HBP magnetic particle chemiluminiscence method detection kit and preparation method thereof
A detection kit and magnetic particle technology, which is applied in the field of heparin-binding protein detection, can solve the problems that the accuracy of the test cannot be guaranteed, the clinical judgment basis cannot be provided, and interferences are removed, so as to reduce the steric hindrance effect and improve the detection sensitivity. and specificity, reducing the effect of non-specific reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Preparation of biotin-labeled HBP capture antibody solution
[0028] Dissolve biotin in dimethyl sulfoxide to 1mg / ml in advance, mix biotin-dimethyl sulfoxide solution and HBP capture antibody at a mass ratio of 1:10, control the volume within 100ul, add carbonate buffer The volume of the solution was made up to 150ul, and incubated at 37°C for 1 hour. After the reaction, the solution was replaced by ultrafiltration using a 50kDa ultrafiltration tube, and the ultrafiltrate was replaced 3 times during the reaction. The final antibody concentrate was collected in 50mM pH7.3 4-hydroxyethylpiperazineethanesulfonic acid (HEPES), 0.5% lauryl polyoxyethylene ether, 50mM potassium chloride, 2% sucrose, 4% arginine, 0.2 In % Proclin-300, the final concentration of biotinylated HBP capture antibody was 2 μg / mL.
[0029] (2) Preparation of biotin-labeled bovine serum albumin solution
[0030] Dissolve biotin in dimethyl sulfoxide to 1mg / ml in advance, mix biotin-dimethyl sul...
Embodiment 2
[0048] Analytical sensitivity of reagents (blank limit)
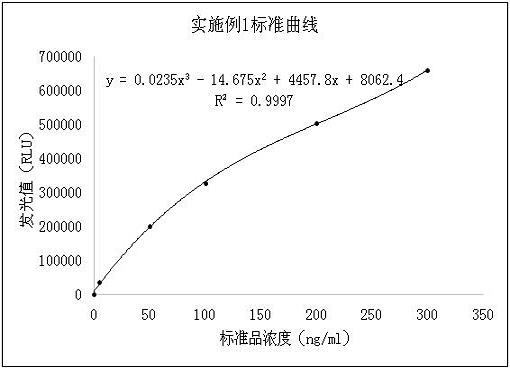
[0049] Using the reagents in Example 1 (4) and (5), select physiological saline as a blank sample test, repeat the test 20 times, test with a semi-automatic chemiluminescence immunoassay analyzer, and record the luminescence value (RLU). The specific data are shown in Table 2 . Calculate the mean value (X) and standard deviation (SD) of 20 test results, bring the calculated value of X±2SD into the standard curve established in Example 1 to calculate the concentration value, and the obtained concentration value is used as the analytical sensitivity of the reagent (blank limit ). The test results are shown in Table 2, the analytical sensitivity (blank limit) of the reagent in (4) is 0.34ng / mL, the analytical sensitivity (blank limit) of the reagent in (5) is 2.11ng / mL, (4 The analytical sensitivity of the reagents in (5) is much lower than that of the reagents in (5), so the reagents in (4) are selected to complete the ...
Embodiment 3
[0052] The specificity of embodiment 3 reagents
[0053] Using the reagents in Example 1 (4) and (5), add calibrator dilution (blank group) and calibration Different concentrations of specific interfering substances bilirubin (D experimental group), triglyceride (G experimental group), total protein (Z experimental group) and rheumatoid factor (L experimental group) were dissolved in the product diluent. The theoretical value reductions of the low-value quality control and high-value quality control in the group were 6.4 and 32 ng / mL. By comparing the test results of the blank group and the experimental group, the smaller the relative deviation between the average value of the five tests and the theoretical value, the better the specificity.
[0054] Table 3 Specificity detection results of Example 1 (4)
[0055]
[0056] Table 4 Specificity detection results of Example 1 (5)
[0057]
[0058] The relative deviation of the five detection results in Example 1 (4) is mu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com