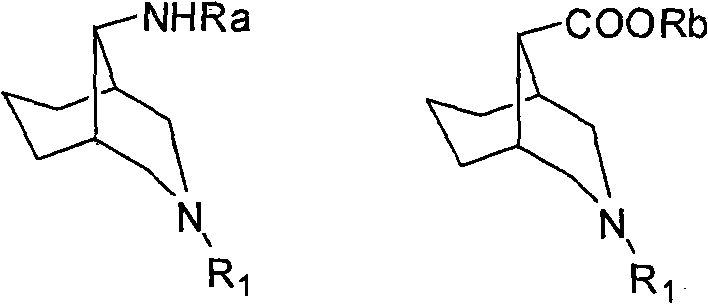
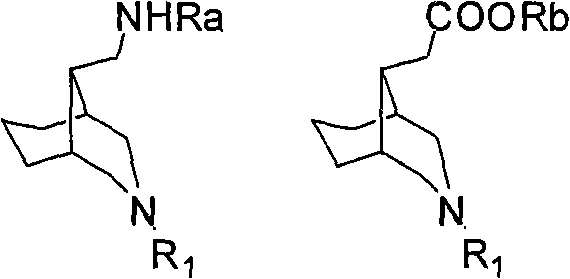
3-azabicyclo (3.3.1) nonane-9-substituted derivative and preparation method thereof
An azabicyclo and derivative technology, which is applied in the field of 3-azabicyclo[3.3.1]nonane-9-substituted derivatives and preparation, can solve the problems of poor water solubility of compounds, limitation of spatial structure extension, etc. Physiological activity, improving selectivity, improving water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
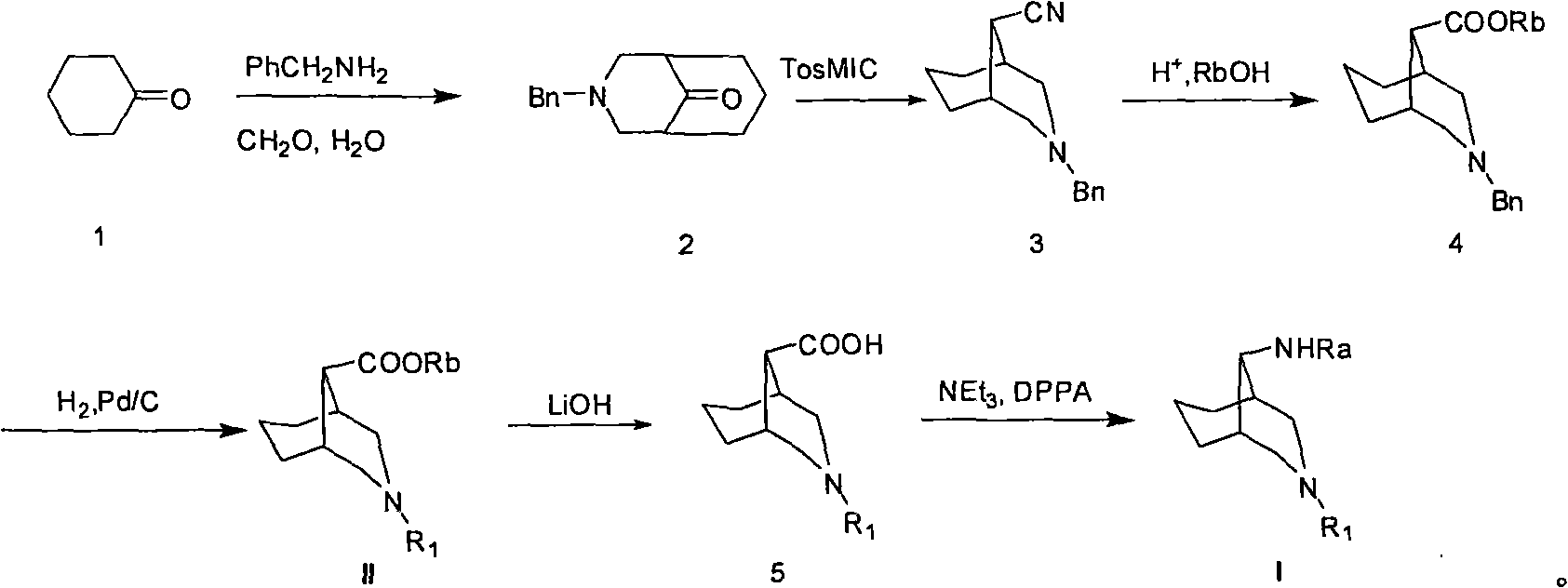
[0058] Example 1: Preparation of 3-benzyl-3-azabicyclo[3.3.1]-9-nonanone
[0059]
[0060] Operation steps: add 210mL (1.6mmol) of benzylamine in a 2L round bottom flask, then add 160mL of concentrated hydrochloric acid under nitrogen protection, add 163mL (1.6mmol) of cyclohexanone, 376mL of 37% formaldehyde to the mixture aqueous solution and 2190 mL of acetic acid. The mixed solution was heated and stirred at 80° C. for two hours, and then concentrated. Diethyl ether and water were added to the residue, the layers were separated, and the aqueous phase was washed with diethyl ether, washed with Na 2 CO 3 The pH of the solid was adjusted to 8, and the mixture was extracted with dichloromethane. The organic phase was dried over anhydrous sodium sulfate and concentrated. The residue was dissolved with 450 mL of ethanol, and 150 mL of acetic anhydride was added. The resulting mixture was stirred for two hours, and 160 mL of concentrated hydrochloric acid was added to the...
Embodiment 2
[0062] Example 2: Preparation of 3-benzyl-9-cyano-3-azabicyclo[3.3.1]nonane
[0063]
[0064] Operation steps: At 0°C, potassium tert-butoxide (81.23g, 725.1mmol) was added to 3-benzyl-3-azabicyclo[3.3.1]-9-nonanone (37.5g, 207.15 mmol), p-toluenesulfonylmethyl isocyanide (72.75 g, 372.9 mmol) and 278 mL of 1,2-dichloroethane were added simultaneously. The mixture was heated at 50°C for 10 hours, and then poured into saturated brine. The mixture was extracted with ethyl acetate and concentrated to give crude product. Crude product is used column chromatography, obtains the 3-benzyl-9-cyano group-3-azabicyclo[3.3.1]nonane (yield 38.1%) of 18.954 grams (wherein petroleum ether / ethyl acetate is used as washing remover).
[0065] HNMR (CDCl3) δ: 7.34 (m, 5H), 3.47 (s, 1H), 3.42 (s, 1H), 3.05 (d, 1H), 2.95 (d, 1H), 2.73 (m, 3H), 2.32 ( dd, J=10.8Hz, J=37.6Hz, 1H), 2.11(m, 3H), 1.85-1.98(m, 1H), 1.52-1.72(m, 3H). MS: 241 (M+1).
Embodiment 3
[0066] Embodiment 3: Preparation of ethyl 3-benzyl-3-azabicyclo[3.3.1]nonane-9-carboxylate
[0067]
[0068] Operation steps: 9-cyano-3-benzyl-3-azabicyclo[3.3.1]nonane (23.954g, 99.8mmol) and 1500mL of HCl gas-saturated ethanol mixture was heated to reflux, and then 12mL of water was added , and the mixture was heated to reflux for 14 hours under nitrogen protection. The reaction mixture was concentrated and washed with saturated NaHCO 3 The solution was adjusted to pH 8. Then dichloromethane and water were added, the layers were separated, and the organic phase was concentrated to obtain a crude product. Column chromatography of the crude product yielded 8.56 g of ethyl 3-benzyl-3-azabicyclo[3.3.1]nonane-9-carboxylate. (Yield 29.9%) (where petroleum ether / ethyl acetate is the eluent).
[0069] HNMR (CDCl3) δ: 7.25 (m, 5H), 3.93-4.19 (m, 2H), 3.28 (d, 2H), 2.90 (d, 1H), 2.66 (m, 2H), 2.04-2.37 (m, 4H) ), 1.31-1.91 (m, 6H), 1.20 (m, 3H). MS: 288 (M+1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com