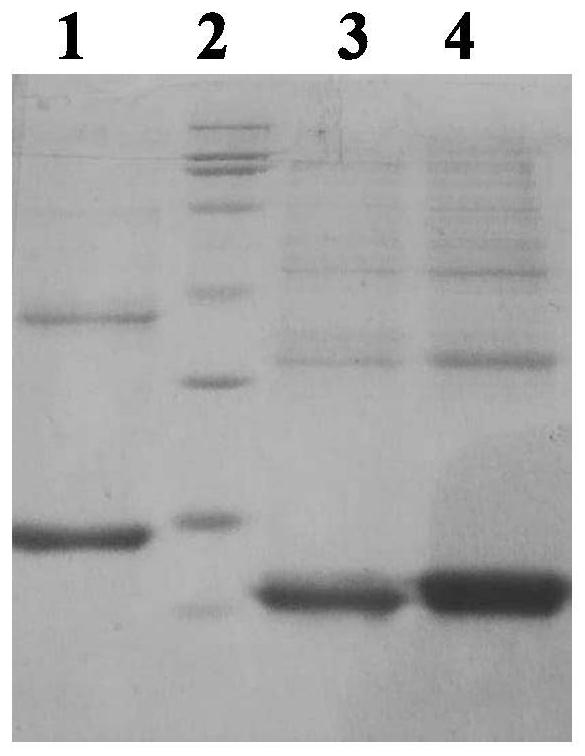
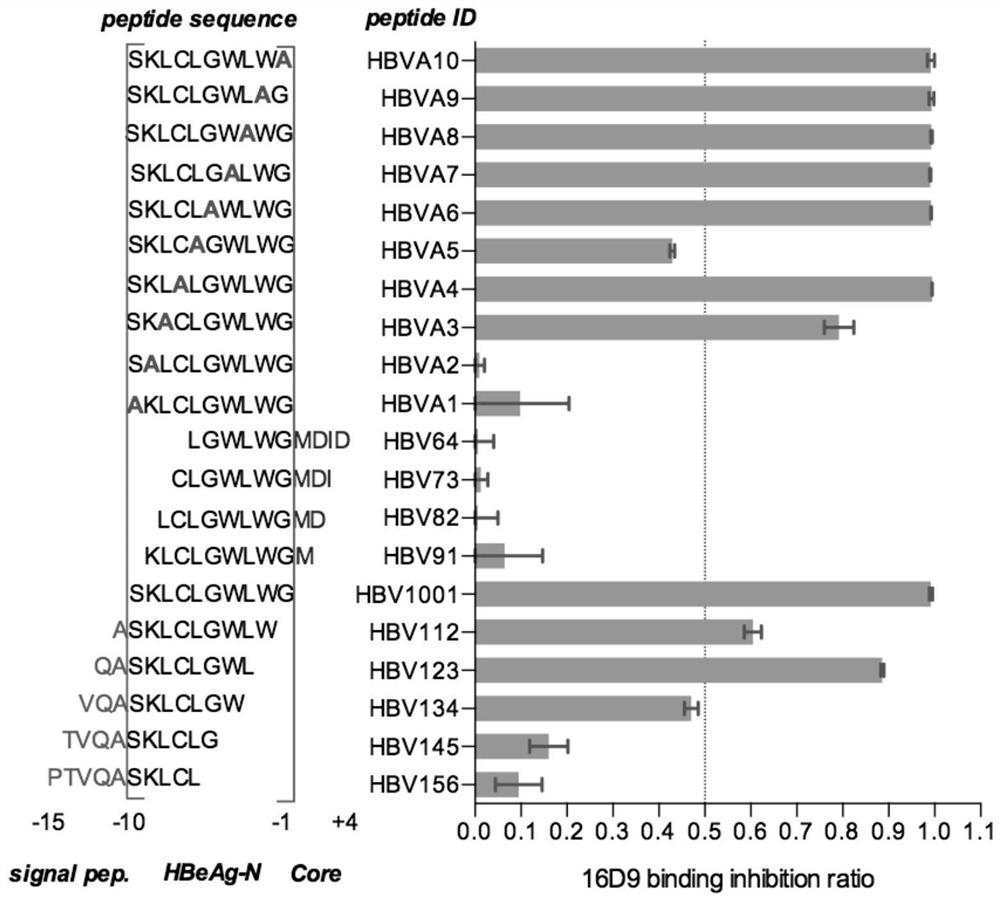
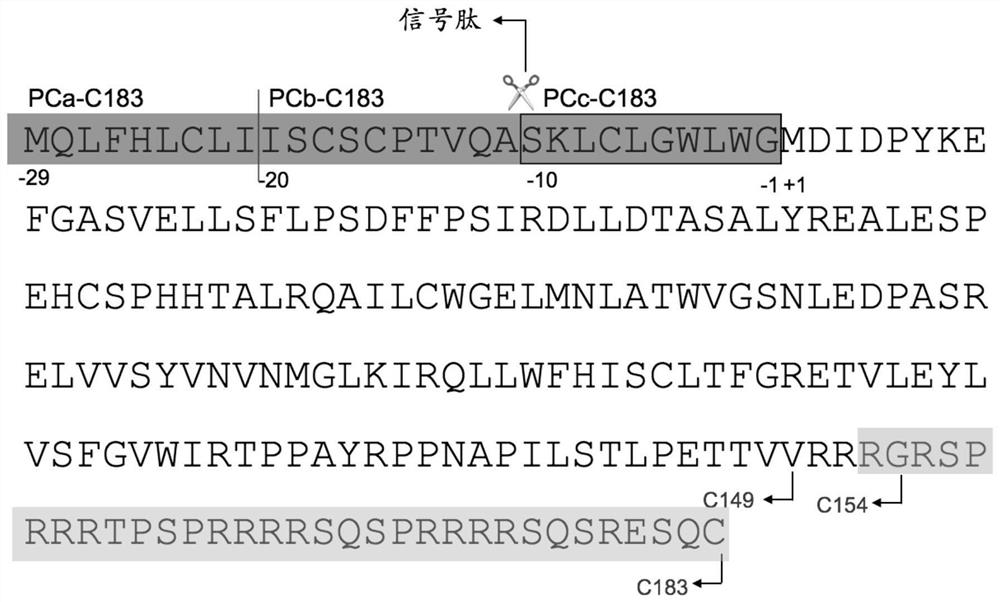
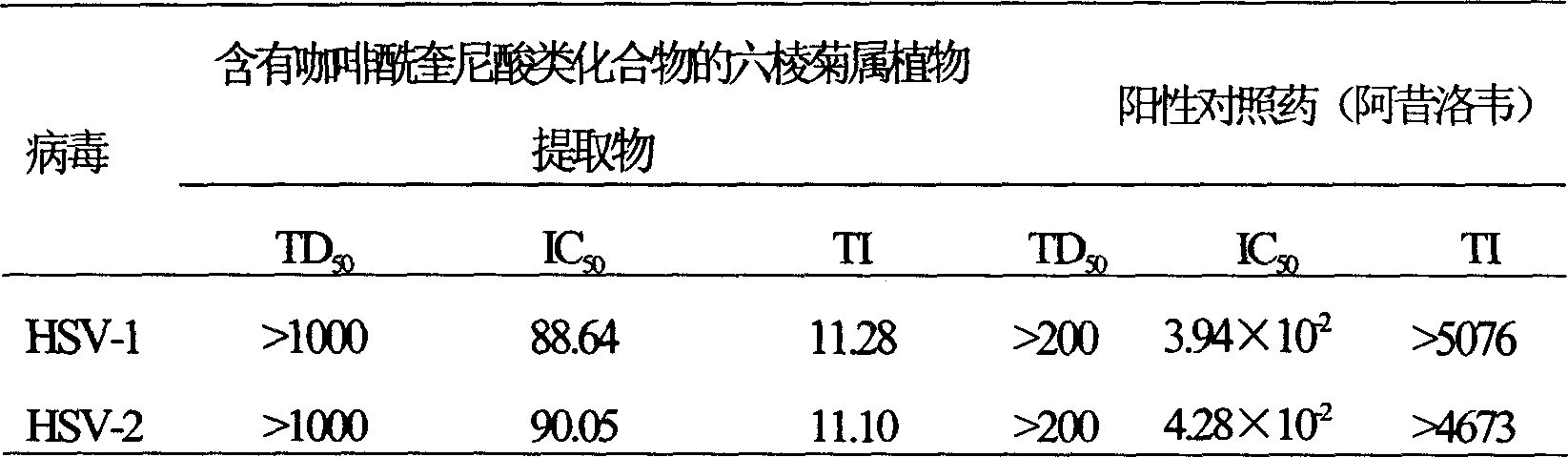
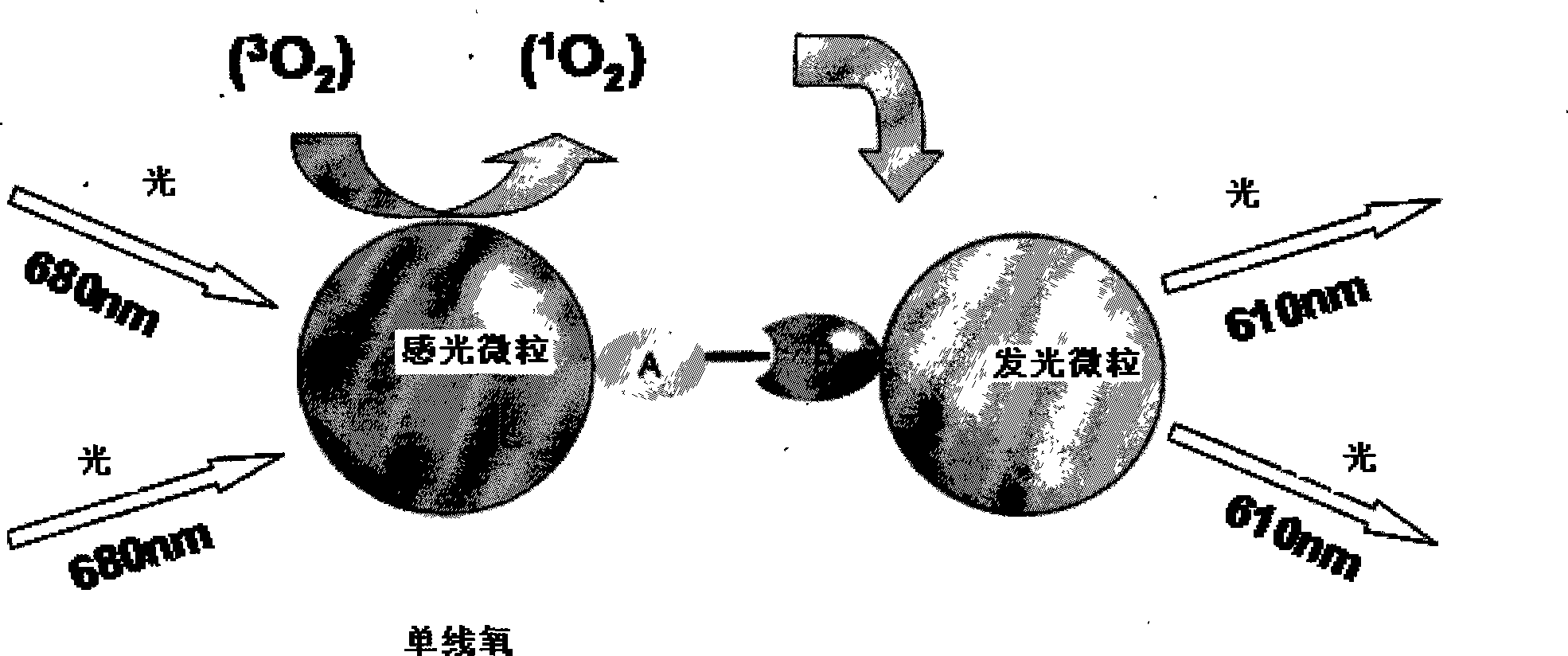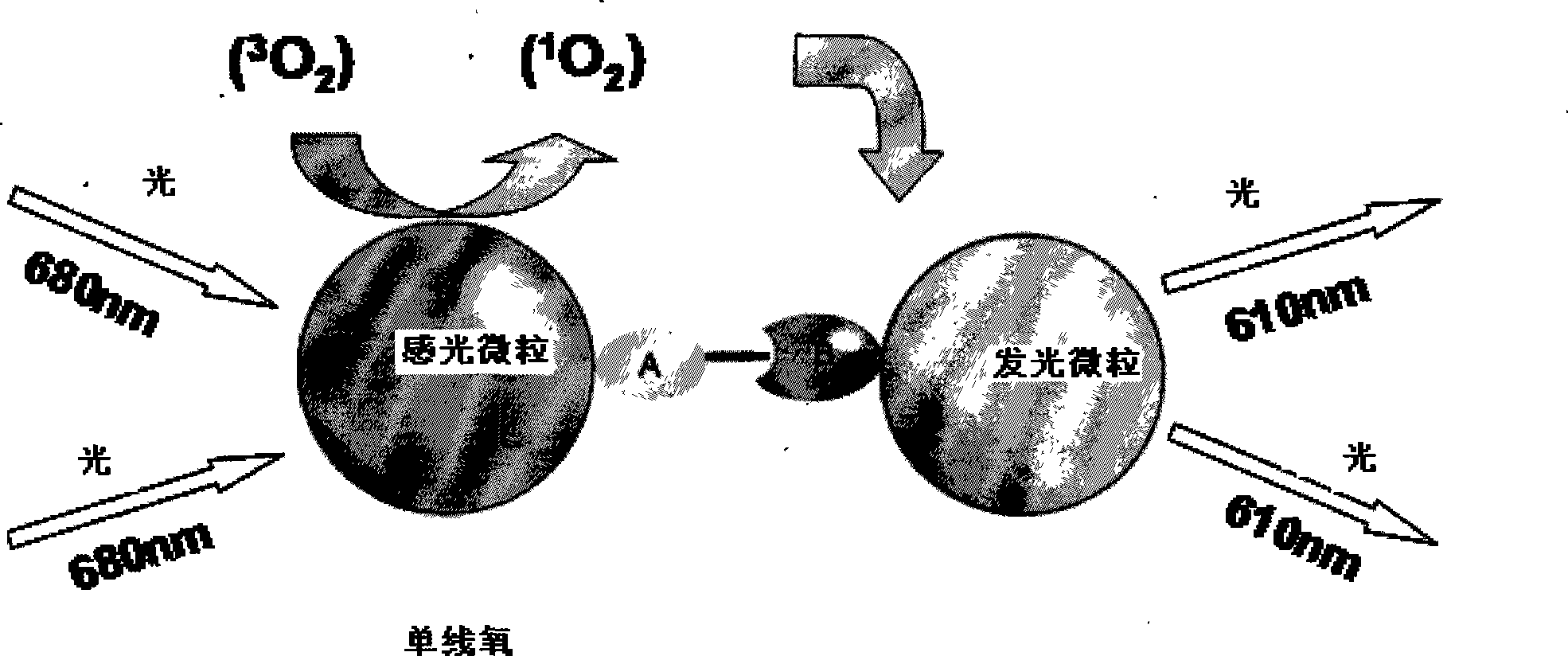Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "Hepatitis B virus e Antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
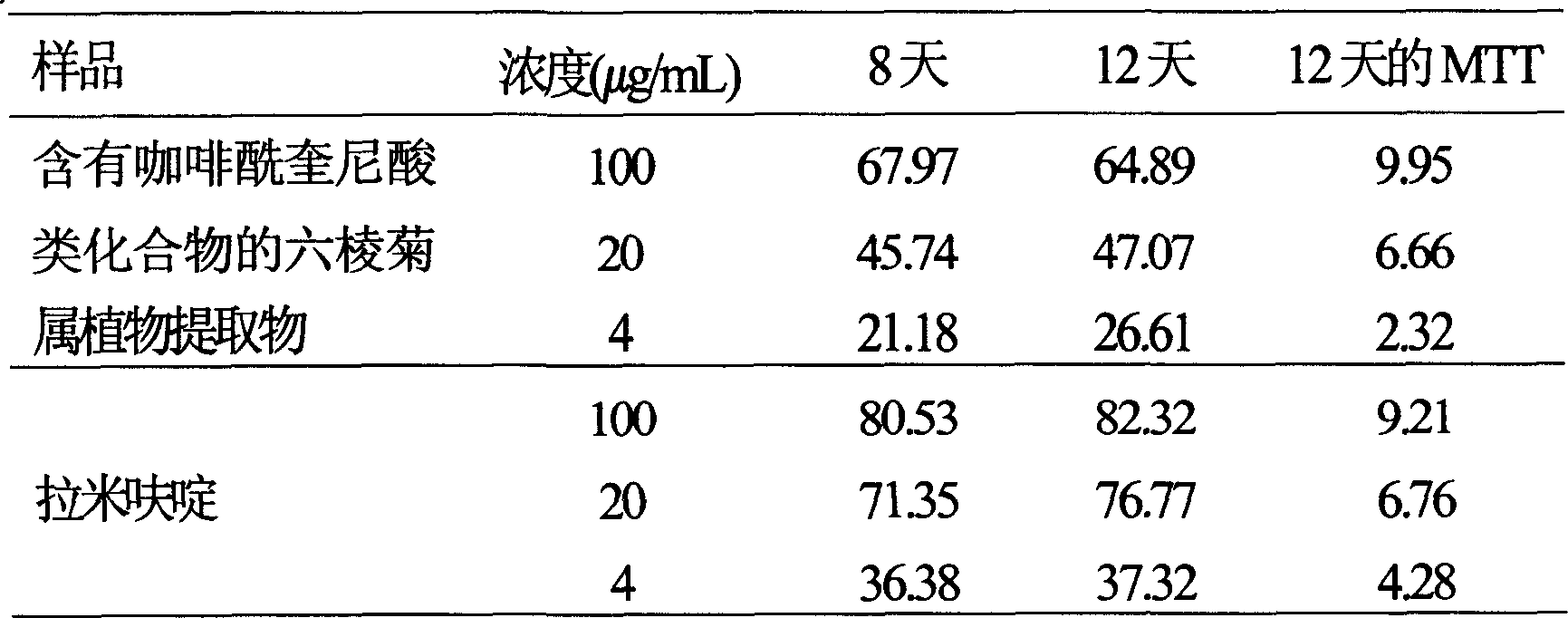
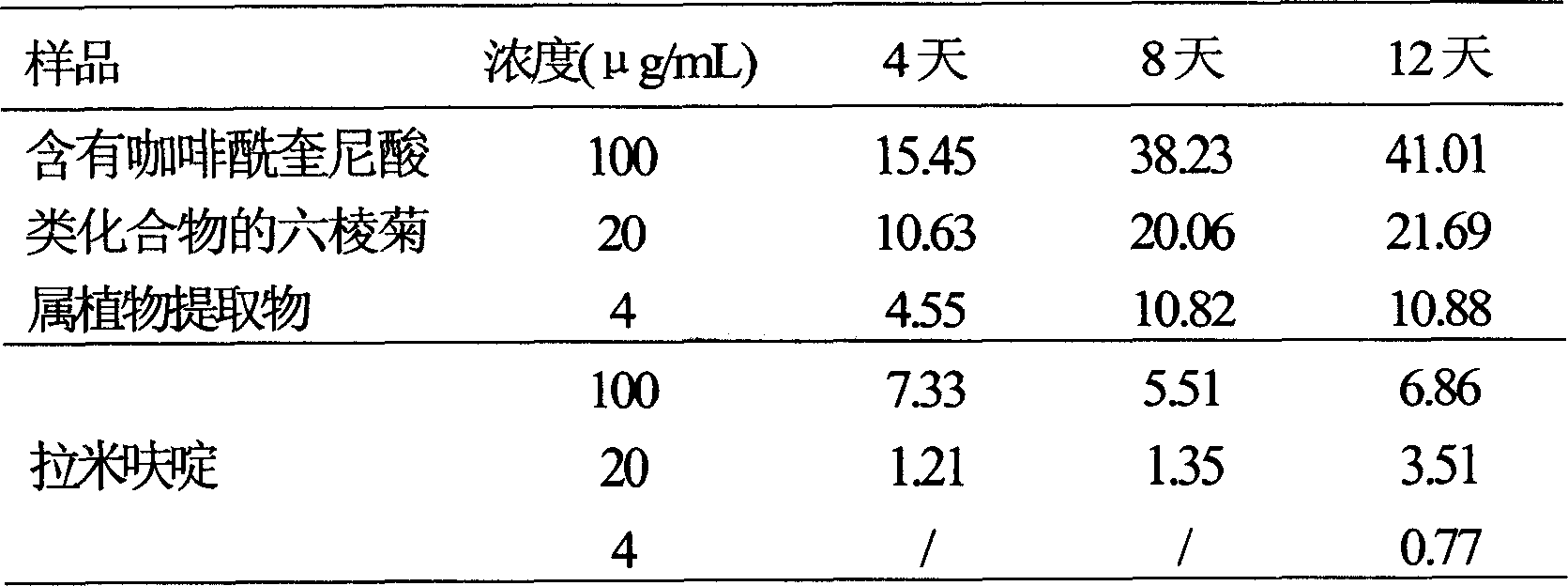
Use of laggera plant abstract in inhibiting herpes simplex virus and hepatitis B virus
InactiveCN1989989AReduced expression functionDigestive systemPharmaceutical delivery mechanismDiseaseCaffeoylquinic acid
The invention involves novel drug use of six-rowed chrysanthemum plant extracts which is used to treating herpes simplex virus (type 1 and / or type 2) and various disease caused by hepatitis B virus infection. The six-rowed chrysanthemum plant extracts is prepared by six-rowed chrysanthemum plant fresh or dry goods through the refining of alcohol-water extraction, column chromatography, alcohol solvent elution, the amount of caffeoyl guinic acid chemical compound is below 30%. The six-rowed chrysanthemum plant extracts prepared in the invention has significant function of inhibiting herpes simplex virus with type 1 (HSV-1), herpes simplex virus type 2 (HSV-2) and hepatitis B virus (HBV) replication, and can reduce effectiveness of HBV e antigen (HBeAg) in the HepG 2.2.15 cell lines, it can be used for treatment various disease caused by said correlate virus infection.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Hepatitis B virus e antigen testing corpuscle, preparation and application thereof
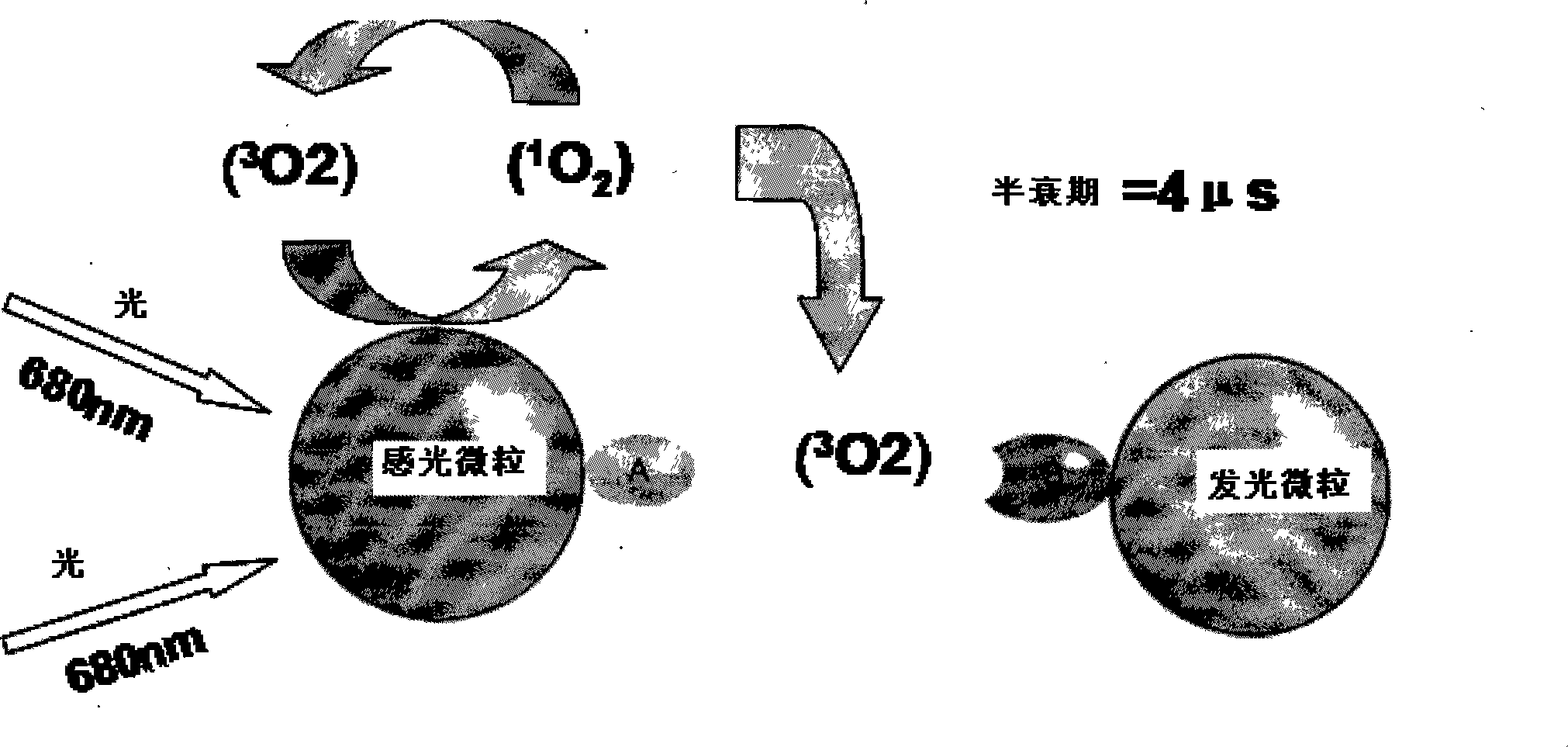
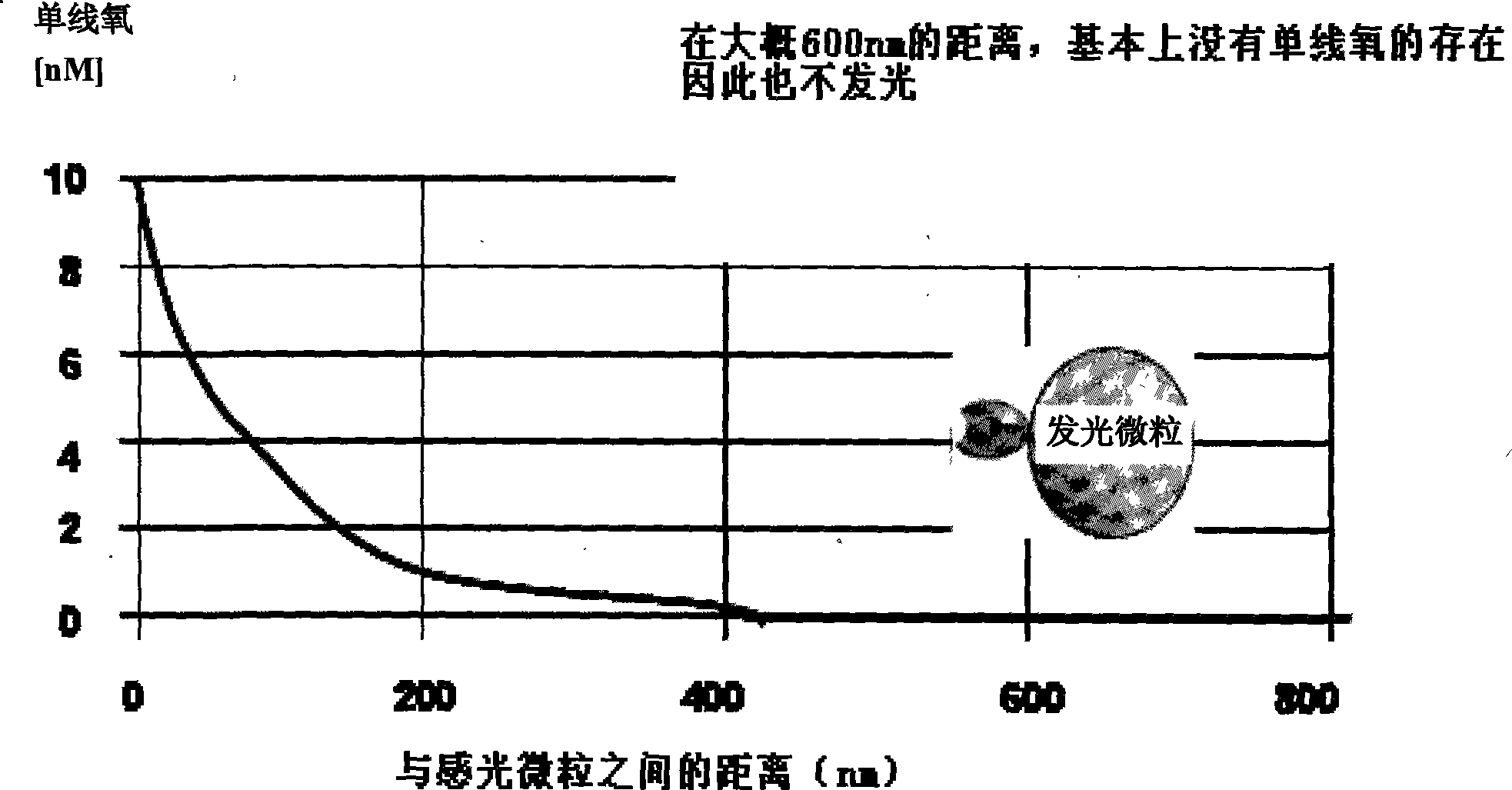
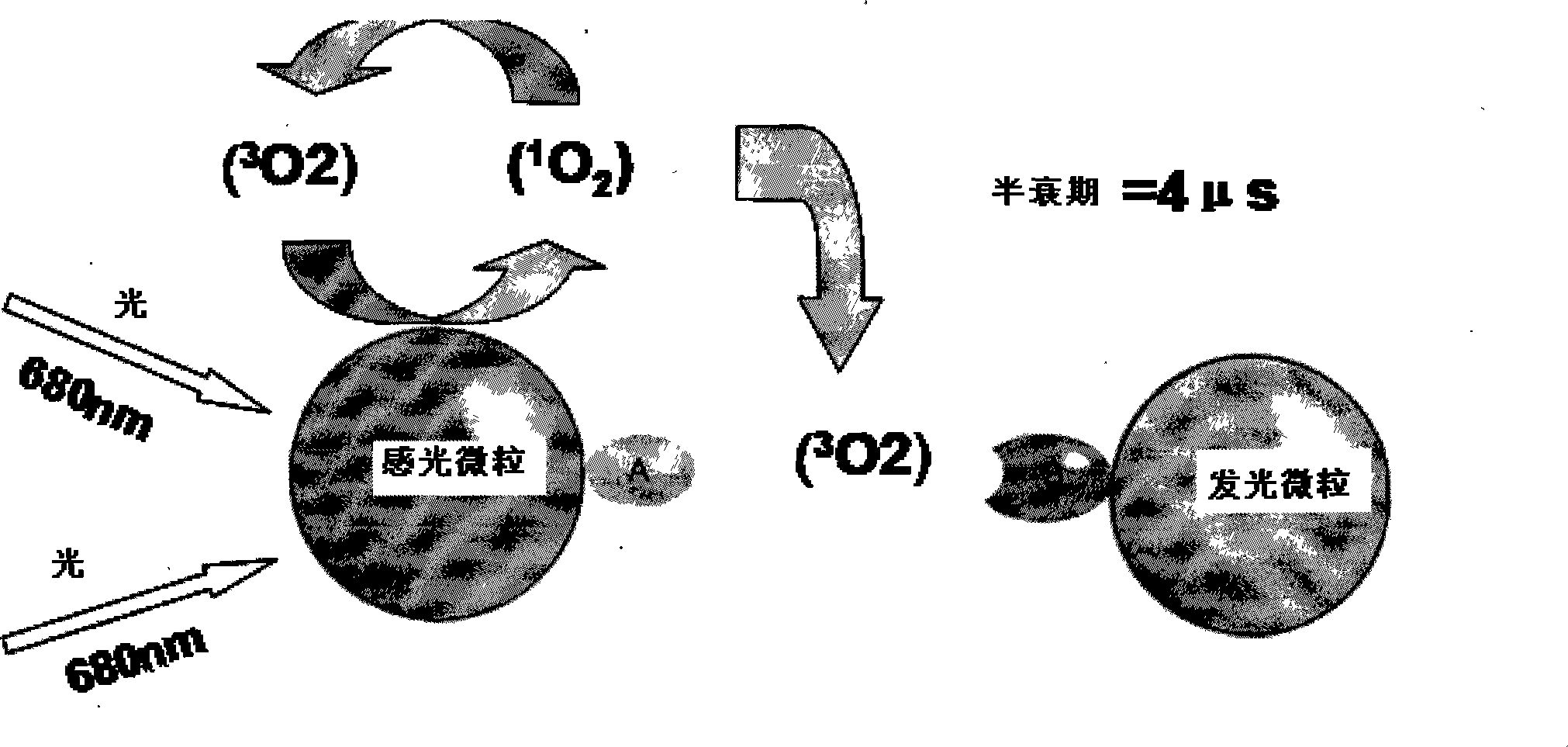
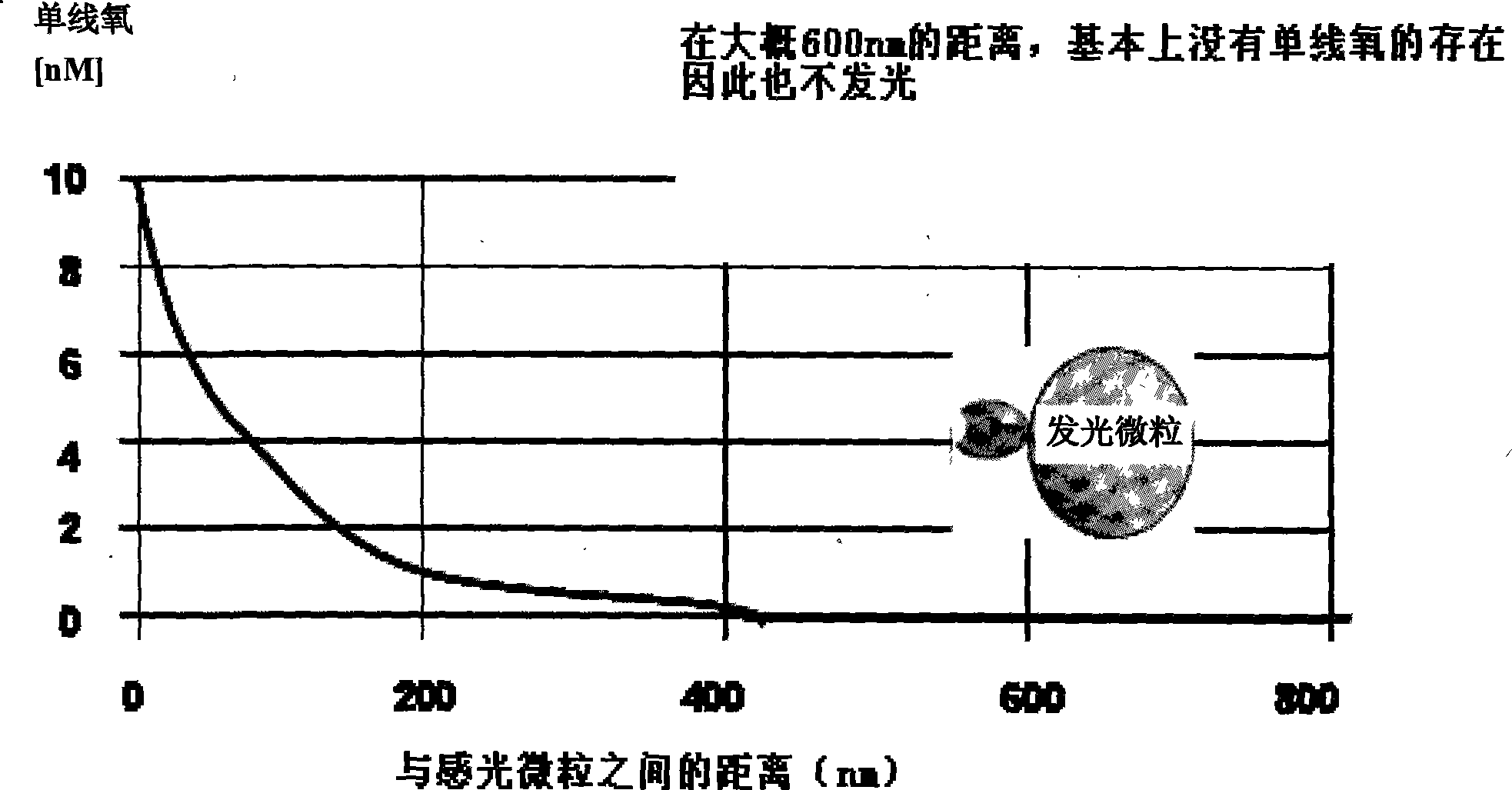
ActiveCN101251540AWide detection rangeReduce sensitivityAnalysis by material excitationAntigenAnti hbe
The invention relates to a diagnosing reagent for hepatitis B, disclosing detection grains for e antigens of the hepatitis B virus, which are of the luminous grains coated by anti-HBE antibodies. The invention also discloses preparation and application for the detection grains for e antigens of the hepatitis B virus; moreover, the invention further discloses an outside-body diagnosis reagent box for detecting e antigens of the hepatitis B in a blood serum sample of human beings as well as a method for utilizing the light excitation chemiluminescence principle to quantitatively and qualitatively detect e antigens of the hepatitis B virus. The reagent box of the invention can be jointly used to diagnose the individual acute or chronic hepatitis B together with other blood serums and clinic information, and screen the hepatitis B for women in the perinatal period so as to judge the risk of newborn babies contaminating the hepatitis B.
Owner:BEYOND DIAGNOSTICS (SHANGHAI) CO LTD
Application of imperatorin in preparing medicament for preventing and treating hepatitis or liver injury
ActiveCN101904839AEasy to manufactureWide variety of sourcesDigestive systemAntiviralsHepatitis B Surface AntigensVirus
The invention relates to the technical field of medicament, providing a novel application of coumarin compound imperatorin in preparing a medicament for preventing and treating virus hepatitis and resisting immunological liver injury or chemical liver injury. Pharmacological tests prove that the imperatorin has stronger activity of inhibiting hepatitis B surface antigen (HbsAg) and hepatitis B virus e antigen (HbeAg) and resisting the immunological liver injury or the chemical liver injury, so the imperatorin can be used for preparing the medicament for preventing and treating the virus hepatitis and resisting the immunological liver injury or the chemical liver injury. The imperatorin has the advantages of convenient preparation, wide sources, low price and high safety. The invention provides the novel application of the imperatorin, and provides a new medicinal source for preventing and treating the virus hepatitis and resisting the immunological liver injury or the chemical liver injury.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Hepatitis B e antigen single quality control product and preparation process thereof
InactiveCN108562754ASufficient sourceEasy to getBiological material analysisBiological testingLaboratory orderThird party
The invention relates to the field of quality control of medical examination, in particular to the preparation of a single quality control substance of a hepatitis B e antigen. The hepatitis B virus eantigen is diluted to a required value by using a specific stabilizer to prepare the quality control product. The preparation process is simple, the production cost is low, added substances and contents are controllable, the product belongs to a liquid type, redissolution is not required, and the composition is stable. Through a biological traceability link program and reference method assignmentof the program, the product can be used as a third-party medical laboratory quality control product during the validity period to meet the needs of clinical infectious disease testing.
Owner:河北睿达模生物科技有限公司
Pure natural functional drink suitable for patient with chronic hepatitis B and preparation method thereof
InactiveCN105494757AGood for healthPlay a preventive roleTea extractionPlant ingredientsAsian ginseng extractFlavouring agent
The invention discloses a pure natural functional drink suitable for a patient with chronic hepatitis B and a preparation method thereof. The pure natural functional drink is prepared from the following components in parts by weight: 0.5 to 2 parts of green tea extract, 0.1 to 0.5 part of grape skin extract, 0.1 to 0.5 part of Chinese yam extract, 0.05 to 0.2 part of Asian ginseng extract, 0.05 to 0.2 part of wild chrysanthemum flower extract, 0.05 to 0.2 part of ginkgo leaf extract, 0.5 to 2 parts of natural flavouring agent, 0.5 to 2 parts of natural sweetening agent, and the balance of water. The invention also discloses the preparation method of the pure nature functional drink for patient with chronic hepatitis B. Tests prove that if the patient with chronic hepatitis B drinks the pure natural functional drink for a long time, the effect of shifting hepatitis B virus e antigen to Yin can be achieved, which can help the patient with chronic hepatitis B to recover his / her health.
Owner:BIO TECH ACADEMY CHINA
Hepatitis B e antigen immune escaping detecting gene chip and kit thereof
ActiveCN101333559AAvoid missing detectionJudgment of infectivityMicrobiological testing/measurementAntigenLeak detection
The invention provides a hepatitis B virus e antigen immune evasion detection gene chip and a kit, belonging to the gene chip technique field of clinical detection, and comprising: a gene chip of a specific DNA probe which detects hepatitis B virus e antigen immune evasion and is fixed on a solid phase carrier as well as a matched reagent; the matched reagent includes a treating fluid for treating serum samples, an amplifying solution for amplifying and labeling the treated samples, a hybridization solution in hybridization reaction with a micro-array, a cleaning solution, an enzyme-labeled working solution and a detection liquid; and the matched reagent and the hepatitis B virus e antigen immune evasion detection gene chip are used jointly in the detection process of the serum samples. The chip and the kit can detect the hepatitis B virus e antigen immune evasion, and avoid the clinical hepatitis B virus e antigen leak detection, thereby being favorable for judging the infectivity and conditions after curing of hepatitis B patients.
Owner:上海裕隆生物科技有限公司
Traditional Chinese medicinal preparation for conditioning and treating chronic hepatitis B
ActiveCN104491574ASymptoms improvedSigns improvedDigestive systemPharmaceutical delivery mechanismAnti hbeHouttuynia
Owner:LANZHOU GUCHI BIO TECH
Nanometer magnetic particle chemiluminescence detection kit for hepatitis B virus e antigen as well as preparation method thereof and detecting method thereof
The invention relates to a nanometer magnetic particle chemiluminescence detection kit for a hepatitis B virus e antigen as well as a preparation method thereof and a detecting method thereof. The kit comprises a solution, which contains a fluorescein labeled hepatitis B virus e antigen antibody, a suspension, which is coated with magnetic particles of a fluorescein antibody, and a solution, which contains an alkaline phosphatase labeled hepatitis B virus e antigen antibody. According to the invention, the hepatitis B virus e antigen can be quantitatively detected with lower cost, higher accuracy and higher precision.
Owner:SUZHOU HAOOUBO BIOPHARML
Hepatitis B virus e antigen quantitative detection kit, preparation method and detection method thereof
InactiveCN105572358AHigh detection sensitivityStrong specificityMaterial analysisAntigenHepatitis B immunization
The present invention discloses a hepatitis B virus e antigen detection kit, which comprises a hepatitis B e antigen calibration substance, an anti-HBe monoclonal antibody coated plate, europium labeled anti-HBe monoclonal antibody, an assay buffer liquid, a fluorescence enhanced liquid and a concentrated washing liquid. The present invention further discloses a preparation method of the kit, and a method for detecting hepatitis B virus e antigen by using the kit. According to the present invention, the kit has advantages of high detection sensitivity, good specificity, simple operation, no pollution, and low cost, can be used for quantitative detection of the HBeAg content in human serum / plasma, and provides precise reference for the clinical hepatitis B individualized management treatment program.
Owner:SUZHOU SYM BIO LIFESCI CO LTD
Application of dehydrocorydaline and scoulerine to medicament production
InactiveCN102078319BGood effectSignificant anti-HBV activityOrganic active ingredientsAntiviralsHepatitis b e antigenHepatitis B Surface Antigens
The invention discloses application of dehydrocorydaline and scoulerine to medicament production. In the invention, a diagnostic kit for hepatitis B virus surface antigen and a diagnostic kit for hepatitis B virus e antigen are adopted to perform tests of resisting hepatitis B surface antigen and hepatitis B e antigen on protoberberine alkaloids scoulerine and dehydrocorydaline respectively. Results prove that the dehydrocorydaline and the scoulerine have remarkable hepatitis B virus resisting activity, and can be used for preparing hepatitis B virus resistant medicament.
Owner:SUN YAT SEN UNIV
Monoclonal antibody for resisting hepatitis B virus e antigen and application thereof
ActiveCN113717283ASensitive highHigh precisionBiological material analysisImmunoglobulins against virusesAntigenHepatitis B immunization
The invention relates to the fields of immunology and molecular virology, in particular to the fields of diagnosis, prevention and treatment of hepatitis B virus. Specifically, the invention relates to a monoclonal antibody of the hepatitis B virus e antigen, and also relates to a detection reagent (especially a chemiluminescence method) of the hepatitis B virus e antigen.
Owner:XIAMEN INNODX BIOTECH CO LTD +1
Method of preparing extract of Mallotus apelta and uses for fighting hepatitis B virus
InactiveCN101584702ASignificant anti-HBV effectAbundant resourcesOrganic active ingredientsDigestive systemDiseaseBULK ACTIVE INGREDIENT
The invention relates to extract activity of Mallotus apelta and uses in prepartion of medicine for treating related diseases caused by hepatitis B. The extract of Mallotus apelta provided by the invention has basic components of apiolin, apiin-7-O-belta-D-glucoside, and flavonoids compounds of 5,7-dihydroxy-6-isopentene group-4'-methoxyl flavonone, flavonoids compounds Mallotus apelta have obvious inhibiting effect on hepatitis B virus surface antigen and hepatitis B virus e antigen ono surface of HepG 2215, and cabaple of inhibiting duplication of Duck Hepatitis B Virus deoxyribonucleic acid, withdraw rebound is weaker than positive reference medicine lamivudine, and hepatitis B is provided with a subsequent inhibiting effect after withdraw. The medicine according to the invention has strong function for fighting hepatitis B virus, clear active ingredient, which is suitable for industrial production, predictablly used for preparing medicine of preparing diseases infected by hepatitis B virus.
Owner:广西中医学院
Use of laggera plant abstract in inhibiting herpes simplex virus and hepatitis B virus
InactiveCN1989989BReduced expression functionDigestive systemPharmaceutical delivery mechanismDiseaseCaffeoylquinic acid
The invention involves novel drug use of six-rowed chrysanthemum plant extracts which is used to treating herpes simplex virus (type 1 and / or type 2) and various disease caused by hepatitis B virus infection. The six-rowed chrysanthemum plant extracts is prepared by six-rowed chrysanthemum plant fresh or dry goods through the refining of alcohol-water extraction, column chromatography, alcohol solvent elution, the amount of caffeoyl guinic acid chemical compound is below 30%. The six-rowed chrysanthemum plant extracts prepared in the invention has significant function of inhibiting herpes simplex virus with type 1 (HSV-1), herpes simplex virus type 2 (HSV-2) and hepatitis B virus (HBV) replication, and can reduce effectiveness of HBV e antigen (HBeAg) in the HepG 2.2.15 cell lines, it can be used for treatment various disease caused by said correlate virus infection.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Hepatitis B virus e antigen (HBeAg) detection kit and preparation method thereof
InactiveCN109541225AImprove accuracyIncreased contact surface areaBiological testingAntigenMicrosphere
The invention discloses a hepatitis B virus e antigen (HBeAg) detection kit and a preparation method thereof. The hepatitis B virus e antigen (HBeAg) detection kit is composed of the following components: HBeAg antibody-coating magnetic microspheres, an HBeAg calibrator, an HBeAg antibody marker bound to lanthanides, an analytical buffer, a cleaning solution, an enhancement solution and an RFID card. Based on combination of the magnetic microspheres and a time-resolved immunoassay method, the drawbacks of long physical adsorption reaction time and slow detection result of an enzyme label plateare overcome, and the reaction time is greatly shortened; and besides, the advantages of high accuracy, high sensitivity, strong specificity, wide linear range, and stable and convenient detection ofa time-resolved detection technology can also be realized. The magnetic microspheres are coated with corresponding antigens or antibodies, due to the stereoscopic characteristic of the magnetic microspheres, the contact surface area of an immune response is greatly increased, the detection time is greatly shortened, and further optimization is made for the traditional two-step method detection, HBeAg detection only requires a one-step method, and a result can be obtained within 30 minutes.
Owner:GUANGZHOU BIOKEY HEALTH TECH CO LTD
Application of benzyl-containing flavonoid lignan in preparation of medicament for treating hepatitis B
InactiveCN101912387AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsAntigenDisease
The invention relates to application of benzyl-containing flavonoid lignan in preparation of a medicament for treating hepatitis B, in particular to application of flavonoid lignan and pharmaceutically-acceptable salt thereof in preparation of the medicament for eliminating hepatitis B e antigen, inhibiting hepatitis B virus desoxyribonucleic acid (HBV DNA) replication and treating viral hepatitis B. The flavonoid lignan can inhibit the activity of hepatitis B virus e antigen (HBeAg); the inhibition intensity of the flavonoid lignan under low concentration of 20 micrograms per milliliter is much higher than that of a positive control front-line medicament lamivudine and an interferon; and the compound with the concentration of 20 micrograms per milliliter displays an inhabitation ratio over 50 percent on the HBV DNA, so that the application of the flavonoid lignan in preparation of the medicament for eliminating the hepatitis B e antigen and the medicament for inhibiting the HBV DNA replication and treating hepatitis B viral diseases can be anticipated.
Owner:DALI UNIV
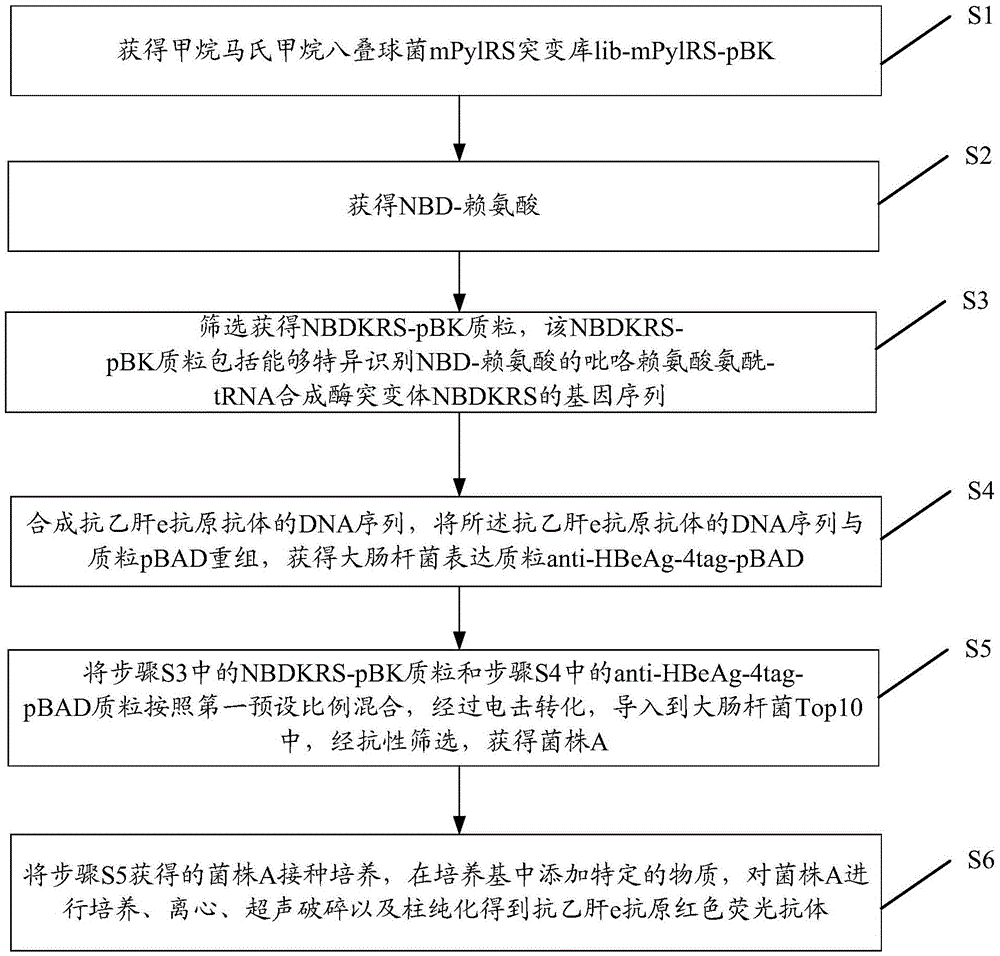
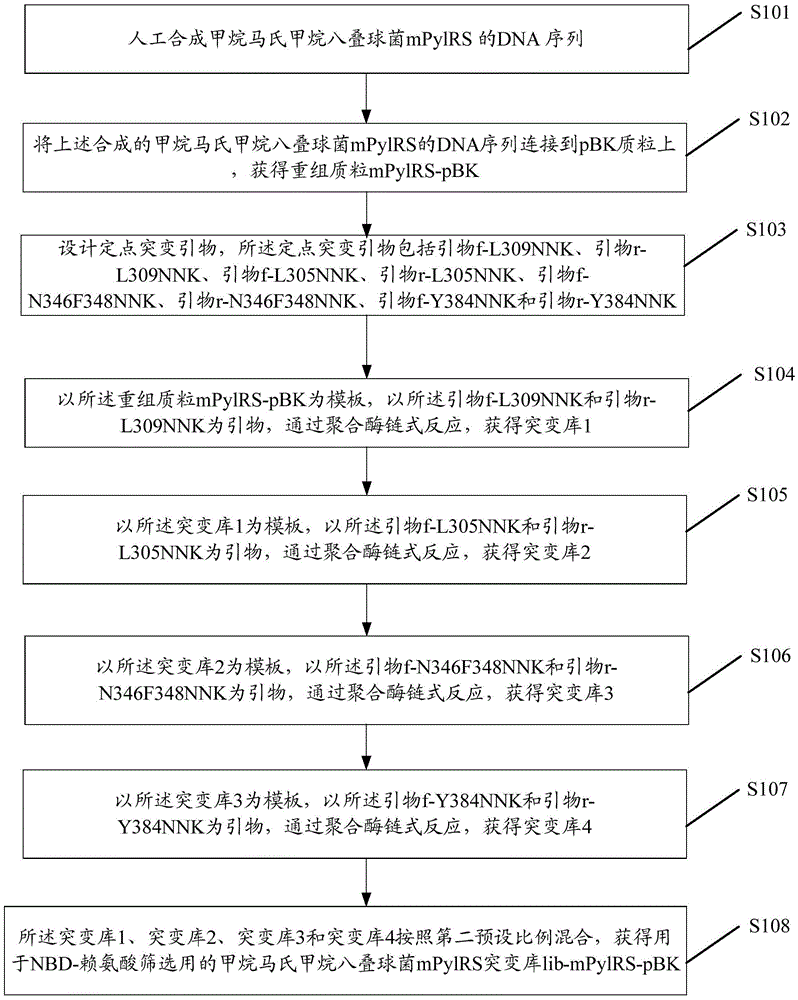
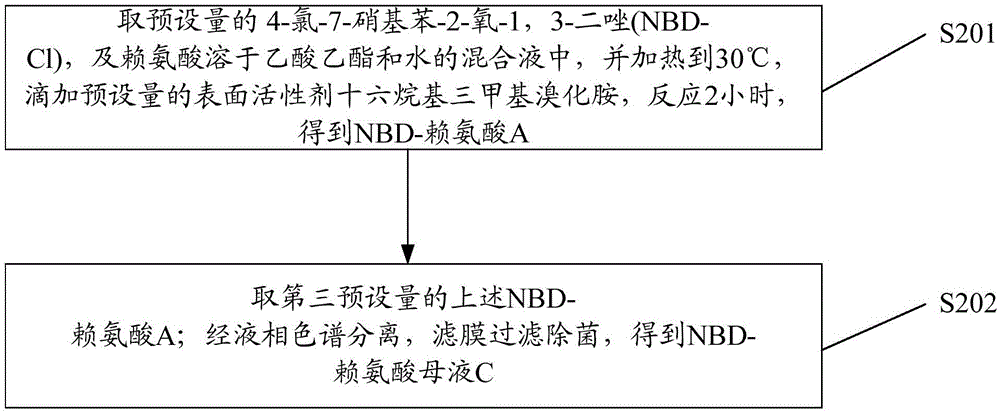
Preparation method of red fluorescent antibody resisting HBeAg (hepatitis B virus e antigen)
InactiveCN105647918AAchieve preparationHigh sensitivityImmunoglobulins against virusesPeptide preparation methodsAntigenFluorescence
The invention discloses a preparation method of a red fluorescent antibody resisting HBeAg (hepatitis B virus e antigen). Genes of an mPylRS (pyrrolysyl-tRNA synthetase) of a synthesized methanosarcina mazei species and a plasmid pBK are recombined, and mPylRS-pBK is obtained; a mutant library lib-mPyl1RS-pBK is established with mPylRS-pBK as a template; lib-mPylRS-pBK is subjected to electroporation and then screened; NBDKRS-pBK plasmids are screened and recombined; DNA (deoxyribonucleic acid) resisting the HBeAg antibody is recombined with pBAD, and anti-HBeAg-4tag-pBAD is obtained; the plasmidsanti-HBeAg-4tag-pBAD and NBDKRS-pBK are transformed in a competent state, and a biological expression system for preparing the red fluorescent antibody resisting HBeAg is obtained. With the adoption of the preparation method, the red fluorescent antibody resisting HBeAg can be prepared, and the detection sensitivity is improved.
Owner:SHENZHEN SHENGBIZHI TECH DEV CO LTD
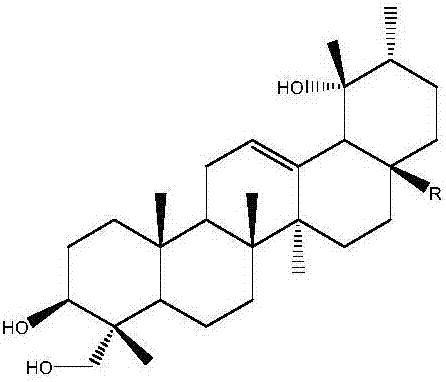
Application of rotundic acid in preparation of anti-hepatitis B virus (HBV) medicine
InactiveCN107281188AGood anti-HBV effectLow effective concentrationOrganic active ingredientsDigestive systemAntigenPositive control
The invention discloses application of rotundic acid in preparation of an anti-hepatitis B virus (HBV) medicine for the first time, and especially discloses application of the rotundic acid in preparation of medicines for inhibiting secretion of HBV surface antigen or HBV E antigen. In-vitro HepG22.2.15 cell pharmacological experiments prove that rotundic acid has excellent anti-HBV effect, and has the advantages of relatively low effective concentration and relatively small cytotoxicity. The rotundic acid has an effect for inhibiting HBsAg and HBeAg, has the inhibition rates for HBsAg and HBeAg respectively being 39 / 5 percent and 40.2 percent at 100mu g / ml, and has the inhibition effect for HBV superior to that of positive control lamivudine, oleanolic acid and ovate leaf holly bark medicinal material extract.
Owner:SUN YAT SEN UNIV +1
Composition of human adipose derived mesenchymal progenitor cells and adipose derived stromal vascular fraction for treating hepatitis B
InactiveCN105663165AGood treatment effectPeptide/protein ingredientsDigestive systemHepatitis B immunizationAntiendomysial antibodies
The invention relates to application of a composition of human adipose derived mesenchymal progenitor cells and an adipose derived stromal vascular fraction in preventing or treating hepatitis B. Specifically, the composition of the human adipose derived mesenchymal progenitor cells and the adipose derived stromal vascular fraction can be used for preparing a pharmaceutical composition for treating the hepatitis B. The pharmaceutical composition, when applied to an object in need, can improve such indexes as hepatitis B surface antigen, hepatitis B surface antibody, hepatitis B virus e antigen, hepatitis B virus e antibody, hepatitis B core antibody and the like, and can significantly reduce hepatitis virus DNA and lower ALT, so that the repair of hepatic function is promoted.
Owner:CELLULAR BIOMEDICINE GRP SHANGHAI +1
Human adipose derived mesenchymal progenitor cells for treating hepatitis B
InactiveCN105663163AEffective treatmentClean up thoroughlyDigestive systemAntiviralsAntigenProgenitor
The invention relates to application of human adipose derived mesenchymal progenitor cells in preventing or treating hepatitis B. Specifically, the human adipose derived mesenchymal progenitor cells can be used for preparing a pharmaceutical composition for treating the hepatitis B. The pharmaceutical composition, when applied to an object in need, can improve such indexes as hepatitis B surface antigen, hepatitis B surface antibody, hepatitis B virus e antigen, hepatitis B virus e antibody, hepatitis B core antibody and the like, and can significantly reduce hepatitis virus DNA and lower ALT, so that the repair of hepatic function is promoted.
Owner:CELLULAR BIOMEDICINE GRP SHANGHAI +1
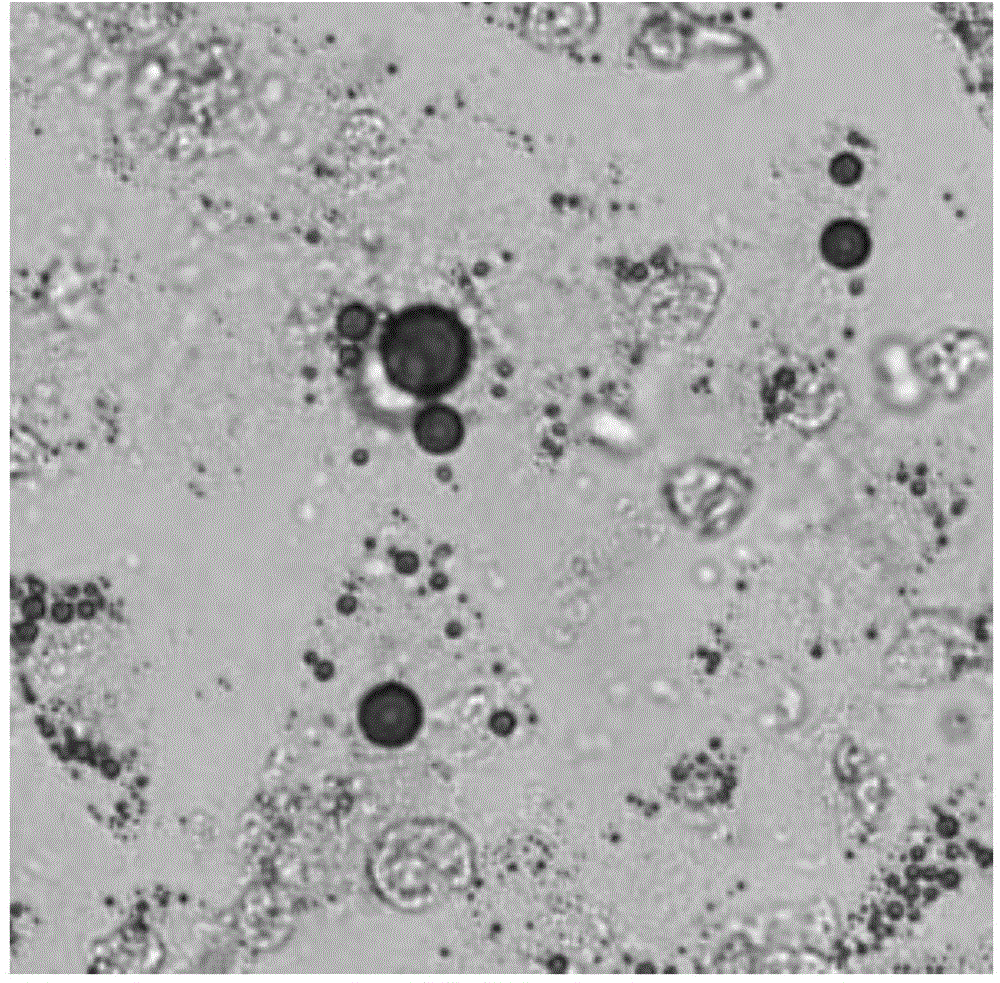
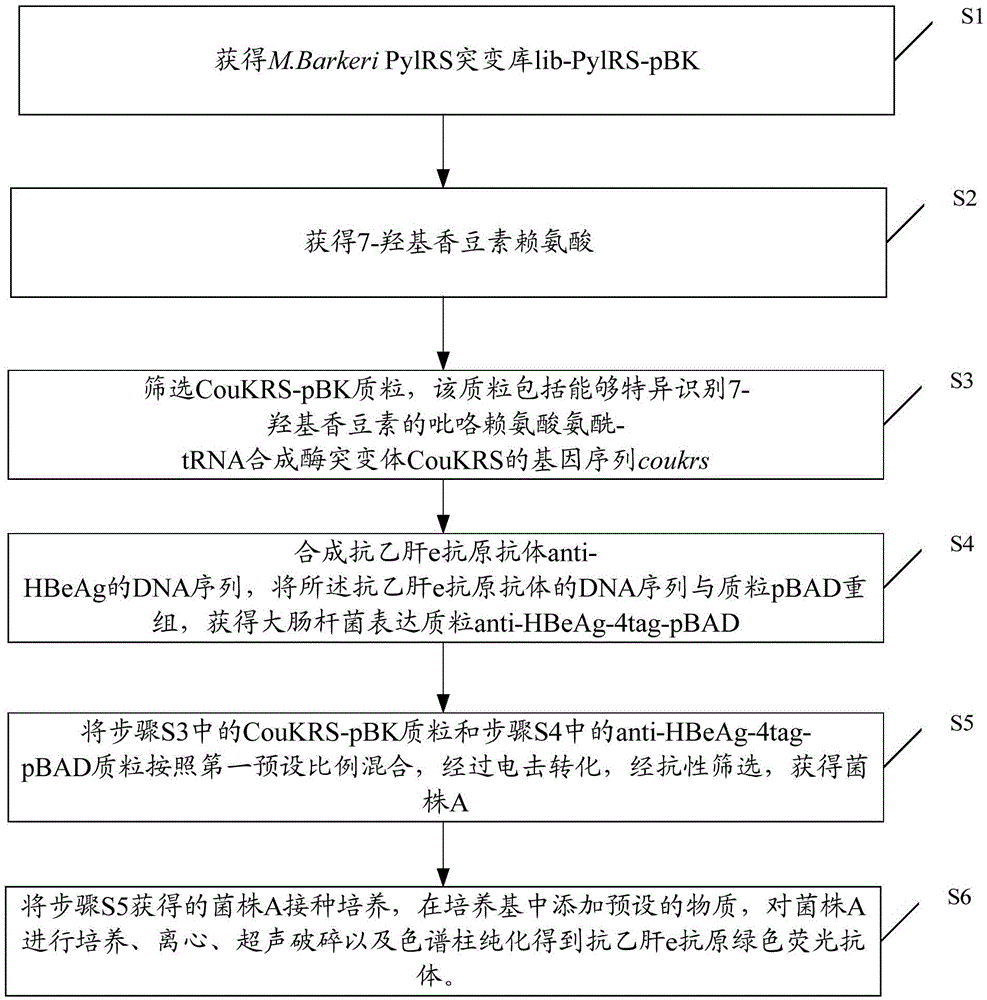
Preparation method of anti-HBeAg (hepatitis B virus e antigen) green fluorescent antibody
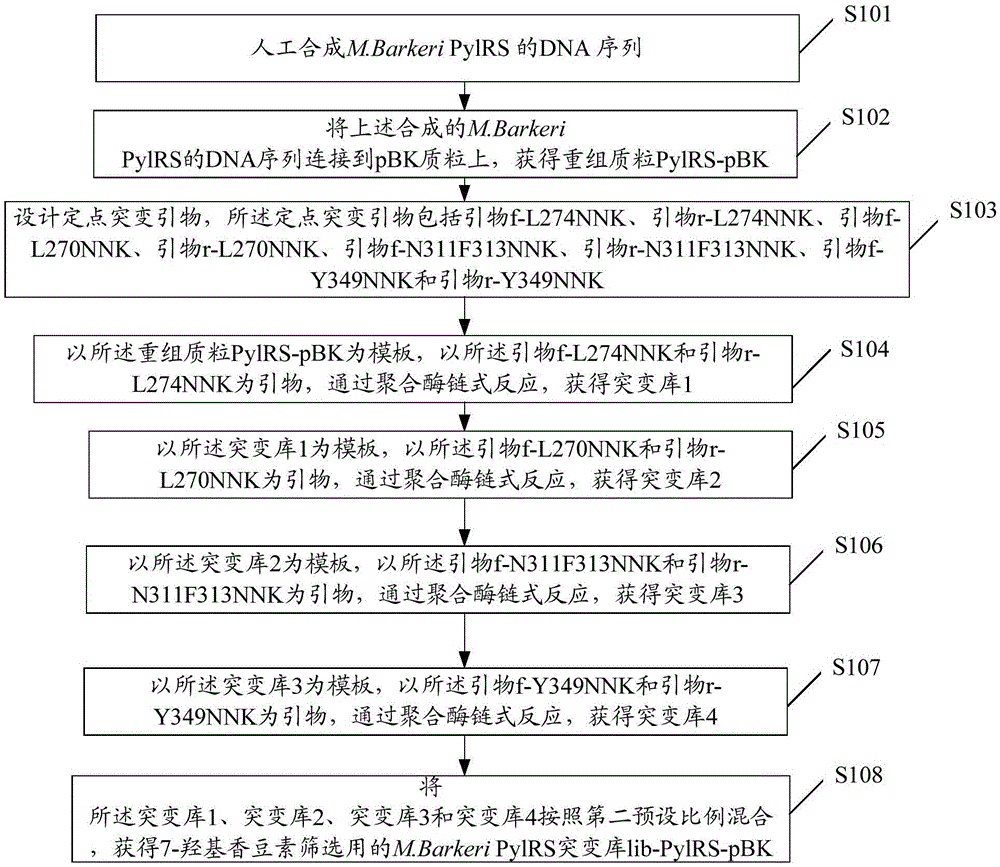
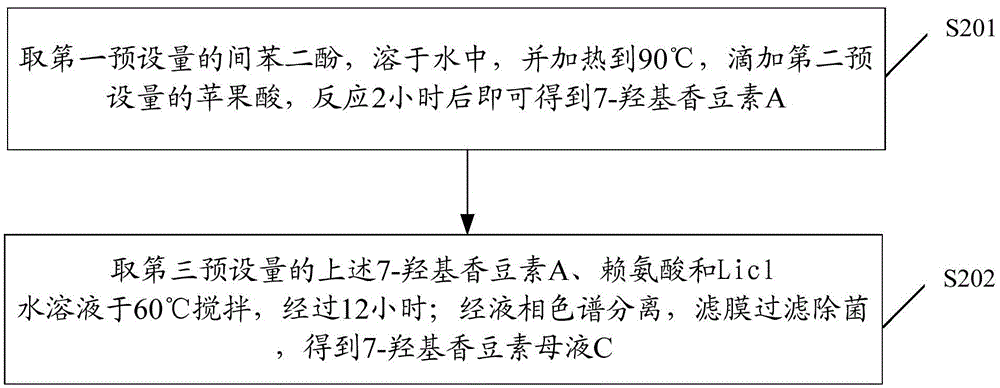
InactiveCN105524167AAchieve preparationHigh sensitivityImmunoglobulins against virusesNucleic acid vectorAntigenFluorescence
The invention discloses a preparation method of an anti-HBeAg (hepatitis B virus e antigen) green fluorescent antibody. DNA (deoxyribonucleic acid) of (PylRS) for M.Barkeri synthesis is recombined with plasmids pBK through genetic engineering means, and PylRS-pBK is obtained; the PylRS-pBK is taken as a template for establishment of a mutation database lib-PylRS-pBK; the lib-PylRS-pBK is subjected to electroporation and screening; CoukRS-pBK plasmids are screened and recombined; DNA of an anti-HBeAg antibody is recombined with pBAD, and anti-HBeAg-4tag-pBAD is obtained; plasmids anti-HBeAg-4tag-pBAD and CoukRS-pBK are converted into a competent state, and a biological expression system for preparing the anti-HBeAg green fluorescent antibody is obtained. The preparation of the anti-HBeAg green fluorescent antibody is realized, and the detection sensitivity is improved.
Owner:BIO TECH ACADEMY CHINA
Special-effect oral liquid for treating hepatitis B
InactiveCN105688030ALiver function returns to normalNormal liver functionAmphibian material medical ingredientsPharmaceutical delivery mechanismDiseaseHepatitis B virus surface
The invention discloses a special-effect oral liquid for treating hepatitis B; the special-effect oral liquid is prepared by decoction and concentration of capillary artemisia, fructus gardeniae, rheum officinale, cortex phellodendri, rhizoma coptidis, dandelion, radix scrophulariae, fructus forsythiae, radix rehmanniae recen, toad venom, brucea javanica, herba lysimachiae, sowthistle tasselflower herb, cortex cinnamomi, rhizoma acori graminei, rhizoma chuanxiong, radix salviae miltiorrhizae, radix curcumae, caulis spatholobi, hawthorn, Chinese yam, radix astragali, jujube, radix morindae officinalis, rhizoma curculiginis, fructus psoraleae, kernels, rhizoma cibotii, radix polygoni multiflori, longan aril, Chinese wolfberry, fructus ligustri lucidi, radix ophiopogonis, bitter apricot kernel, lotus seed, ganoderma lucidum, radix saposhnikoviae, radix angelicae tuhuo, radix puerariae, radix bupleuri, rhizoma cimicifugae, mung beans, pericarpium citri reticulatae, and endothelium corneum gigeriae galli. The pure traditional Chinese medicine preparation has stronger functions of cleaning hepatitis B viruses, regulating immunity, repairing damaged liver cells, improving hepatic microcirculation, and promoting hepatitis B virus surface antibodies to be turned into positive and hepatitis B surface antigens, hepatitis B e antigens, hepatitis B e antibodies and hepatitis B core antibodies to be turned into negative, has the advantages of exact curative effect, short treatment course and quick acting, and allows the disease not to relapse after the disease is cured.
Owner:仲从开
Hepatitis B virus e antigen testing corpuscle, preparation and application thereof
ActiveCN101251540BWide detection rangeReduce sensitivityAnalysis by material excitationAntigenAnti hbe
The invention relates to a diagnosing reagent for hepatitis B, disclosing detection grains for e antigens of the hepatitis B virus, which are of the luminous grains coated by anti-HBE antibodies. The invention also discloses preparation and application for the detection grains for e antigens of the hepatitis B virus; moreover, the invention further discloses an outside-body diagnosis reagent box for detecting e antigens of the hepatitis B in a blood serum sample of human beings as well as a method for utilizing the light excitation chemiluminescence principle to quantitatively and qualitatively detect e antigens of the hepatitis B virus. The reagent box of the invention can be jointly used to diagnose the individual acute or chronic hepatitis B together with other blood serums and clinic information, and screen the hepatitis B for women in the perinatal period so as to judge the risk of newborn babies contaminating the hepatitis B.
Owner:BEYOND DIAGNOSTICS (SHANGHAI) CO LTD
Hepatitis B e antigen immune escaping detecting gene chip and kit thereof
The invention provides a hepatitis B virus e antigen immune evasion detection gene chip and a kit, belonging to the gene chip technique field of clinical detection, and comprising: a gene chip of a specific DNA probe which detects hepatitis B virus e antigen immune evasion and is fixed on a solid phase carrier as well as a matched reagent; the matched reagent includes a treating fluid for treating serum samples, an amplifying solution for amplifying and labeling the treated samples, a hybridization solution in hybridization reaction with a micro-array, a cleaning solution, an enzyme-labeled working solution and a detection liquid; and the matched reagent and the hepatitis B virus e antigen immune evasion detection gene chip are used jointly in the detection process of the serum samples. The chip and the kit can detect the hepatitis B virus e antigen immune evasion, and avoid the clinical hepatitis B virus e antigen leak detection, thereby being favorable for judging the infectivity and conditions after curing of hepatitis B patients.
Owner:上海裕隆生物科技有限公司
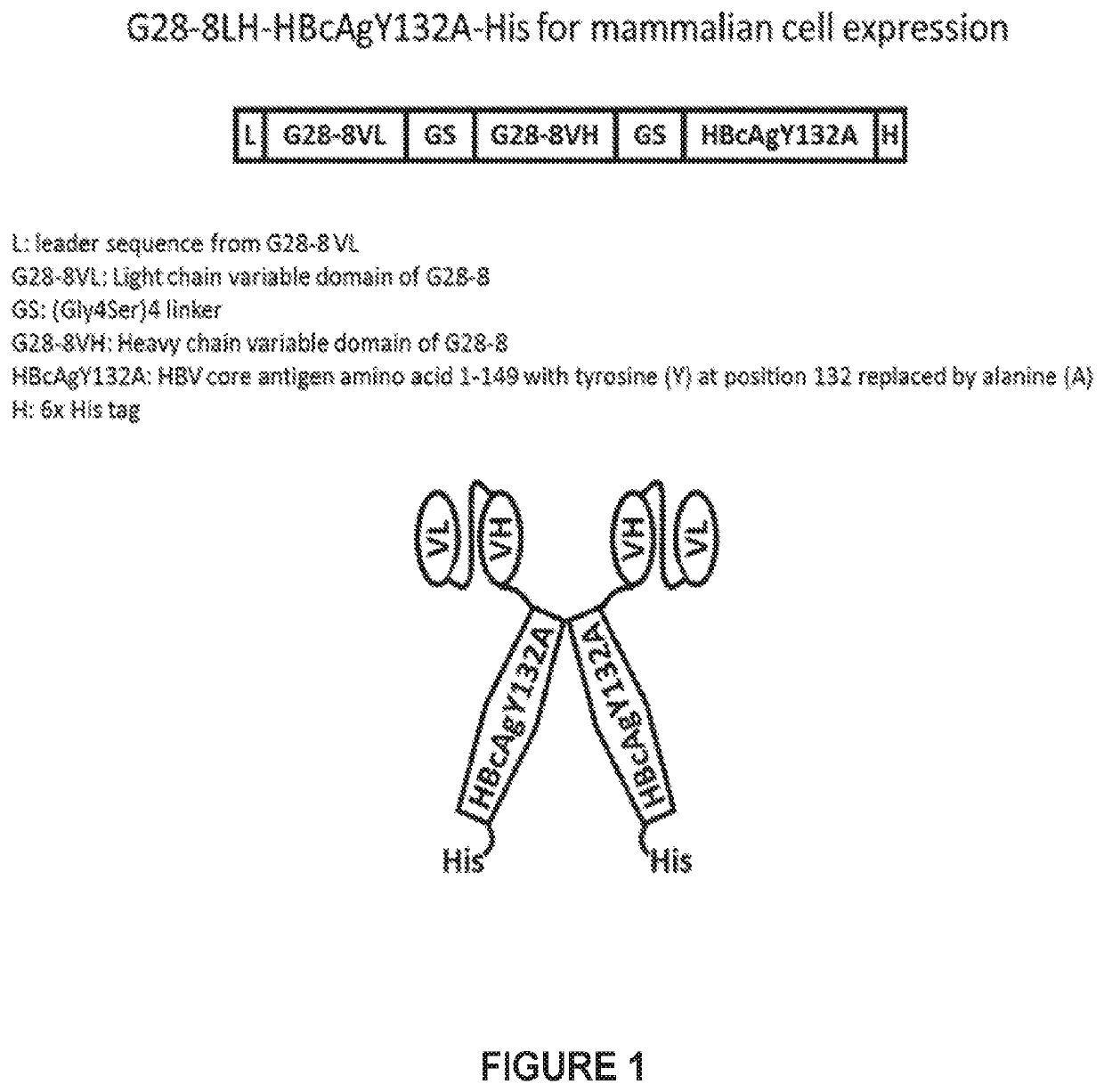
Therapeutic Vaccine for Hepatitis B Virus (HBV) using the HBV Core Antigen
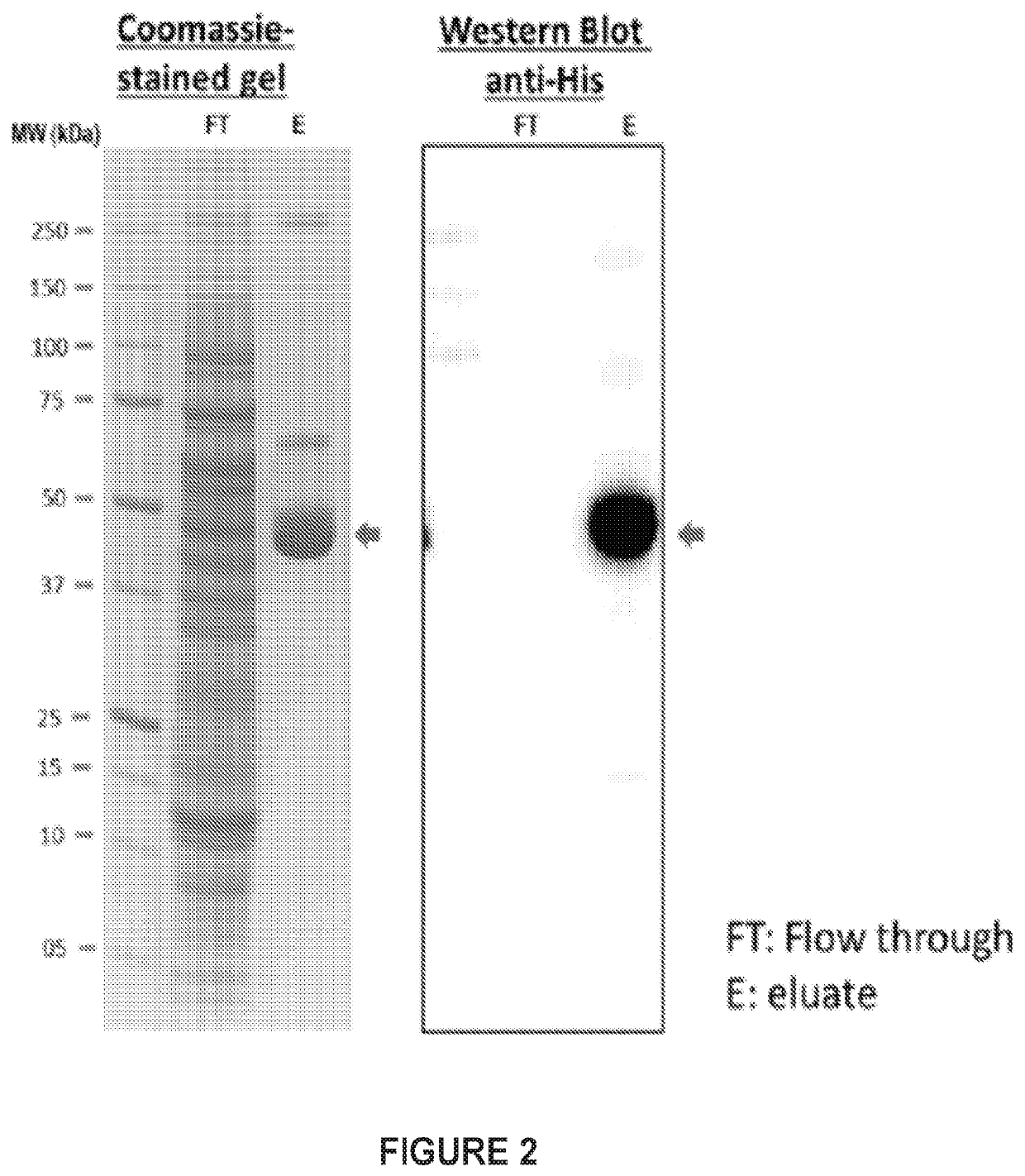
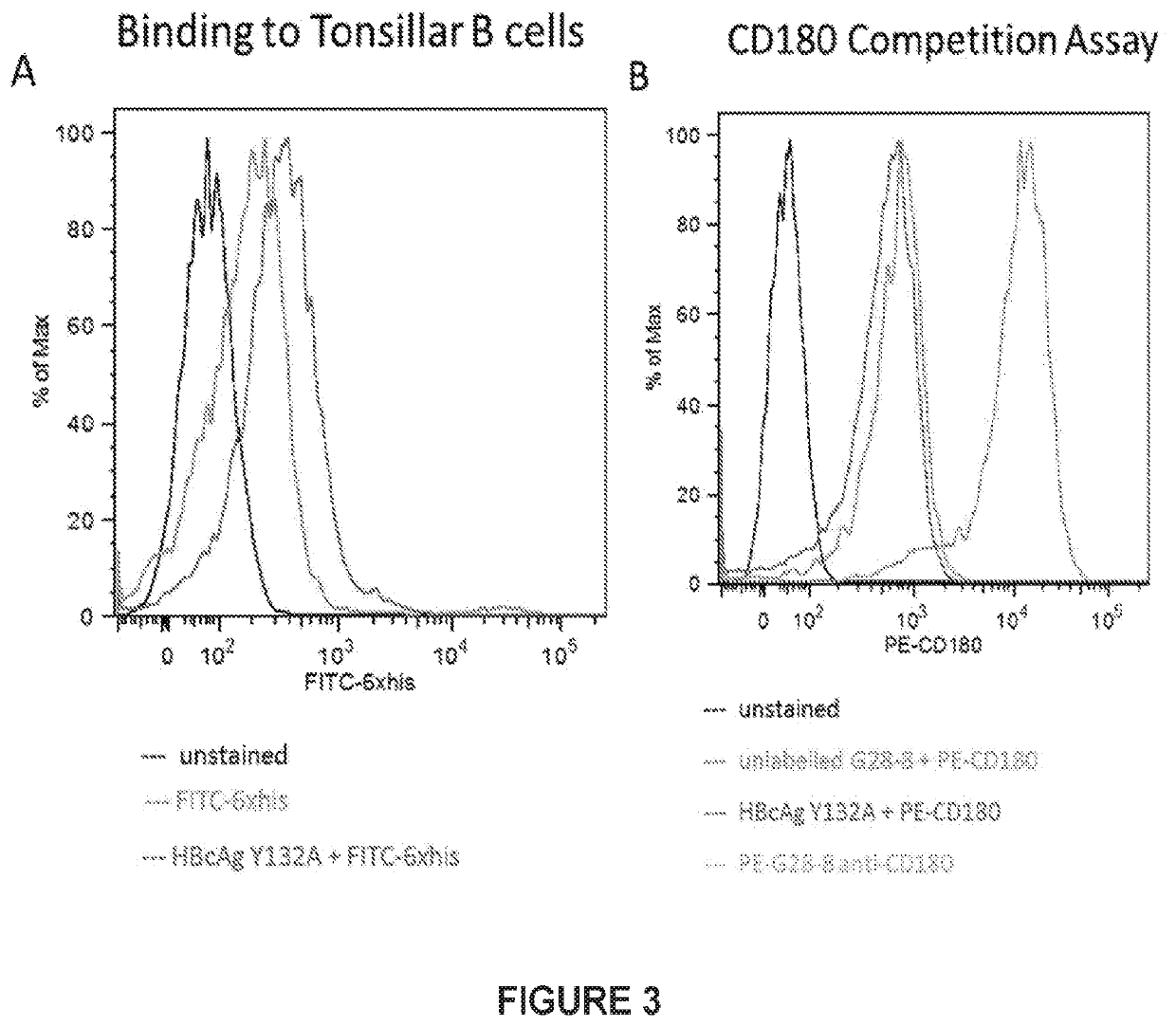
ActiveUS20200306367A1Control developmentAntibody mimetics/scaffoldsViral antigen ingredientsHepatitis B virus core AntigenDisease
Provided herein are compositions of CD1280 binding proteins and a Hepatitis B virus core antigen (HBcAg) and / or a Hepatitis B virus E antigen (HBeAg), or antigenic fragments or mutants thereof, attached to the CD180 binding protein, and methods for using the compositions to treat or limit the development of hepatitis-B virus (HBV)-related disorders.
Owner:ABACUS BIOSCIENCE INC +1
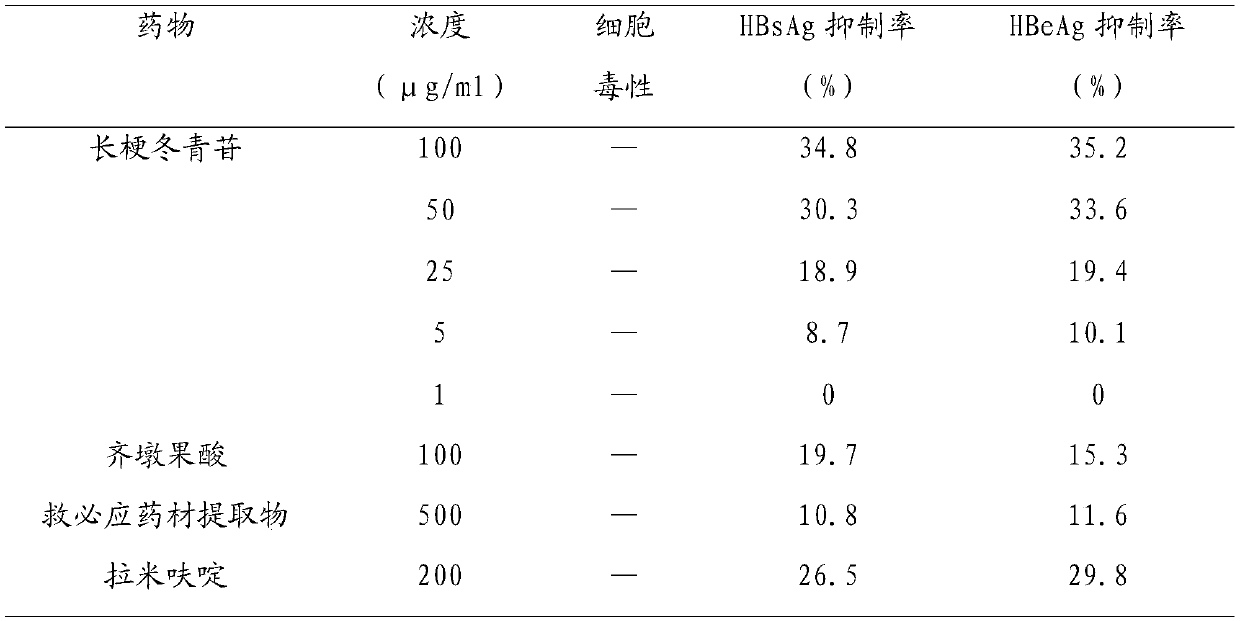
Application of pedicel glucoside in the preparation of anti-hepatitis B virus medicine
ActiveCN107095877BGood anti-HBV effectLow effective concentrationOrganic active ingredientsDigestive systemHepatitis B Virus AntigenAntigen
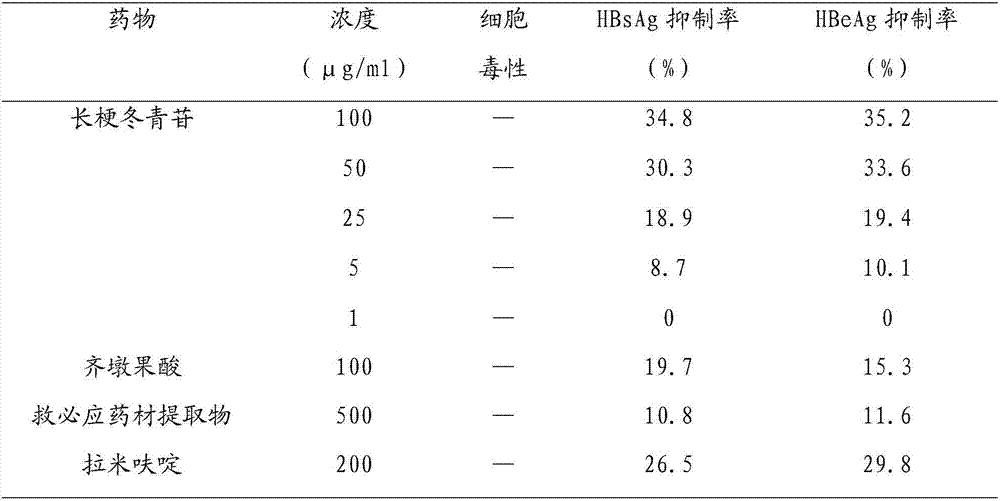
A novel application of pedunculoside in preparation of anti-(hepatitis-B-virus) medicines is disclosed for the first time by the invention. The application includes an application of the pedunculoside in preparation of medicines inhibiting hepatitis B surface antigen secretion or hepatitis B virus E antigen secretion. In-vitro HepG22.2.15 cell pharmacological experiments prove that: the pedunculoside has good anti-HBV functions and has characteristics of a low effective concentration and low cell toxicity; the pedunculoside can inhibit HBsAg and HBeAg; when the concentration is 100 [mu]g / mL, the HBsAg inhibiting rate and the HBeAg inhibiting rate are 34.8% and 35.2% respectively; and an HBV inhibiting function of the pedunculoside is higher than HBV inhibiting functions of positive controls which are lamivudine, oleanolic acid and a ilex rotunda bark extract product.
Owner:SUN YAT SEN UNIV +1
Method of preparing extract of Mallotus apelta and uses for fighting hepatitis B virus
InactiveCN101584702BSignificant anti-HBV effectAbundant resourcesOrganic active ingredientsDigestive systemBULK ACTIVE INGREDIENTHepatitis B virus surface Antigen
The invention relates to anti-HBV activity of Mallotus apelta extract and uses in prepartion of medicine for treating related diseases caused by hepatitis B. The extract of Mallotus apelta provided by the invention has basic components of apiolin, apiin-7-O-belta-D-glucoside, and flavonoids compounds of 5,7-dihydroxy-6-isopentene group-4'-methoxyl flavonone, flavonoids compounds Mallotus apelta have obvious inhibiting effect on hepatitis B virus surface antigen and hepatitis B virus e antigen ono surface of HepG 2215, and cabaple of inhibiting duplication of Duck Hepatitis B Virus deoxyribonucleic acid, withdraw rebound is weaker than positive reference medicine lamivudine, and hepatitis B is provided with a subsequent inhibiting effect after withdraw. The medicine according to the invention has strong function for fighting hepatitis B virus, clear active ingredient, which is suitable for industrial production, predictablly used for preparing medicine of preparing diseases infected by hepatitis B virus.
Owner:广西中医学院
Nano magnetic particle chemiluminescence assay kit for hepatitis B virus e antigen and preparation method thereof
ActiveCN103048454BHigh precisionAnalysis of small differences between batchesMaterial analysisAntigenFluorescein
The invention relates to a nanometer magnetic particle chemiluminescence detection kit for a hepatitis B virus e antigen as well as a preparation method thereof and a detecting method thereof. The kit comprises a solution, which contains a fluorescein labeled hepatitis B virus e antigen antibody, a suspension, which is coated with magnetic particles of a fluorescein antibody, and a solution, which contains an alkaline phosphatase labeled hepatitis B virus e antigen antibody. According to the invention, the hepatitis B virus e antigen can be quantitatively detected with lower cost, higher accuracy and higher precision.
Owner:SUZHOU HAOOUBO BIOPHARML
Application of benzyl-containing flavonoid lignan in preparation of medicament for treating hepatitis B
InactiveCN101912387BConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsAntigenDisease
The invention relates to application of benzyl-containing flavonoid lignan in preparation of a medicament for treating hepatitis B, in particular to application of flavonoid lignan and pharmaceutically-acceptable salt thereof in preparation of the medicament for eliminating hepatitis B e antigen, inhibiting hepatitis B virus desoxyribonucleic acid (HBV DNA) replication and treating viral hepatitis B. The flavonoid lignan can inhibit the activity of hepatitis B virus e antigen (HBeAg); the inhibition intensity of the flavonoid lignan under low concentration of 20 micrograms per milliliter is much higher than that of a positive control front-line medicament lamivudine and an interferon; and the compound with the concentration of 20 micrograms per milliliter displays an inhabitation ratio over 50 percent on the HBV DNA, so that the application of the flavonoid lignan in preparation of the medicament for eliminating the hepatitis B e antigen and the medicament for inhibiting the HBV DNA replication and treating hepatitis B viral diseases can be anticipated.
Owner:DALI UNIV
Application of pedunculoside in preparation of anti-(hepatitis-B-virus) medicines
ActiveCN107095877AGood anti-HBV effectLow effective concentrationOrganic active ingredientsDigestive systemAntigenPositive control
A novel application of pedunculoside in preparation of anti-(hepatitis-B-virus) medicines is disclosed for the first time by the invention. The application includes an application of the pedunculoside in preparation of medicines inhibiting hepatitis B surface antigen secretion or hepatitis B virus E antigen secretion. In-vitro HepG22.2.15 cell pharmacological experiments prove that: the pedunculoside has good anti-HBV functions and has characteristics of a low effective concentration and low cell toxicity; the pedunculoside can inhibit HBsAg and HBeAg; when the concentration is 100 [mu]g / mL, the HBsAg inhibiting rate and the HBeAg inhibiting rate are 34.8% and 35.2% respectively; and an HBV inhibiting function of the pedunculoside is higher than HBV inhibiting functions of positive controls which are lamivudine, oleanolic acid and a ilex rotunda bark extract product.
Owner:SUN YAT SEN UNIV +1
Reagent kit for detecting hepatitis B virus e antigen and use
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com