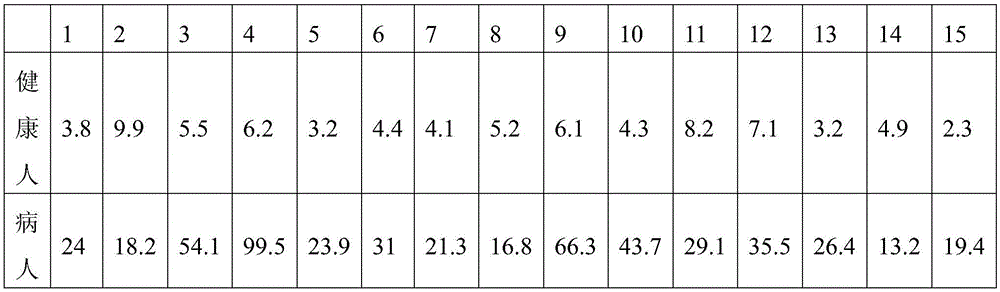
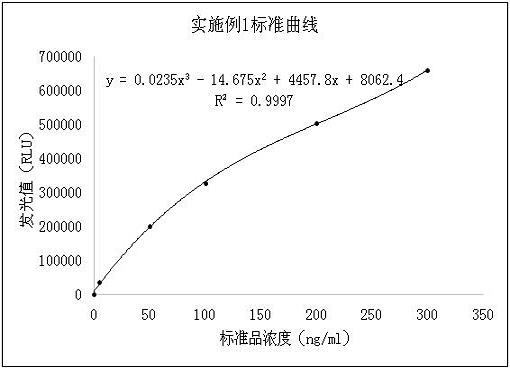
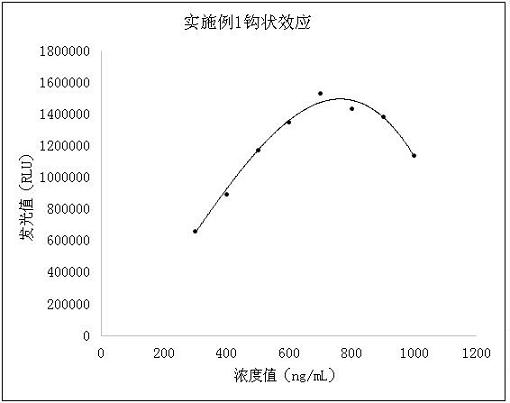
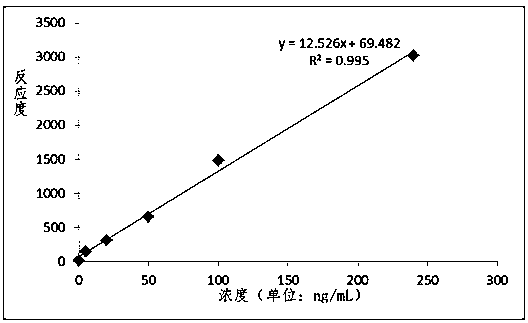
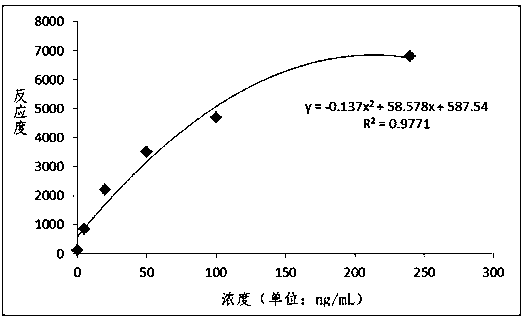
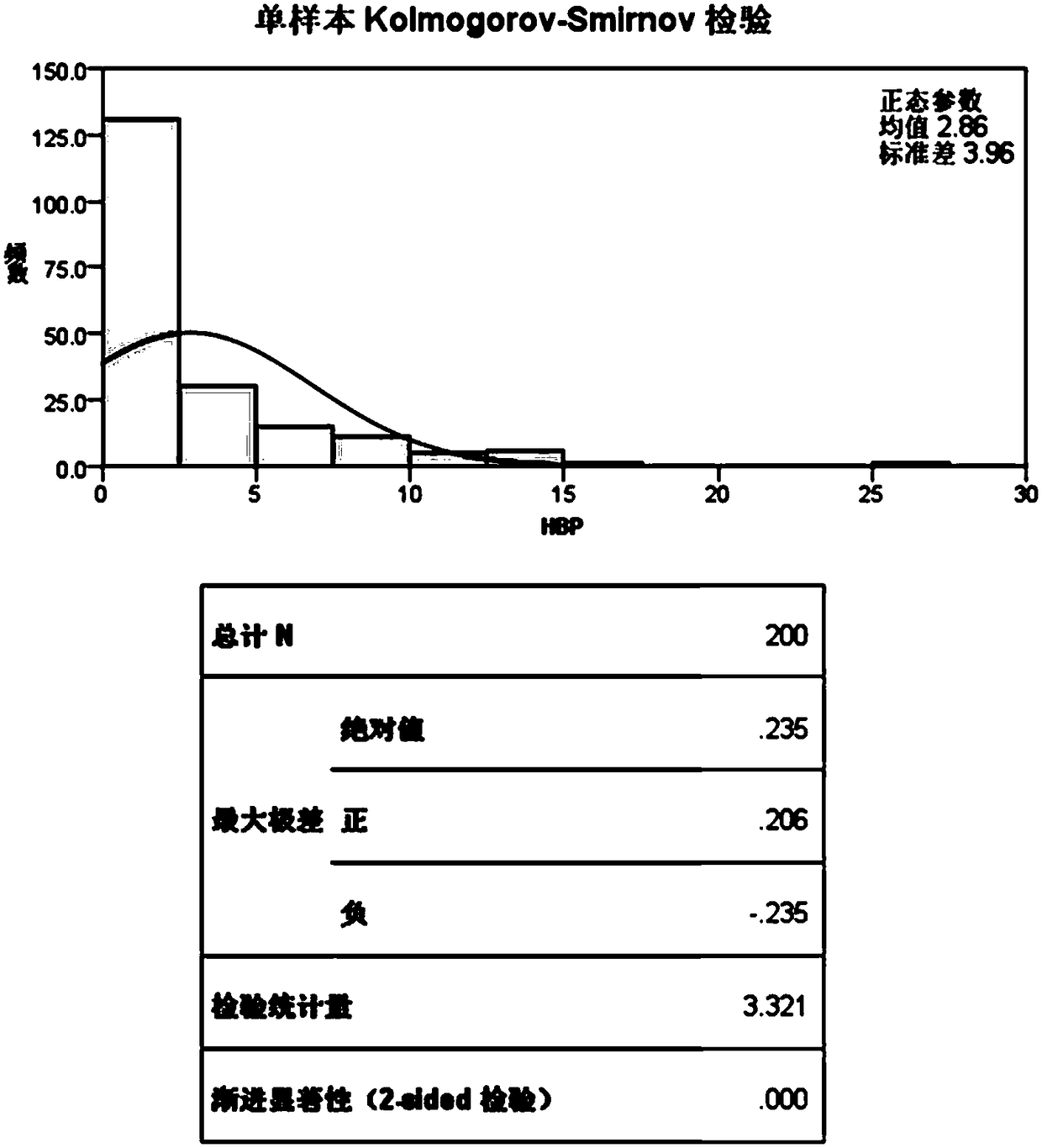
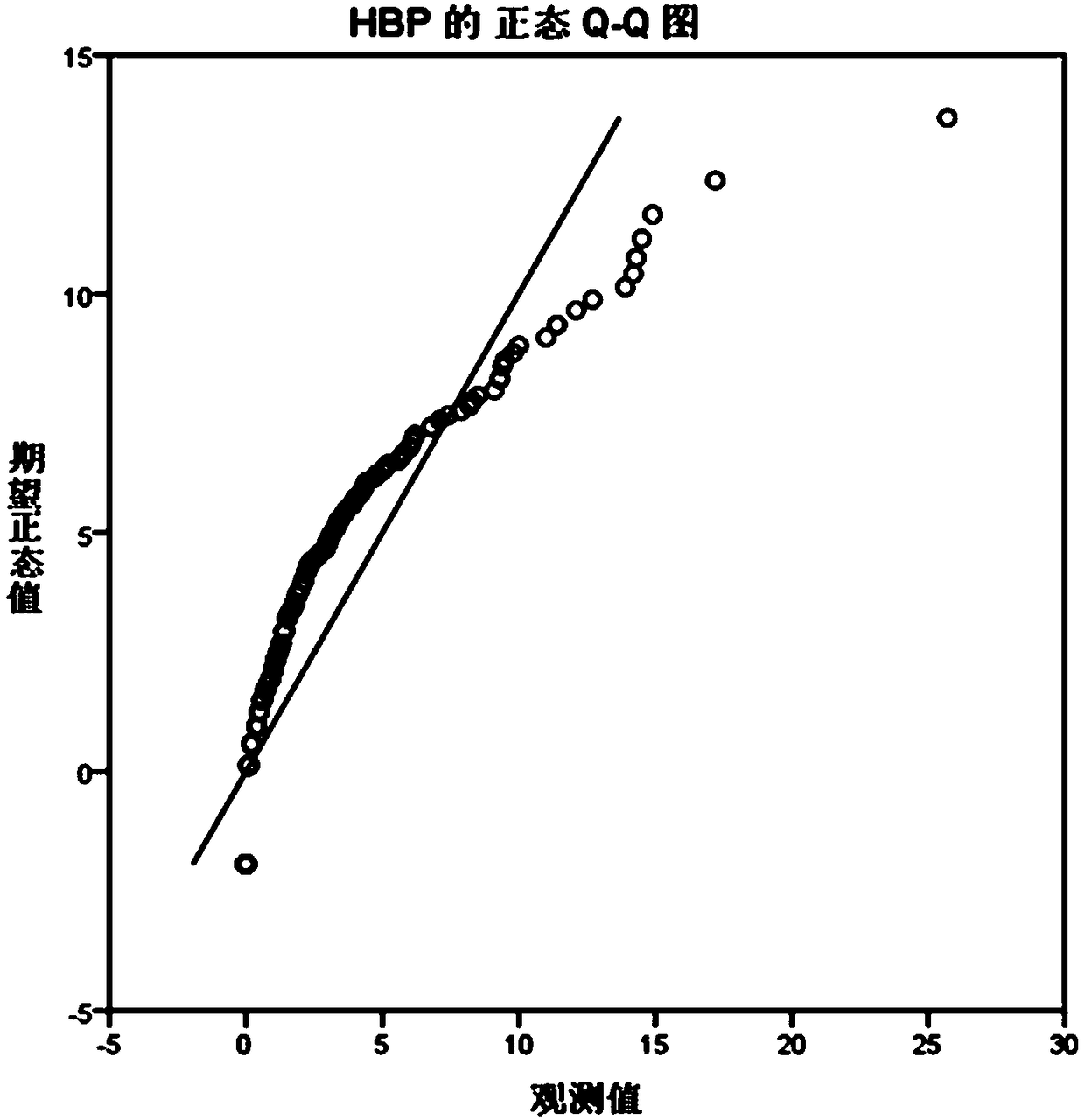
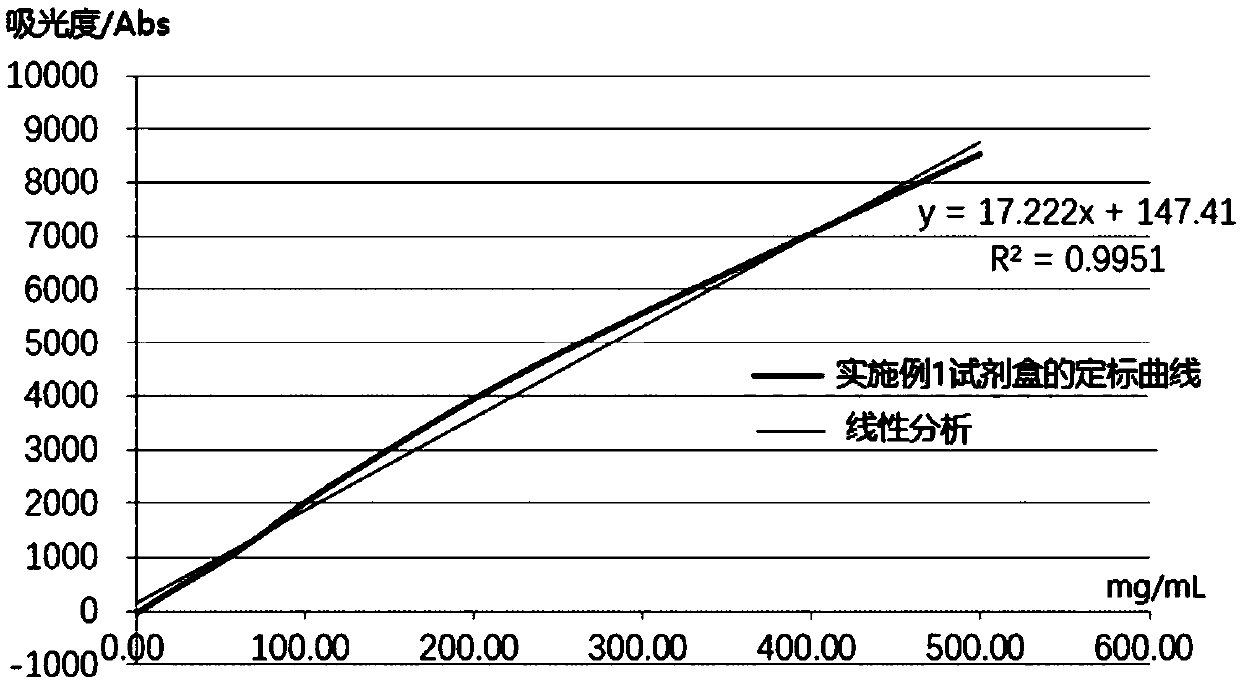
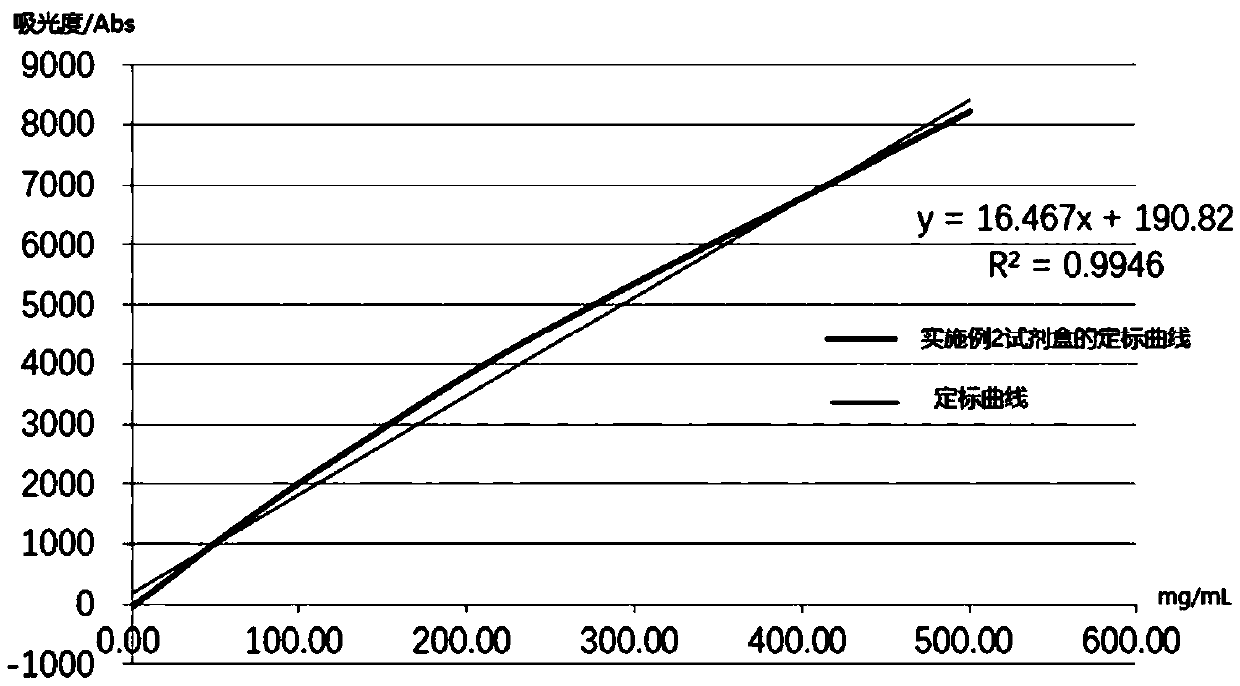
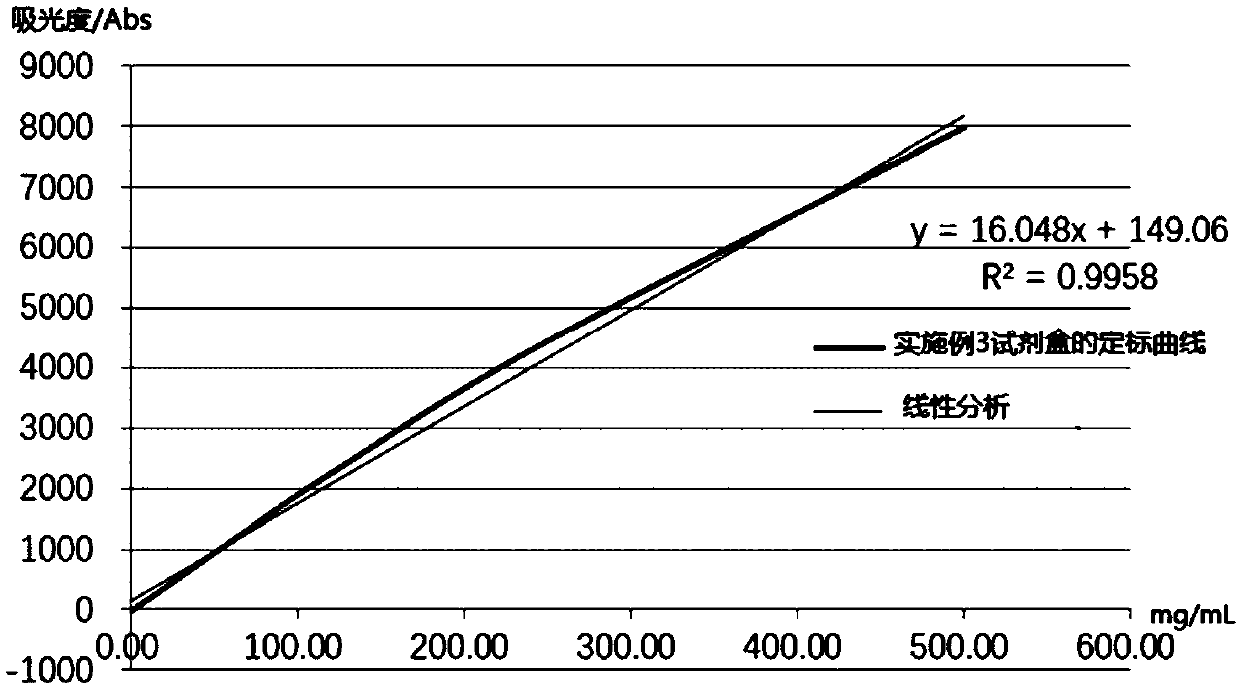
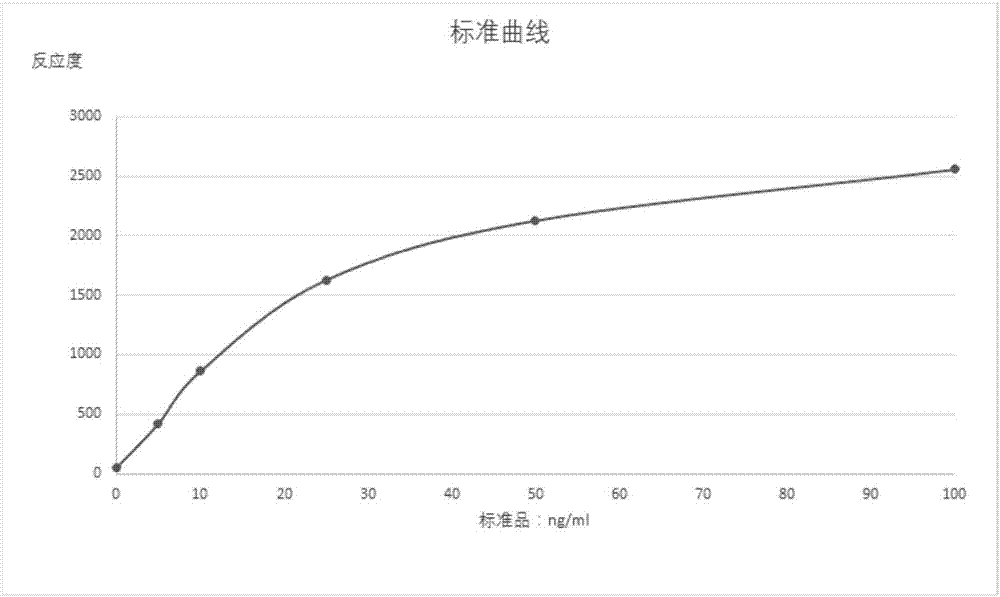
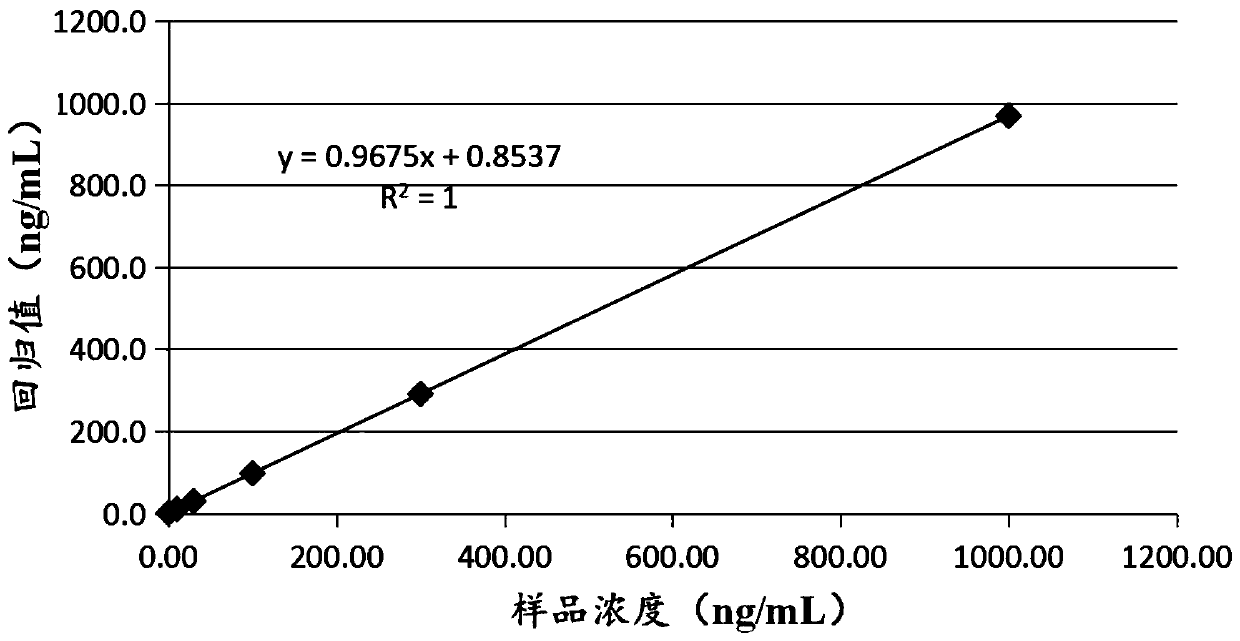
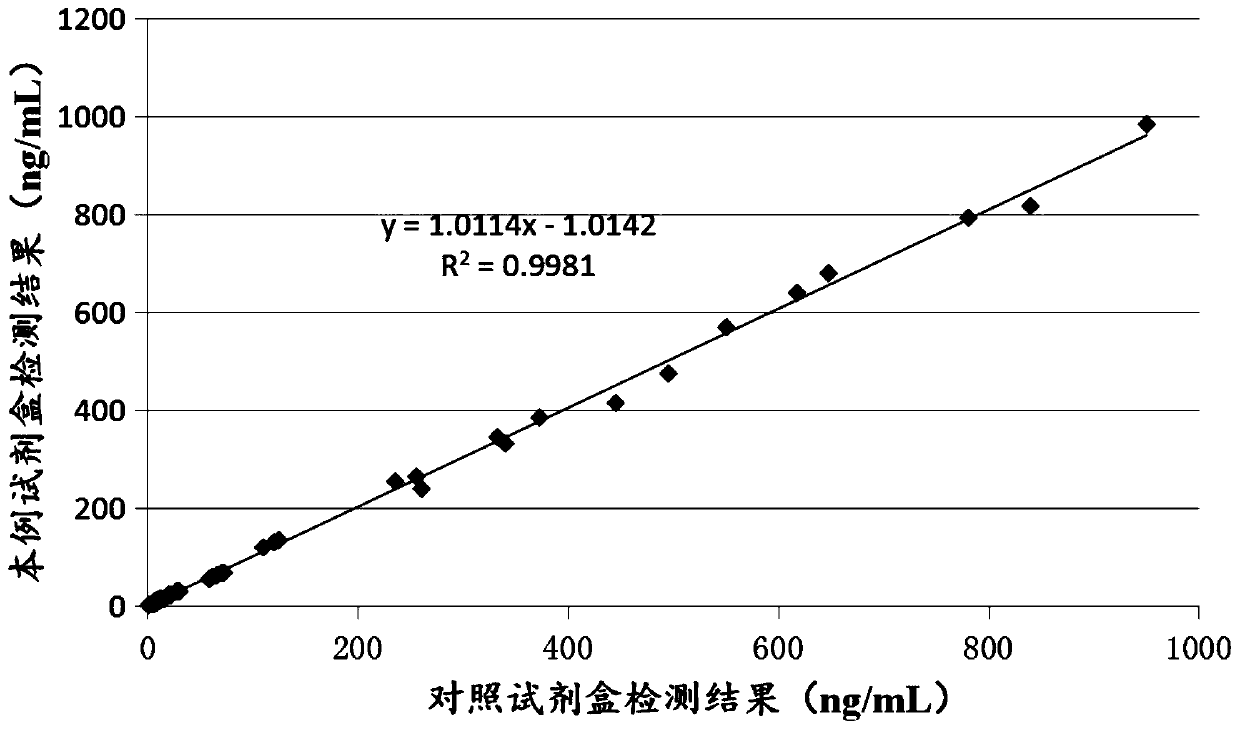
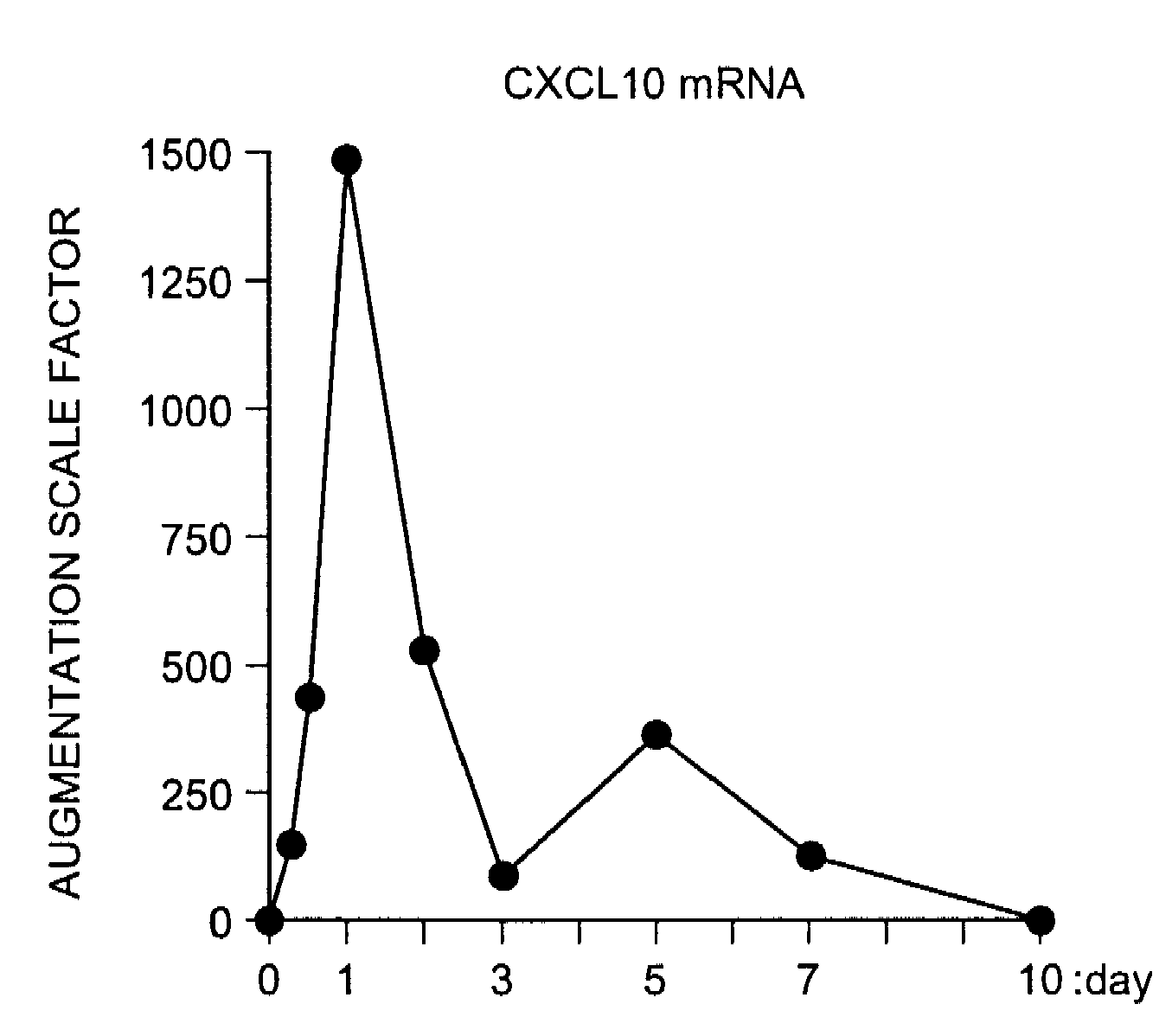
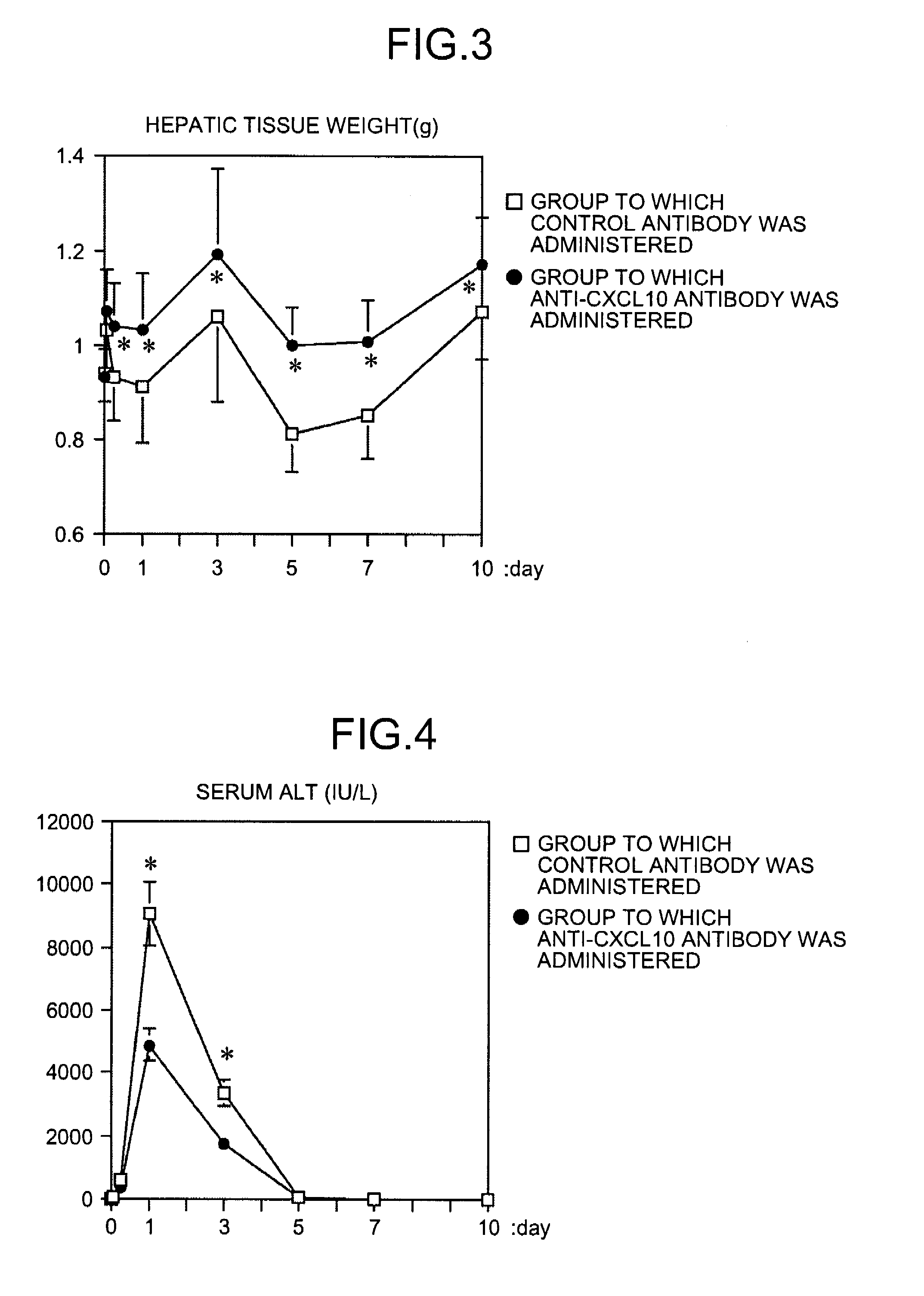
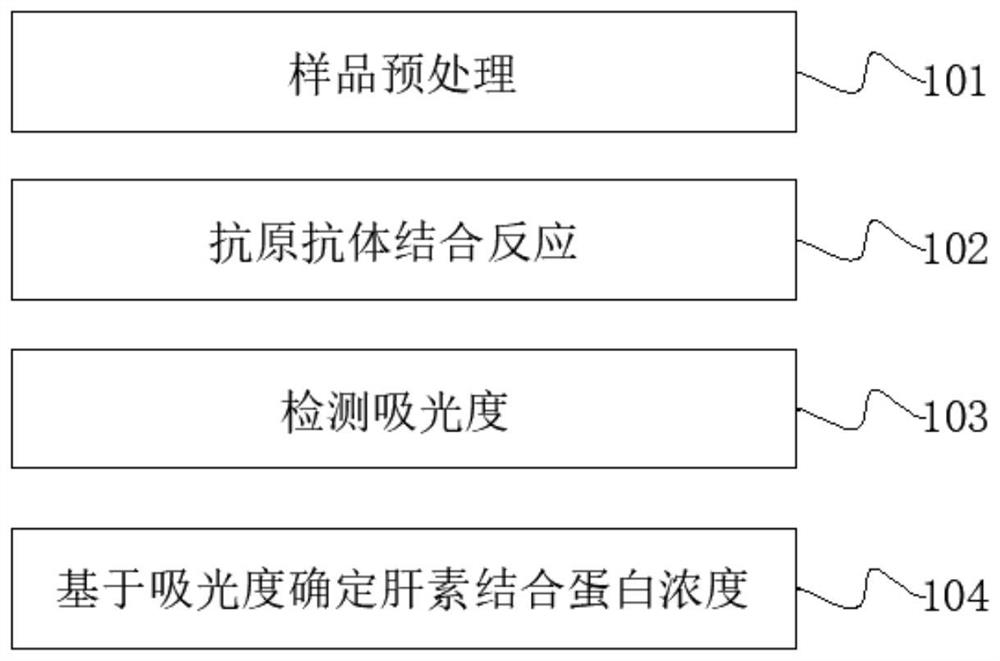
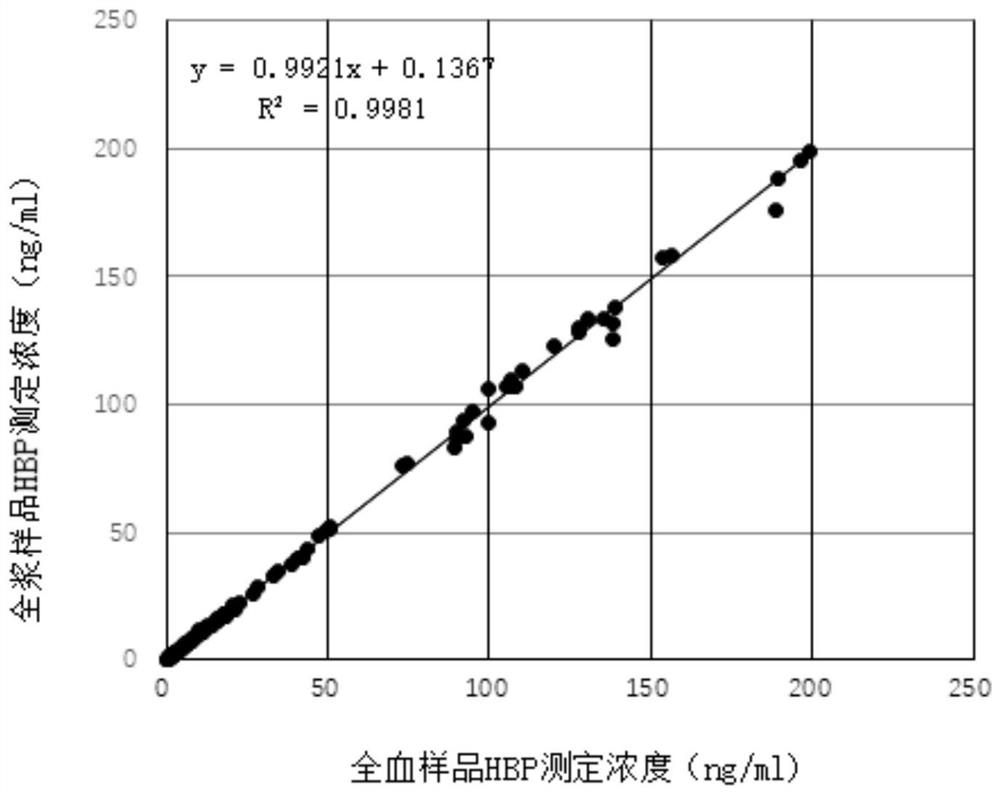
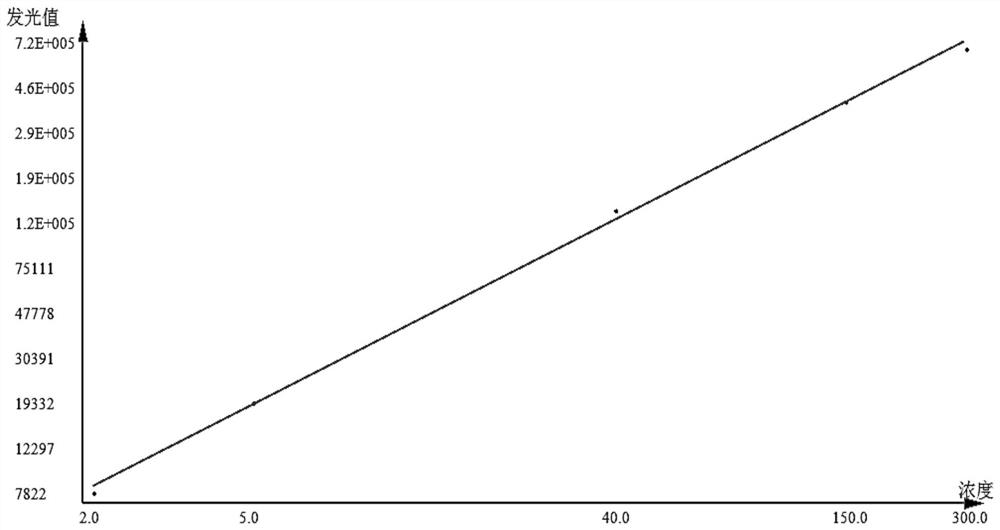
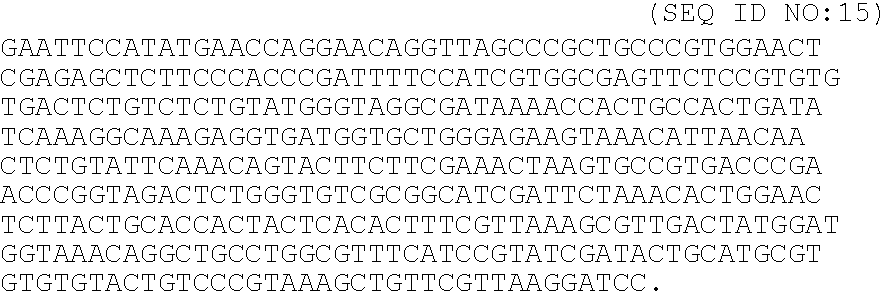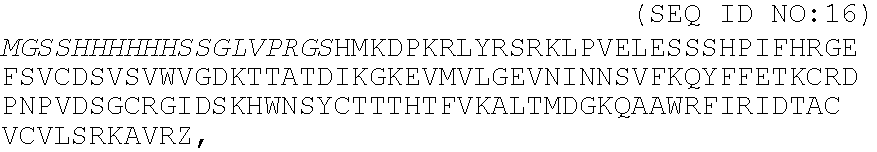Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
46 results about "Heparin-binding protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Heparin-binding protein. A proinflammatory, antimicrobial peptide released by neutrophils. It increases the permeability of blood vessels. It is found in high concentrations in plasma of patients with septic shock.
Enzyme-mediated modification of fibrin for tissue engineering: incorporation of proteins
InactiveUS6960452B2Improve fidelityHigh activityPowder deliveryOrganic active ingredientsDrug biological activityBiology
Owner:ETH ZZURICH +1
Heparin-binding protein modified with heparan sulfate sugar chains, process for producing the same and pharmaceutical compositions containing the same
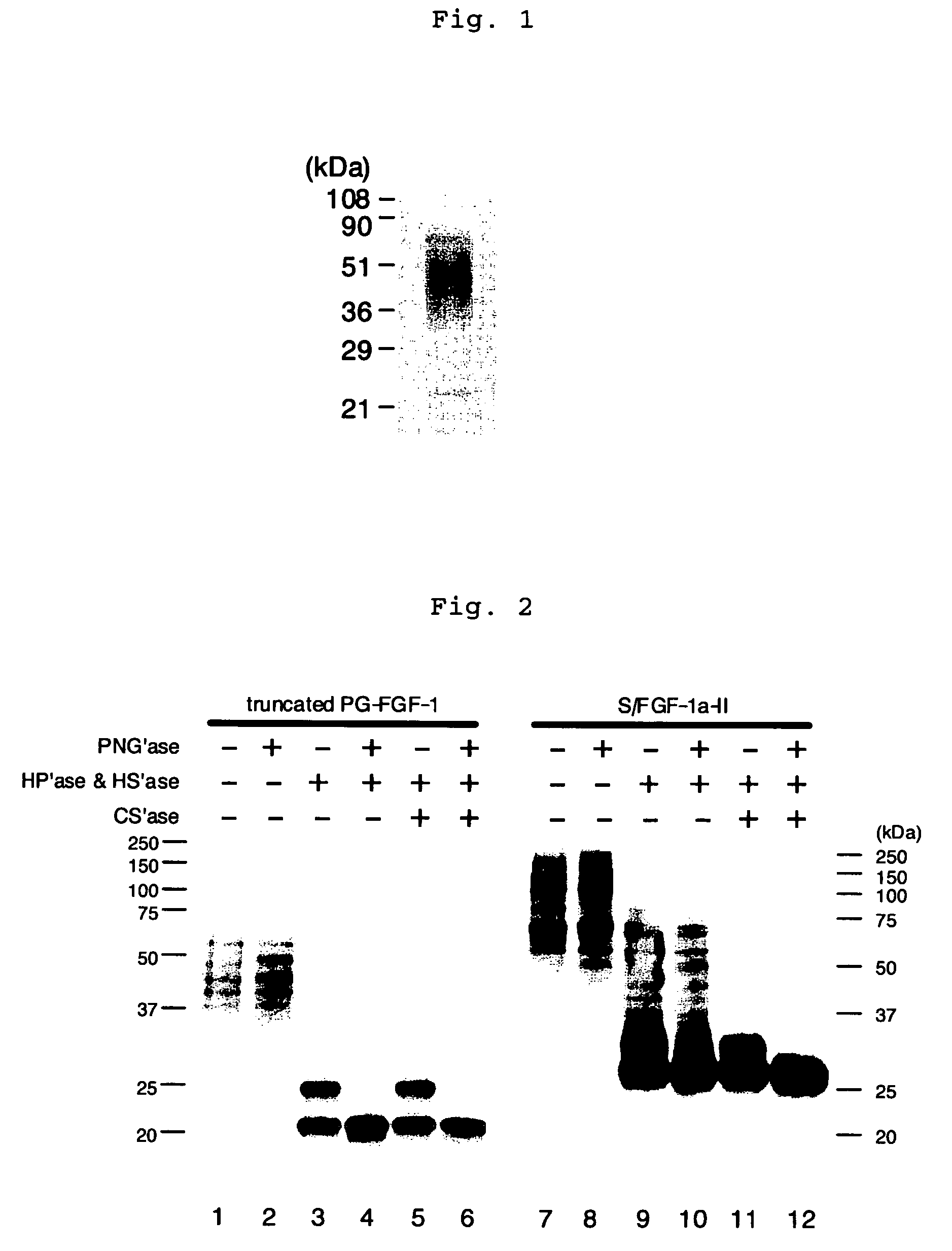
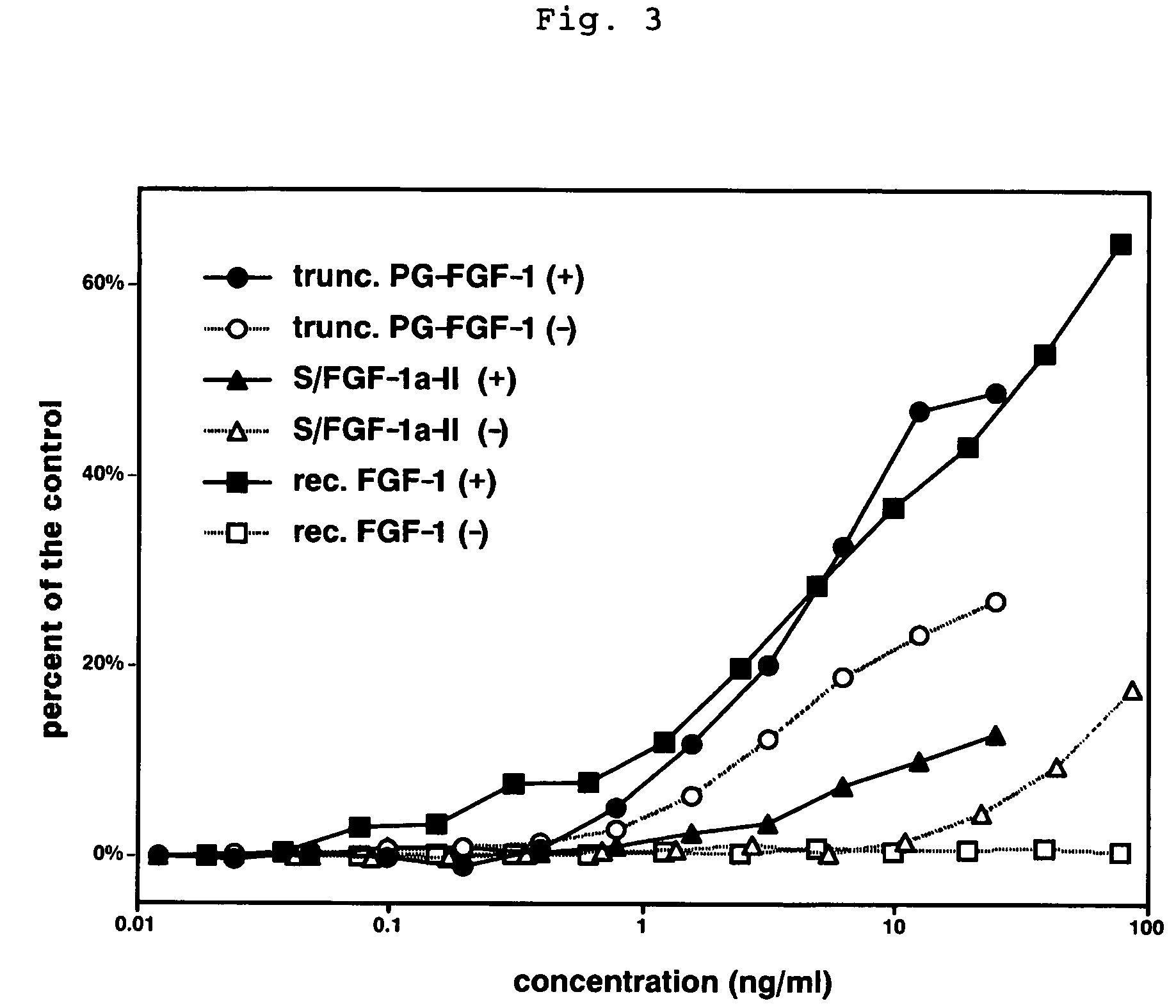
A heparin-binding protein having covalently bonded heparan sulfate sugar chains within its molecule is produced by ligating a cDNA encoding a peptide which can be modified with heparan sulfate sugar chains selectively to a cDNA encoding a heparin-binding protein and producing in an animal cell the gene product of the resultant ligated cDNA. This heparan sulfate sugar chain-modified heparin-binding protein is functionalized by covalently bonding thereto glycosaminoglycan sugar chains containing little chondroitin sulfate. For example, this heparin-binding protein is excellent in stabilities, such as thermostability, acid resistance, alkali resistance and in vivo stability. Further, the heparan sulfate sugar chain-modified heparin-binding protein is effective in cell proliferation and tissue regeneration, and has effect of regulating the physiological functions of FGFs. Thus, this heparin-binding protein is extremely useful as a medicine for preventing or treating various FGF-related diseases.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Heparin binding protein detection test paper for immunomicrosphere chromatography detection
InactiveCN108267592ASimple clinical operationHigh sensitivityBiological testingMicrosphereFluorescence
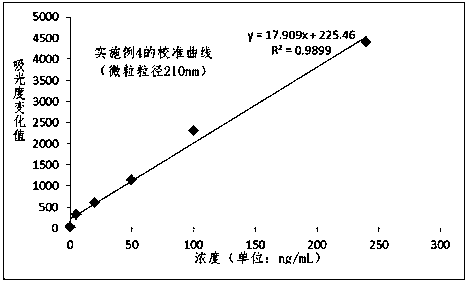
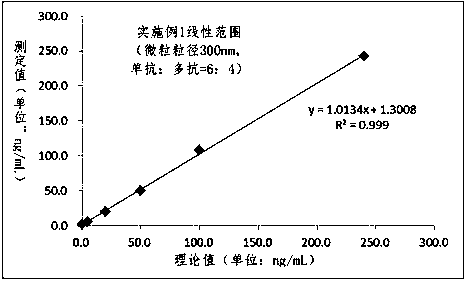
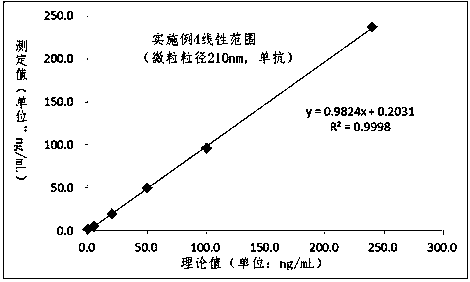
The invention relates to the technical field of clinical infectious disease marker detection, in particular to heparin binding protein detection test paper for immunomicrosphere chromatography detection. A method includes the following steps that a calibration curve is prepared; a detection buffer solution containing rabbit anti-human heparin binding protein antibody is prepared, fluorescent or colorful latex microsphere-marked chicken anti-rabbit antibody is prepared, a sample pad coats a solid phase, a detection line coated with a mouse anti-human heparin binding protein antibody and a reaction pad coated with a goat anti-rabbit antibody quality control line are prepared, a separation membrane is assembled, a test paper strip is assembled, a clinical sample is detected, and the dried test paper strip is placed in an aluminum foil bag. The heparin binding protein detection test paper for fluorescence chromatography detection has high cost effectiveness, and the level of heparin binding protein in body fluids of patients is quantitatively detected and evaluated. The method is used for a POCT detection system and screening or detection of changes of heparin binding protein in the body fluids of the patients to determine a detection method of infectious disease markers so that the heparin binding protein detection technology can become a clinical conventional detection project.
Owner:PRO MED BEIJING TECH
Kit for detecting heparin binding protein through immunofluorescence chromatography and preparation method of kit
The invention relates to the technical field of medical supplies, and particularly discloses a kit for detecting heparin binding protein through immunofluorescence chromatography and a preparation method of the kit. The kit comprises a first buffer solution, a second buffer solution and a reagent card. The first buffer solution is a phosphate buffer solution containing rabbit anti-human heparin binding protein antibodies, wherein the concentration of the rabbit anti-human heparin binding protein antibodies is 0.83-2 nanograms per microliter. The second buffer solution is a phosphate buffer solution containing fluorescently-labeled affinipure chicken anti-rabbit, wherein the concentration of fluorescently-labeled affinipure chicken anti-rabbit is 0.05-0.2 microgram per microliter. The reagent card comprises a plastic cushion plate. A nitrocellulose membrane is arranged on the plastic cushion plate. A detection line and a quality control line are arranged on the nitrocellulose membrane in a spaced and left-right mode, a glass cellulose membrane is arranged on the portion, on the left side of the detection line, of the nitrocellulose membrane. A piece of water absorption paper is arranged on the portion, on the right side of the quality control line, of the nitrocellulose membrane. A blood filter membrane is arranged on the glass cellulose membrane. The detection line is wrapped by rabbit anti-human heparin binding protein antibodies. The quality control line is wrapped by goat anti-rabbit antibodies. The kit has a good linear range and is high in flexibility and accuracy.
Owner:河南生生医疗器械有限公司
HBP magnetic particle chemiluminiscence method detection kit and preparation method thereof
ActiveCN111679086AReduce dosageLow costChemiluminescene/bioluminescenceDisease diagnosisBovine serum albuminStreptavidin
The invention discloses a magnetic particle chemiluminiscence detection kit for heparin-binding protein, a preparation method of the magnetic particle chemiluminiscence detection kit and application of the magnetic particle chemiluminiscence detection kit to immunological detection of the heparin-binding protein. The magnetic particle chemiluminiscence detection kit comprises a streptavidin-coatedmagnetic particle suspension solution, a biotin-labeled heparin-binding protein capture antibody and biotin-labeled bovine serum albumin mixed solution, a luminous marker coupled heparin-binding protein detection antibody solution, a heparin-binding protein series calibration product and a quality control product. The kit provided by the invention utilizes the advantages of a magnetic particle chemiluminescence detection method, and has the characteristics of simple operation, strong specificity, high sensitivity, wide linear range and the like when a full-automatic chemiluminescence immunoassay analyzer is used for detecting the heparin-binding protein in a specimen.
Owner:SUZHOU KANGHESHUN MEDICAL TECH
Heparin binding protein detection kit
The invention provides a heparin binding protein detection kit. A double-antibody sandwich method is adopted and is characterized by comprising the following steps: coating micropores of an ELISA plate with HBP monoclonal antibodies, diluting HBP in plasma as antigen substance by using a sample diluting solution, and combining the antigen substance with the HBP monoclonal antibodies coating a solid phase to form a solid phase-antibody-antigen complex in a first incubation period; fully washing uncombined components by using a washing buffer solution, adding an enzyme conjugate for incubation, and combining the enzyme conjugate with the solid phase-antibody-antigen complex; further washing, removing uncombined components, adding a substrate for incubation, catalyzing the substrate via enzyme to prepare a coloured product, adding a stop solution for stopping reacting, and detecting optical density values of various pores via an enzyme labeling instrument, wherein the size of the optical density values is in positive correlation with the concentration of HBP in the plasma. The heparin binding protein detection kit is a novel immune detection kit for quantitatively detecting heparin binding protein in human plasma in vitro and can contribute strength to medical examination and human health.
Owner:安徽同致生物工程股份有限公司
Detection kit for heparin binding protein and preparation method thereof
The invention discloses a detection kit for heparin binding protein and a preparation method thereof. The kit is a latex-enhanced immunoturbidimetry detection reagent. The latex-enhanced immunoturbidimetric detection reagent is prepared from the following main components of: buffering solution, a surface active agent, diluted liquid R1 of salt and preservative, and reaction liquid R2 containing latex particles labeled by HBP (Heparin Binding Protein) antibodies, buffering solution, the salt, a stabilizer, a suspending agent and the preservative, HBP calibration products and quality control products. The invention also discloses a method for detecting the concentration of the heparin binding protein (HBP) in a blood sample by using the kit and utilizing the transmitting or scattering turbidimetric principle. The detection kit disclosed by the invention adopts dual reagents, is simple in operation, high in sensitivity and wide in linear range, can be widely applied to various transmitting or scattering analyzers including common biochemical analyzers and specific protein analyzers and the like.
Owner:SUZHOU KANGHESHUN MEDICAL TECH
Kit and method for detecting heparin binding protein by dry-type quantitative immunofluorescence technology
InactiveCN108956998ASimple and fast operationAccurate operationBiological material analysisBiological testingBlood plasmaBiology
The invention relates to the technical field of immunoassay, and especially relates to a kit for detecting a heparin binding protein by a dry-type quantitative immunofluorescence technology, and a method for detecting the heparin binding protein. The kit uses the dry-type quantitative immunofluorescence technology to in vitro quantitatively detect the heparin binding protein in human blood plasma,is a novel immunoassay kit, overcomes personal errors of an EIA technology in the technical aspects (such as skill, temperature, time and result judgment) of an operator, can provide a powerful experiment diagnosis basis for clinic diagnosis, curative effect observation and prognosis judgment by quantitatively and objectively reflecting the existence of the HBP, and is deeply widely accepted by doctors and patients. The method for detecting the heparin binding protein has the characteristics of high specificity, good repeatability, high detection sensitivity, wide linear range, high automation degree, and simplicity and rapidness in operation; and compared with the EIA method, the method in the invention has the advantages of rapidness, simplicity, accuracy, and small interference.
Owner:HANGZHOU JOINSTAR BIOTECH
Growth factor modified extracellular matrix material and methods for preparation and use thereof
ActiveUS20080299171A1Promote wound repairPromote repairPeptide/protein ingredientsMetabolism disorderCell-Extracellular MatrixECM Protein
Described are tissue graft constructs that include submucosa and other extracellular matrix materials that incorporate a number of exogenous proteins. Further described are methods for making tissue graft constructs that include stripping endogenous heparin binding proteins from a porcine graft material and thereafter binding one or more human growth factors to the native heparin molecules that are retained within the graft material. Such graft materials may be used in methods for the treatment of wounds in patients.
Owner:COOK BIOTECH
Human heparin binding protein assay kit with high sensitivity and wide detection range
InactiveCN109613259AWide detection rangeGuaranteed SensitivityBiological testingMicrosphereConcentration gradient
The embodiments of the invention relate to the field of immunoassay, and in particular to a human heparin binding protein assay kit with high sensitivity and wide detection range. The human heparin binding protein assay kit comprises: a reagent 1, a reagent 2 and human HBP calibrators with different concentration gradients. The reagent 2 comprises a carboxyl latex microsphere labeled only with human HBP monoclonal antibody and a carboxyl latex microsphere labeled only with human HBP polyclonal antibody. The average particle diameter of the carboxyl latex microsphere labeled only with the humanHBP monoclonal antibody > the average particle diameter of the carboxyl latex microsphere labeled only with the human HBP polyclonal antibody. The human heparin binding protein assay kit provided bythe invention can complete a single sample test within 10 minutes, and indexes of precision, accuracy and anti-interference are excellent. The invention can be used clinically to predict an organ dysfunction caused by sepsis, and can be used as an early diagnostic marker for sepsis, especially serious bacterial infection.
Owner:BEIJING BEIER BIOENG
Method of rapidly detecting concentration of heparin-combined protein in blood
The invention discloses a method of rapidly detecting concentration of heparin-combined proteins in blood. By means of a latex enhanced turbidimetric immunoassay method, an heparin-combined protein antibody is coated with latex particles to produce an antibody-latex particle composite; when the antibody-latex particle composite is specifically combined with the heparin-combined proteins in blood, the concentration of the heparin-combined proteins is measured by detecting the absorbance of a blood sample. The method is simple in operations, has high accuracy, allows accurate quantification and is suitable for detection of the heparin-combined proteins.
Owner:NINGBO ACCUTECH BIOSCI LTD
Haparin-Binding Protein Modified with Heparan Sulfate Sugar Chains, Process for Producing the Same and Pharmaceutical Compositions Containing the Same
A heparin-binding protein having covalently bonded heparan sulfate sugar chains within its molecule is produced by ligating a cDNA encoding a peptide which can be modified with heparan sulfate sugar chains selectively to a cDNA encoding a heparin-binding protein and producing in an animal cell the gene product of the resultant ligated cDNA. This heparan sulfate sugar chain-modified heparin-binding protein is functionalized by covalently bonding thereto glycosaminoglycan sugar chains containing little chondroitin sulfate. For example, this heparin-binding protein is excellent in stabilities, such as thermostability, acid resistance, alkali resistance and in vivo stability. Further, the heparan sulfate sugar chain-modified heparin-binding protein is effective in cell proliferation and tissue regeneration, and has effect of regulating the physiological functions of FGFs. Thus, this heparin-binding protein is extremely useful as a medicine for preventing or treating various FGF-related diseases.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Kit for detecting human heparin binding protein and preparation method thereof
PendingCN110806487AHigh detection sensitivityWide detection linear rangeBiological testingMicrosphereMonoclonal antibody
The application discloses a kit for detecting human heparin binding protein and a preparation method thereof. The kit for latex enhanced immunoturbidimetry detection comprises a reagent 1, a reagent 2and a calibration product. The reagent 2 contains HBP-monoclonal-antibody-labeled large-sphere latex microspheres and HBP-monoclonal-antibody-labeled small-sphere latex microspheres, wherein a particle size difference between the large-sphere latex microsphere and the small-sphere latex microsphere is at least 200 nm and a surface charge difference between the small-sphere latex microspheres andthe large-sphere latex microspheres is at least 100 [mu] eq / g. According to the application, the large-sphere and small-sphere latex microspheres with the particle size difference value of at least 200nm and the surface charge difference value of at least 100 [mu]eq / g are used for HBP monoclonal antibody labeling to realize latex enhanced immunoturbidimetry detection, so that the detection sensitivity can be improved and the wider detection linear range can be obtained. A high-sensitivity and full-scale detection kit is provided for HBP clinical detection.
Owner:SHENZHEN AMTECH BIOENGINEERING LTD INC
Agent for promoting hepatic cell replication and agent for improving insulin resistance
InactiveUS20090081787A1Easy to copyUseful in treatmentMetabolism disorderDigestive systemCXCL10Subfamily
The present invention aims at providing a medicament and a method for promoting replication of hepatocytes and a medicament and a method for ameliorating insulin resistance. An effective amount of a neutralizing agent for CXCL10 belonging to a subfamily of chemokines which are heparin-binding proteins is administered to the hepatocytes to promote the replication of the hepatocytes. As the neutralizing agent for CXCL10, a factor which is specifically bound to CXCL10 and inhibits an activity of CXCL10 or a factor which inhibits CXCL10 expression is suitably used. By administering the neutralizing agent to impaired hepatic tissue, it is possible to restore and regenerate the hepatic tissue. Meanwhile, by administering an effective amount of the neutralizing agent for CXCL10 to the hepatocytes, the insulin resistance in type II diabetes and metabolic syndrome is ameliorated.
Owner:STELIC INST OF REGENERATIVE MEDICINE
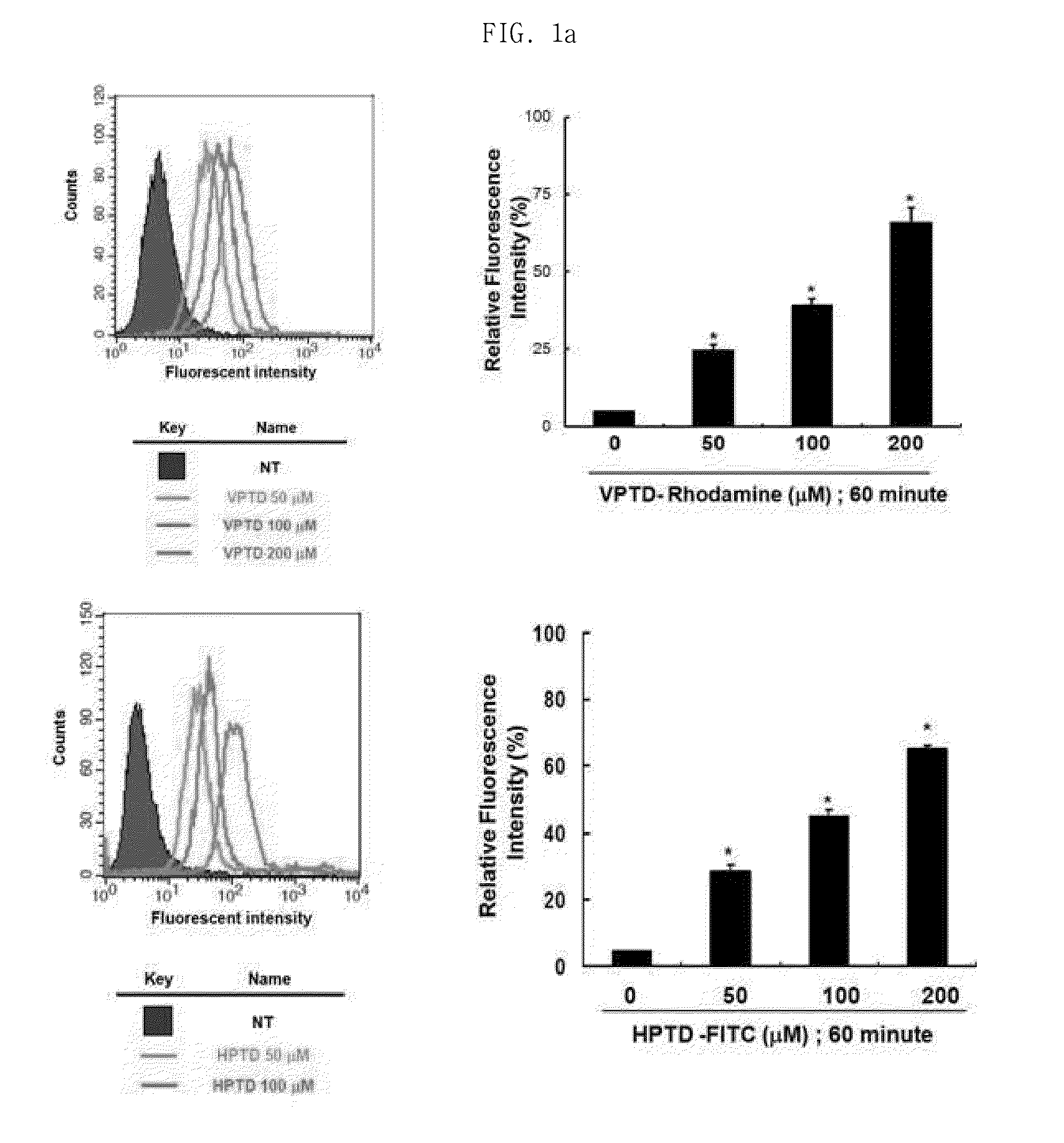
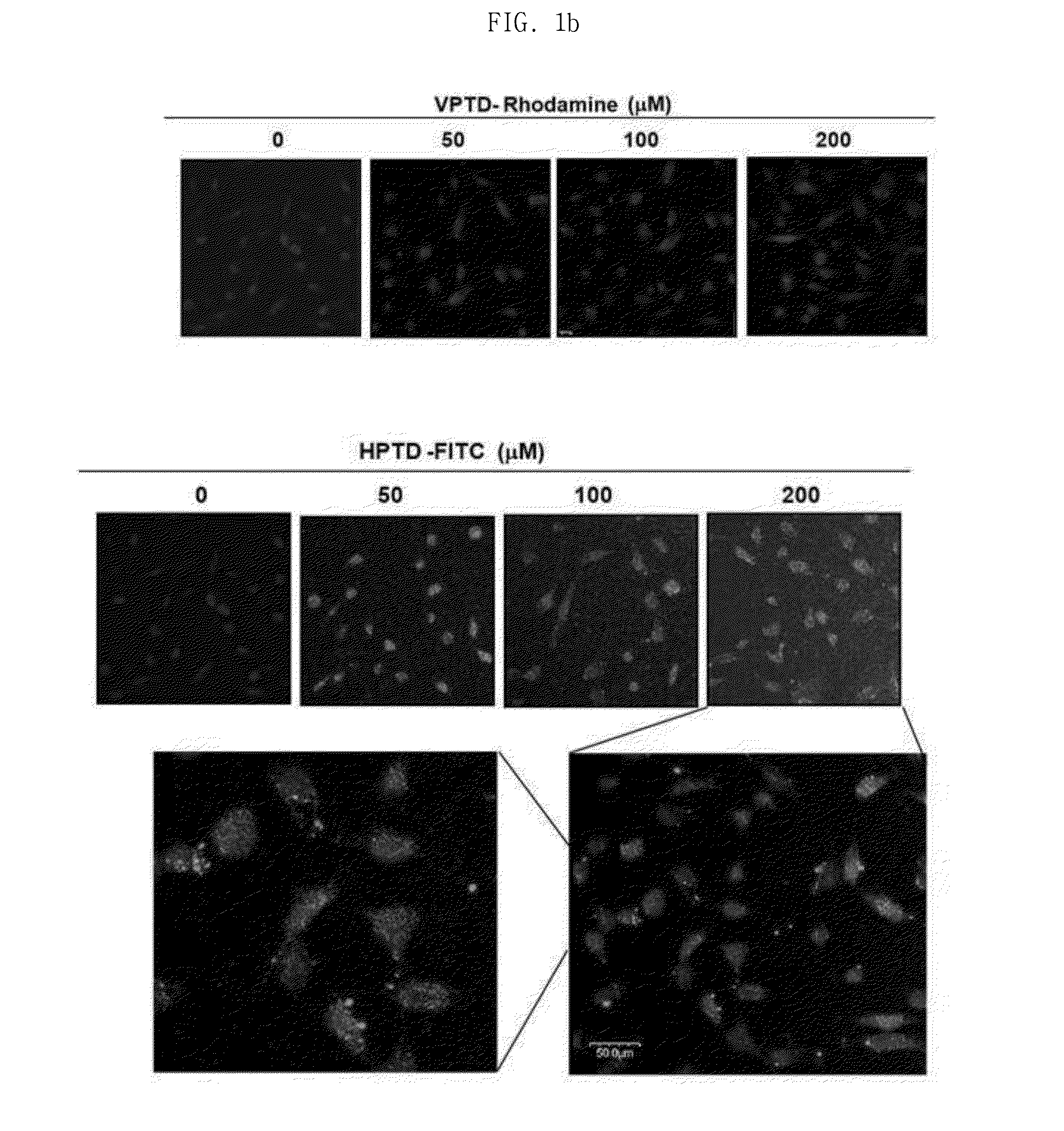
Peptide having cancer selective translocation function and use thereof
InactiveUS20150165060A1Minimizes problemQuality improvementPolypeptide with localisation/targeting motifOrganic active ingredientsSide effectProtein transduction domain
The present invention relates a peptide having cancer selective translocation function, and the use thereof, and more particularly to a VEGF-binding protein transduction domain (VPTD) represented as SEQ ID NO: 1 or a heparin-binding protein transduction domain (HPTD) represented as SEQ ID NO: 2, which bind specifically to VEGF and heparin, which are overexpressed specifically in tumor cells or tumor tissues, and to a conjugate comprising a drug linked to the peptide.The peptide and the peptide-drug conjugate bind specifically to VEGF and heparin in tumor cells or tumor tissue and accumulate in the tumor cells or tumor tissue, and thus can be used for the accurate diagnosis and treatment of cancer. Also, the non-specific distribution of the peptide or the conjugate in the body after administration is inhibited, and thus the side effects thereof can be minimized. Accordingly, the peptide or the conjugate is useful for the diagnosis and treatment of cancer.
Owner:SEOUL NAT UNIV R&DB FOUND +1
Dry immunofluorescence quantitative method heparin binding protein (HBP) detection kit
PendingCN111896730AImprove storage conditionsSolve instabilityMaterial analysisReagent stripCellulose
Owner:QINGDAO HIGHTOP BIOTECH
Reagent and method for measuring thrombin-antithrombin complex
ActiveUS20180238871A1Small amountAccurate measurementDisease diagnosisBiological testingDextranAmmonium chloride mixture
Owner:MITSUBISHI CHEM MEDIENCE
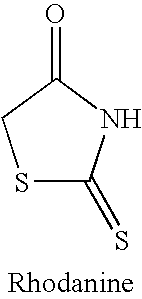
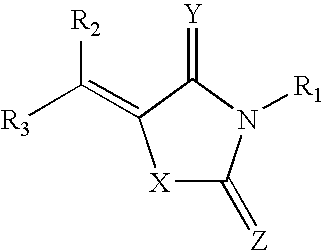
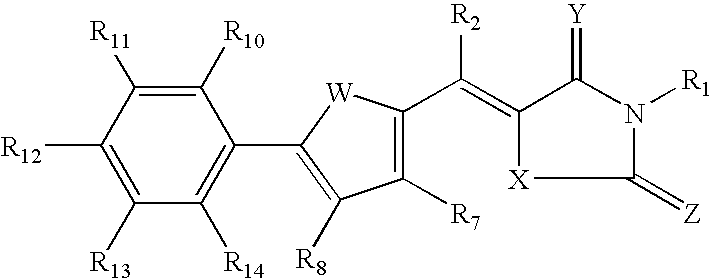
Rigidified Compounds for Modulating Heparanase Activity
Disclosed are novel rigidified compounds having a rhodanine-like residue and at least one aryl or heteroaryl residue linked to the rhodanine-like residue, whereby a core structure of these compounds, as defined in the specification, is characterized as having one or zero free-to-rotate bonds. Also disclosed are pharmaceutical compositions containing these rigidified compounds and uses thereof for modulating the activity of heparanase and hence in the treatment of heparanase-associated diseases and disorders, and uses thereof for modulating the activity of heparin-binding proteins and hence in the treatment of heparin-binding proteins-associated diseases and disorders as well as in the treatment of medical conditions that are at least partially treatable by rhodanine or a rhodanine analog.
Owner:INSIGHT BIOPHARMLS
Single-reagent heparin binding protein detection reagent kit and method for preparing same
InactiveCN108051439AChange the degree of scatteringChange absorbanceMaterial analysis by observing effect on chemical indicatorScattering properties measurementsPreservativeLatex particle
The invention discloses a single-reagent heparin binding protein (HBP) detection reagent kit and a method for preparing the same. The single-reagent heparin binding protein detection reagent kit is asingle-reagent detection reagent kit. Main components of the single-reagent detection reagent kit include reaction liquid, standard products with HBP and quality control products. The reaction liquidcomprises HBP antibody labeling latex particles and further comprises buffer solution, surfactants, salt, stabilizers, suspending agents and preservatives. The invention further discloses a method fordetecting the concentration of the HBP in blood samples by the aid of the single-reagent heparin binding protein detection reagent kit. The single-reagent heparin binding protein detection reagent kit and the methods have the advantage that the single-reagent heparin binding protein detection reagent kit comprises a single reagent, is easy to operate, high in sensitivity and wide in linear rangeand can be widely applied to various transmission or scattering analyzers including universal biochemical analyzers, specific protein analyzers and the like.
Owner:SUZHOU KANGHESHUN MEDICAL TECH
Heparin-binding proteins modified with sugar chains, method of producing the same and pharmaceutical compositions containing same
InactiveUS20060079451A1Function increaseImprove in vivo stabilityOrganic active ingredientsNervous disorderSugarBULK ACTIVE INGREDIENT
A heparin-binding protein functionalized by covalently bonding thereto a sugar chain, a method for producing the protein and a pharmaceutical composition containing the protein as an active ingredient, as well as a method of functionalizing a natural protein having no sugar chain by covalently bonding thereto a sugar chain.
Owner:MALVERN INSTRUMENTS
Expression of heparin-binding protein in recombinant mammalian cells
The present invention relates to a method for producing a mammalian heparin-binding protein in a mammalian cell that can be cultured under anaerobic conditions after introducing a nucleic acid encoding as heparin-binding protein into said cell with comprising (a) introducing into said mammalian cell a nucleic acid encoding said heparin-binding protein; (b) culturing the cell of step (a) under conditions conducive to expression of said HBP; and (c) recovering said HBP from the culture medium. Furthermore, the invention relates to the recombinant mammalian cell used in the method.
Owner:LEUKOTECH
Kit for quantitatively detecting HBP by using magnetic micro-particle chemiluminiscence, preparation method and detection method thereof
PendingCN112255416AGood repeatabilityGood precisionChemiluminescene/bioluminescenceBiological material analysisAntigenQuantitative determination
The invention relates to a kit for quantitatively detecting HBP by using magnetic micro-particle chemiluminiscence, a preparation method and a detection method thereof. The kit comprises an HBP calibrator, an HBP reagent 1, an HBP reagent 2, an HBP magnetic separation reagent and an HBP quality control material, wherein the HBP calibrator comprises six levels, and is composed of HBP antigens withdifferent concentrations and a Tris buffer solution containing bovine serum albumin, the HBP reagent 1 is prepared from biotin ester labeled HBP-Ab and a Tris buffer solution containing bovine serum albumin, the HBP reagent 2 comprises an alkaline phosphatase labeled HBP-Ab and a Tris buffer solution containing bovine serum albumin, the HBP magnetic separation reagent comprises streptavidin labeled magnetic micro particles and a Tris buffer solution containing bovine serum albumin, and the HBP quality control material comprises two quality control levels, and is composed of HBP antigens with different concentrations and a Tris buffer solution containing bovine serum albumin. The kit can be prepared at a low cost, and can realize accurate and high-precision quantitative determination of human heparin binding protein (HBP).
Owner:BEIJING LEADMAN BIOCHEM
Human heparin binding protein immunoturbidimetry detection reagent and application thereof
The embodiment of the invention discloses a kit for detecting heparin binding protein in a sample. The kit comprises a pretreatment reagent for pretreating a sample and a recognition reagent for recognizing heparin binding protein. The pretreatment reagent comprises a hemolytic agent and an electrolyte, the hemolytic agent is TritonX-100 with the concentration of 1-3 mg / mL, the electrolyte is sodium chloride with the concentration of 30-60 mg / mL, and the pH value of the pretreatment reagent is 5.5-6.5. The kit can be used for detecting the concentration of the heparin binding protein by using a whole blood sample, the blood sampling amount required for detection is small, and the detection time is short. The embodiment of the invention further discloses application of the kit in quantitative detection of the heparin binding protein and a method.
Owner:苏州优诺康生物技术有限公司
Heparin binding protein antibody as well as kit and application thereof
ActiveCN114133452AGood effectHigh sensitivityBiological material analysisGenetic engineeringMonoclonal antibody preparationMonoclonal
The invention relates to a pair of monoclonal antibodies which are used in pairs and can be specifically combined with a human heparin binding protein, a human heparin binding protein detection kit prepared from the monoclonal antibodies, and application of the monoclonal antibodies in detection of the human heparin binding protein. The monoclonal antibody pair for detecting the human heparin binding protein has the advantages of high sensitivity, wide linear range and universality of antibodies on different methodology detection platforms.
Owner:杭州伊佰新生物技术有限公司
Kit for high-specificity detection of heparin binding protein and application thereof
PendingCN113777326AAvoid interferenceImprove stabilityChemiluminescene/bioluminescenceDisease diagnosisAntigen bindingBiotin
The invention discloses a kit for high-specificity detection of heparin binding protein and application thereof, and relates to the technical field of immunodetection. The kit comprises an HBP binding antibody connected with fluorescein isothiocyanate, an HBP labeled antibody labeled with a luminous marker and a solid-phase carrier with the surface coated with a fluorescein isothiocyanate antibody, a sample is detected through a fluorescein isothiocyanate and anti-fluorescein isothiocyanate system, interference of endogenous biotin is well avoided, the specificity is high, the stability is good, and the kit has more advantages in detection limit.
Owner:北京森美希克玛生物科技有限公司
Heparin binding protein determination kit
ActiveCN112858696AStrong specificityGood repeatabilityDisease diagnosisBiological testingClinical diagnosisInternal medicine
The invention discloses a heparin binding protein determination kit. The heparin binding protein determination kit comprises a first reagent, a second reagent and a test strip, the heparin binding protein determination kit can quantitatively and objectively reflect the existence of HBP, and provides a more powerful experimental diagnosis basis for clinical diagnosis, curative effect observation and prognosis judgment. The heparin binding protein determination kit provided by the invention is high in specificity, good in repeatability, high in detection sensitivity, wide in linear range and high in stability.
Owner:江西英大生物技术有限公司
Heparin-binding proteins modified with sugar chains, method of producing the same and pharmaceutical compositions containing the same
InactiveUS7005415B1Function increaseImprove in vivo stabilityOrganic active ingredientsNervous disorderPharmaceutical drugBULK ACTIVE INGREDIENT
A heparin-binding protein functionalized by covalently bonding thereto a sugar chain, a method for producing the protein and a pharmaceutical composition containing the protein as an active ingredient, as well as a method for functionalizing a natural protein having no sugar chain by covalently bonding thereto a sugar chain.
Owner:NAT INST OF ADVANCED IND SCI & TECH
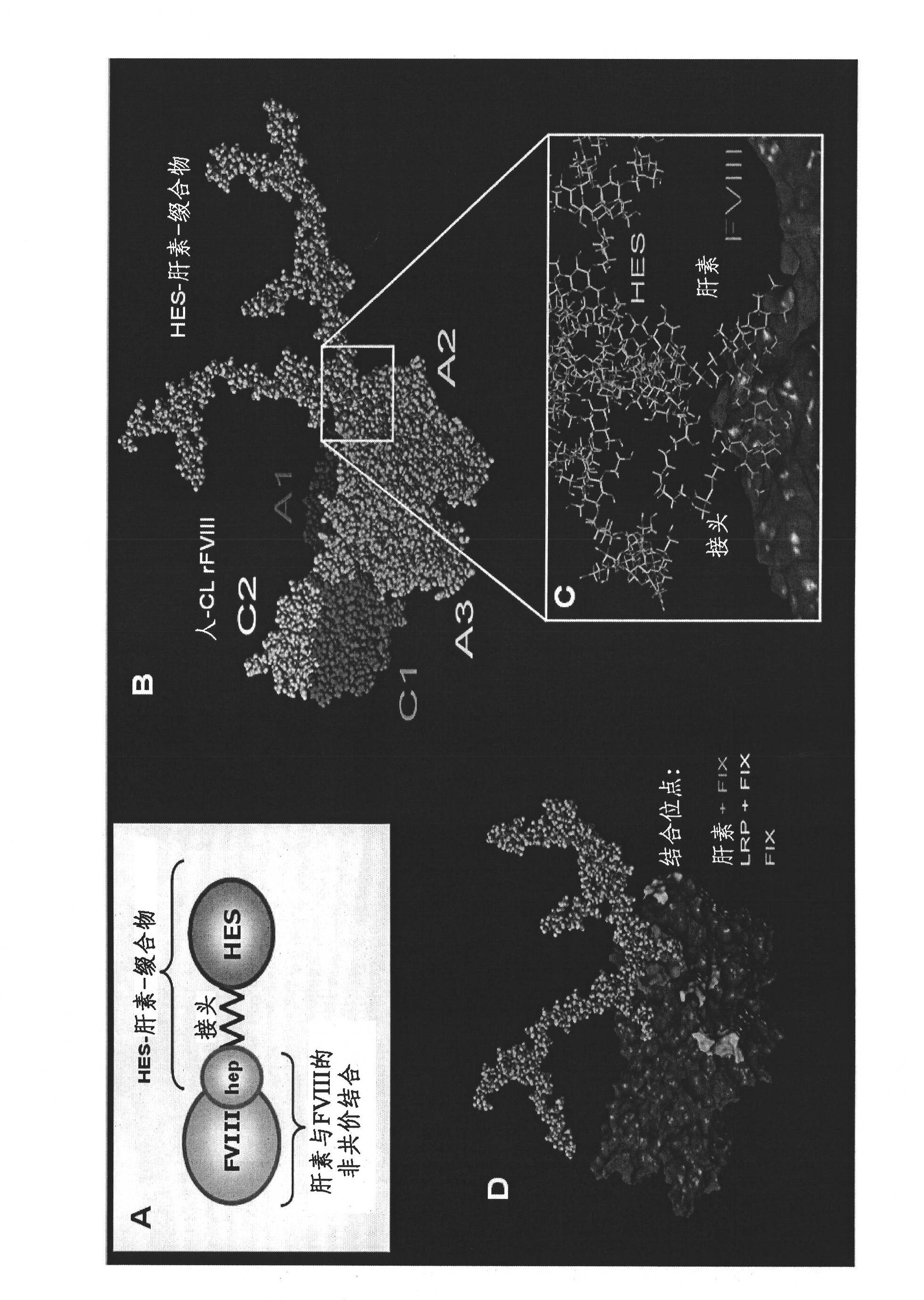
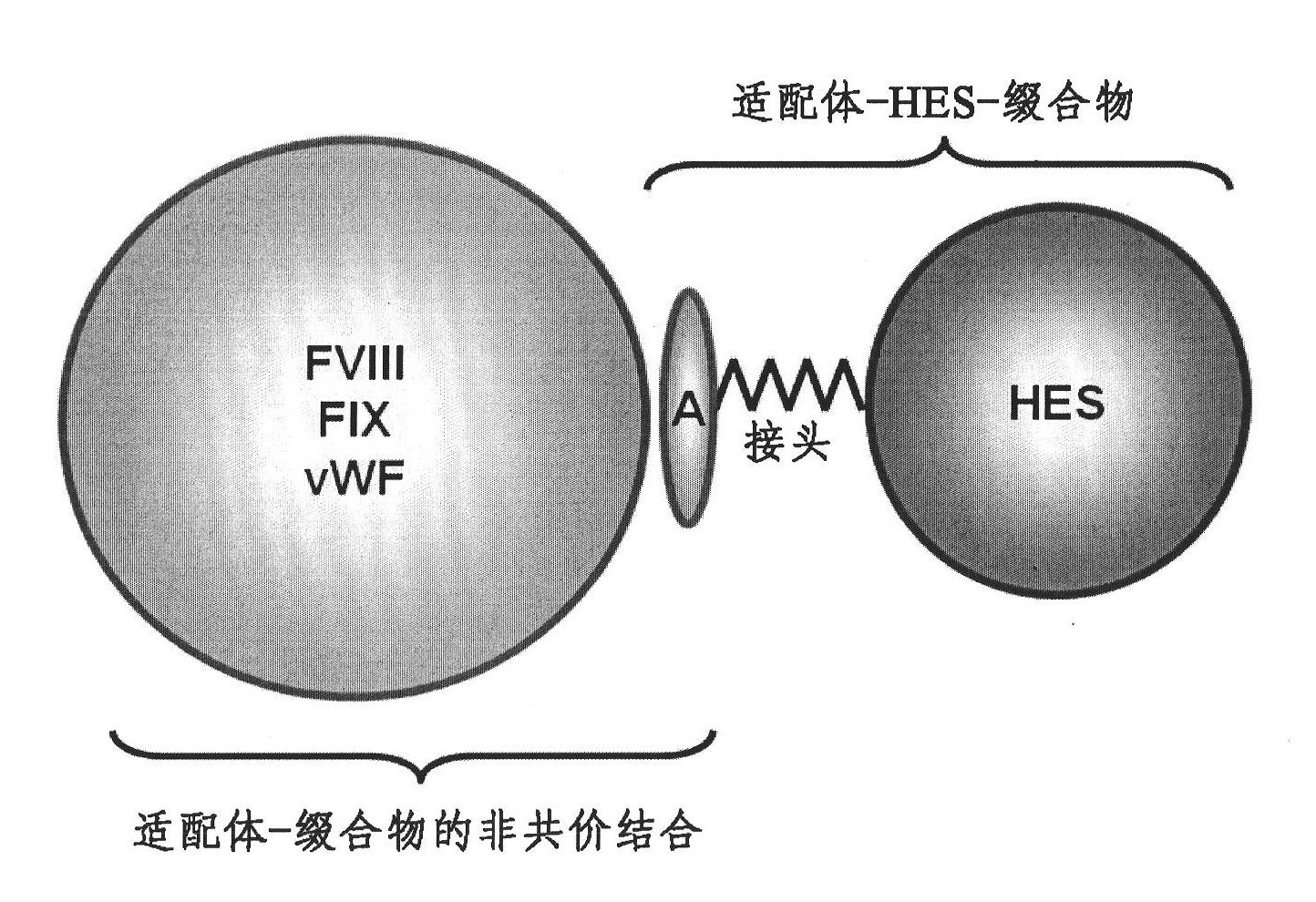
Complex comprising both heparin binding proteins and heparin-hydroxyalkyl starch conjugates
InactiveCN102083469ABinding affinityExtended half-lifePeptide/protein ingredientsPharmaceutical non-active ingredientsProtein targetSoluble polymer
A complex comprises at least one target protein and at least one binding molecule having a binding affinity for said target protein, wherein said molecule having a binding affinity is covalently or non-covalently bound to at least one water-soluble polymer.
Owner:OCTAPHARMA
Growth factor modified extracellular matrix material preparation and methods for preparation and use thereof
ActiveUS8591930B2Promote repairPromote tissue repairPeptide/protein ingredientsDepsipeptidesMaterials preparationCell-Extracellular Matrix
Described are tissue graft constructs that include submucosa and other extracellular matrix materials that incorporate a number of exogenous proteins. Further described are methods for making tissue graft constructs that include stripping endogenous heparin binding proteins from a porcine graft material and thereafter binding one or more human growth factors to the native heparin molecules that are retained within the graft material. Such graft materials may be used in methods for the treatment of wounds in patients.
Owner:COOK BIOTECH
Heparin-binding protein assay kit as well as preparation method and assay method thereof
PendingCN111929443AStrong specificityHigh detection sensitivityChemiluminescene/bioluminescenceDisease diagnosisImmune profilingEnzyme immunoassays
The invention discloses a heparin-binding protein assay kit applying a magnetic particle chemiluminescence enzyme immunoassay method. The heparin-binding protein assay kit comprises avidin-labeled magnetic particles, a biotin-labeled HBP antibody 1, a horseradish peroxidase-labeled HBP antibody 2, an HBP calibrator, a concentrated washing liquid and a substrate. The invention also discloses a preparation method and a determination method of the kit. The novel immunoassay kit disclosed by the invention is used for quantitatively assaying HBP in human body fluid in vitro, overcomes personal errors of a traditional enzyme immunoassay (EIA) method in the aspect of operation, can quantitatively and objectively reflect the existence of HBP, and provides a more powerful experimental diagnosis basis for clinical diagnosis, curative effect observation and prognosis judgment. The heparin binding protein determination method provided by the invention is high in specificity, good in repeatability,high in detection sensitivity, wide in linear range, high in automation degree and simple, convenient and rapid to operate; compared with an EIA method, the method has the advantages of rapidness, simplicity, convenience, accuracy and small interference.
Owner:HANGZHOU JOINSTAR BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com