Process Validation Apparatus and Methods
A technology for verifying equipment and equipment, applied in biochemical equipment and methods, sanitation equipment for toilets, microbiological determination/inspection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
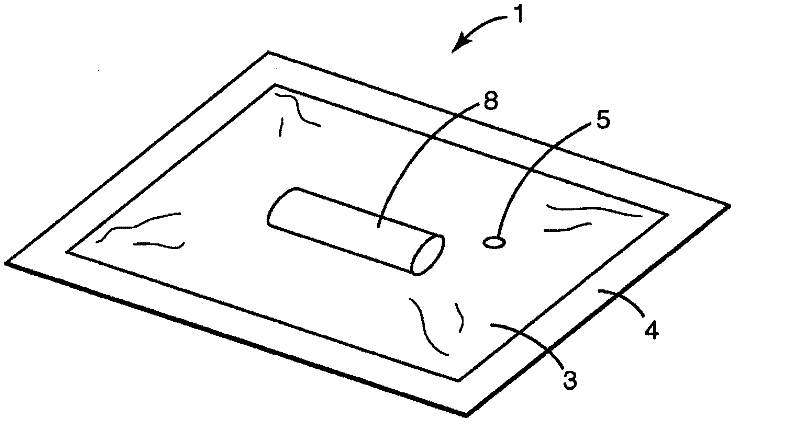
[0264] Sterilant inlet pipe
[0265] Attest TM Rapid 5 Test Pack Plus #41382 contains Attest TM 1292 Rapid Biological Indicators (Attest BIs) (Attest TM 1292 Rapid Biological Indicator) and Comply TM SteriGage TM 1243 Steam Chemical Integrator (SteriGage) (Comply TM SteriGage TM 1243 Vapor Chemical Integrator), both available from 3M Company (St. Paul, MN), provided in a heat-sealable polyfoil pouch to increase the amount of time required for the inactivation of the biological and chemical indicators inside the test pack. time. in US 4,636,472 figure 2 The structure of the Attest Test Pack is shown in . Attest Rapid 5 Test Packs are designed to monitor a 4-minute 132°C (270°F) 4-pulse pre-vacuum sterilizer, so the indicator is deactivated within 4 minutes. CadPak N sachets were obtained from TechniPac Inc. (Le Sueur, Minnesota). The pouch is a multilayer pouch constructed of layers of nylon, polyethylene, foil, and polyethylene. Sachets have tubing at one ...
example 2
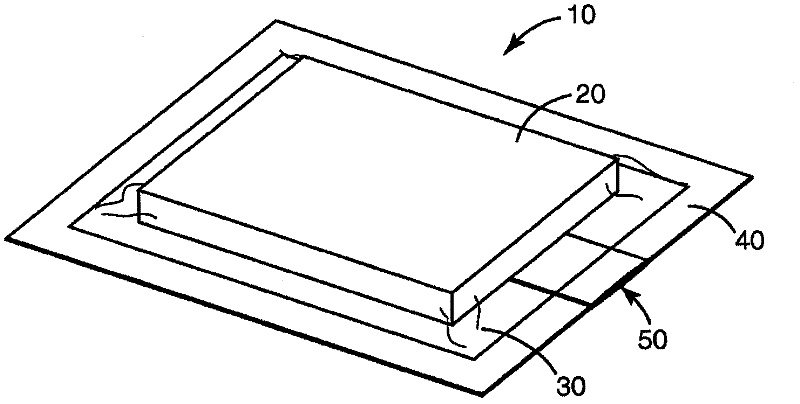
[0275] Sterilant inlet multi-layer piping
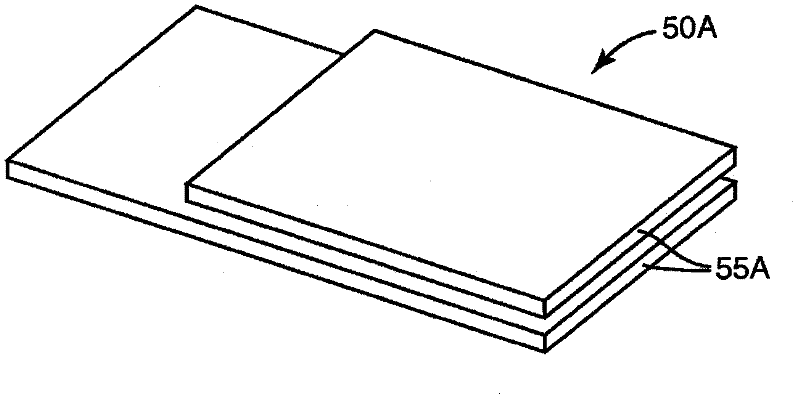
[0276] In this example the same configuration as described in Example 1 was used except that the tubing was altered to reduce the time to deactivate the biological and chemical indicators. In this example, the tubing is a multi-ply construction using two 10 mil indexed sheets with a tissue sheet sandwiched between the indexed sheets to enlarge the discharge opening, as in Figure 4 shown. The tubing is held together with the tape around the exterior creating an opening between the two index paginated sheets and the tissue. The length of the pipe is approximately 14 cm, and the estimated surface area is 9 cm for widths of 0.64 cm and 0.95 cm, respectively 2 and 13.3cm 2 .
[0277] Table 2 shows the results. The data shows that the multi-layer drain construction can reduce the time to deactivate the biological and chemical indicators inside the test pack. This configuration should be useful for monitoring a 10 minute sterilizat...
example 3
[0282] Sachets with small diameter openings
[0283] In this example, the Attest Rapid 5 Test Pack was heat sealed to the inside of a clear polyester and polypropylene laminate film commercially available under the tradename Material 123 from Alcan Packaging. The pouch is discharged by punching a hole in the membrane with an 18 gauge needle. Three different configurations are compared. The package tested has a hole on the top of the package, as in Image 6 shown; with two holes, one on the top and one on the bottom of the bag; and one hole on the top with a membrane channel below the hole, as Figure 7A with Figure 7B shown. Exposure conditions and BIs and CIs were tested as described in Example 1. The packages were exposed for 5 minutes, 10 minutes, 15 minutes and 20 minutes in a 132°C pre-vacuum sterilizer.
[0284] The results in Table 3 show that sachets with pinhole openings in the membrane significantly increased the exposure time required to inactivate the biol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com