Heparin binding protein antibody as well as kit and application thereof
A heparin-binding protein and antibody technology, which is applied in the field of immunoassay, can solve problems such as narrow linear range, low sensitivity of HBP paired antibody, and poor versatility of the methodological detection platform, achieving the effect of high sensitivity and wide linear range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
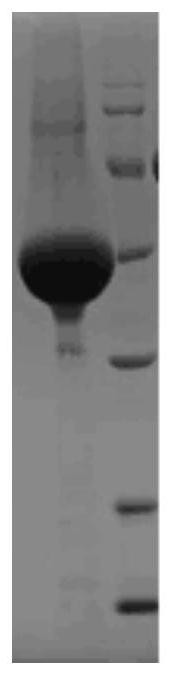
[0037] Embodiment 1, preparation HBP recombinant antigen
[0038] Plasmid construction: According to the HBP mature protein sequence SEQ ID No.1 of UniProtKB number P20160, the sequence was optimized according to the preferred codons of human genes, and the gene was entrusted to Shanghai Sangong Biosynthesis as shown in SEQ ID No.2. The 5' end of the gene has EcoRI restriction site, with BamHI restriction site at the 3' end, the synthetic gene is included in the puck57 plasmid. The puck57-HBP plasmid was digested with EcoRI and BamHI, and the excised fragments were recovered from the agarose electrophoresis gel and connected to the pcDNA3.1 vector after double digestion with EcoRI and BamHI. The positive clone pcDNA3.1-HBP obtained was passed through After the sequence identification was correct, a large amount of LB medium was inoculated, the bacteria were collected, and the endotoxin-free plasmid was extracted with the kit Endo-Free Plasmid Maxi Kit (OMEGA Company, product n...
Embodiment 2
[0041] Embodiment 2, preparation of HBP monoclonal antibody
[0042] Immunization of animals: Dilute the HBP recombinant antigen prepared in Example 1 to 1 mg / ml with 20 mM PBS pH 7.4, mix with adjuvant at a ratio of 1:1, and emulsify. Eight-week-old Balb / c mice were immunized with emulsified HBP antigen, 100ug per mouse, with complete Freund's adjuvant for the initial immunization, and incomplete Freund's adjuvant for booster immunization. After three injections of immunization, the tail vein blood was collected to determine the titer, and those with a titer over 1 million were selected for cell fusion. Impulse immunization was performed 72 hours before fusion, and 30-50ug of HBP antigen was injected into the tail vein.
[0043] Fusion cells: kill the mice to be fused, take the spleen, grind it in a 200-mesh sieve, and wash it with RPMI1640 medium at the same time, transfer the cell suspension to a 50ml centrifuge tube, wash it 4 times with RPMI1640 medium, Centrifuge at 15...
Embodiment 3
[0045] Embodiment 3, HBP antibody pairing
[0046] 23 monoclonal antibodies were labeled with sodium periodate method (refer to Guo Chunxiang, Guo Xiqiong. Introducing a simple, fast and efficient horseradish peroxidase-labeled antibody with sodium periodate method, Shanghai Journal of Immunology. 1983, 3(02)) Specifically: Dissolve 5mg of HRP in 0.5ml of water, add 0.5ml of fresh 0.06M NaIO4 aqueous solution, mix well, and place at 4°C for 30min. After taking it out, add 0.5ml of 0.16M ethylene glycol aqueous solution, and after standing at room temperature for 30min, add 1ml of aqueous solution containing 5mg of purified antibody, mix well and put in a dialysis bag to dialyze against 0.05M, pH9.51 carbonate buffer overnight. After taking it out, add 0.2ml of NaBH4 solution (5mg / ml) and put it in the refrigerator for 2h. Add an equal volume of saturated ammonium sulfate, place in the refrigerator for 30 minutes, centrifuge, resuspend the precipitate with 2.5ml 20mMPBS, add a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com