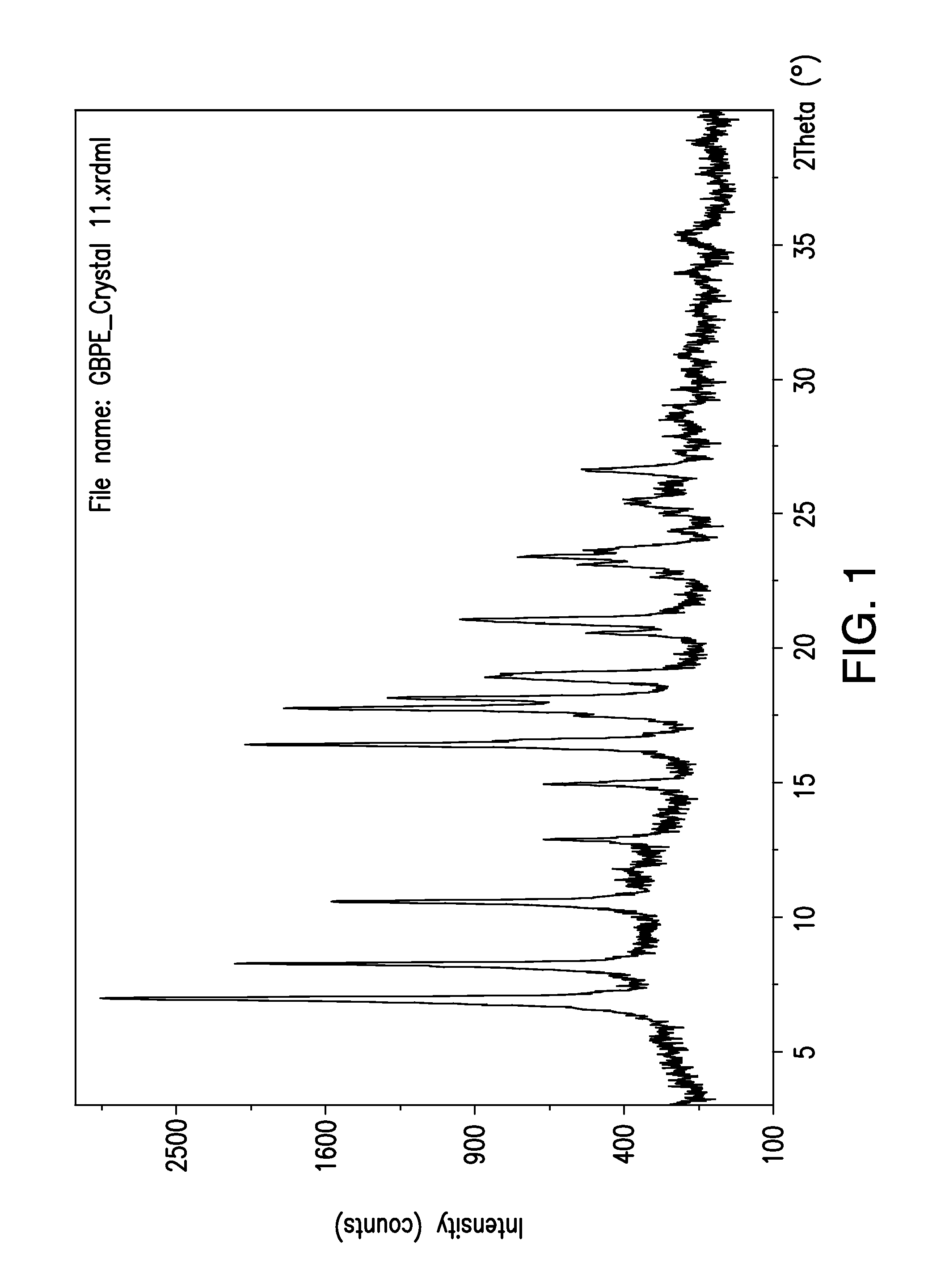
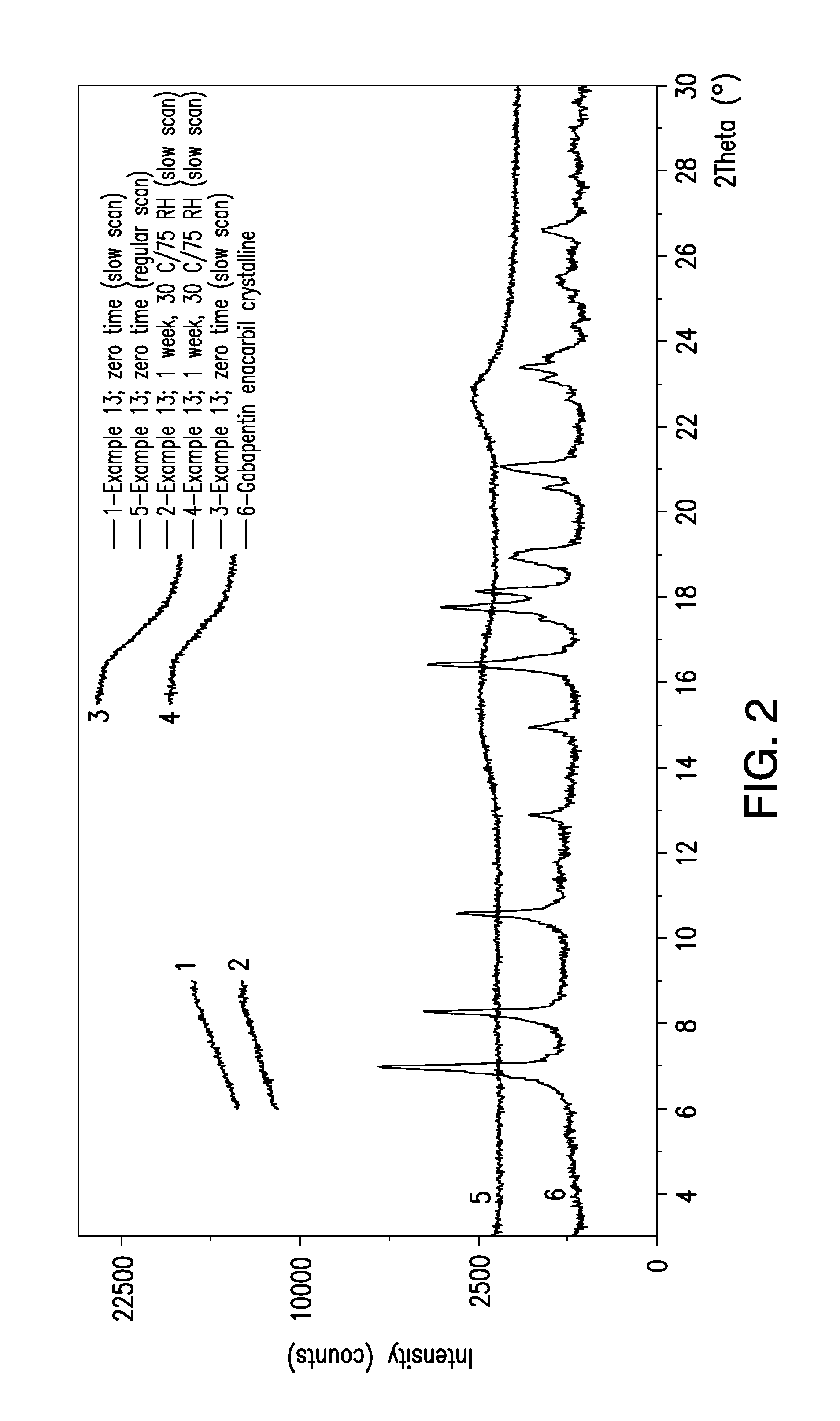
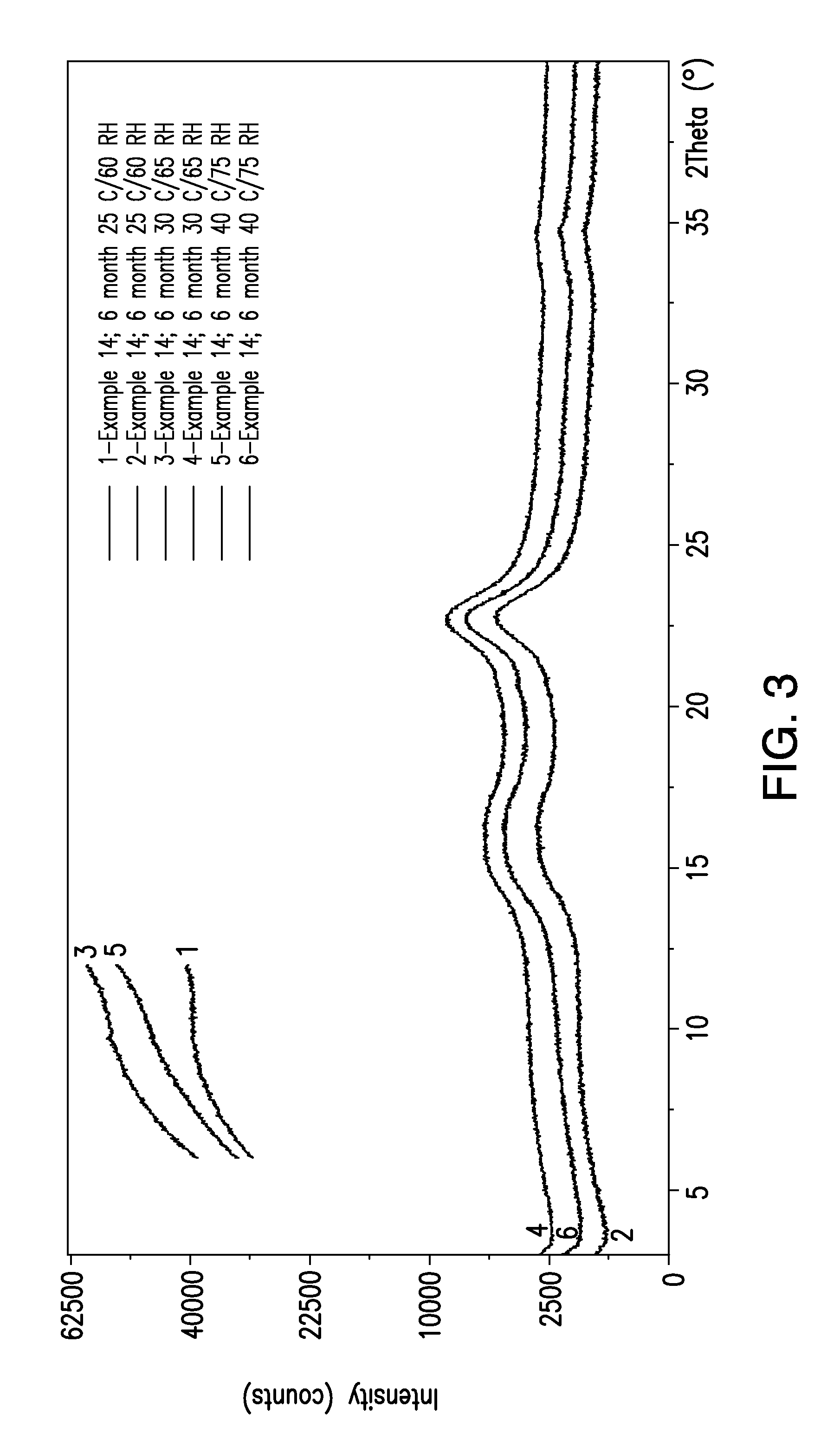
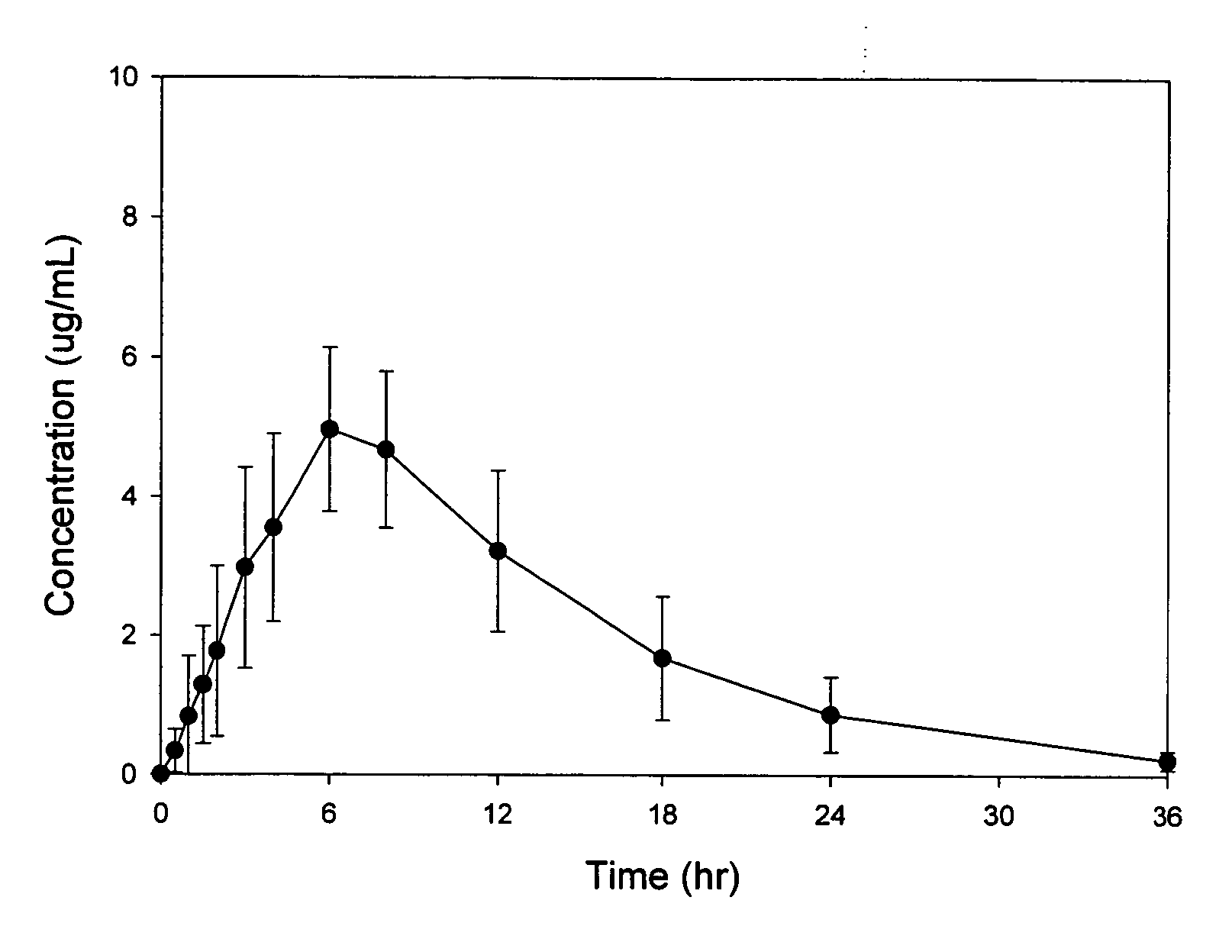
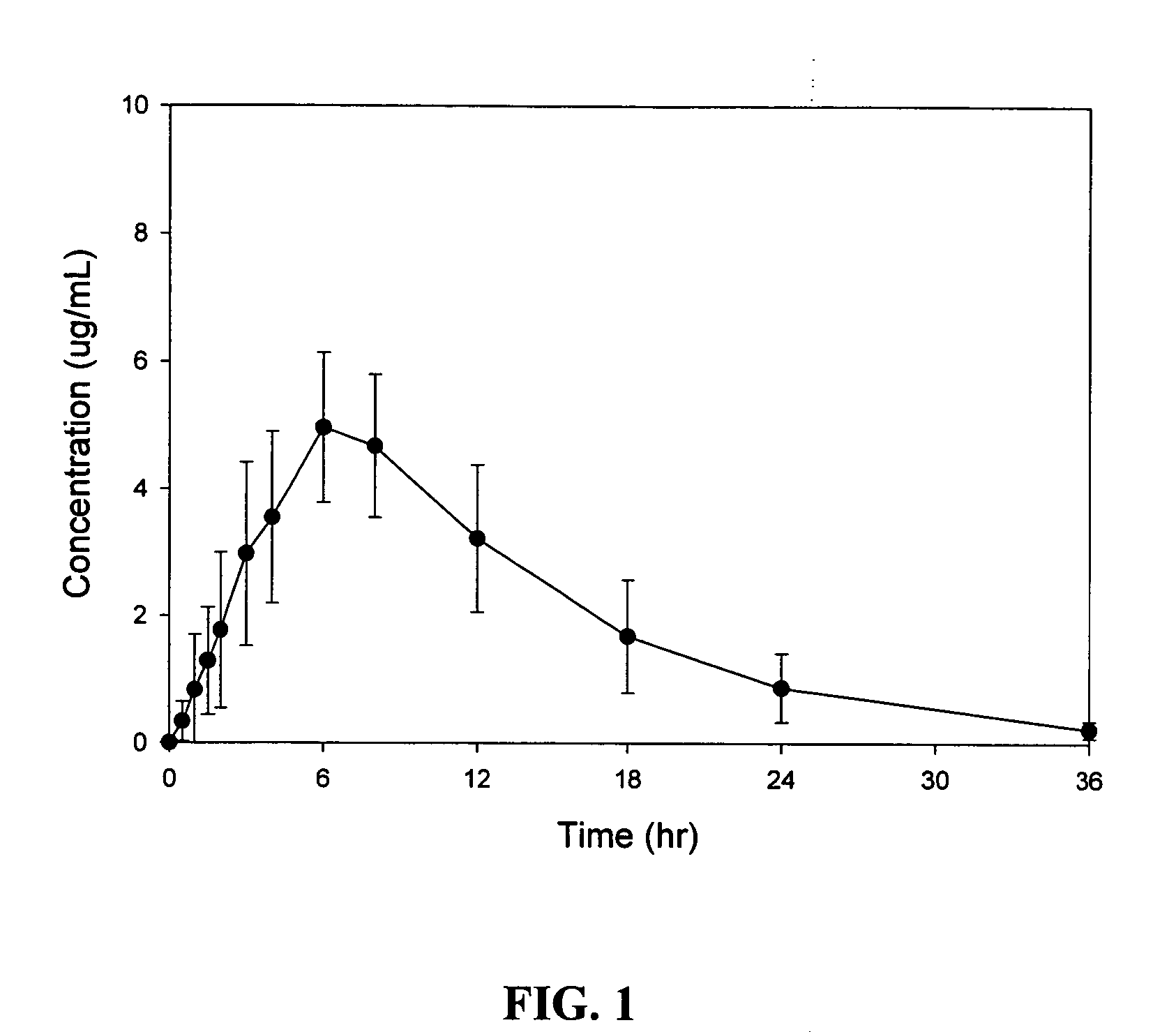
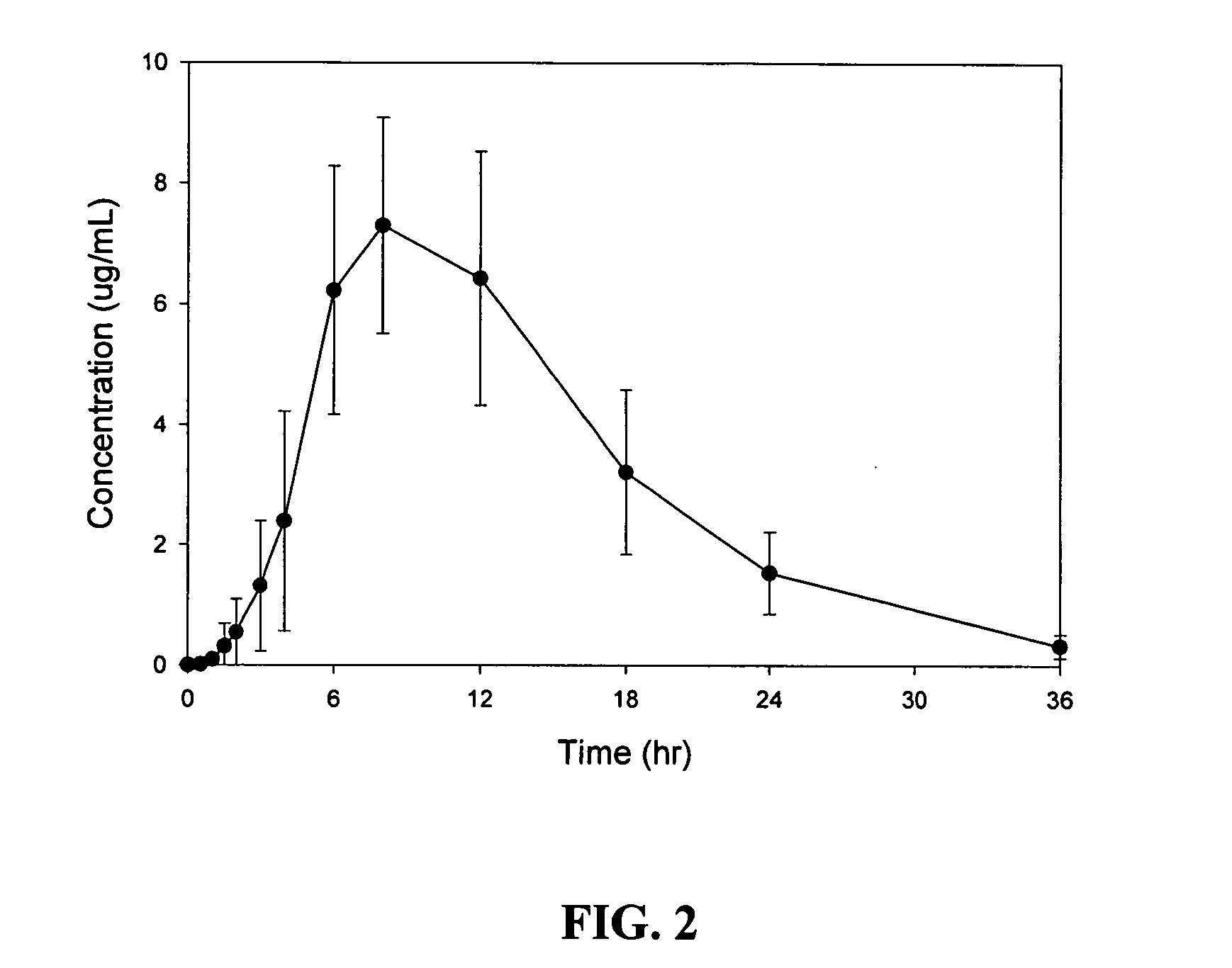
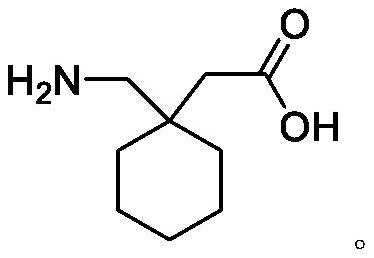
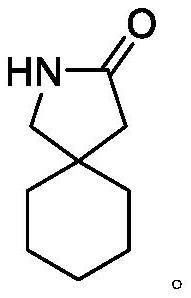
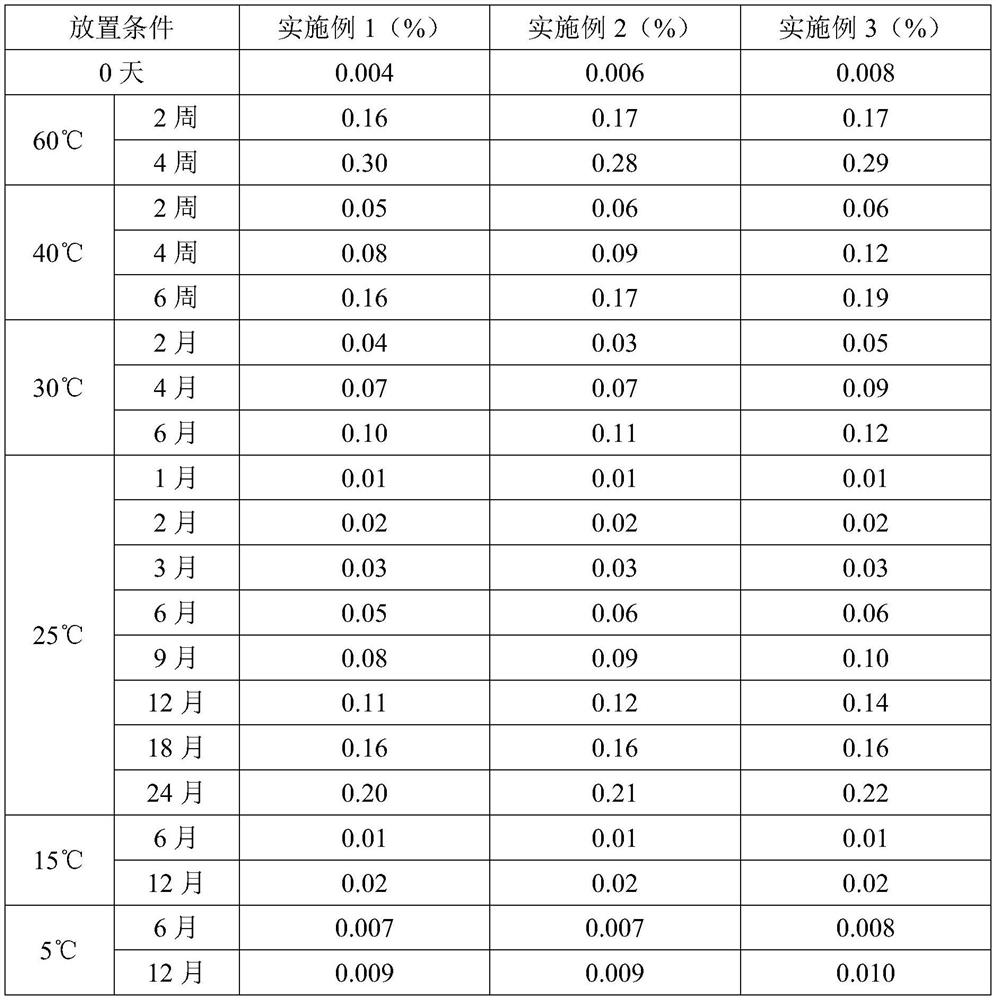
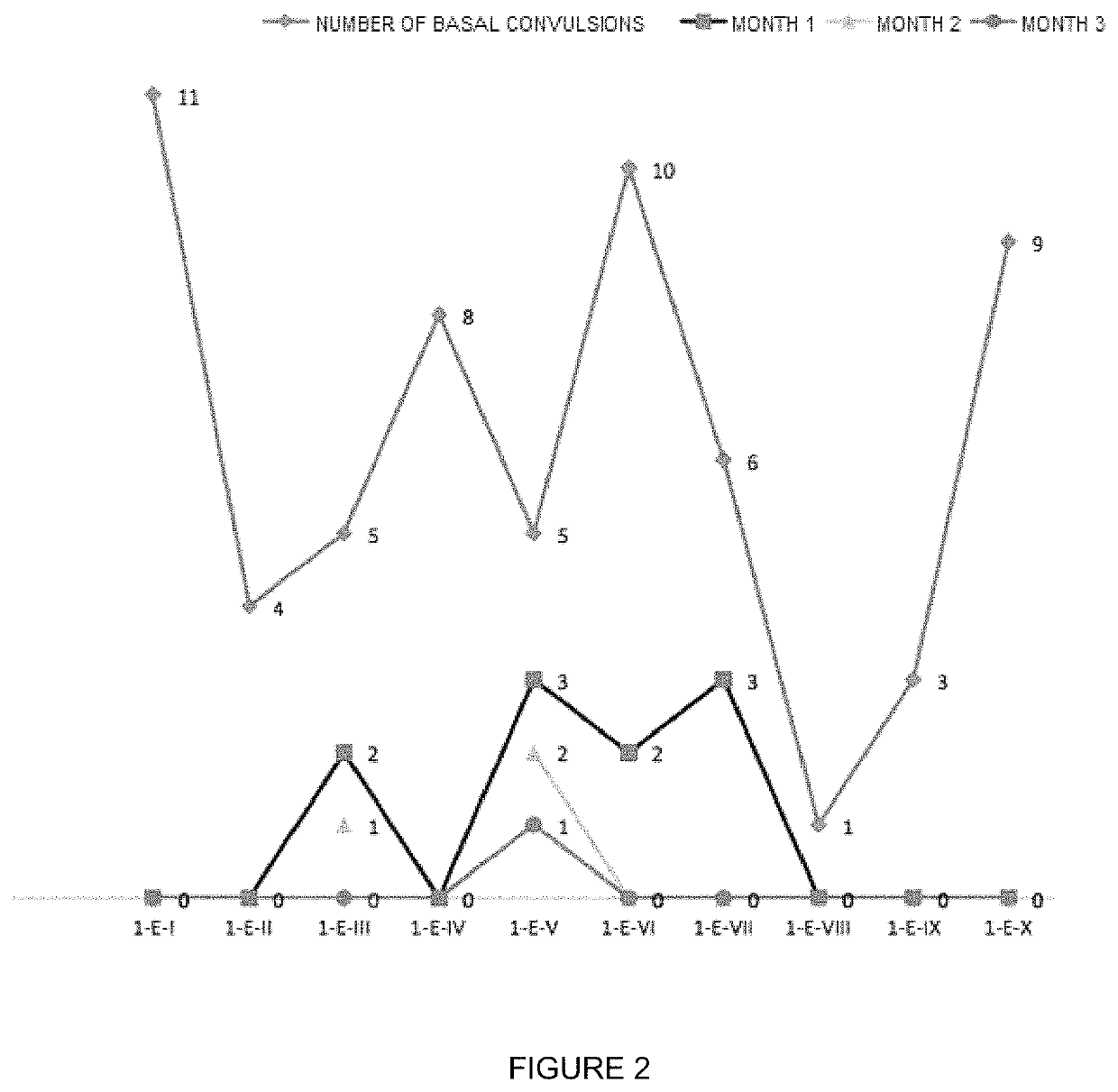
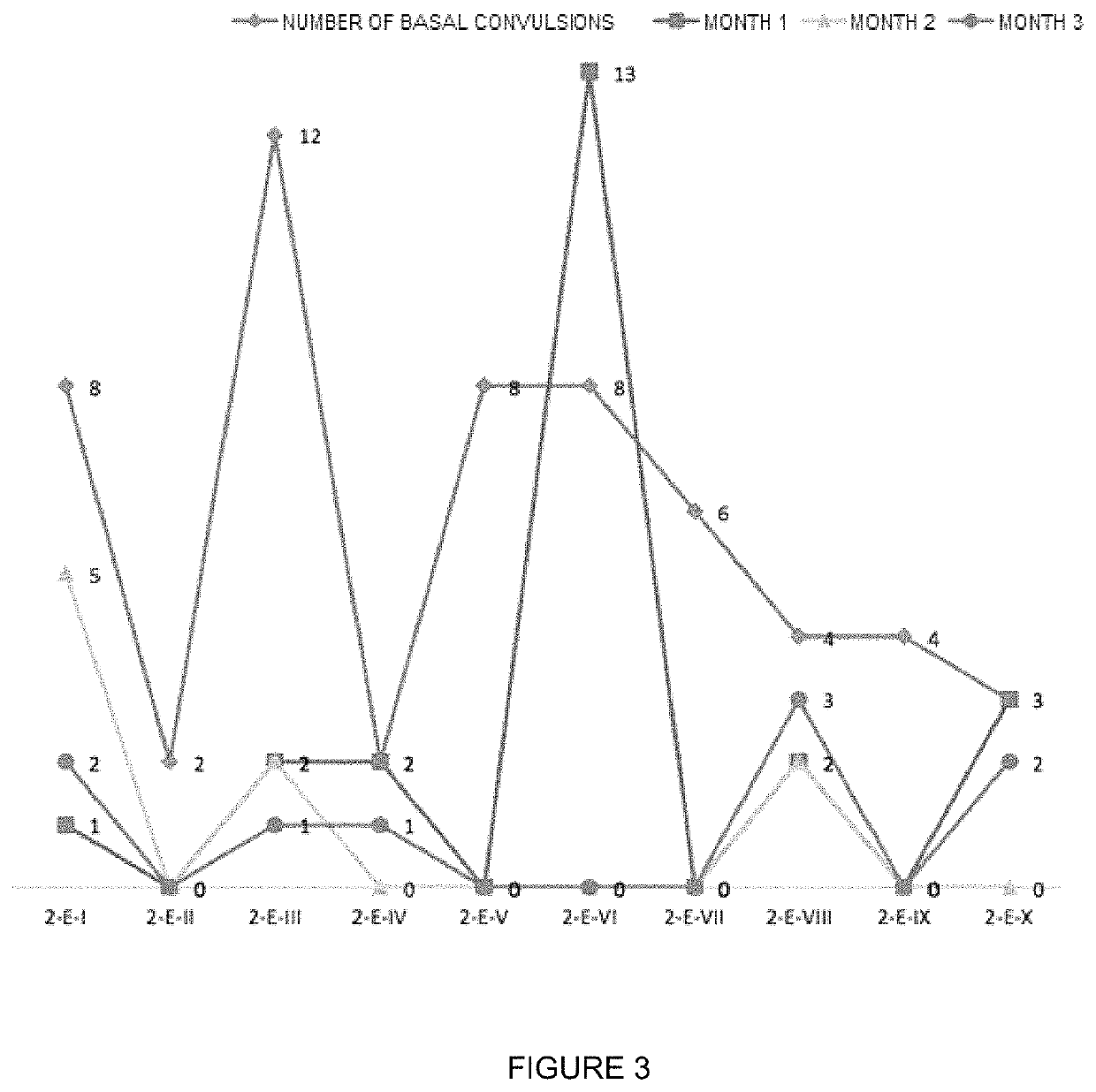
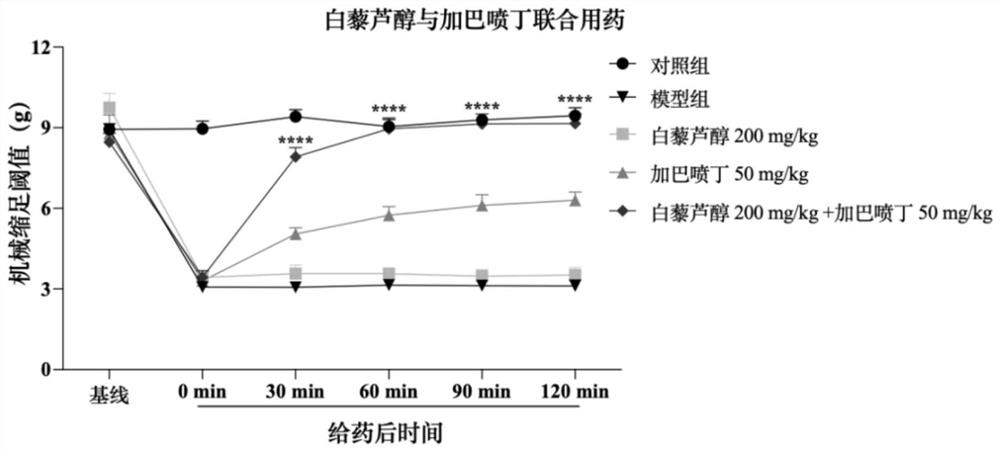
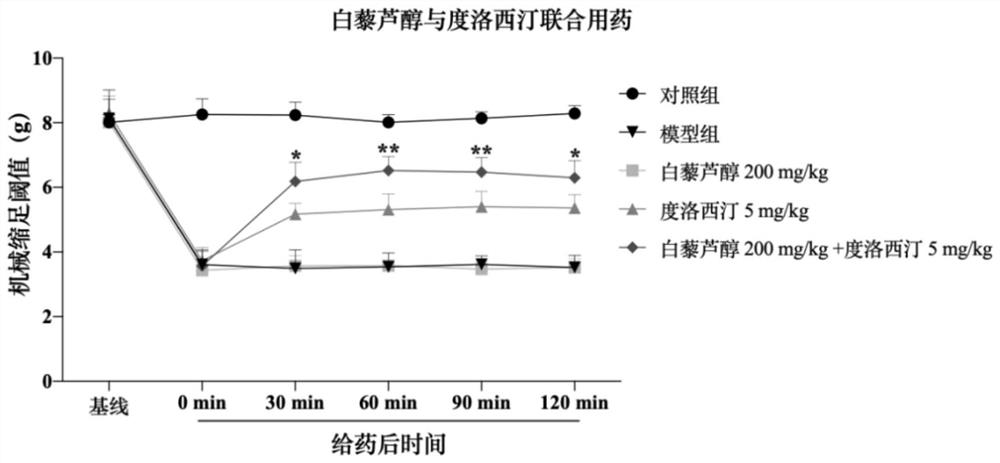
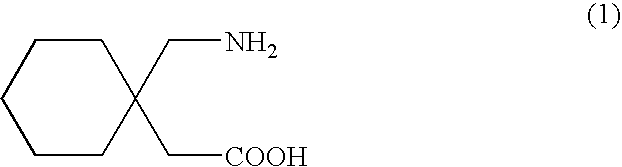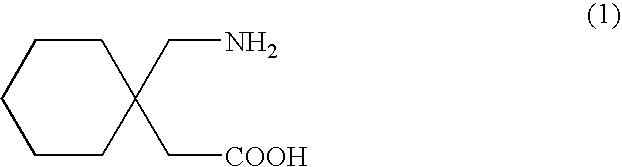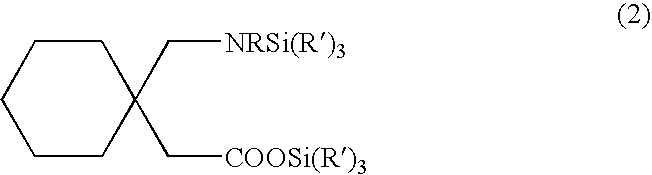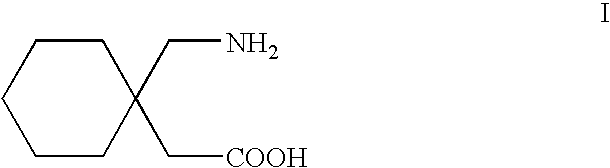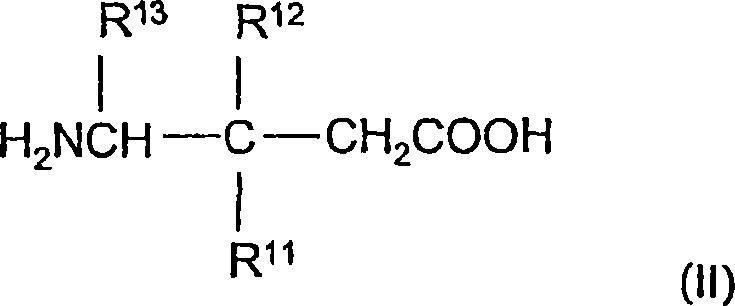Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
49 results about "Gabapentin enacarbil" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat moderate to severe restless legs syndrome. It is also used to relieve nerve pain following shingles (a painful rash due to herpes zoster infection).
Synergistic combinations
The instant invention relates to a combination of an alpha-2-delta ligand and a PDEV inhibitor for use in therapy, particularly in the curative, prophylactic or palliative treatment of pain, particularly neuropathic pain. Particularly preferred alpha-2-delta ligands are gabapentin and pregabalin. Particularly preferred PDEV inhibitors are sildenafil, vardenafil and tadalafil.
Owner:PFIZER INC
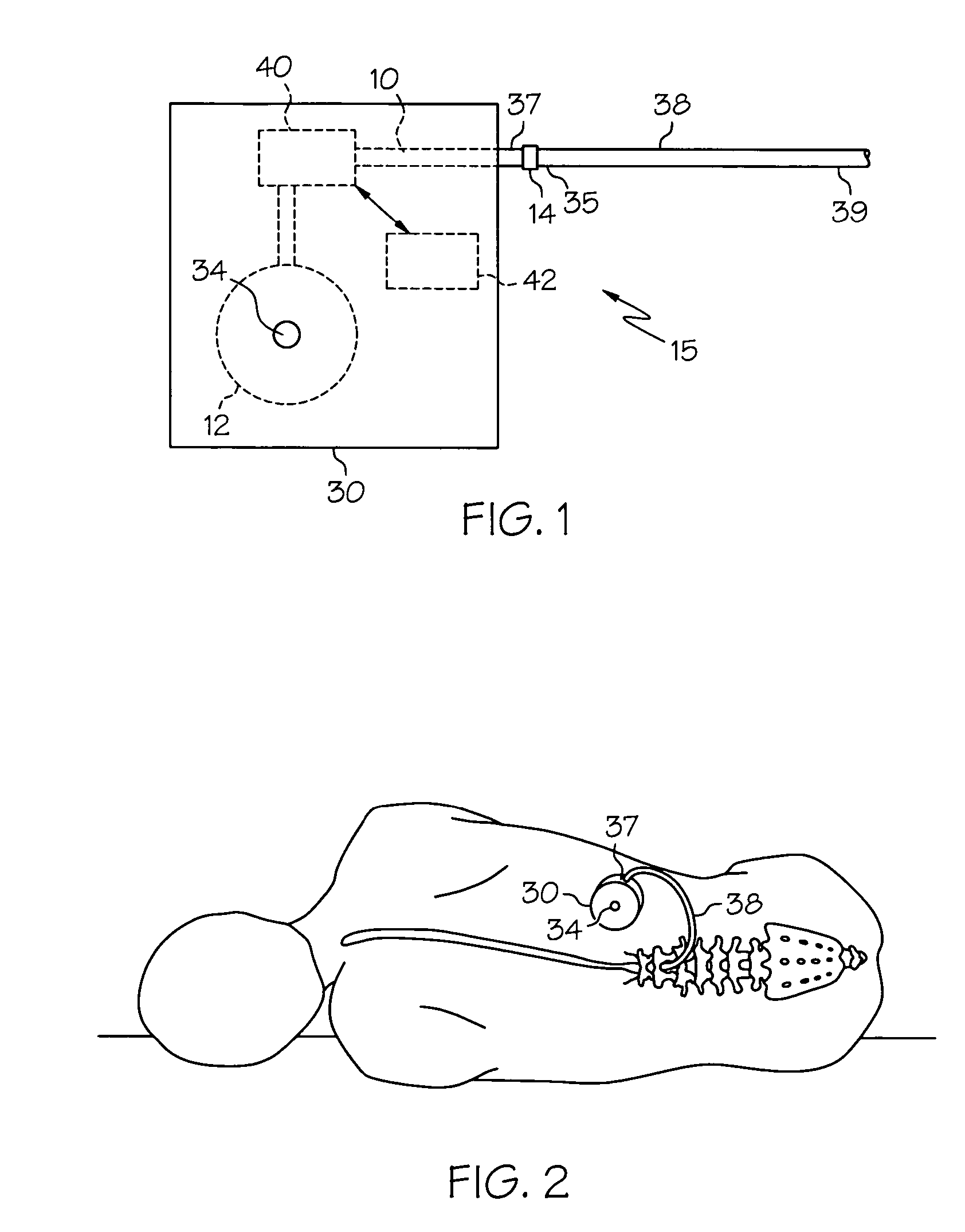
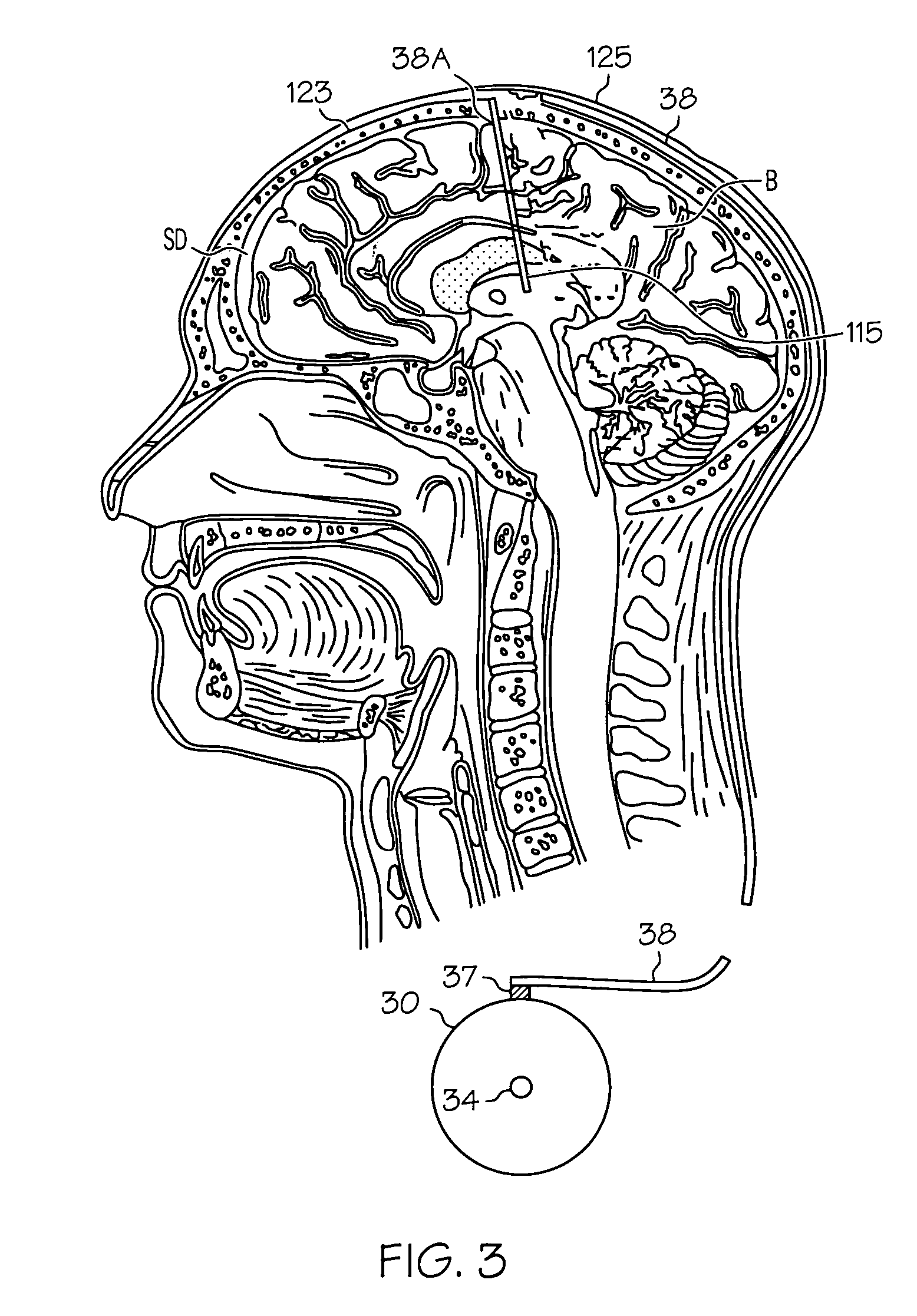
Pump systems including injectable gabapentin compositions
InactiveUS20050004219A1Reduced hypertonicityReducing solvent tonicityOrganic active ingredientsBiocideGabapentinTonicity
A system including a reservoir, a pump coupled to the reservoir, a catheter coupled to the pump and adapted for delivering a therapeutic agent to a cerebrospinal fluid of a patient; and an injectable gabapentin composition housed in the reservoir and deliverable through the catheter, is discussed. The injectable gabapentin composition may have reduced tonicity. One such injectable gabapentin composition contains greater than about 30 mg / ml gabapentin and has a tonicity of less than about 900 mOsm. Another such injecable gabapentin composition contains less that 0.9% sodium chloride.
Owner:MEDTRONIC INC
Process for producing gabapentin or pharmaceutical grade
The invention describes a process for the preparation of pharmaceutical grade gabapentin, consisting of neutralizing an alcoholic solution of gabapentin hydrochloride with basic ion exchange resins and thereafter directly isolating the gabapentin, without requiring either the formation or the isolation of intermediates other than the pharmaceutical grade product.
Owner:MEDICHEM
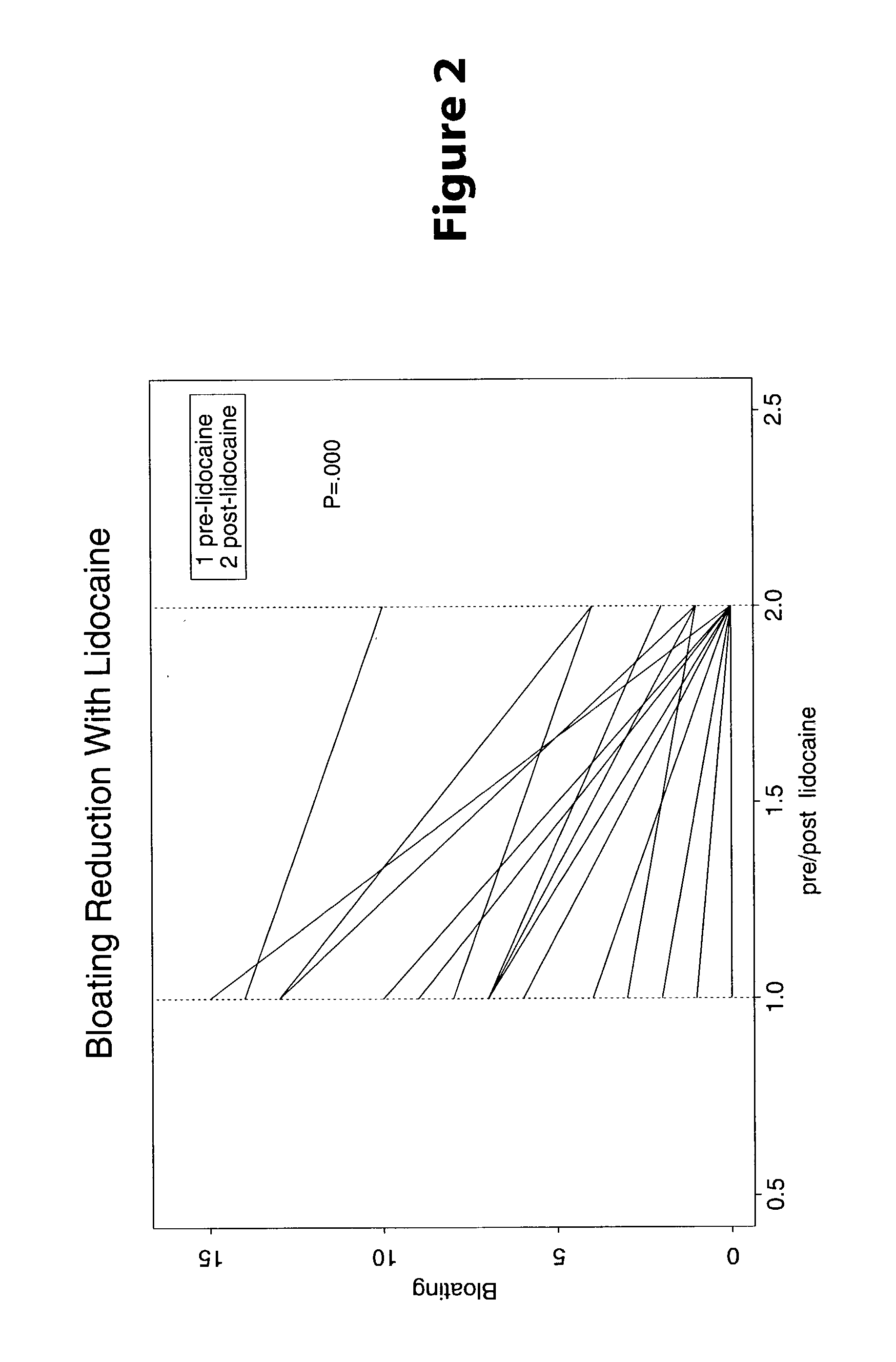
Treatment of visceral pain, E.G., irritable bowel syndrome with nerve-acting agents
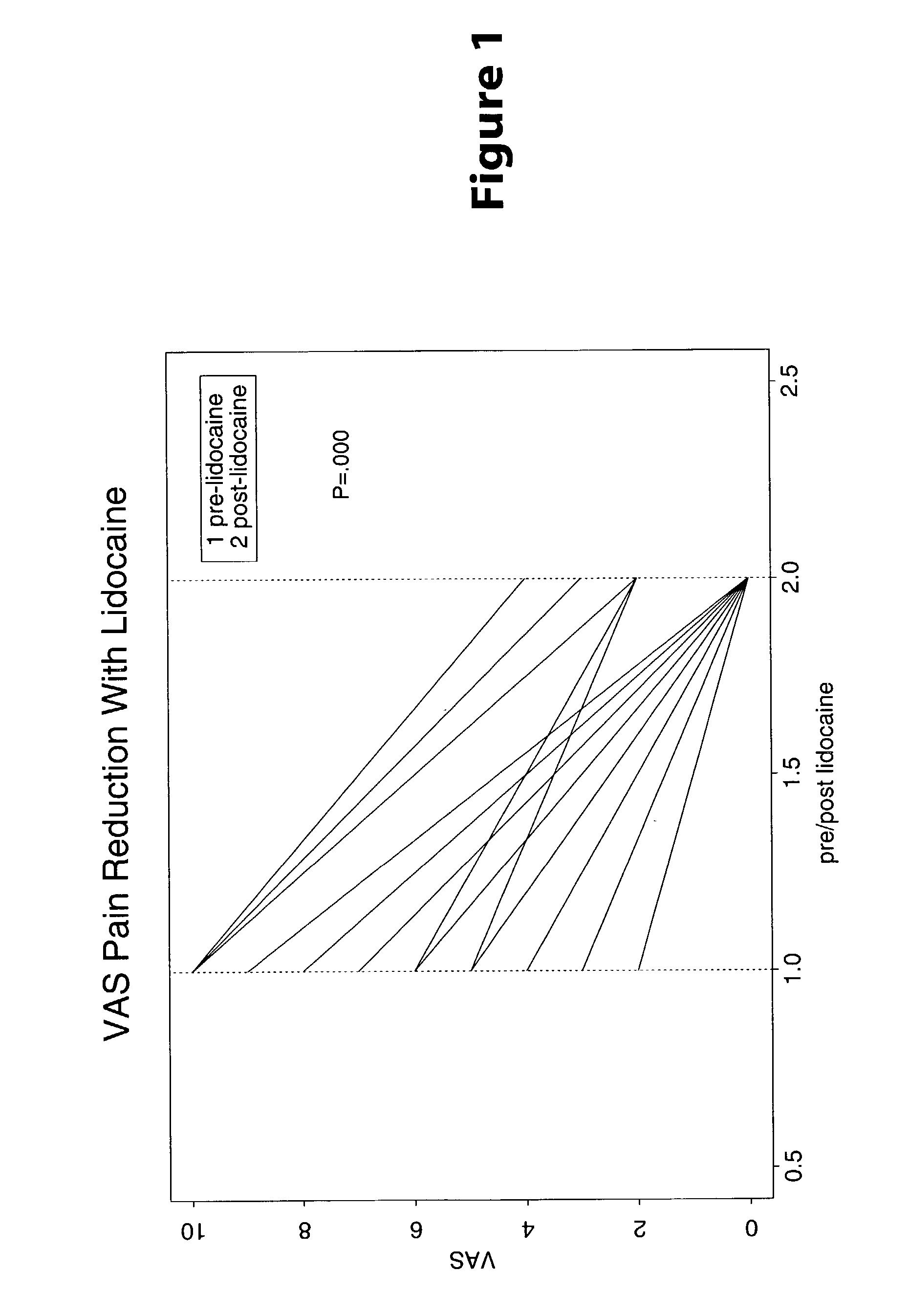
Methods are provided for use in treating humans suffering from irritable bowel syndrome. In the subject methods, an effective amount of a nerve-acting agent, e.g., lidocaine, topiramate, mexiletine and gabapentin, etc., is administered to a human suffering from irritable bowel syndrome. Also provided are pharmaceutical compositions and kits for use in practicing the subject methods.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
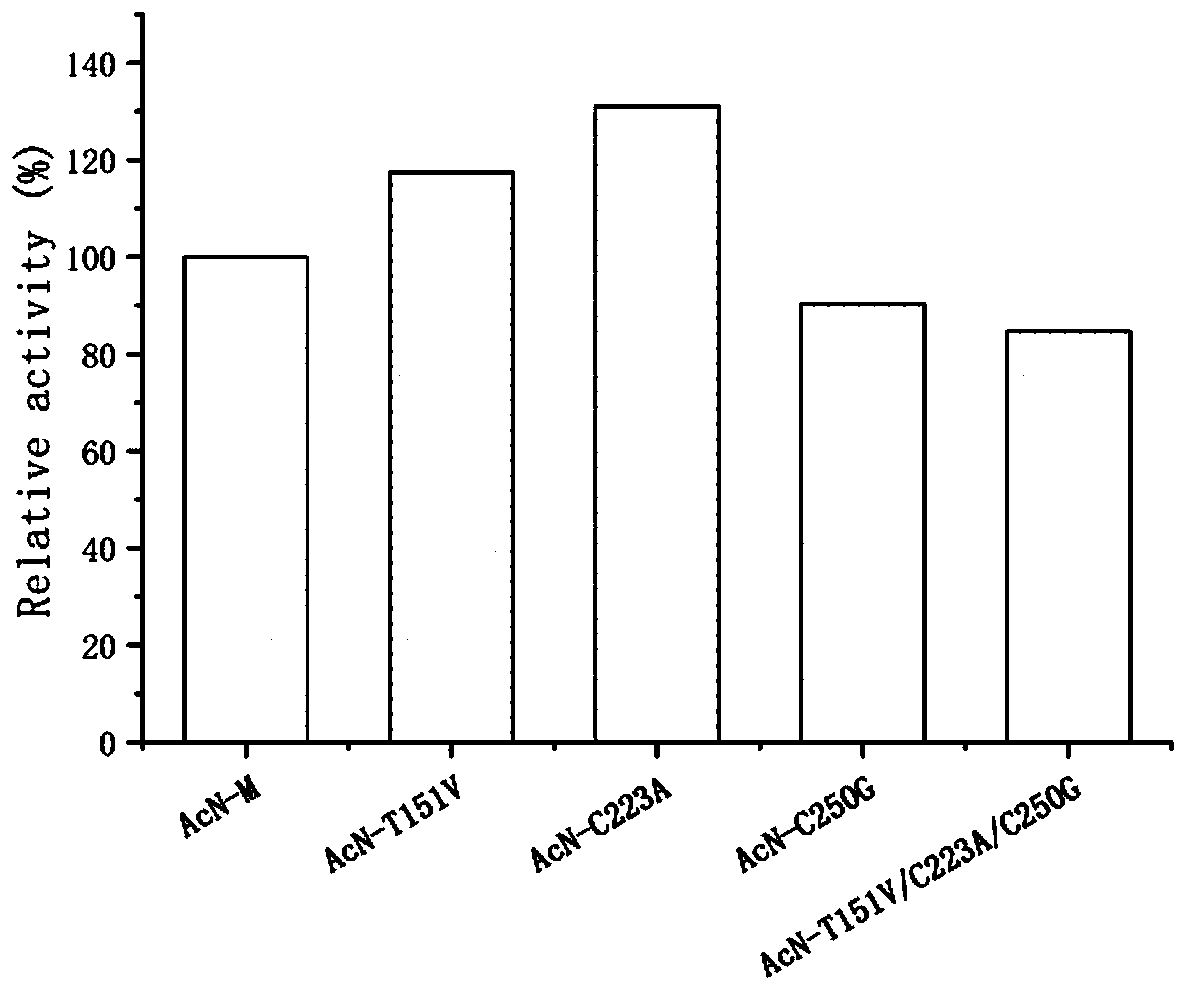
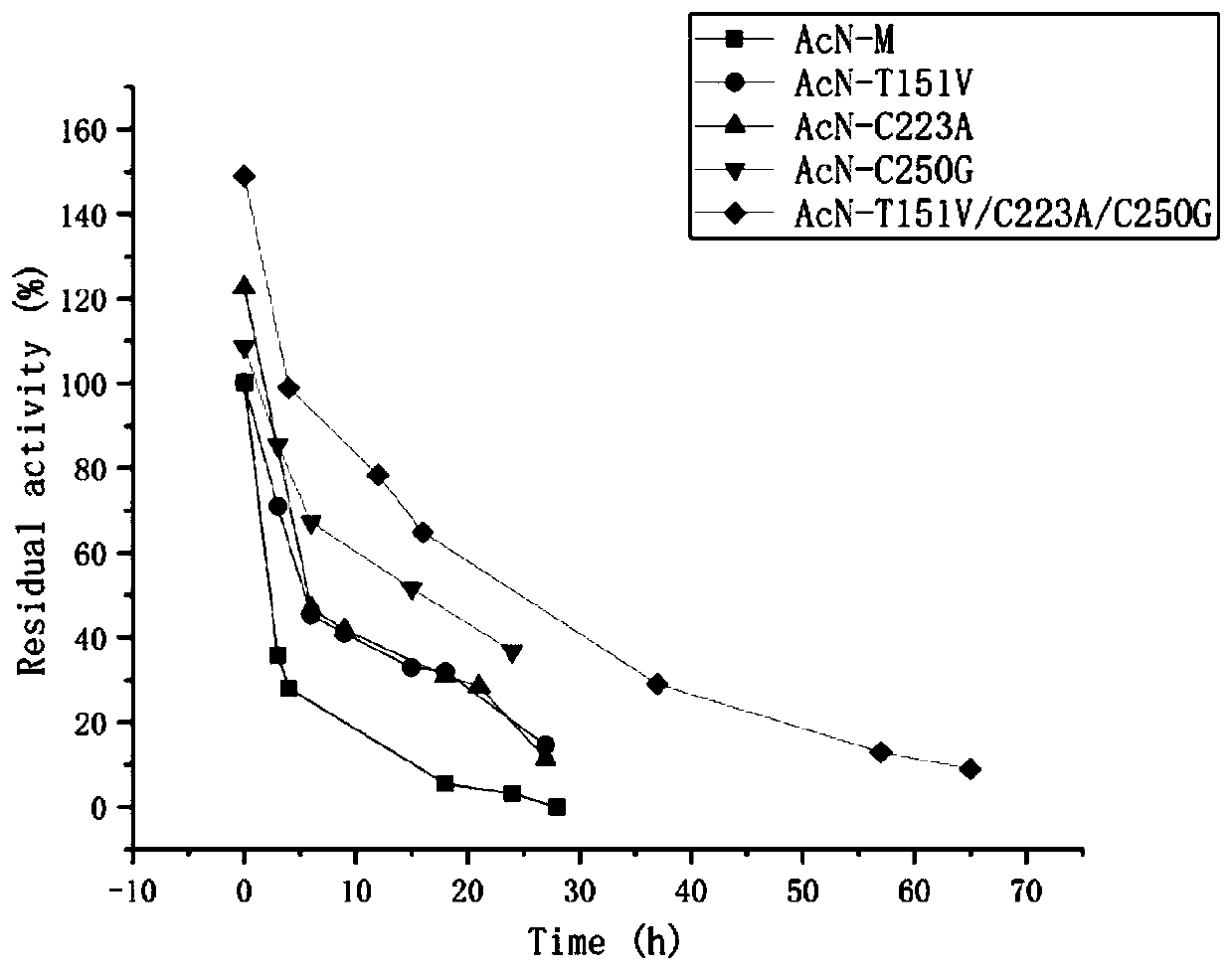
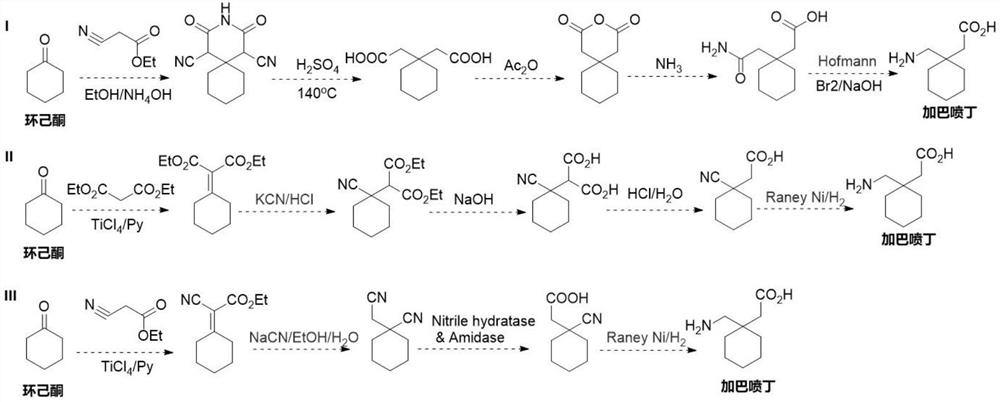
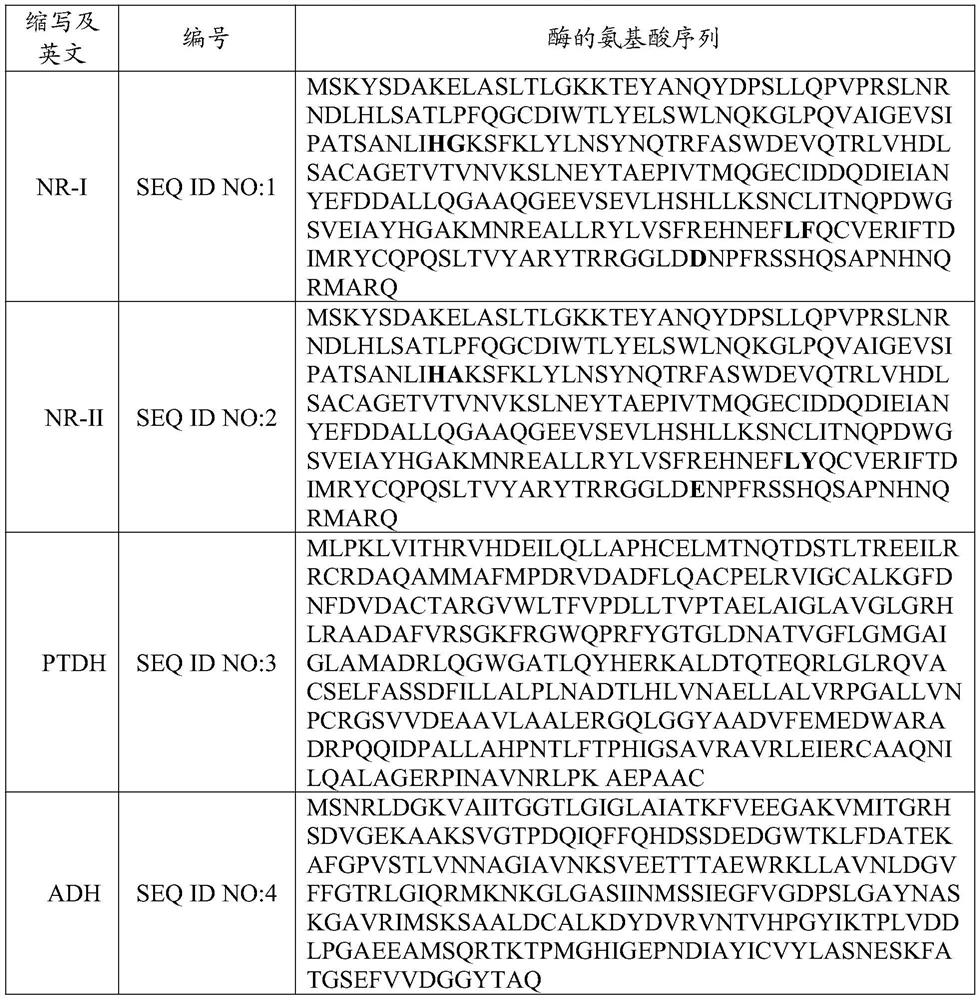
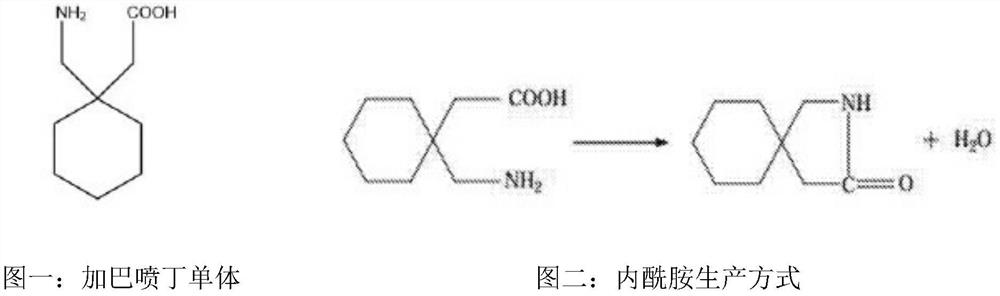
Nitrilase mutant and application thereof in preparation of anti-epileptic drug intermediate
The invention discloses a nitrilase mutant and an application thereof in preparation of an anti-epileptic drug intermediate. The mutant is obtained by mutating one or more of 151th amino acid, 223th amino acid and 250th amino acid of an amino acid sequence shown in SEQ ID No.2. The thermal stability of the nitrilase mutant AcN-T151V / C223A / C250G is improved by 1.73 times, 1M 1-cyanocyclohexyl acetonitrile is hydrolyzed by use of recombinant escherichia coli containing the nitrilase mutant at the temperature of 35 DEG C to generate 1-cyanocyclohexylacetic acid, and the yield of a final product reaches 95%. When 1.2 M 1-cyanocyclohexyl acetonitrile is hydrolyzed at the temperature of 35 DEG C, the final yield reaches 97%. Gabapentin is synthesized by use of the nitrilase mutant, and the yieldof the final product reaches 80%.
Owner:ZHEJIANG UNIV OF TECH
Interconnected medicine precursor of gabapentin and pregabalin and its medicinal use
The present invention relates to the interconnected medicine precursor of gabapentin and pregabalin and its non-toxic pharmaceutically acceptable salt and their use in preparing medicines for treating epilepsy, neurogenic pain and / or anxiety neurosis.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Compositions and dosage forms for enhanced absorption of gabapentin and pregabalin
A complex comprised of gabapentin or pregabalin and a transport moiety, such as an alkyl sulfate, is described. The complex has an enhanced absorption in the gastrointestinal tract, particularly the lower gastrointestinal tract. The complex, and compositions and dosage forms prepared using the complex, provide for absorption by the body of the drug through a period of ten to twenty-four hours, thus enabling a once-daily dosage form for gabapentin or pregabalin.
Owner:ALZA CORP
Application of 7-O-beta-D-acetylation sugar-coumarin compounds in treating chronic neuropathic pains
The invention relates to application of 7-O-beta-D-acetylation sugar-coumarin compounds in treating chronic neuropathic pains. The 7-O-beta-D-acetylation sugar-coumarin compounds comprise a pharmaceutically acceptable salt, ester or a solvate thereof, and the application relates to the application of the salt, the ester or the solvate in preparing medicaments for treating the chronic neuropathic pains, wherein a glycosyl part is arbitrary pyranose or furanose, the hydroxy of which is totally acetylated; a representative compound is 7-O-beta-D-acetylation glucose-coumarin; and the structural formula is disclosed in the specification. On a classical sciatic nerve chronic compression injury (CCI) model, through single intragastric administration and continuous intragastric administration for 7 days, the compound shows definite treatment effect of resisting nervous pains. At a dose point appointed by an experiment, the compound can obviously improve the mechanical stimulus pain threshold, the nervous pain resistant efficacy is equivalent to that of Gabapentin which is a contrast medicament, and the duration is superior to that of the Gabapentin. Proved by research results, the compound can be used for treating the chronic neuropathic pains.
Owner:YUNNAN UNIV
Gabapentin tablets and methods for their preparation
The present invention is generally directed to methods for preparing stable gabapentin tablets by wet granulation. A wet granulation method for preparing gabapentin tablets includes forming a mixture by dry mixing of a first portion of a binder with the gabapentin, one or more excipients, or a combination of the gabapentin and the one or more excipients; and adding a second portion of the binder to the mixture, wherein the second portion of the binder is in the form of a solution or dispersion.
Owner:RANBAXY LAB LTD
Processes for the preparation of Gabapentin
InactiveUS20060020034A1Simple preparation processLow production costOrganic active ingredientsBiocideGabapentinMedicinal chemistry
The present invention relates to new processes for the preparation of gabapentin by the desilylation of a silylated gabapentin or by the silylation-desilylation of an acid addition salt of gabapentin with a silylating agent.
Owner:SANDOZ AG
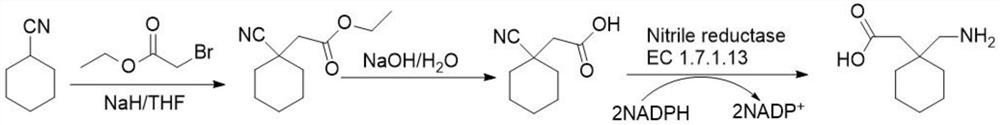
Preparation method of cyano reductase and gabapentin
The invention relates to the technical field of medicine synthesis, in particular to a preparation method of cyano reductase and gabapentin. The method comprises the following steps: by taking sodium hydride as a catalyst, carrying out condensation reaction on cyclohexyl methyl cyanide and 2-ethyl bromoacetate to generate 2-cyano-2-cyclohexyl ethyl acetate; under an alkaline condition, carrying out hydrolysis reaction on 2-cyano-2-cyclohexyl ethyl acetate to obtain 2-cyano-2-cyclohexyl acetic acid; and converting the 2-cyano-2-cyclohexyl acetic acid into gabapentin under the action of the cyano reductase. Aiming at the defects (the problem of traditional chemical preparation of gabapentin) of a literature scheme, cyano reductase is introduced, and a route is systematically optimized, so that reaction steps are reduced, and meanwhile, reaction conditions are milder and more environment-friendly. According to the route, the overall yield of gabapentin can be remarkably increased, and meanwhile, the gabapentin better meets the requirements of current green production.
Owner:SHENZHEN READLINE BIOTECH CO LTD
Processes for the preparation of gabapentin
The present invention relates to a process of preparing gabapentin from its hydrochloride salt by the use of alkylamine and choice of solvent. The resulting gabapentin can be isolated either as Form II or Form III, characterized by their spectra, by simply choosing an appropriate solvent in the process.
Owner:DIVI S LAB LTD
Gabapentin tablet and preparation method thereof
InactiveCN102688216AGood molding effectImprove physical strengthNervous disorderPeptide/protein ingredientsCelluloseAdhesive
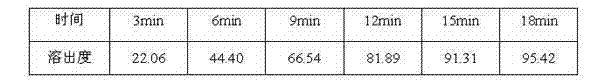
The invention belongs to the field of medicine preparation and relates to a gabapentin tablet which is prepared by wet granulation and prepared mainly from the following raw materials by weight percentage: 80.0% or more of gabapentin, 4.0-17.0% of low-substituted hydroxypropyl cellulose, 0.5-2.5% of adhesive, 0.1-2.5% of stabilizer and 0.5-1.5% of lubricant. The invention further provides a preparation method of the gabapentin tablet. The gabapentin raw material adopted in the invention has good formability and high physical strength after being crushed. Besides, the gabapentin raw material can be prepared by adopting an ordinary wet granulation method and has the characteristics of simple preparation and low cost. The gabapentin tablet provided by the invention has the advantages of very high dissolution in vitro, high content of gabapentin, less application auxiliary materials and high convenience for use, thus meeting modern medicine's requirements of 'small in three aspects' and 'convenient in three aspects', and can be prepared by adopting an ordinary wet granulation method and has the characteristics of simple preparation and low cost.
Owner:HANGZHOU LIUCHA PHARMA
Phenolic acid salts of gabapentin in liquid and/or semi-solid dosage forms and methods of use
The present invention relates to pharmaceutical compositions of gabapentin tannate, processes for production of those compositions and methods of use of those compositions. The present invention provides a novel process for preparation of the tannate salt of gabapentin in liquid or semi-solid dosage form for human and veterinary pharmaceutical use. Tannate salts of active pharmaceutical ingredients are used in sustained release applications and to improve certain organoleptic properties such as taste. The process may utilize either natural or synthetic tannic acid.
Owner:KIEL LAB
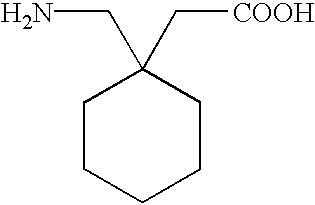
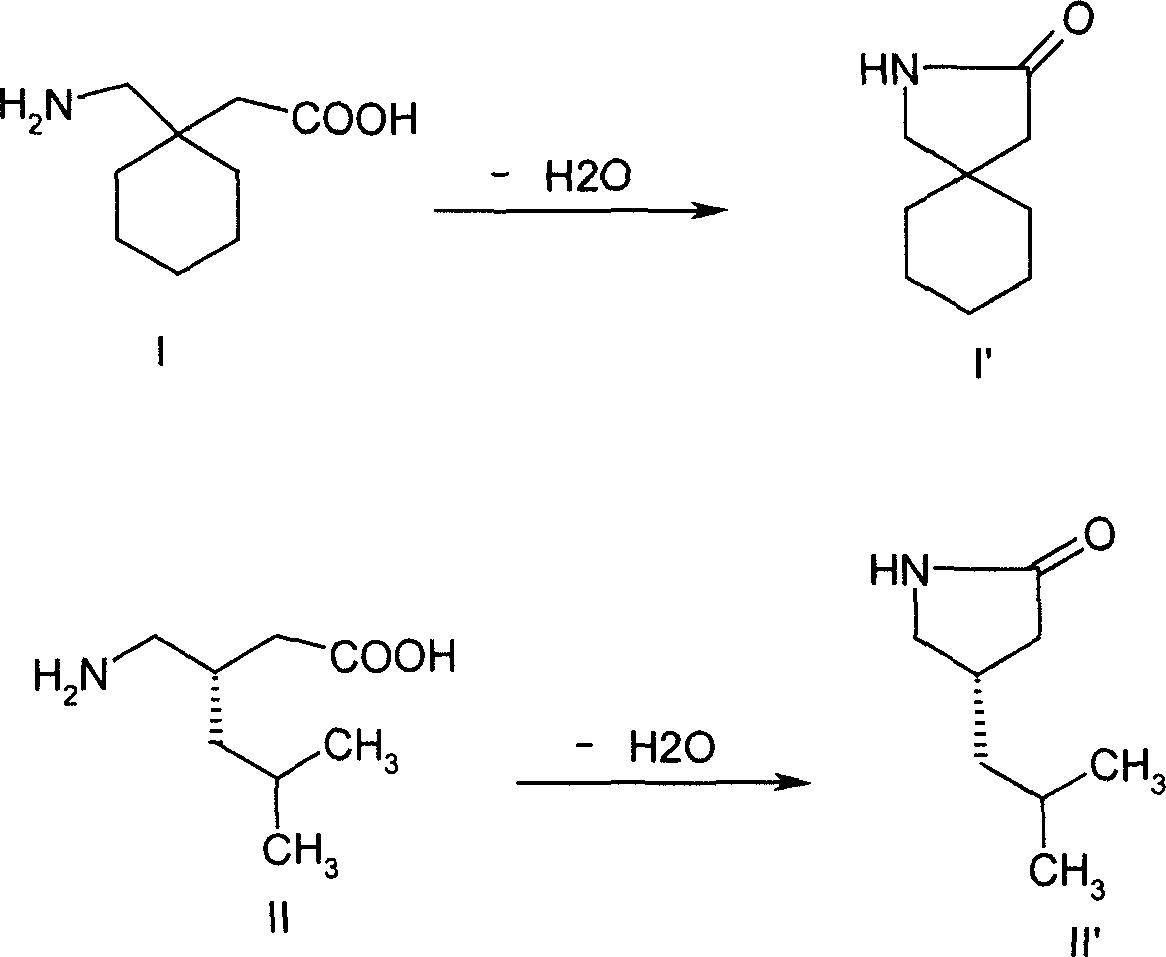
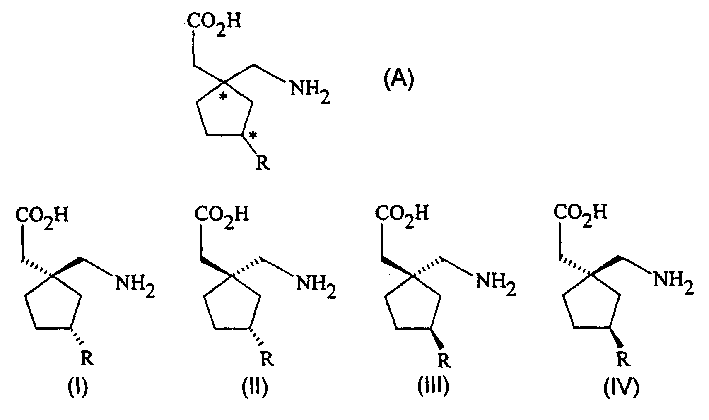
Stereoselective synthesis of cyclic amino acids
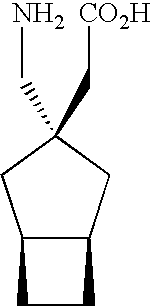
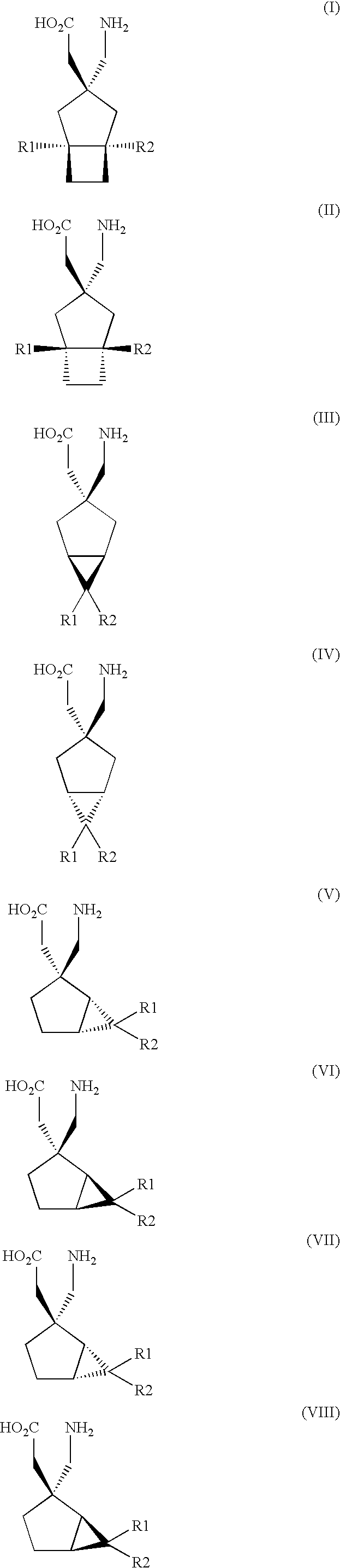
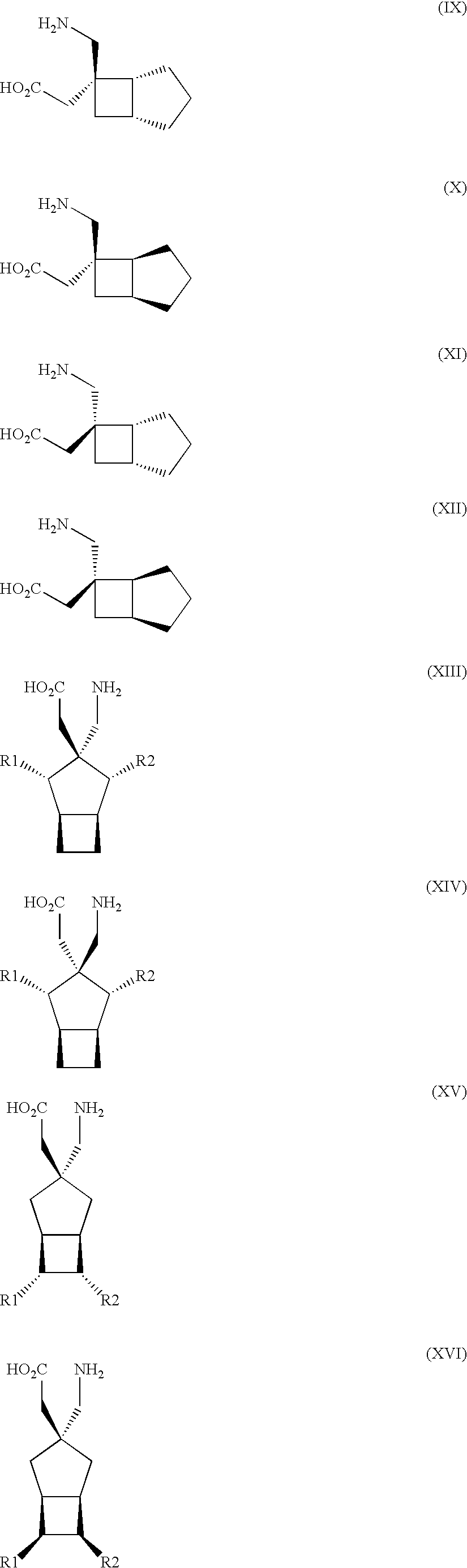
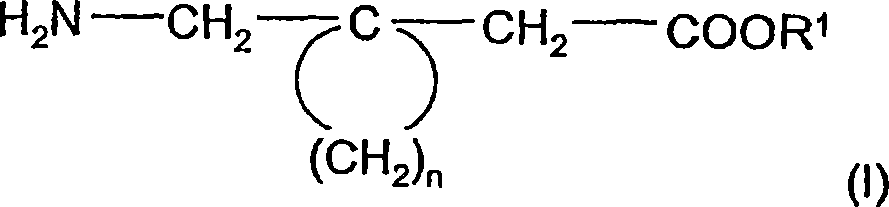
The instant invention is a route to stereospecific 3-substituted 5-membered ring isomers of Formula (A). The final products are useful as agents in the treatment of epilepsy, faintness attacks, hypokinesia, cranial disorders, neurodegenerative disorders, depression, anxiety, panic, pain, neuropathological disorders, gastrointestinal disorders such as irritable bowel syndrome (IBS), inflammation especially arthritis, sleep disorders, premenstrual syndrome, and hot flashes. The invention provides novel routes to synthesize stereoselectively analogs of gabapentin (Neurontin3) of Formulas (I), (II), (III) and (IV) wherein R is C1-C10 alkyl or C3-C10 cycloalkyl and pharmaceutically acceptable salts thereof.
Owner:WARNER-LAMBERT CO
Sustained release oral dosage forms of gabapentin
InactiveCN1668284AProlong gastric residence timeOrganic active ingredientsNervous disorderProlonged-release tabletPlasma concentration
The present invention relates to sustained release oral dosage forms of gabapentin and at least one rate controlling polymer, and a process for the preparation of the sustained release oral dosage forms, and a process for the preparation thereof. The sustained release tablet includes gabapentin or a pharmaceutically acceptable salt or hydrates thereof and at least one rate-controlling polymer such that the tablet provides therapeutically effective plasma levels of gabapentin for a period of up to about 12 hours.
Owner:RANBAXY LAB LTD
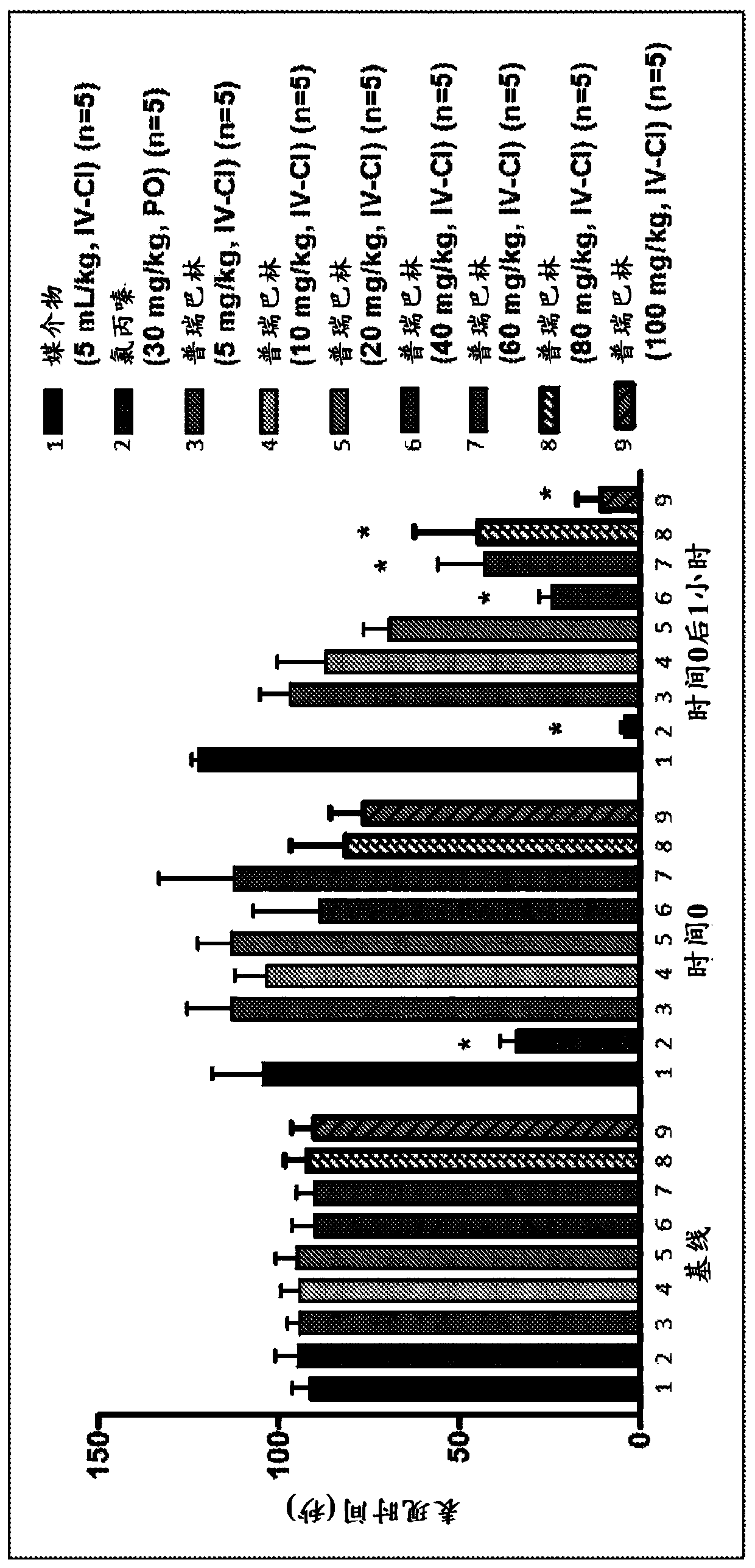
Processes for the preparation and purification of gabapentin enacarbil
Owner:TEVA PHARM USA INC
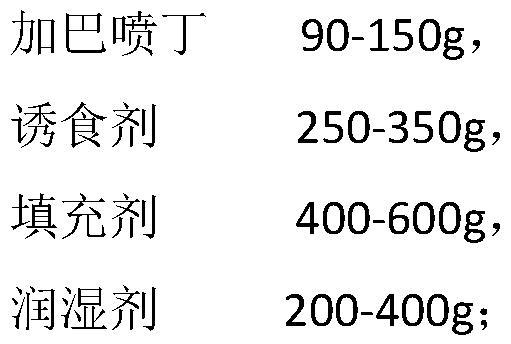
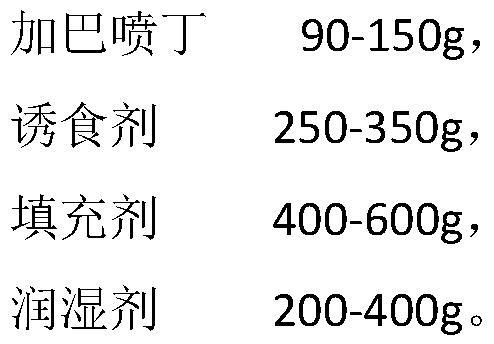
Gabapentin soft chewable tablet preparation, and preparation method and application thereof
PendingCN114028352AAct as a stabilizerThe role of the stabilizer hasOrganic active ingredientsNervous disorderPotato starchPolythylene glycol
The invention provides a gabapentin soft chewable tablet preparation, and a preparation method and application thereof. The gabapentin soft chewable tablet preparation comprises gabapentin, a phagostimulant, a filler and a wetting agent, the wetting agent is a mixture of glycerol and polyethylene glycol, the content of polyethylene glycol is 20-40 wt%, the phagostimulant is selected from chicken liver powder, beef powder or chicken powder and the like, and the filler comprises common auxiliary materials such as microcrystalline cellulose, corn starch, potato starch or pregelatinized starch. The preparation method comprises the following steps: mixing gabapentin, the phagostimulant and the filler in a wet type granulator, then dropwise adding the wetting agent into the wet type granulator, and carrying out granulation, granule finishing and tabletting. According to the invention, feeding and administration are convenient, and the subjective initiative of pet eating can be improved by adding the phagostimulant; and soft tablets chewable in the mouth can be prepared through the high viscosity property of a moisturizer such as glycerin, and meanwhile the moisturizer such as glycerin can just play a role of a stabilizer for gabapentin.
Owner:上海汉维生物医药科技有限公司
Utilize the method for directly synthesizing gabapentin with 1-cyano cyclohexyl acetic acid
ActiveCN107235850BHigh yieldAchieve conversionOrganic compound preparationAmino-carboxyl compound preparationBiotechnologyPtru catalyst
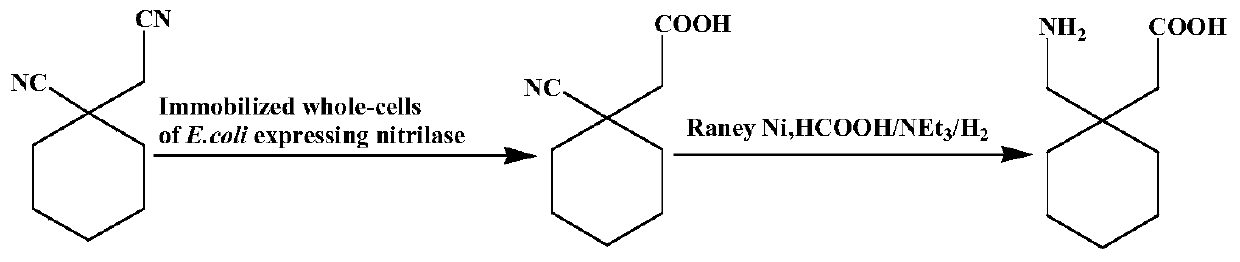
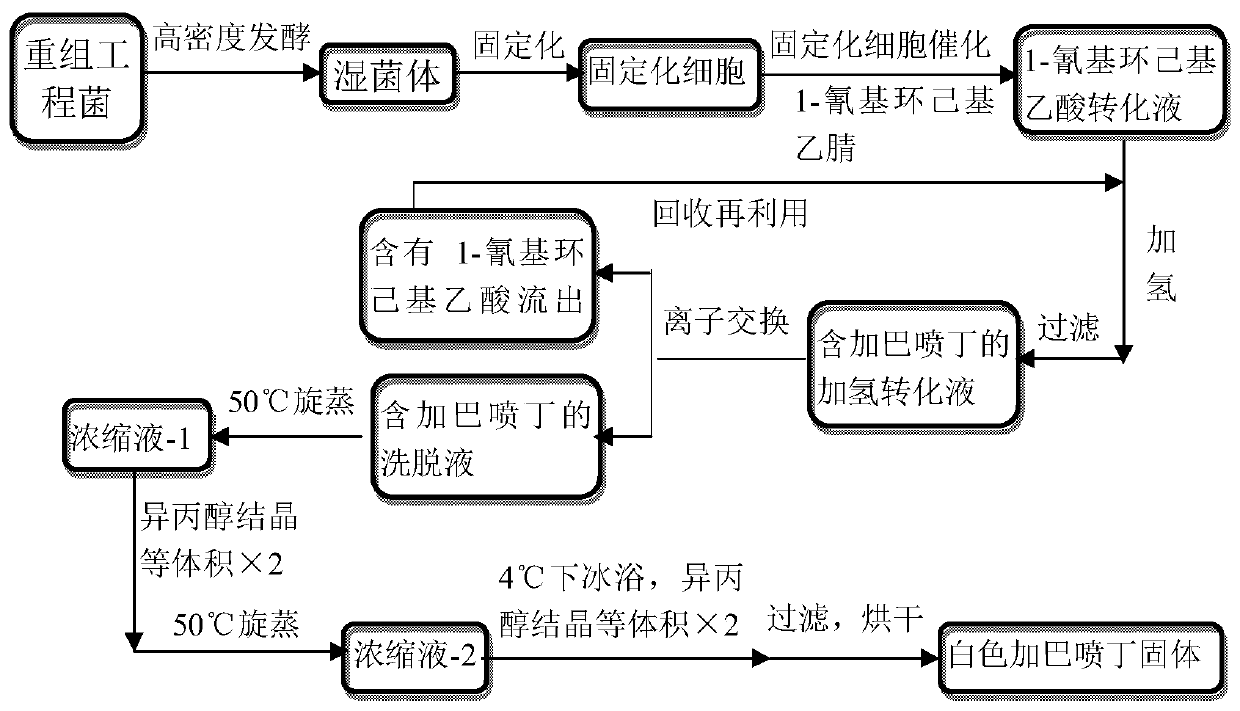
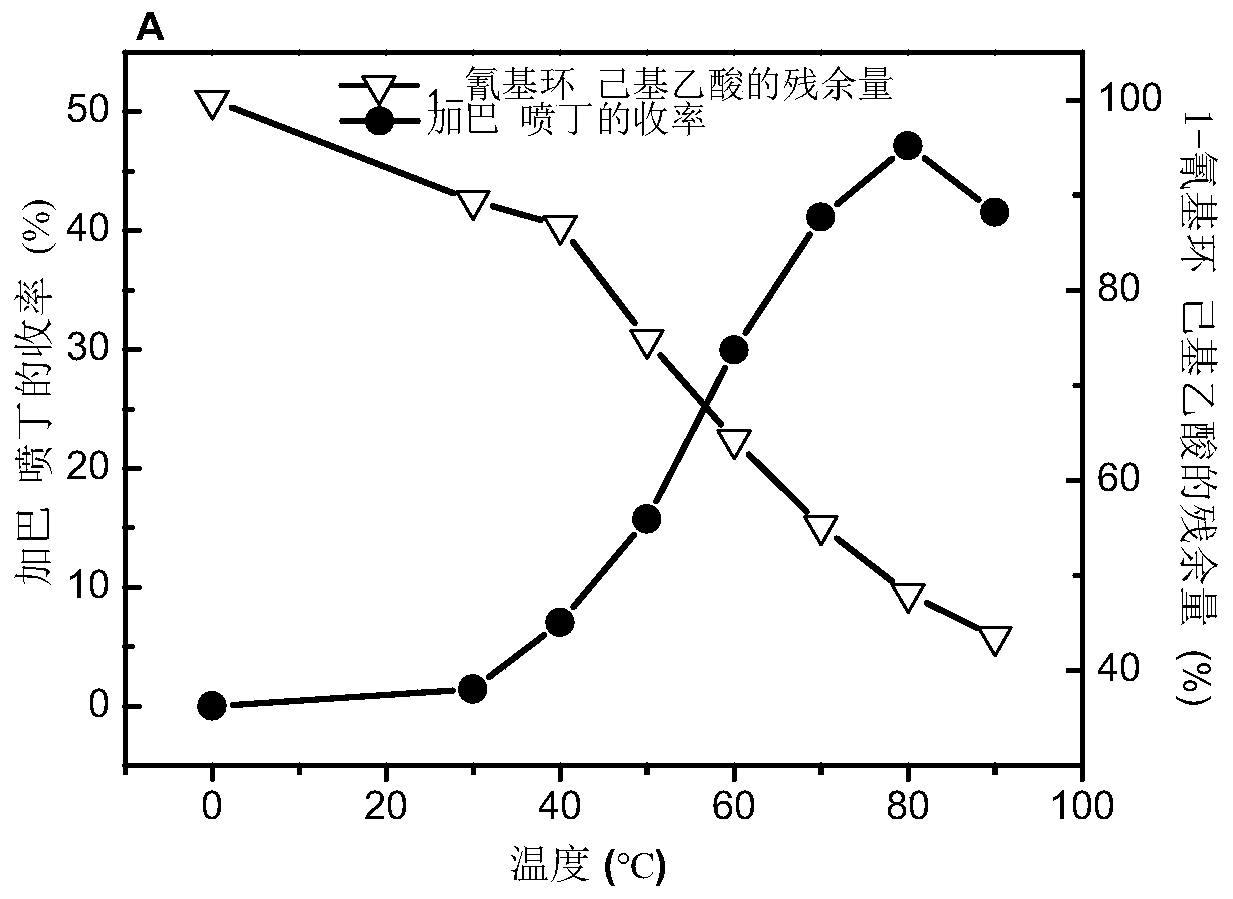
The invention discloses a method for directly synthesizing gabapentin by virtue of 1-cyanocyclohexyl acetic acid. The method comprises the following steps: dispersing immobilized cells of nitrilase genetically engineered bacteria into deionized water, adding 1-cyanocyclohexyl acetonitrile, stirring the completely react at 20-50 DEG C and 10rpm-350rpm, and carrying out suction filtration, so as to obtain filtrate a; adding the filtrate a into a hydrogenation reaction kettle, adding a catalyst and an aid, introducing nitrogen to completely react at 20-150 DEG C and 300rpm-1100rpm, and separating and purifying reaction liquid, so as to obtain gabapentin. By adding the aid and changing a heating strategy, the reaction is carried out for one batch under the optimal condition, and then the yield of gabapentin reaches 53.3%; 1-cyanocyclohexyl acetic acid is recycled and hydrogenated for five cycles, the yield of gabapentin reaches above 80% and is increased by 20% than that of a reported chemical process.
Owner:ZHEJIANG UNIV OF TECH
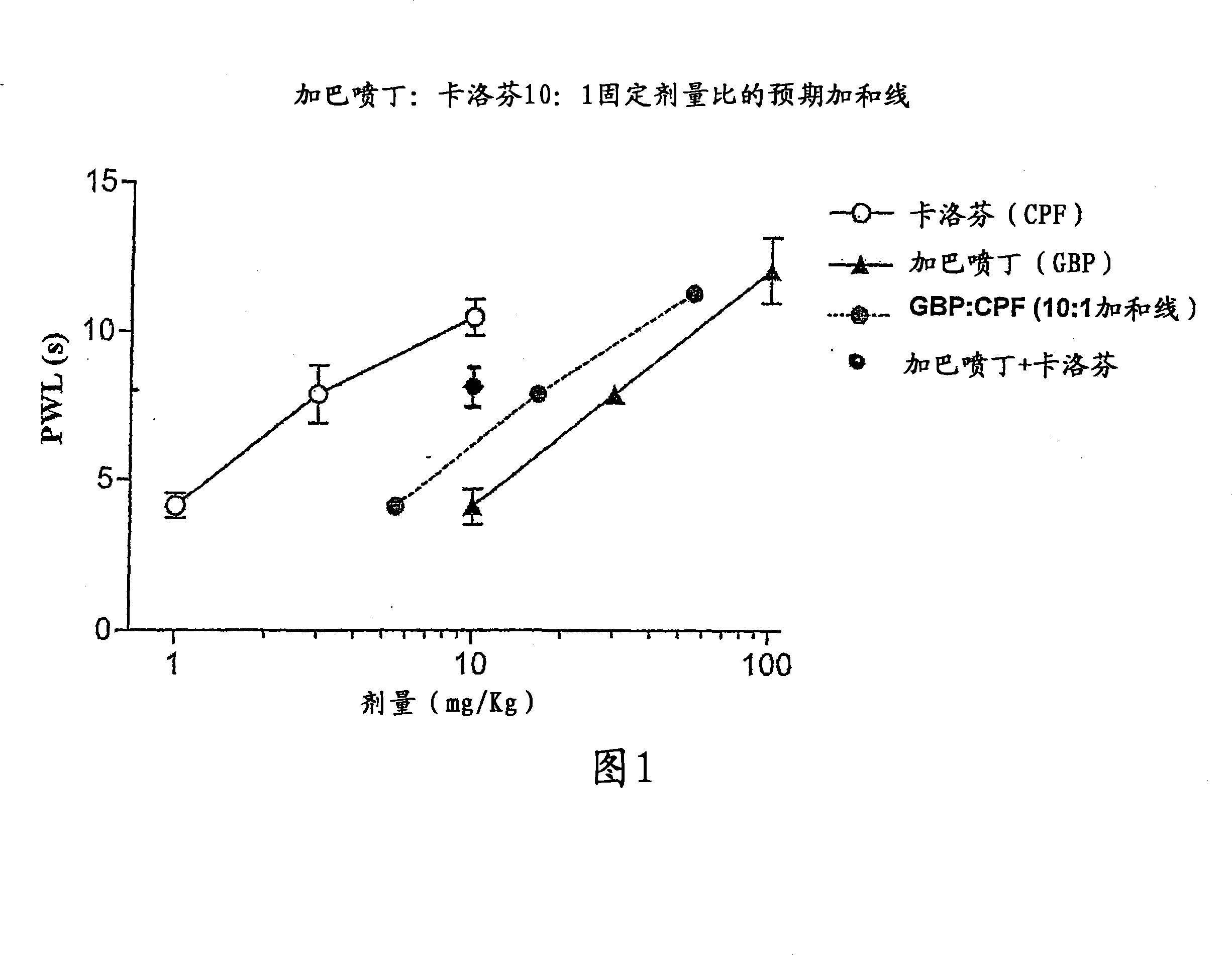
Synergistic combinations of non-steroidal antiinflammatory drugs with alpha-2 delta-ligands
InactiveCN101180045AOrganic active ingredientsPeptide/protein ingredientsPregabalinAntiinflammatory drug
Owner:PFIZER INC
Acetaminophen-pregabalin combinations and methods of treating pain
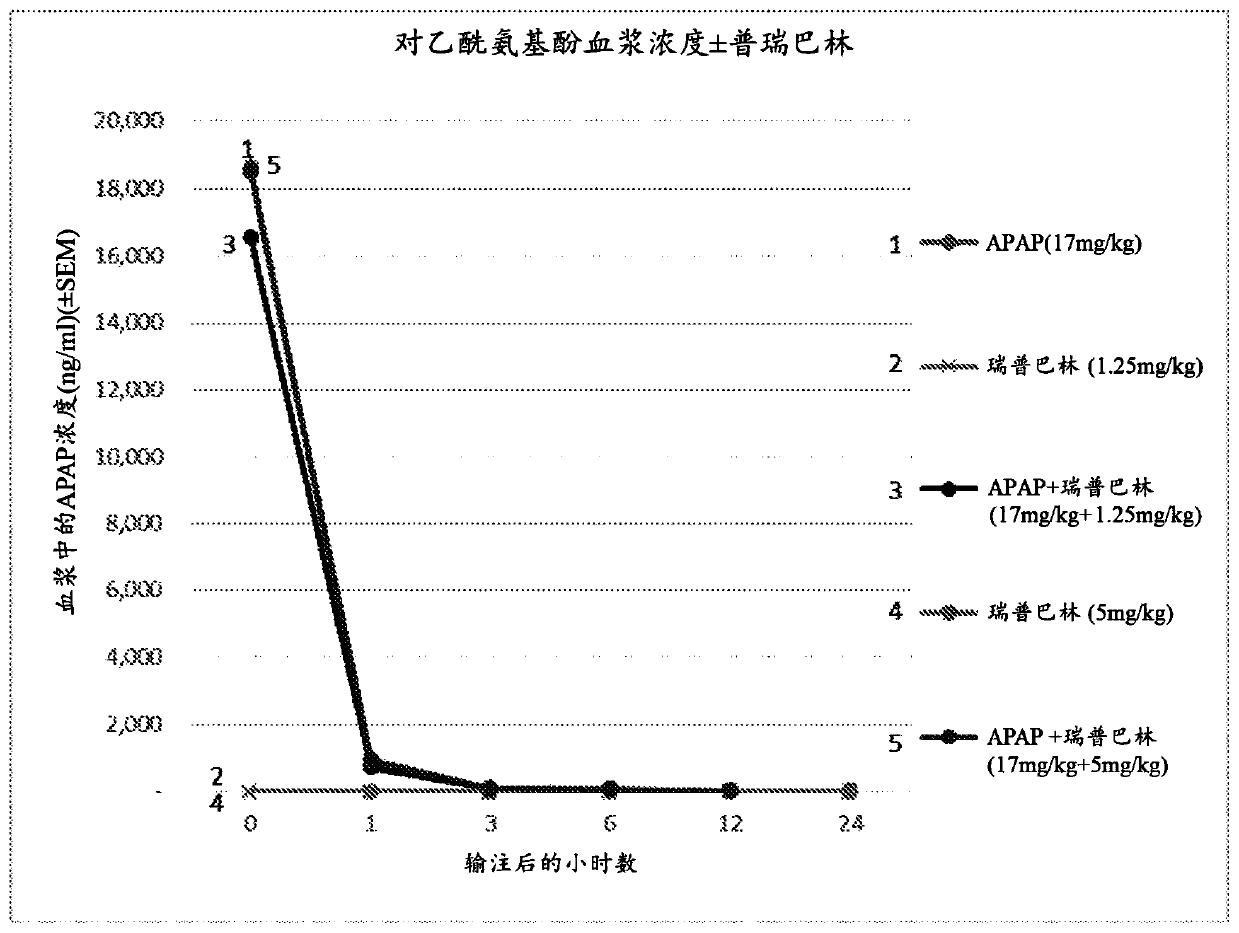
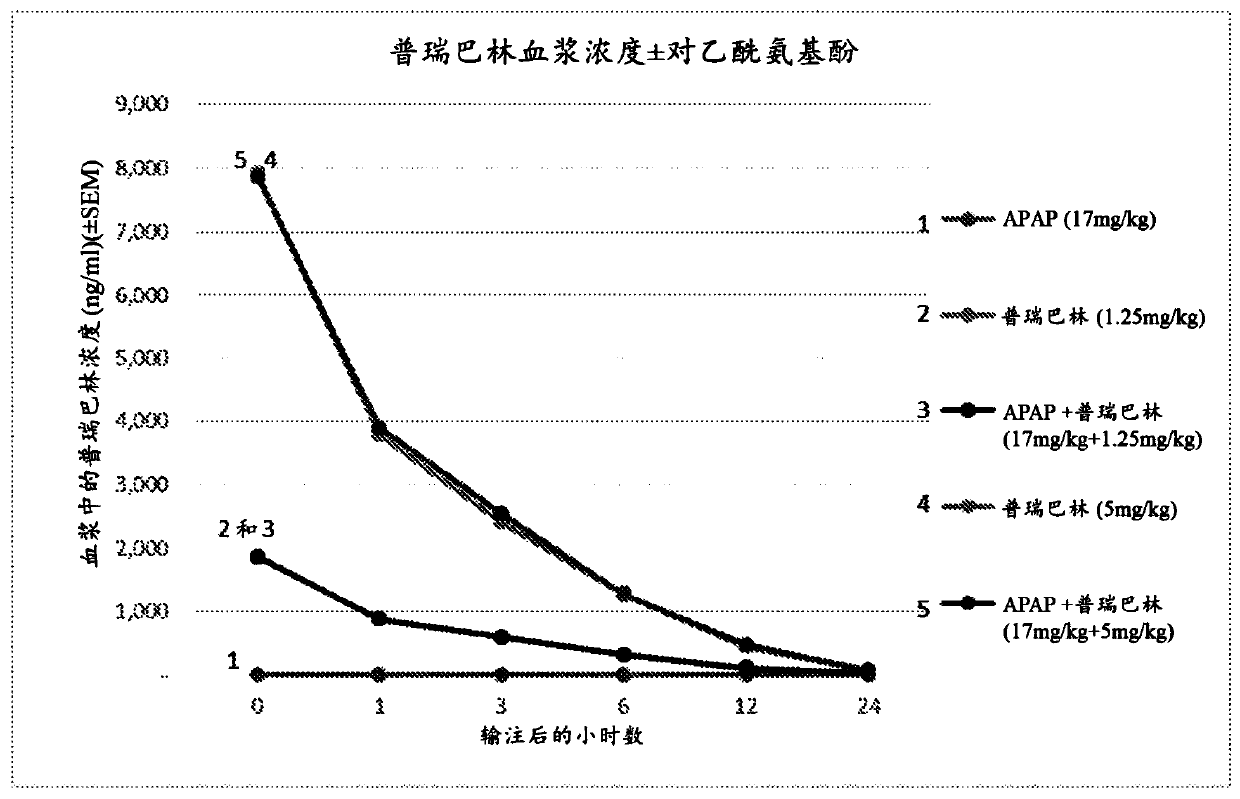
Compositions and methods for an injectable liquid formulation for reducing consumption or need of a postoperative analgesic compound by a patient are presented. The pharmaceutical formulation can contain a non-opioid analgesic and a gabapentinoid, and can be administered prior to surgery to reduce postoperative pain.
Owner:NEVAKAR INJECTABLES INC
Salt of gabapentin and 2, 6-pyridine dicarboxylic acid as well as preparation method and application thereof
ActiveCN112321442ASimple manufacturing methodHigh purityNervous disorderPeptide/protein ingredientsDicarboxylic acidPyridine
The invention relates to a salt of gabapentin and 2, 6-pyridine dicarboxylic acid as well as a preparation method and application thereof. The salt of gabapentin and 2, 6-pyridine dicarboxylic acid has the advantages of being high in purity, small in hygroscopicity, simple in preparation method and good in reproducibility, the chemical stability is remarkably improved, the production process of amedicine is optimized, and the druggability of the medicine is improved.
Owner:CHINA PHARM UNIV
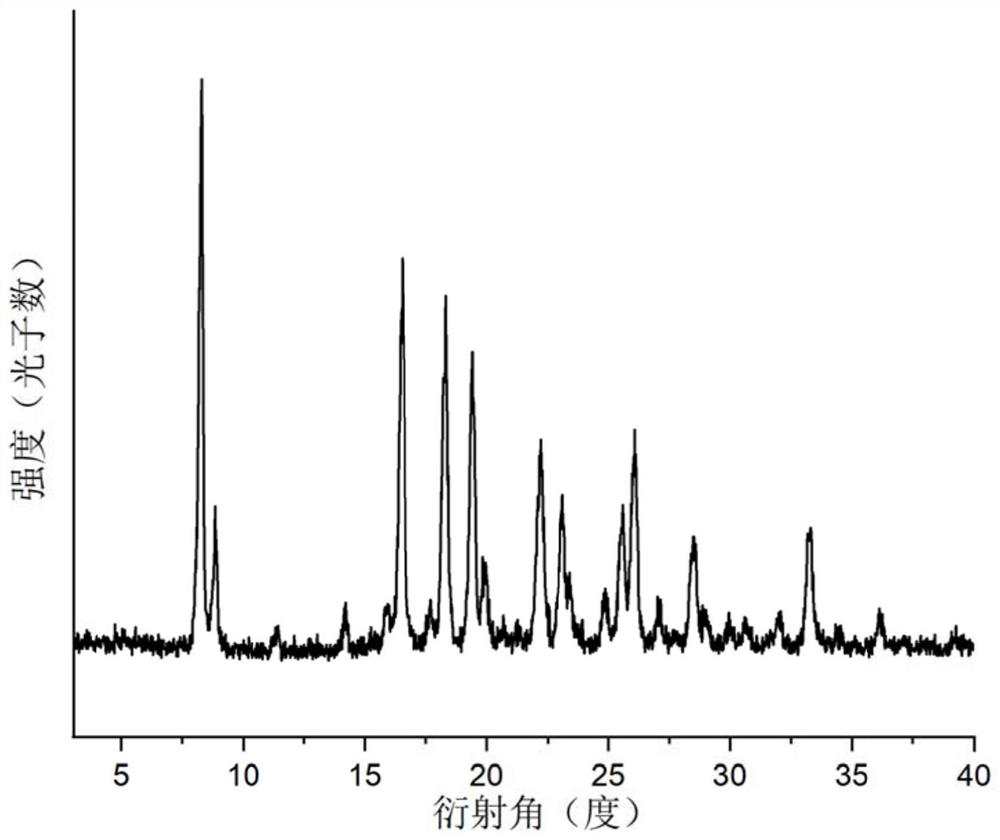
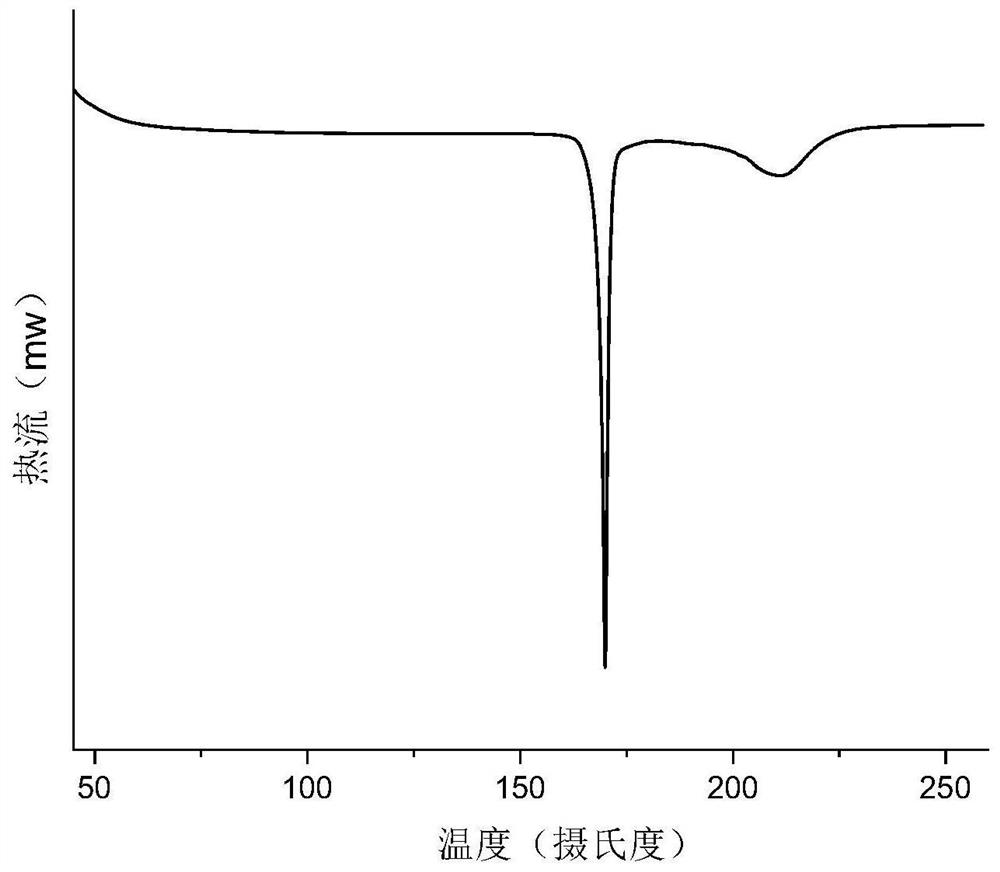
Gabapentin enacarbil compositions
The present invention provides a stabilized composition comprising a non-crystalline gabapentin enacarbil and at least one crystallization-inhibiting compound. In particular, the present invention provides a stabilized composition of gabapentin enacarbil, wherein the gabapentin enacarbil is maintained in a non-crystalline form by the composition, for example, as an amorphous form. The invention also provides, among other things, methods of making the stabilized composition, or use of the stabilized composition for making a medicament.
Owner:TEVA PHARM USA INC
GABA analog prodrug sustained release oral dosage forms
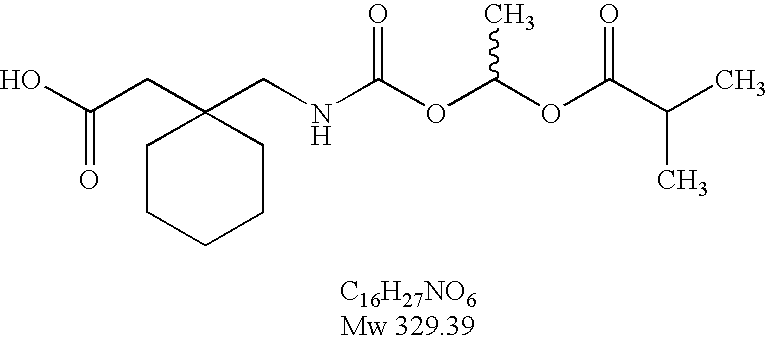
Sustained release oral dosage forms of a gabapentin prodrug, 1-{[(α-isobutanoyloxyethoxy)carbonyl]aminomethyl}-1-cyclohexane acetic acid, are disclosed. The dosage forms are useful for treating or preventing diseases and disorders for which gabapentin is therapeutically effective.
Owner:XENOPORT
TPGS micelle oral liquid containing gabapentin compound and preparation method of TPGS micelle oral liquid
PendingCN114306234ANo bitternessReduce generationOrganic active ingredientsNervous disorderPharmaceutical drugFatty acid
The invention relates to TPGS micelle oral liquid containing a gabapentin compound. The gabapentin compound is prepared from gabapentin and fatty acid or prepared from gabapentin, fatty acid salt and hydrochloric acid. The prepared TPGS micelle oral liquid containing the gabapentin compound can effectively reduce generation of condensation compound lactam in gabapentin molecules, improves drug stability, masks bitter taste of gabapentin, and improves compliance of patients.
Owner:江苏百奥信康医药科技有限公司
Nitrilase mutant and its application
ActiveCN107177576BImprove thermal stabilityHigh activityBacteriaHydrolasesEscherichia coliProtein molecules
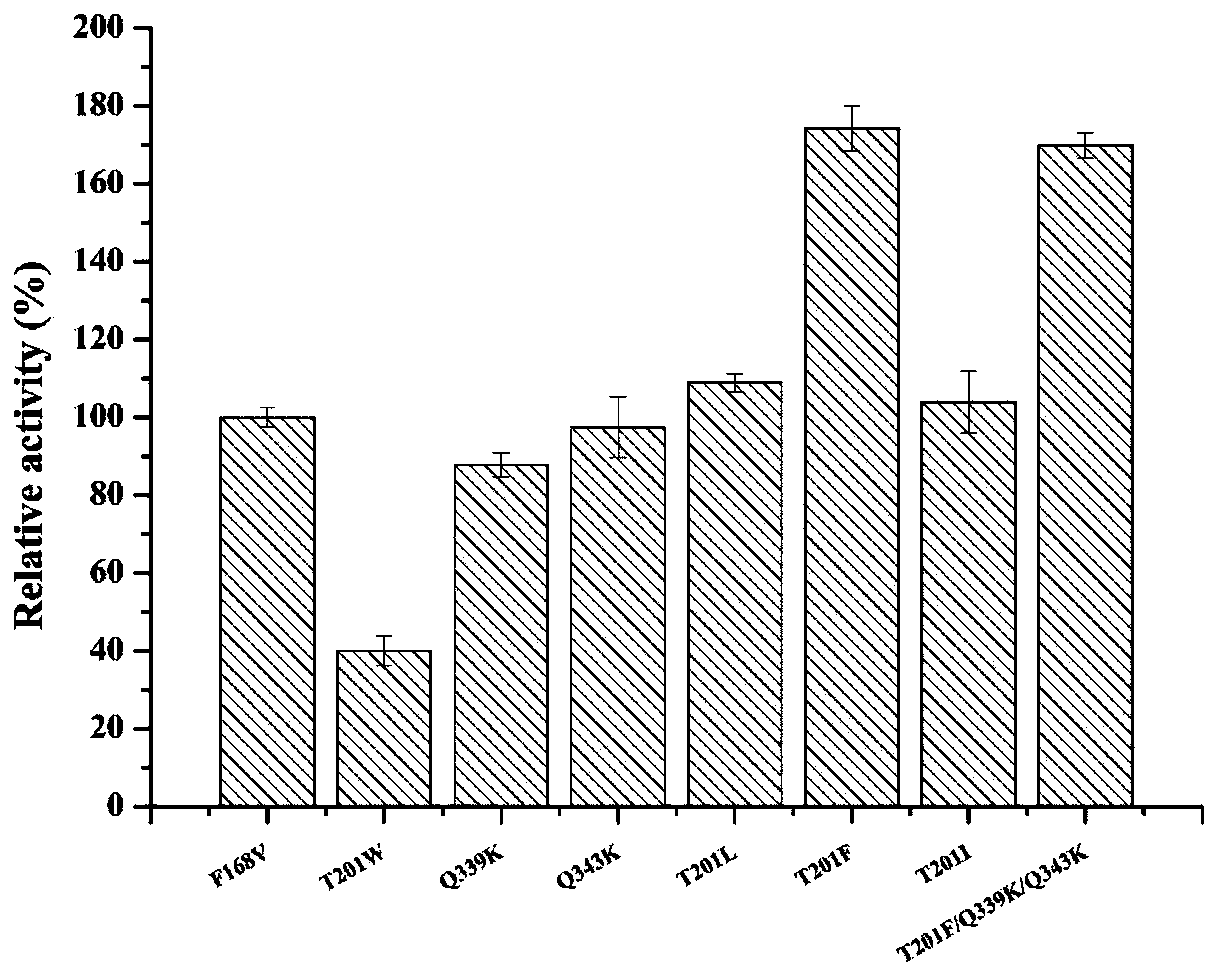
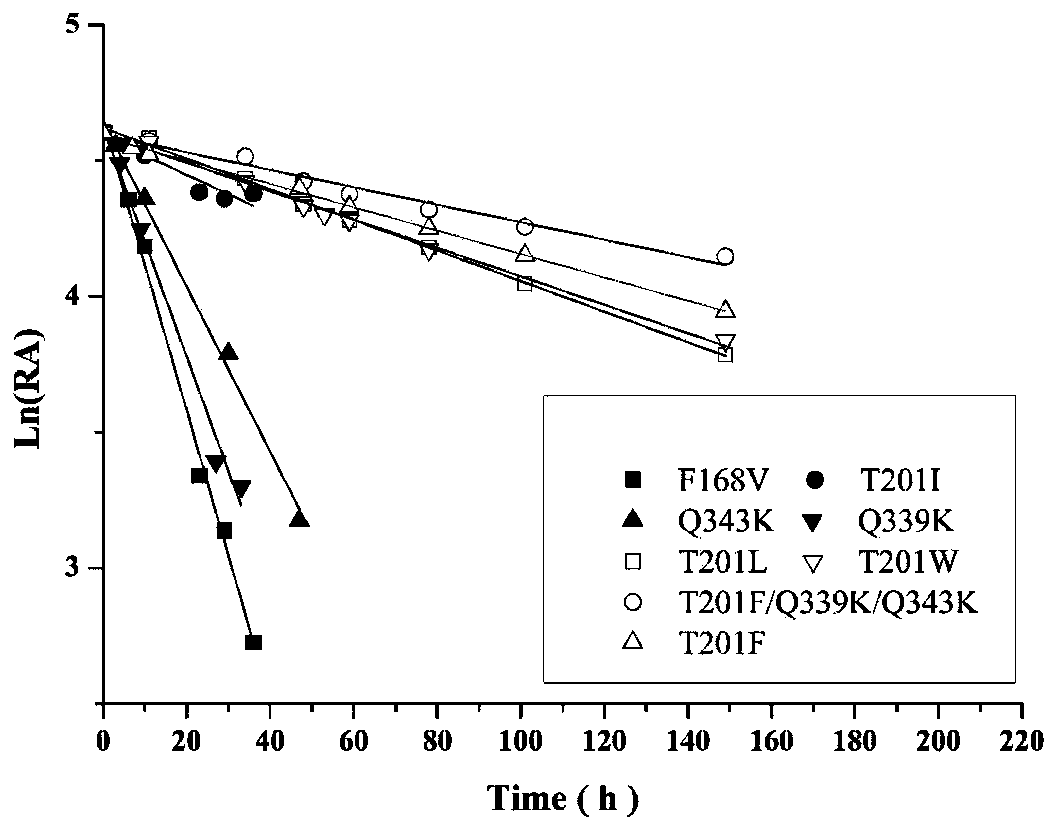
The invention discloses nitrilase mutant and application thereof. The mutant is obtained by carrying out mutation one or more of the sits including 201 site, 339 site and 343 site of an amino acid sequence as shown in SEQ ID No.2. By modification of protein molecules, the thermal stability of nitrilase AcN pure enzyme is improved by 14 times to a maximum extent, recombinant escherichia coli which contains the nitrilase mutant is used for hydrolyzing 1-cyanocyclohexane acetonitrile at the high temperature (45 DEG C), and the reaction time is shortened to be one third of the original reaction time. Therefore, the acquired mutant has good application prospect in efficient catalysis of the 1-cyanocyclohexane acetonitrile to synthesize 1-cyanocyclohexane acetic acid as a gabapentin intermediate.
Owner:ZHEJIANG UNIV OF TECH
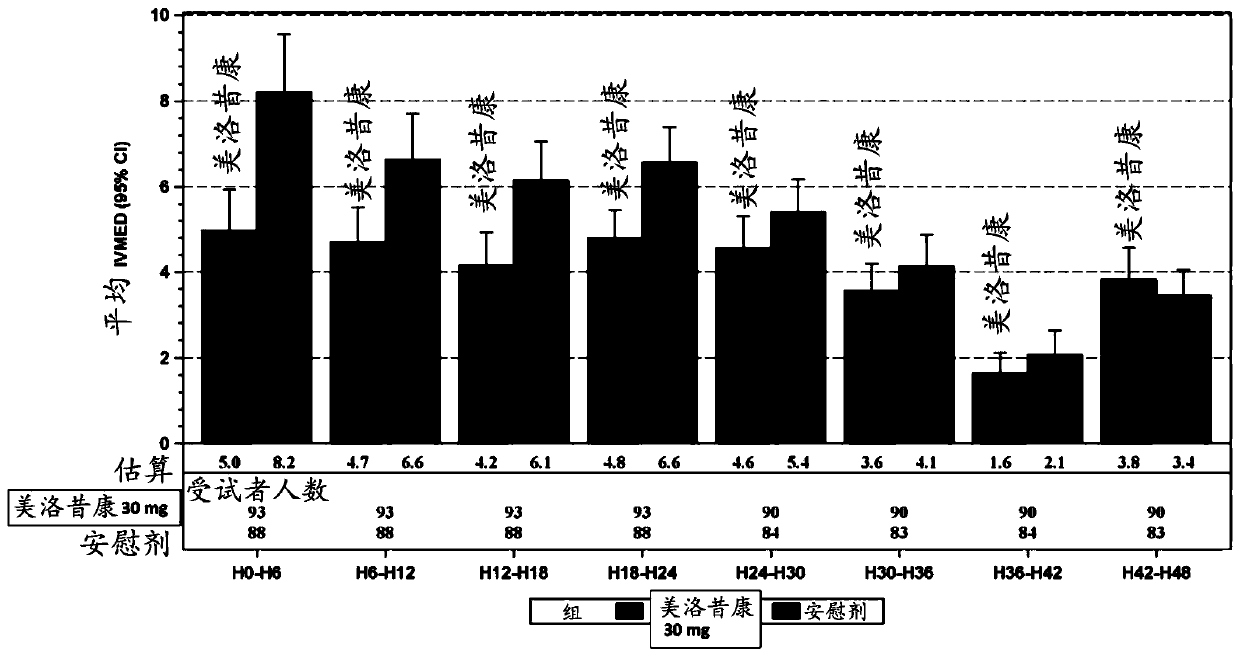
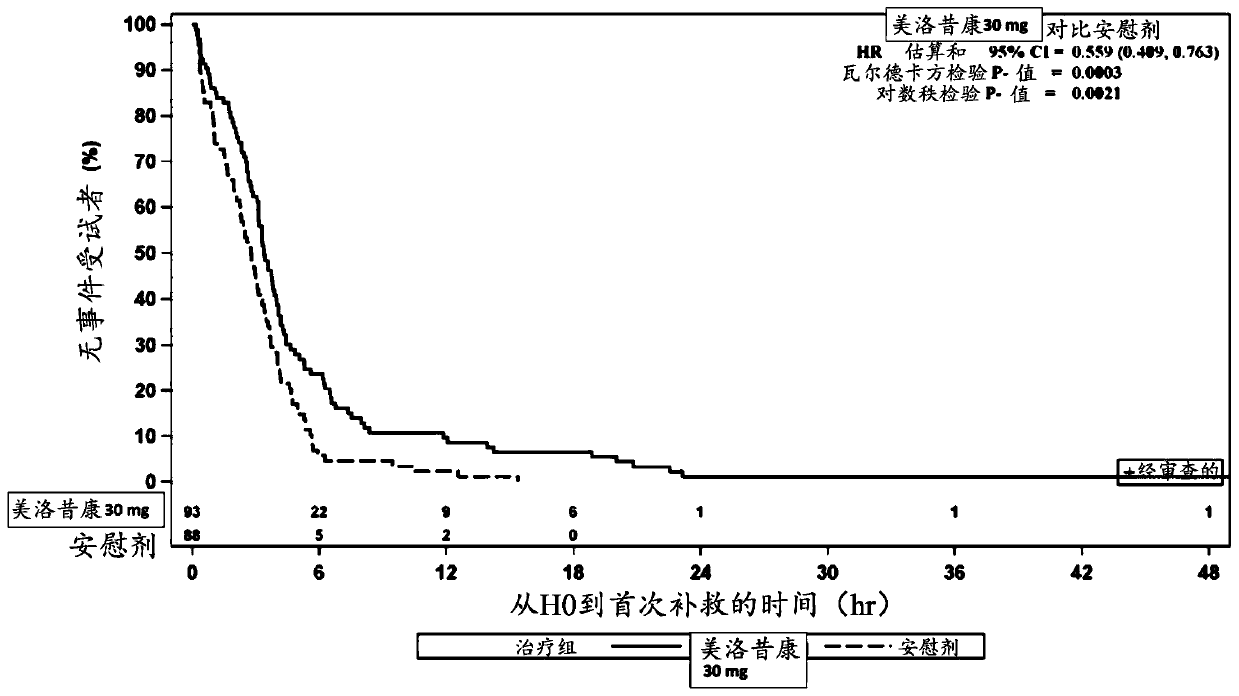
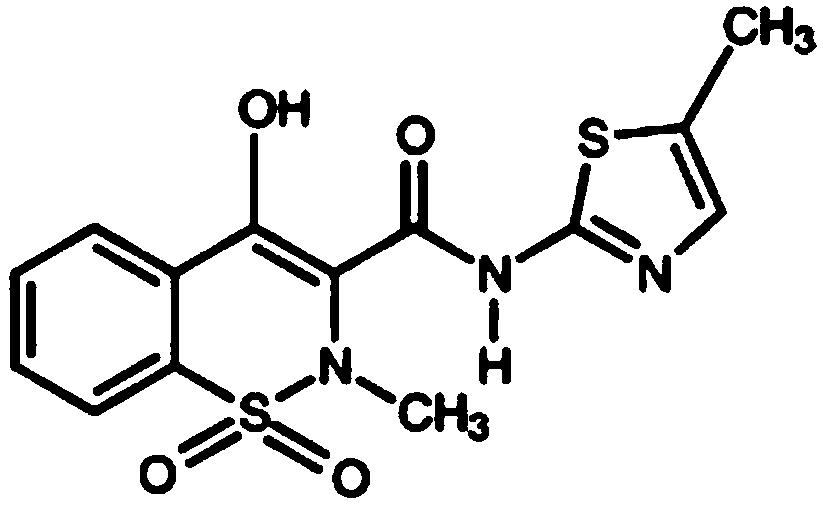
Methods of administering intravenous meloxicam pre-operatively and in combination with other drugs
The disclosure provides methods of treating pain in a patient who will be subjected to a surgical procedure, comprising administering meloxicam to the patient prior to start of the surgical procedure.In some embodiments, meloxicam is nanocrystalline meloxicam. The disclosure further provides methods of treating pain in a patient in need thereof, comprising administering meloxicam intravenously tothe patient in combination with acetaminophen and / or gabapentin.
Owner:BAUDAX BIO INC
Oral veterinary composition with gabapentin
Owner:CHAIT AUERBACH JAIME SAMUEL
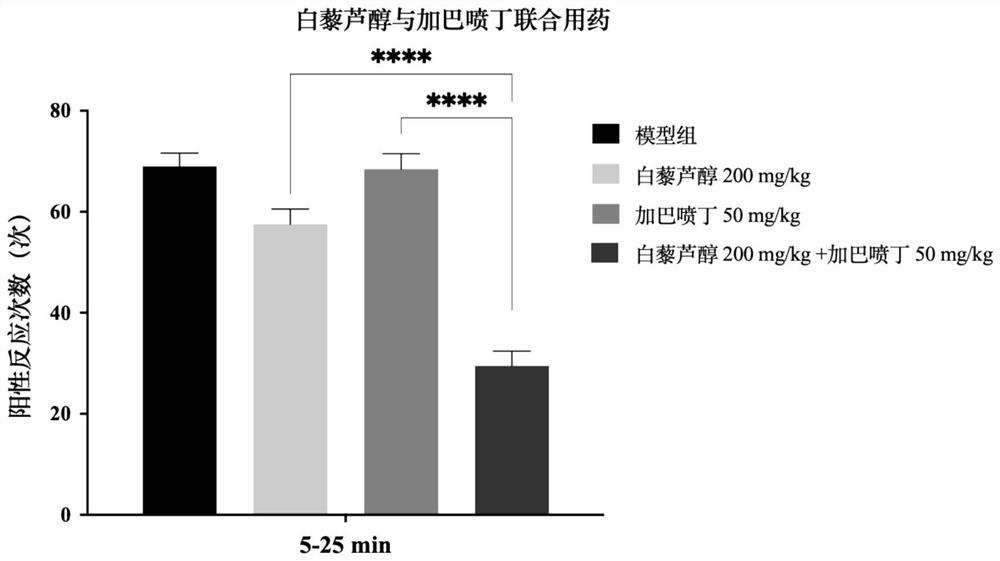
Pharmaceutical composition with synergistic analgesic effect
PendingCN114053257ALower doseReduce or avoid adverse reactionsHydroxy compound active ingredientsAntipyreticDuloxetineCo medication
The invention belongs to the technical field of medicines, and particularly relates to a pharmaceutical composition with a synergistic analgesic effect, and the pharmaceutical composition comprises resveratrol and one of gabapentin and duloxetine. According to the invention, resveratrol and gabapentin or duloxetine are combined for medication, a large number of experimental studies show that the combined medication can generate a very obvious synergistic analgesic effect, and experiments show that the dose combination does not affect the movement balance ability and muscle coordination of an acting object. Therefore, the analgesic effect of the combination of the resveratrol and the gabapentin or duloxetine is superior to that of the gabapentin or duloxetine which is independently used, so that the pharmaceutical composition has a better application prospect; in addition, the use dosage of gabapentin or duloxetine can be greatly reduced through pharmaceutical composition, guarantee is provided for compliance and tolerance of clinical medication, and adverse reactions of gabapentin or duloxetine are reduced or avoided.
Owner:JIANGSU OCEAN UNIV
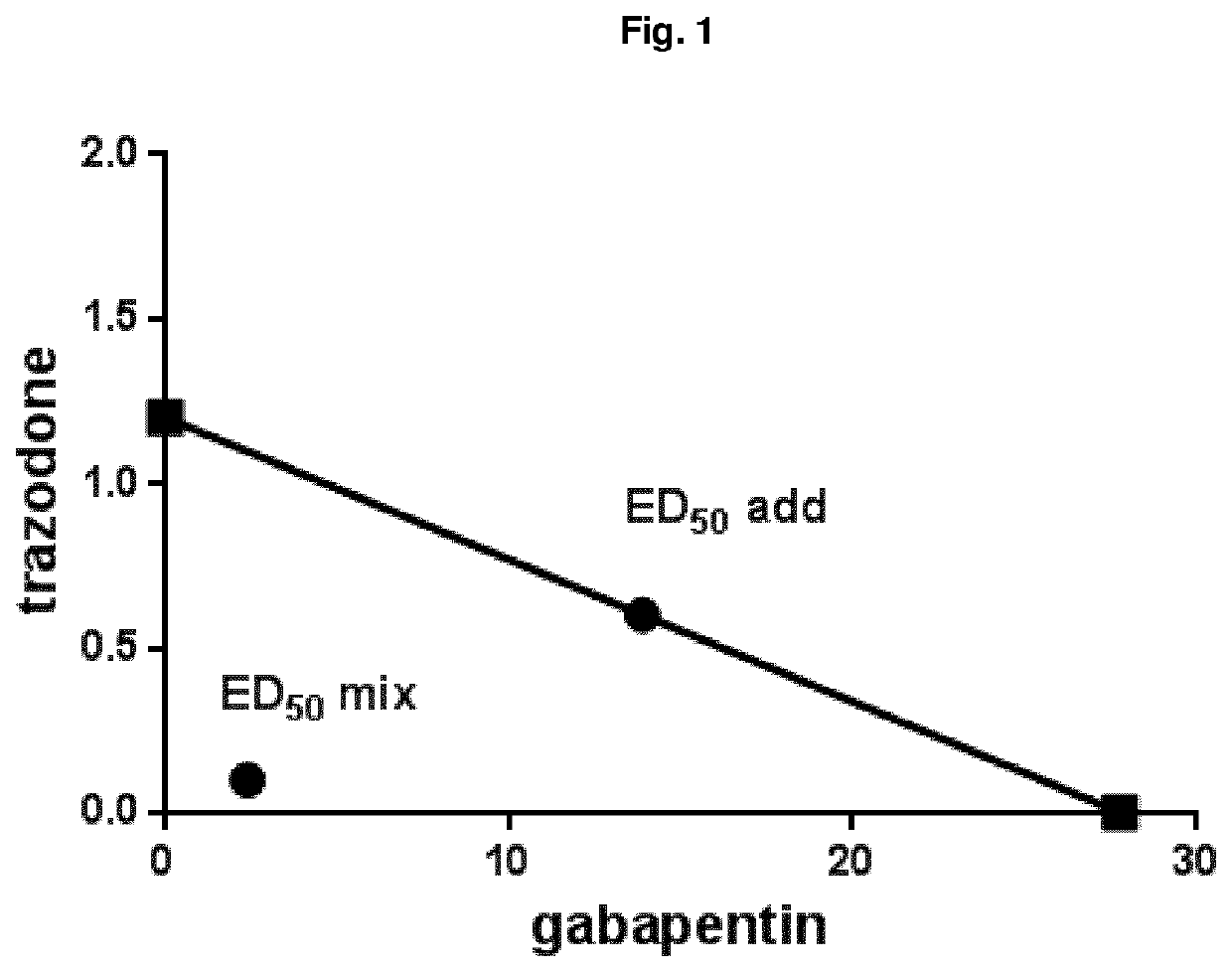
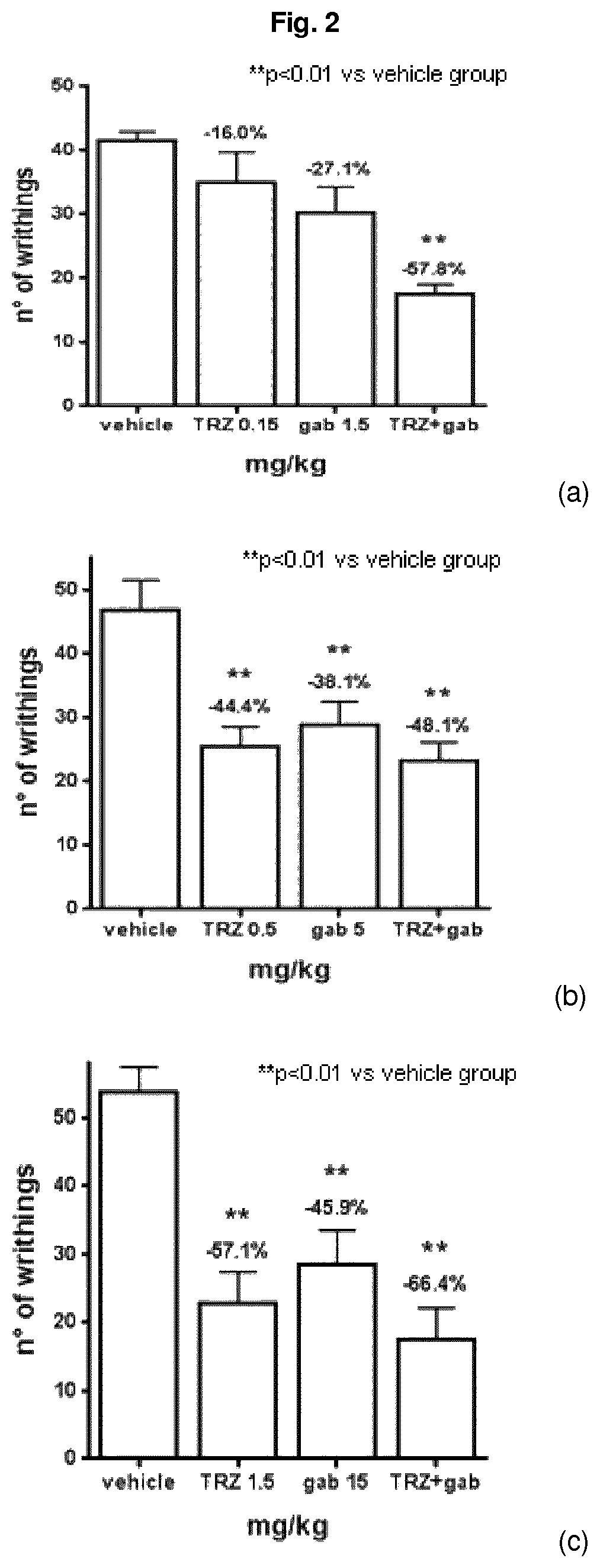
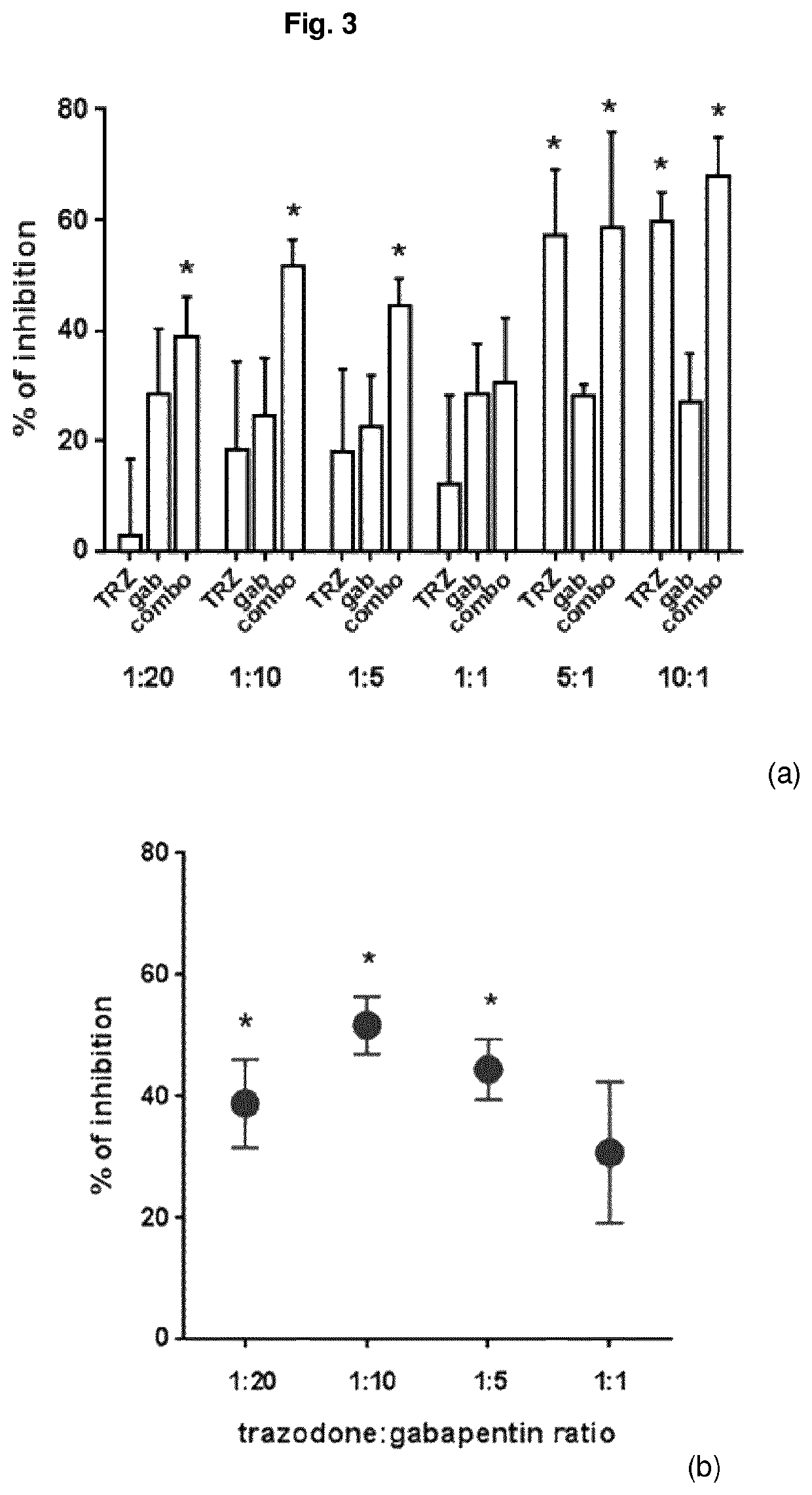
Combination of trazodone and gabapentin for the treatment of pain
ActiveUS10786498B2Relieve painOrganic active ingredientsNervous disorderPharmaceutical drugTreatment pain
Owner:AZIENDE CHIMHE RIUNITE ANGELINI FRANCESCO A C R A F
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com