Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
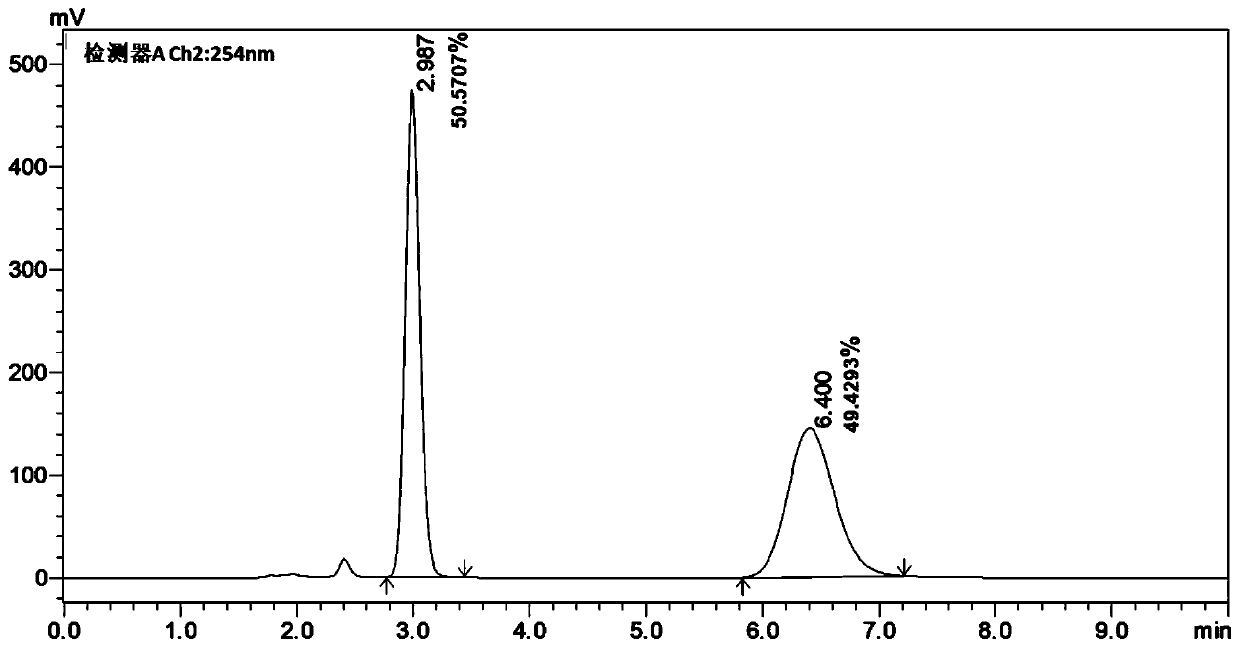
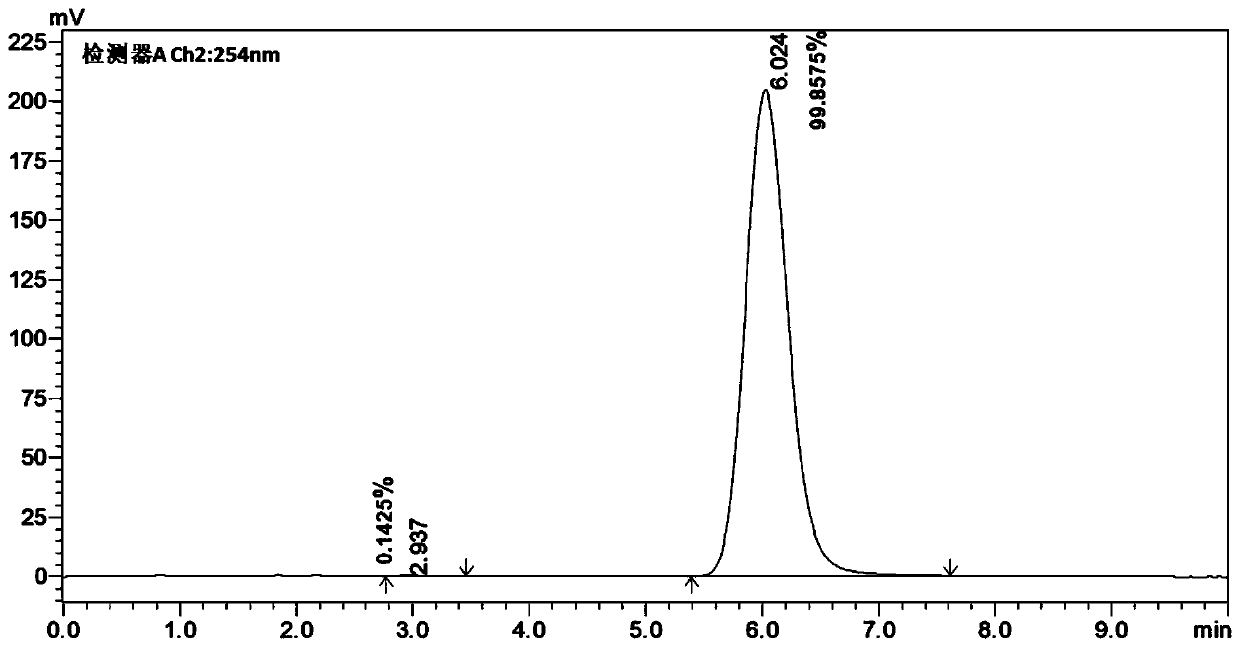
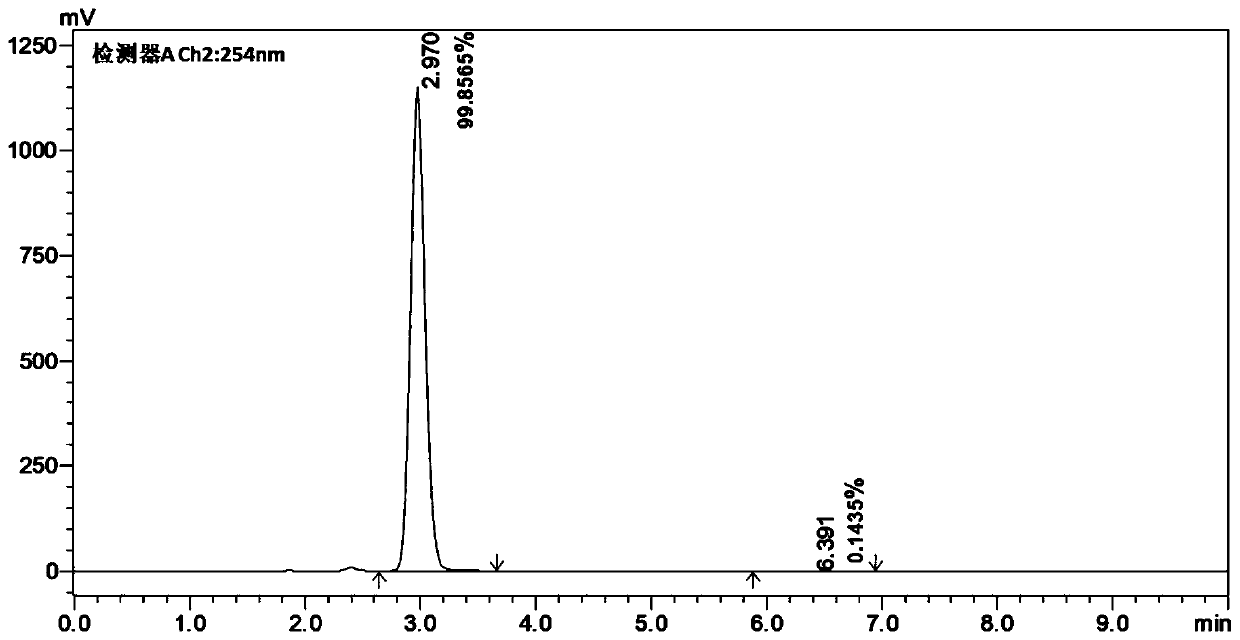
45 results about "Bicyclic molecule" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
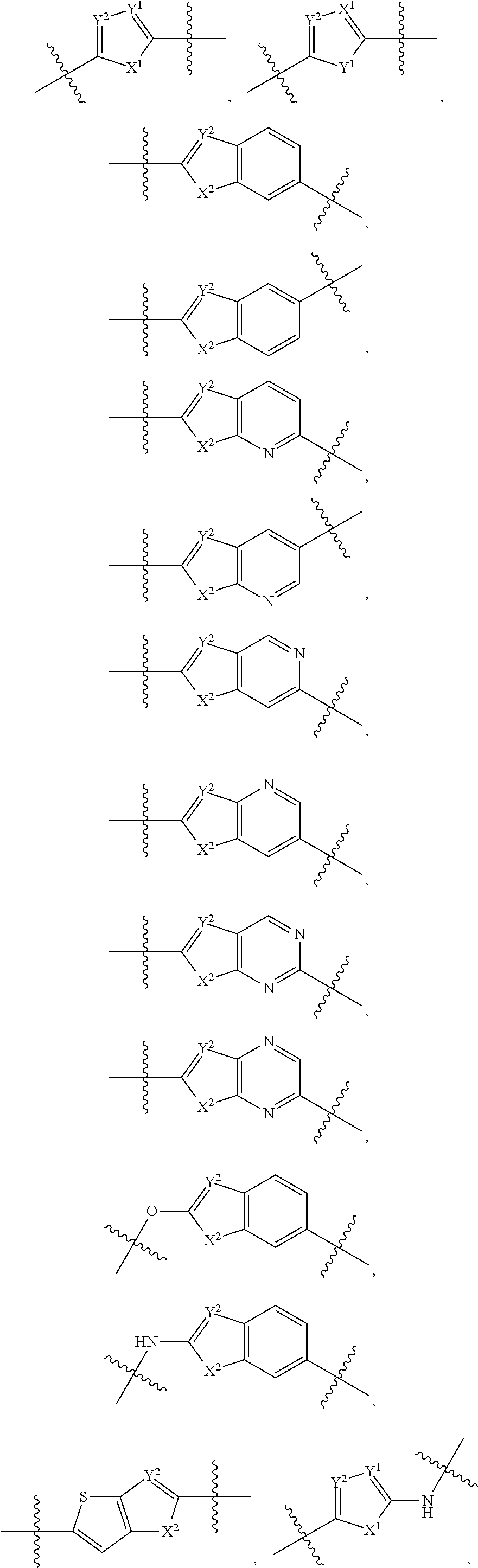
A bicyclic molecule (bi = two, cycle = ring) is a molecule that features two joined rings. Bicyclic structures occur widely, for example in many biologically important molecules like α-thujene and camphor. A bicyclic compound can be carbocyclic (all of the ring atoms are carbons), or heterocyclic (the rings atoms consist of at least two different elements), like DABCO. Moreover, the two rings can both be aliphatic (e.g. decalin and norbornane), or can be aromatic (e.g. naphthalene), or a combination of aliphatic and aromatic (e.g. tetralin).
Photochromic composition
ActiveUS20160222285A1Improved color developabilityIncrease speedSpectales/gogglesLayered productsMolecular compositionPolyrotaxane
A photochromic composition comprising (A) a polyrotaxane having a composite molecular structure composed of an axial molecule and a plurality of cyclic molecules clathrating the axial molecule and (B) a photochromic compound.
Owner:TOKUYAMA CORP
Material for curable solvent-based topcoating material, and coating material and coating film comprising or formed from the same
ActiveUS20090042034A1Improve scratch resistanceAvoid crackingSynthetic resin layered productsInksPolyrotaxaneSolvent based
[Object] To provide a coating material for a curable solvent-based topcoating material, having excellent marring resistance, chipping resistance, producing no crack and excellent in performances such as weatherability, stain resistance, adhesion and the like, and a curable solvent-type topcoating material using such a material.[Solving Means] A coating material is blended preferably in an amount of 60 to 90% by mass relative to paint film forming components thereby to form a curable solvent-based topcoating material. The coating material includes an oleophilic polyrotaxane which includes a cyclic molecule, a linear molecule including the cyclic molecule with piercing through the cyclic molecule, and blocking groups which are placed at both end terminals of the linear molecule to prevent the cyclic molecule from leaving from the linear molecule, at least one of the above-mentioned liner molecule and the cyclic molecule having hydrophobic modification group.
Owner:NISSAN MOTOR CO LTD +1
Modified hydrophilic polyrotaxane and cross-linked polyrotaxane
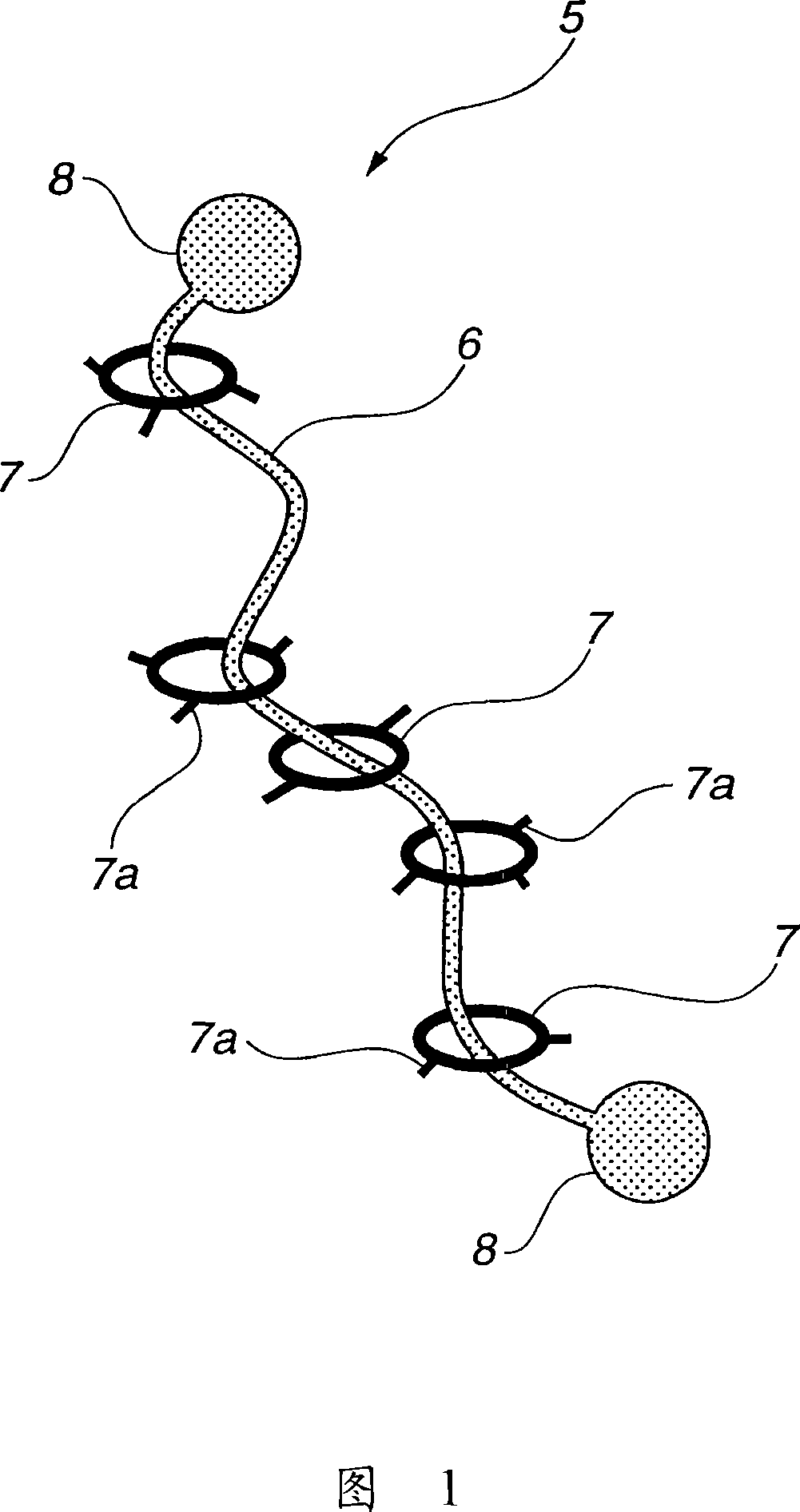
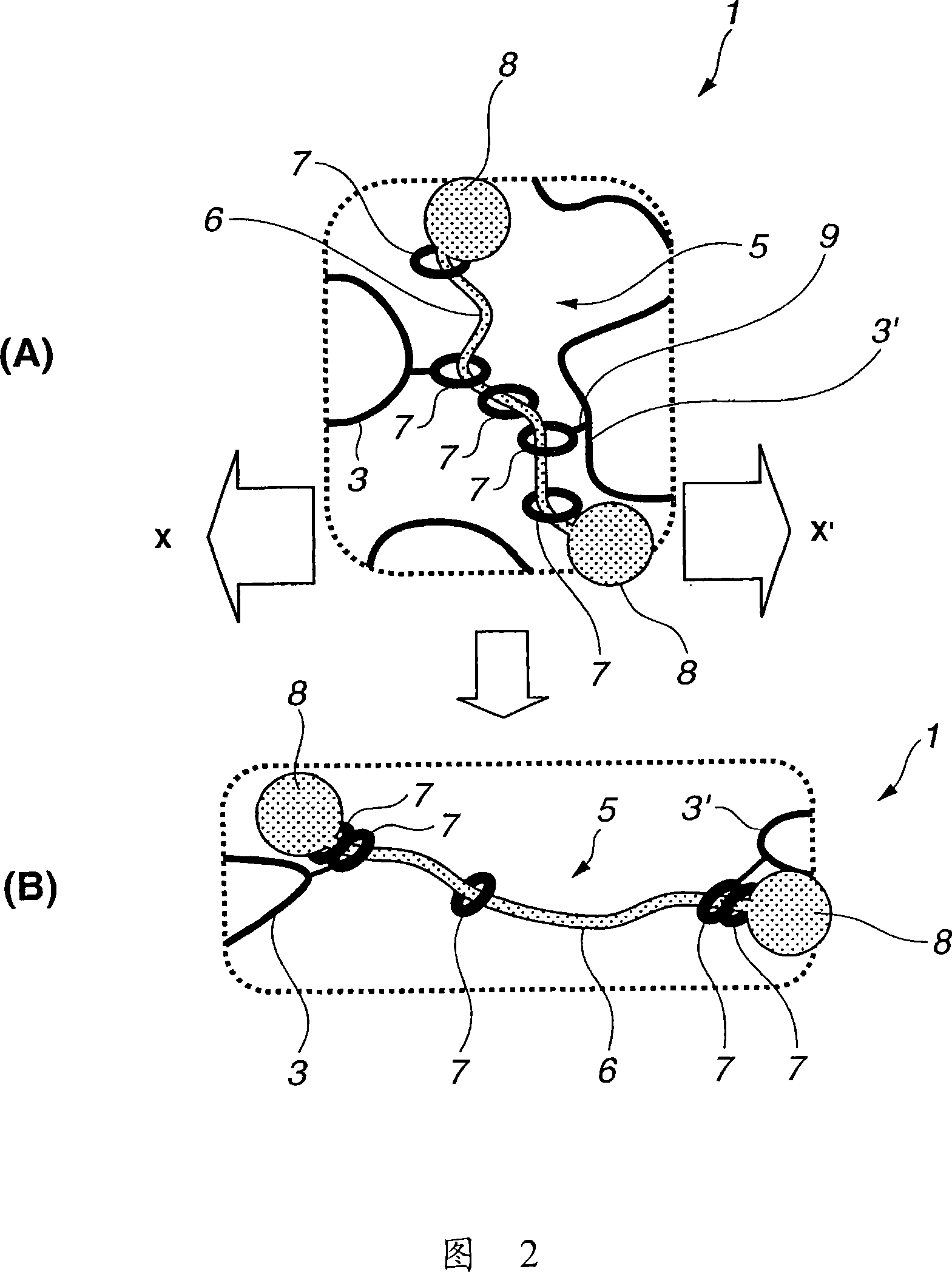
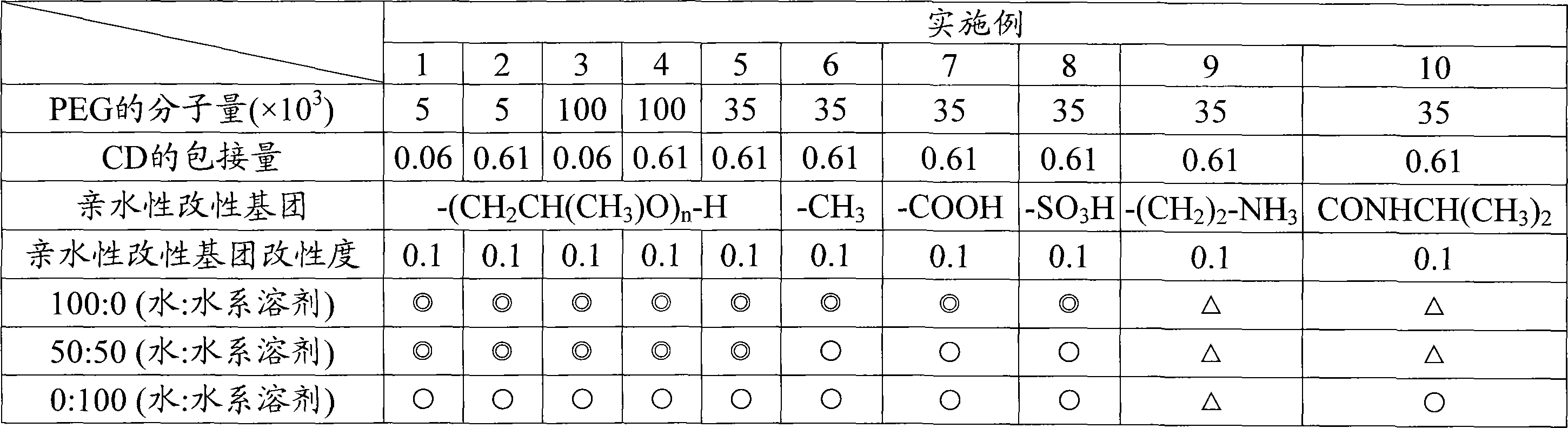
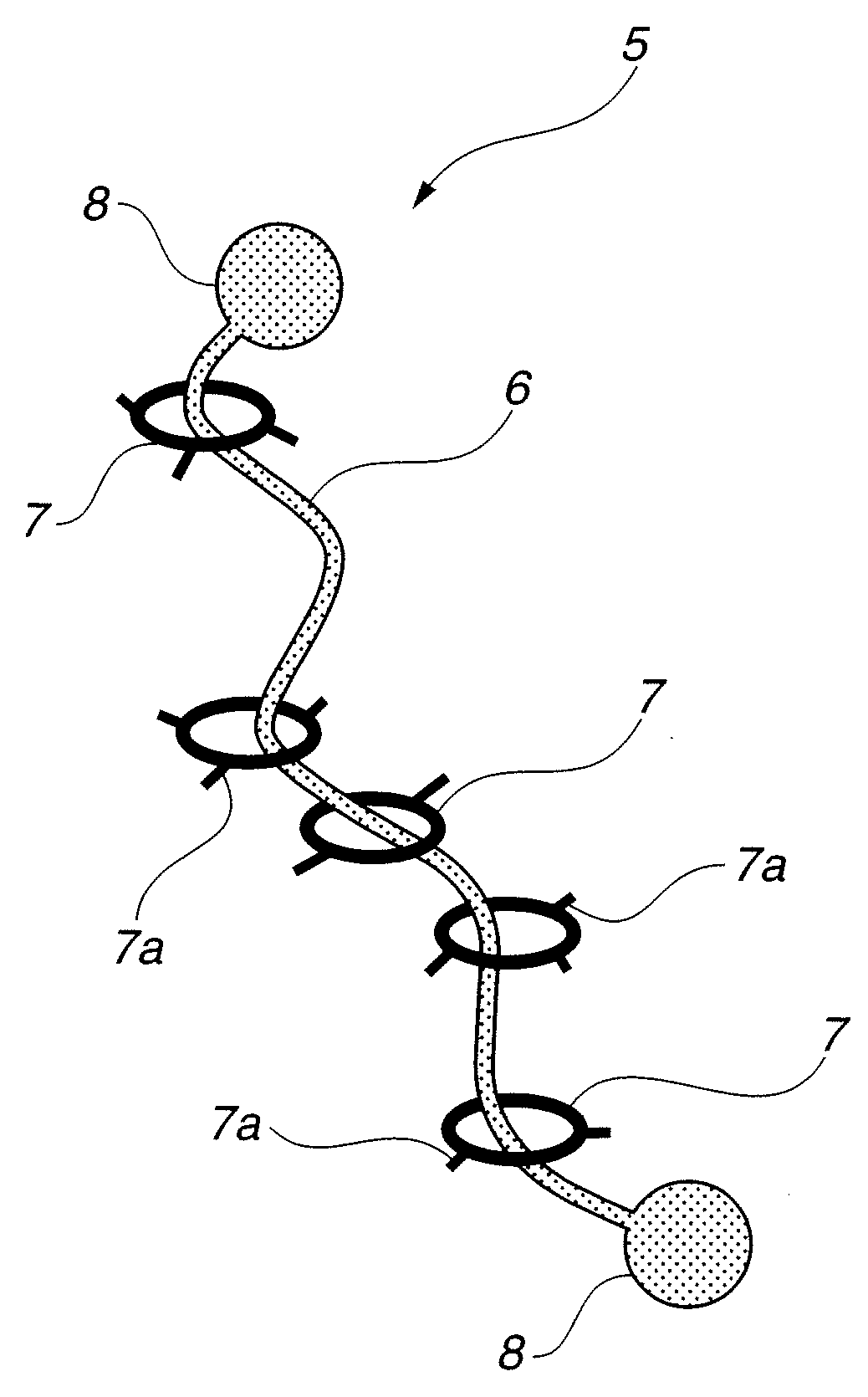
To provide a modified hydrophilic polyrotaxane which is soluble in water or an aqueous solvent, a cross-linked polyrotaxane produced using the modified hydrophilic polyrotaxane, and a solvent for dissolving the modified hydrophilic polyrotaxane therein. A modified hydrophilic polyrotaxane having a cyclic molecule, a linear molecule which includes the cyclic molecule with piercing through the cyclic molecule, and capping groups which are placed at both end termini of the linear molecule and serve to prevent the cyclic molecule from leaving from the linear molecule. The cyclic molecule is cyclodextrin, and each of all or some of the hydroxyl groups in the cyclodextrin is modified with a hydrophilic modification group. A cross-linked polyrotaxane comprising the modified hydrophilic polyrotaxane and a polymer linked through a cyclic molecule. A solvate for dissolving the modified hydrophilic polyrotaxane therein comprising water, an aqueous solvent or a combination thereof.
Owner:NISSAN MOTOR CO LTD +1
Modified hydrophilic polyrotaxane and cross-linked polyrotaxane
[Object] To provide a modified hydrophilic polyrotaxane which is soluble in water or a water-like solvent, a cross-linked polyrotaxane using this, and a solvent for dissolving the modified hydrophilic polyrotaxane.[Solving Means] A modified hydrophilic polyrotaxane has a cyclic molecule, a linear molecule including the cyclic molecule with piercing through the cyclic molecule, and blocking groups which are placed at both end terminals of the linear molecule to prevent the cyclic molecule from leaving from the linear molecule. The cyclic molecule is cyclodextrin, and each of all or a part of the hydroxyl groups in the cyclodextrin is modified with a hydrophilic modification group.A cross-linked polyrotaxane is formed by combining this modified hydrophilic polyrotaxane and a polymer through the cyclic molecule.A solvent for dissolving this modified hydrophilic polyrotaxane contains at least one of water and a water-like solvent.
Owner:NISSAN MOTOR CO LTD +1
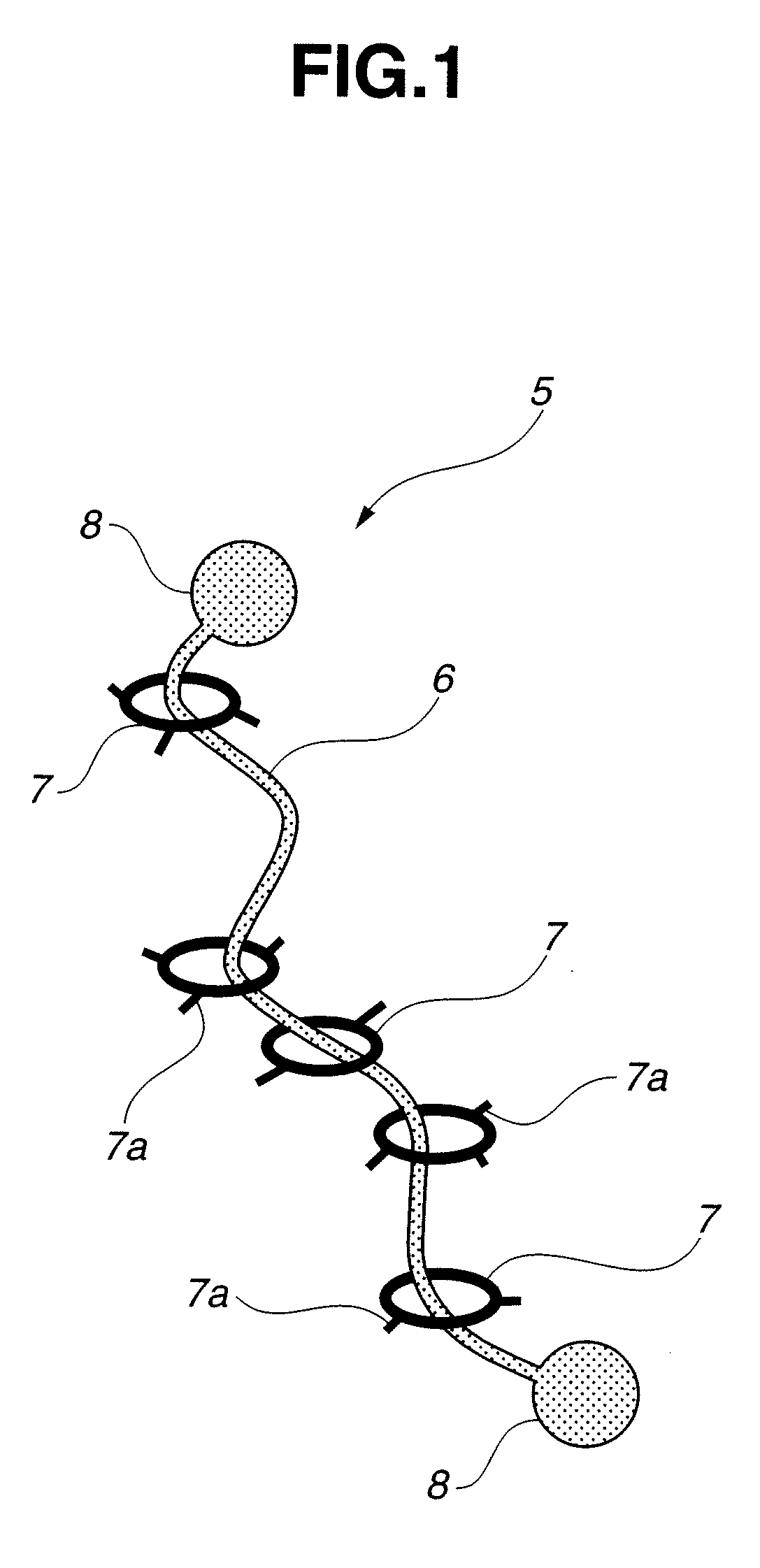
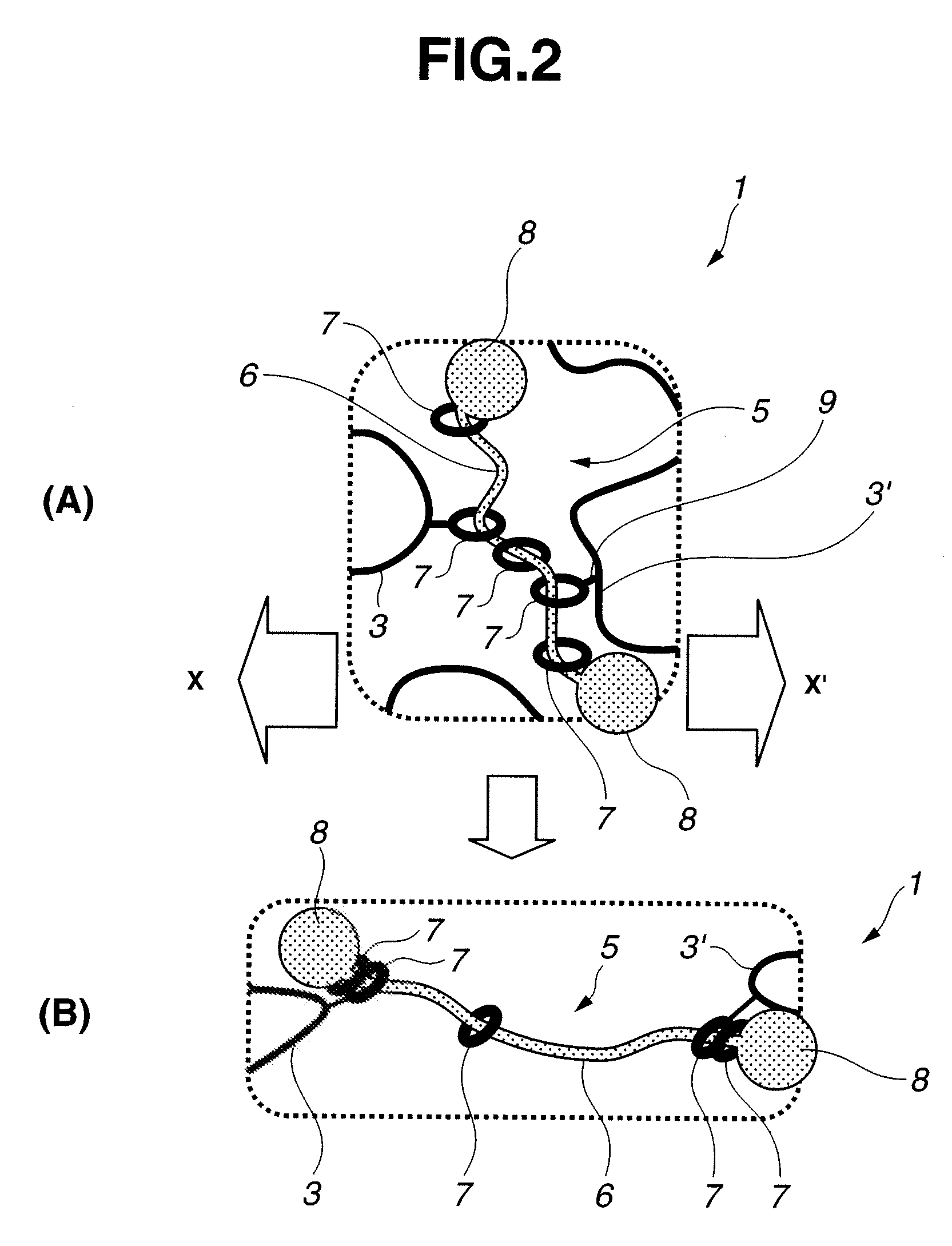
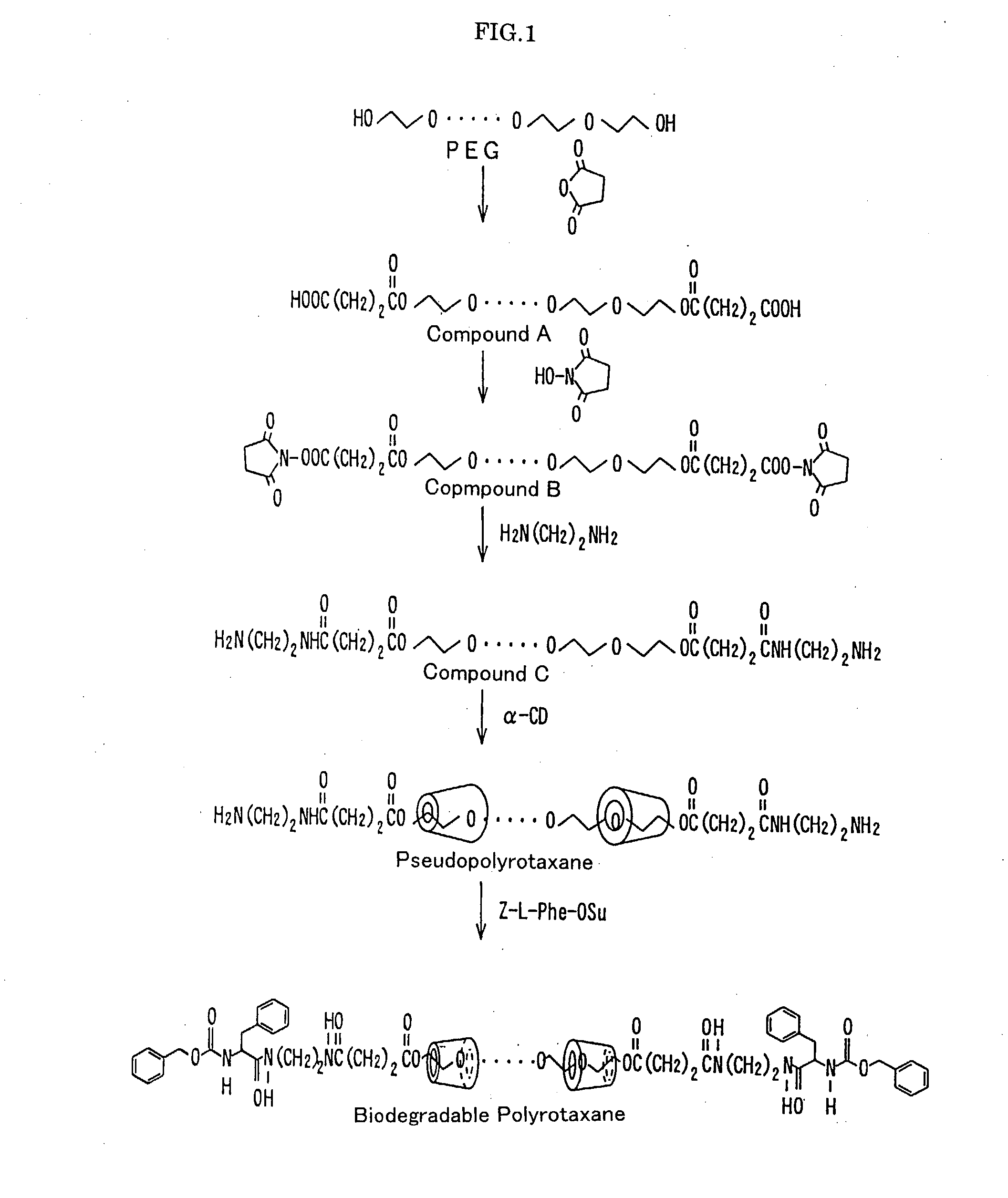
Base material for tissue reconstruction, implantable material, and methods of preparing the same
InactiveUS20030124168A1Fast bondingPromote decompositionSurgical adhesivesArtificial cell constructsPolyrotaxaneTissue reconstruction
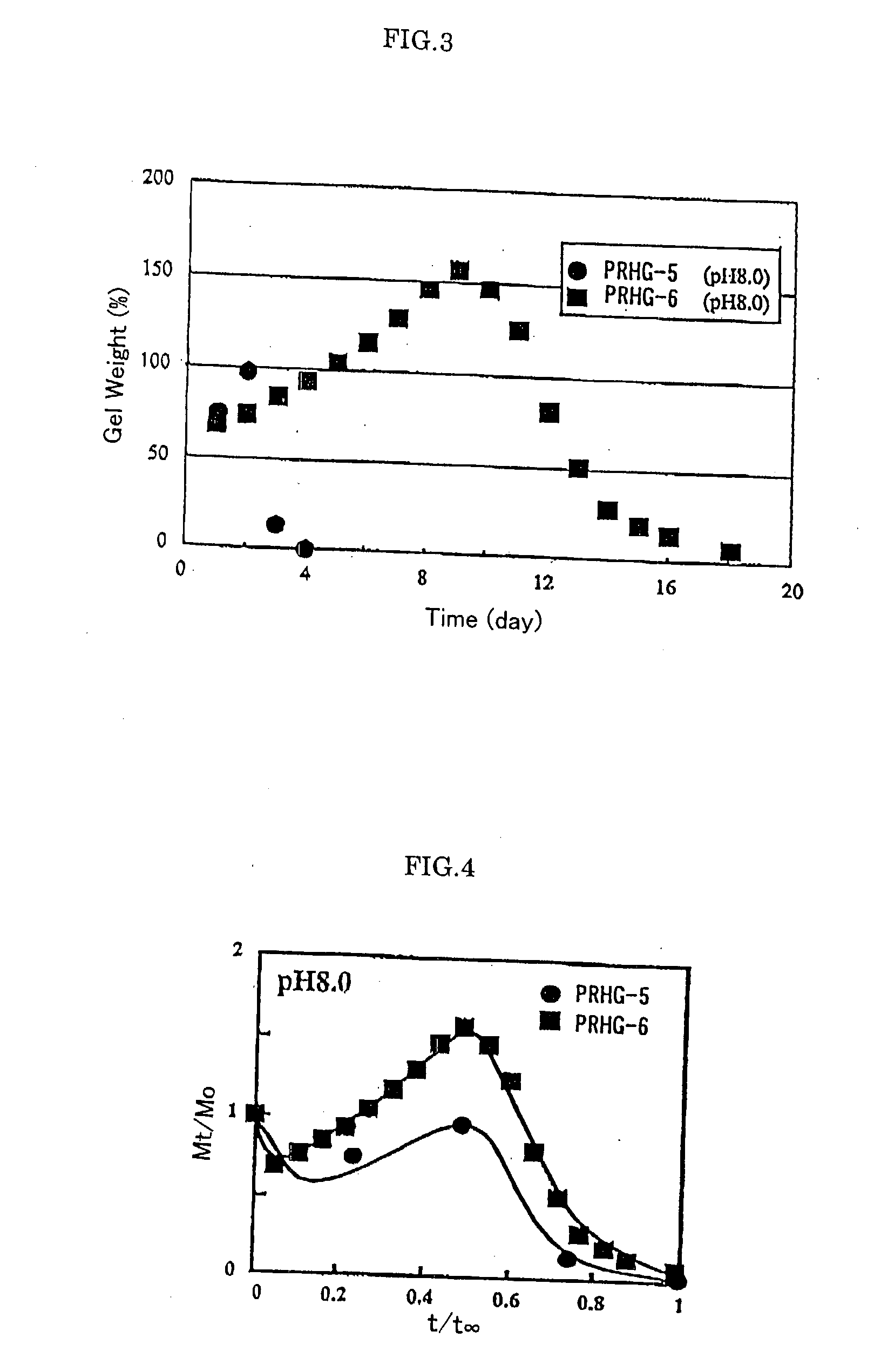
Base materials for tissue regeneration which enable the tissue regeneration, have mechanical properties needed in the tissue regeneration and can disappear via degradation in vivo after the completion of the tissue regeneration; and implantable materials with the use of the same. The above-described base materials for tissue regeneration comprise polyrotaxane, wherein biocompatible groups having bulky substituents have been introduced via hydrolyzable bond into both ends of a linear molecule penetrating plural cyclic molecules, or a polyrotaxane hydrogel having a network structure formed by crosslinking the cyclic molecules to each other, the biocompatible groups to each other, or the cyclic molecules to the biocompatible groups in each polyrotaxane molecule.
Owner:JAPAN TISSUE ENG +1
Aza-bridged-ring compound
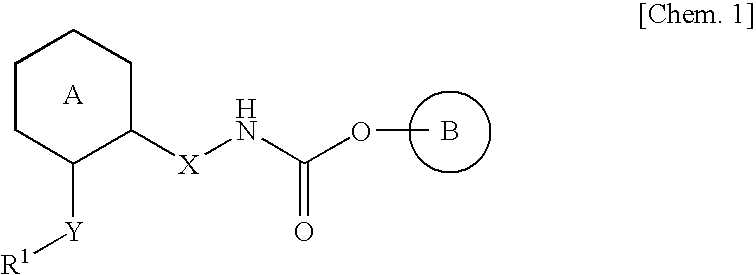
[Problems] Provided is a compound which has an antagonistic action on a muscarinic M3 receptor and is useful as an active ingredient of a prophylactic and / or therapeutic agent for an inflammatory disease such as a chronic obstructive pulmonary disease (COPD), asthma and the like.[Means for Solving Problems] The present inventors have made studies on a compound having an antagonistic action on the binding of a muscarinic M3 receptor, and they have found that an aza-bridged-ring compound or a salt thereof has an antagonistic action on the binding of a muscarinic M3 receptor, thereby completing the present invention. The aza-bridged-ring compound of the present invention has an antagonistic action on the binding of a muscarinic M3 receptor, and can be used as a prophylactic and / or therapeutic agent for an inflammatory disease such as a chronic obstructive pulmonary disease (COPD), asthma and the like.
Owner:ASTELLAS PHARMA INC
Material for Room Temperature Curable Solvent-Borne Overcoating Material,Coating Material Using Same and Coating Film
ActiveUS20090047532A1Equal resistanceHigh viscoelasticityLiquid surface applicatorsSynthetic resin layered productsWeather resistancePolyrotaxane
[Object] To provide a coating material for a room temperature curable solvent-borne overcoating material having chipping resistance and not allowing occurrence of cracks, and a room temperature curable solvent-borne overcoating material using the same, the room temperature curable solvent-borne overcoating material being excellent in abrasion resistance and in performance such as weather resistance, contamination resistance and adhesion.[Solving Means] A coating material is formed of a lipophilic polyrotaxane including a cyclic molecule, a linear molecule piercing through the cyclic molecule to include it, and blocking groups disposed at both end terminals of the linear molecule to prevent departure of the cyclic molecule. In the lipophilic polyrotaxane, at least one of the linear molecule and the cyclic molecule has a hydrophobic modification group. A room temperature curable solvent-borne overcoating material is formed to contain the coating material preferably within a range of from 60 to 100% by mass relative to a film-forming component.
Owner:NISSAN MOTOR CO LTD +1
Material for Curable Aqueous Overcoating Material and Coating Material Using Same
InactiveUS20100129677A1Improved abrasionImprove chip resistanceLiquid surface applicatorsSynthetic resin layered productsPolyrotaxaneHydrophile
There is provided a curable aqueous overcoating composition containing 1 to 90 mass % of hydrophilic polyrotaxane with respect to the coating film forming component, where the hydrophilic polyrotaxane has a cyclic molecule, a linear molecule included in the cyclic molecule in a skewered manner and blocking groups arranged on opposite ends of the linear molecule to prevent elimination of the cyclic molecule from the linear molecule and at least one of the linear molecule and the cyclic molecule has a hydrophilic modifying group.
Owner:NISSAN MOTOR CO LTD +1
Material for curable aqueous overcoating material and coating material using same
InactiveCN101278019AImprove wear resistanceImprove crush resistanceLiquid surface applicatorsSynthetic resin layered productsEndcappingPolyrotaxane
A curable aqueous overcoating material is obtained by blending 1-90% by mass of a hydrophilic polyrotaxane relative to the coating film-forming component. The hydrophilic polyrotaxane has a cyclic molecule, a linear molecule passing through the cyclic molecule and enclosed thereby, and blocking groups arranged on both ends of the linear molecule for preventing elimination of the cyclic molecule, and at least one of the linear molecule and the cyclic molecule has a hydrophilic modification group.
Owner:NISSAN MOTOR CO LTD +1
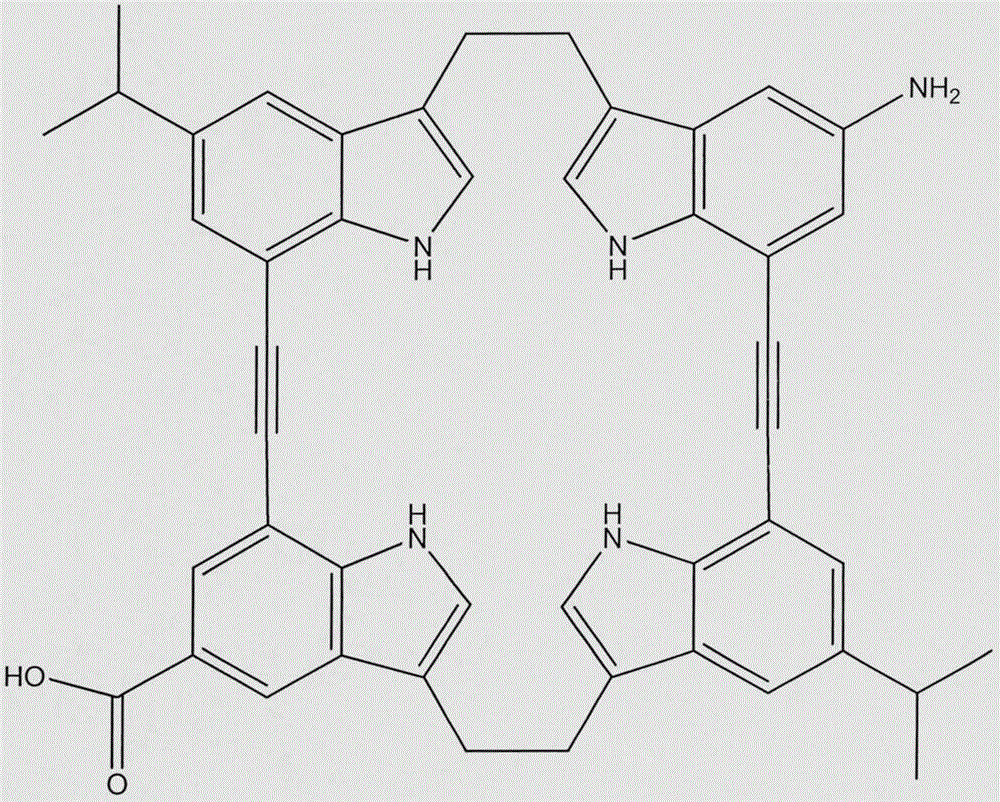
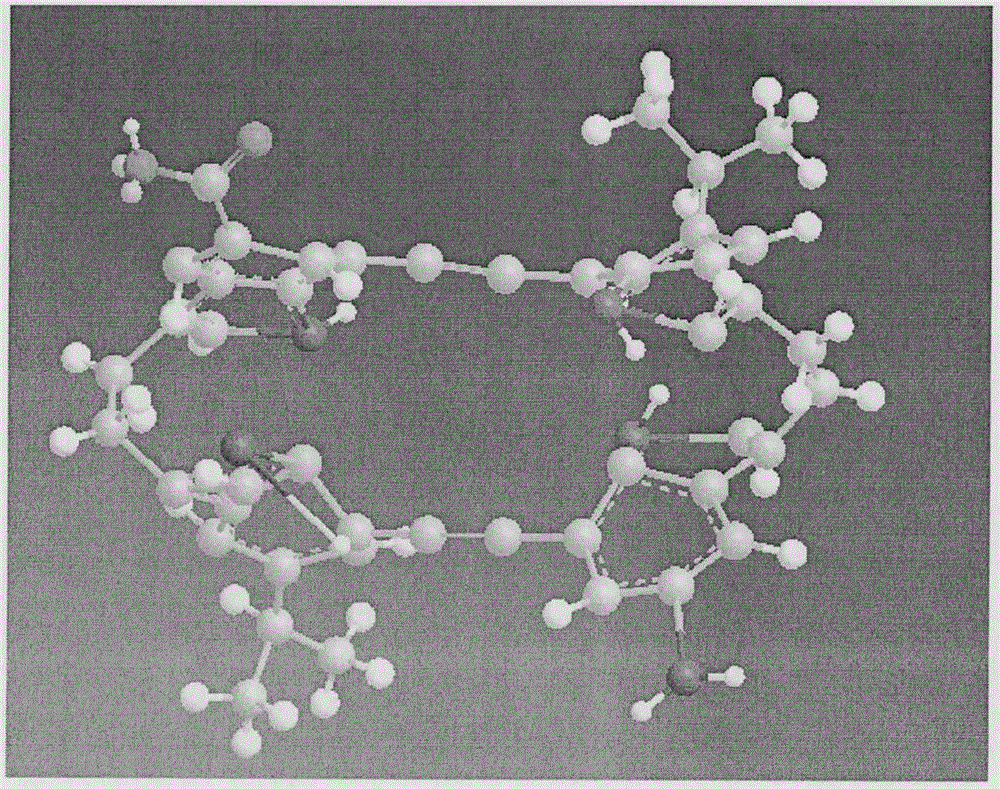
Macrocyclic molecule, preparation method and application thereof
ActiveCN109705131AImprove recognition efficiencyAvoid competitionOrganic chemistryComponent separationBicyclic moleculeMolecular recognition
The invention provides a macrocyclic molecule, a preparation method and an application thereof. A chiral macrocyclic molecule, as the formula I or II, has the function of chiral recognition; an internal compensation macrocyclic molecule, as the formula III, has the function of recognizing a strong-hydrophilic neutral molecule.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA
Self-restoring macromolecular material and production method for same
InactiveUS20170233533A1Excellent stress relaxationLoss of material propertyPolyrotaxaneBicyclic molecule
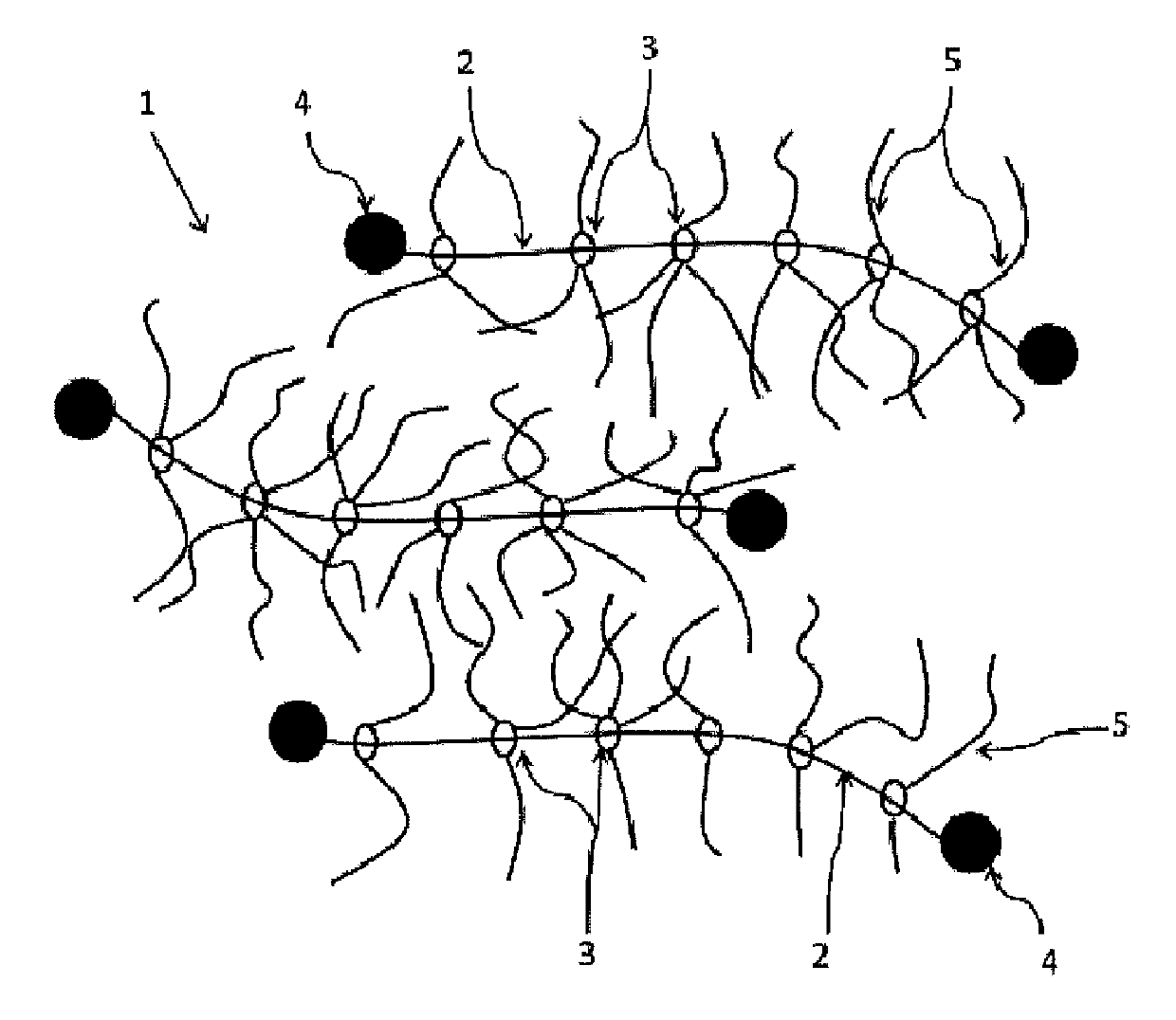
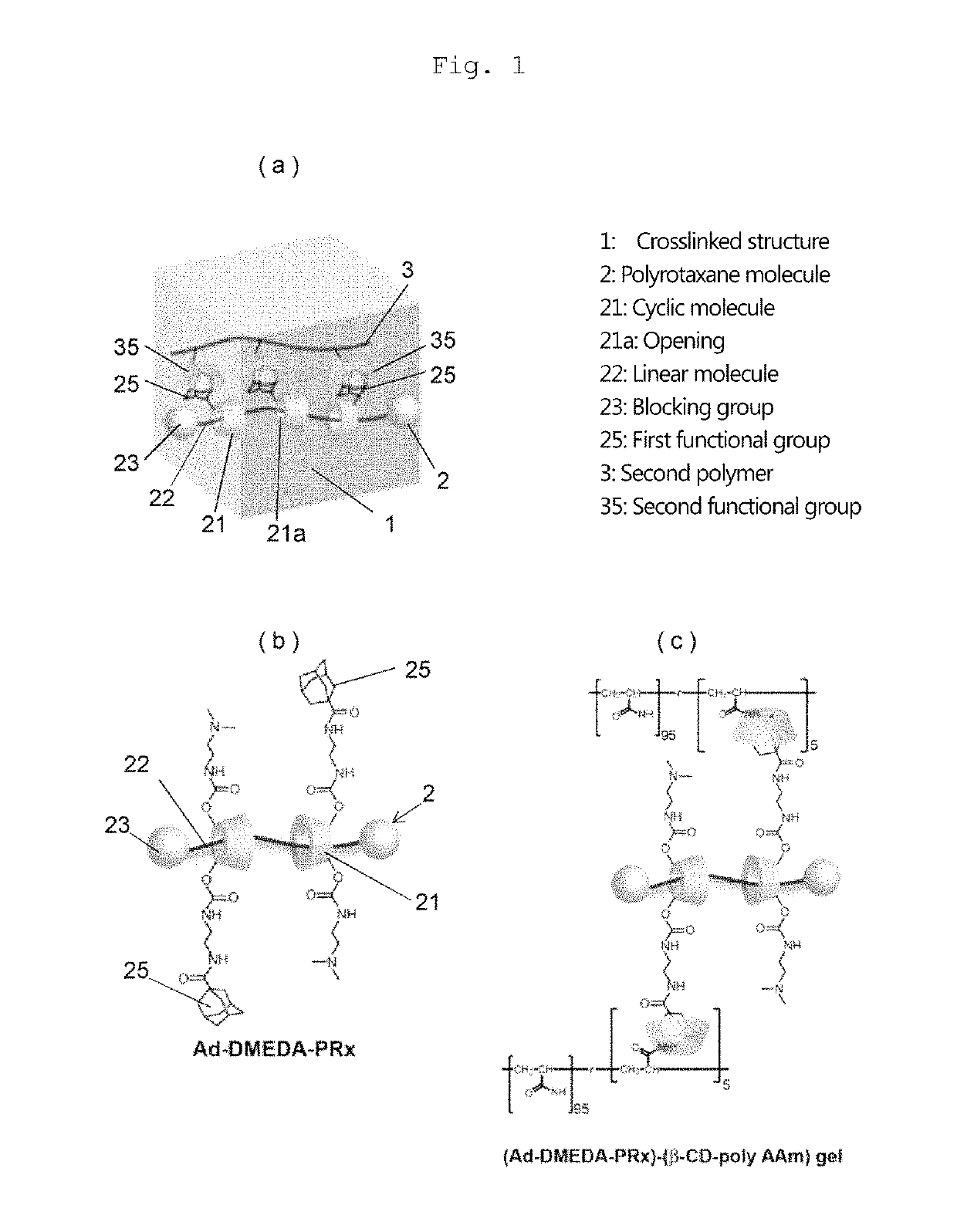
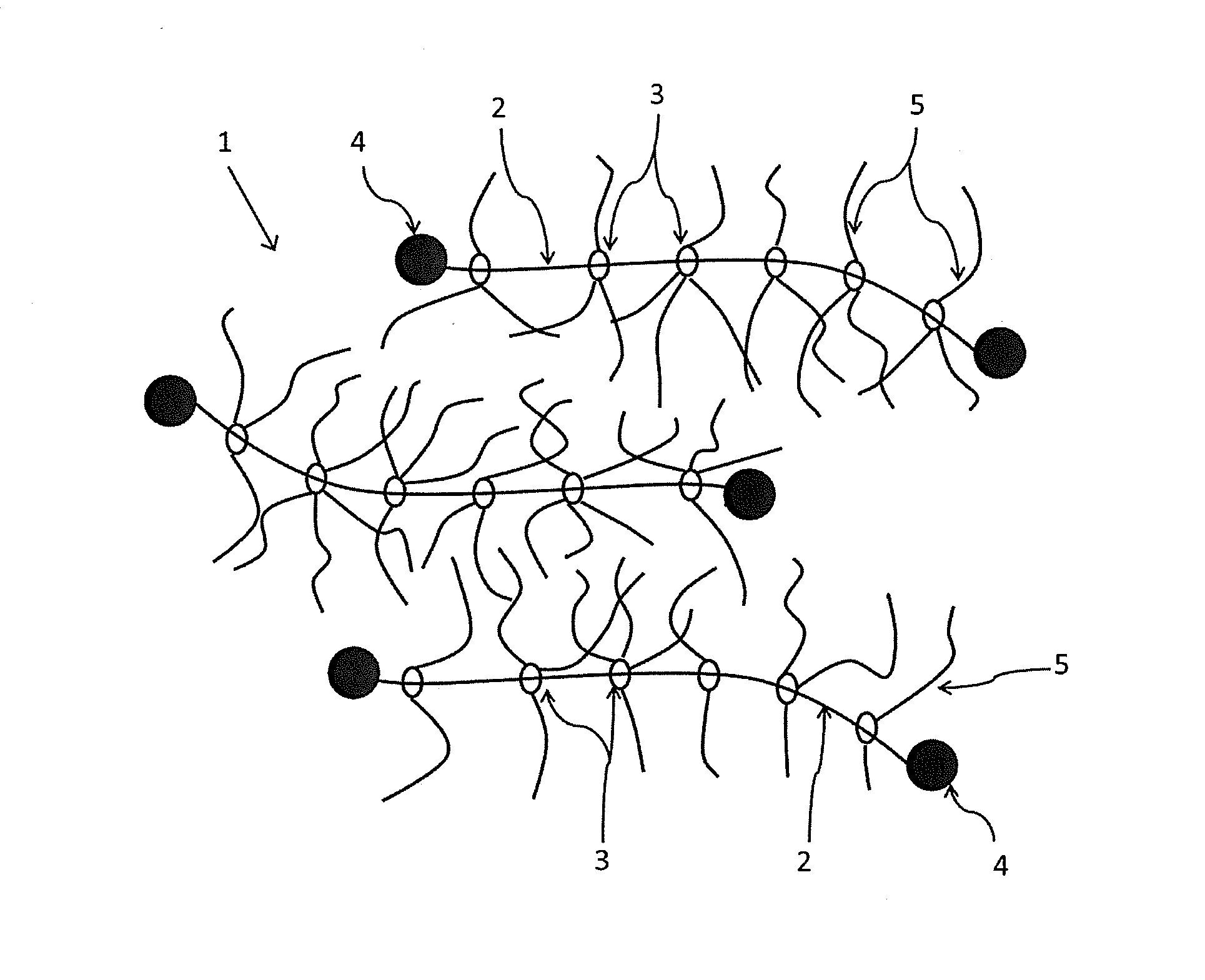
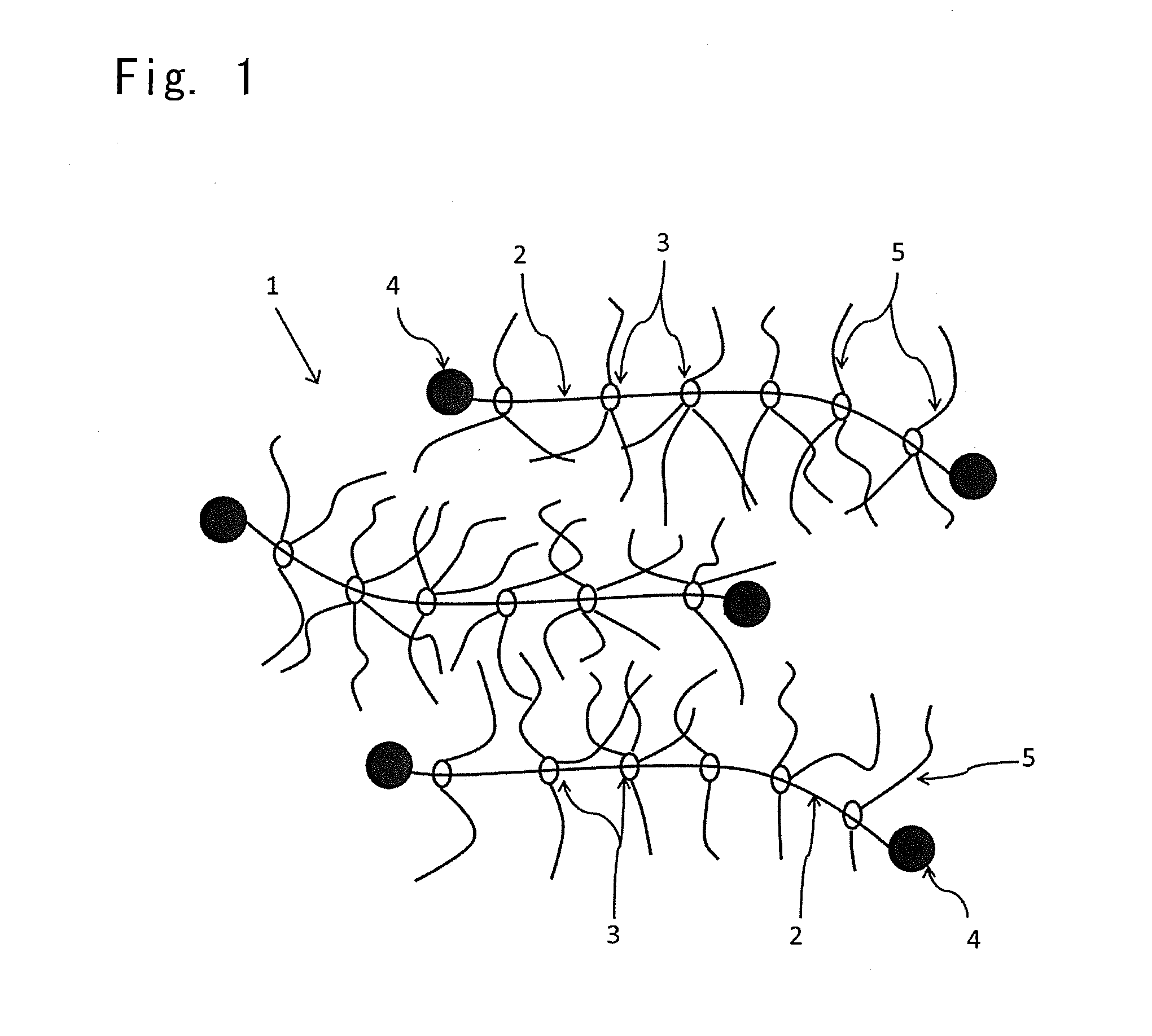
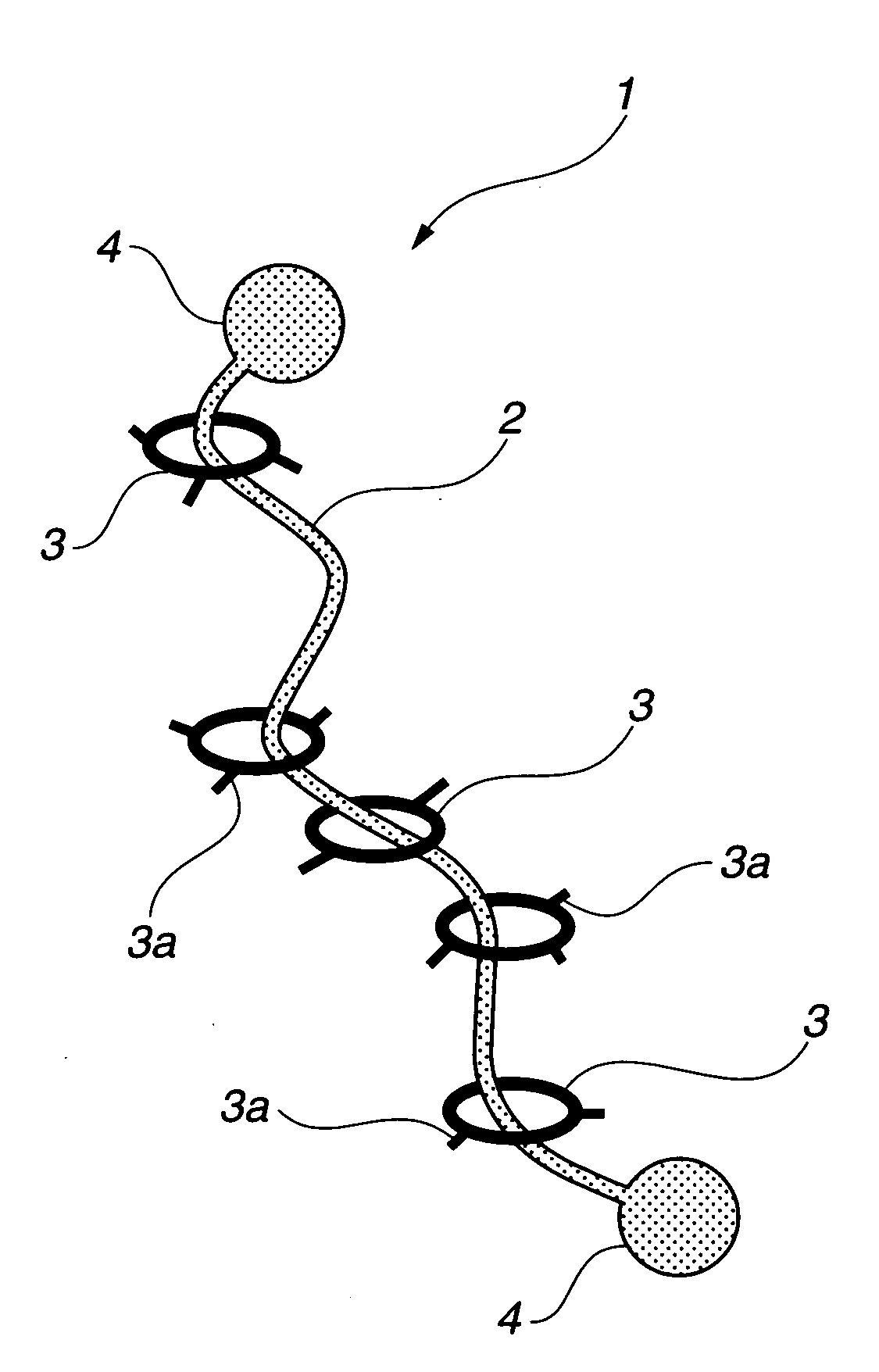
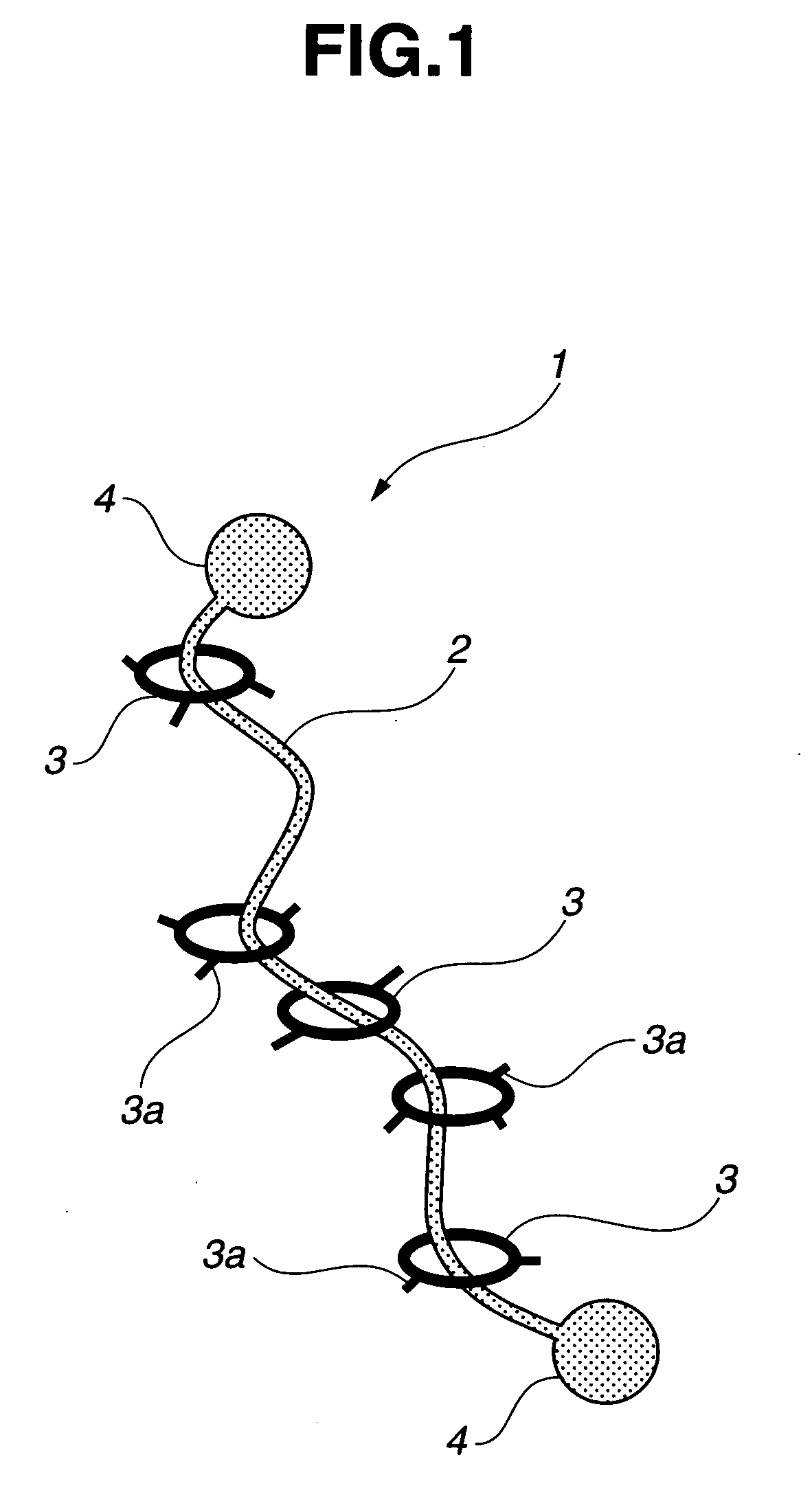
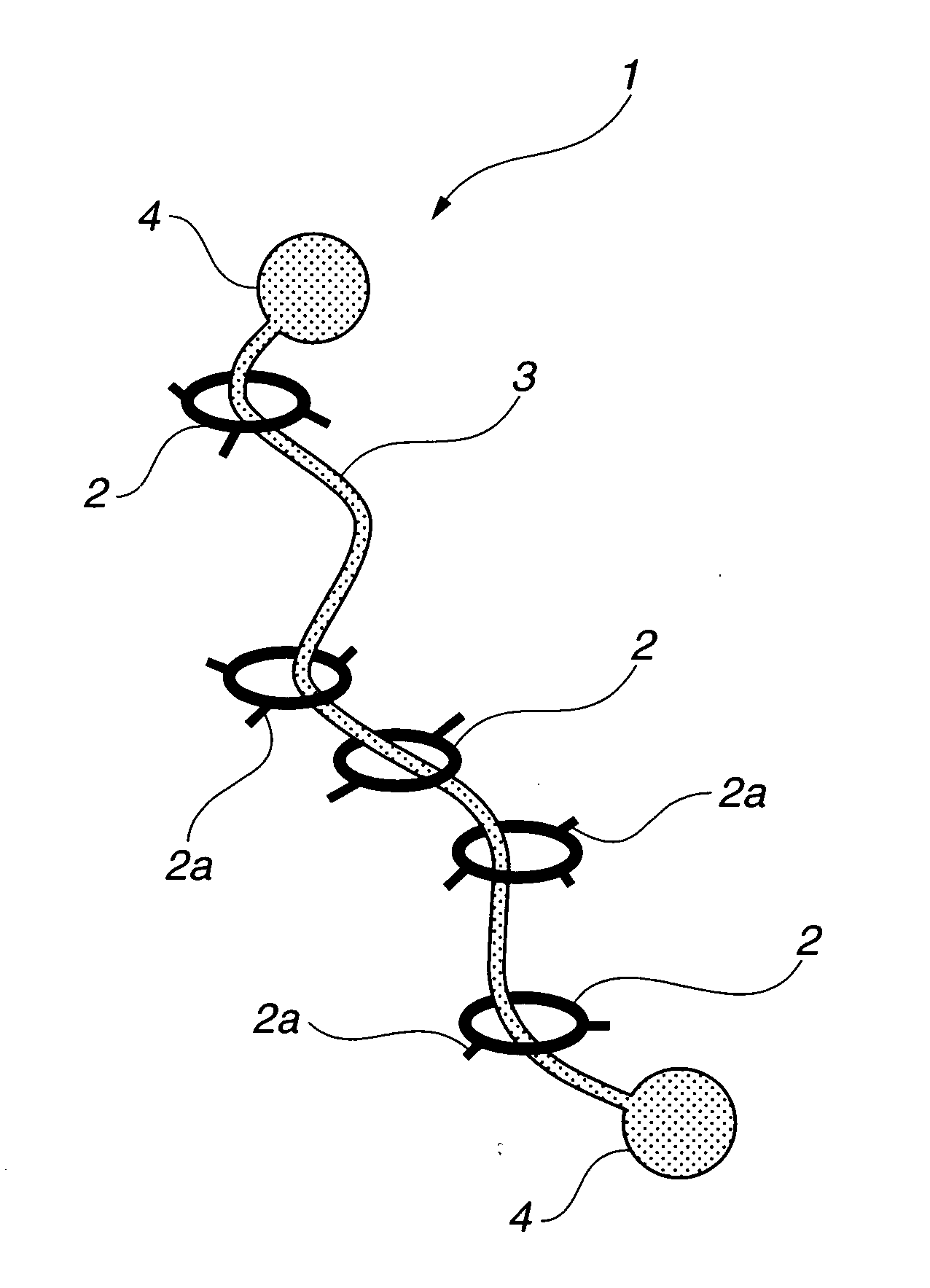
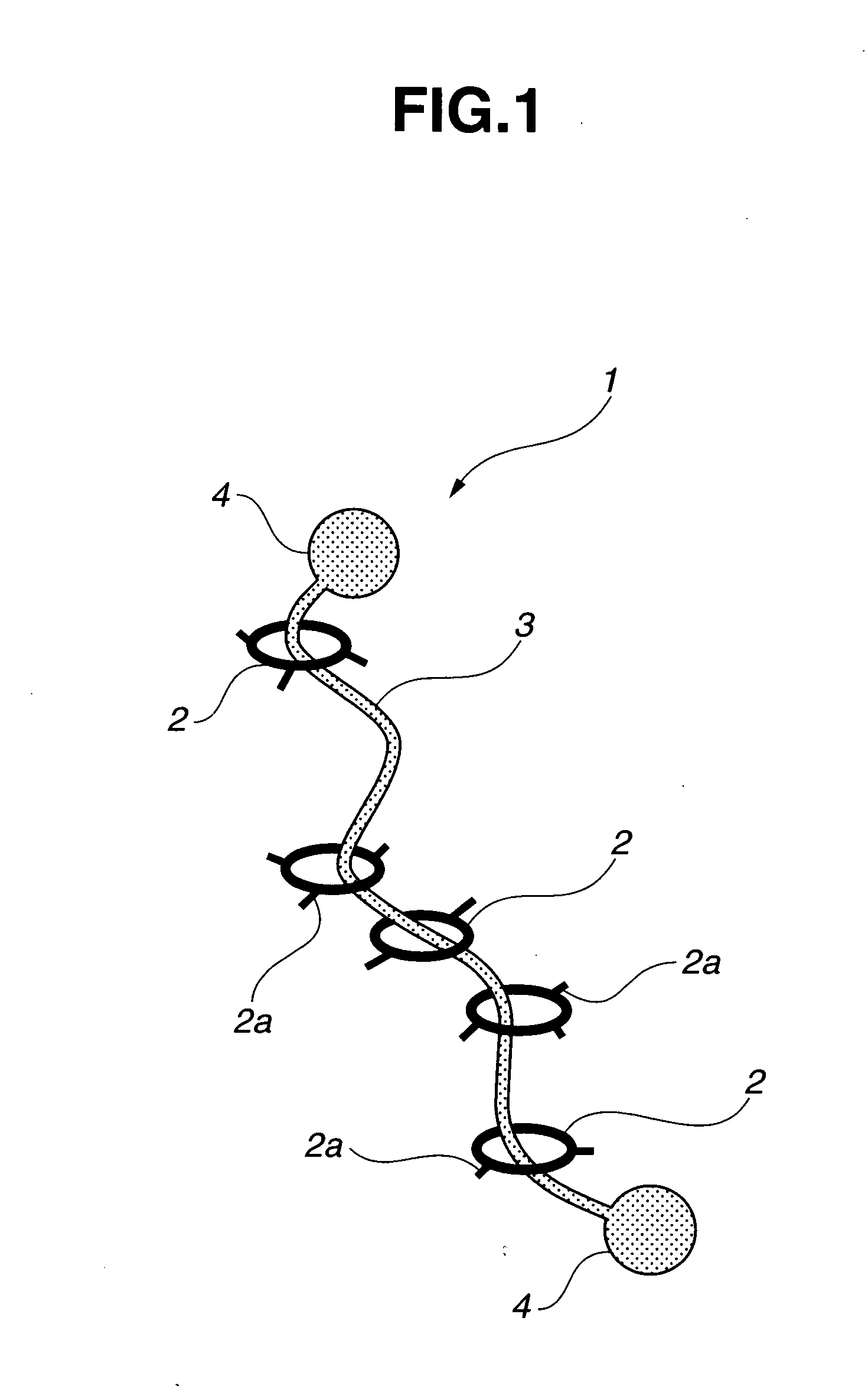
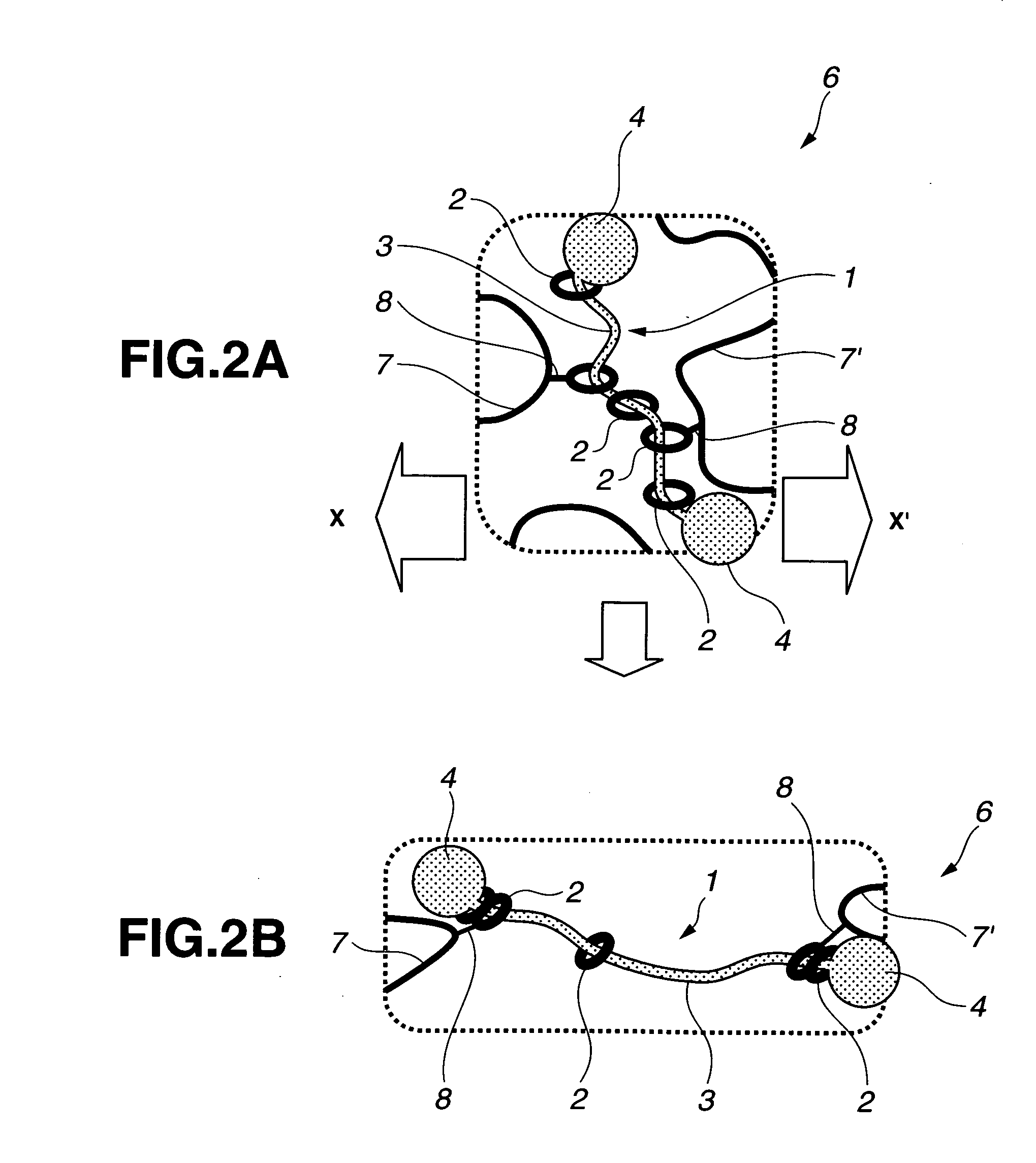
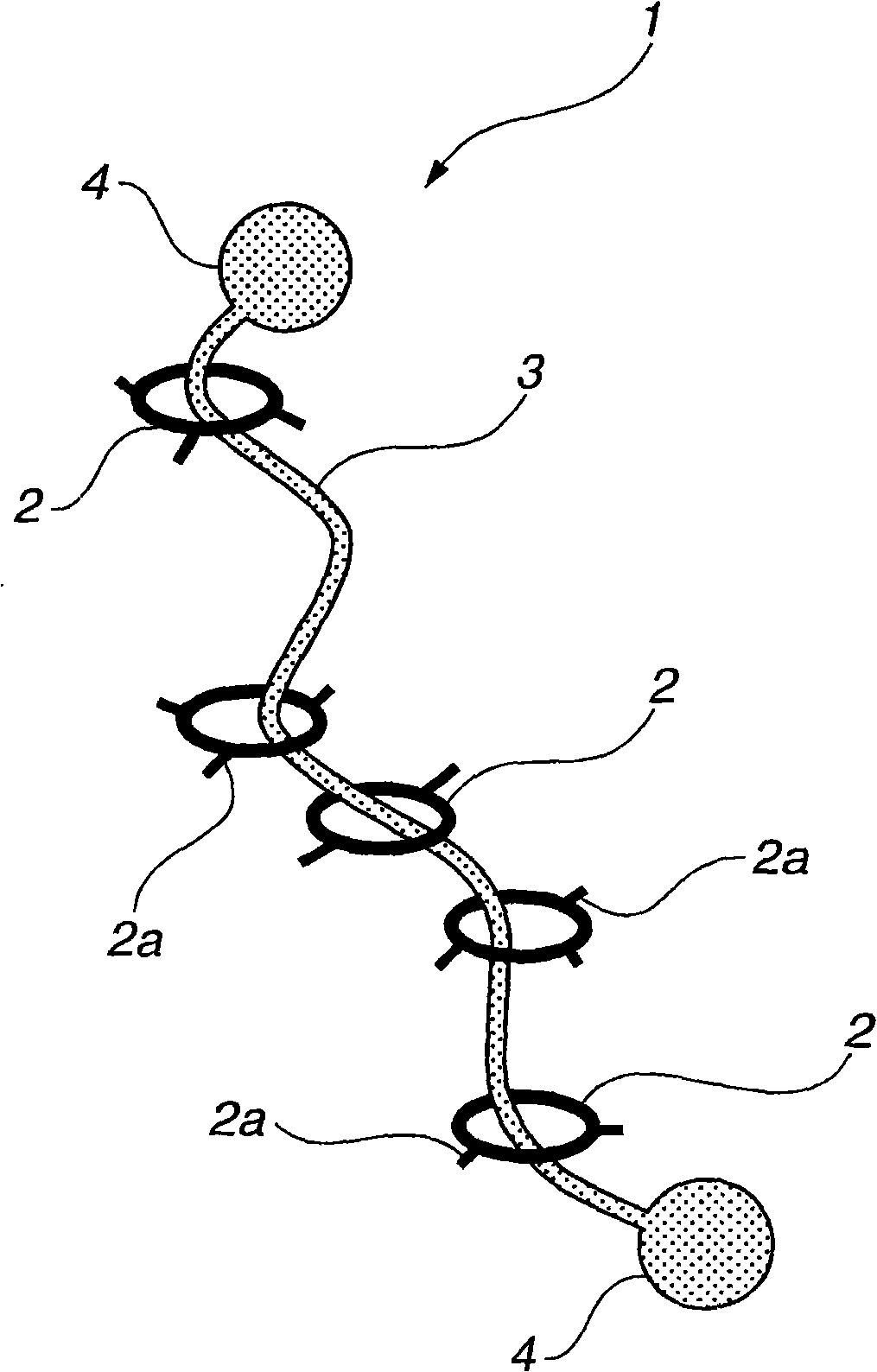
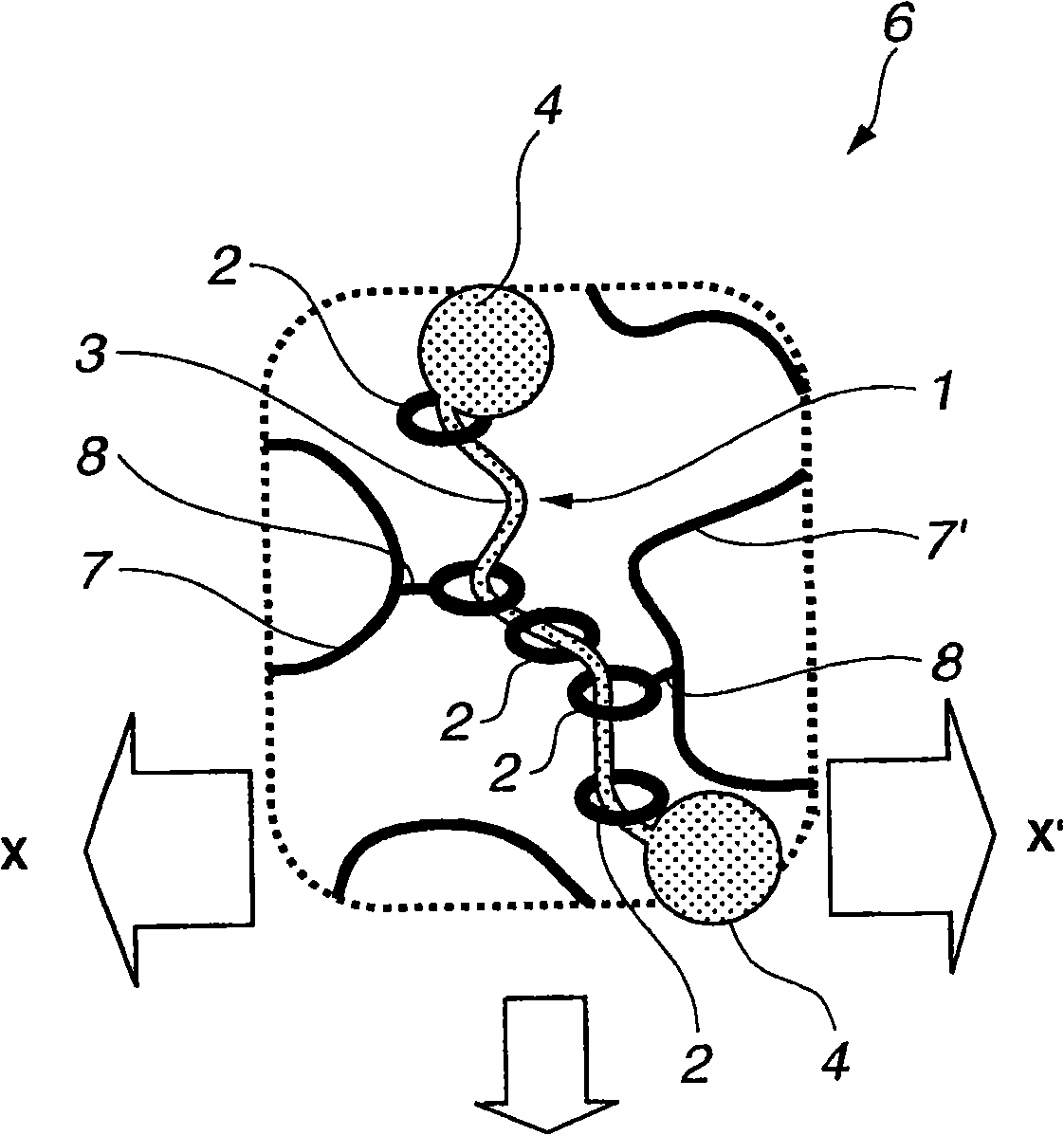
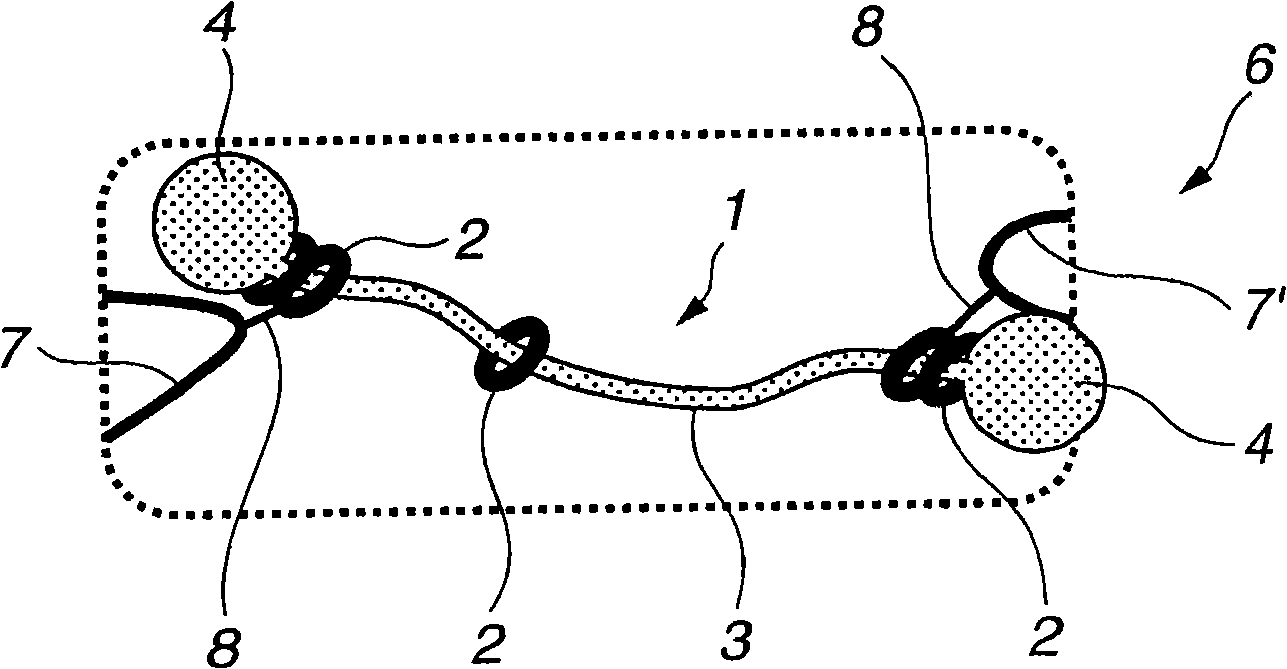
Provided is a self-restoring macromolecular material that not only has excellent stress relaxation but that can also be easily restored to its original state, even when damaged or severed. Also provided is a method for producing the self-restoring macromolecular material. The self-restoring macromolecular material contains a crosslinked structure that is formed by crosslinking a polymer containing at least a polyrotaxane molecule. The polyrotaxane molecule is formed so as to include a cyclic molecule 21 and a linear molecule that passes through an opening 21a of the cyclic molecule. The crosslinked structure 1 is crosslinked via a reversible bond between the cyclic molecule of the polyrotaxane molecule and a polymer molecule other than the polyrotaxane molecule.
Owner:OSAKA UNIV
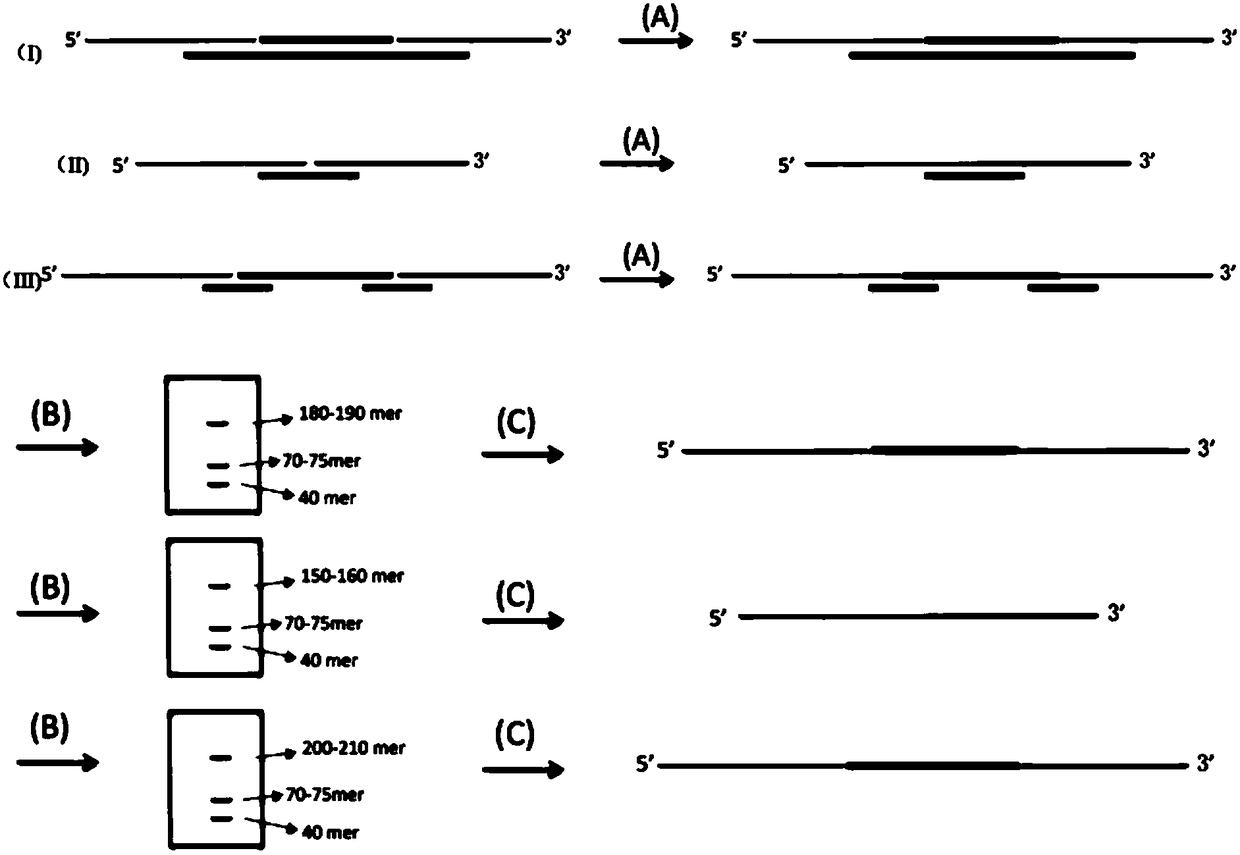
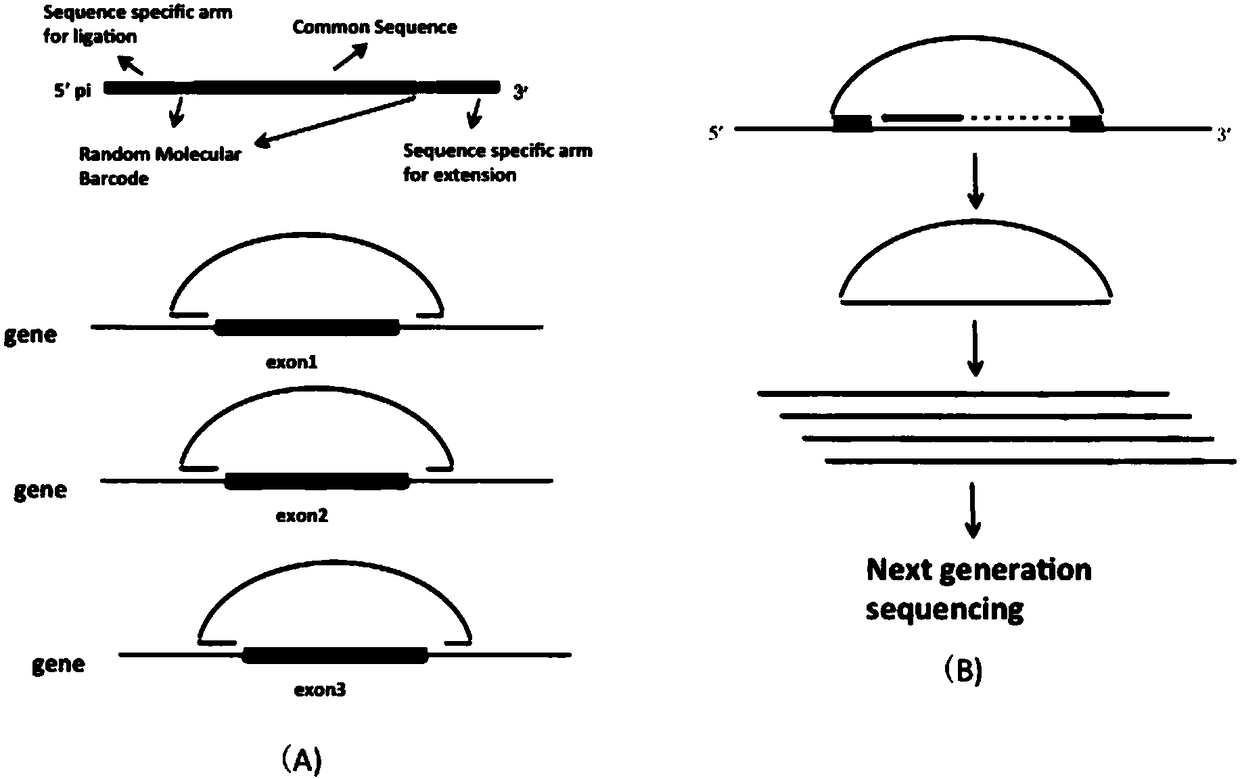
Preparation method of nucleic acid targeted capture sequencing library based on long chain molecule inversion probe
ActiveCN108396057AIncrease the lengthImprove capture efficiencyMicrobiological testing/measurementLibrary creationConnexonPhosphorylation
The invention discloses a preparation method of a nucleic acid targeted capture sequencing library based on a long chain molecule inversion probe. The preparation method comprises the following steps:a, synthesizing a capture probe A, a capture probe B and a connexon C; b, adding phosphorylated probes A and B and the connexon C into a ligase reaction system, simultaneously adding DNA (Deoxyribonucleic Acid) ligase so as to connect the A with the B under the bridging effect of the C; c, combining multiple connection mixtures for different target areas, and separating and purifying the connected product by denaturing electrophoresis or a nucleic acid purification kit to obtain the long chain molecular inversion probe; d, mixing the long chain molecular inversion probe with DNA or cDNA of ato-be-tested sample, hybridizing, adding DNA polymerase, DNA ligase, dNTP and a Mg2<+>-containing buffer solution into a buffer solution, extending the long chain molecular inversion probe and formingclosed molecules under the action of the DNA ligase; e, adding exonuclease to degrade non-cyclic DNA molecules; f) carrying out PCR (Polymerase Chain Reaction) amplification by using primers corresponding to a common sequence region of the long-chain molecular inversion probe to obtain the sequencing library of a targeted region.
Owner:CHONGQING CANCER INST
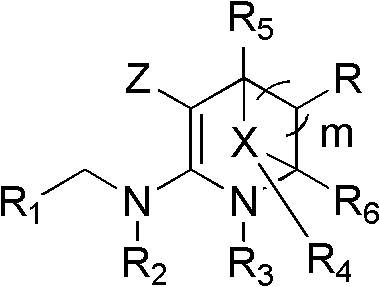
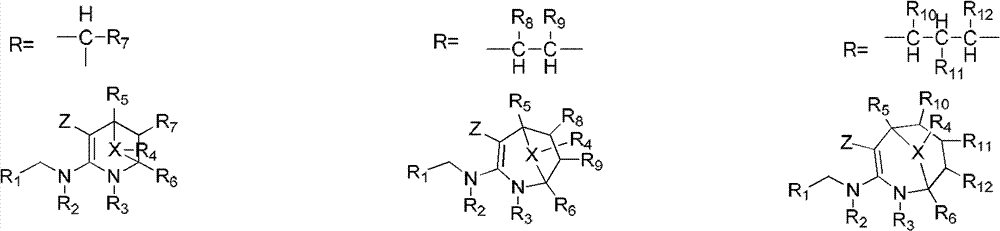
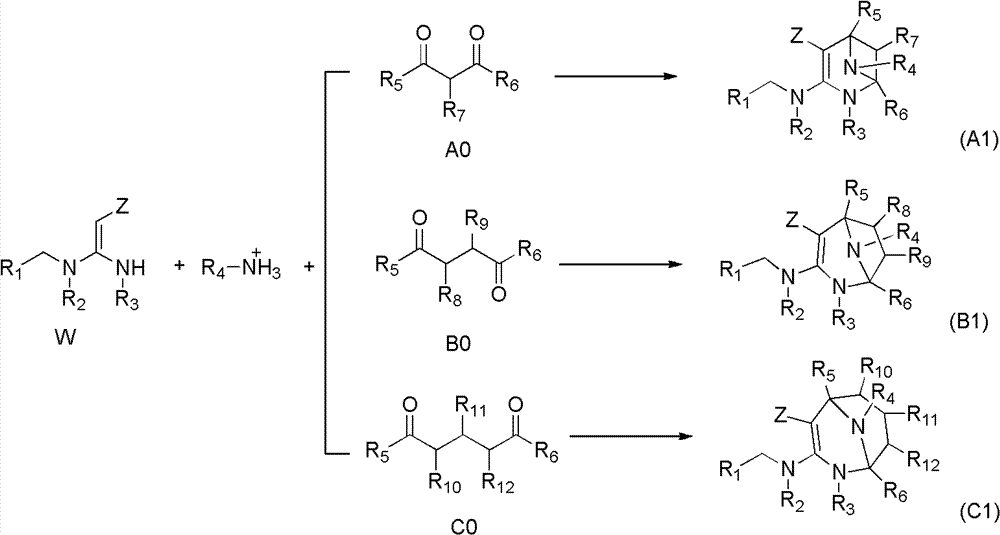
Nitrogen (sulfur) containing bridge ring compound with insecticidal activity, preparation method and application
InactiveCN103242323AHigh insecticidal activityExpand insecticidal spectrumBiocideOrganic chemistryBridged-Ring CompoundsAgriculture
The invention relates to a nitrogen (sulfur) containing bridge ring compound with insecticidal activity, a preparation method and an application. Specifically, the invention discloses a compound with a general formula I or an optical isomer, a cis-trans-isomer or an agricultural pharmacology acceptable salt of the compound, and a preparation method thereof, wherein R1, R2, R3, R4, R5, R6, R, m, X and Z are respectively as defined in the specification. The invention also discloses an agricultural composition comprising the above compound and an application thereof. The above compound has high insecticidal activity for homoptera and lepidoptera agriculture and forestry insects, such as aphids, plant hoppers, whiteflies, cotton bollworms, prodenia litura, armyworm, etc. The general formula 1 is shown in the specification.
Owner:EAST CHINA UNIV OF SCI & TECH
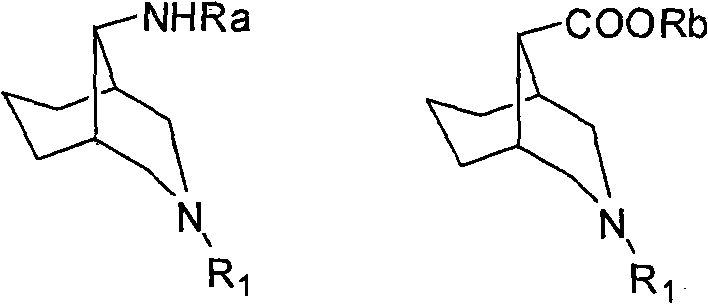
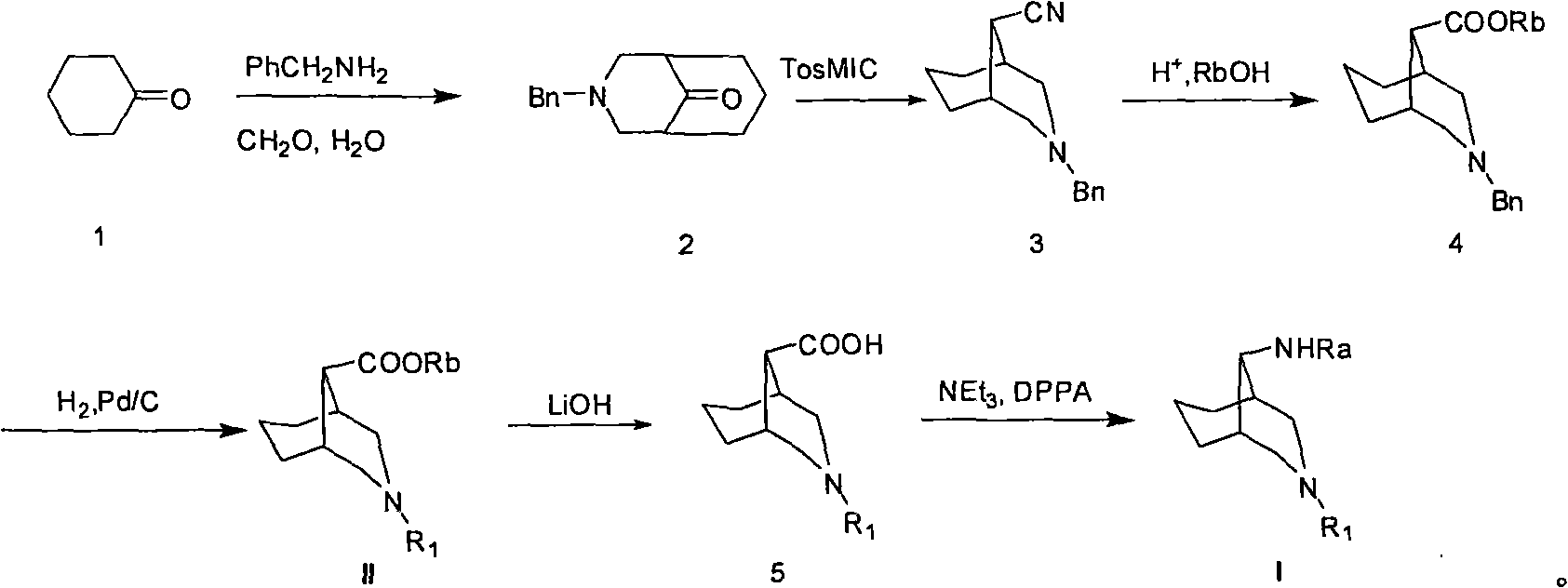
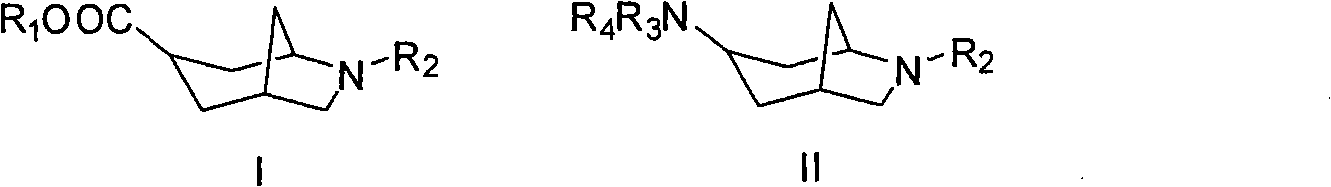
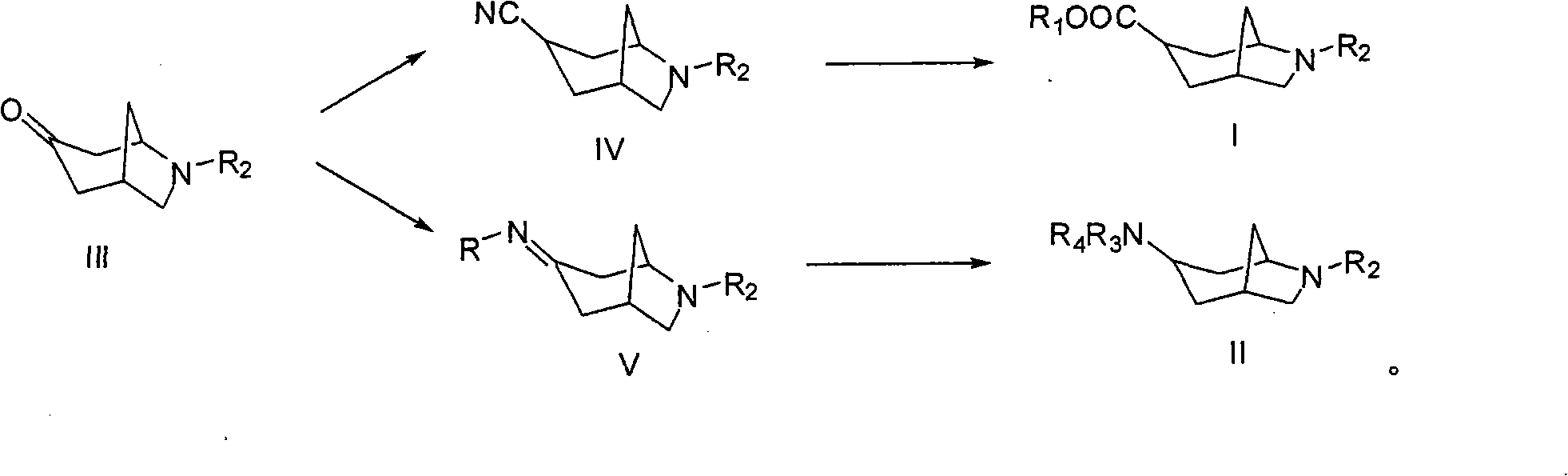
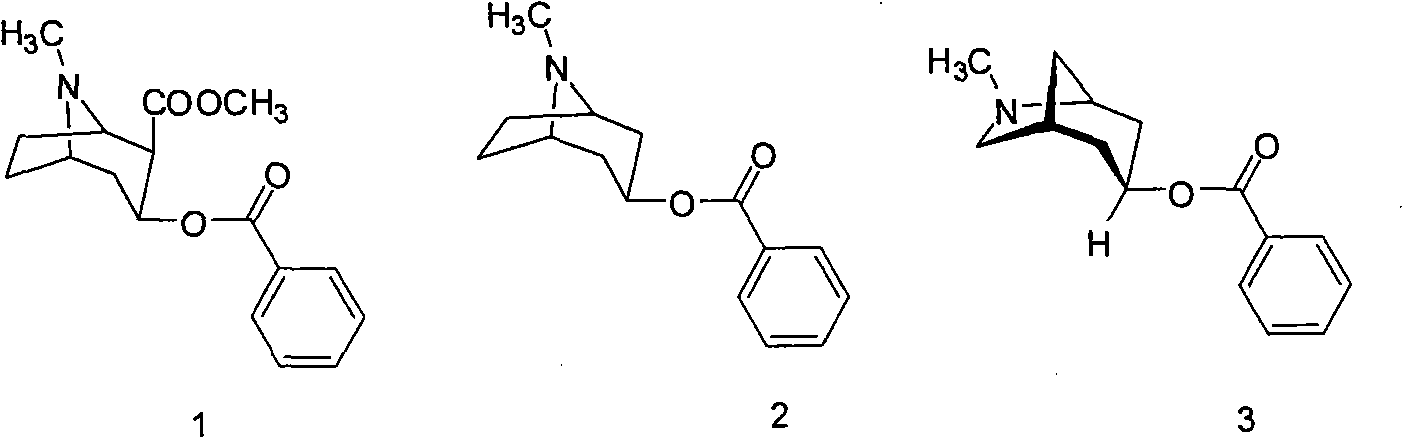
3-azabicyclo (3.3.1) nonane-9-substituted derivative and preparation method thereof
The invention relates to a 3-azabicyclo (3.3.1) nonane-9-substituted derivative and a preparation method thereof, which mainly solve the technical problems that the extension of the prior endocyclic compound with 3-azabicyclo (3.3.1) nonane structure in a space structure is limited and the water solubility of the compound is poor. The chemical structural formula of the 3-azabicyclo (3.3.1) nonane-9-substituted derivative is shown as above, and the optimal compound structure in the formula (1) is I, II, III, and IV. The invention is mainly used as a template compound in medicine research.
Owner:无锡药明康德新药开发股份有限公司 +1
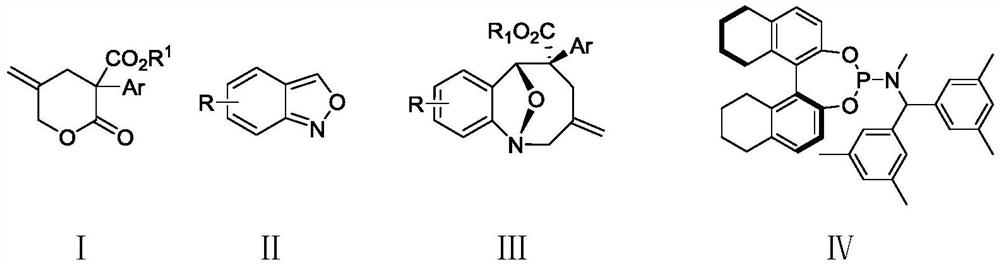
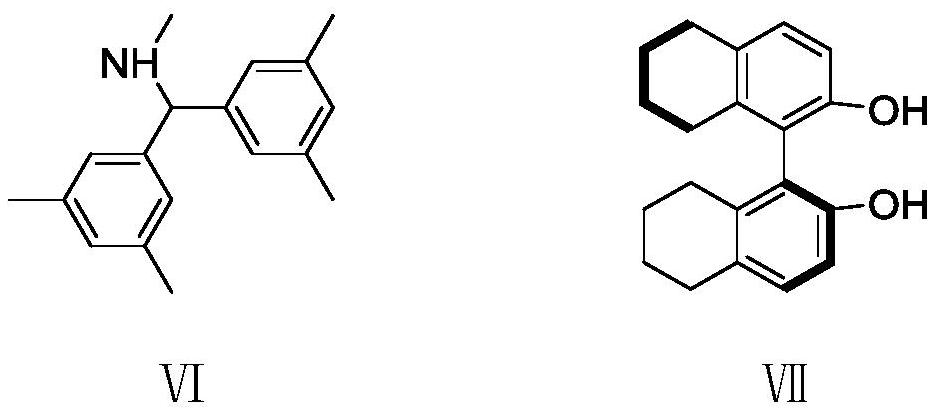
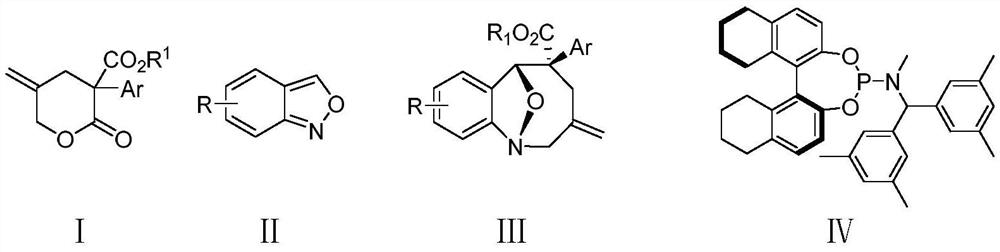
Method for synthesizing eight-membered bridged ring compound through palladium-catalyzed asymmetric cycloaddition reaction
ActiveCN112940002AEfficient asymmetric synthesisLow priceGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystPalladium catalyst
The invention provides a method for synthesizing an eight-membered bridged ring compound by palladium-catalyzed asymmetric cycloaddition reaction. The method comprises the following steps: in an organic solvent, under catalysis of a palladium catalytic system and in the presence of an additive, carrying out [4 + 4] cycloaddition reaction on a gamma-methylene-delta-valerolactone compound I and a benzo [C] isoxazole compound II, thereby obtaining the eight-membered bridged ring compound III. The palladium catalysis system is composed of a palladium catalyst and a chiral ligand. According to the method disclosed by the invention, the eight-membered bridged ring compound can be generated with high stereoselectivity and regioselectivity, and the eight-membered ring compound is subjected to efficient asymmetric synthesis; and the method for preparing the eight-membered ring compound through the palladium-catalyzed [4 + 4] cycloaddition reaction has the advantages of being convenient to operate, wide in substrate application range, cheap and easily available in reaction raw materials and the like.
Owner:SHANDONG UNIV
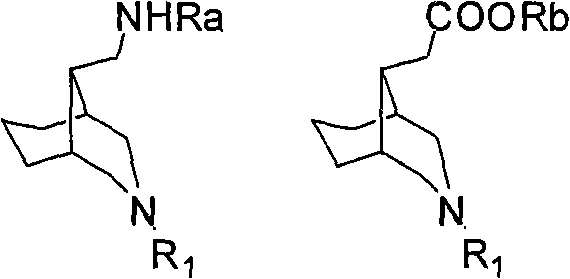
6-azabicyclo (3.2.1) nonane-3-substituted derivative and preparation method thereof
The invention relates to a 6-azabicyclo (3.2.1) nonane-3-substituted derivative and a preparation method thereof, which mainly solve the technical problems that the extension of the prior endocyclic compound with 6-azabicyclo (3.23.1) nonane structure in a space structure is limited and the water solubility of the compound is poor. The reaction formula of the 6-azabicyclo (3.2.1) nonane-3-substituted derivative is shown as above. 3-nitrile-6-azabicyclo (3.2.1) nonane compound IV is obtained by adopting 6-azabicyclo (3.2.1) nonane-3-ketone III as raw materials through nitrile addition, and then the compound IV is reacted with ethanol under acid condition to generate ester compound I; or the compound III is reacted with oxyammonia, aminos, hydrazines, amides, sulfonamides, hydrazides or sulfonyl hydrazides to generate compound V, and the compound V is reduced to generate compound II.
Owner:无锡药明康德新药开发股份有限公司 +1
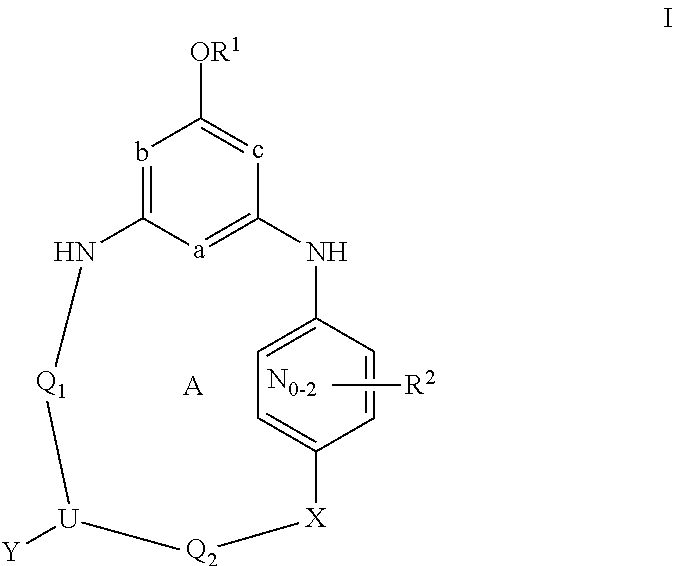
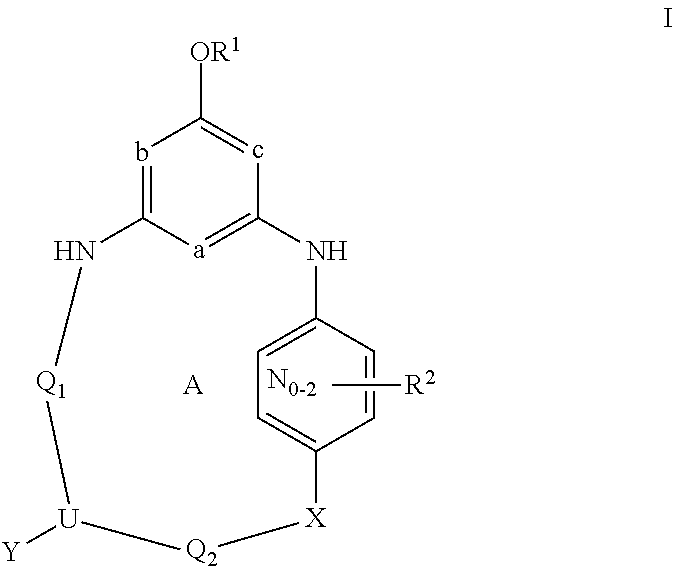
Bridged ring compounds as hepatitis C virus (HCV) inhibitors and pharmaceutical applications thereof
Owner:SUNSHINE LAKE PHARM CO LTD
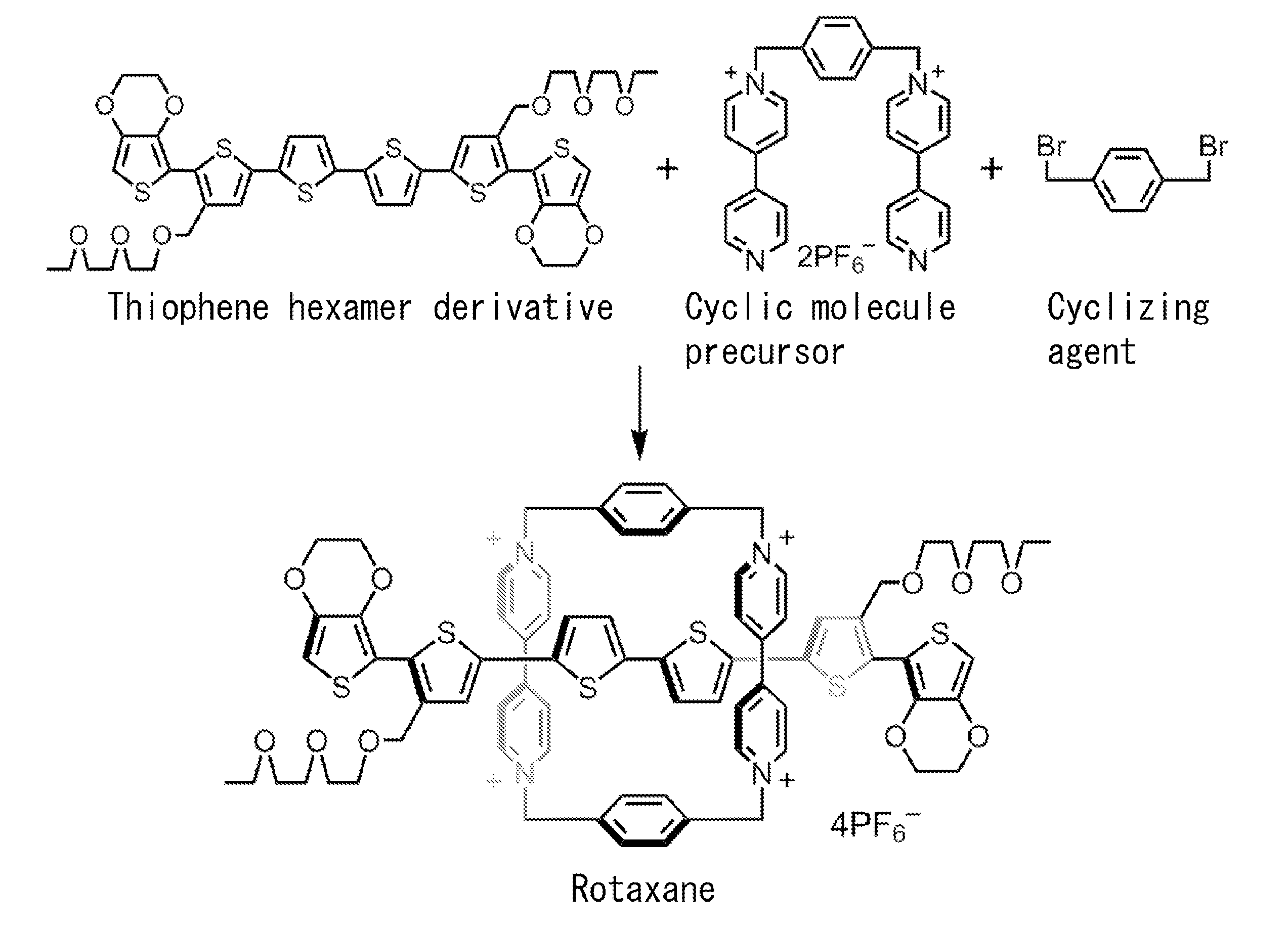
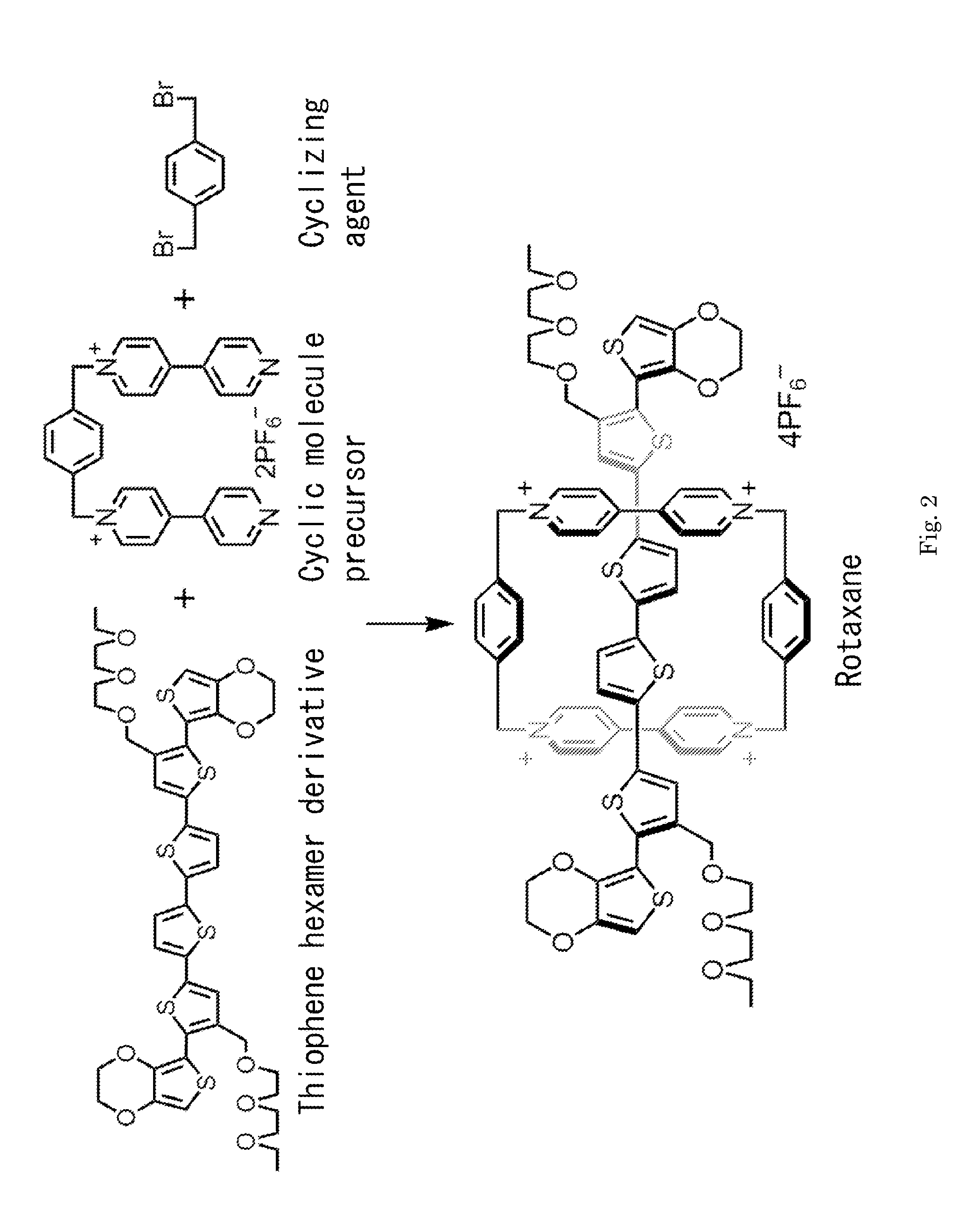
Electrically conductive polyrotaxane
InactiveUS20120083582A1Improve conductivityElectrolysis componentsOrganic chemistryPolyrotaxaneOligomer
The conductive polyrotaxane of the present invention contains an electron-accepting cyclic molecule in each of its repeating unit. Since the electron-accepting cyclic molecule remains stable because of molecular interaction with a π-conjugated oligomer molecule, the electron-accepting cyclic molecule is not dissociated during rotaxane polymerization reaction, and thus a conductive polyrotaxane of stable quality can be obtained.
Owner:NAT INST FOR MATERIALS SCI
Bridged Ring compounds As Hepatitis C Virus (HCV) Inhibitors And Pharmaceutical Applications Thereof
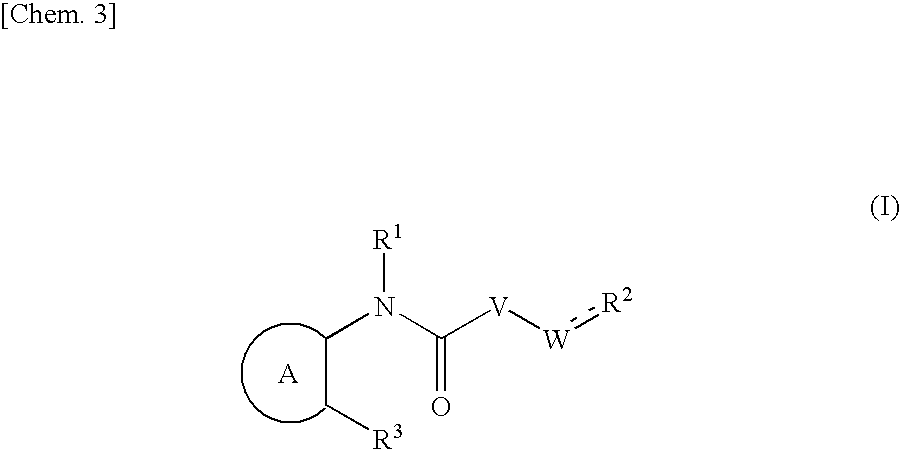
Provided herein is a compound having Formula (I), or a stereoisomer, a geometric isomer, a tautomer, an N-oxide, a hydrate, a solvate, a metabolite, a pharmaceutically acceptable salt or a prodrug thereof, which can be used for treating HCV infection or a HCV disorder. Also provided herein are pharmaceutical compositions comprising the compounds disclosed herein, which can be used for treating HCV infection or a HCV disorder.
Owner:SUNSHINE LAKE PHARM CO LTD
1,3-disubstituted-3-diazabicyclo[3,3,1] nonane derivative and preparation method thereof
ActiveCN102875467AIncrease polarityIncrease diversityOrganic chemistryRespiratory disorderChemical structureSide chain
The invention relates to a 1,3-disubstituted-3-diazabicyclo[3,3,1] nonane derivative and a preparation method thereof, mainly solving the technical problems that bridge-ring compounds with 3-diazabicyclo[3,3,1] nonane structure are limited in space structural extension, which is not good for rapid screening of compound activity and SAR analysis. The derivative disclosed herein is represented by the following formula, wherein R1 represents a protecting group of a substituted functional group or an amino group, and is selected form H, C1-C10 straight chain or one of alkyl, alkanoyl and alkylsulphonyl which contain substituent side chains; and G is one of hydroxymethyl, hydroxyl, ester, and formamido.
Owner:WUXI BIOLOGICS CO LTD +1
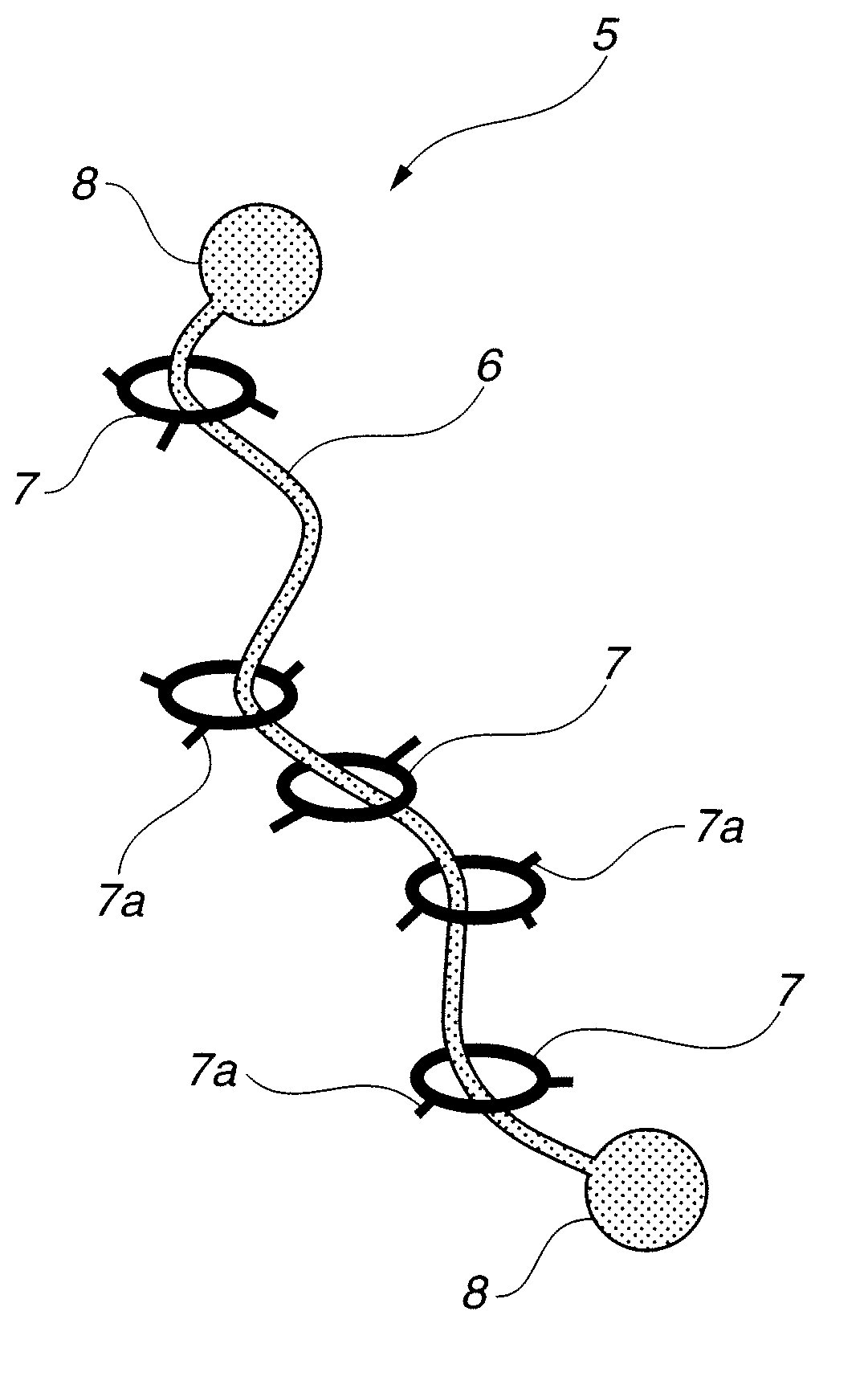
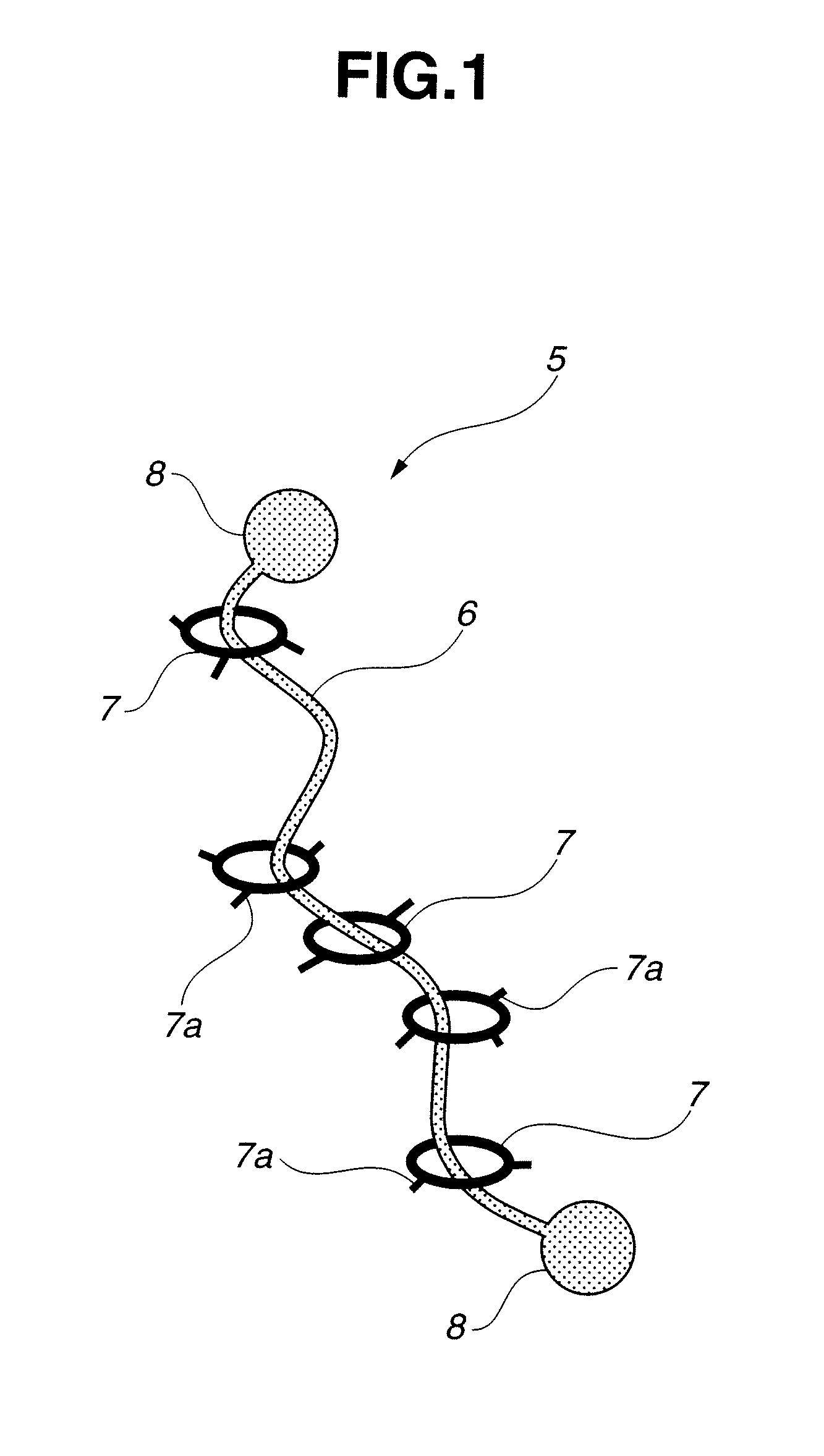
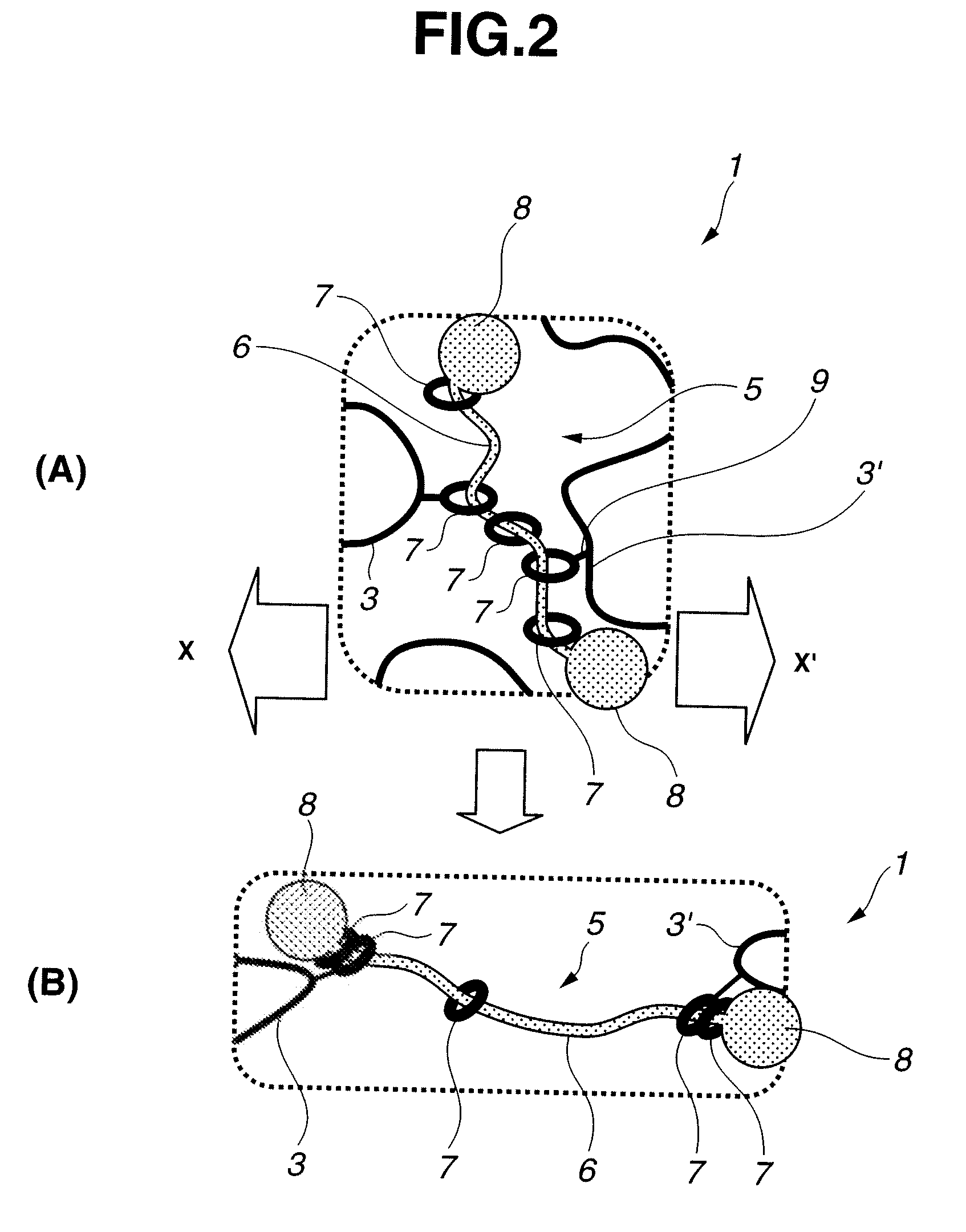
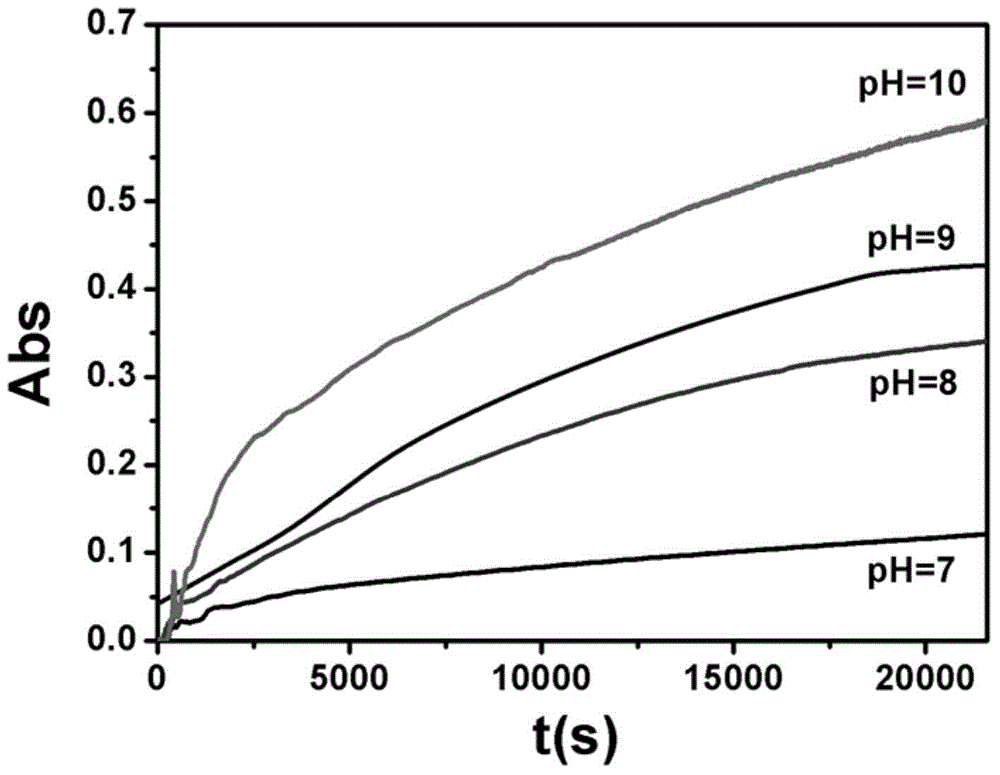
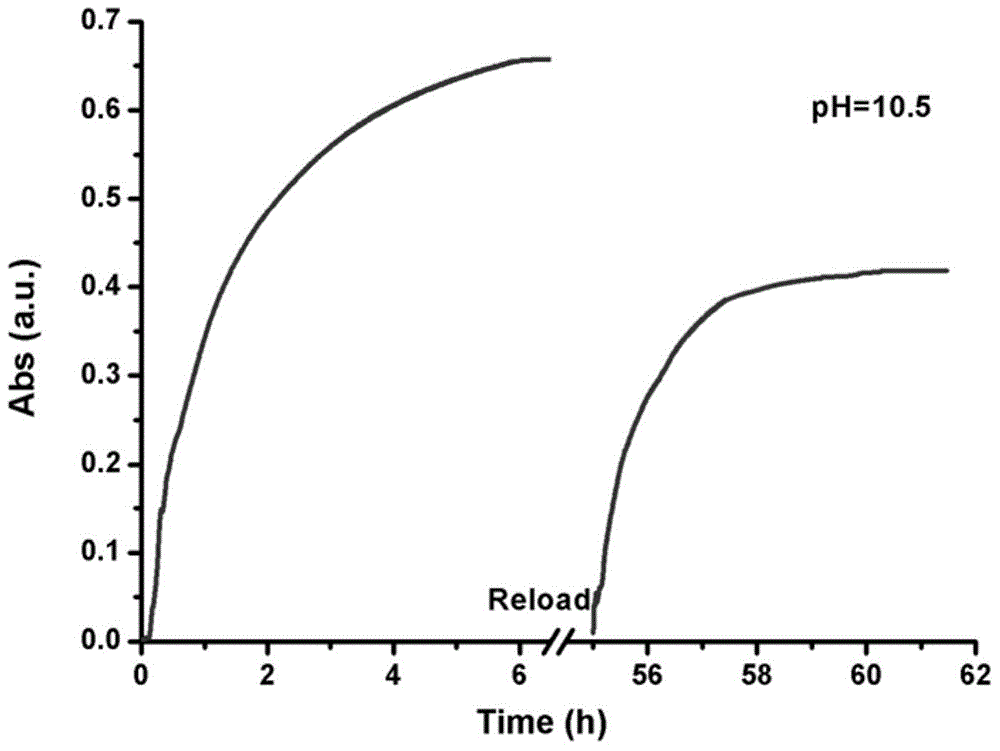
Intelligent nanometer container capable of realizing reversible movement of macrocyclic molecules under stimulation of pH values and preparation method thereof
InactiveCN105983390AEasy to operateHigh sensitivityOther chemical processesBicyclic moleculeUnder-stimulation
The invention discloses an intelligent nanometer container capable of realizing reversible movement of macrocyclic molecules under stimulation of pH values and a preparation method thereof. Compared with conventional nanometer containers, the intelligent nanometer container provided by the invention has the advantages of simple operation, high sensitivity, a wide application scope, etc. The major characteristic of the intelligent nanometer container is that macrocyclic molecules can stay at different positions under different pH values. Controllable release and stage release are realized through reversible movement of macrocyclic molecules on a branched chain, so the intelligent nanometer container has good application prospects.
Owner:NANJING UNIV OF SCI & TECH
1,3,7-tri-substituted-diazabicyclo[3,3,1] nonane derivative and preparation method thereof
ActiveCN102875550AIncrease polarityIncrease diversityOrganic chemistryBulk chemical productionChemical structureBicyclic molecule
The invention relates to a 1,3,7-tri-substituted-diazabicyclo[3,3,1] nonane derivative and a preparation method thereof, mainly solving the technical problems that bridge-ring compounds with 1,3,7-tri-substituted-diazabicyclo[3,3,1] nonane structure are limited in space structural extension, which is not good for rapid screening of compound activity and SAR analysis. The derivative disclosed herein is represented by the following formula, wherein R1 represents a protecting group of a substituted functional group or an amino group, and is selected form H or benzyl; R2 represents a protecting group for substituting a functional group or an amino group, and is selected form H or carbobenzoxy; and G is one of hydroxymethyl, hydroxyl, ester, and formamido.
Owner:CHANGZHOU HEQUAN PHARMA CO LTD
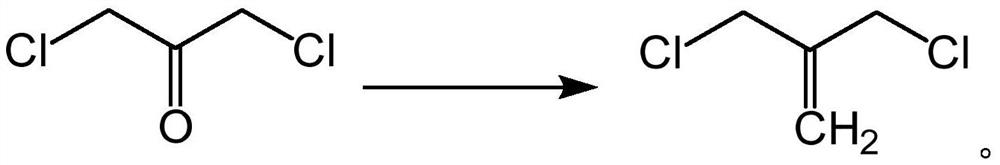
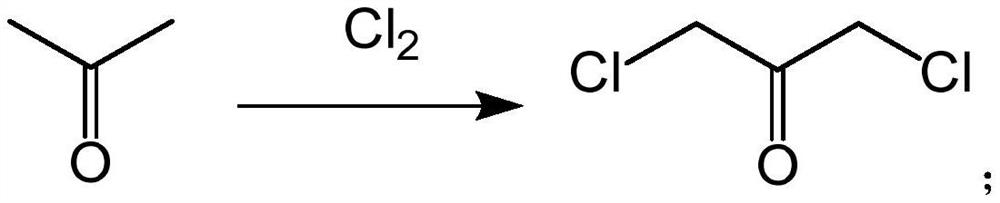
3-chloro-2-chloromethyl propylene, preparation method and application thereof
PendingCN113024348AHigh yieldRaw materials are easy to getOrganic compound preparationCarbonyl compound preparationPtru catalystBiochemical engineering
The invention relates to 3-chloro-2-chloromethyl propene, a preparation method and application thereof. The preparation method comprises the step that 1, 3-dichloroacetone is subjected to a carbonization reaction under the action of a catalyst to obtain 3-chloro-2-chloromethyl propene. According to the invention, 1, 3-dichloroacetone serves as a raw material and is subjected to a carbonization reaction under the action of a catalyst to obtain a product 3-chloro-2-chloromethyl propene in one step, wherein the raw materials are easy to obtain, the steps are simple, a large amount of wastewater is not generated in the preparation process, the method is environment-friendly, the yield of the final product is high and can reach 51-65%, and the 3-chloro-2-chloromethyl propylene can be applied to the synthesis of bridged ring compounds.
Owner:济南尚博医药股份有限公司
1,3-disubstituted-3-azabicyclo [3,2,1] octane derivatives and preparation method thereof
ActiveCN102452981AChange fat solubilityAlter metabolic performanceOrganic chemistryAntineoplastic agentsChemical structureTert-Butyloxycarbonyl protecting group
The invention relates to 1,3-disubstituted-3-azabicyclo [3,2,1] octane substituted derivates and a preparation method thereof. The invention mainly solves the technical problems that the extension of the existing bicyclic compound with a 3-azabicyclo [3,2,1] octane structure is limited in a spatial structure, thereby being not favorable for quickly screening compound activity and carrying out SAR (structure-activity relationship) analysis. The chemical structural formula of the derivates is as shown in the specification, wherein R1 is a substituted functional group or amino protecting group and is selected from H, C1-C10 linear chains or one of alkyl, benzyl, tertbutyloxycarbonyl, alkanoyl, sulfonyl, carbamide and thiocarbamide containing substituent lateral chains; and G is selected from hydroxyl, C1-C10 linear chains or one of alkoxyl, methylamino and dimethylamino containing substituent lateral chains.
Owner:上海药明康德新药开发有限公司
3-trifluoromethyl-2,5-diazabicyclo[2.2.1] heptane derivant and preparation method thereof
InactiveCN102167702AChange solubilityAlter biological metabolic stabilityOrganic chemistryBulk chemical productionSolubilitySide chain
The invention discloses a 3-trifluoromethyl-2,5-diazabicyclo[2.2.1] heptane derivant and a preparation method thereof, mainly aiming to solve the technical problems that bridged compounds with structure of 2,5-diazabicyclo[2.2.1] heptane are restrained in extension of a space structure, and the compounds have poor water solubility. The chemical structural formula is as follows in the specification, wherein X is NR1 or O, R1 is a functional group or amino-substituted protective group, and is selected from one of H, C1-10 linear-chain or substituent-side-chain-containing alkyl, benzyl, 2,4-dimethoxybenzyl, 4-methoxybenzyl, tertiarybutoxy carbonyl, carboxybenzyl, alkylacyl, aroyl, alkylsulfonyl or aryl sulfonyl, and R2 is a functional group or amino-substituted protective group, and is selected from one of H, C1-10 linear-chain or substituent-side-chain-containing alkyl, benzyl, 2,4-dimethoxybenzyl, 4-methoxybenzyl, carboxybenzyl, alkylacyl, aroyl, alkylsulfonyl or aryl sulfonyl.
Owner:上海药明康德新药开发有限公司
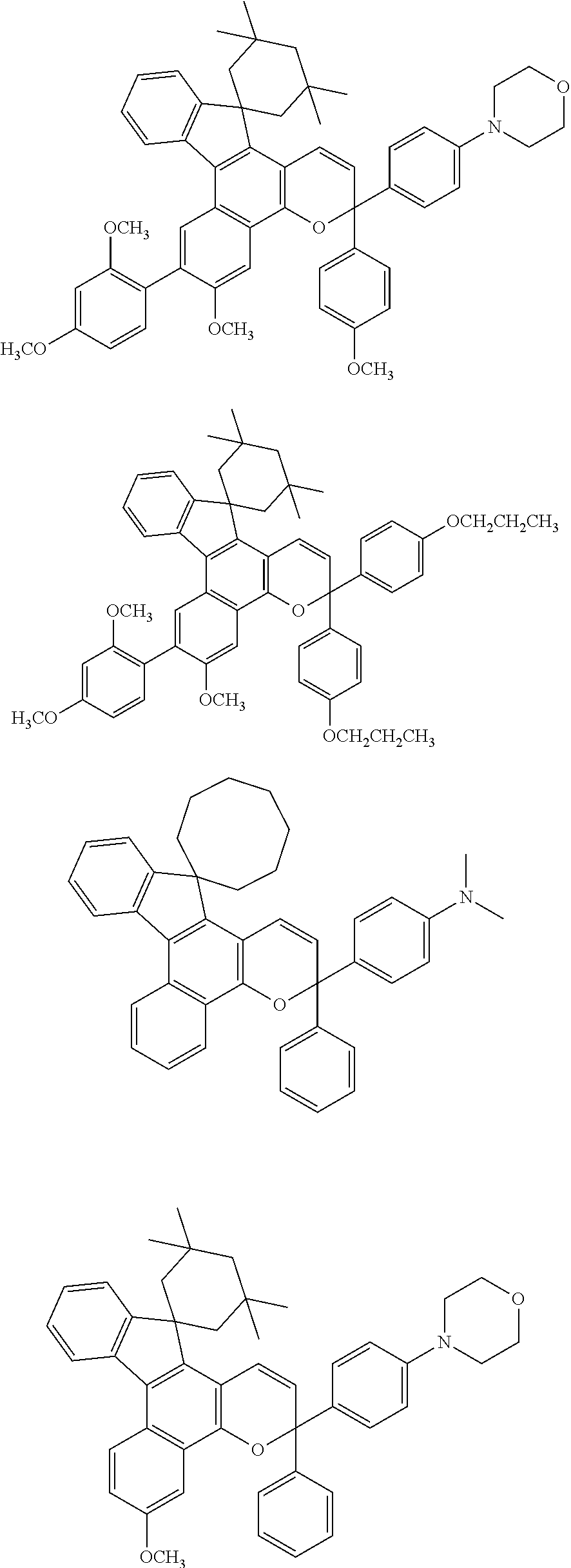
Tunable chiral film optical filter
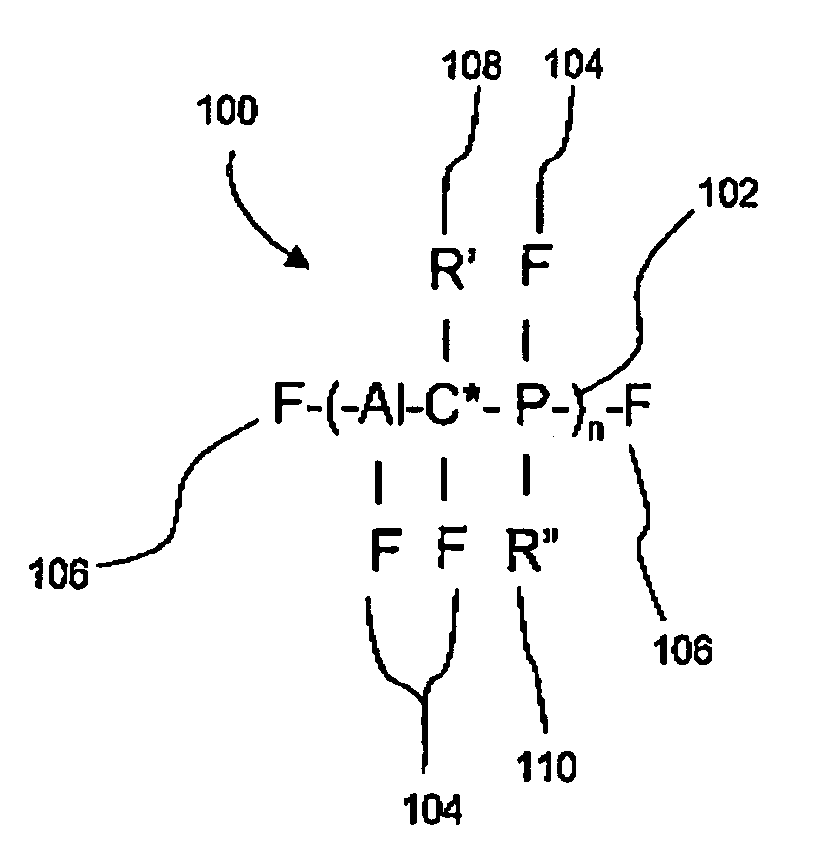
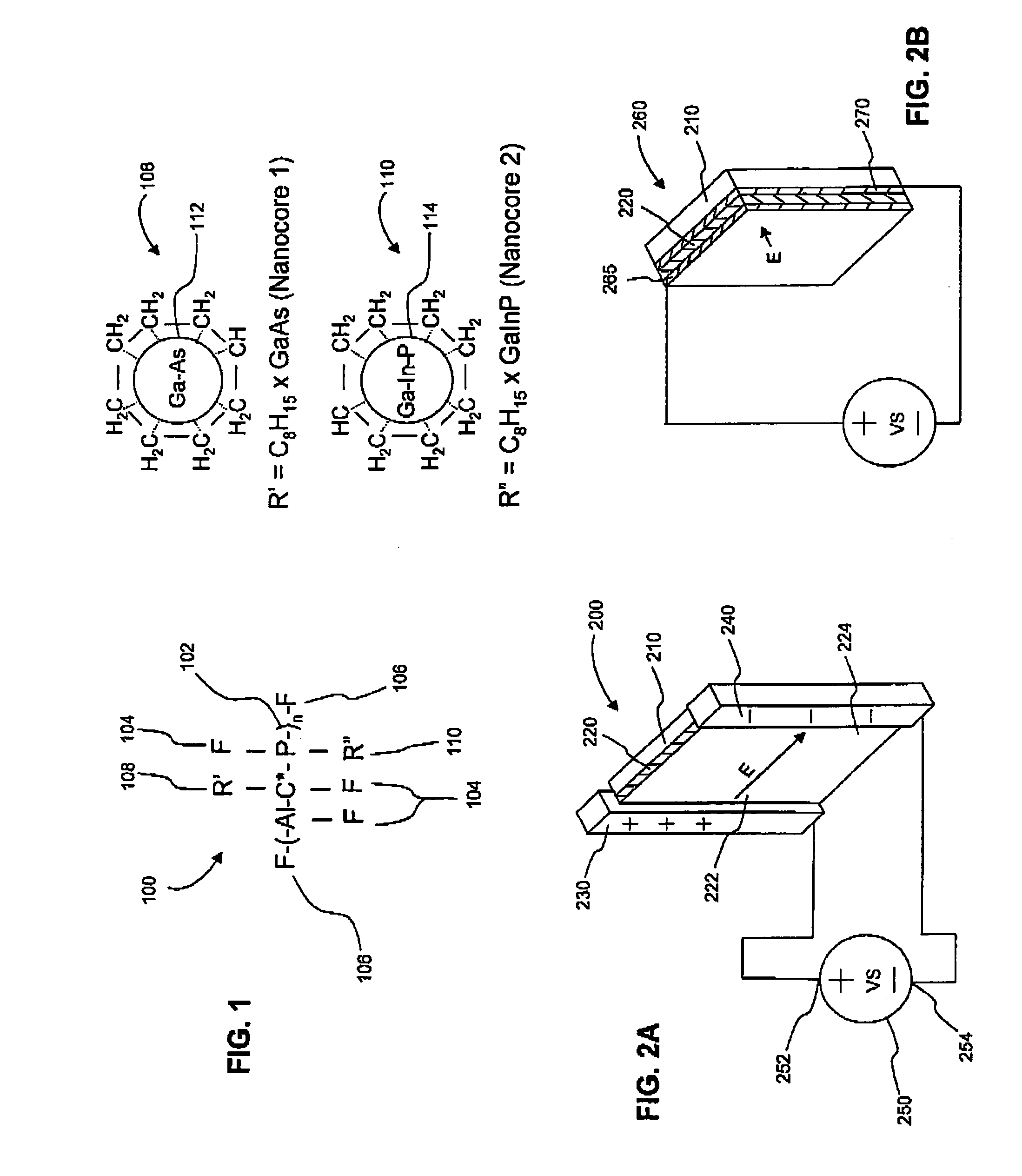
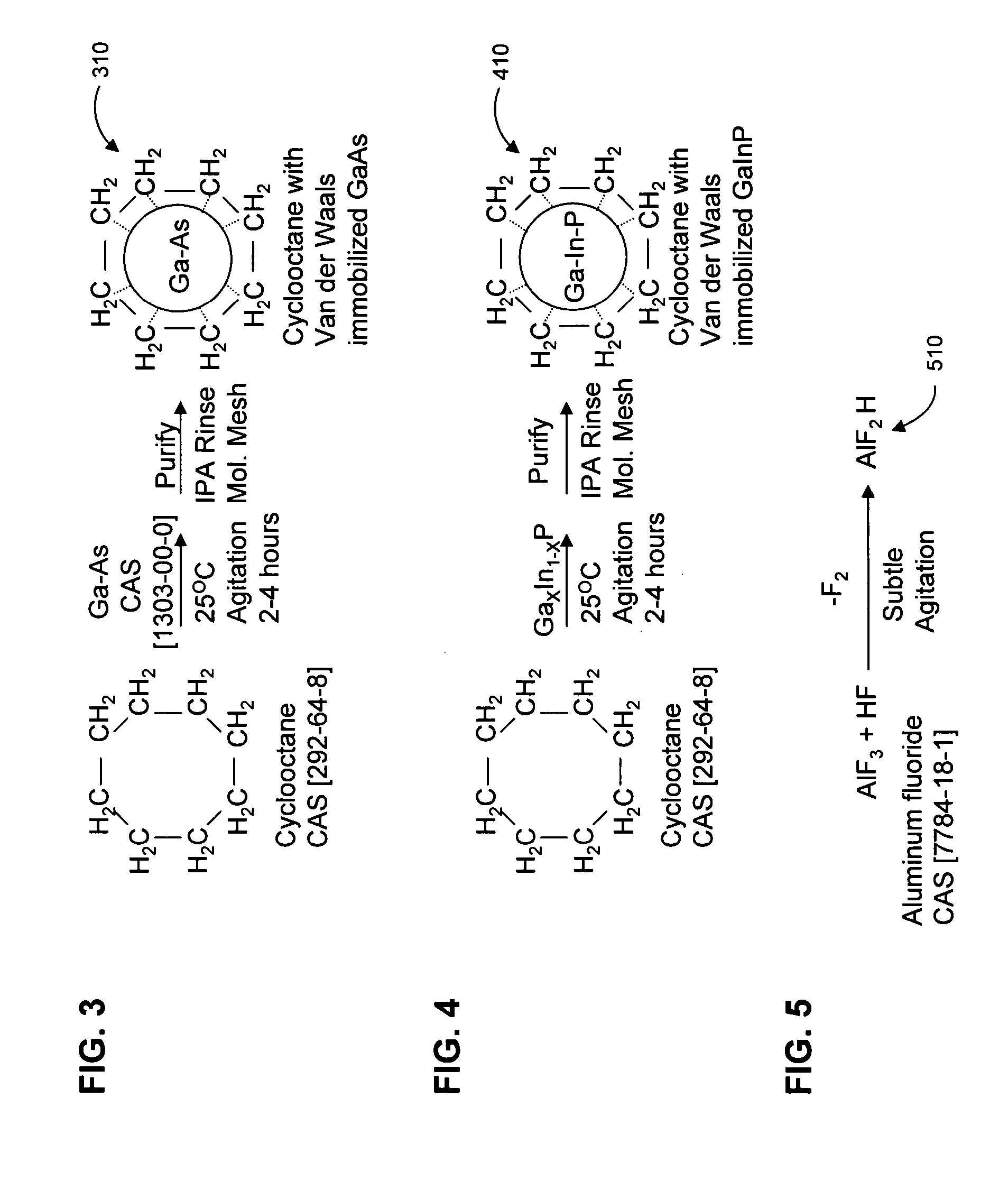
An optically active composition (100) for optical applications has been identified. The optically active composition (100) can include at least one cyclic molecule having a nanocore (112) disposed within the cyclic molecule to form a filled ring (108). The composition (100) is optically transmissive for at least one photonic wavelength that would not otherwise be transmitted by the composition (100) if the nanocore were absent from the cyclic molecule. The cyclic molecule can be a carbon ring, an aromatic ring, or a heterocyclic ring. The filled ring (108) can be attached to a chiral molecule which is a repeat unit (102) in a polymeric backbone. A second filled ring (110) which causes the composition to be optically transmissive at a second wavelength also can be attached to the chiral molecule (102) as well. An electric field can be applied to the filled ring (108) to adjust the wavelength at which filled ring (108) is transmissive.
Owner:HARRIS CORP
Polyrotaxane, production method therefor, and optical composition containing said polyrotaxane
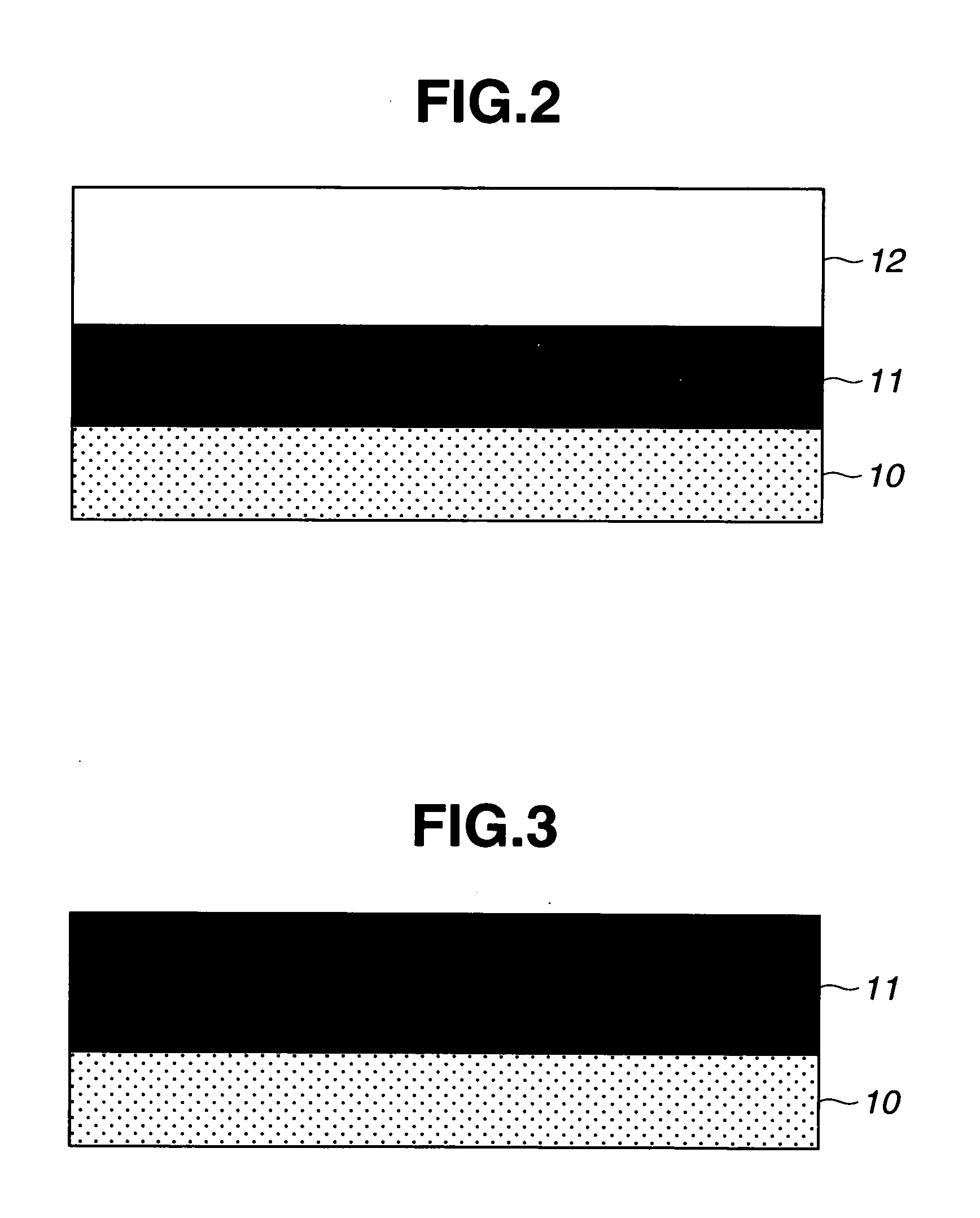
ActiveUS10494488B2Increased durabilityTenebresent compositionsOptical partsPolyrotaxaneChemical composition
The present invention provides an optical composition from which an optical article having reduced poor appearance such as cloudiness and optical strain during lens base material production can be obtained, and when a photochromic compound is added, a photochromic cured body having excellent photochromism and mechanical strength can also be formed, and a polyrotaxane used therefor. The polyrotaxane has a composite molecular structure formed of an axle molecule and a plurality of cyclic molecules clathrating the axle molecule, satisfying at least one of (X) and (Y). (X): A side chain having a secondary or tertiary hydroxyl group is introduced into at least part of the cyclic molecule of the polyrotaxane. (Y): A side chain having a group represented by -A (A is an organic group, and contains at least one hydroxyl group) is introduced into at least part of the cyclic molecule of the polyrotaxane, and a pKa of the hydroxyl group of the compound represented by H-A is 6 or more and less than 14.
Owner:TOKUYAMA CORP
Self-restoring macromolecular material and production method for same
InactiveUS10329386B2Excellent stress relaxationLoss of material propertyPolyrotaxaneStress relaxation
Provided is a self-restoring macromolecular material that not only has excellent stress relaxation but that can also be easily restored to its original state, even when damaged or severed. Also provided is a method for producing the self-restoring macromolecular material. The self-restoring macromolecular material contains a crosslinked structure that is formed by crosslinking a polymer containing at least a polyrotaxane molecule. The polyrotaxane molecule is formed so as to include a cyclic molecule 21 and a linear molecule that passes through an opening 21a of the cyclic molecule. The crosslinked structure 1 is crosslinked via a reversible bond between the cyclic molecule of the polyrotaxane molecule and a polymer molecule other than the polyrotaxane molecule.
Owner:OSAKA UNIV
Preparation of macrocyclic molecule-modified nano silica capillary chromatographic column
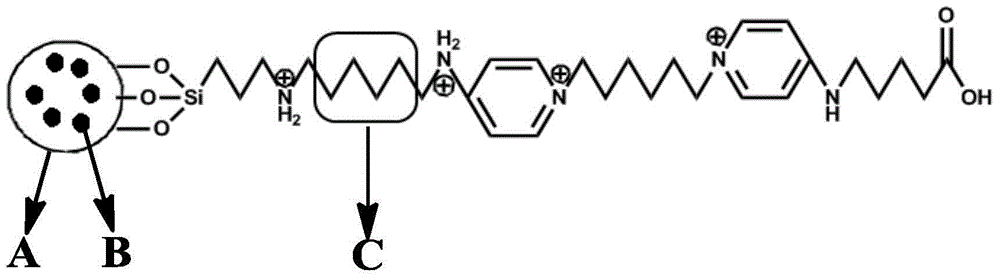
InactiveCN106268711AHigh selectivityEasy to separateIon-exchange process apparatusOther chemical processesInorganic ChemicalNitrogen
The invention belongs to the interdisciplinary field of inorganic chemistry and organic chemistry and discloses preparation of a macrocyclic molecule-modified nano silica capillary chromatographic column. Nano silica is modified first with silylation reagent APTS, macrocyclic molecules are then reacted with dichloride oxalate, separating and purifying are carried out to obtain macrocyclic molecule acyl chloride, and the macrocyclic molecule acyl chloride is then reacted with the modified nano silica to obtain the macrocyclic molecule-modified nano silica; the macrocyclic molecule-modified nano silica is charged into a capillary chromatographic column through a column charging pump, nitrogen flushing is carried out, and the temperature 280 DEG C is held for 12 h to obtain the capillary chromatographic column. The capillary chromatographic column prepared herein has an important application prospect in terms of separation, purification and chiral enantiomer separation.
Owner:ANQING NORMAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
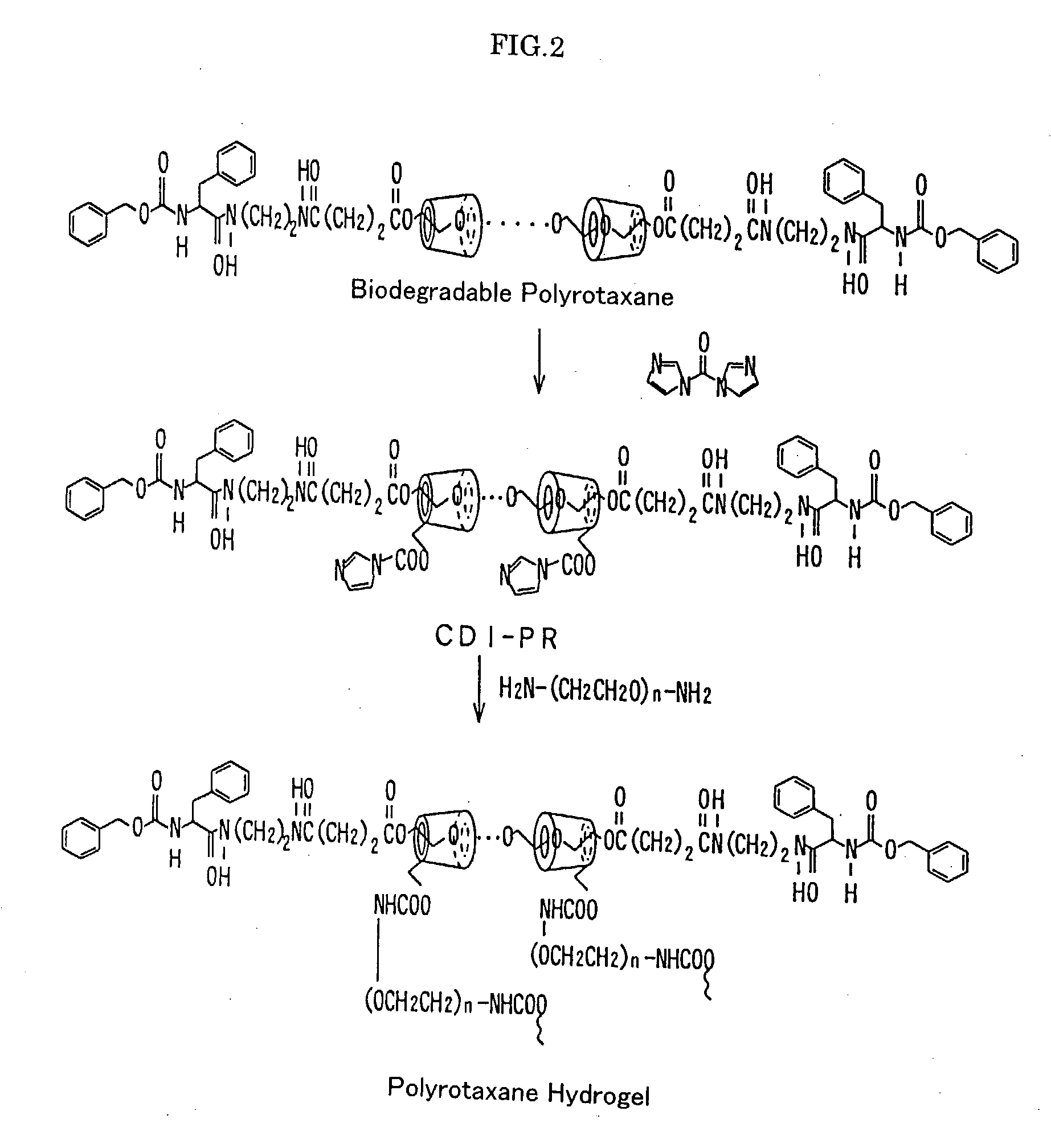



![1,3-disubstituted-3-diazabicyclo[3,3,1] nonane derivative and preparation method thereof 1,3-disubstituted-3-diazabicyclo[3,3,1] nonane derivative and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/05551e65-594a-469a-aa73-c08f67411d63/139211DEST_PATH_IMAGE003.PNG)
![1,3-disubstituted-3-diazabicyclo[3,3,1] nonane derivative and preparation method thereof 1,3-disubstituted-3-diazabicyclo[3,3,1] nonane derivative and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/05551e65-594a-469a-aa73-c08f67411d63/176597DEST_PATH_IMAGE004.PNG)
![1,3-disubstituted-3-diazabicyclo[3,3,1] nonane derivative and preparation method thereof 1,3-disubstituted-3-diazabicyclo[3,3,1] nonane derivative and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/05551e65-594a-469a-aa73-c08f67411d63/201110193553X100001DEST_PATH_IMAGE001.PNG)
![1,3,7-tri-substituted-diazabicyclo[3,3,1] nonane derivative and preparation method thereof 1,3,7-tri-substituted-diazabicyclo[3,3,1] nonane derivative and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2ca5a438-a7bf-443f-9c27-b67801e85408/2011101935544100001DEST_PATH_IMAGE001.PNG)
![1,3,7-tri-substituted-diazabicyclo[3,3,1] nonane derivative and preparation method thereof 1,3,7-tri-substituted-diazabicyclo[3,3,1] nonane derivative and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2ca5a438-a7bf-443f-9c27-b67801e85408/2011101935544100001DEST_PATH_IMAGE003.PNG)
![1,3,7-tri-substituted-diazabicyclo[3,3,1] nonane derivative and preparation method thereof 1,3,7-tri-substituted-diazabicyclo[3,3,1] nonane derivative and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2ca5a438-a7bf-443f-9c27-b67801e85408/2011101935544100001DEST_PATH_IMAGE005.PNG)
![1,3-disubstituted-3-azabicyclo [3,2,1] octane derivatives and preparation method thereof 1,3-disubstituted-3-azabicyclo [3,2,1] octane derivatives and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e2e5d4eb-9e49-410c-a39f-d3e0f35661d4/60869DEST_PATH_IMAGE015.png)
![1,3-disubstituted-3-azabicyclo [3,2,1] octane derivatives and preparation method thereof 1,3-disubstituted-3-azabicyclo [3,2,1] octane derivatives and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e2e5d4eb-9e49-410c-a39f-d3e0f35661d4/71517DEST_PATH_IMAGE013.png)
![1,3-disubstituted-3-azabicyclo [3,2,1] octane derivatives and preparation method thereof 1,3-disubstituted-3-azabicyclo [3,2,1] octane derivatives and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e2e5d4eb-9e49-410c-a39f-d3e0f35661d4/97777DEST_PATH_IMAGE019.png)
![3-trifluoromethyl-2,5-diazabicyclo[2.2.1] heptane derivant and preparation method thereof 3-trifluoromethyl-2,5-diazabicyclo[2.2.1] heptane derivant and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2547f914-1758-41be-bd07-a8060bdb397e/FSA00000037763800011.png)
![3-trifluoromethyl-2,5-diazabicyclo[2.2.1] heptane derivant and preparation method thereof 3-trifluoromethyl-2,5-diazabicyclo[2.2.1] heptane derivant and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2547f914-1758-41be-bd07-a8060bdb397e/FSA00000037763800012.png)
![3-trifluoromethyl-2,5-diazabicyclo[2.2.1] heptane derivant and preparation method thereof 3-trifluoromethyl-2,5-diazabicyclo[2.2.1] heptane derivant and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2547f914-1758-41be-bd07-a8060bdb397e/FSA00000037763800021.png)