Complex comprising both heparin binding proteins and heparin-hydroxyalkyl starch conjugates
A technology for complexes and conjugates, applied in the field of preparing the complexes, capable of solving problems such as incomplete, uneven connection, inactivation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0183] Example 1: Synthetic chemistry: HES-heparanoid-conjugates
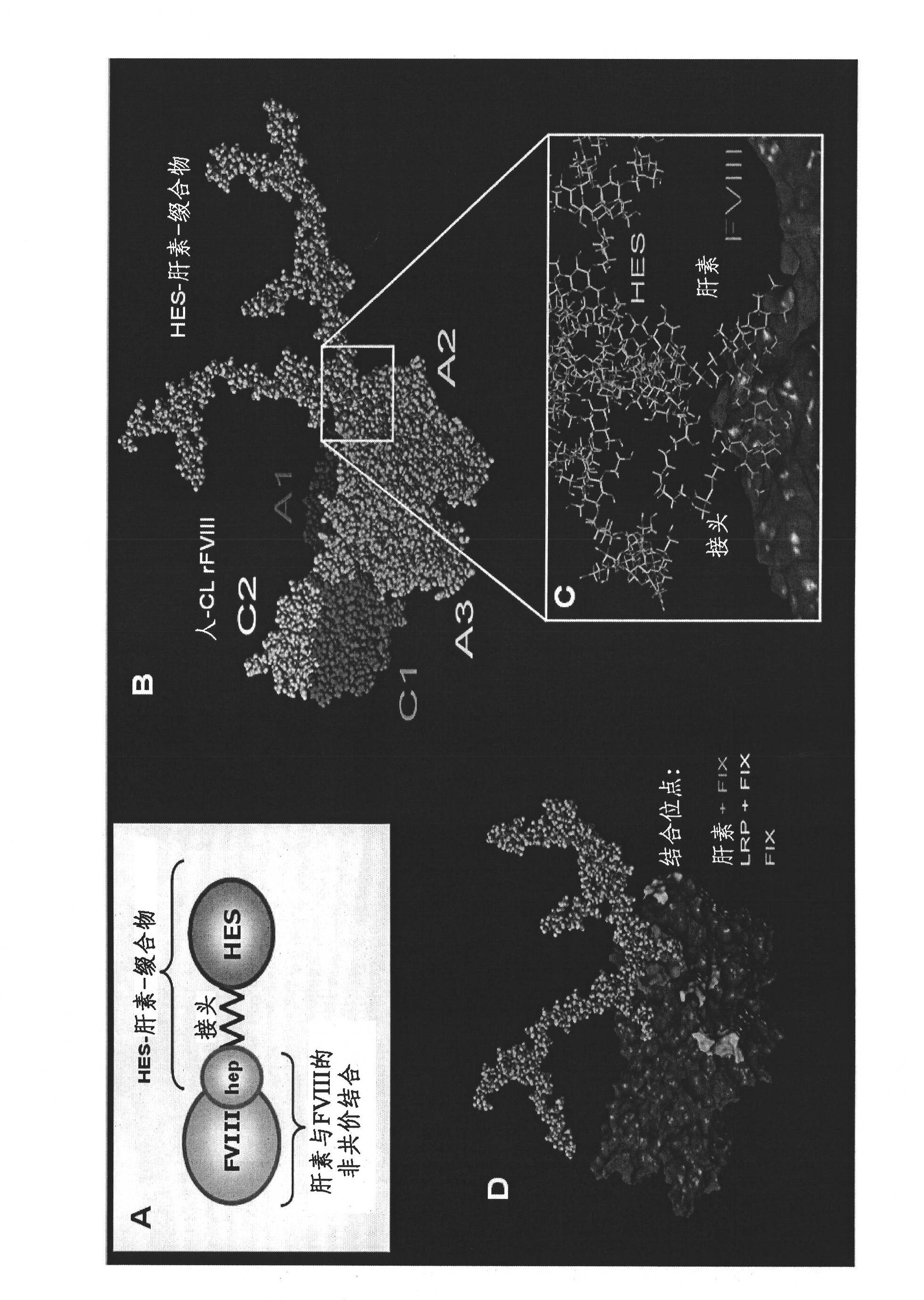
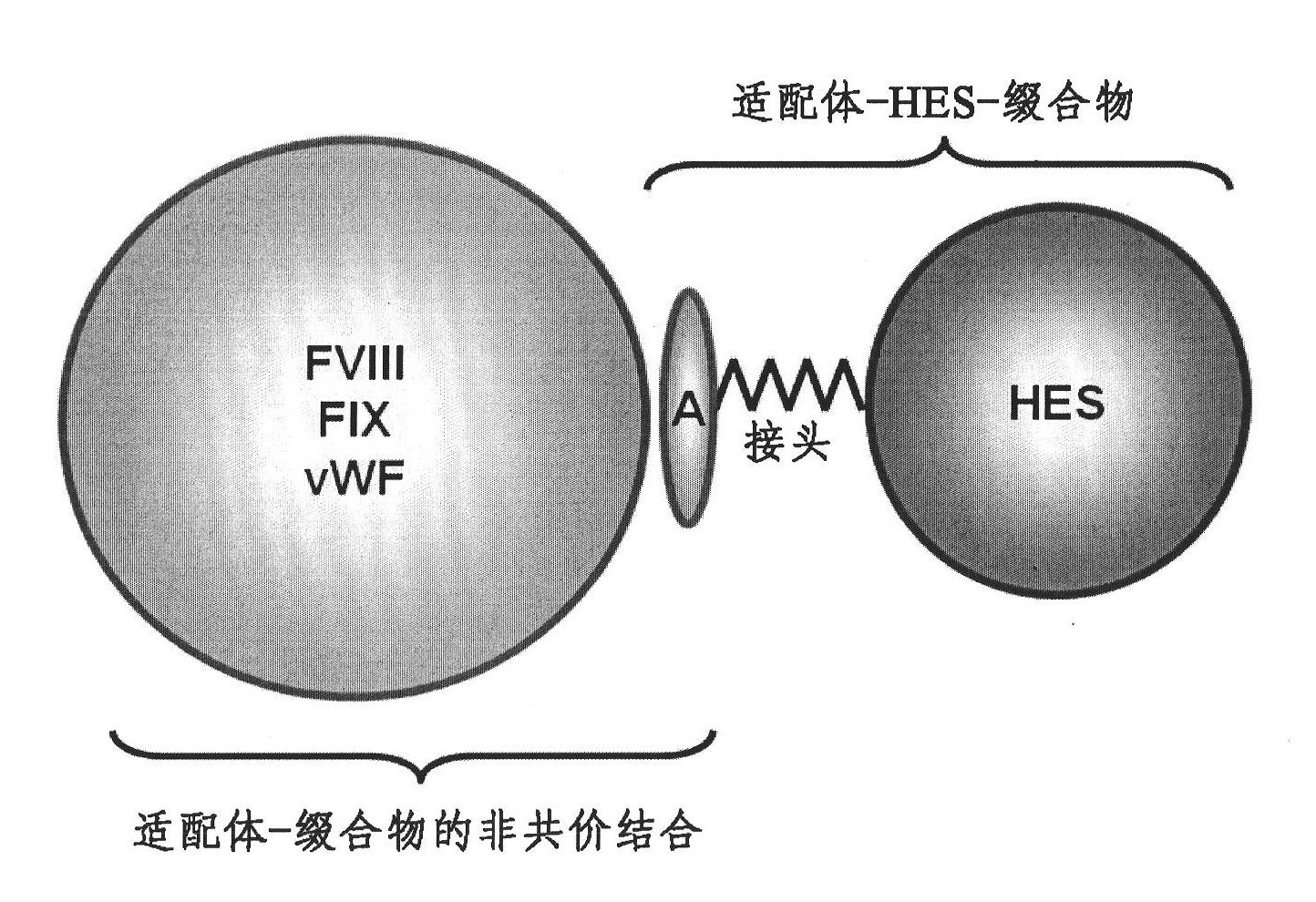
[0184] One approach to prolonging the in vivo half-life of recombinant Factor VIII is based on hydroxyethyl starch as a macromolecular carrier non-covalently attached via selective binding sites on Factor VIII. For such non-covalently linked heparins, derivatives or mimetics thereof can be used as binding molecules for Factor VIII. HES binds to these heparanoids to form large complexes that cover and protect parts of the protein surface.
[0185] The proof of principle was evaluated by a factor VIII binding study performed by surface plasmon resonance followed by a large-scale in vivo study using a covalent conjugate of heparinoid and hydroxyethyl starch (1:1) . figure 2 The three-dimensional structure of Factor VIII in a complex containing heparan-HES (1:1) conjugate is shown.
[0186] To obtain this 1:1 conjugate, both heparin and HES were chemically modified at only one position with a complementary fu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com