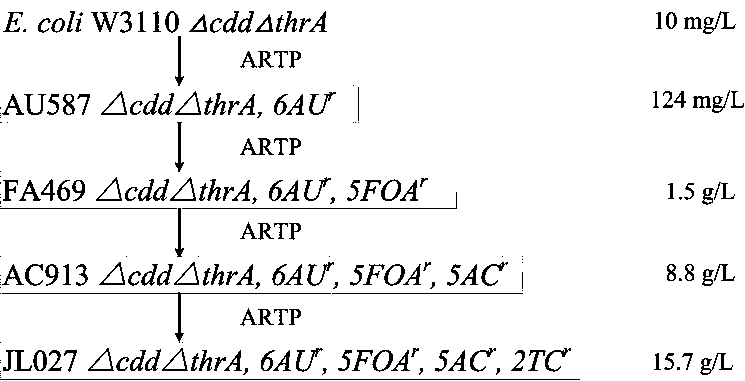
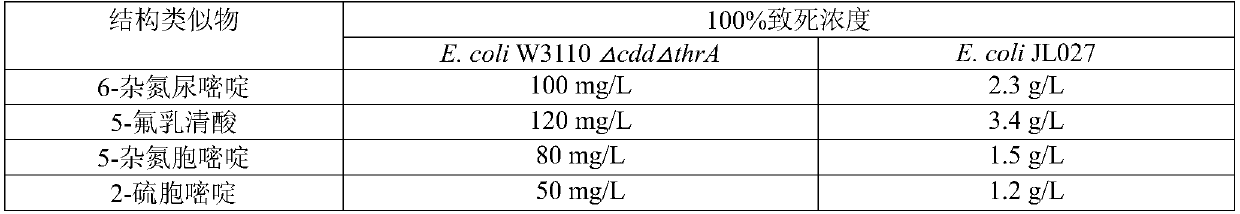
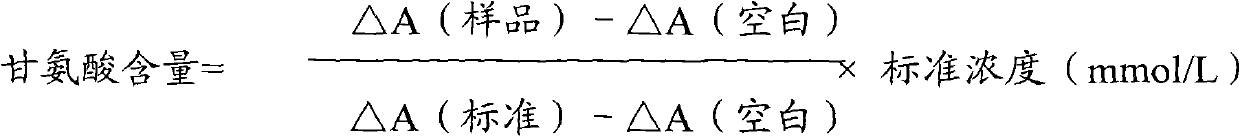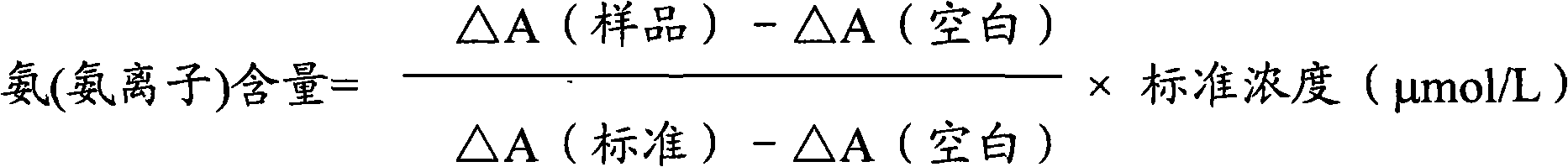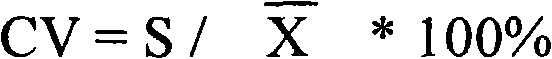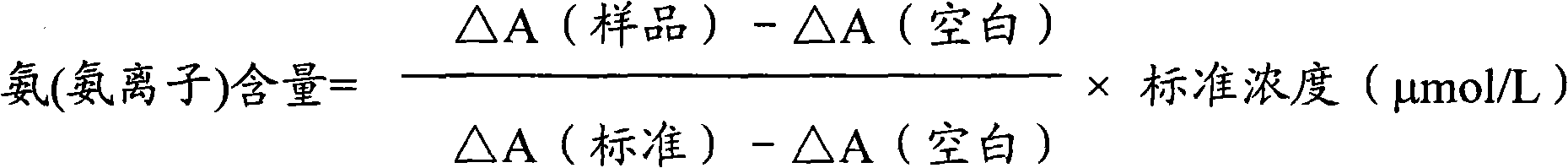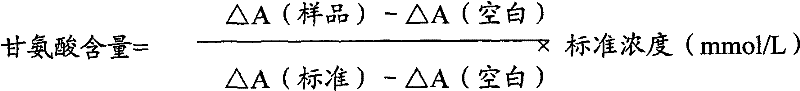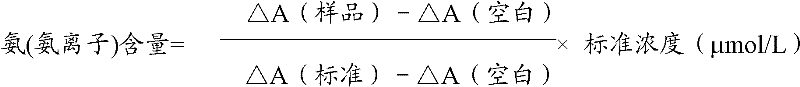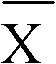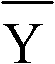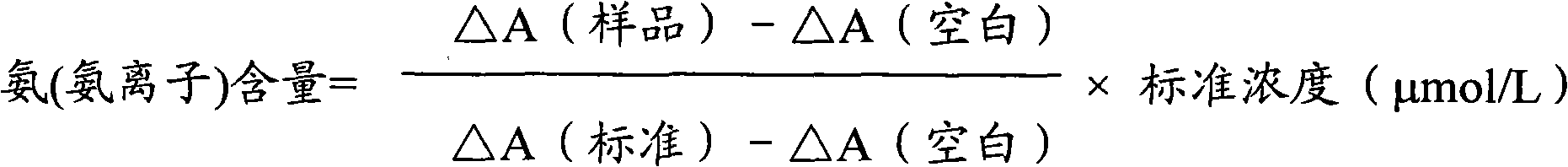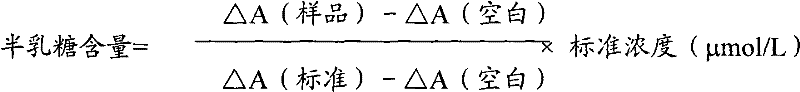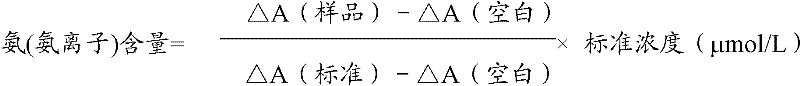Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
65 results about "Carbamoyl phosphate synthetase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
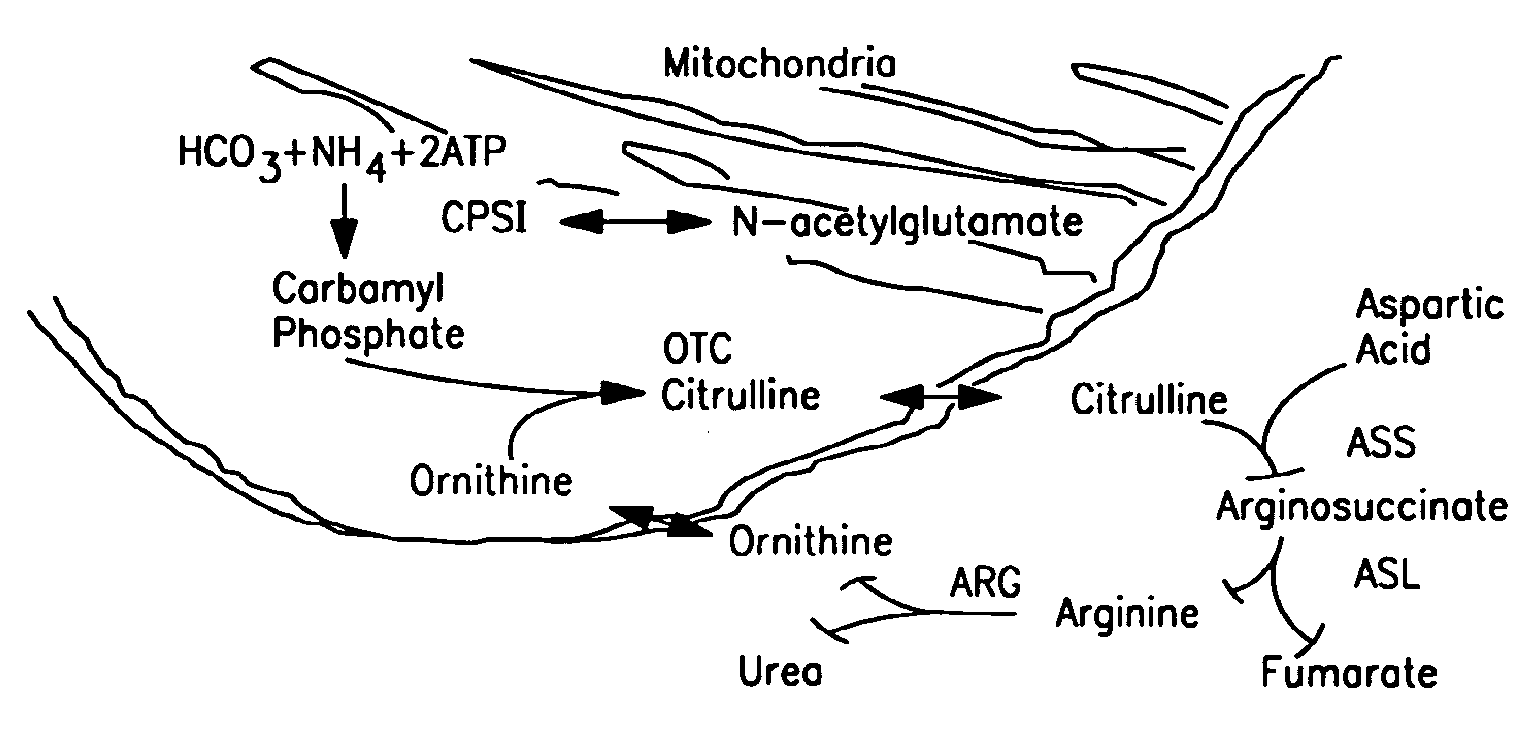
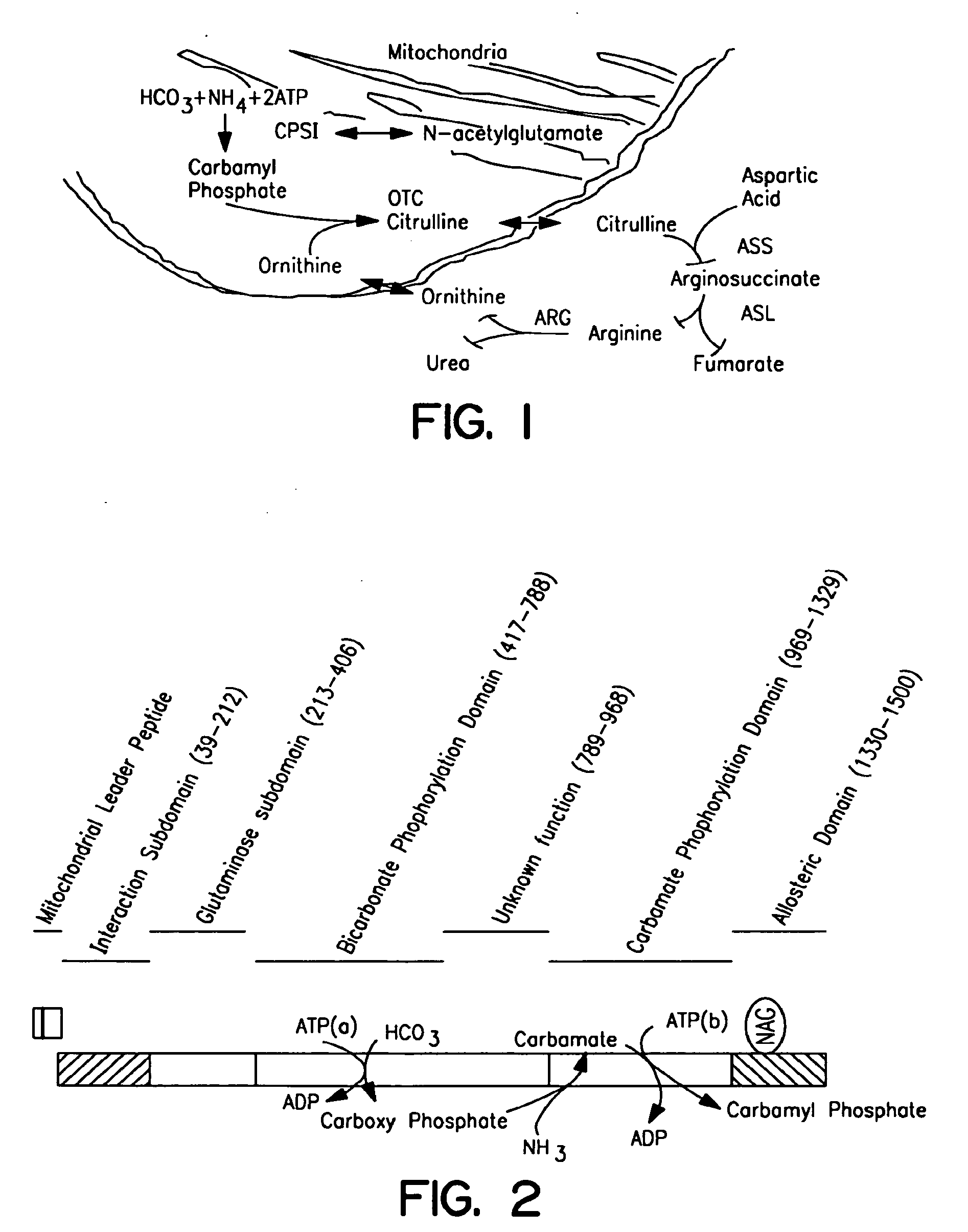
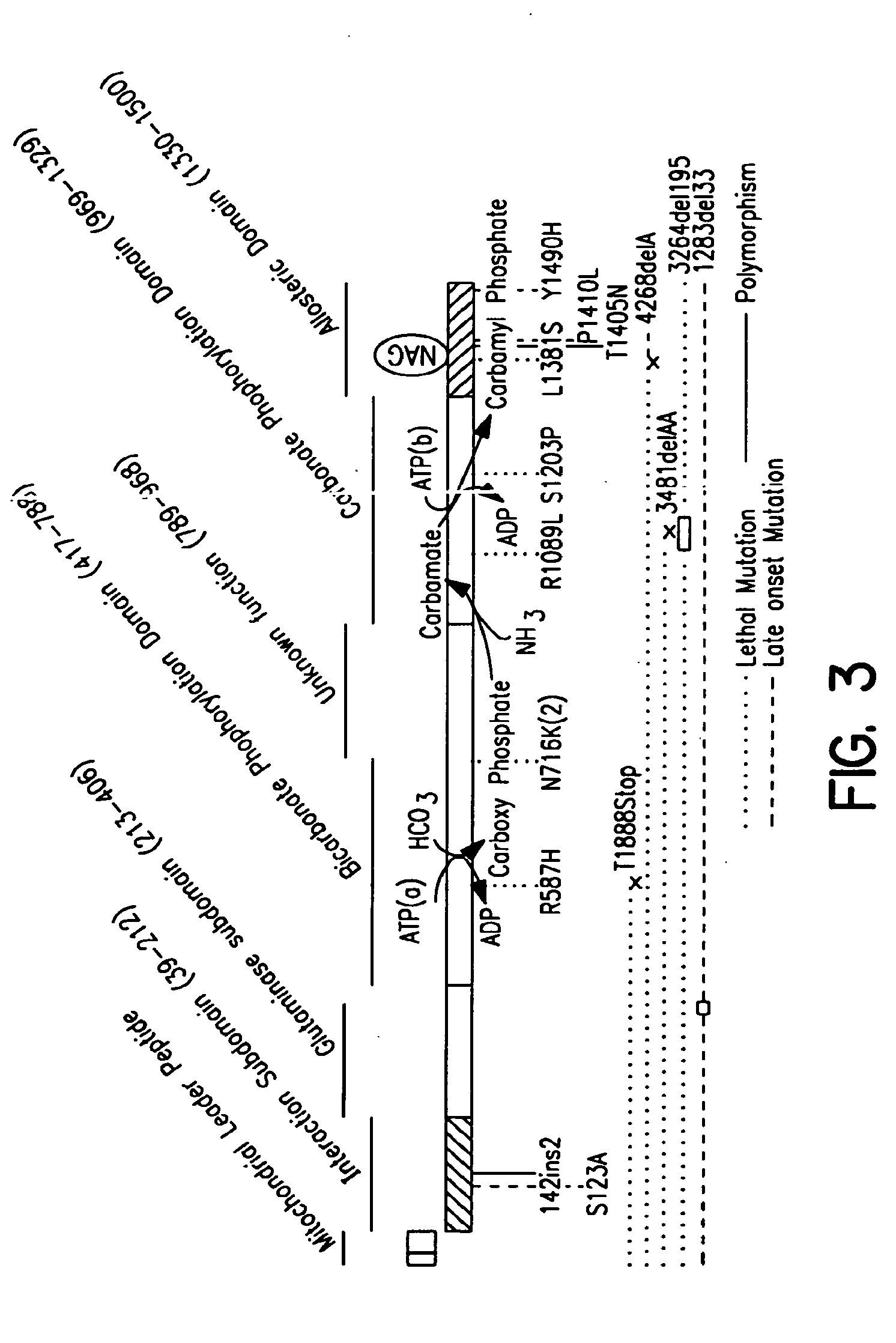
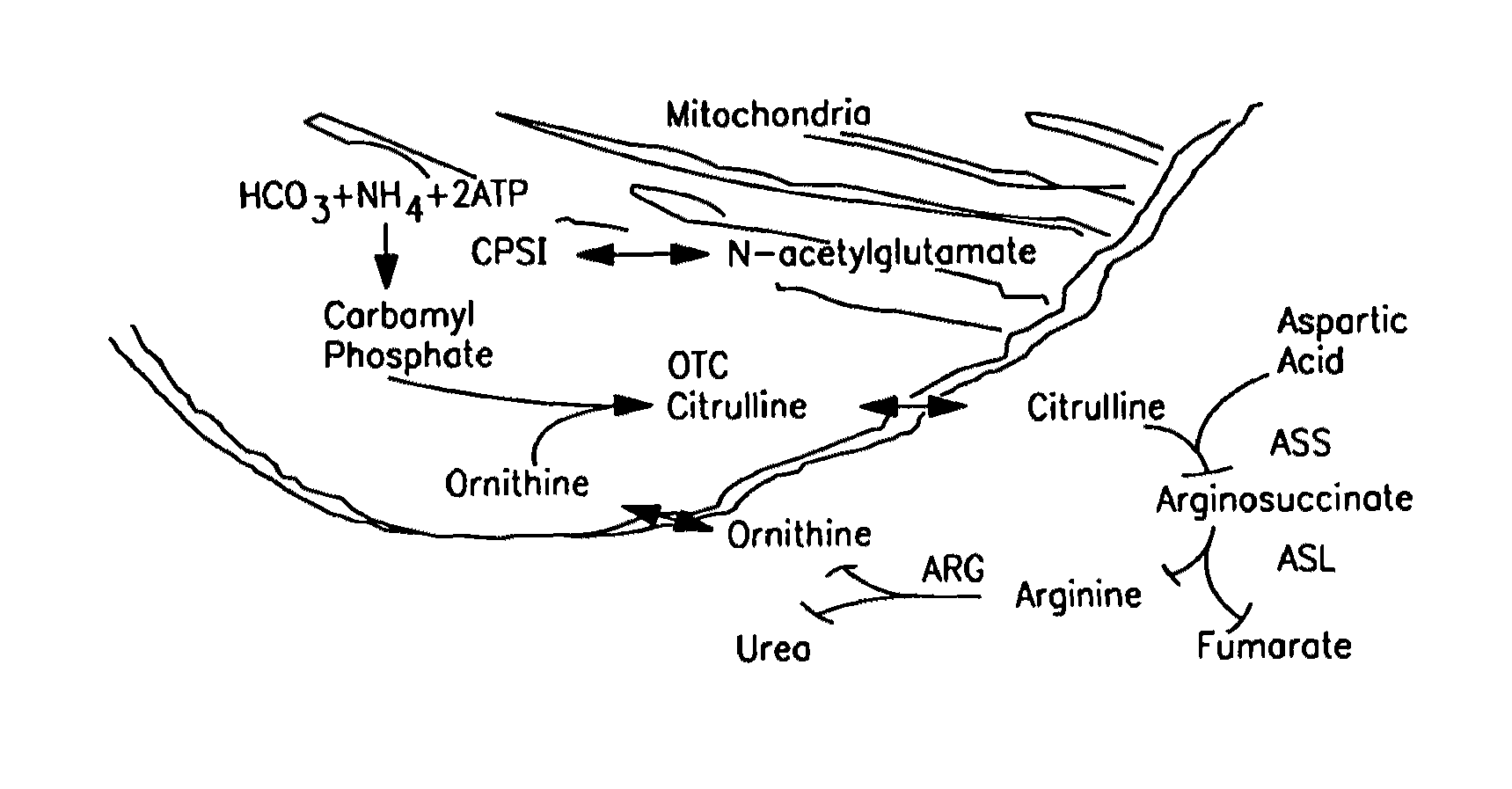
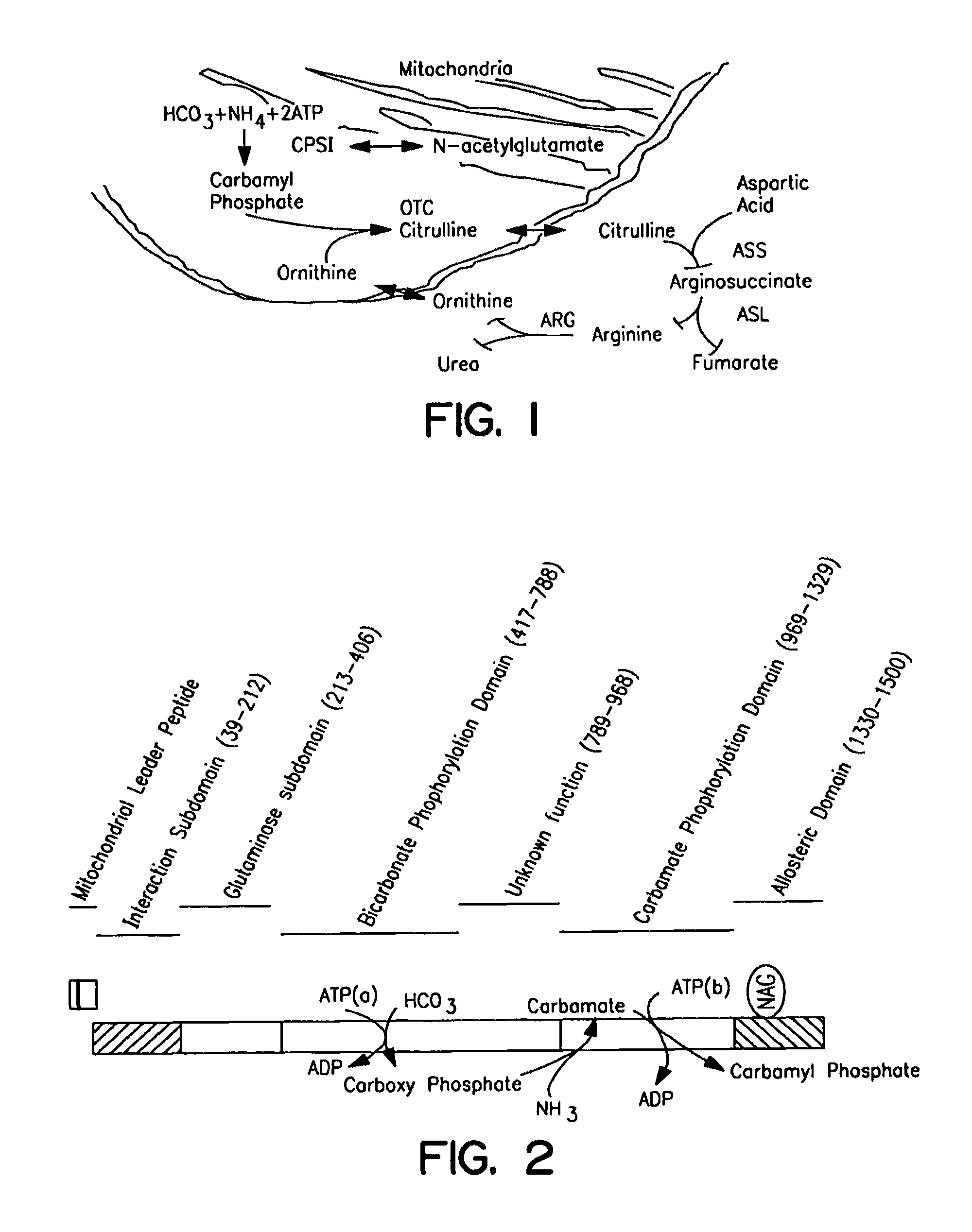
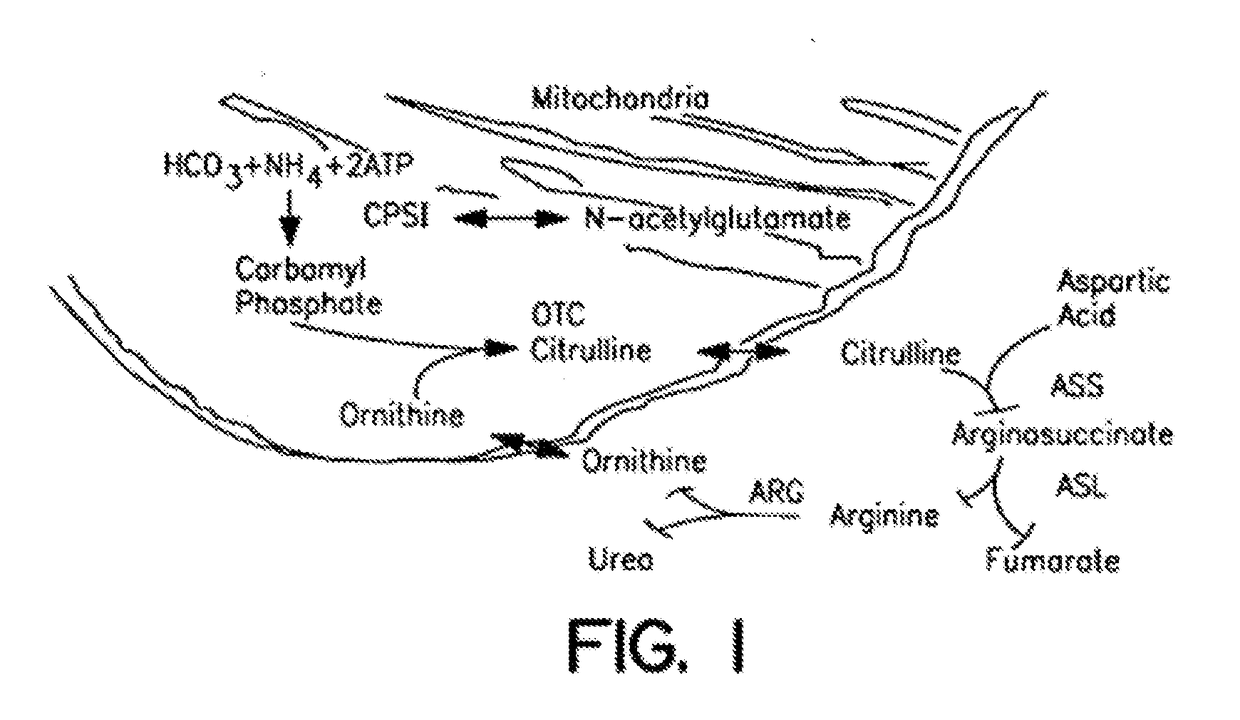
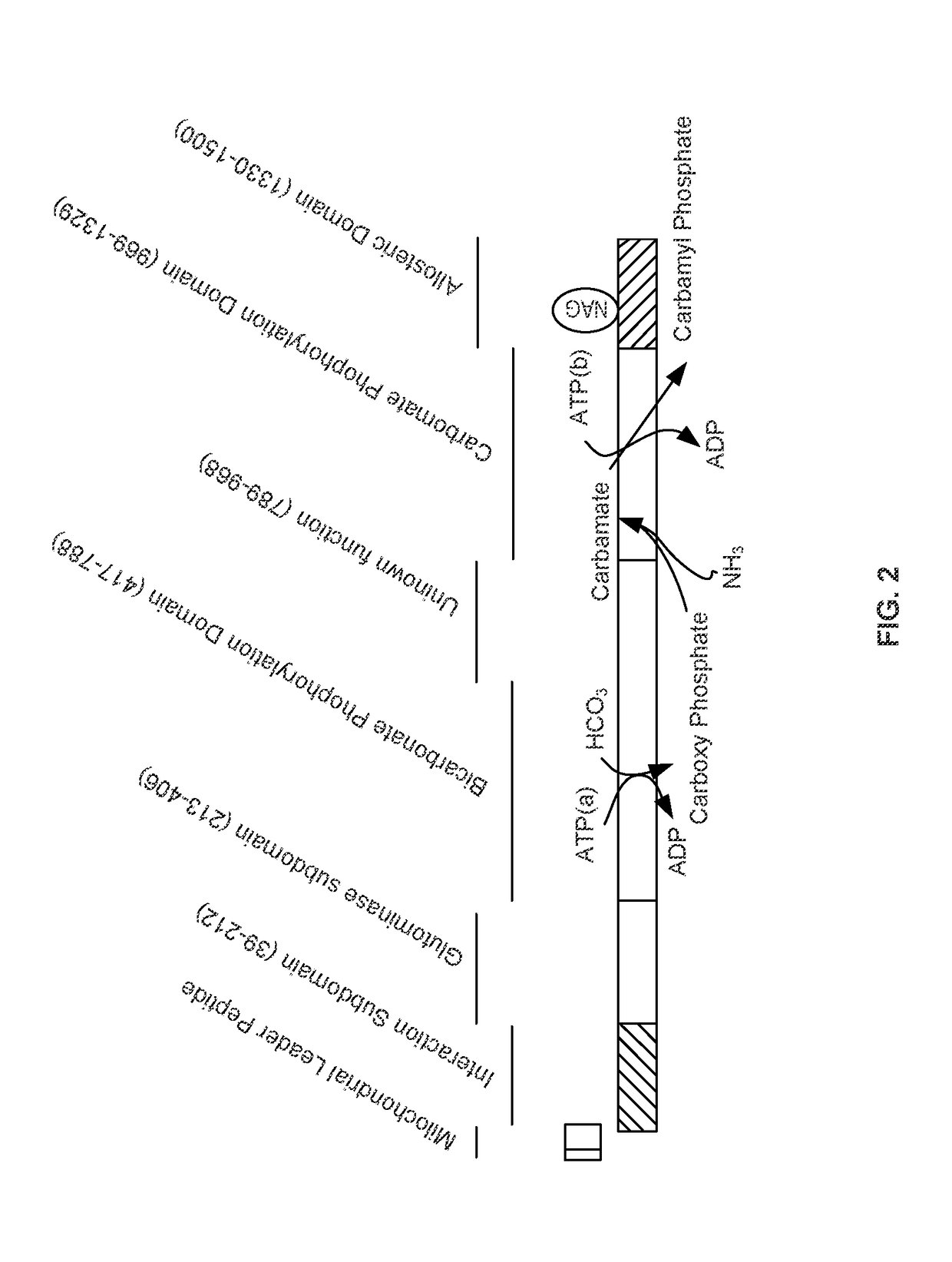
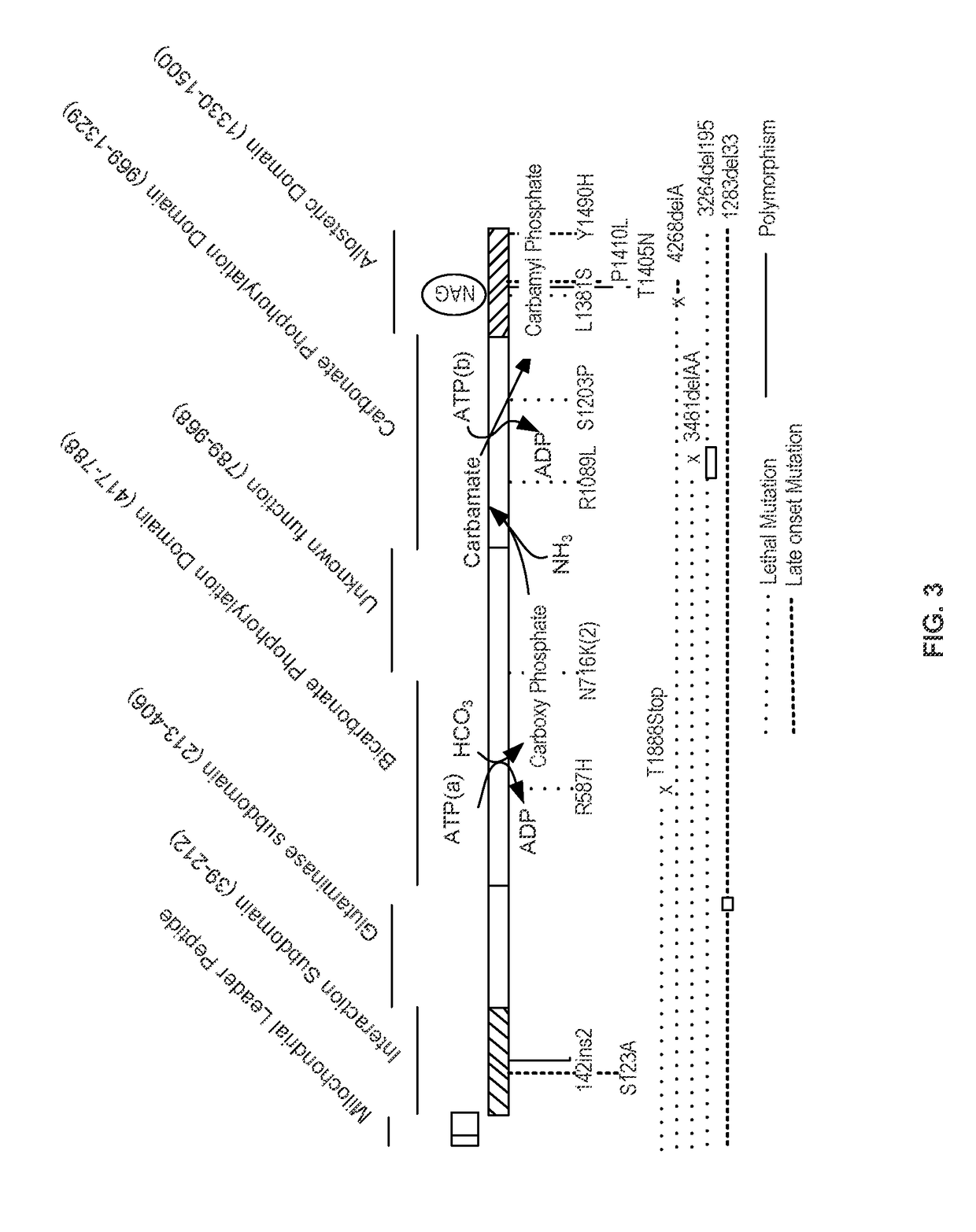
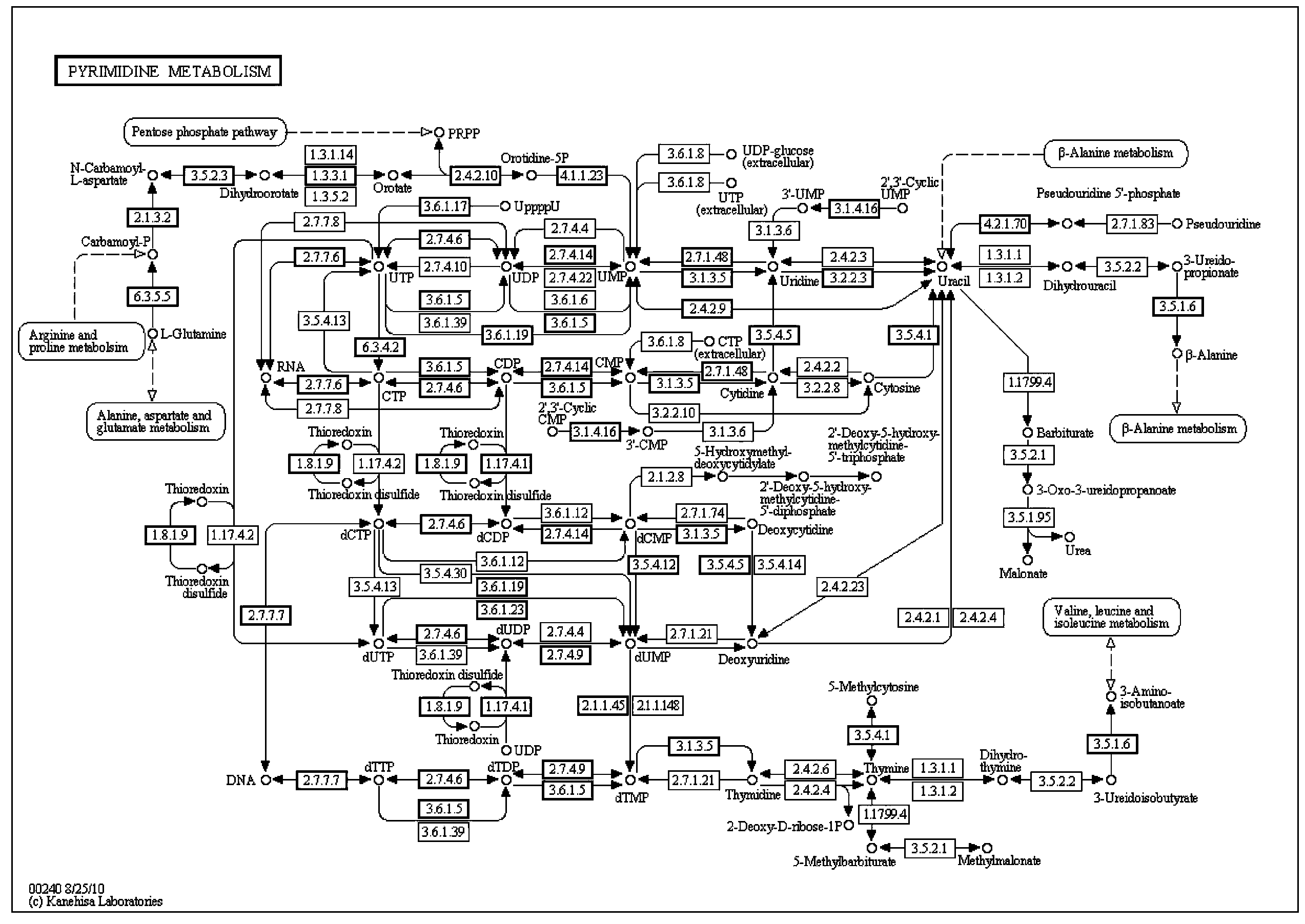
Carbamoyl phosphate synthetase catalyzes the ATP-dependent synthesis of carbamoyl phosphate from glutamine (EC 6.3.5.5) or ammonia (EC 6.3.4.16) and bicarbonate. This enzyme catalyzes the reaction of ATP and bicarbonate to produce carboxy phosphate and ADP. Carboxy phosphate reacts with ammonia to give carbamic acid. In turn, carbamic acid reacts with a second ATP to give carbamoyl phosphate plus ADP.
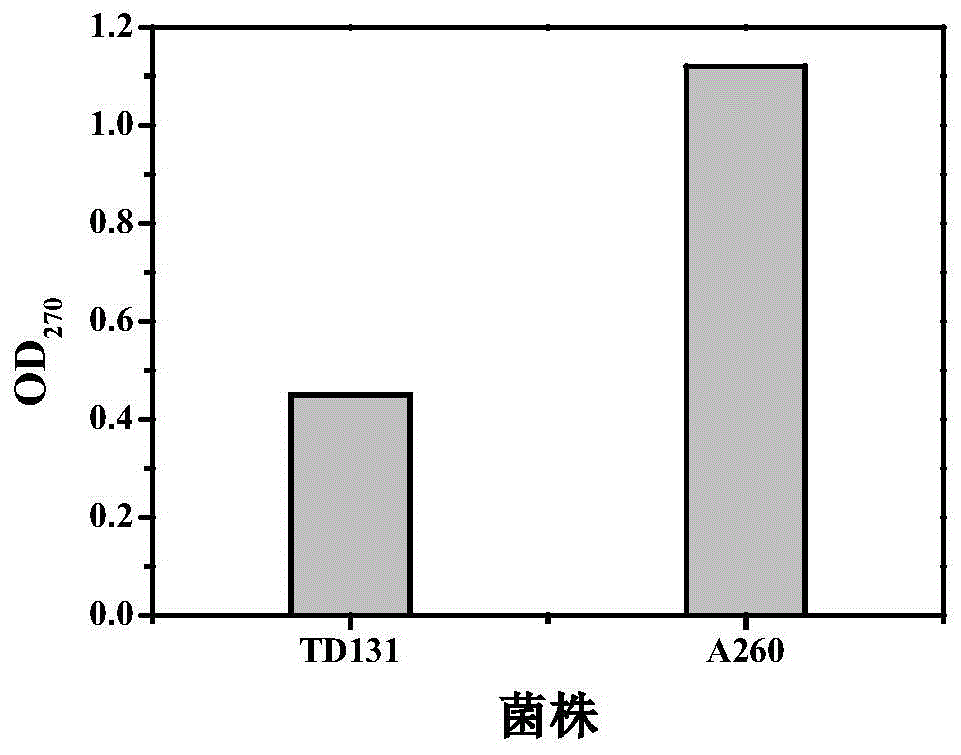
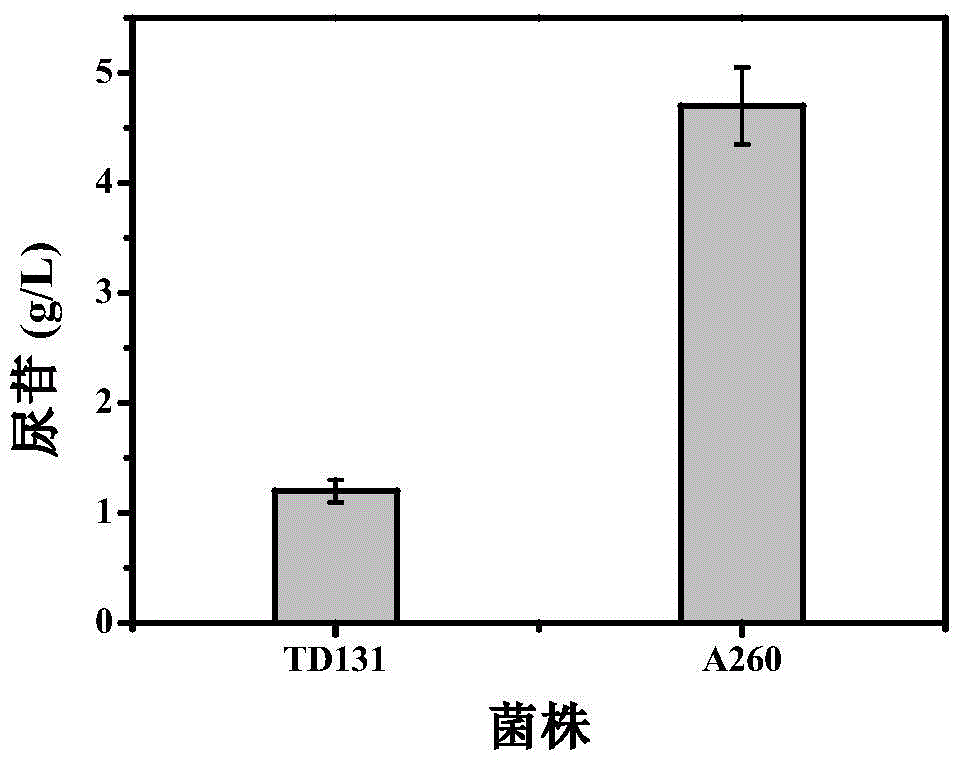
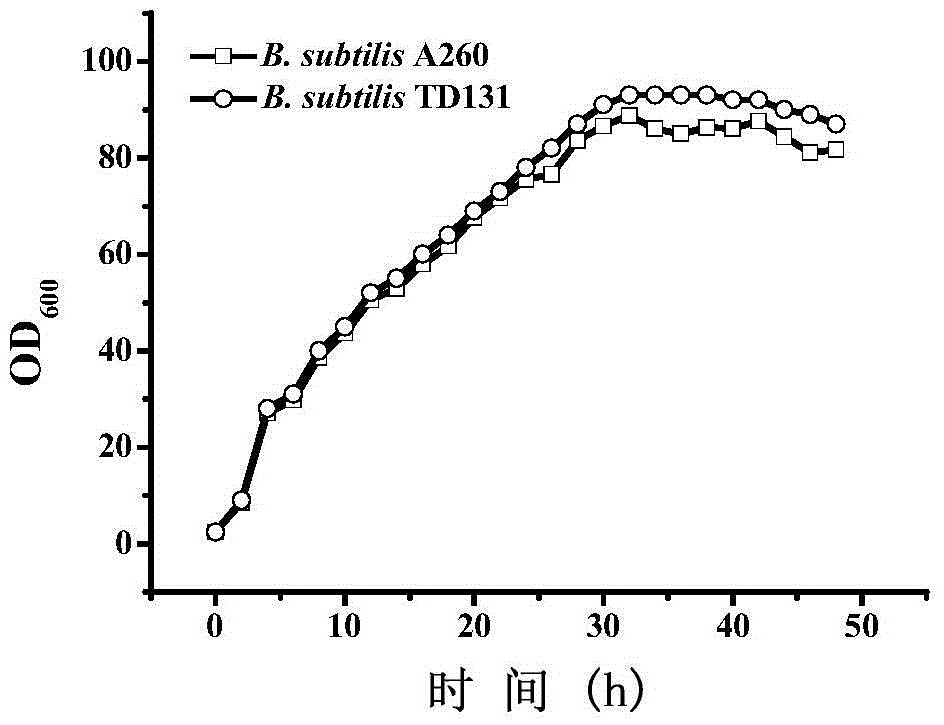
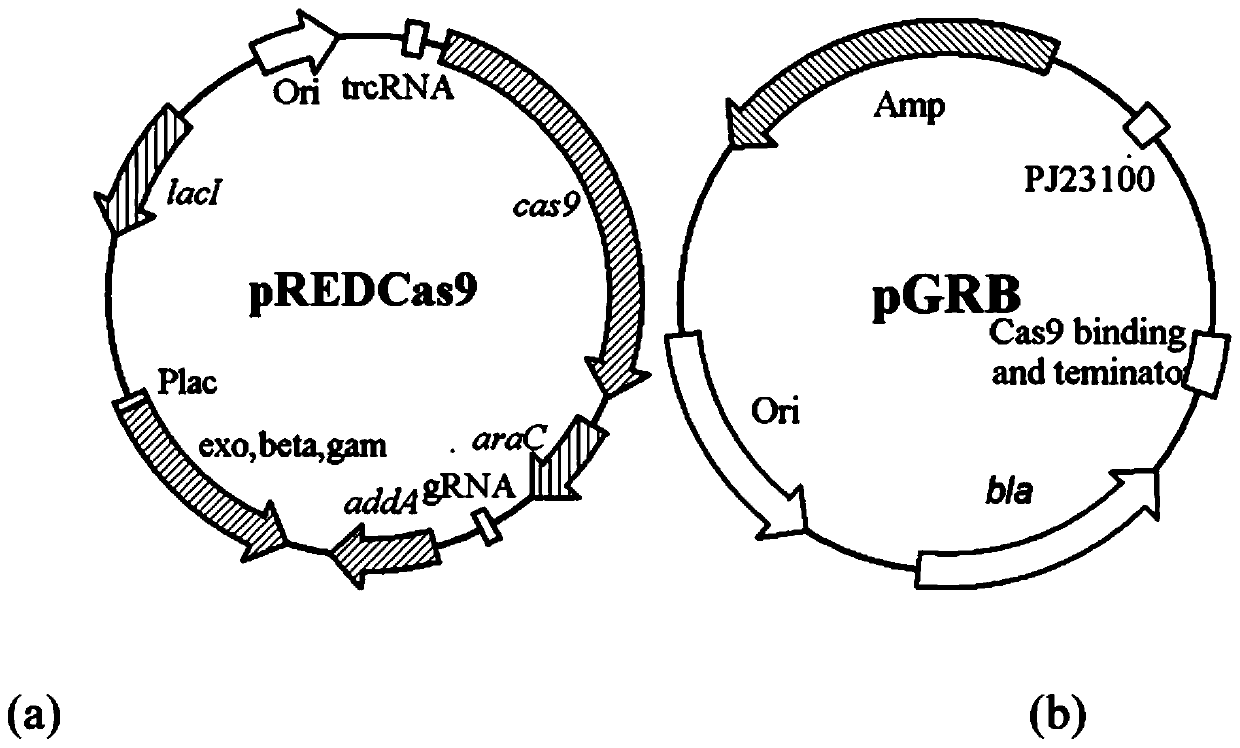
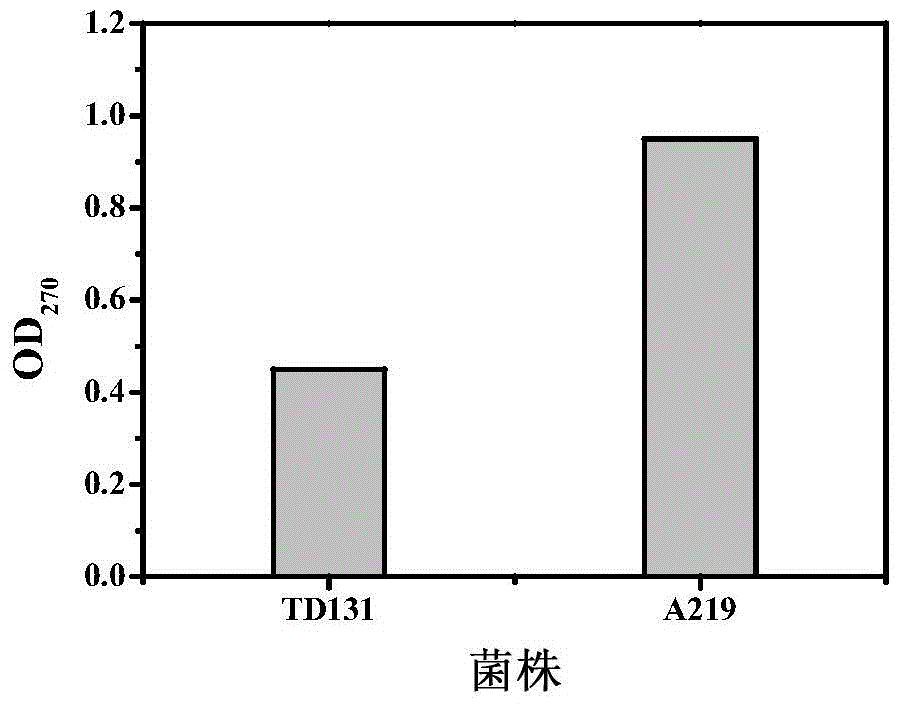
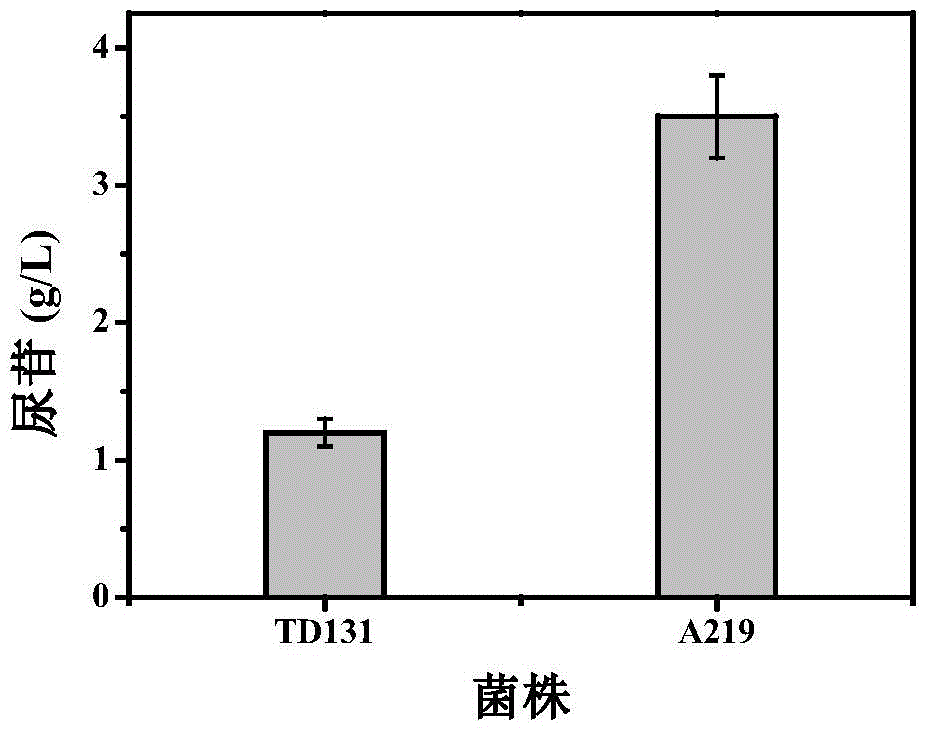
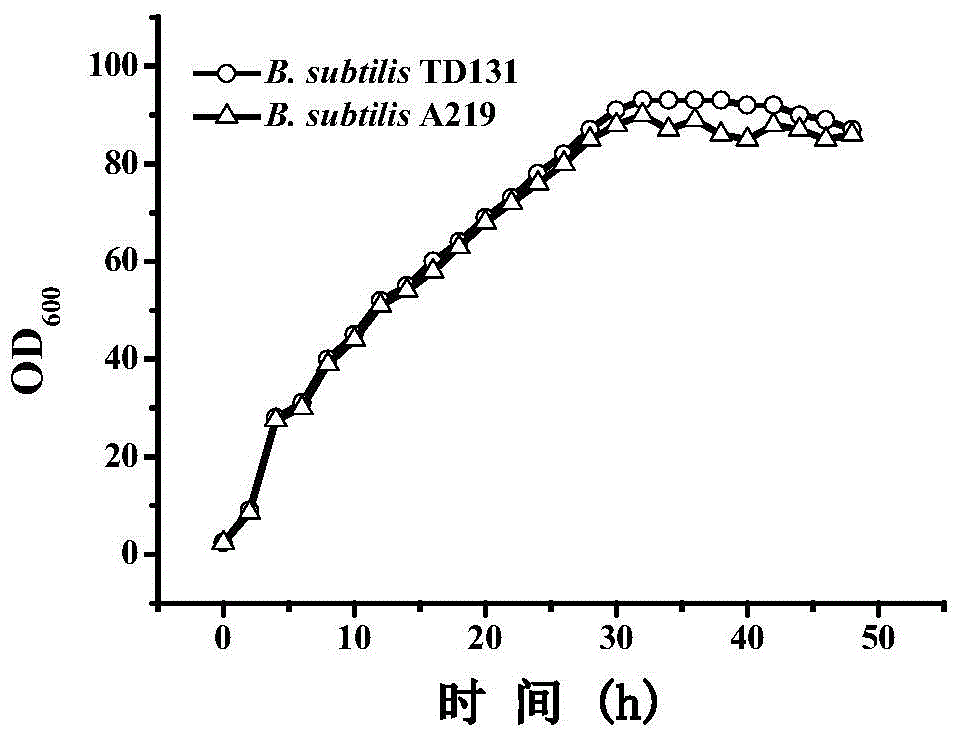
Pyrimidine nucleoside high-yielding strain and carbamyl phosphate synthetase adjusting site thereof
ActiveCN105671007AImprove toleranceBacteriaMicroorganism based processesCarbamyl PhosphateStructural analog
The invention belongs to the technical field of enzyme engineering, and concretely relates to a pyrimidine nucleoside high-yielding strain and a carbamyl phosphate synthetase adjusting site thereof. The invention provides a pyrimidine nucleoside production Bacillus subtilis mutant strain and a carbamyl phosphate synthetase encoding gene. A key regulation site related to carbamyl phosphate synthetase end product feedback inhibition is defined, and provides reference for breeding of later pyrimidine nucleoside high-yielding strains. The Bacillus subtilis mutant strain allows the output of fermentation process produced nucleoside pyrimidine uridine to reach 14-16g / L, and has substantially improved tolerance to different pyrimidine nucleoside structure analogs.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Genetically engineered bacterium for producing L-arginine and construction method and application thereof
ActiveCN110964683AShorten the growth cycleClear genetic backgroundBacteriaHydrolasesEscherichia coliCarbamyl Phosphate
The invention discloses a genetically engineered bacterium for producing L-arginine and a construction method and application thereof. According to the invention, a gene for encoding carbamyl phosphate synthetase and a gene for encoding L-arginine biosynthetic pathway enzyme are integrated in escherichia coli; a synthetic route of arginine in escherichia coli and metabolic flux related to argininein a whole amino acid metabolic network are analyzed and reconstructed to obtain genetically engineered bacteria which are clear in genetic background, do not carry plasmids, are not mutated and canstably and efficiently produce L-arginine; and the genetically engineered bacteria have good L-arginine production capacity.
Owner:NINGXIA EPPEN BIOTECH
Therapeutic methods employing nitric oxide precursors
Isolated polynucleotide molecules and peptides encoded by these molecules are used in the analysis of human carbamyl phosphate synthetase I phenotypes, as well as in diagnostic and therapeutic applications, relating to a human carbamyl phosphate synthetase I polymorphism. By analyzing genomic DNA or amplified genomic DNA, or amplified cDNA derived from mRNA, it is possible to type a human carbamyl phosphate synthetase I with regard to the human carbamyl phosphate synthetase I polymorphism, for example, in the context of diagnosing and treating hepatic veno-occlusive disease (HVOD) associated with bone marrow transplants.
Owner:VANDERBILT UNIV
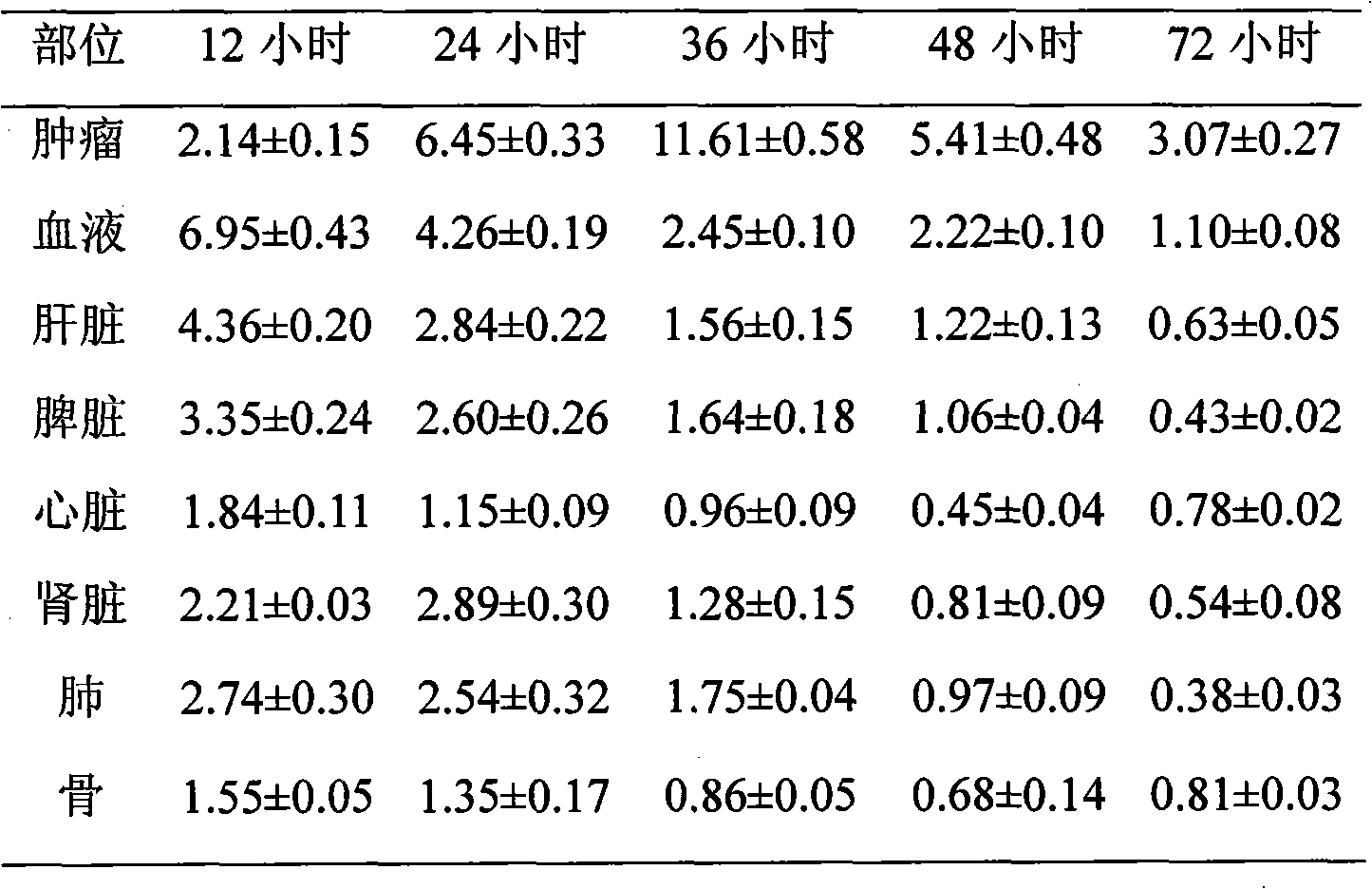
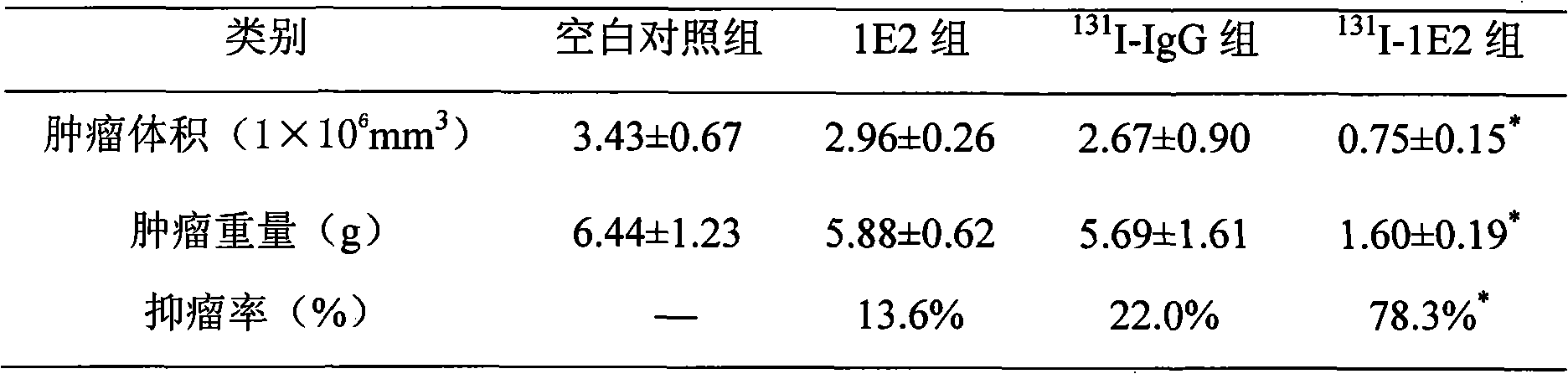
131I labeled anti-tumor humanized monoclonal antibody 1E2 and use thereof
InactiveCN101407545AImprove targetingGood inhibitory effectIn-vivo radioactive preparationsImmunoglobulins against animals/humansAntigenPhage antibodies
The invention provides an anti-lung cancer human resource monoclonal antibody 1E2 marked by <131>I. The human resource 1E2 antibody, Na <131>I and chloramine T are used as raw materials, and purified 1E2 antibody marked by the <131>I can be prepared by using the chloramine T method, wherein, the human source 1E2 antibody is obtained by taking paracanser lymph nodes of a non-small cell lung cancer patient, extracting total RNA in lymph node tissues, and then a phage antibody library containing the 1E2 antibody is obtained by using the constructing technology of the phage antibody library, and the phage antibody 1E2 is adsorbed by using a carbamyl phosphate synthetase antigen, and a human source 1E2 antibody which is specific to the carbamyl phosphate synthetase is finally obtained by washing, screening and amplifying. The invention applies radiation immunity treatment technology, combines advantages of radionuclide and anti-tumor cell human source monoclonal antibody to one, improves the targeting ability and curative effect of medicines to tumor cells, reduces side effects and poor immune reactions, and is wider in application range and suitable for treatments of the non-small cell lung cancer of each stage.
Owner:CHONGQING MEDICAL UNIVERSITY
Therapeutic methods employing nitric oxide precursors
Isolated polynucleotide molecules and peptides encoded by these molecules are used in the analysis of human carbamyl phosphate synthetase I phenotypes, as well as in diagnostic and therapeutic applications, relating to a human carbamyl phosphate synthetase I polymorphism. By analyzing genomic DNA or amplified genomic DNA, or amplified cDNA derived from mRNA, it is possible to type a human carbamyl phosphate synthetase I with regard to the human carbamyl phosphate synthetase I polymorphism, for example, in the context of diagnosing and treating hepatic veno-occlusive disease (HVOD) associated with bone marrow transplants.
Owner:VANDERBILT UNIV
Pyrimidine nucleoside high-yielding strain and carbamyl phosphate synthetase adjusting site thereof
InactiveCN105671008AImprove toleranceBacteriaMicroorganism based processesCarbamyl PhosphateStructural analog
The invention belongs to the technical field of enzyme engineering, and concretely relates to a pyrimidine nucleoside high-yielding strain and a carbamyl phosphate synthetase adjusting site thereof. The invention provides a pyrimidine nucleoside production Bacillus subtilis mutant strain and a carbamyl phosphate synthetase encoding gene. A key regulation site related to carbamyl phosphate synthetase end product feedback inhibition is known, and provides reference for breeding of later pyrimidine nucleoside high-yielding strains. The Bacillus subtilis mutant strain allows the output of fermentation process produced nucleoside pyrimidine uridine to reach 12.5-14.5g / L, and has substantially improved tolerance to different pyrimidine nucleoside structure analogs.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Escherichia coli mutant strain with high cytidine production and method for fermentation production of cytidine
ActiveCN111321103AEffective deregulation of feedbackDefeedback regulationBacteriaHydrolasesEscherichia coliPhenylalanine
The present invention relates to an escherichia coli mutant strain with high cytidine production and a method for fermentation production of cytidine, and belongs to the technical field of fermentation. The escherichia coli mutant strain is obtained by mutagenic screening and has the following characteristics: 1, alanine at 182nd position of carbamyl phosphate synthetase is mutated to valine, andserine at 948th position is mutated to phenylalanine; 2, isoleucine at 86th position, glycine at 93rd position and cysteine at 109th position of aspartic acid carbamoyl transferase regulatory subunitare deleted; 3, asparagine at 72nd position of uridine kinase is mutated to alanine and aspartic acid at 159th position is mutated to asparagine; and 4, aspartic acid at 147th position of cytidine triphosphate synthase is mutated to glutamic acid, and glutamic acid at 149th position is mutated to alanine. The mutant strain is fermented in a 50-L fermentation tank, cytidine yield reaches (90 plus or minus 2) g / L, sugar-acid conversion rate reaches (30 plus or minus 1)%, and the cytidine yield and sugar-acid conversion rate are both the highest values reported.
Owner:HENAN JULONG BIOLOGICAL ENG CO LTD +1
Method for raising expression of carbamoyl phosphate synthetase in HepGL liver cancer cells
InactiveCN103710360AHigh promoter activityImprove expression levelVector-based foreign material introductionForeign genetic material cellsFhit geneBiology
The invention discloses a method for raising expression of carbamoyl phosphate synthetase in HepGL liver cancer cells. The method comprises steps: CPS1 genome DNAs extracted from HepGL liver cancer cells are subjected to bisulfite treatment; the amplified CPS1 promoter area of the treated DNAs is subjected to methylation specific gene amplification; the amplified methylation sequences are subjected to sequencing; TALENs expression plasmids and non-methylation homologous plasmids which are homologous with the methylation loci are constructed according to base sequences around methylation loci, wherein, the homologous non-methylation sequence in the homologous plasmids is a mutation sequence wherein CG is mutated into CA; the TALENs expression plasmids and the homologous plasmids are subjected to transfection into HepGL liver cancer cells, HepGL liver cancer cells with transfection generation are screened, and subjected to Q-PCR and western blotting processing, and the expression amounts of mRNA of CPS1 and proteins in the cells are detected. The method can enhance the ammonia metabolism capability of HepGL liver cancer cells.
Owner:ZHUJIANG HOSPITAL SOUTHERN MEDICAL UNIV
Homocysteine diagnosing/measuring reagent (kit) and homocysteine concentration measuring method
InactiveCN101750392AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsSodium bicarbonateAdditive ingredient
The invention relates to a homocysteine diagnosing / measuring reagent (kit) using the technologies of an enzyme colorimetric method and an enzyme linkage method. At the same time, the invention also relates to a homocysteine concentration measuring method, the reagent composition and reagent ingredients, which belong to the technical field of medical detection and measurement. The reagent (kit) mainly comprises the following ingredients: a buffer solution, cozymase, sodium bicarbonate (carbon dioxide), adenyl pyrophosphate, glyceraldehyde-3-phosphate, homocysteine desulfhydrase, carbamyl phosphate synthetase, glyceraldehyde-3-phosphate dehydrogenase and stabilizing agents. The method has the following steps: mixing samples and the reagent by the certain volume percent for making the samples and the reagent take a series of enzymatic reaction, and then, placing reactants under an ultraviolet / visible light analyzer to detect the rising degree of the light absorbancy in the position with the main wave length of 340 nm, so the concentration of the homocysteine can be measured and calculated.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Method and kit for measuring glycine
InactiveCN102087267AMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementGlycineCarbamyl Phosphate
The invention relates to a method for measuring glycine content by utilizing a double amplification method, an enzyme colorimetric method and an enzyme linked method technology, and composition and components of reagents. The measuring technical principle is that the measurement is finished according to a series of catalytic reactions of glycine dehydrogenase, carbamyl phosphate synthetase and glyceraldehyde-3-phosphate dehydrogenase. The invention also relates to a kit for measuring glycine. The measuring method has high sensitivity and small error; therefore, the method and the kit for measuring the glycine can be widely applied to food inspection.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Reagent (kit) for diagnosing/determining amino acid and method for determining concentration of amino acid
InactiveCN101750337AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsSodium bicarbonateEnzymatic Colorimetry
The invention relates to a reagent (kit) for diagnosing / determining amino acid by using an enzyme multiplication method, an enzyme colorimetric method and an enzyme link method, and also discloses a method for determining the concentration of the amino acid, a composition and components of the reagent, belonging to the technical field of medicine / food / environmental test determination. The reagent (kit) comprises the main components: a buffer solution, a coenzyme, sodium bicarbonate (carbon dioxide), adenosine triphosphoric acid, glyceraldehyde-3-phosphate, an amino acid dehydrogenase, a carbamyl phosphate synthetase, a glyceraldehyde-3-phosphate dehydrogenase and a stabilizing agent. A sample and the reagent are mixed according to a certain volume ratio to generate a series of enzymatic reactions, and reactants are placed under an ultraviolet / visible light analyzer for detecting the degree of absorbance rise at the dominant wavelength of 340 nm, thereby measuring and calculating the concentration of the amino acid.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
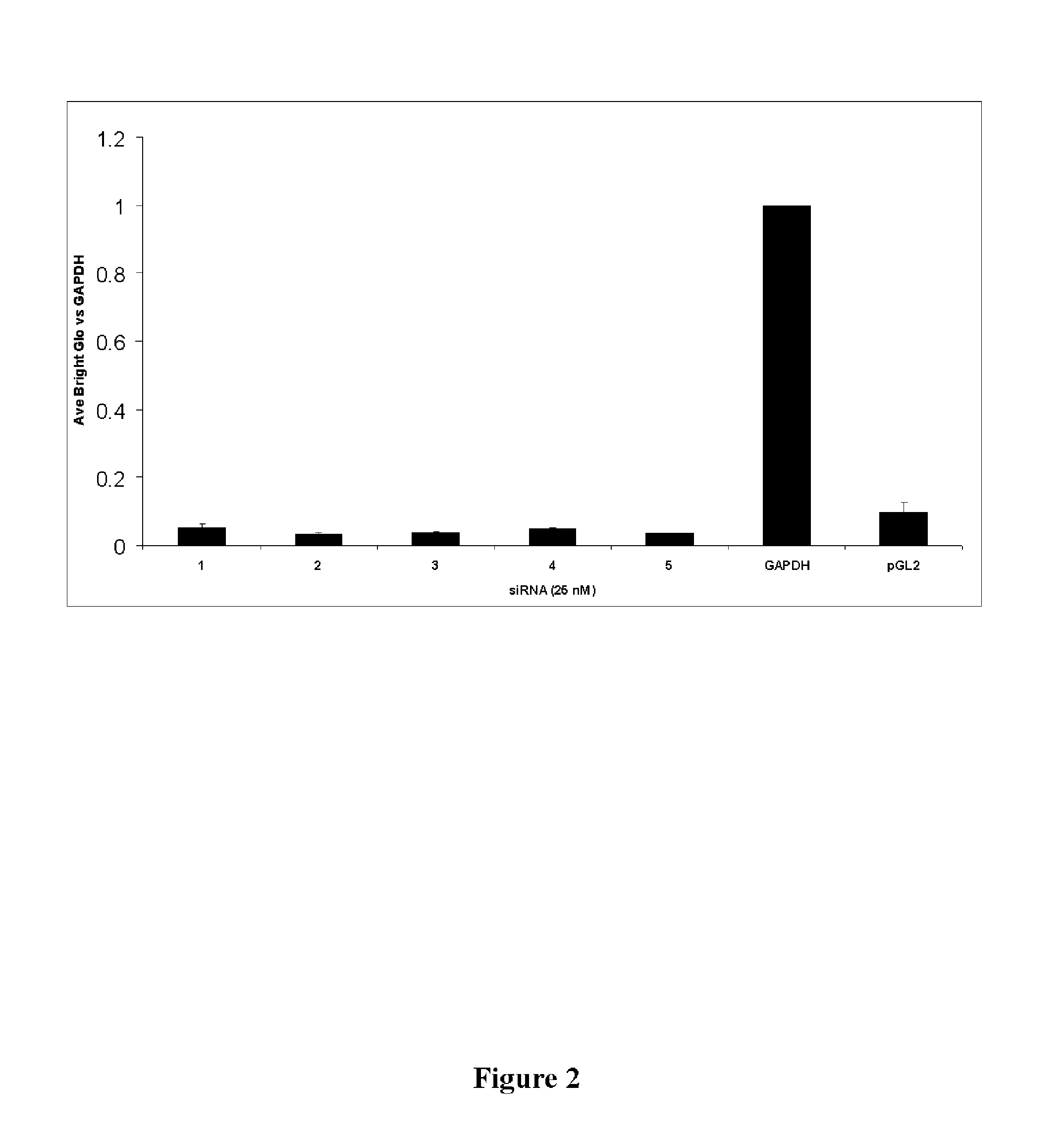
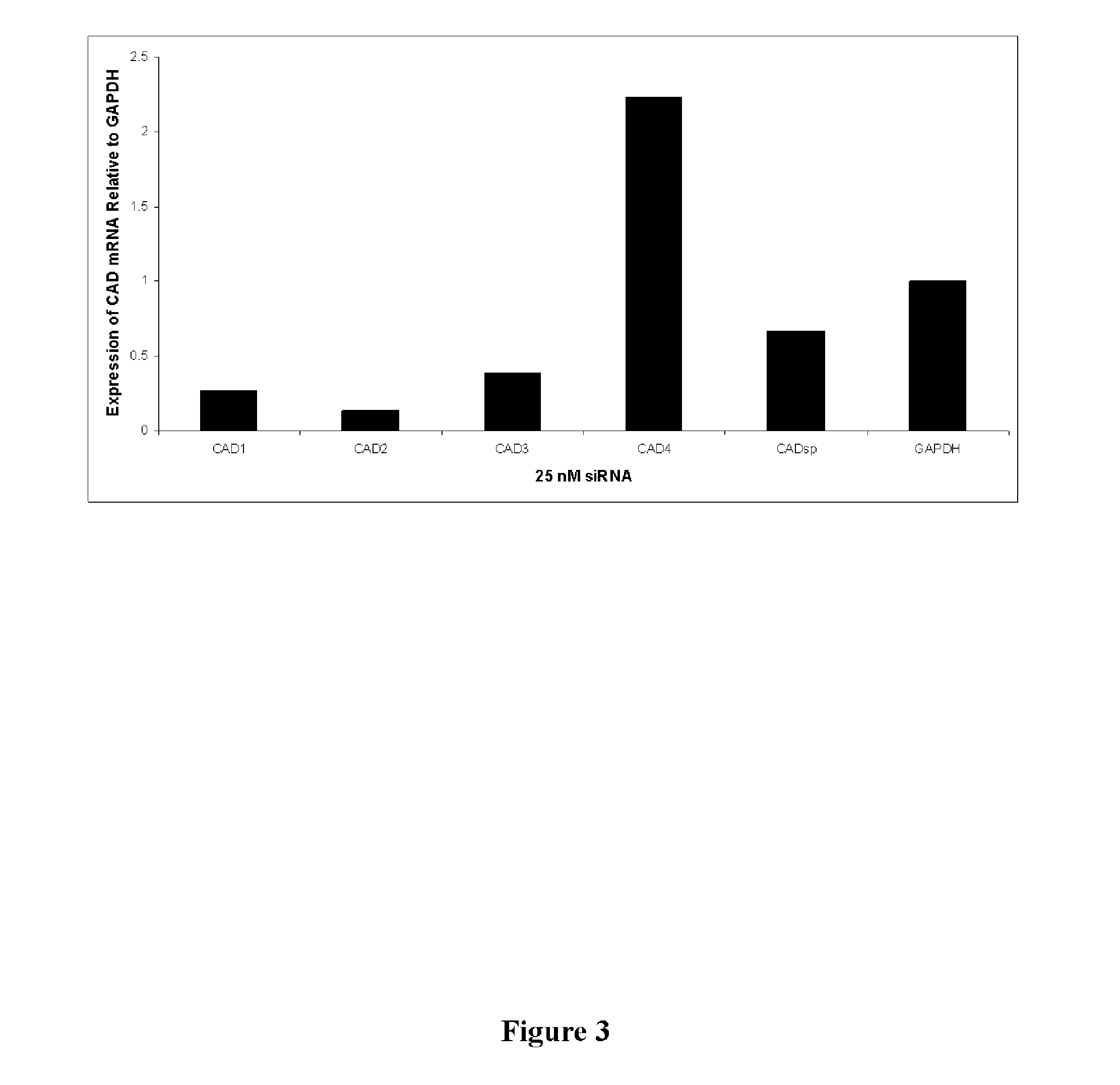
dsRNA FOR TREATING VIRAL INFECTION
InactiveUS20100183704A1Reduced activityLower Level RequirementsOrganic active ingredientsSugar derivativesDiseaseAspartate Transcarbamylase
The invention relates to double-stranded ribonucleic acids (dsRNAs) targeting gene expression carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, and dihydroorotase (CAD), and their use for treating infection by positive stranded RNA viruses such as hepatitis C virus (HCV). Each dsRNA comprises an antisense strand having a nucleotide sequence which is less that 30 nucleotides in length, generally 19-25 nucleotides in length, and which is substantially complementary to at least a part of the CAD target mRNA. A plurality of such dsRNA may be employed to provide therapeutic benefit. The invention also relates to a pharmaceutical composition comprising the dsRNA together with a pharmaceutically acceptable carrier, and including a delivery modality such as fully encapsulated liposomes or lipid complexes. The invention further includes methods for treating diseases caused by positive stranded RNA virus infection using the pharmaceutical compositions; and methods for inhibiting the propagation of positive stranded RNA viruses in and between cells.
Owner:NOVARTIS AG
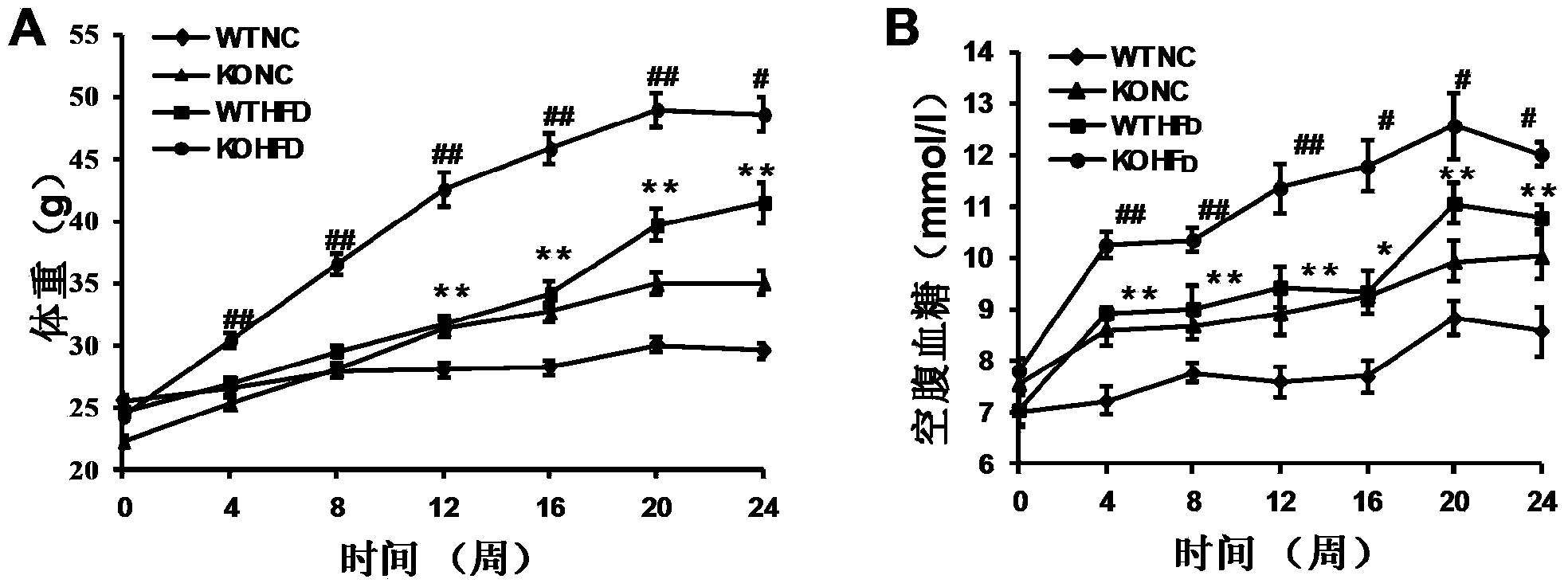
Function and application of thymidylate synthetase CAD (carbamoyl-phosphate synthetase II, aspartate transcarbamylase and dihydroorotase) gene in treating fatty liver and type-II diabetes mellitus
ActiveCN104069511AImprove fatty liverImprove type 2 diabetesPeptide/protein ingredientsMetabolism disorderIntraperitoneal routeStaining
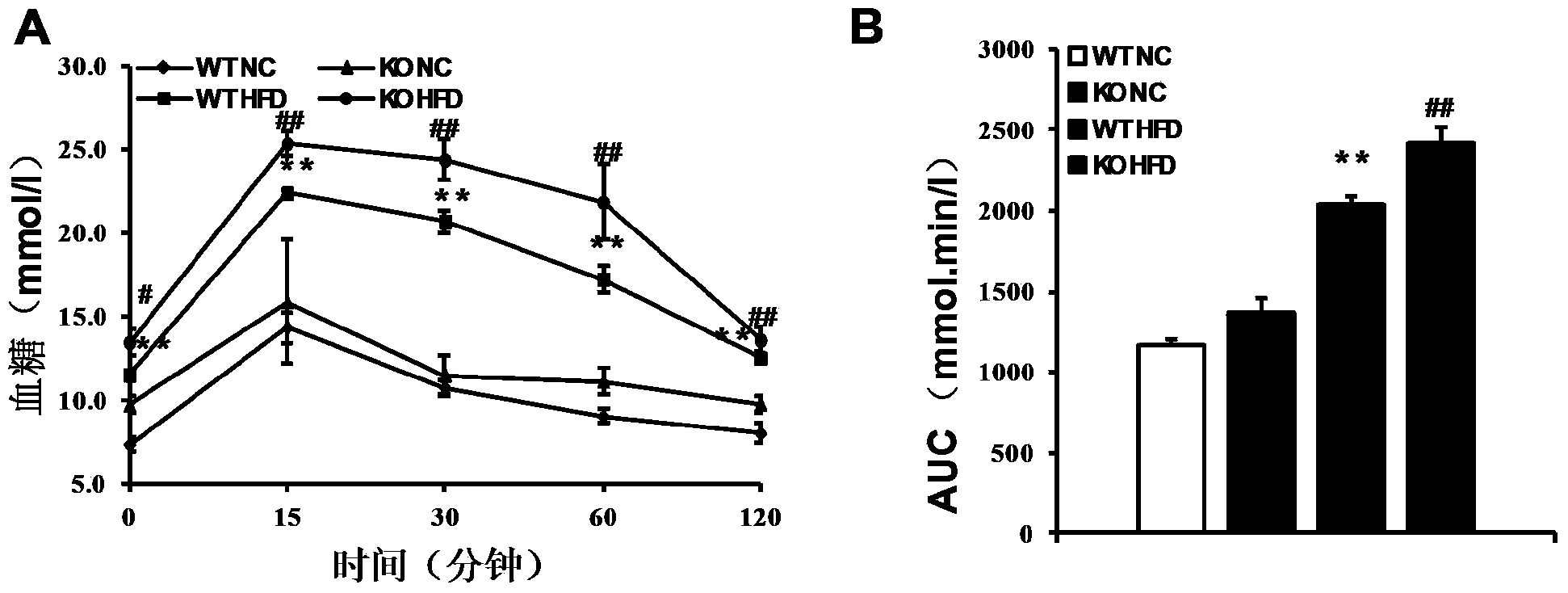
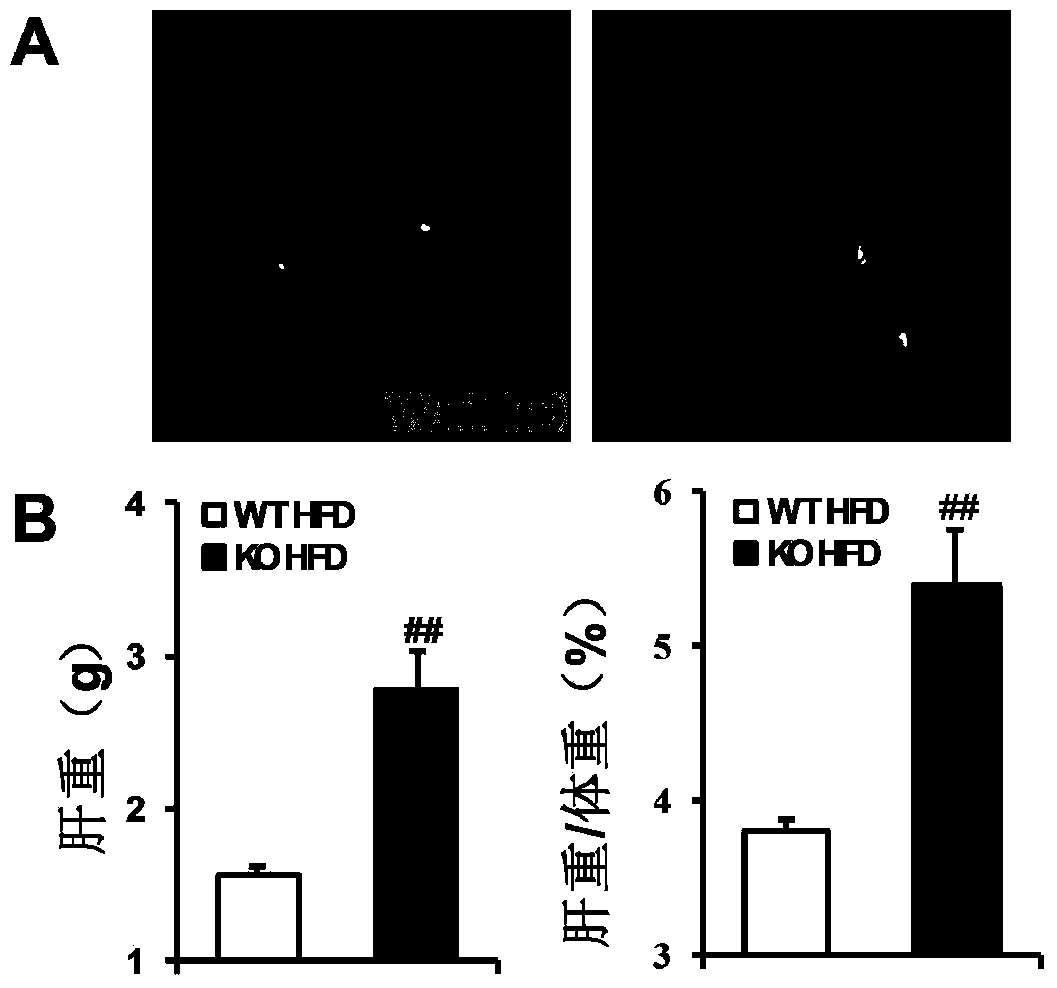
The invention discloses a function and application of thymidylate synthetase CAD (carbamoyl-phosphate synthetase II, aspartate transcarbamylase and dihydroorotase) genes in treating fatty liver and type-II diabetes mellitus, and belongs to the field of gene functions and application. Functions of CAD genes are studied by a high-fat diet (HFD) induced model, and results discover that the levels of body weight and fasting blood-glucose of a CAD gene knock-out mouse in an HFD group are higher than those of a WT (Wild Type) mouse in a control group; glucose tolerance tests by intraperitoneal injection discover that the tolerance of the CAD gene knock-out mouse on glucose is remarkably weakened; the integral liver appearance, liver weight, liver / weight ratio and lipid component pathological staining result of the mouse indicate that the fatty liver disease of the CAD knock-out mouse in the HFD group is remarkably severe, the lipid accumulation is remarkably increased, and these results indicate that CAD gene knock-out can remarkably deteriorate fatty liver and type-II diabetes mellitus. Regarding the effects of CAD, the CAD can be used for preparing medicaments for preventing, relieving and / or treating fatty liver and / or type-II diabetes mellitus.
Owner:武汉惠康基因科技有限公司
Ammonia (ammonia ion) determination method and ammonia (ammonia ion) diagnosis/determination kit
InactiveCN102466720AMicrobiological testing/measurementColor/spectral properties measurementsContent determinationAmmonia
The invention relates to an ammonia (ammonia ion) content determination method employing an enzymatic colorimetric method and an enzyme-linked technology. The invention also relates to the composition and components of a reagent. The technological principle of the determination is that: the determination is achieved with a series of catalytic reactions of glutamate oxidase, carbamoyl phosphate synthetase and hydrogen cyanide synthetase. The invention also relates to an ammonia (ammonia ion) diagnosis / determination kit. The determination method provided by the invention is advantaged in high sensitivity and low error. Therefore, the determination method and the kit provided by the invention can be widely applied in clinical medicine / food examination.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Glycine Determination Method and Glycine Determination Kit
InactiveCN102298017AMicrobiological testing/measurementColor/spectral properties measurementsGlycineEnzyme assay
The invention relates to a method for measuring glycine content using enzyme colorimetry and enzyme-linked method technology, the composition and components of reagents, and the technical principle of its measurement is based on glycine transaminase, carbamoyl phosphate synthetase, malonate semialdehyde dehydrogenation A series of catalyzed reactions of the enzyme is completed, and the invention also relates to a glycine determination kit. The determination method of the invention has high sensitivity and small error, so the determination method and the kit of the invention can be widely used in food inspection.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination method of galactose and galactose diagnosis/measurement kit
InactiveCN102297949AMicrobiological testing/measurementColor/spectral properties measurementsGalactokinaseEnzyme assay
The present invention relates to the determination method of galactose content, the composition and the component of reagent by enzyme colorimetric method and enzyme-linked method technology, the technical principle of its determination is based on galactokinase, carbamoyl phosphate synthetase, malonate semialdehyde A series of catalytic reactions of the dehydrogenase is completed, and the invention also relates to a galactose diagnosis / measurement kit. The assay method of the present invention has high sensitivity and small error, so the assay method and kit of the present invention can be widely used in clinical medicine / food inspection.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Therapeutic methods employing nitric oxide precursors
Isolated polynucleotide molecules and peptides encoded by these molecules are used in the analysis of human carbamyl phosphate synthetase I phenotypes, as well as in diagnostic and therapeutic applications, relating to a human carbamyl phosphate synthetase I polymorphism. By analyzing genomic DNA or amplified genomic DNA, or amplified cDNA derived from mRNA, it is possible to type a human carbamyl phosphate synthetase I with regard to the human carbamyl phosphate synthetase I polymorphism, for example, in the context of diagnosing and treating hepatic veno-occlusive disease (HVOD) associated with bone marrow transplants.
Owner:VANDERBILT UNIV
Measurement method for ammonia (ammonia ions) and diagnosis/measurement kit for ammonia (ammonia ions)
InactiveCN102466712AMicrobiological testing/measurementColor/spectral properties measurementsCarbamyl PhosphateAmmonia
The invention relates to a content measurement method for ammonia (ammonia ions), reagent composition and components. The measurement method makes use of an enzymatic colorimetric method and a couple reaction method, and has a technical principle based on a series of catalytic reactions of glutamate oxidase, carbamyl phosphate synthetase, and melate dehydrogenase. The invention also relates to a diagnosis / measurement kit for ammonia (ammonia ions). The measurement method of the invention has high sensitivity and minor errors, so that the measurement method and kit provided in the invention can be widely applied in clinical medicine / food inspection.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Cordyceps Chinese Hirsutella carbamyl phosphate synthetase, coding gene and application thereof
ActiveCN103031281BIncrease productionEnhance expressive abilityBacteriaMicroorganism based processesNucleotideBiology
The invention relates to a carbamyl phosphate synthetase from Bailing production bacterium Cordyceps Chinese Hirsutella for synthesizing metabolic carbamyl phosphate from L-glutamic acid, a coding gene and application thereof. The amino acid sequence of the carbamyl phosphate synthetase has more than 90% of homology with sequence disclosed as SEQ ID NO.1; and the nucleotide sequence of the coding gene has more than 90% of homology with sequence disclosed as SEQ ID NO.2. The invention researches the metabolic pathway of L-glutamic acid synthesized carbamyl phosphate synthetase in details in principle. The cloned DNA (deoxyribonucleic acid) comprising the nucleotide sequence provided by the invention can be transformed into engineering bacterium by transduction, conversion and conjugal transfer. By adjusting the expression of the carbamyl phosphate biosynthesized gene, the host carbamyl phosphate is endowed with high expressivity, thereby providing an effective way for enhancing the yield of the carbamyl phosphate and having great application prospects.
Owner:ZHEJIANG UNIV OF TECH +1
Glycine Determination Method and Glycine Determination Kit
InactiveCN102298035AMicrobiological testing/measurementColor/spectral properties measurementsGlycineEnzyme assay
The present invention relates to the measuring method of the content of glycine, the composition and the composition of the reagent by using enzyme cycle amplification method, enzyme colorimetric method and enzyme-linked method technology, the technical principle of its measuring is based on glycine transaminase, carbamoyl phosphate synthetase, gluten The series of catalyzed reactions of amino acid synthase is completed, and the invention also relates to a glycine determination kit. The determination method of the invention has high sensitivity and small error, so the determination method and the kit of the invention can be widely used in food inspection.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination method of galactose and galactose diagnosis/measurement kit
InactiveCN102297948AMicrobiological testing/measurementColor/spectral properties measurementsGalactokinaseELISA unit
The invention relates to a method for measuring galactose content using enzyme colorimetry and enzyme-linked method technology, the composition and components of reagents, and the technical principle of its measurement is based on galactose kinase, carbamoyl phosphate synthase, hydrogen cyanide synthase The series of catalytic reactions are completed, and the present invention also relates to a galactose diagnosis / measurement kit. The assay method of the present invention has high sensitivity and small error, so the assay method and kit of the present invention can be widely used in clinical medicine / food inspection.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Ammonia (ammonia ion) determination method and ammonia (ammonia ion) diagnosis/determination reagent kit
InactiveCN102539677AMicrobiological testing/measurementColor/spectral properties measurementsFood inspectionAmmonia kinase
The invention relates to a method for determination of ammonia (ammonia ion) content by using an enzymatic colorimetric method and an enzyme couple reaction technique and composition and components of a reagent. A determination technology principle of the method lies in that the determination is completed according to series catalytic reactions of ammonia kinase, carbamyl phosphate synthetase and malonic acid-semialdehyde dehydrogenase. The invention further relates to an ammonia (an ammonia ion) diagnosis / determination reagent kit. The determination method is high in sensitivity and small in error. Therefore, the determination method and the reagent kit can be widely applied to clinical medicine / food inspection.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Reagent (kit) for diagnosing/determining amino acid and method for determining concentration of amino acid
InactiveCN101762491AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsSodium bicarbonatePhosphoric acid
The invention relates to a reagent (kit) for diagnosing / determining amino acid by utilizing the techniques of an enzyme colorimetric method and an enzyme coupling method, and also relates to a method for determining the concentration of amino acid and a composition and components of the reagent, belonging to the technical field of detection and determination of medicine / foodstuff / environment. The reagent (kit) mainly comprises the following components: a buffer solution, coenzyme, sodium hydrogen carbonate (carbon dioxide), adenosine triphosphoric acid, glyceraldehyde-3-phosphoric acid, amino acid oxidase, carbamyl phosphate synthetase, glyceraldehyde-3-phosphoric acid dehydrogenase and a stabilizer. A sample and the reagent are mixed according to a certain volume ratio to generate a series of enzymic reactions, then a reactant is placed under an ultraviolet / visible light analyzer, and the rising degree of absorbance in the position of 340 nm of main wave length is detected so that the concentration of the amino acid is measured and calculated.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination method of ammonia (ammonia ions) and diagnosis/determination kit for ammonia (ammonia ions)
InactiveCN102565332AMicrobiological testing/measurementColor/spectral properties measurementsCarbamyl PhosphateEnzymatic Colorimetry
The invention relates to a determination method of ammonia (ammonia ions) content by means of technologies of enzymatic colorimetry and enzyme-linked assay, and composition and components of a reagent. The technical principle of determination is based on a series of catalytic reactions of ammonia kinase, carbamyl phosphate synthetase, and malic dehydrogenase. The invention further relates to a diagnosis / determination kit for ammonia (ammonia ions). The determination method provided by the invention has high sensitivity and small error. Therefore, the determination method and the kit provided by the invention can be widely applied to clinical medical / food inspection.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Assay method of ammonia (ammonia ion) and ammonia (ammonia ion) diagnosis/assay kit
InactiveCN102466716AMicrobiological testing/measurementColor/spectral properties measurementsBenzoic acidAssay
The invention relates to an assay method for assaying ammonia (ammonia ion) content with an enzymatic colorimetry and an enzyme-linked immunosorbent assay technology, and component and composition of a reagent, the technology principle of the assay is completed according to the series of catalytic reactions of glutamate oxidase, carbamyl phosphate synthetase, chlorobenzoic acid-1 and 2-dioxygenase, and the invention also relates to an ammonia (ammonia ion) diagnosis / assay kit. The assay method disclosed by the invention is high in sensitivity, and is small in error, so the assay method and the kit, disclosed by the invention, can be widely applied to clinical medicine / food inspection.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Glycine Determination Method and Glycine Determination Kit
InactiveCN102298013ASuitable for testingMicrobiological testing/measurementColor/spectral properties measurementsGlycineELISA unit
The present invention relates to the determination method of the content of glycine, the composition and the component of the reagent using double amplification method, enzyme colorimetric method and enzyme-linked method technology, and the technical principle of its determination is based on hydrogen cyanide synthase, carbamoyl phosphate synthesis The series of catalyzed reactions of the enzyme and glutamate synthetase are completed, and the invention also relates to a glycine determination kit. The determination method of the invention has high sensitivity and small error, so the determination method and the kit of the invention can be widely used in food inspection.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination method of galactose and galactose diagnosis/measurement kit
InactiveCN102297981AMicrobiological testing/measurementColor/spectral properties measurementsGalactokinaseLactose
The invention relates to a method for measuring galactose content using enzyme colorimetry and enzyme-linked method technology, the composition and components of reagents, and the technical principle of its measurement is based on galactose kinase, carbamoyl phosphate synthetase, pyruvate dehydrogenase The series of catalytic reactions are completed, and the present invention also relates to a galactose diagnosis / measurement kit. The assay method of the present invention has high sensitivity and small error, so the assay method and kit of the present invention can be widely used in clinical medicine / food inspection.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Ammonia (ammonia ion) determination method and ammonia (ammonia ion) diagnosis/determination kit
InactiveCN102565353AMicrobiological testing/measurementColor/spectral properties measurementsCarbamyl PhosphateEnzymatic Colorimetry
The invention relates to a method for determining the content of ammonia (ammonia ion) by using technologies of enzymatic colorimetry and enzyme-linked immunosorbant assay, and compositions of a reagent. A technical principle for determination is that the determination is finished according to a series of catalytic reactions of ammonia kinase, carbamyl phosphate synthetase and fatty acid synthase. The invention also relates to an ammonia (ammonia ion) diagnosis / determination kit. The determination method provided by the invention is high in sensitivity and small in error, so that the determination method and the kit can be widely applied to clinical medical / food inspection.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination method of galactose and galactose diagnostic/assay kit
InactiveCN102297983AMicrobiological testing/measurementColor/spectral properties measurementsHydrogenGalactokinase
The invention relates to a method for measuring galactose content using enzyme colorimetry and enzyme-linked method technology, the composition and components of reagents, and the technical principle of the measurement is based on galactose kinase, carbamoyl phosphate synthase, phosphogluconate The series of catalyzed reactions of the hydrogenase is completed, and the invention also relates to a galactose diagnosis / measurement kit. The assay method of the present invention has high sensitivity and small error, so the assay method and kit of the present invention can be widely used in clinical medicine / food inspection.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Ammonia (ammonia ion) determination method and ammonia (ammonia ion) diagnosis/determination reagent kit
InactiveCN102539664AMicrobiological testing/measurementColor/spectral properties measurementsCarbamyl PhosphateEnzymatic Colorimetry
The invention relates to a method for determination of ammonia (ammonia ion) content by using an enzymatic colorimetric method and an enzyme couple reaction technique and composition and components of a reagent. A determination technology principle of the method is completed according to series catalytic reactions of ammonia kinase, carbamyl phosphate synthetase and formate dehydrogenase. The invention further relates to an ammonia (ammonia ion) diagnosis / determination reagent kit. The determination method is high in sensitivity and small in error. Therefore, the determination method and the reagent kit can be widely applied to clinical medical science / food inspection.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com