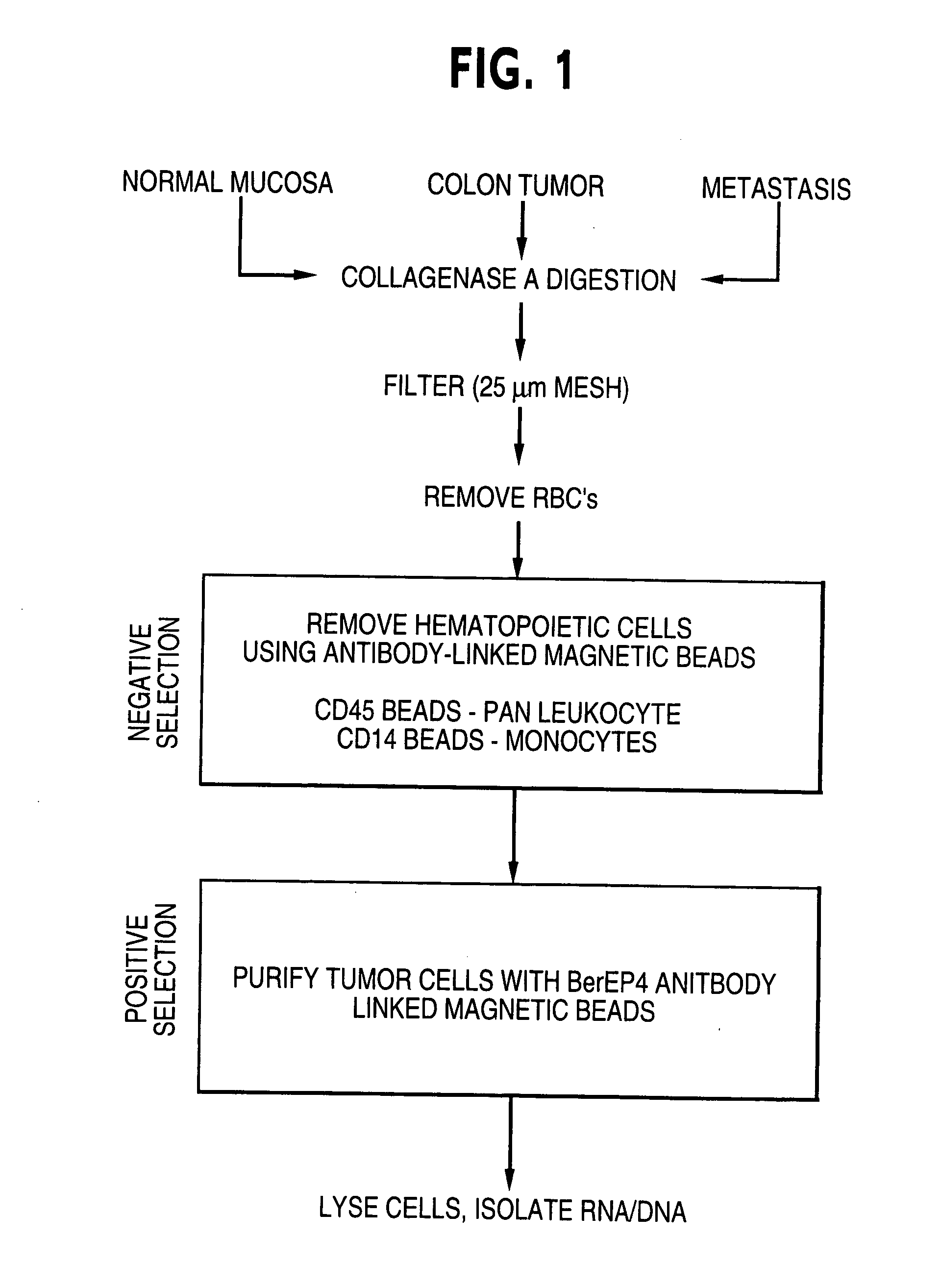
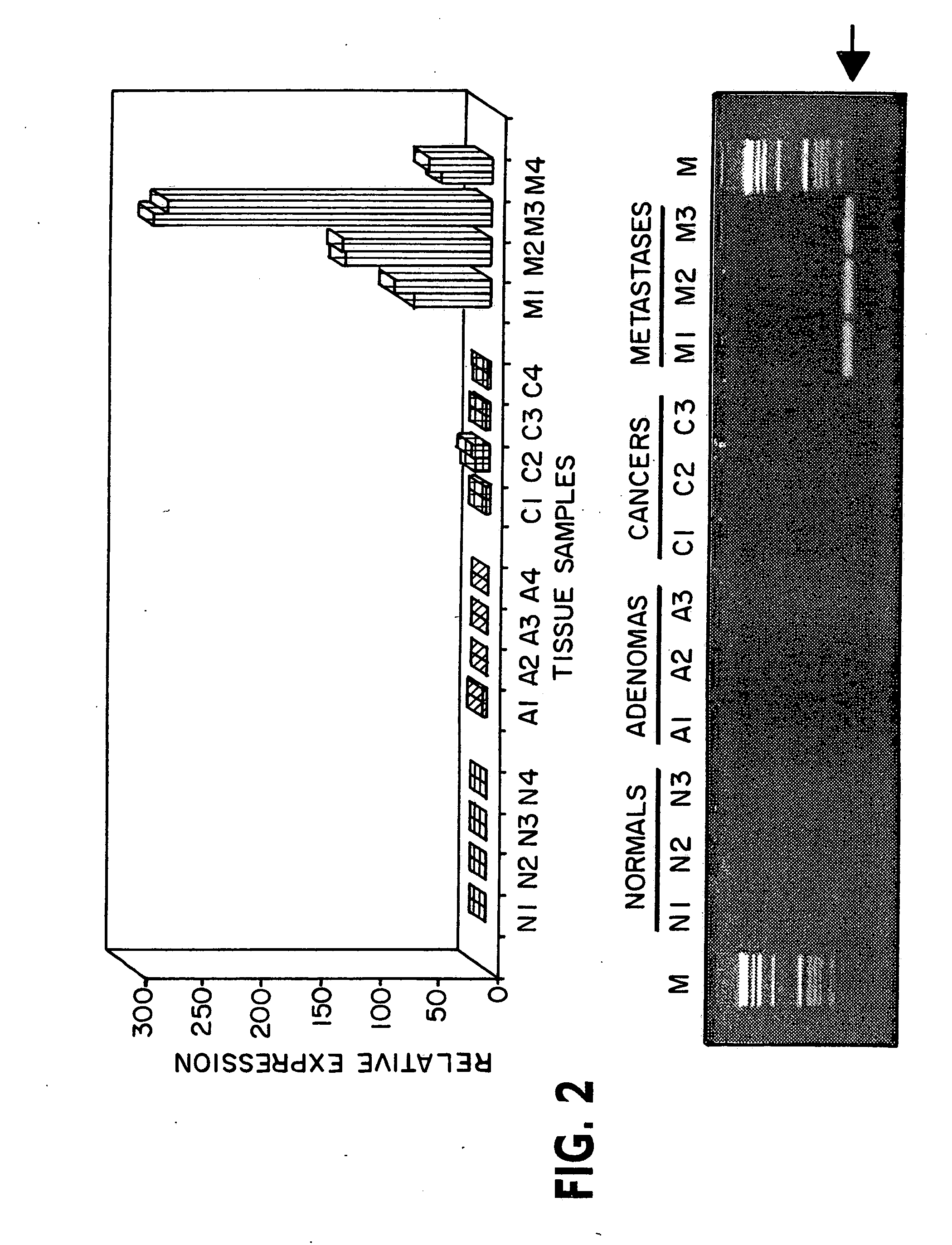
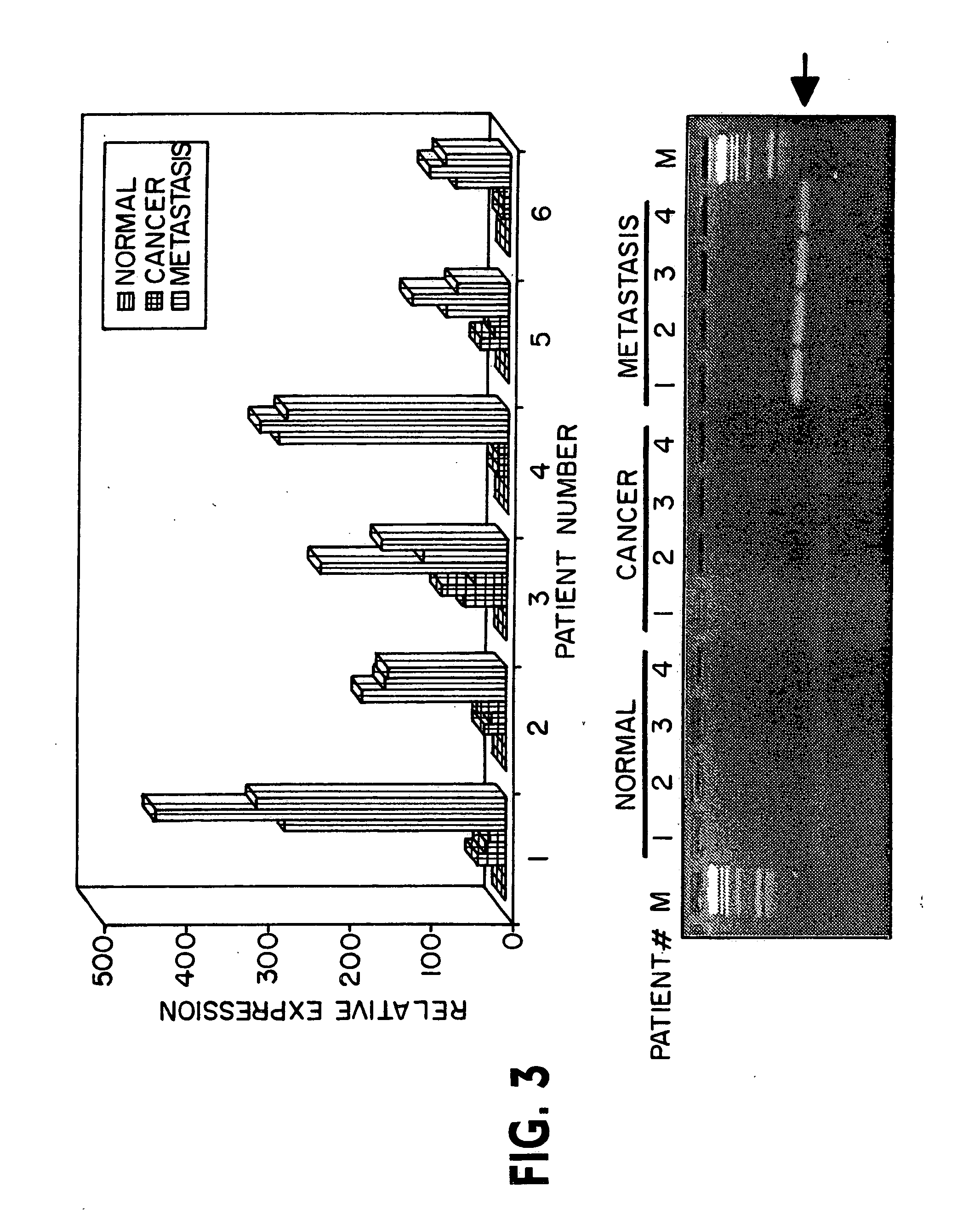
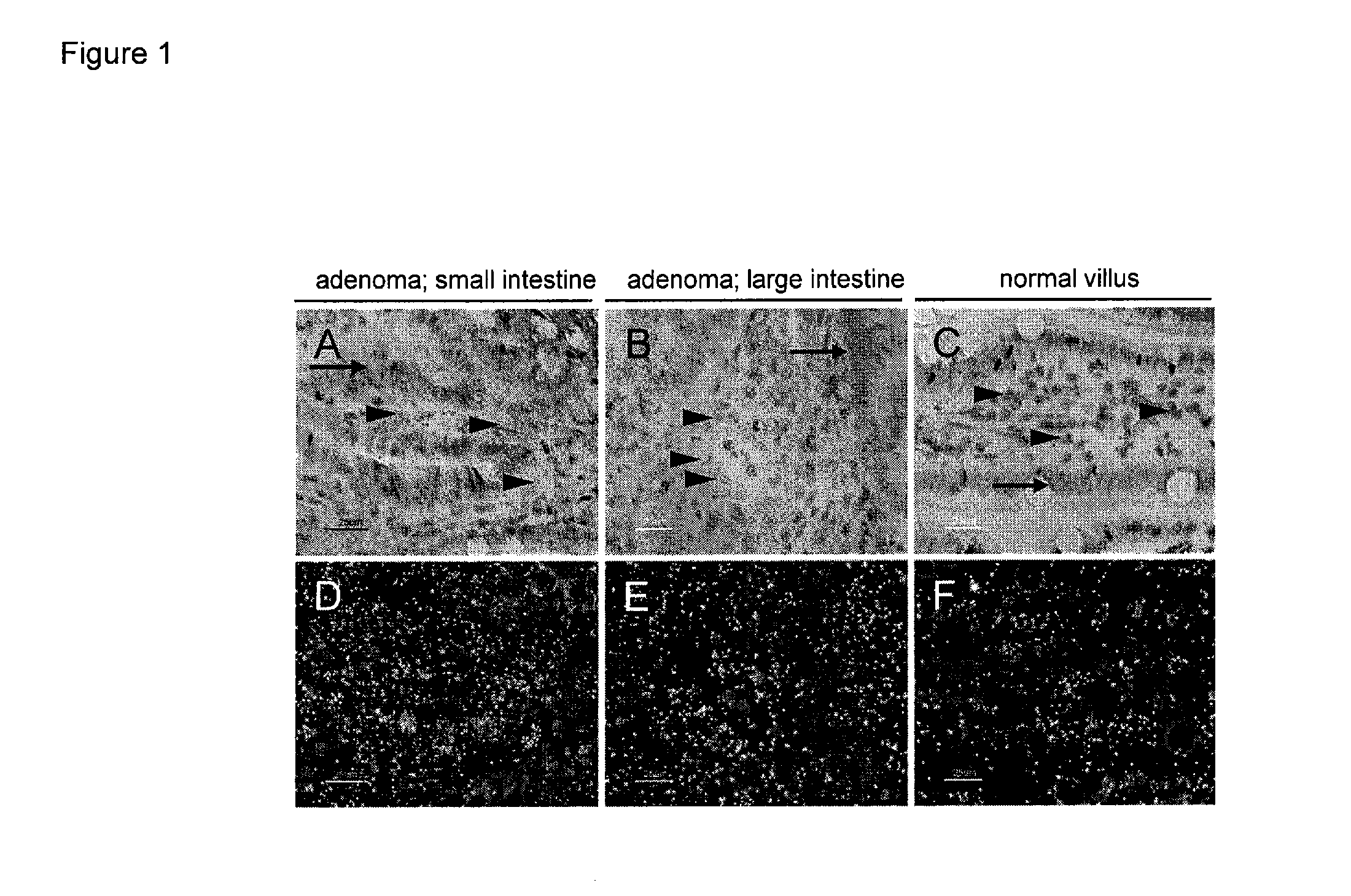
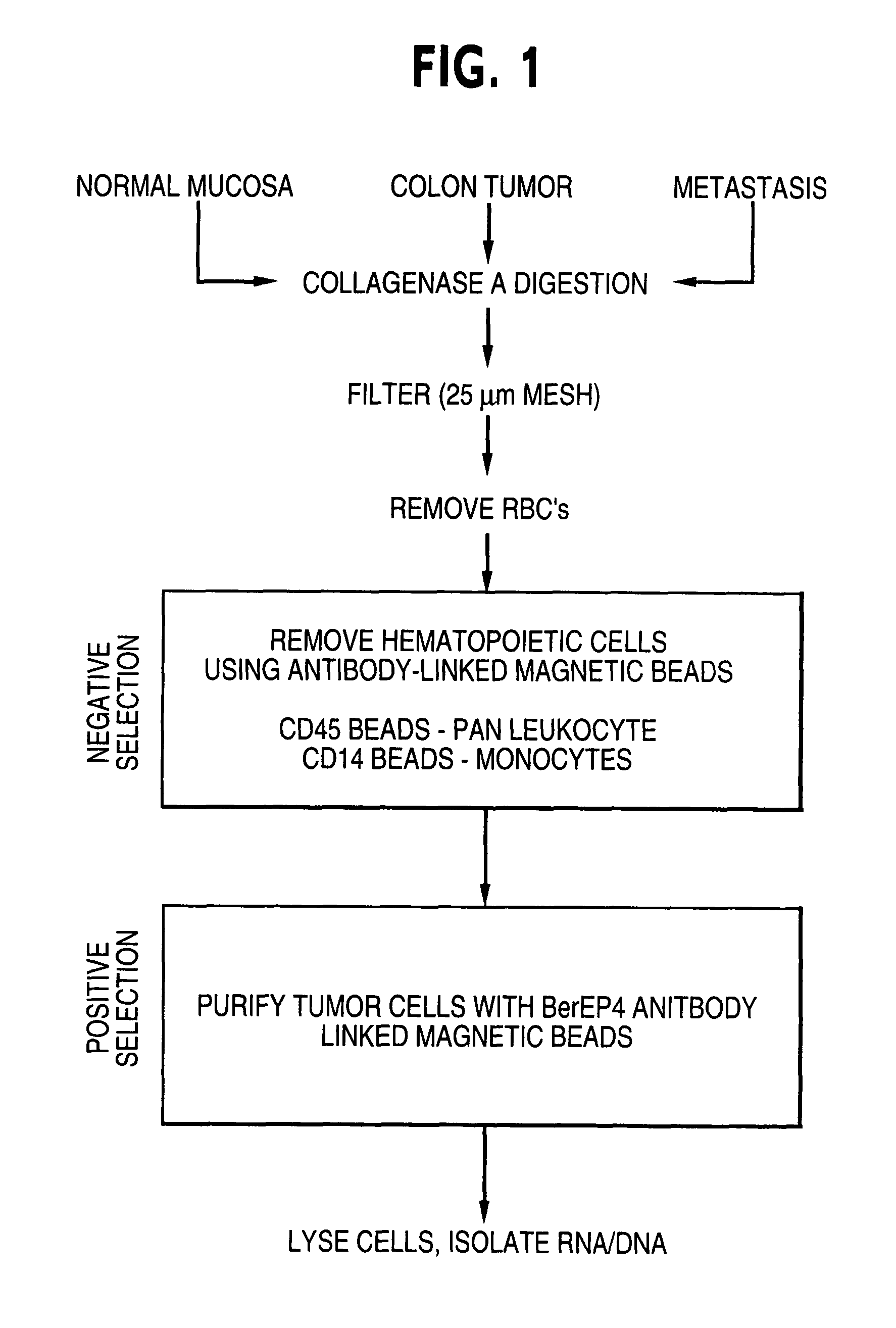
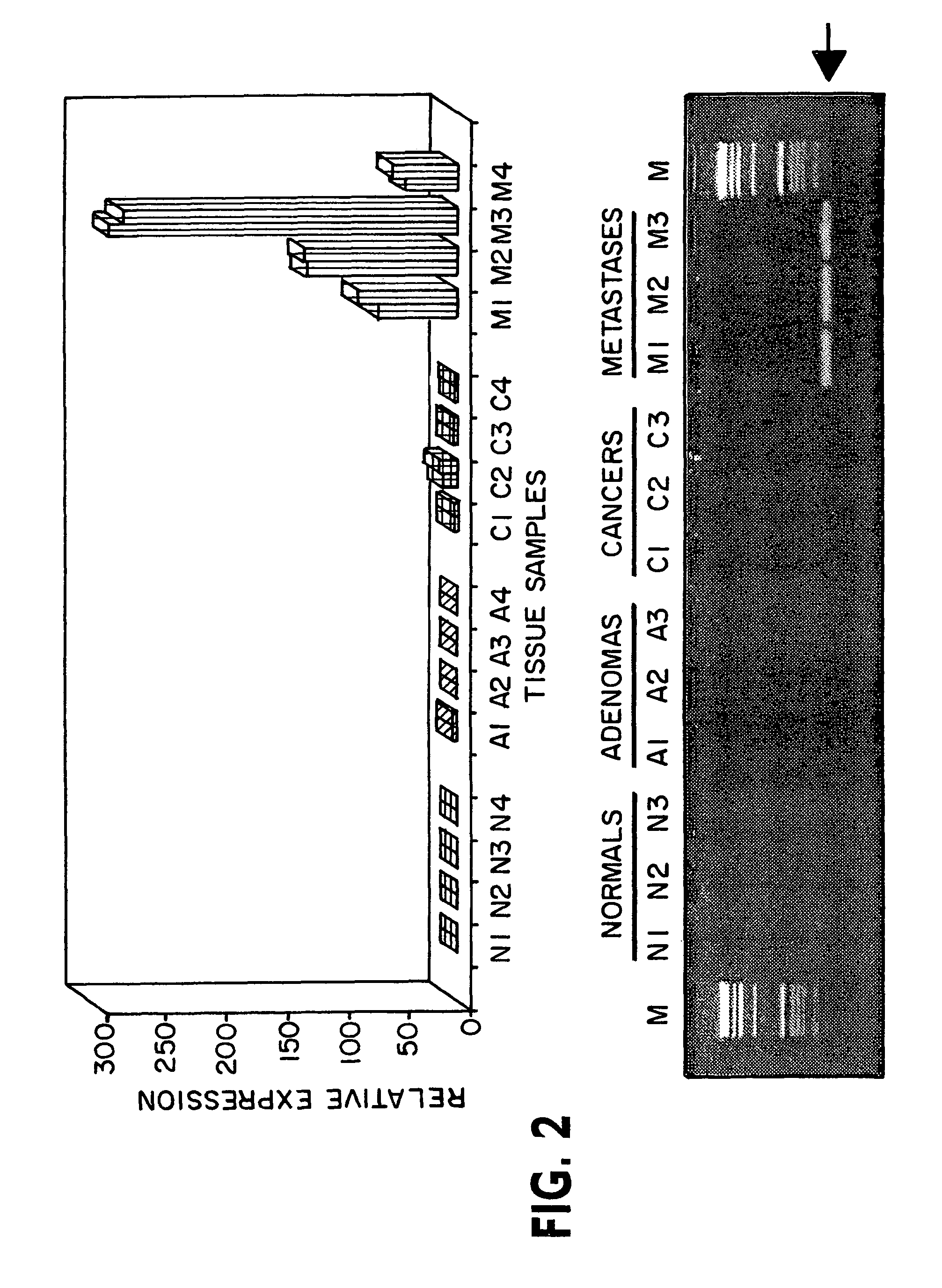
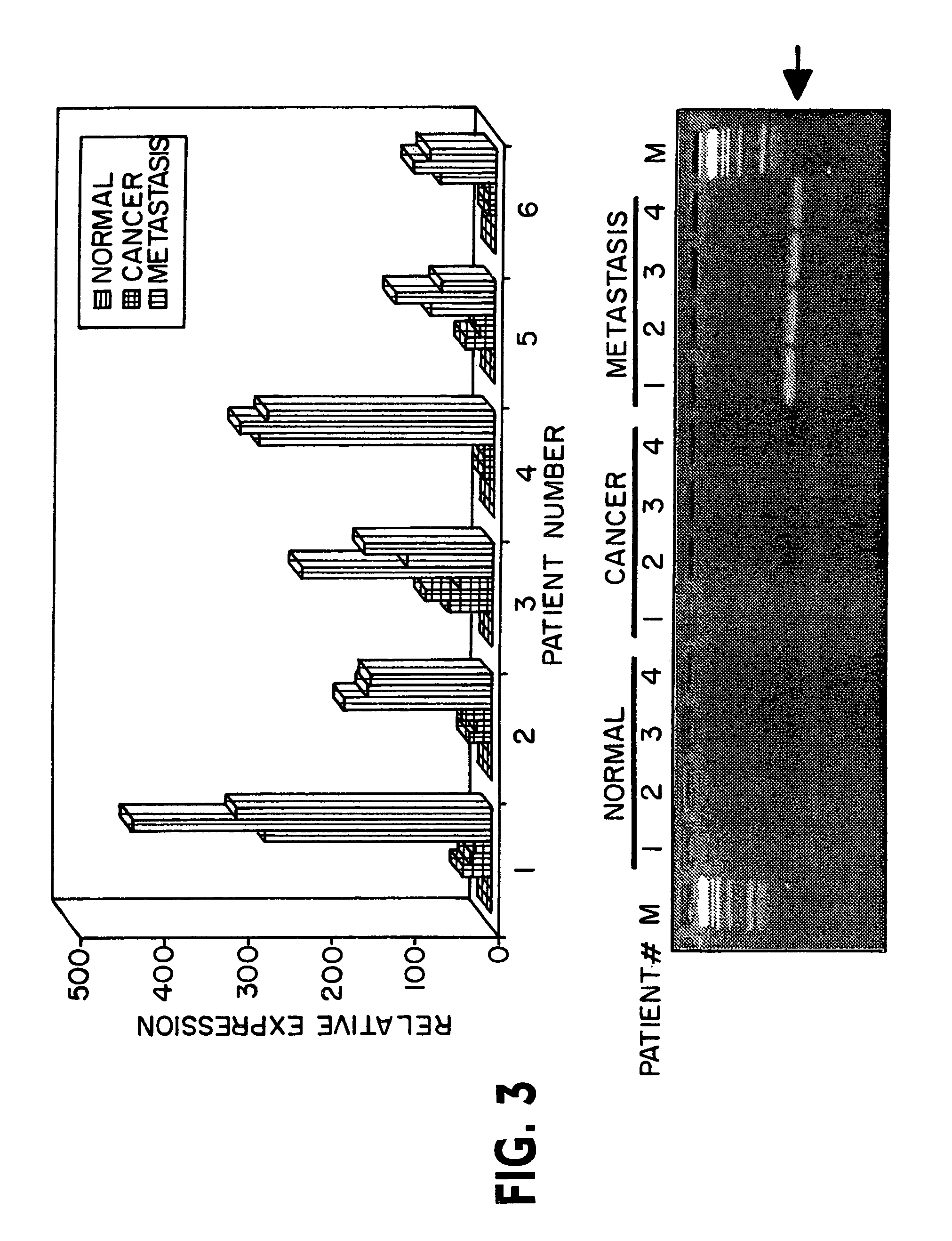
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
52 results about "Non metastatic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
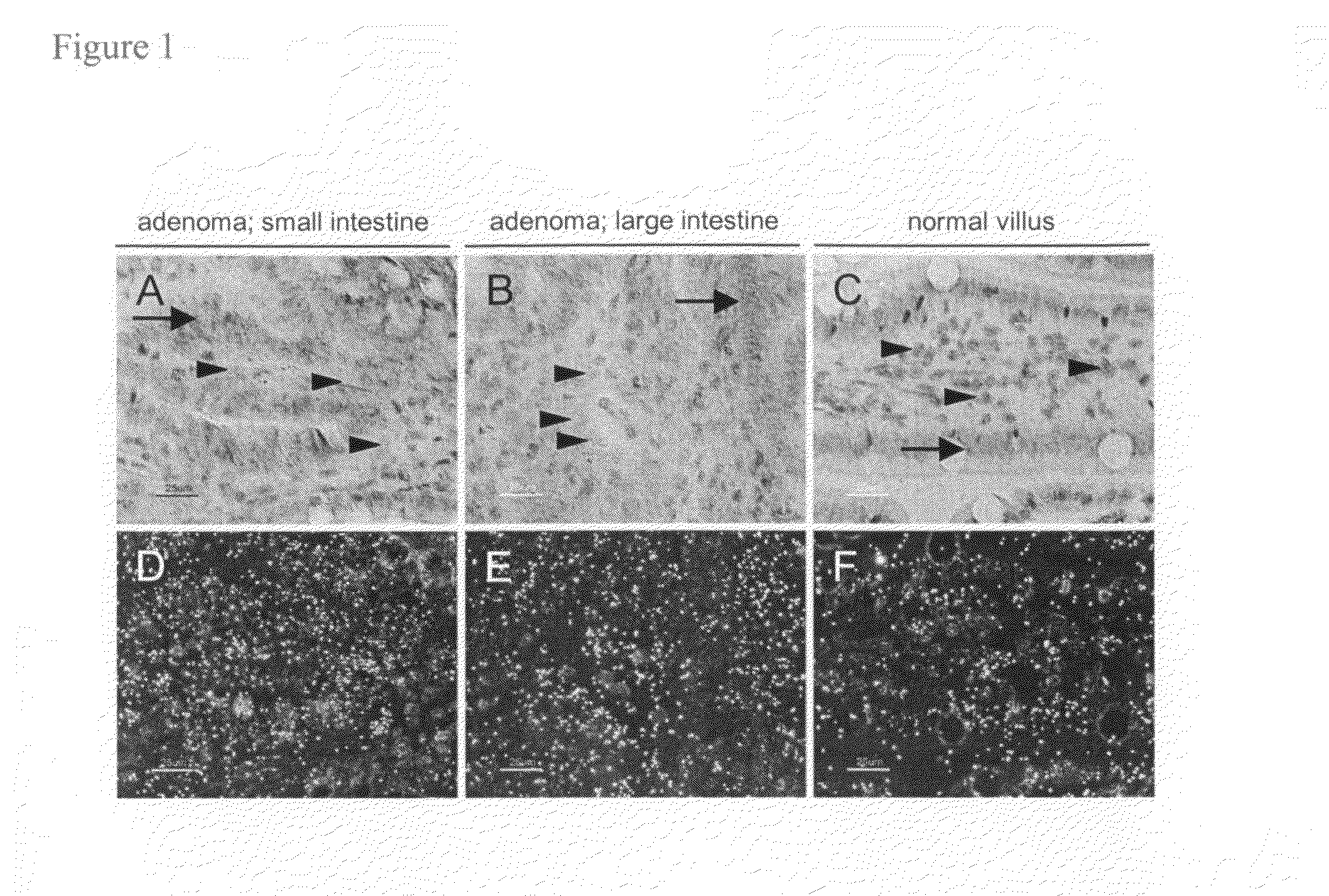
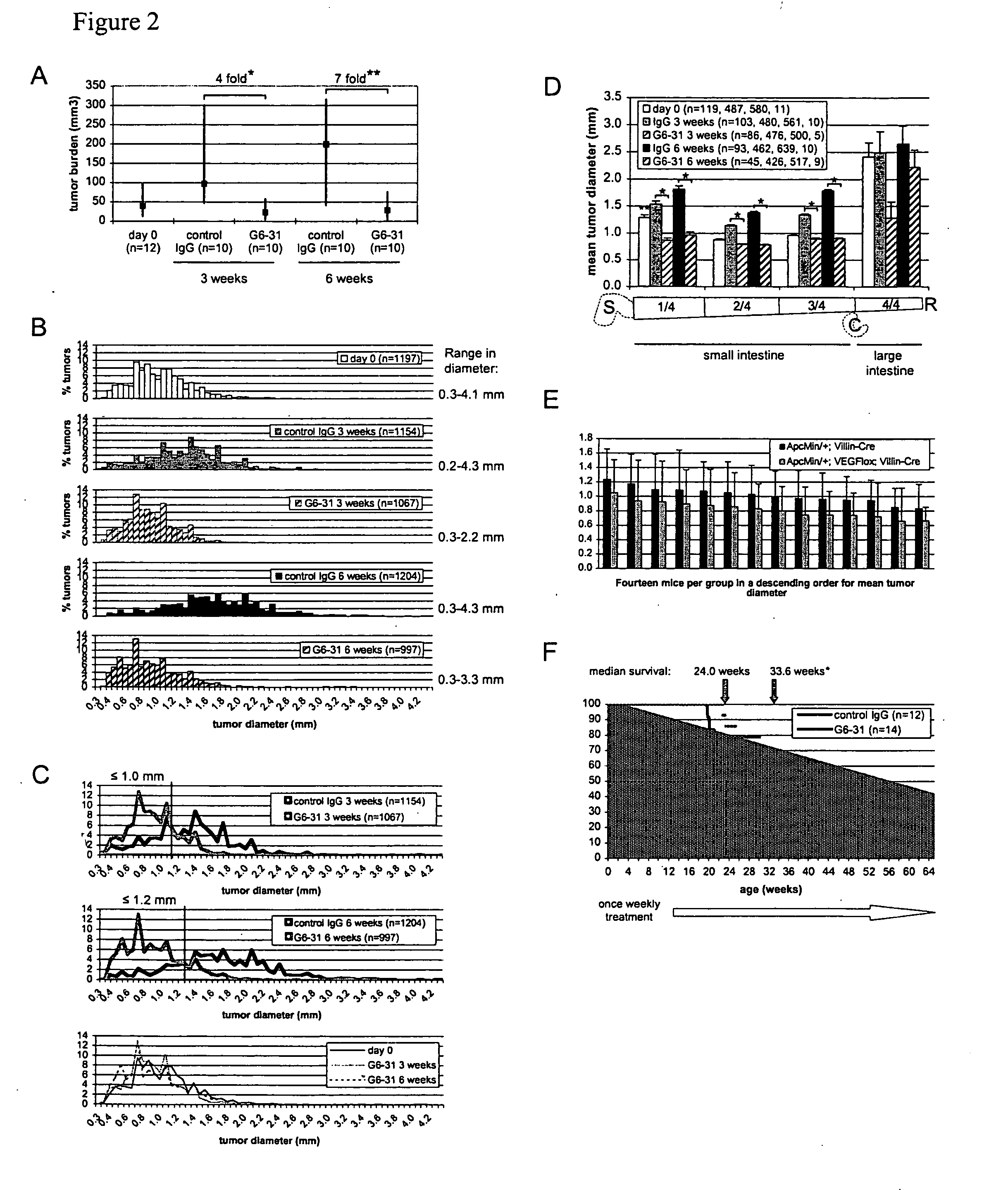
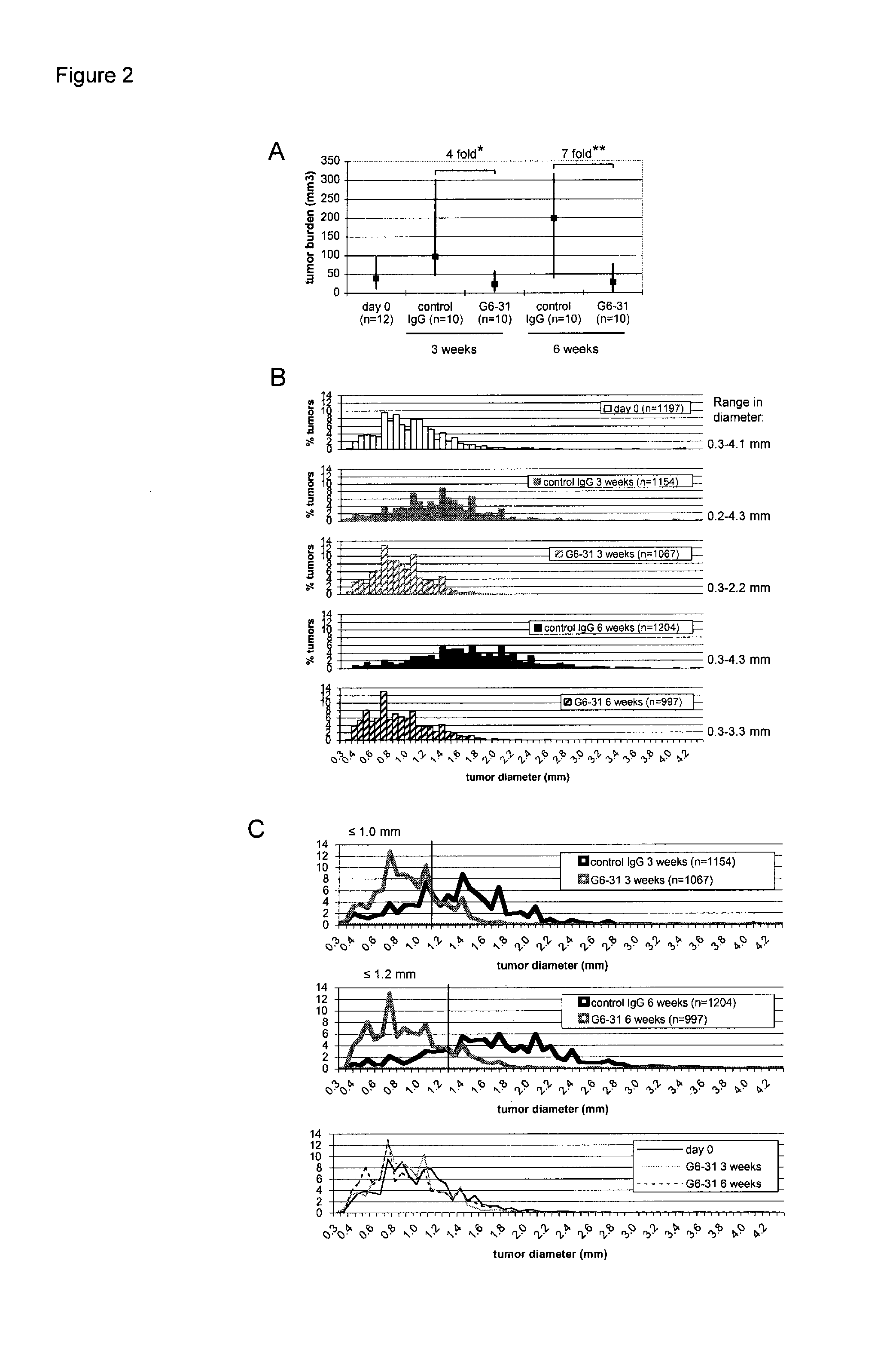
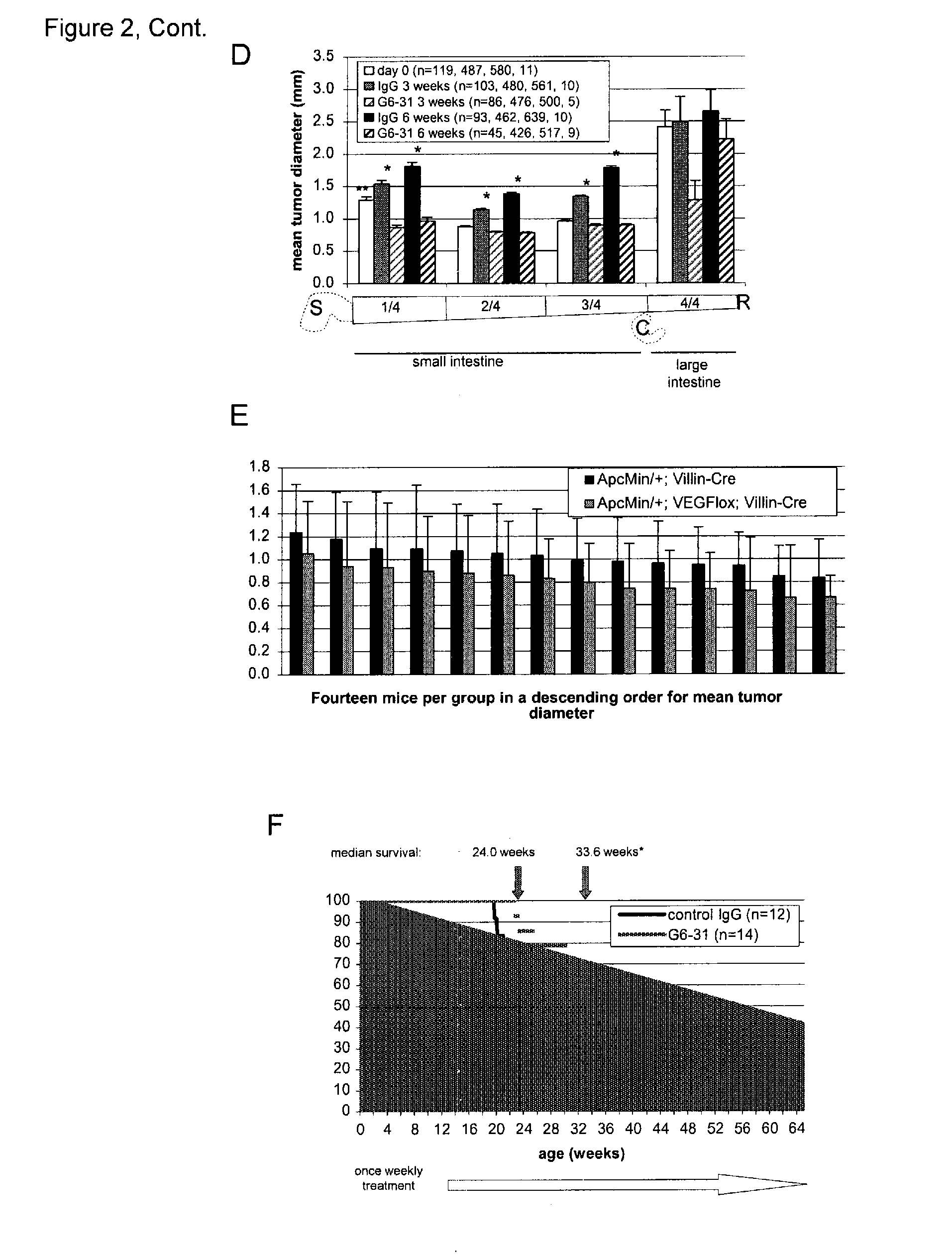
VEGF-specific antagonists for adjuvant and neoadjuvant therapy and the treatment of early stage tumors
InactiveUS20080248033A1Reduce and prevent likelihoodPrevent relapseOrganic active ingredientsPeptide/protein ingredientsStaging tumorsStage tumor
Disclosed herein are methods of treating benign, pre-cancerous, or non-metastatic tumors using an anti-VEGF-specific antagonist. Also disclosed are methods of treating a subject at risk of developing benign, pre-cancerous, or non-metastatic tumors using an anti-VEGF-specific antagonist. Also disclosed are methods of treating or preventing recurrence of a tumor using an anti-VEGF-specific antagonist as well as use of VEGF-specific antagonists in neoadjuvant and adjuvant cancer therapy.
Owner:GENENTECH INC
Recombinant human Claudin18.2 tumor vaccine and preparation method thereof
InactiveCN101584860AImprove securitySimple manufacturing methodMicroorganism based processesAntibody medical ingredientsSide effectMouse Stomach
The invention belongs to the field of medicinal biotechnology, and in particular relates to a recombinant human Claudin18.2 tumor vaccine and a preparation method thereof. The invention aims to solve the problems of immunotherapy for stomach cancer, pancreatic cancer, esophagus cancer and metastatic and non-metastatic ovarian cancer, such as high after-excision recurrence rate, strong chemo-treatment and radiation treatment toxic side effect and high monoclonal antibody therapy cost. The invention adopts a technical proposal that the recombinant human Claudin18.2 tumor vaccine has a sequence of HMKSSQYIKANSKFIGEFDQWSTQDLYNNPVTAVFNYQGLWRSCVRESSGFTECRGYFTLLGLPAMLQAV. Animal experiments prove that rhClaudin18.2 fusion protein can induce high-titre neutralizing antibody in the bodies of tumor-bearing mice by over 1:10,000; the antibody can be combined with human KATOIII and PANC-1 tumor cells and mouse stomach cancer MFC and pancreatic cancer MPC-83 cells; and the protein serving as a tumor vaccine can suppress the growth of the stomach cancer MFC and pancreatic cancer MPC-83 cells in the bodies of the mice.
Owner:西安杰诺瓦生物科技有限公司
Phosphatase associated with metastasis
ActiveUS20050047996A1Propensity of the tissue to metastasizeIn-vivo radioactive preparationsHydrolasesAbnormal tissue growthEpithelium
Among the genes identified, in a comparison of the global gene expression profile of metastatic colorectal cancer to that of primary cancers, benign colorectal tumors, and normal colorectal epithelium, the PRL-3 protein tyrosine phosphatase gene was of particular interest. It was expressed at high levels in each of 18 cancer metastases studied but at lower levels in non-metastatic tumors and normal colorectal epithelium. In three of twelve metastases examined, multiple copies of the PRL-3 gene were found within a small amplicon located at chromosome 8q24.3. These data suggest that the PRL-3 gene is important for colorectal cancer metastasis and provides a new therapeutic target for these intractable lesions.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Vegf-specific antagonists for adjuvant and neoadjuvant therapy and the treatment of early stage tumors
InactiveUS20110052576A1Reduce the burden onSmall sizeOrganic active ingredientsPeptide/protein ingredientsStaging tumorsAdjuvant
Disclosed herein are methods of treating benign, pre-cancerous, or non-metastatic tumors using an anti-VEGF-specific antagonist. Also disclosed are methods of treating a subject at risk of developing benign, pre-cancerous, or non-metastatic tumors using an anti-VEGF-specific antagonist. Also disclosed are methods of treating or preventing recurrence of a tumor using an anti-VEGF-specific antagonist as well as use of VEGF-specific antagonists in neoadjuvant and adjuvant cancer therapy.
Owner:GENENTECH INC
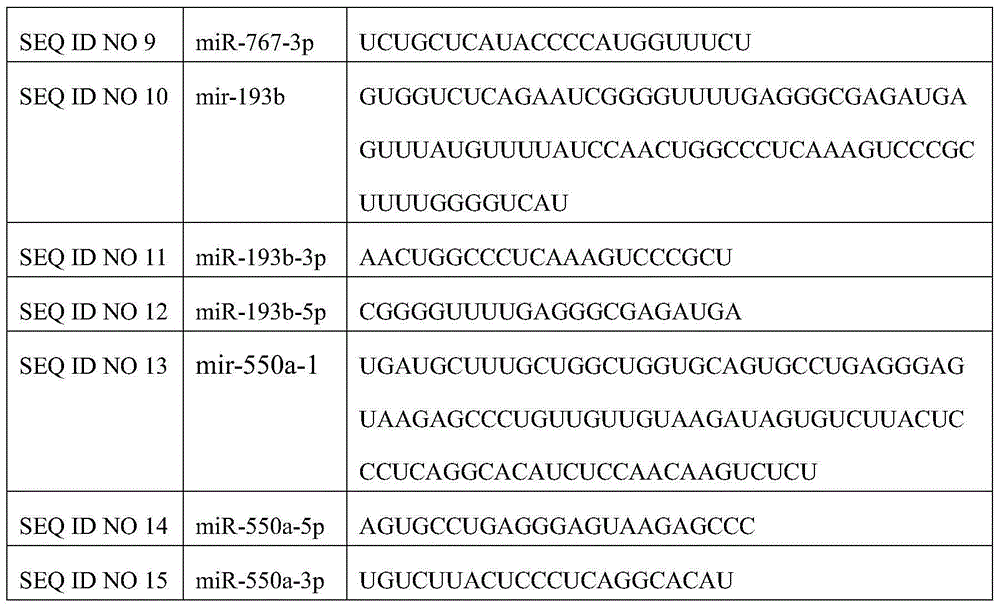
Composition related to lung adenocarcinoma metastasis and application thereof
InactiveCN104784704AOrganic active ingredientsGenetic material ingredientsSequence analysisLymphatic Spread
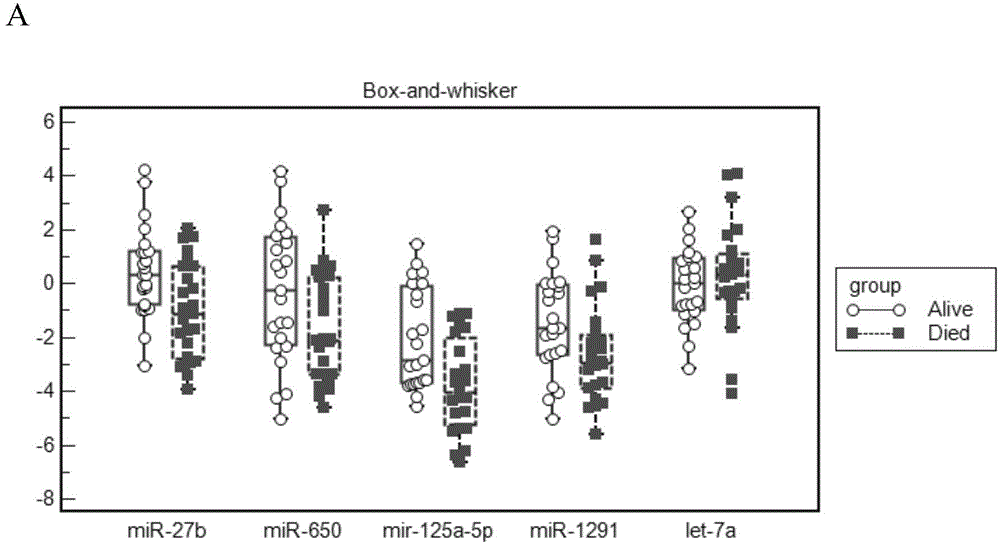
The invention relates to a composition related to lung adenocarcinoma metastasis and application thereof and particularly relates to novel application of the composition composed of one or more of the mir-193a, mir-3942, mir-767, mir-193b and mir-550a-1 and / or matures miRNA for diagnosing the lung adenocarcinoma metastasis. By performing sequencing analysis on the lung adenocarcinoma metastatic group and the non-metastatic group, a reserved drone gene is chosen to perform molecular biology verification. The result shows that the miRNA provided by the invention is closely related to the lung adenocarcinoma metastasis. The composition can be used for clinical diagnosis and preventive detection and has good practical application value.
Owner:BEIJING MEDINTELL BIOMED CO LTD
Alpha enolase-directed diagnostics and therapeutics for cancer and chemotherapeutic drug resistance
InactiveUS20070077583A1High sensitivityHigh expressionOrganic active ingredientsBiocideAlpha-enolaseInduction chemotherapy
Disclosed are methods for detecting a neoplasm and / or chemotherapeutic drug resistance or angiogenic potential in neoplastic cells by detecting an increase in the expression of α-enolase in such cells, or in the case of metastatic potential on the surface of such cells, as compared to the level of expression of α-enolase protein in a normal or non-MDR neoplastic cell or on the surface of a non-metastatic neoplastic cell. In addition, methods and a composition are disclosed for increasing the sensitivity of a neoplasm to a chemotherapeutic drug treatment regime, for inhibiting angiogenesis and metastatic potential in chemotherapeutic drug resistant or neoplastic cells, and for inducing apoptosis in chemotherapeutic drug resistant or neoplastic cells.
Owner:AURELIUM BIOPHARMA
Micro RNA biomarker for predicting early non-metastatic colorectal cancer prognosis and detection method
The invention belongs to the technical field of biomedicine and relates to a micro RNA biomarker for predicting early non-metastatic colorectal cancer prognosis and a detection method. The early non-metastatic colorectal cancer refers to Dukes' A and Dukes' B glandular cancers. Particularly, the invention relates to a diagnostic kit for identifying molecular markers of one or more mammalian target cells for prognosis of different early non-metastatic colorectal cancers. The kit comprises multiple nucleic acid molecules, a miRNA sequence is encoded by each nucleic acid molecule, one or more in the multiple nucleic acid molecules have differential expressions in target cells and one or more control cells, and one or more nucleic acid molecules with differential expressions represent nucleic acid expression characteristics together, wherein the nucleic acid expression characteristics refer to indications for identifying prognosis of different early non-metastatic colorectal cancers. The invention further relates to a corresponding method for preventing or treating the disease by using the biomarker and detection method and a pharmaceutical composition.
Owner:上海兰卫医学检验所股份有限公司
Method For Early Detection of Lung Cancer
InactiveUS20140038194A1High sensitivityWide dynamic rangeMicrobiological testing/measurementStage I Lung CancerMir 106a
The invention provides a blood-based noninvasive early lung cancer detection method, which investigates a panel of miRNA levels in a blood or plasma sample. The panel of miRNA includes miR-17, miR-21, miR-24, miR-106a, miR-125b, miR-128, miR-155, miR-182, miR-183, miR-197, miR-199b, miR-203, miR-205, miR-210, miR-221, and a combination thereof. Preferably, the panel of miRNA may include miR-21, miR-128, miR-155, miR-182, miR-183, and miR-197. The inventive method can not only detect stage I lung cancer patients with high accuracy, but also differentiate between all stages of lung cancer patients and lung cancer-free individuals, metastatic and non-metastatic lung cancer patients and monitor the significant changes of miRNA levels during chemotherapy.
Owner:UNIVERSITY OF MISSOURI
Compositions and methods for the prevention and treatment of cancer
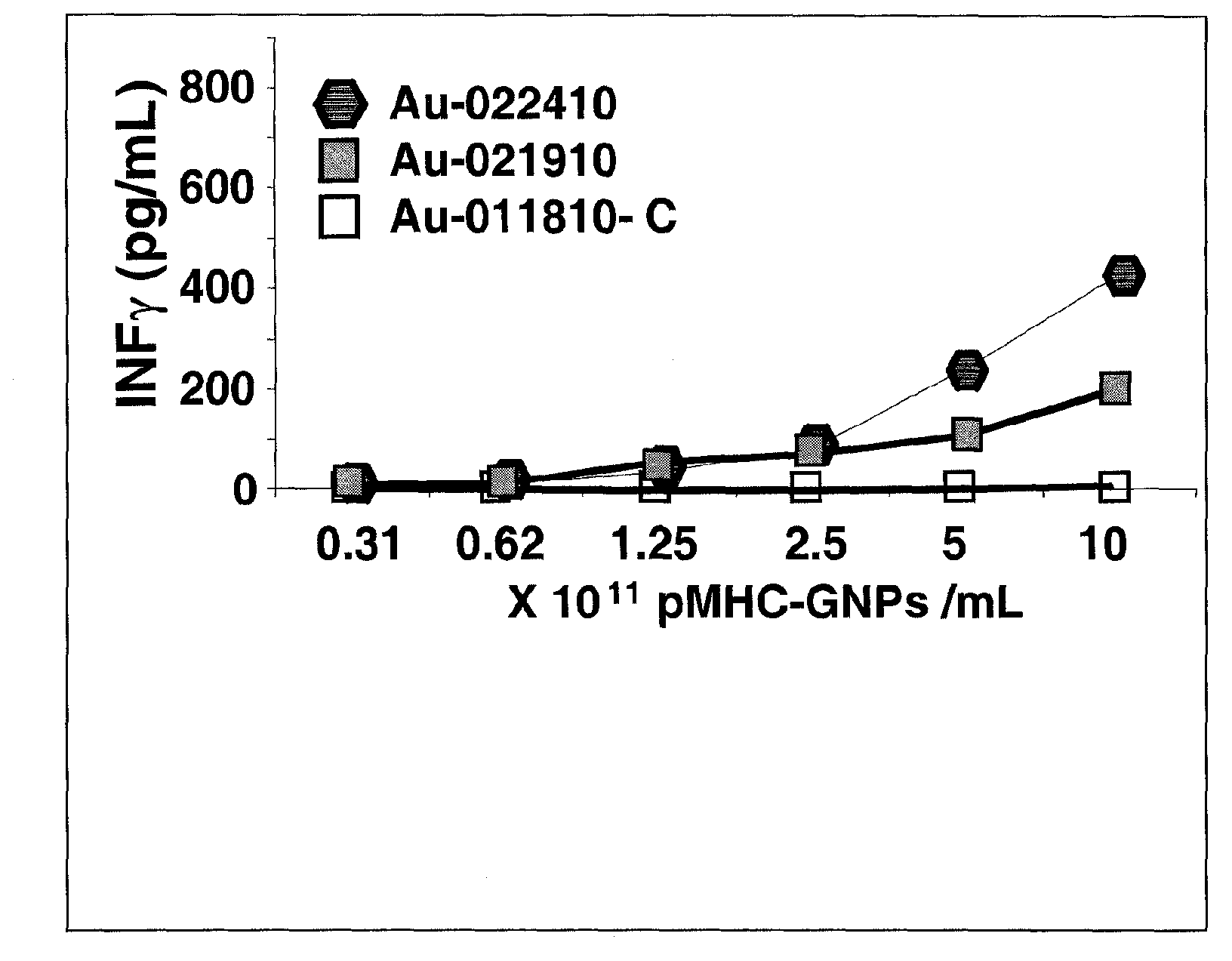
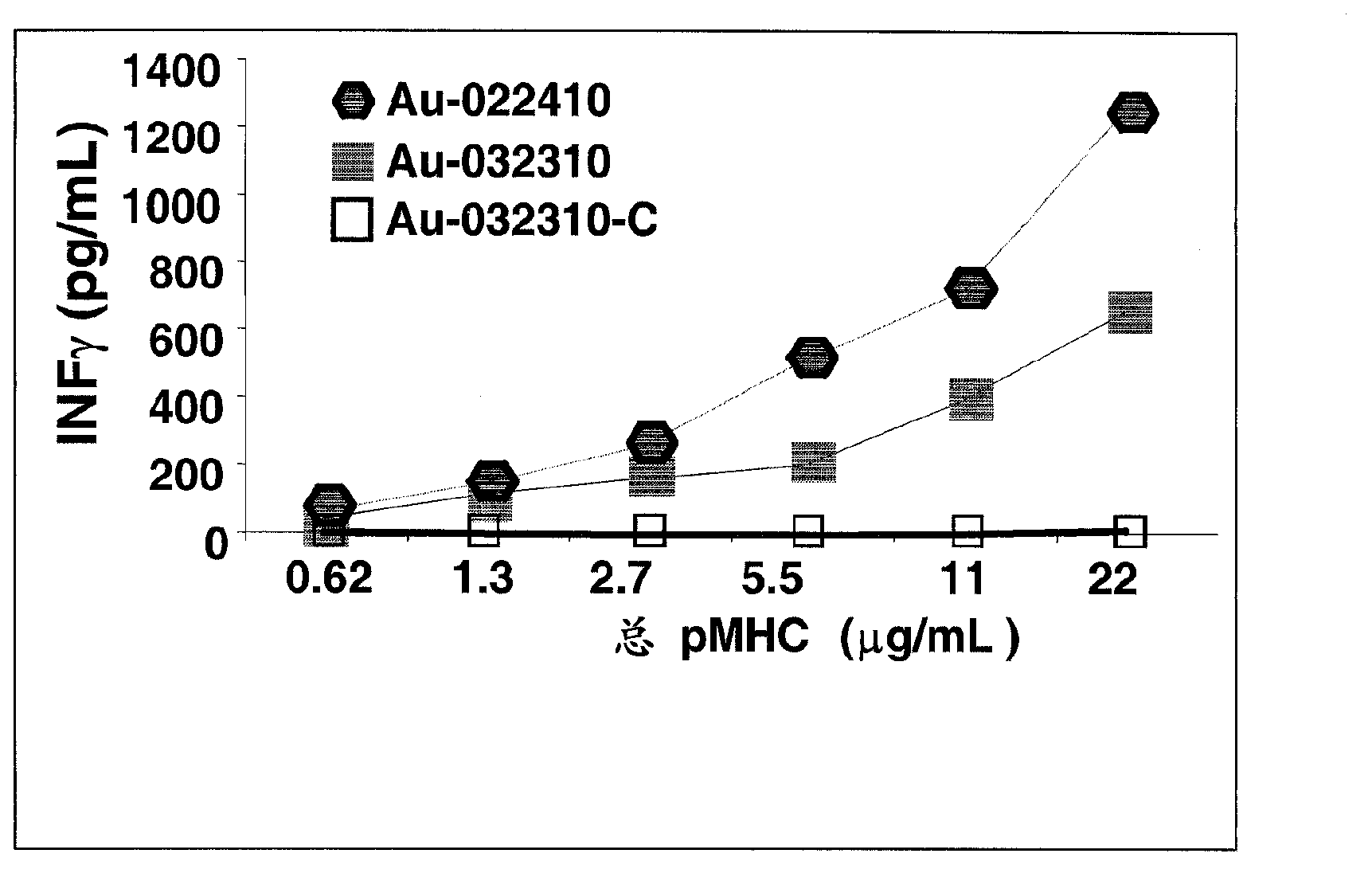
Conventional cancer immunotherapy falls short at efficiently expanding T cells that specifically target cancerous cells in numbers sufficient to significantly reduce the tumor size or cancerous cell number in vivo. To overcome this limitation, provided herein are nanoparticles coated with MHC class I and / or class II molecules presenting tumor-specific antigens and co-stimulatory molecules and their use to expand antigen-specific anti-tumorigenic T cellsto levels not achieved in current immunotherapeutic techniques. These antigen-specific anti-tumorigenic T cells include cytotoxic T cells, effector T cells, memory T cells, and helper T cells that are necessary to initiate and maintain a substantial immune response against metastatic or non- metastatic cancerous, pre-cancerous, or neoplastic cells in vivo.; The present invention describes a systemic approach to targeting cancerous or pre-cancerous cells that are circulating cells, as in lymphomas, migratory metastatic cells, and solid tumors.
Owner:UTI LLP
Methylated biomarkers related to gastric cancer lymph node metastasis detection or combination and application thereof
ActiveCN114317738AAccurate predictionAccurate diagnosisMicrobiological testing/measurementDNA/RNA fragmentationDNA methylationOncology
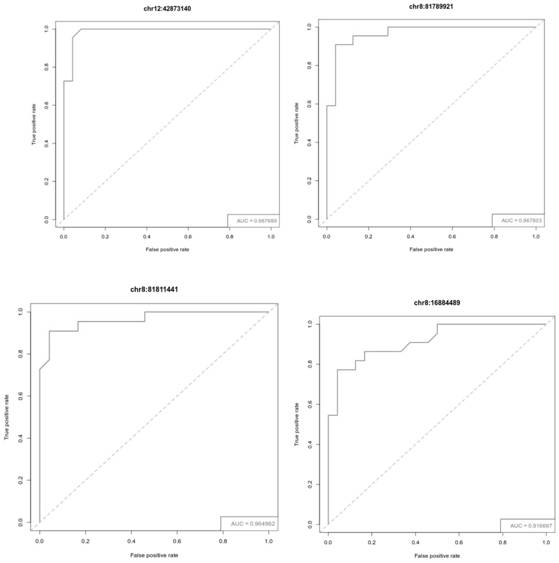
The invention relates to a methylated biomarker or a combination thereof for detecting gastric cancer lymph node metastasis and non-metastasis and application of the methylated biomarker. The methylated biomarker is selected from at least one of chr12: 42873140, chr8: 81789921, chr8: 81811441 and chr8: 16884489. According to the invention, the appropriate DNA methylation biomarker is screened and used for detecting gastric cancer lymph node metastasis and non-metastasis, and the DNA methylation biomarker can achieve the purpose of accurate prediction. The novel DNA methylation marker developed by the invention can be used for identifying whether the lymph node metastasis of the early gastric cancer occurs or not, can be used for assisting clinical accurate diagnosis and guiding treatment, and also can be used for technical research in laboratories.
Owner:ANCHORDX MEDICAL CO LTD
MicroRNA markers for discriminating metastatic and non-metastatic squamous cell lung carcinoma
The invention relates to 21 microRNA markers for discriminating metastatic and non-metastatic squamous cell lung carcinoma. More specifically, the invention relates to a class of microRNA markers for discriminating metastatic and non-metastatic squamous cell lung carcinoma. It has been proved by examination that, the specific microRNA markers can effectively discriminate metastatic and non-metastatic squamous cell lung carcinoma tissue. The invention also relates to a chip and a test kit for detecting the microRNA markers.
Owner:SHANGHAI INST OF ONCOLOGY
Epithelial-mesenchymal transition in circulating tumor cells (CTCS) negatives for cytokeratin (CK) expression in patients with non-metastatic breast cancer
The inventors of the present invention have surprisingly discovered that EGFR expression in nonmetastatic breast cancer patients with CK-negative CTCs could induce EMT process. A simultaneous detection of both EGFR and EMT markers (VIM and Slug) in CTCs might improve prognostic or predictive information in patients with operable breast cancer.
Owner:SERVICIO ANDALUZ DE SALUD (SAS) +1
Molecular marker differentiating metastatic squamous cell lung carcinoma from non-metastatic squamous cell lung carcinoma
InactiveCN105755154AAchieve early diagnosisReduce mortalityMicrobiological testing/measurementBiological material analysisLymphatic SpreadDrug target
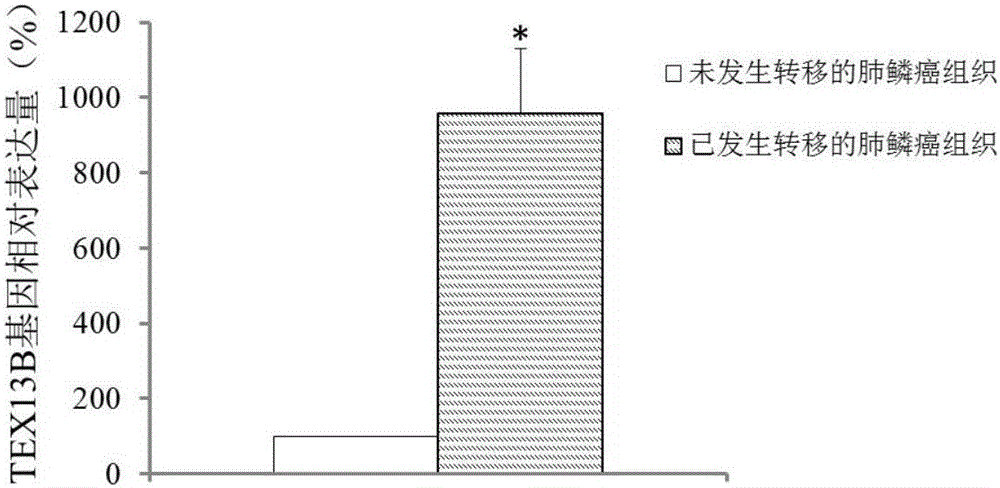
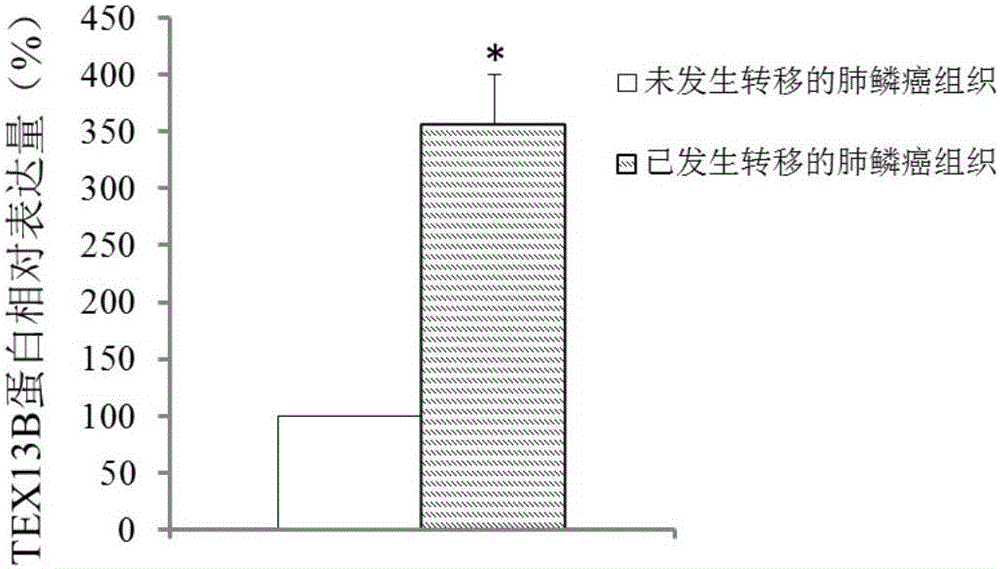
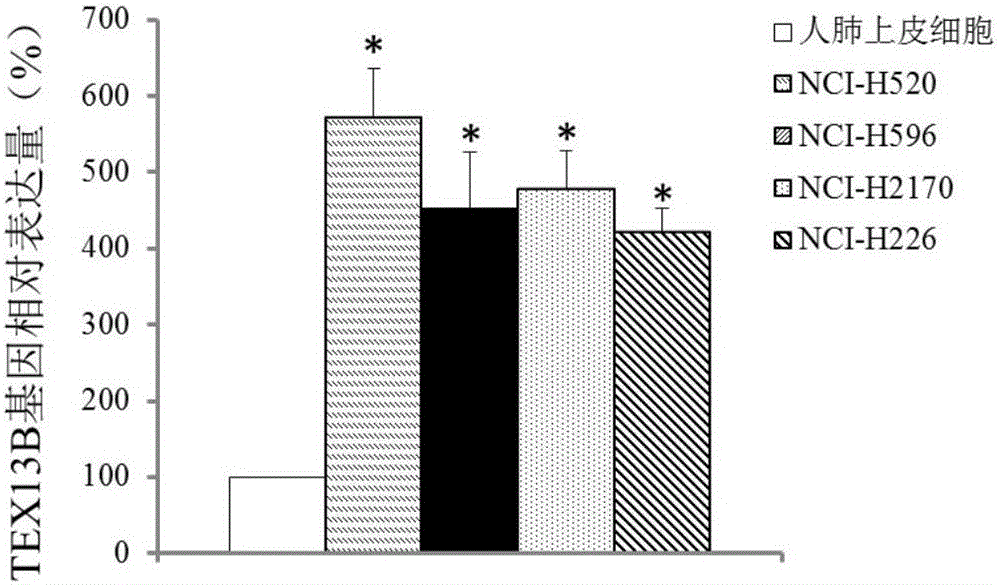
The invention discloses application of TEX13B gene and an expression product thereof to preparation of diagnostic tools for squamous cell lung carcinoma metastasis. According to the invention, a gene chip and a QPCR experiment prove that the TEX13B gene has difference in the expression of squamous cell lung carcinoma non-metastatic samples and squamous cell lung carcinoma metastatic samples, so that the TEX13B gene is believed to be a molecular marker for diagnosing squamous cell lung carcinoma metastasis. A culture in vitro cell experiment proves that restraining the expression of the TEX13B gene can restrain the adhesion, migration and invasion of lung squamous carcinoma, so that the TEX13B gene is believed to be a drug target for treating squamous cell lung carcinoma metastasis. As a new molecular marker, the TEX13B gene and the expression product thereof have a wide clinical application prospect.
Owner:BEIJING MEDINTELL BIOMED CO LTD
Application of a product detecting the expression level of a biomarker in carcinogenesis state indicating
InactiveCN108548929ABroaden your mindComplete formMicrobiological testing/measurementBiological testingSerum igeAdenocarcinoma lung cancer
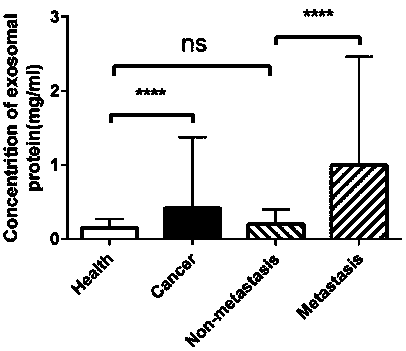
The invention belongs to the field of medical and health, and particularly relates to application of a product detecting the expression level of a biomarker in carcinogenesis state indicating. The biomarker is LBP, a gene encoding LBP, or a polypeptide or polypeptide fragment synthesizing the LBP. It is proposed by the invention for the first time that the level of exosome protein LBP in serum ofpatients with non-small cell lung cancer is significantly higher than that of a healthy contrast group, and the level of exosome protein LBP of patients with metastatic non-small cell lung cancer is significantly higher than that of patients with non-metastatic non-small cell lung cancer, and therefore, the level of the exosome protein LBP in serum can be utilized for distinguishing patients withnon-small cell lung cancer from a contrast case, distinguishing metastatic non-small cell lung cancer, non-metastatic non-small cell lung cancer, lung adenocarcinoma and squamous-cell lung cancer.
Owner:谢丽
HNC (Head and Neck Cancer) prognosis biomarker based on lymph node microbial flora and application of HNC prognosis biomarker
PendingCN113684242AGood forecastThe value of good practical applicationHealth-index calculationMicrobiological testing/measurementFloraMicrobiome
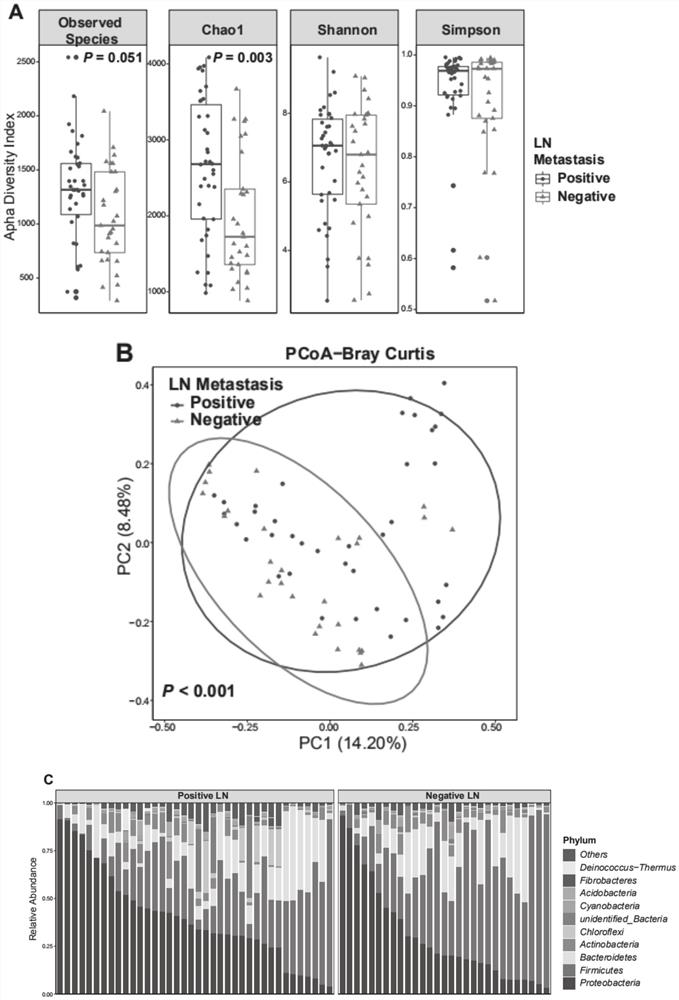
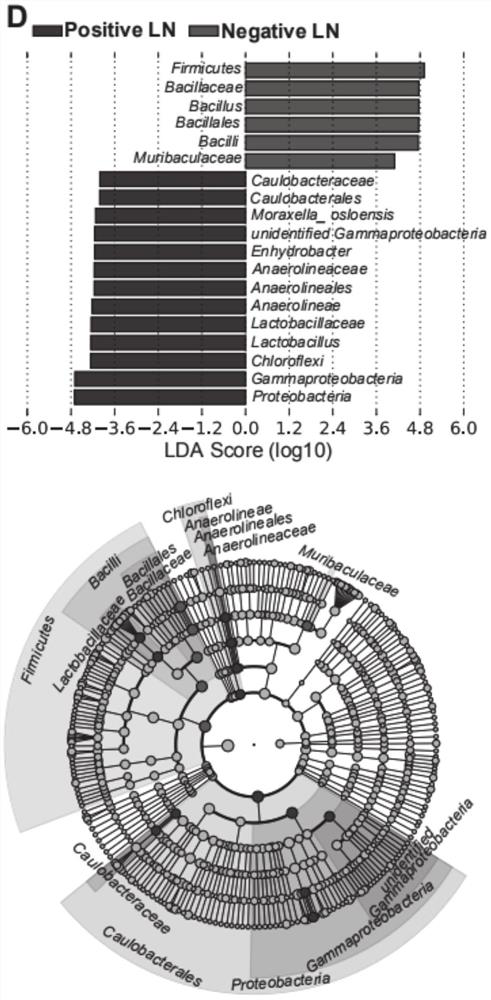
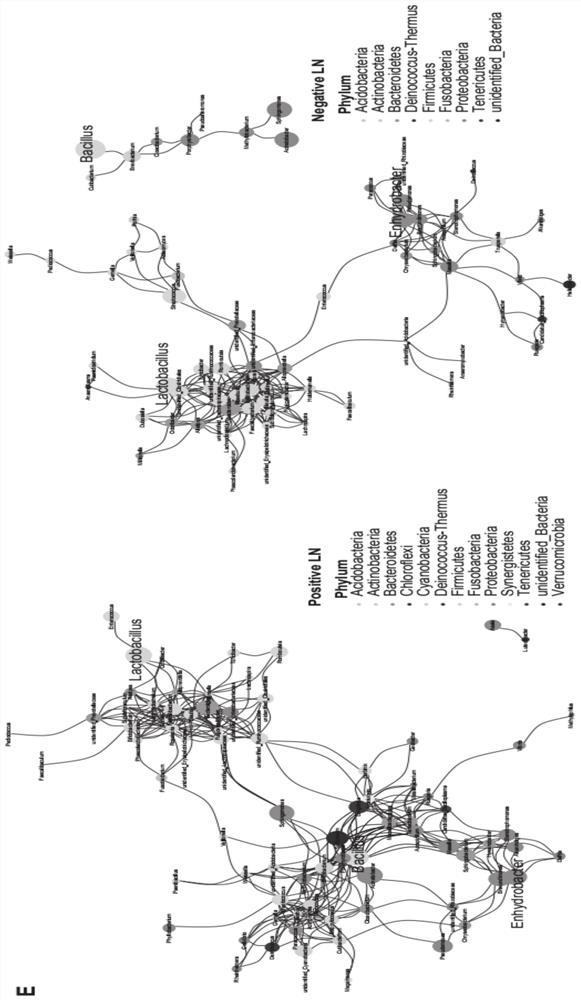
The invention provides an HNC (Head and Neck Cancer) prognosis biomarker based on a lymph node microbial flora and application of the HNC prognosis biomarker, and belongs to the technical field of disease prognosis and molecular biology. According to the invention, the 16S ribosomal RNA gene of the lymph node microbial flora of an HNC patient is subjected to high-throughput sequencing, so that it is proved that different species exist in flora species of metastatic lymph nodes and non-metastatic lymph nodes in the HNC patient; furthermore, through a microbial flora symbiotic relationship network, it is found that the flora interrelation between the species of the metastatic lymph node and the species of the non-metastatic lymph node of the HNC patient is also different at the genus level; the result discovers the characteristics of the microbiome of the metastatic lymph node and the non-metastatic lymph node of the HNC patient for the first time; and meanwhile, it is known by analysis that the flora difference characteristics (diversity index values and relative abundance of different species) have a good prediction effect on the lifetime (total lifetime and three-year lifetime) of the HNC patient, so that the invention has a good practical application value.
Owner:SHANDONG UNIV +1
Phosphatase associated with metastasis
ActiveUS7745165B2Propensity of the tissue to metastasizeHydrolasesMicrobiological testing/measurementEpitheliumAbnormal tissue growth
Among the genes identified, in a comparison of the global gene expression profile of metastatic colorectal cancer to that of primary cancers, benign colorectal tumors, and normal colorectal epithelium, the PRL-3 protein tyrosine phosphatase gene was of particular interest. It was expressed at high levels in each of 18 cancer metastases studied but at lower levels in non-metastatic tumors and normal colorectal epithelium. In three of twelve metastases examined, multiple copies of the PRL-3 gene were found within a small amplicon located at chromosome 8q24.3. These data suggest that the PRL-3 gene is important for colorectal cancer metastasis and provides a new therapeutic target for these intractable lesions.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Methods for determining prognosis for breast cancer patients
InactiveUS20180104232A1Easy treatmentIncrease in motilityMicrobiological testing/measurementAntibody ingredientsAdjuvantTissue sample
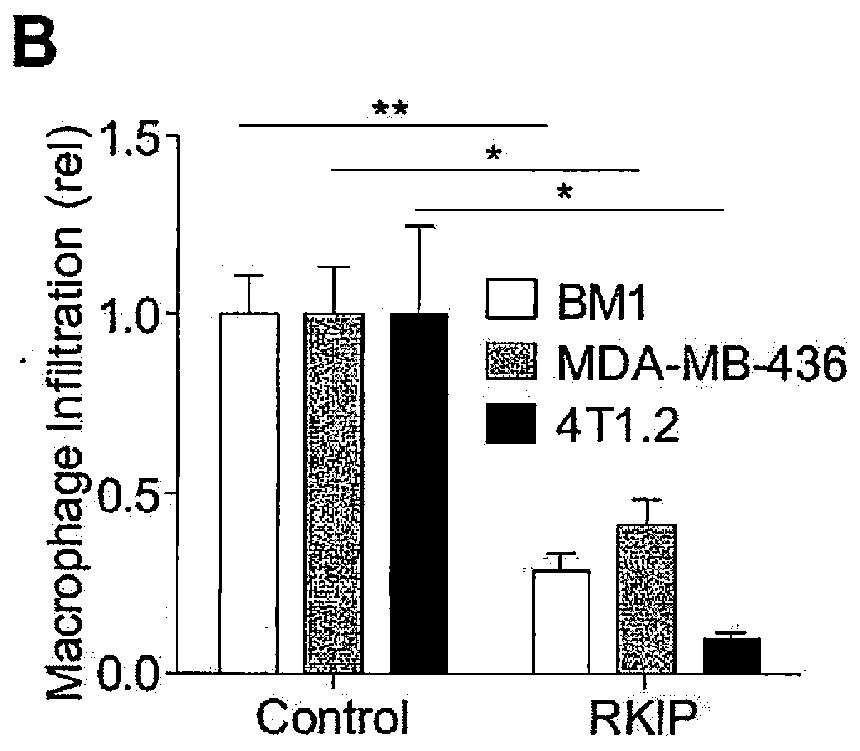
Described herein are methods for treating breast cancer and for providing a prognosis for metastatic-free survival of breast cancer patients. Aspects relate to treating a patient determined to be at high risk for developing or having metastatic breast cancer comprising administering adjuvant or neoadjuvant therapy to the patient determined to be at high risk for developing or having metastatic breast cancer, wherein the patient was determined to be at high risk for developing or having metastatic breast cancer by determining that the expression level of RKIP was reduced and / or the expression level of one or more of HMGA2, CCL5, TN-FR2, GRN, and CCL7 was elevated in a biological sample from the patient compared to a control non-metastatic tissue sample.
Owner:UNIVERSITY OF CHICAGO
Applications of CTx to preparation of medicaments for diagnosing colorectal cancer
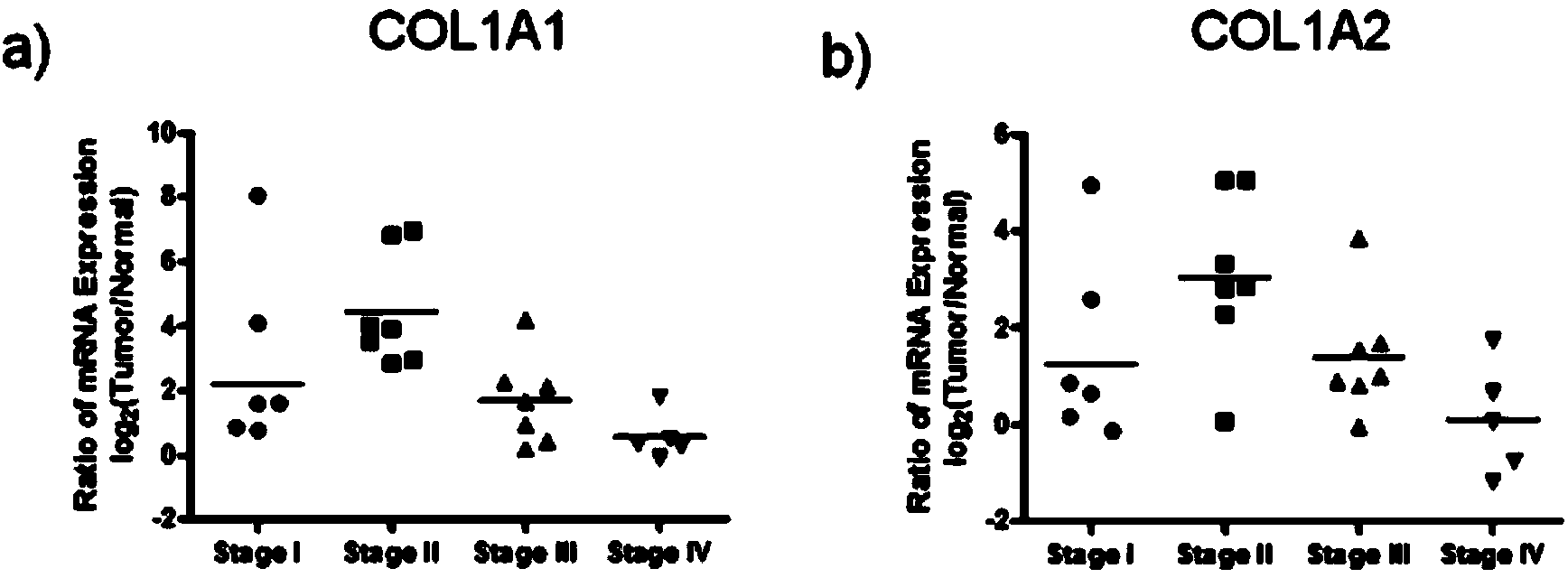
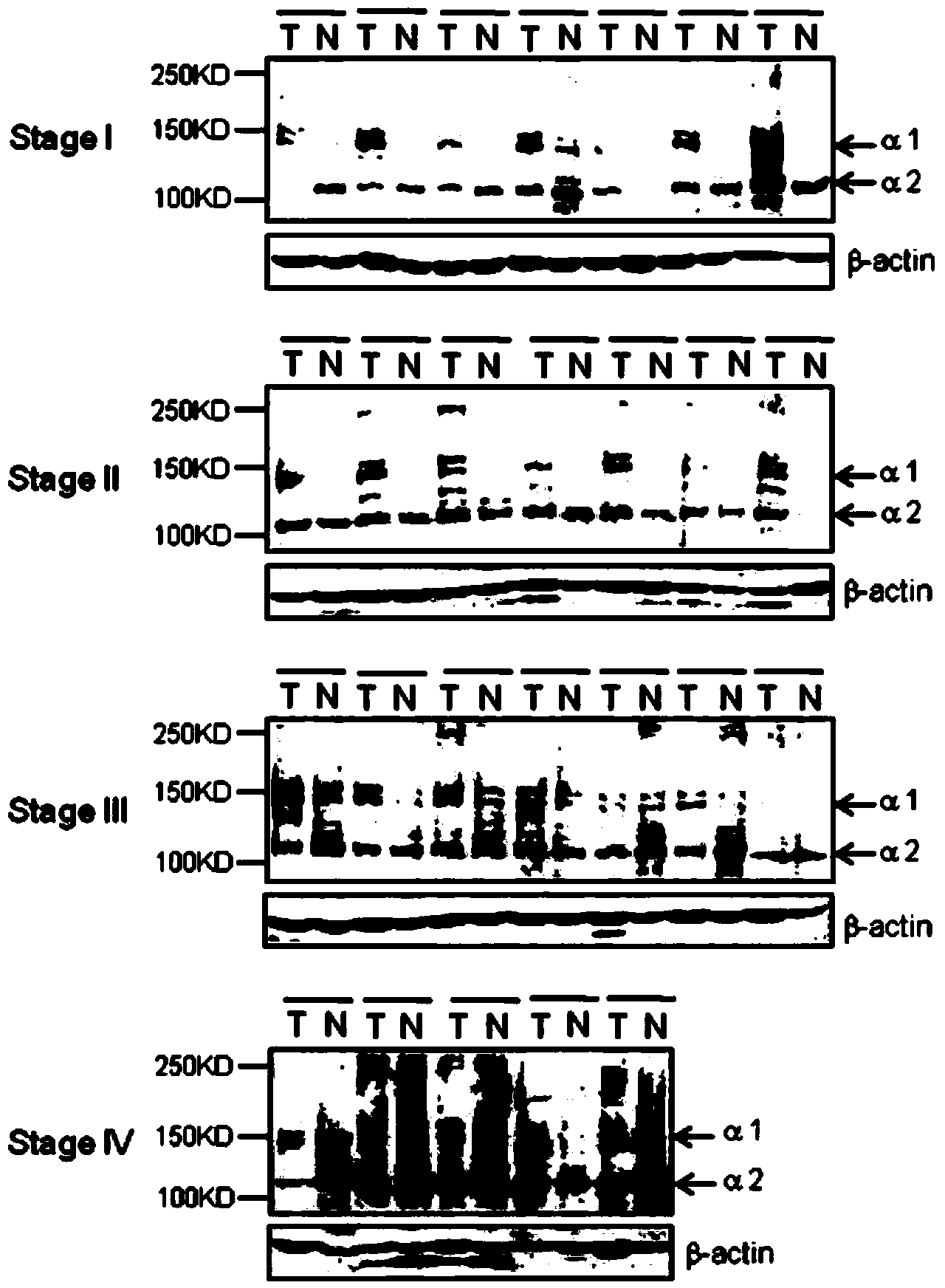
The invention relates to the technical field of biology, and especially relates to applications of CTx to preparation of medicaments for diagnosing colorectal cancer, wherein CTx is a fragment at the carboxyl telopeptide of an extracellular matrix component type I collagen. The invention provides applications of CTx to prepare and screen medicaments for diagnosing colorectal cancer and screen medicaments for treating colorectal cancer. Colorectal cancer tissues and serum clinic specimens are selected for researching the clinic value of type I collagen on generation, development, staging prognosis and the like of colorectal cancer. Through a large number of experiments, inventors of the invention discover that the expression level change of CTx in serums of colorectal cancer patients at different stages is in positive correlation with pathology staging, and compared with conventional alimentary canal tumour oncofetal antigens, CTx is capable of better differencing the metastatic colorectal cancer patients and the non-metastatic colorectal cancer patients. Additionally, the inventors also discover that CTx is correlated with 3-year tumor-free survival of the colorectal cancer patients, and the high expression of CTx indicates poor tumor-free survival and prognosis of the colorectal cancer patients.
Owner:SHANGHAI JIAO TONG UNIV
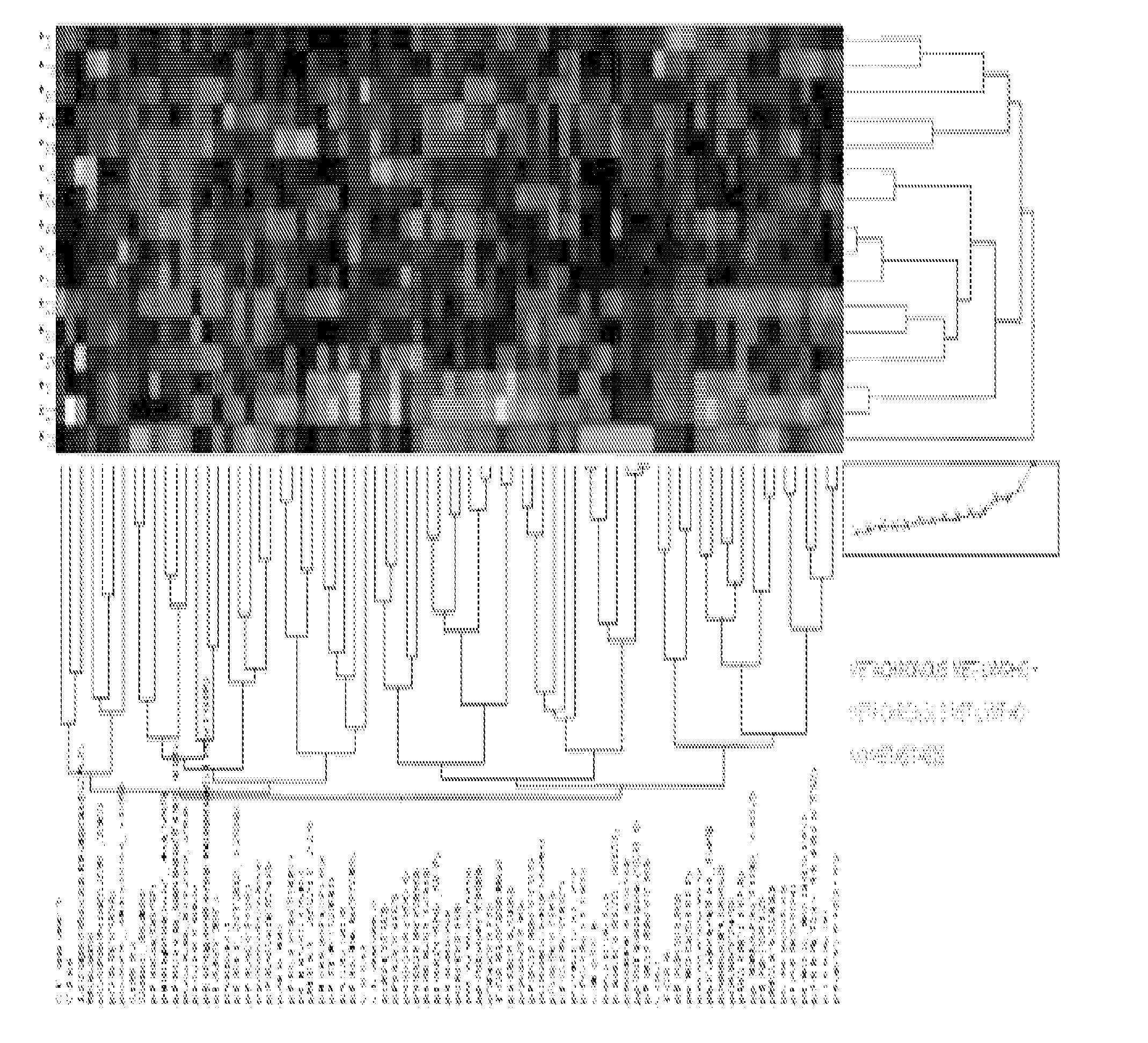
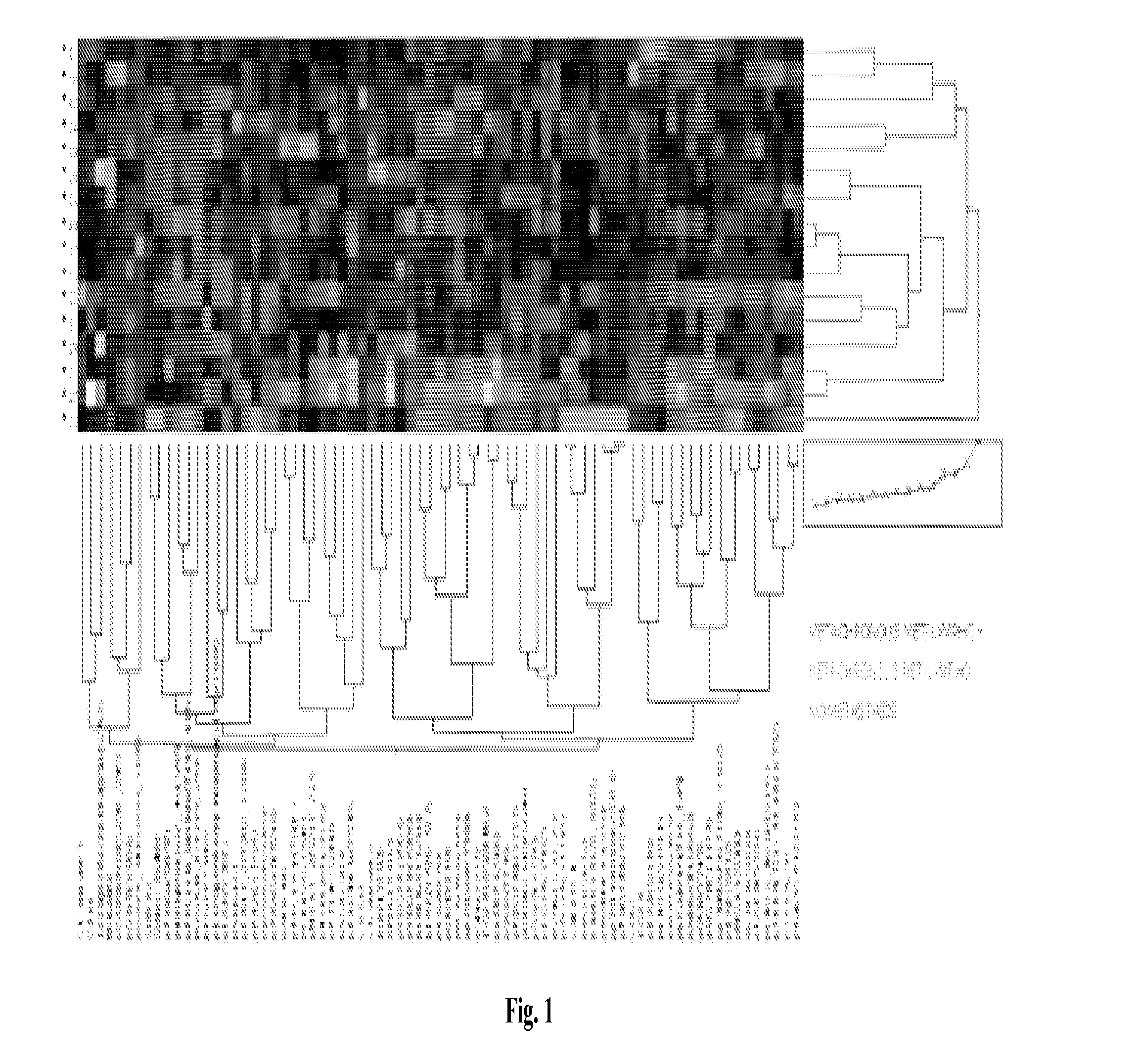
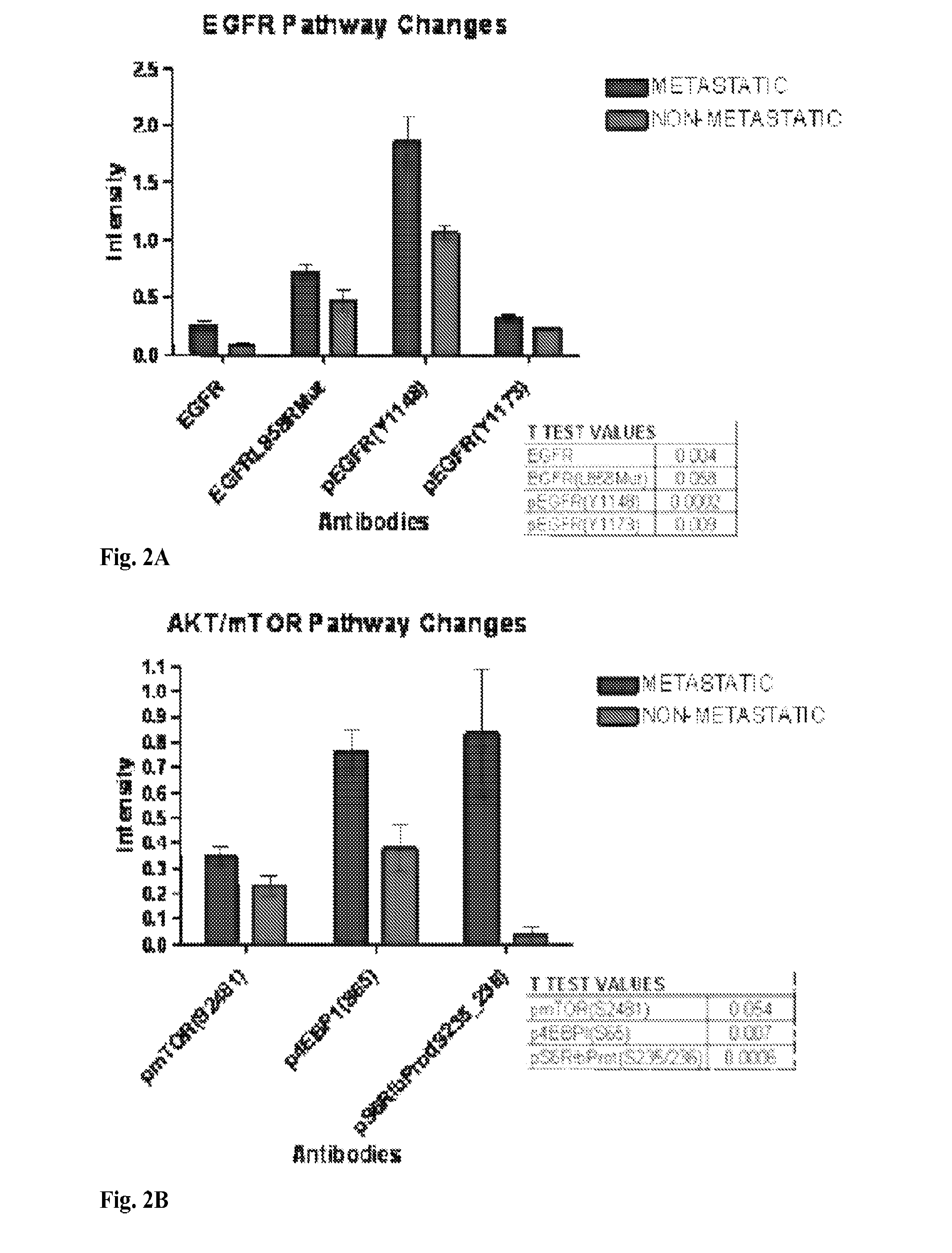
Signal pathway alterations and drug target elevations in primary metachronous metastatic colorectal cancer compared to non-metastatic disease
The present invention relates to the identification and diagnostic use of biomarkers in primary colorectal cancer tumors whose activation level are predictive of the likelihood of the onset of metastatic disease. These biomarkers may be used to determine the suitability of a patient for aggressive and / or targeted treatments. Kits and compositions of the invention are also provided.
Owner:GEORGE MASON INTPROP INC
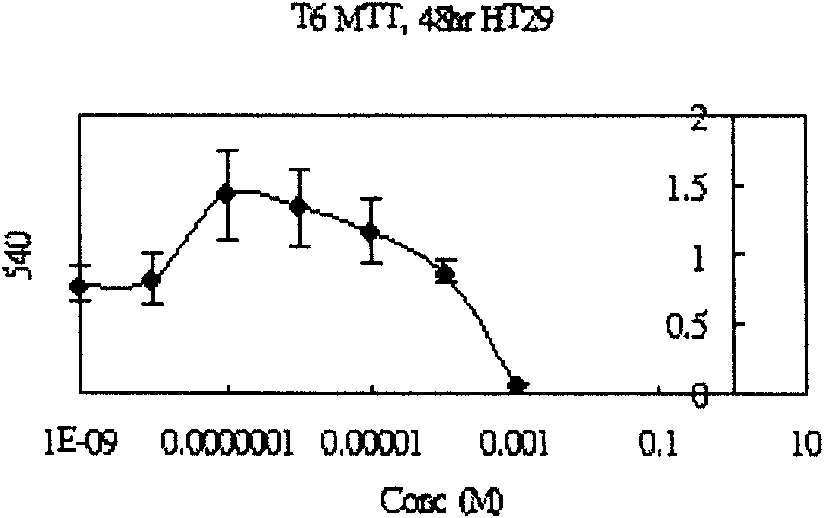
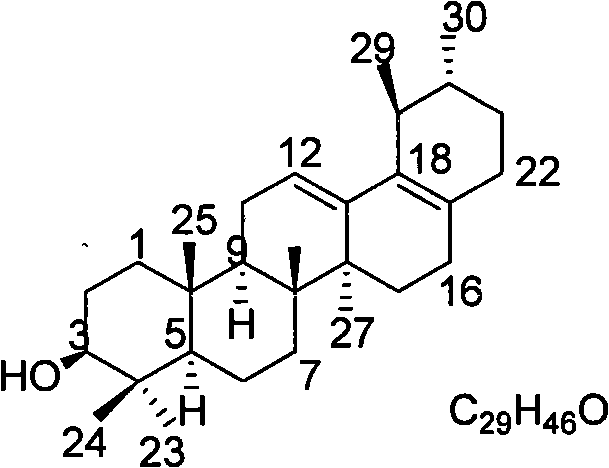
Medicament for treating colon cancer and preparation method thereof
InactiveCN101810624AColon cancer tumor suppressor effect is goodElevated interleukin-2Organic active ingredientsAntineoplastic agentsChromatographic separationSide effect
The invention discloses a medicament for treating colon cancer and a preparation method thereof. The medicament is prepared from medicinal active ingredients and pharmaceutic adjuvant, wherein the medicinal active ingredients comprise 3 beta-hydroxyl-28-deletion-12,17(18)-diene ursane. The preparation method comprises the following steps of: (1) adding methanol or ethanol into crushed charred sanguisorba root serving as a medicinal raw material, filtering the mixture, concentrating filtrate under normal pressure or reduced pressure to obtain extractum liquidum; (2) dispersing the extractum liquidum obtained by the step (1) with water, and extracting with chloroform to obtain extractum; (3) carrying out silicagel column chromatographic separation on the extractum obtained by the step (2) recovering a solvent, placing the solvent to separate white powder out, filtering the mixture to obtain the 3 beta-hydroxyl-28-deletion-12,17(18)-diene ursane; and (4) preparing the medicament from 20 to 90 weight percent of the 3 beta-hydroxyl-28-deletion-12,17(18)-diene ursane, and the balance of the pharmaceutic adjuvant by a conventional preparation method. The medicament mainly treats non-metastatic symptoms of the colon cancer, has no toxic or side effects, and is used by matching postoperative chemotherapy so as to relieve pain of patients.
Owner:SHANDONG ACAD OF CHINESE MEDICINE
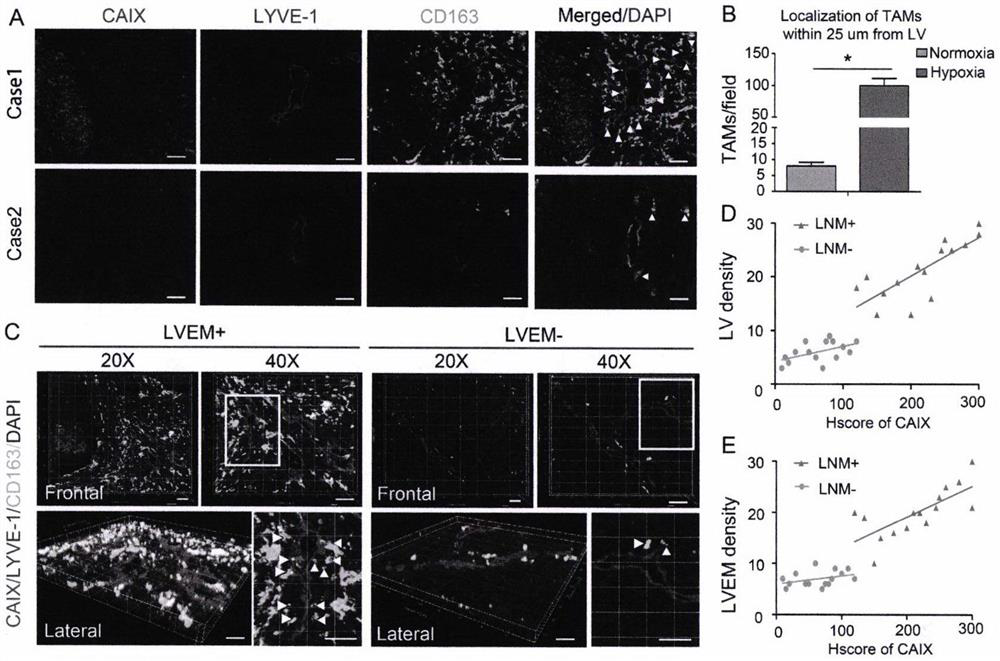
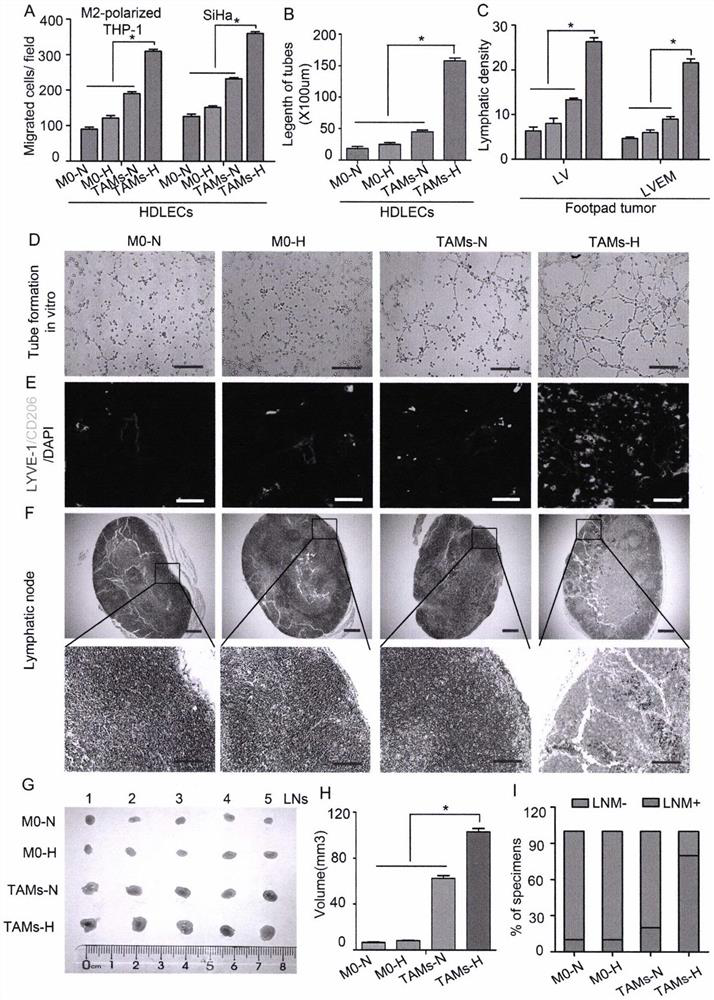
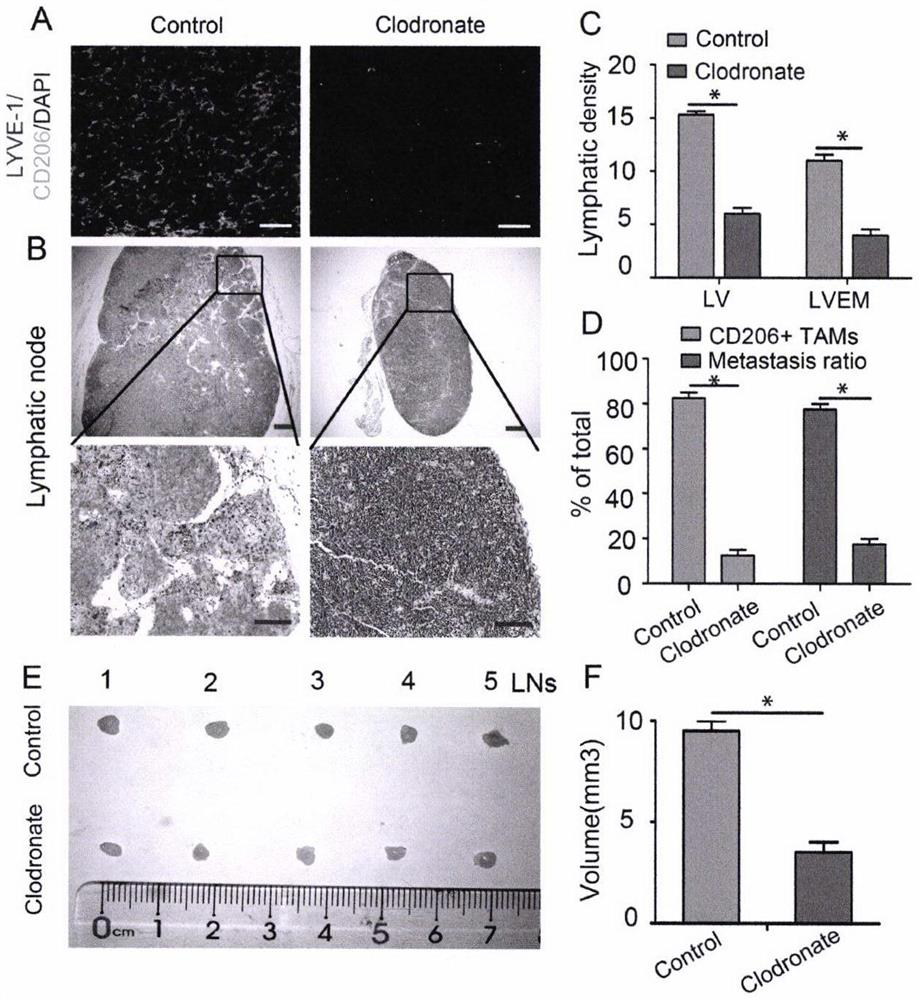
Detection and application of novel cervical cancer metastasis marker
PendingCN112067808AAccurate Auxiliary DiagnosisAccurate AssistDisease diagnosisNode metastasisLymphatic vessel
The invention belongs to the field of gene engineering, and discloses detection and an application of a novel cervical cancer metastasis marker. The cervical cancer metastasis marker is a lymphatic metastasis mode LVEM wrapped by tumor-associated macrophages, and is applied to diagnosis for distinguishing metastatic and non-metastatic early cervical cancers. Aiming at defects that an existing method for evaluating preoperative early cervical cancer lymph node metastasis is not high in sensitivity, has limitation on detection of micro-metastatic lymph nodes, is not high in timeliness and economic practicability, has false negative or false positive, is not high in sensitivity and specificity of the existing tumor marker and the like, based on a fact that an expression level of the LVEM canserve as an index for evaluating the lymph node metastasis state of a cervical cancer patient, the LVEM is developed as a marker, the expression level of the LVEM in cervical cancer tissue is detectedthrough a multicolor immunofluorescence technology, and the lymph node metastasis state of the cervical cancer patient can be more sensitively and specifically evaluated before an operation.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV (GUANGZHOU RESPIRATORY CENT)
MicroRNA markers for discriminating metastatic and non-metastatic squamous cell lung carcinoma
InactiveCN102851283BNucleotide librariesMicrobiological testing/measurementLung squamous cell carcinomaMicroRNA
The invention relates to 21 microRNA markers for discriminating metastatic and non-metastatic squamous cell lung carcinoma. More specifically, the invention relates to a class of microRNA markers for discriminating metastatic and non-metastatic squamous cell lung carcinoma. It has been proved by examination that, the specific microRNA markers can effectively discriminate metastatic and non-metastatic squamous cell lung carcinoma tissue. The invention also relates to a chip and a test kit for detecting the microRNA markers.
Owner:SHANGHAI INST OF ONCOLOGY
Vegf-specific antagonists for adjuvant and neoadjuvant therapy and the treatment of early stage tumors
Disclosed herein are methods of treating benign, pre-cancerous, or non- metastatic tumors using an anti-VEGF-specific antagonist. Also disclosed are methods of treating a subject at risk of developingbenign, pre-cancerous, or non- metastatic tumors using an anti-VEGF-specific antagonist. Also disclosed are methods of treating or preventing recurrence of a tumor using an anti-VEGF-specific antagonist as well as use of VEGF-specific antagonists in neoadjuvant and adjuvant cancer therapy.
Owner:GENENTECH INC
Medical eye protection paste
A medical eye protection paste comprises an isolated layer, a gel layer and a supporting layer, the gel layer is arranged between the isolated layer and the supporting layer. The medical eye protection paste has the use for lock-and-preserve moisture to protect eyes, the supporting layer loads the gel layer as a matrix, the isolated layer has the use for isolating the gel layer from the outside world and for the gel layer to be contaminated. According to the invention, the gel layer has the advantages of having good initial adhesion, permanent adhesion, non-metastatic and no wiredrawing, wherein setting time is suitable, the medical eye protection paste can guarantee that eyelid of patient is adhered, eyelash or eyebrow of patient is not adhered, and according to the need, suitable plastering agent is selected, structure that the medical eye protection paste is suitable for separating eyelid to avoid skin injury is provided.
Owner:HANGZHOU MEDZONE BIO-TECH CO LTD
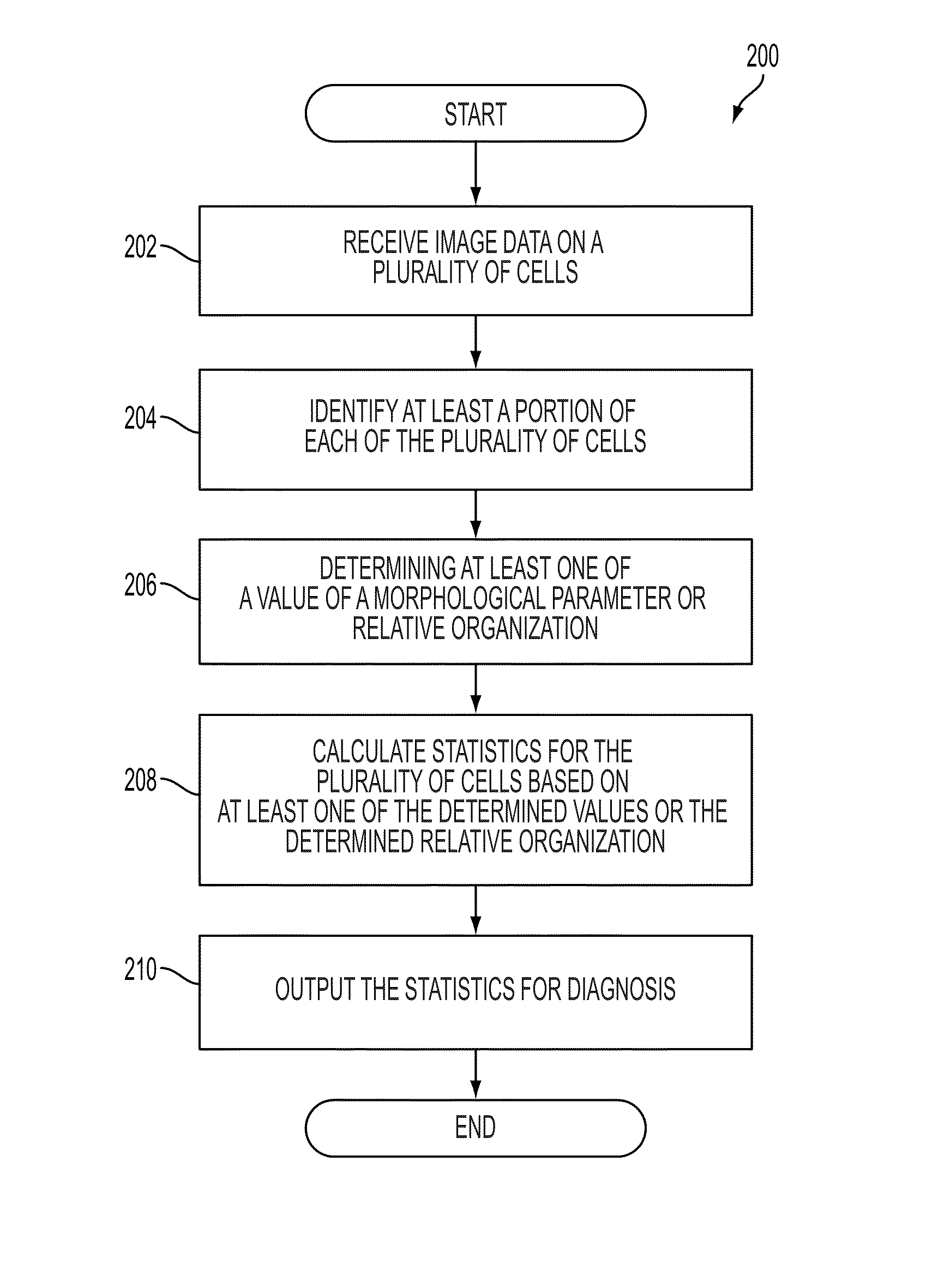
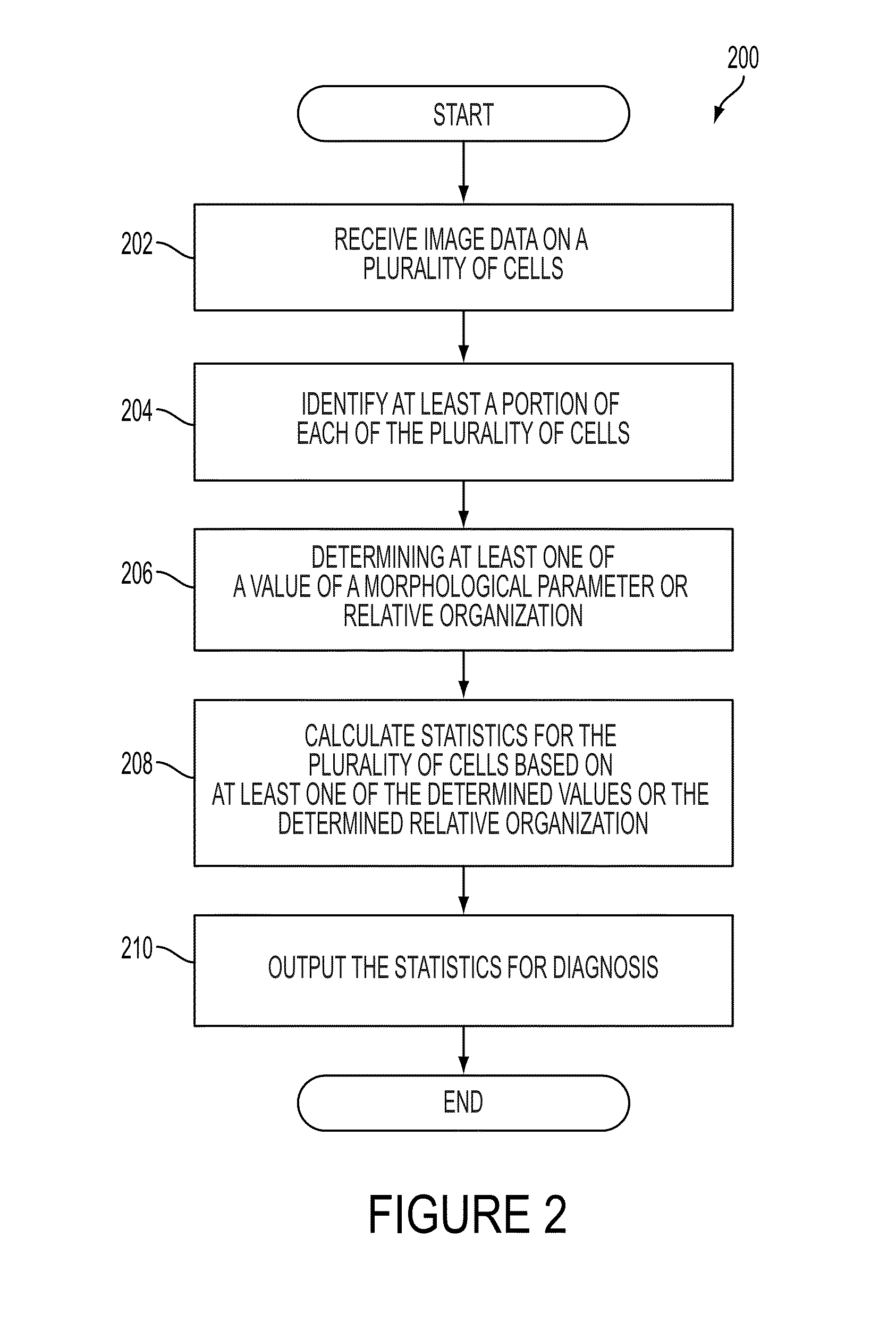
System and device for characterizing cells
A diagnostic device includes a microscope configured to obtain image data on a plurality of cells and a computing device. The computing device is configured to receive the image data, identify at least a portion of each of the plurality of cells based on the received image data, determine at least one of a value of a morphological parameter for each identified at least a portion of the plurality of cells or a relative organization among the identified at least a portion of the plurality of cells, and calculate statistics for the plurality of cells based on the at least one of the determined values of the morphological parameter or the determined relative organization, the statistics including information suitable for distinguishing metastatic cells from non-metastatic cells. The diagnostic device further includes an output device configured to output the statistics for diagnosis.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
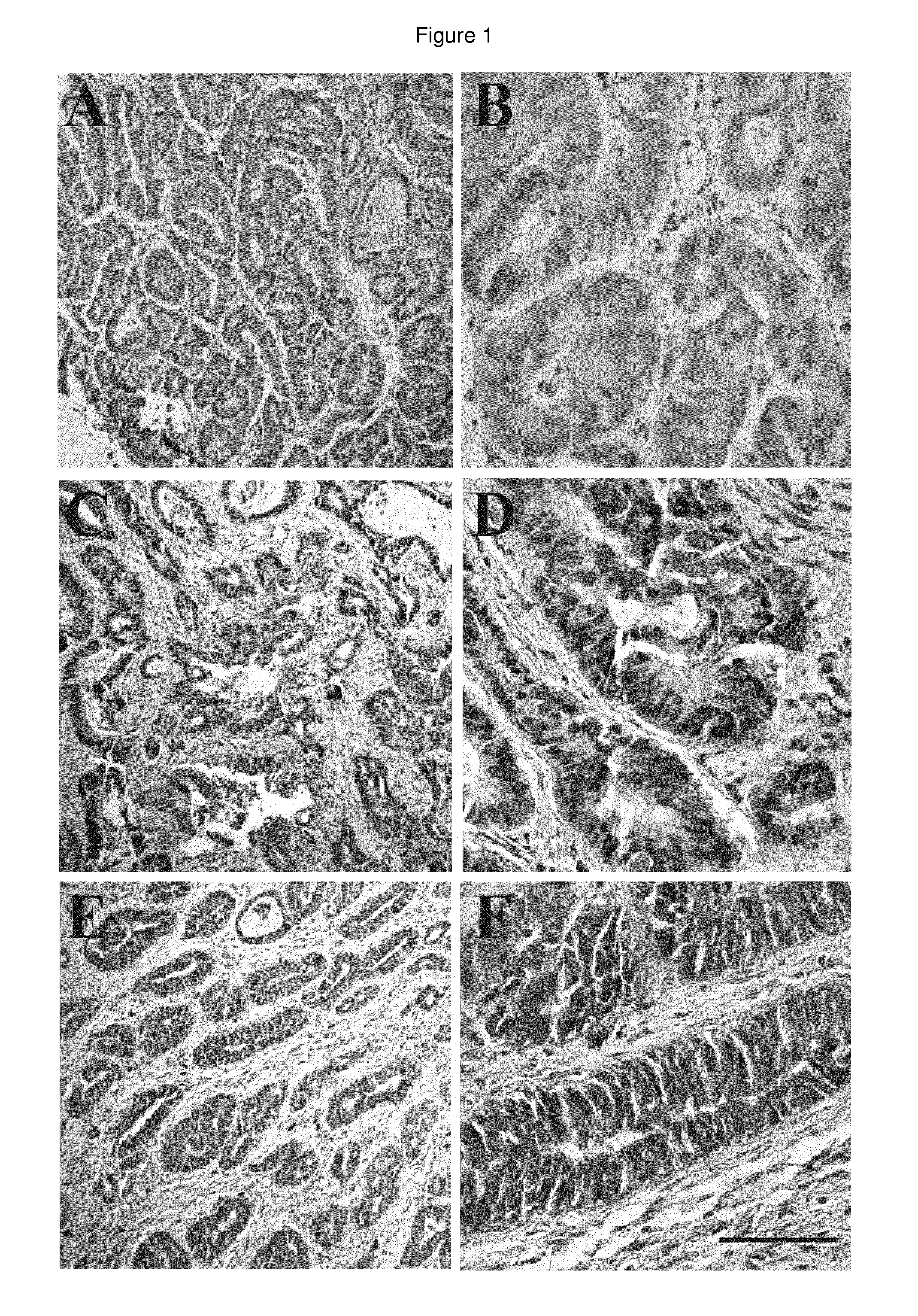
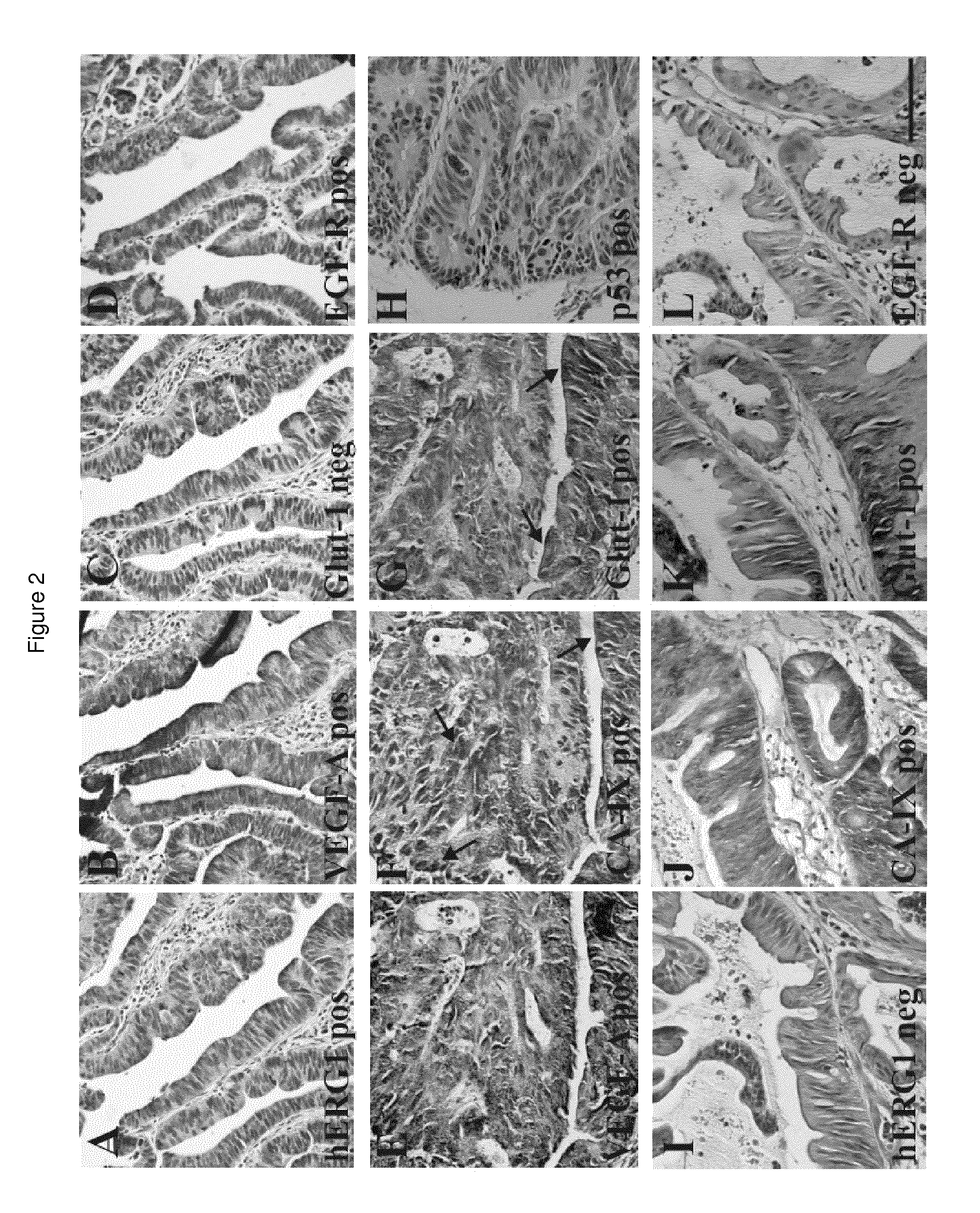
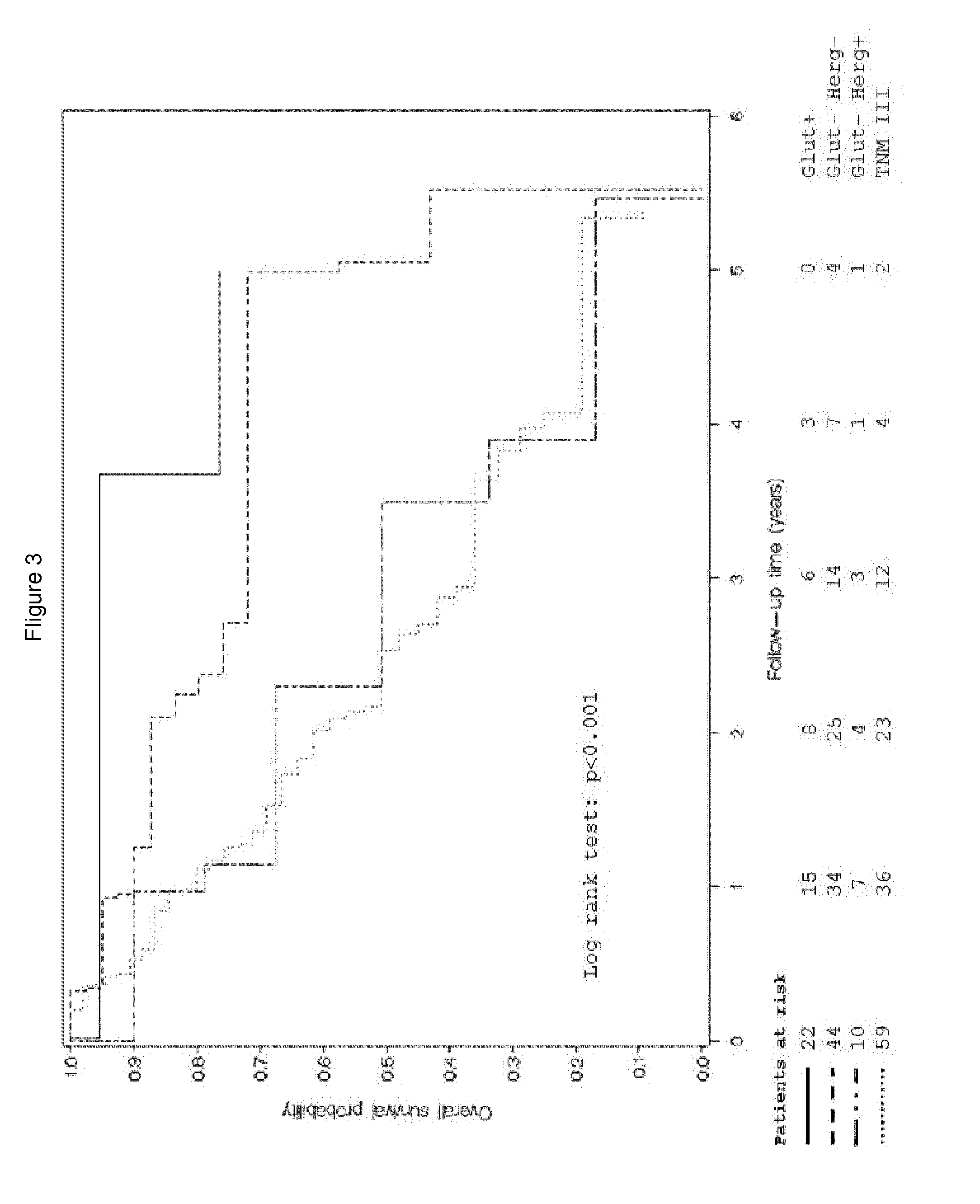
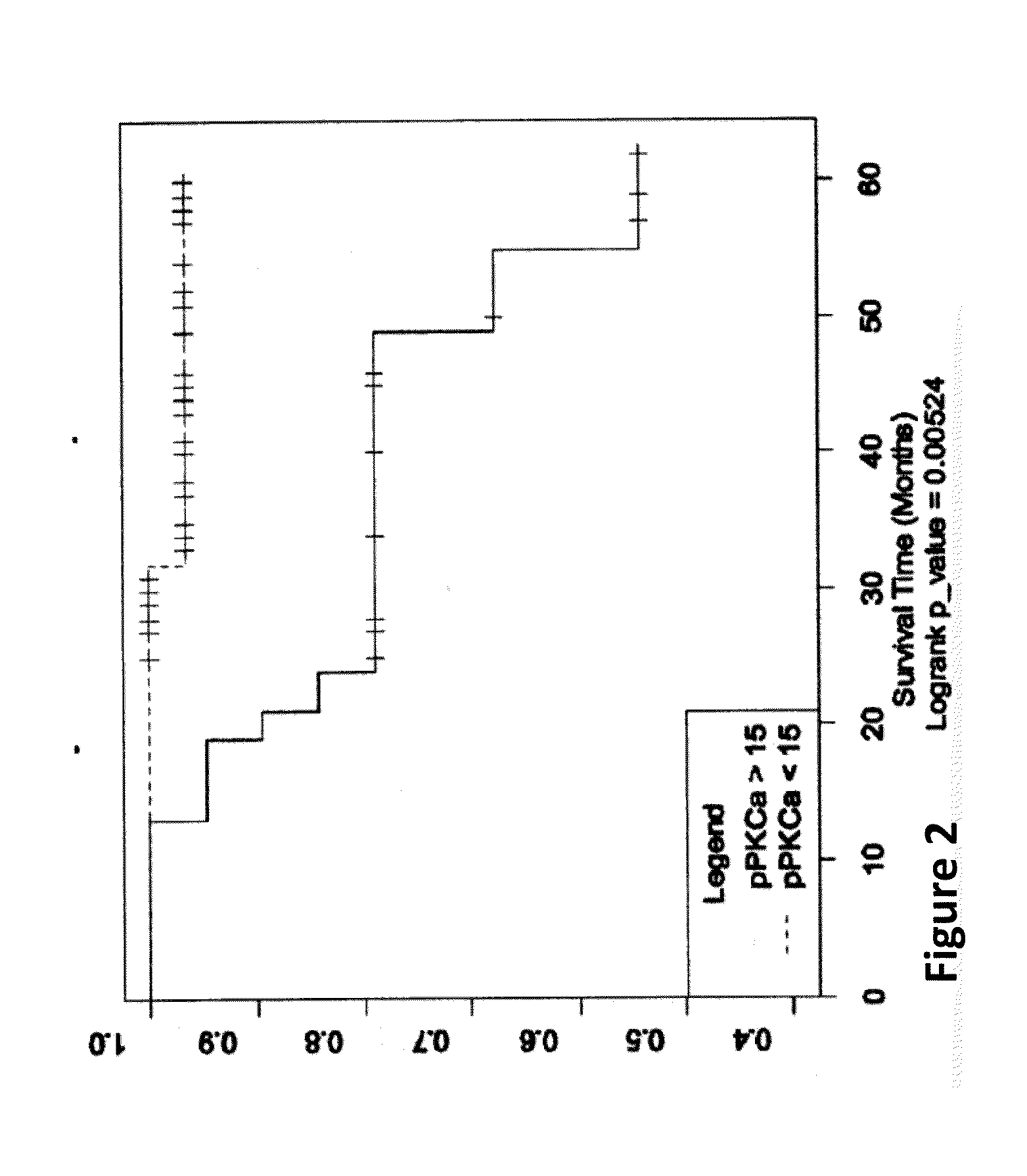
Herg1 and glut-1 in colorectal cancer
The present invention describes the determination of the prognostic impact of hERG1 potassium channels along with other hypoxia-related biomolecular parameters, in patients who underwent radical surgery with curative intent for non metastatic CRC.
Owner:UNIVERSITY OF FLORENCE
Kit for early warning of colorectal cancer metastasis
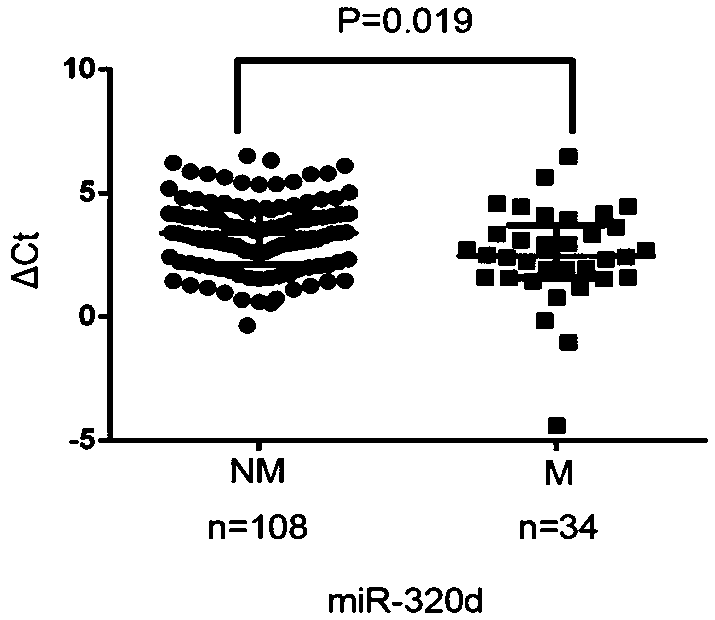
The invention relates to development for early warning of a colorectal cancer (CRC) metastasis kit based on exosome microRNA:miR-320d, and belongs to the field of medical treatment and public health.A biomarker for early warning of colorectal cancer metastasis is serum exosome miR-320d which includes a reverse transcription reaction system, a fluorescent quantitative PCR reaction system and an internal reference system. Compared with patients with non-metastatic colorectal cancer, the expression level of the miR-320d in the colorectal cancer metastatic serum exosome is increased significantly, ROC curve analysis shows that AUC is 0.633, the sensitivity is 62.0%, and the specificity is 64.7%.
Owner:谢丽
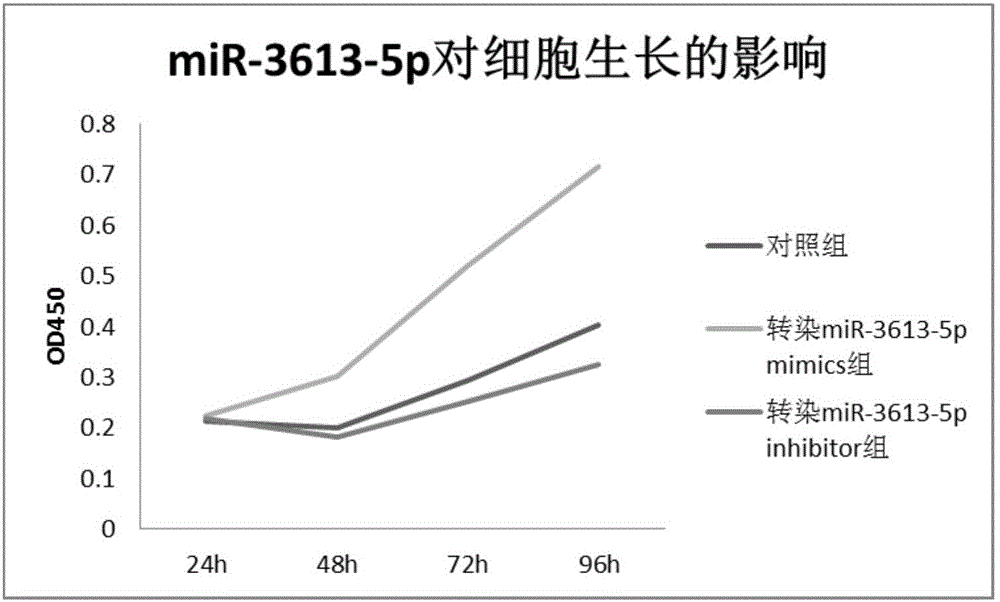
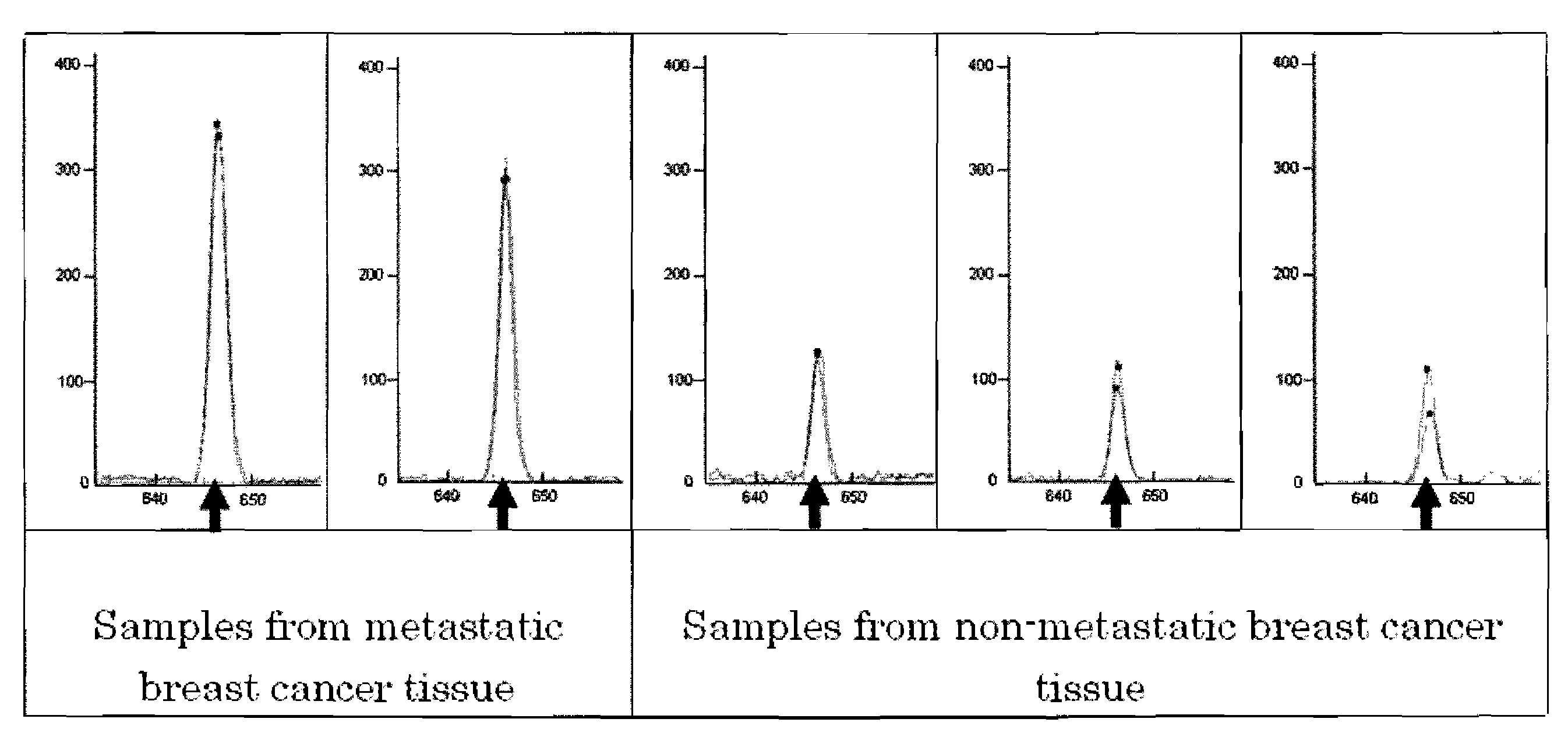
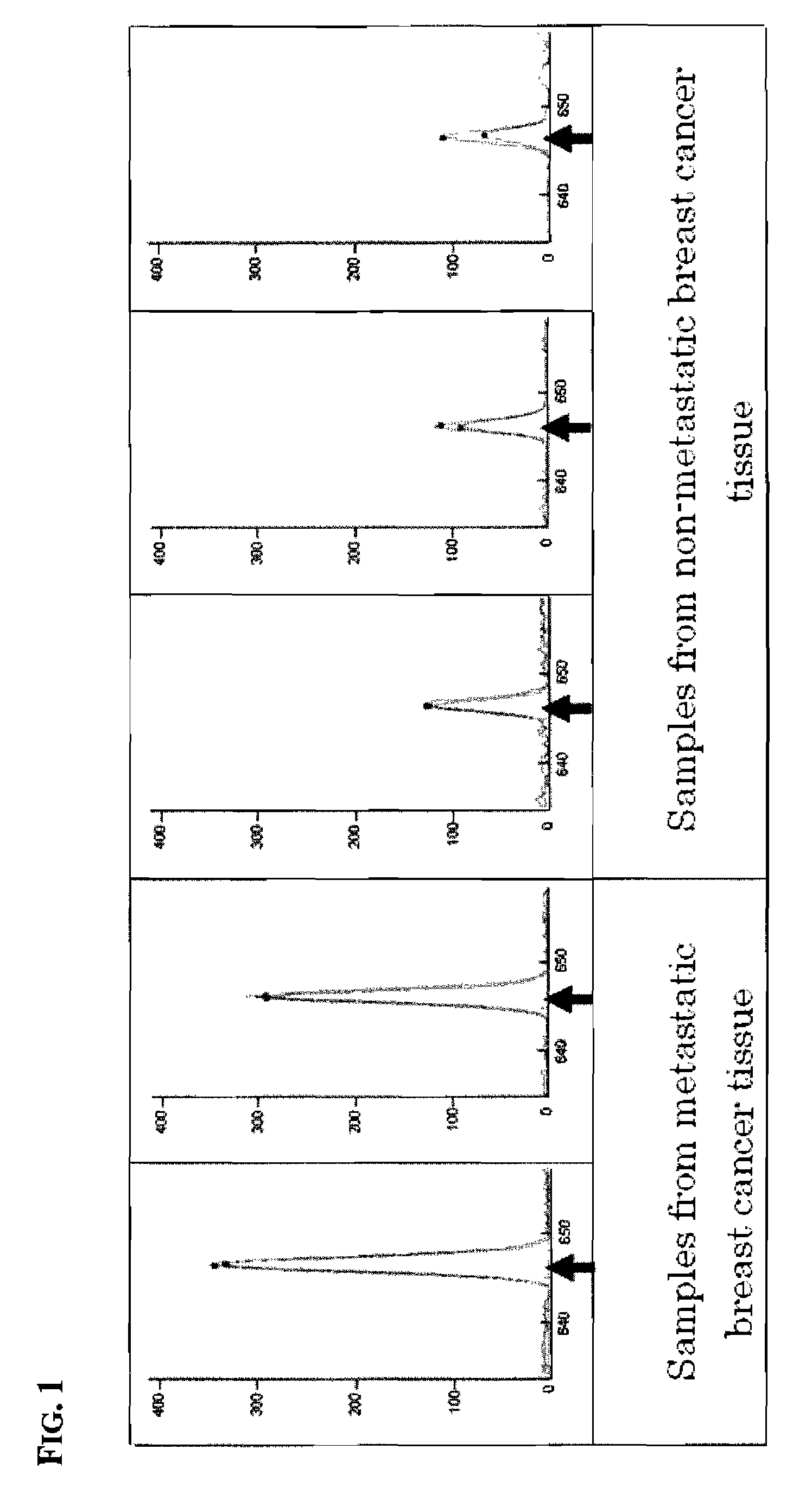
Novel application of mir-3613 and mature miRNA thereof
InactiveCN107435073AOrganic active ingredientsGenetic material ingredientsTranscriptome SequencingBiology
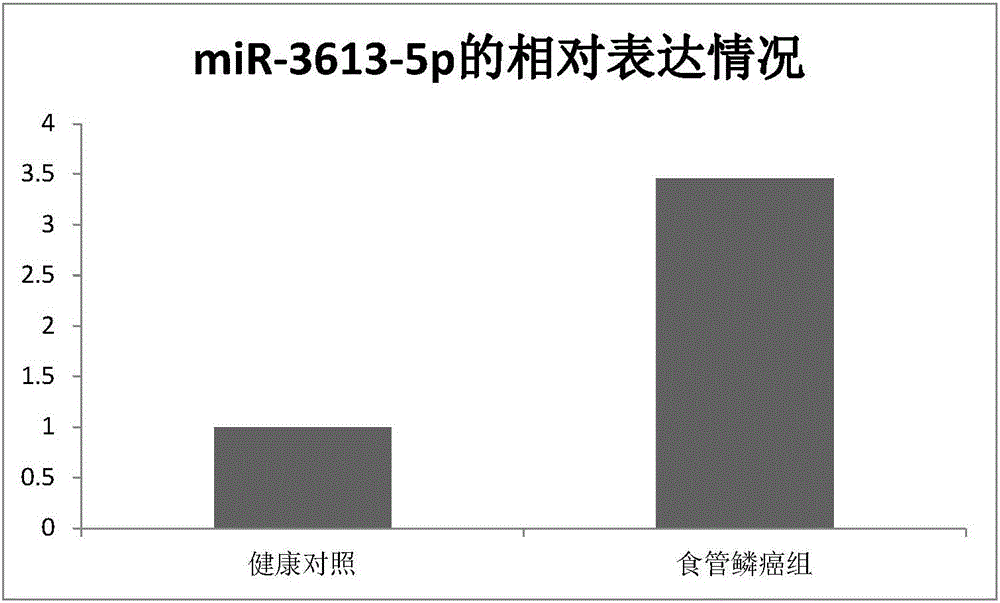
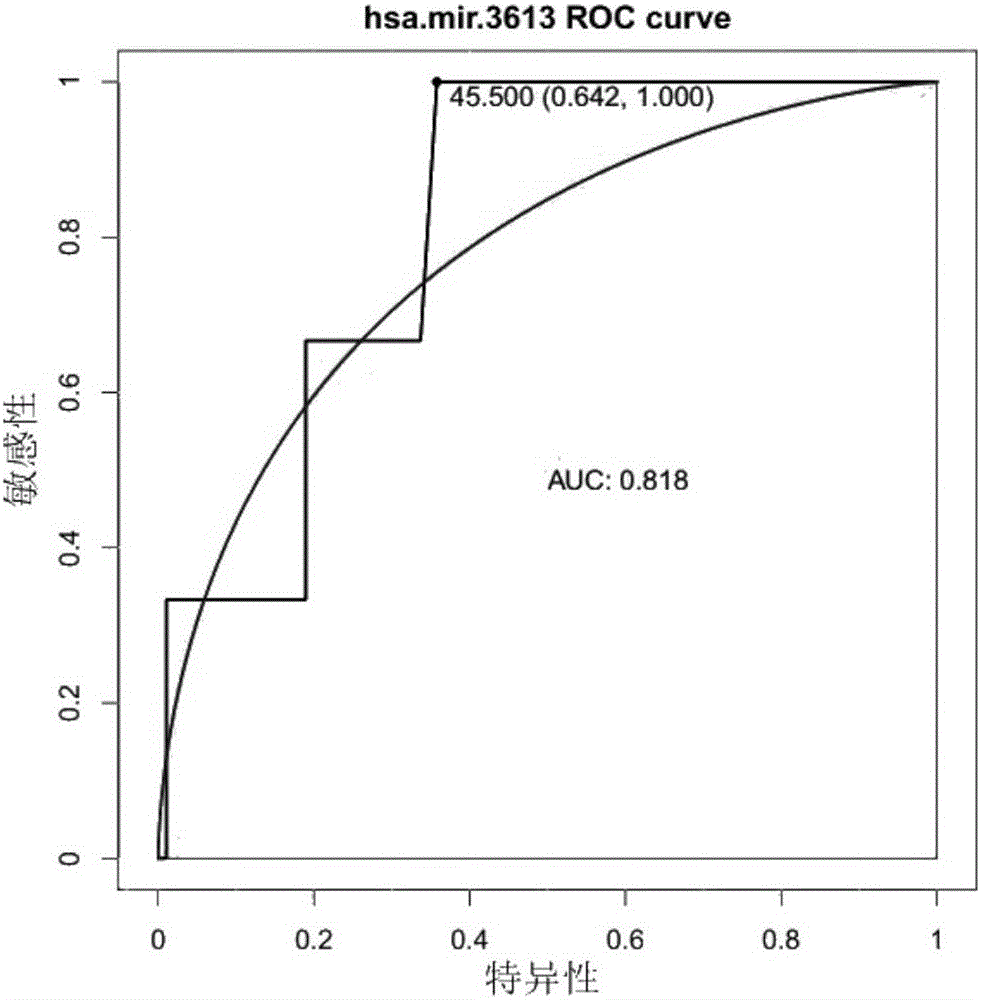
The invention relates to a novel application of mir-3613 and mature miRNA thereof. Based upon transcriptome sequencing on samples of metastatic esophageal squamous carcinoma tissues, non-metastatic esophageal squamous carcinoma tissues and para-carcinoma tissues, as well as analysis which is implemented in accordance with a bio-informatics method, some molecular markers related to esophageal squamous carcinoma are discovered, and the expression quantity of miR-3613-5p in the carcinoma tissues is higher than that of miR-3613-5p in the para-carcinoma tissues; and furthermore, a fluorescent quantitative PCR experimental result is consistent with a high-throughput sequencing result. The invention offers potential molecular markers for the precise diagnosis of the esophageal squamous carcinoma in the clinical field; and an important practical application value is achieved.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
Signal pathway alterations and drug target elevations in primary metachronous metastatic colorectal cancer compared to non-metastatic disease
The present invention relates to the identification and diagnostic use of biomarkers in primary colorectal cancer tumors whose activation level are predictive of the likelihood of the onset of metastatic disease. These biomarkers may be used to determine the suitability of a patient for aggressive and / or targeted treatments. Kits and compositions of the invention are also provided.
Owner:GEORGE MASON INTPROP INC +1
Genes for prognosis of cancer
InactiveUS20090011423A1Reduce riskImprove patient ' quality of lifeMicrobiological testing/measurementOncologyLymph node metastasis
To provide a novel method for determining the risk of lymph node metastasis of breast cancer uses as an index the difference in the expression levels of marker genes between metastatic breast cancer tissue (or cell) and non-metastatic breast cancer tissue (or cell). The method involves (1) measuring the expression level of a gene having a base sequence in human metastatic breast cancer tissue (or cell), (2) measuring the expression level of the same gene in human non-metastatic breast cancer tissue (or cell), and (3) comparing the expression level of (1) with the expression level of (2) and determine the risk of lymph node metastasis of breast cancer based on the difference between the expression levels.
Owner:MESSENGERSCAPE +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com