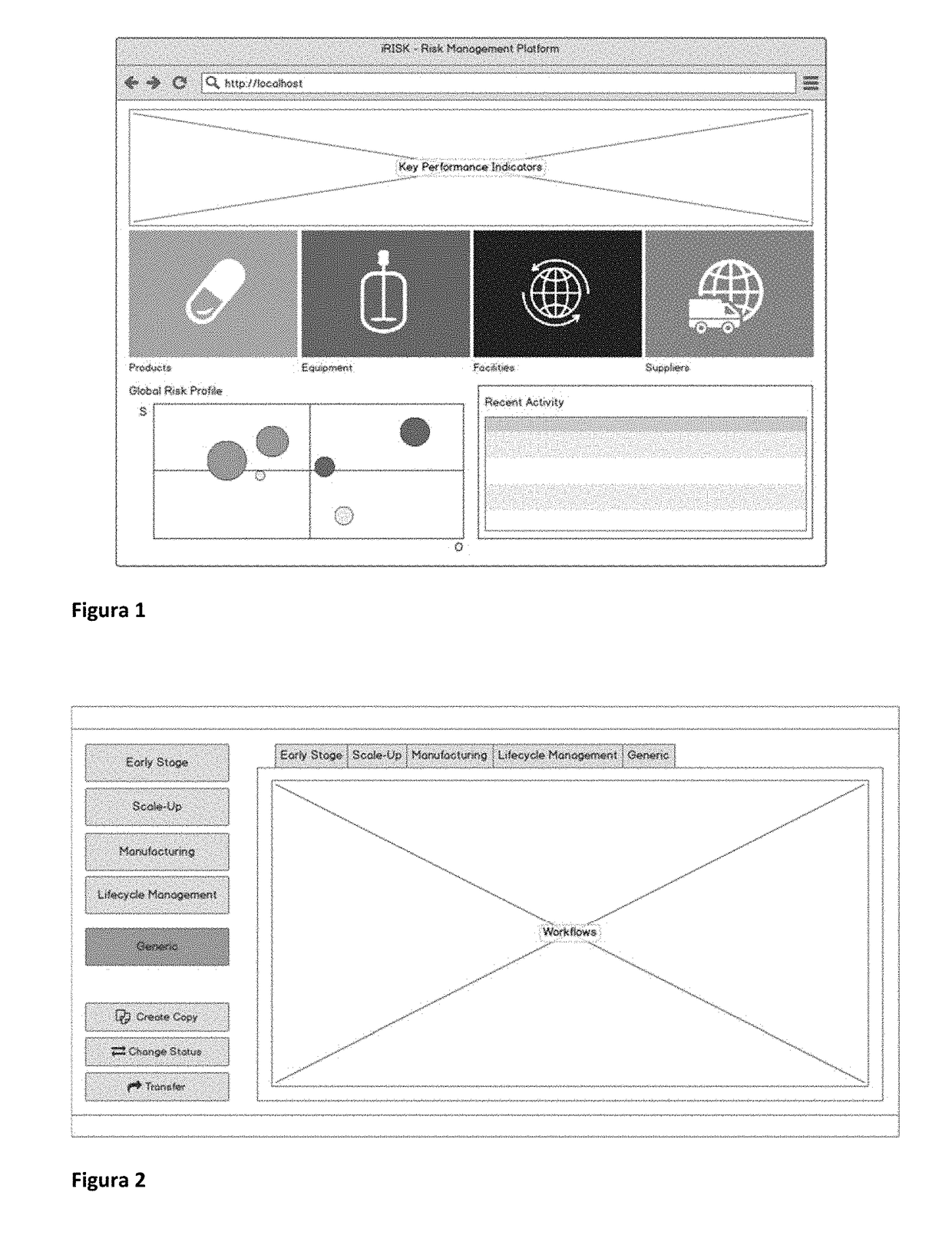
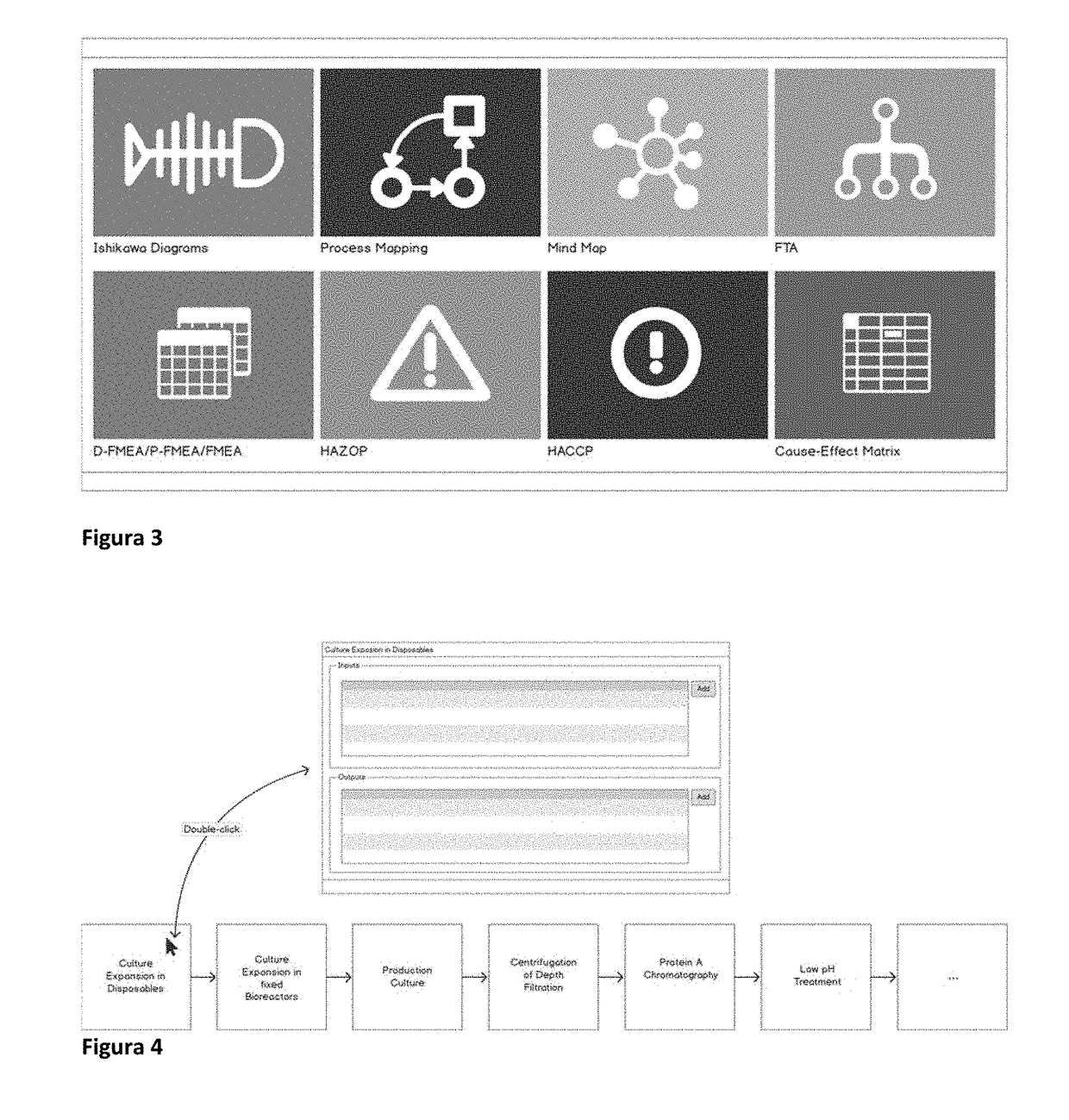
A method for building risk-management workflows (‘Step A’), comprising several risk analysis tools seamless integrated (‘Step B’), to be applied to process design, process and equipment qualification, manufacturing stages and supply management (‘Step C’) of multi-step processing of chemical, pharmaceutical or biologic products (‘Step D’), for risk identification, assessment, mitigation and management over lifecycle (‘Step E’), thus supporting ongoing process verifications, product quality reviews, and knowledge-based process and product continuous improvement (‘Step F’). Workflows (‘Step A’) can be specific of certain stages (‘Step C’), products (‘Step D’), production equipment or facilities used to produce products, but can and should be combined to support the lifecycle management aspects of steps ‘E’ and ‘F’. The use of workflows (‘Step A’) with ‘Step B’ features combined, supports the type of activities in steps ‘E’ and ‘F’, provides a knowledge-management framework (‘Step F’) applicable across multiple products and platform technologies, that supports a science-based justification to decisions taken at defined lifecycle stages (‘Step C’).
Owner:4TUNE CONSULTADORIA E SERVICOS DE ENGENHARIA IND LDA
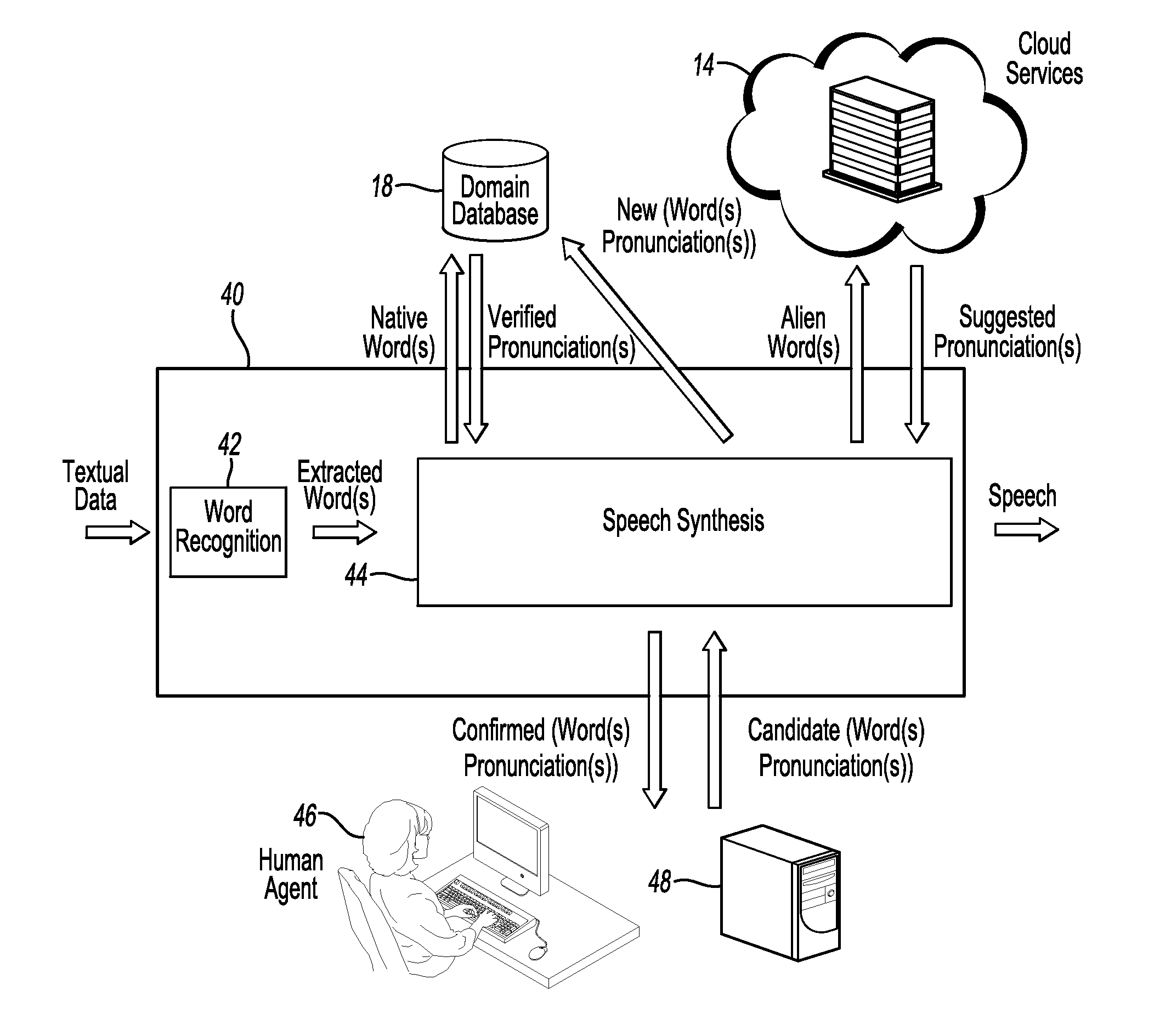
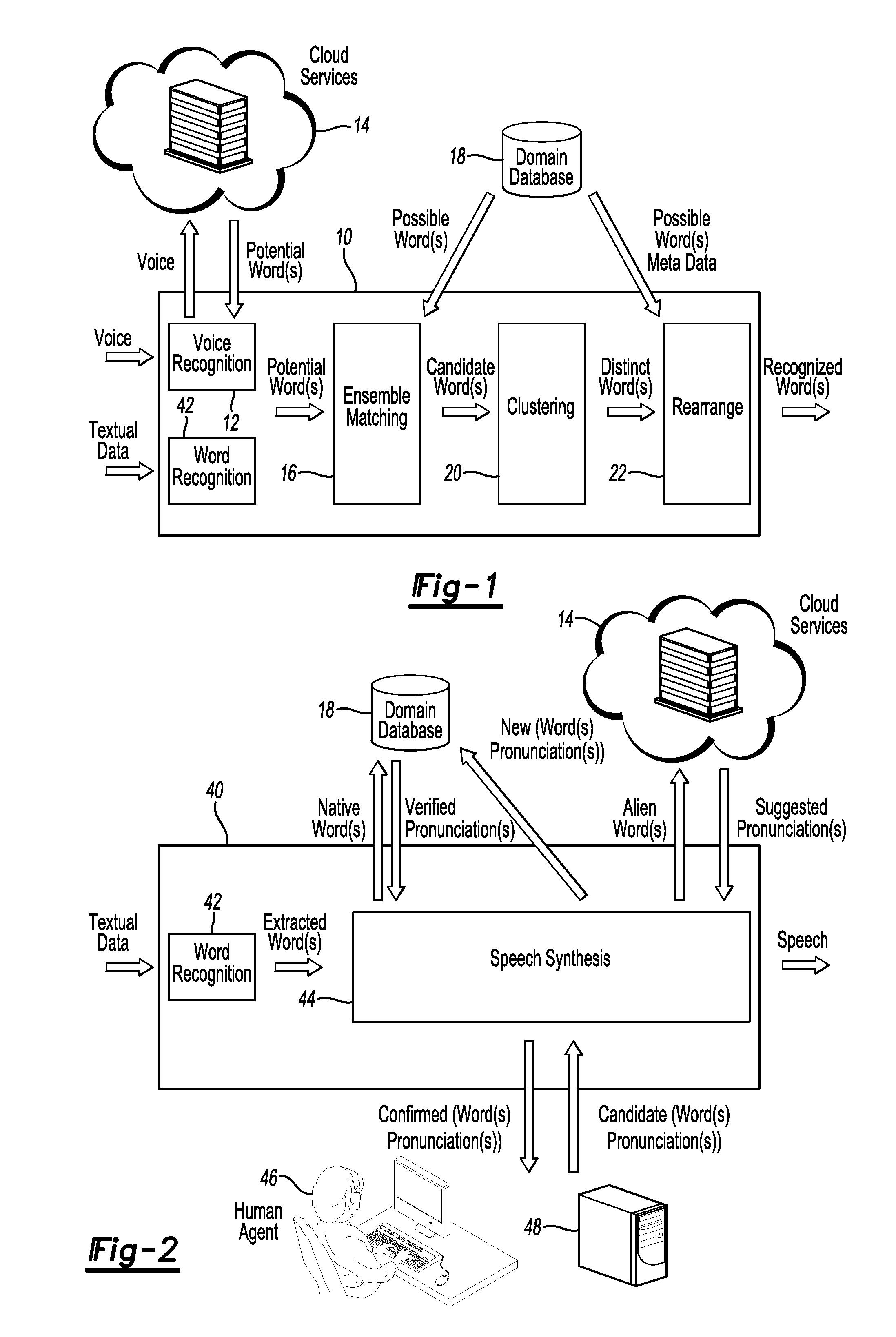
Enhanced human machine interface through hybrid word recognition and dynamic speech synthesis tuning
InactiveUS20150206539A1Improve user experienceExact matchSpeech recognitionSpeech synthesisHuman–machine interfaceA domain
A human machine interface enables human users to interact with a machine by inputting auditory and / or textual data. The interface and corresponding method perform efficient look up of words, corresponding to inputted human data, which are stored in a domain database. The robustness of a speech synthesis engine is enhanced by updating the deployed pronunciation vocabulary dynamically. The architecture of the preferred embodiment of the former method includes a combination of ensemble matching, clustering, and rearrangement methods. The latter method involves retrieving suggested phonetic pronunciations for words unknown to the speech synthesis engine and verifying those through a manual or autonomous process.
Owner:RIDETONES
Rule merging in system for monitoring adherence by developers to a software code development process
ActiveCN102253829AQuality improvementImprove satisfactionError detection/correctionSpecific program execution arrangementsSoftware development processSubject-matter expert
The invention relates to the rule merging in system for monitoring adherence by developers to a software code development process. In a rule-based system for monitoring process adherence, first and second processing patterns are received and merged to provide a merged processing pattern. Each processing pattern, which may be expressed in a state graph representation, embodies at least a portion of a desired software code development process. Optionally, the merged processing pattern may be presented to a subject-matter expert to obtain feedback thereon. The merged processing pattern may then be converted into an executable process verification rule for use in monitoring process adherence. In an embodiment, development process event data is compared to the executable process verification rules. Violations of the rules result in the generation of failure indications that may be stored and subsequently reported as needed. In this manner, efficiency of automated process adherence monitoring systems may be improved when determining the level of compliance by developers with one or more software code development processes.
Owner:ACCENTURE GLOBAL SERVICES LTD
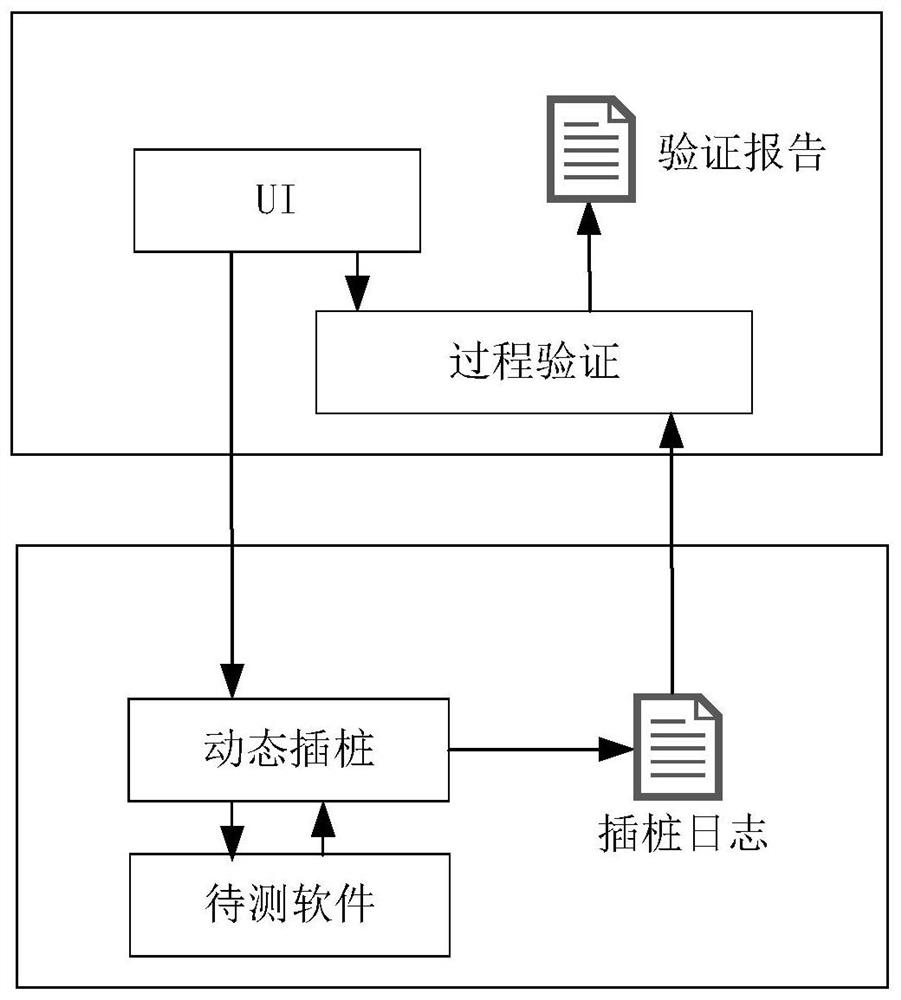
Program running process conformance verification method
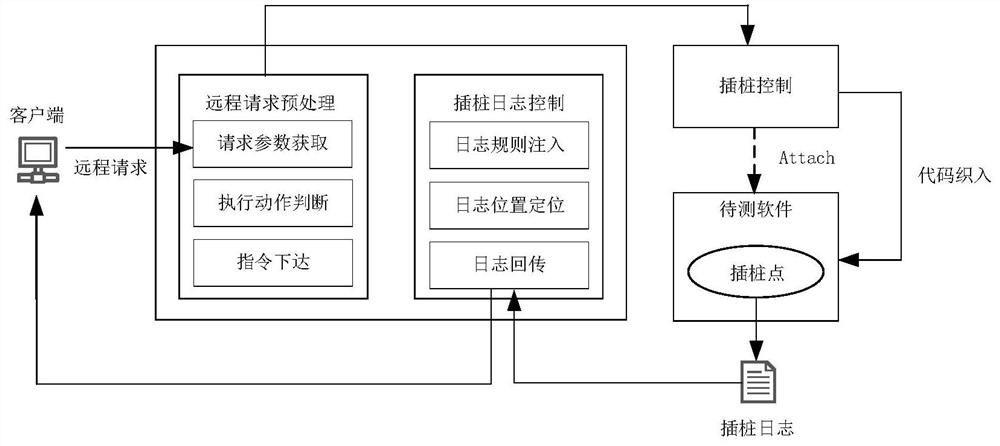
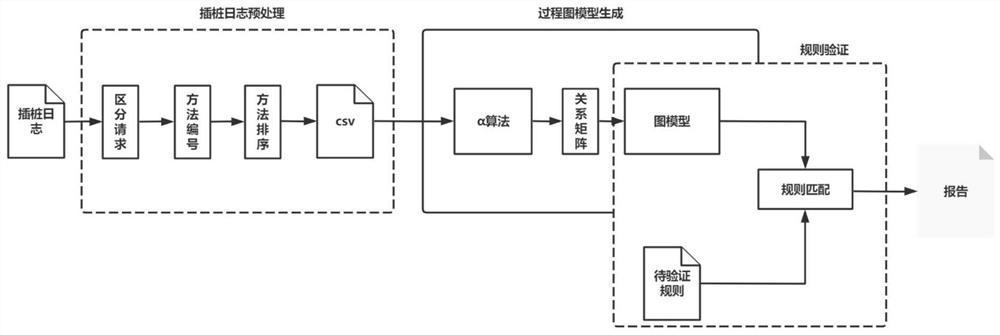
ActiveCN112579437AFilling the Gaps in Compliance VerificationSoftware testing/debuggingData modelingValidation methods
The invention provides a program running process conformance verification method, which comprises two links of dynamic instrumentation and process verification. The method comprises the steps of receiving and analyzing an instruction input by a user, executing an instrumentation start-stop process and returning a process data log during dynamic instrumentation work; receiving the process data logduring the process verification, extracting the relationship between methods in the log to establish a process diagram model, and sequentially matching the process diagram model with the standard rule, thereby verifying the standard conformity of the program running process. According to the program running process verification method, the internal execution process information during program running is visually and quantitatively displayed through methods such as instrumentation point burying and data modeling, the program running process verification method is provided, and the blank of software running process conformity verification is filled up.
Owner:中国科学院电子学研究所苏州研究院 +1
Process Validation Apparatus and Methods
InactiveCN102271717AMicrobiological testing/measurementLavatory sanitoryEngineeringProcess validation
Owner:3M INNOVATIVE PROPERTIES CO
Lighting test module
ActiveCN109064957AThe number of strips is flexible and variableSimple processStatic indicating devicesImage resolutionEngineering
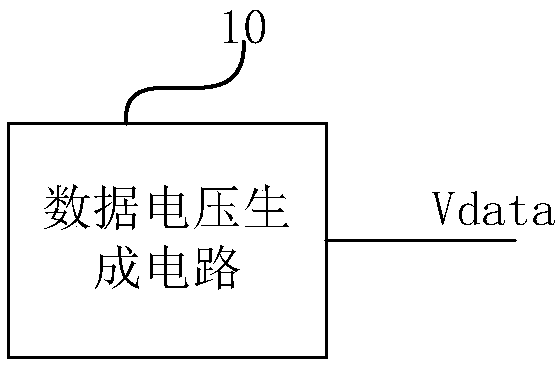
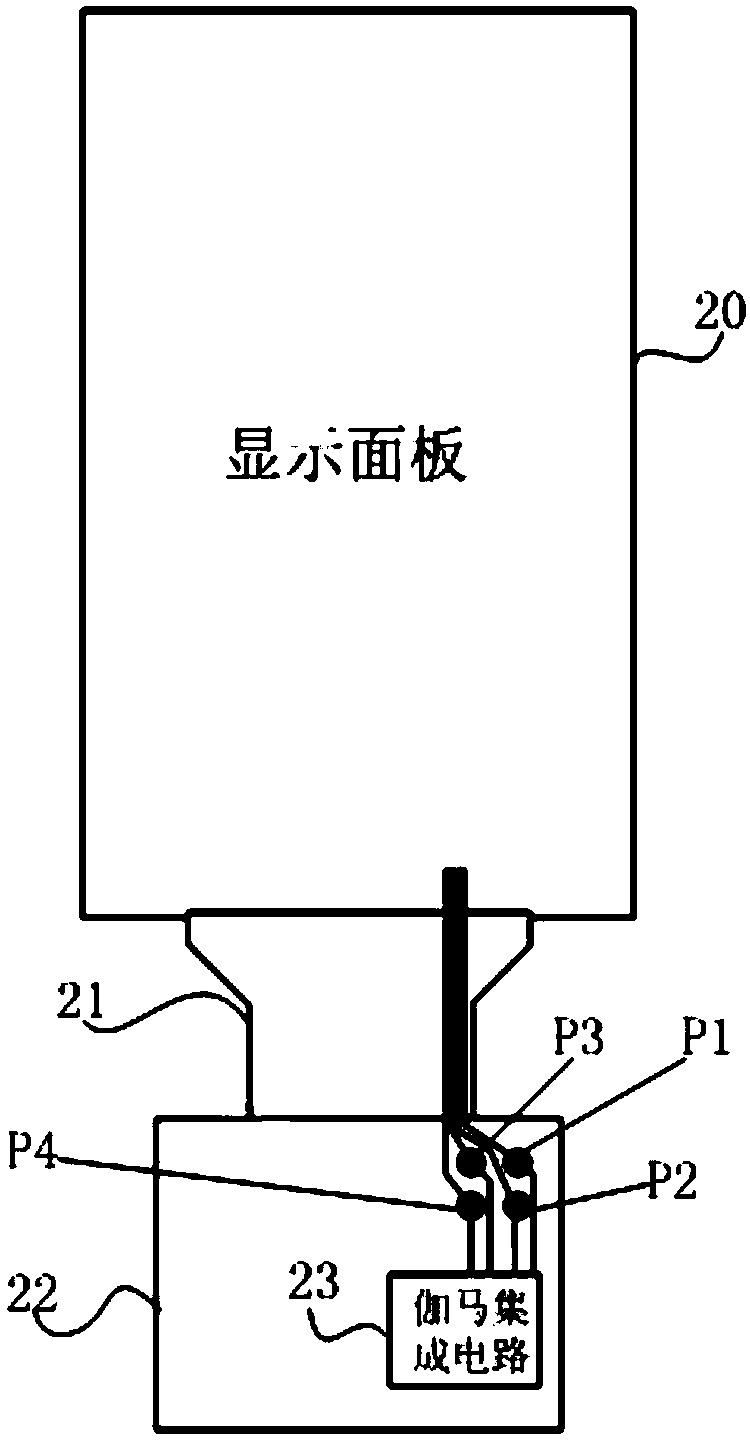
The invention provides a lighting test module. The lighting test module is used for performing the lighting test for a display panel, the lighting test module comprises a data voltage generating circuit arranged on a peripheral circuit board, wherein the data voltage generating circuit is used for providing a data voltage for preset data lines contained by the display panel during the lighting test. The lighting test module is advantaged in that during the lighting test, the data voltage generating circuit providing the data voltage is arranged outside the display panel and is subjected to hardwareization, the data voltage is provided by the data voltage generating circuit for the preset data lines, the quantity of the preset data lines is flexible and variable, the lighting test module isespecially suitable for unconventional, low resolution display panel development or process validation, and the process is simple during process validation of the display panel.
Owner:BOE TECH GRP CO LTD
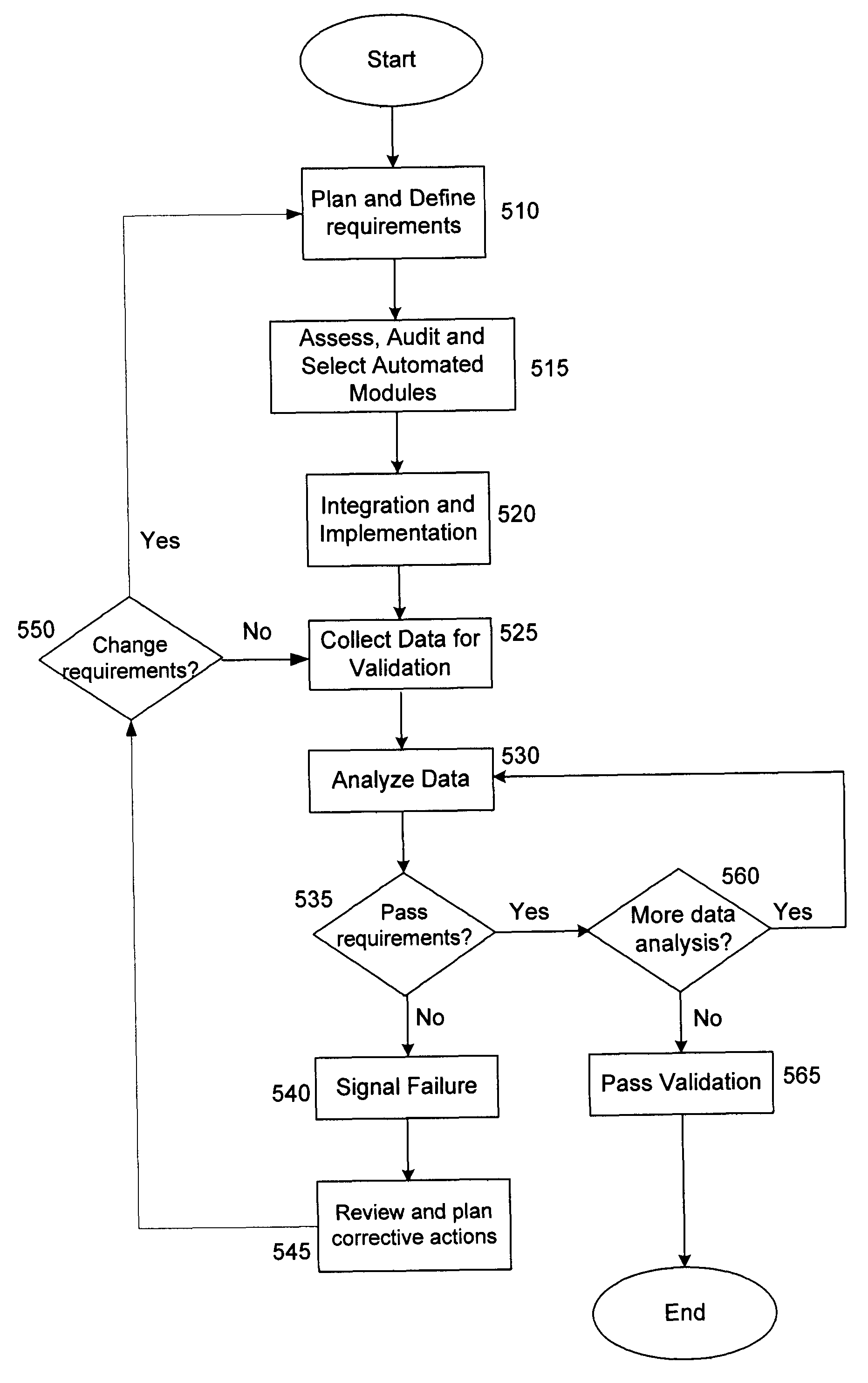
Method and system for process design validation
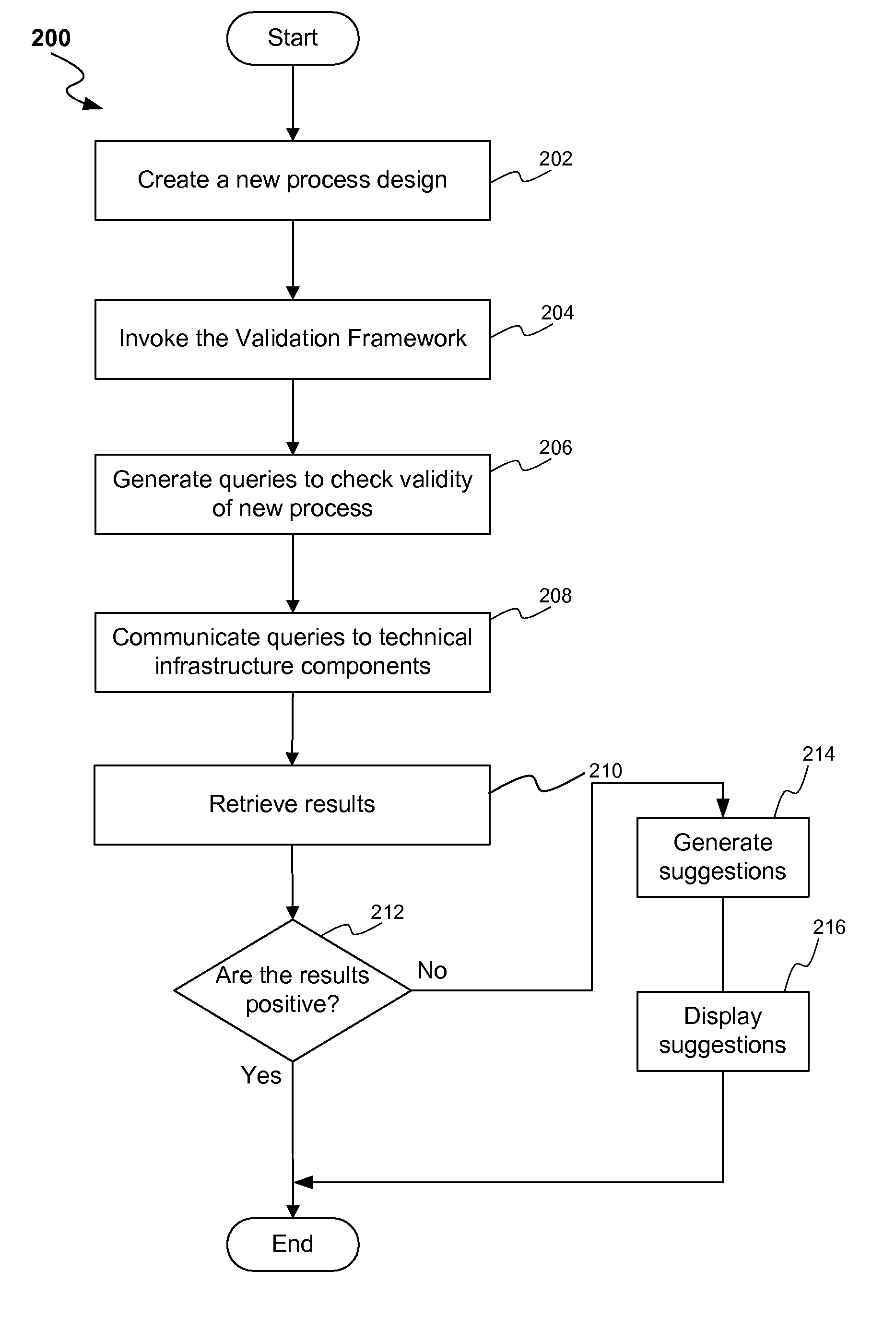
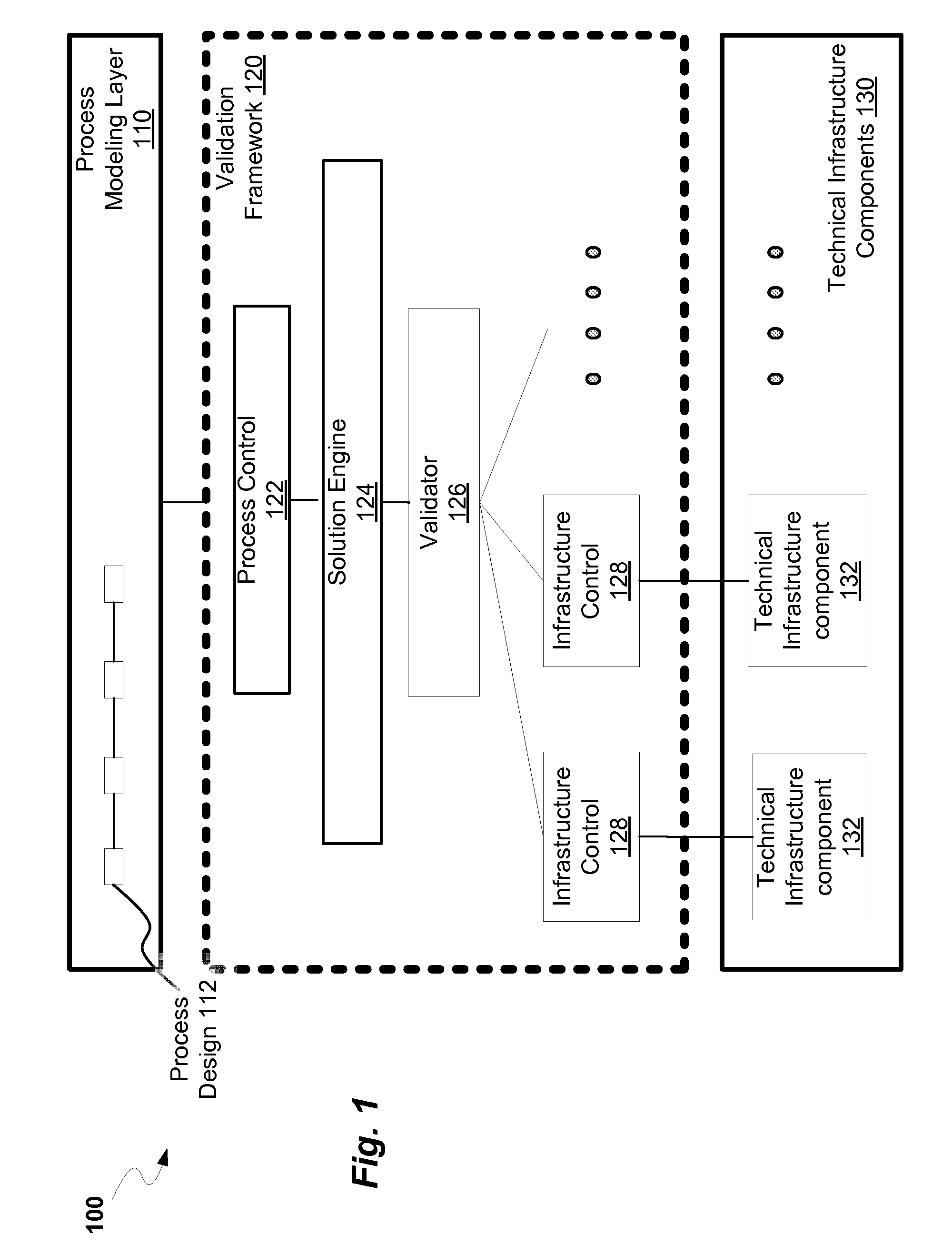
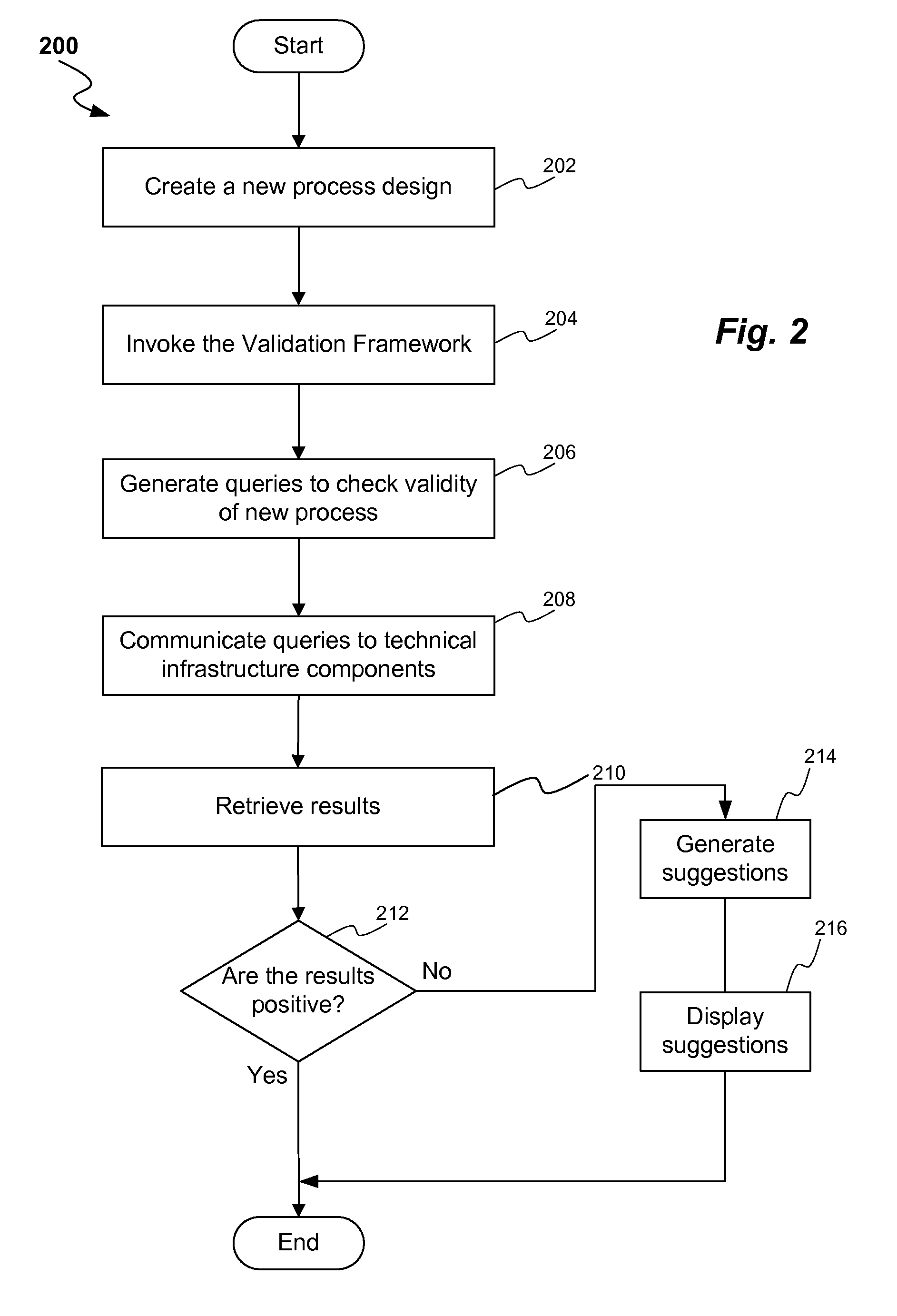
ActiveUS20080300813A1Structural/machines measurementElectrical measurementsProcess designProcess validation
A method and system for process design validation is provided. A process validation is first triggered. A process design is evaluated against a technical infrastructure component and if a non-positive result is achieved as a result of process design validation, suggestions are generated in order to achieve a positive result.
Owner:SAP AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com