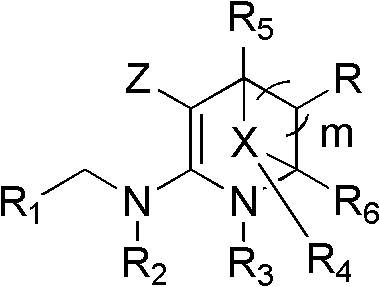
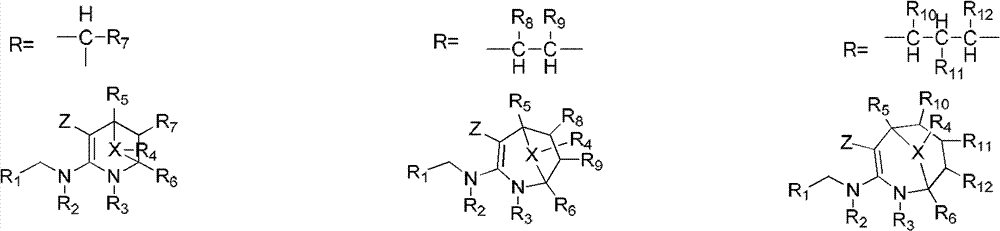
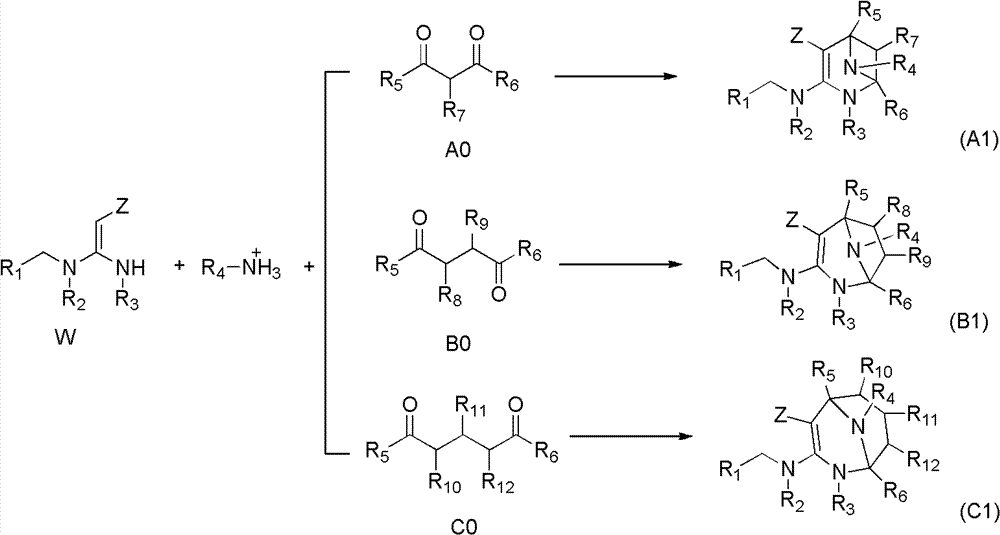
Nitrogen (sulfur) containing bridge ring compound with insecticidal activity, preparation method and application
A compound and carbonyl technology, applied in the field of nitrogen-containing bridged ring neonicotinoid insecticides, can solve the problems of limited drug selectivity and narrow insecticidal spectrum, achieve significant insecticidal activity, expand insecticidal spectrum, and improve insecticidal activity. The effect of insect activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0106] The preparation method of the compound of the present invention
[0107] The compound represented by the general formula 1 of the present invention can be prepared by the following method, but the conditions of the method, such as reactants, solvents, bases, the amount of the compound used, reaction temperature, reaction time required, etc. are not limited to the following explanations. The compound of the present invention can also be conveniently prepared by optionally combining various synthetic methods described in the specification or known in the art. Such a combination can be easily performed by those skilled in the art to which the present invention belongs.
[0108] The acid used in the reaction is protonic acid or Lewis acid, including (but not limited to): hydrochloric acid, acetic acid, sodium dihydrogen phosphate, p-toluenesulfonic acid, trifluoroacetic acid, boron trifluoride, aluminum trichloride, trichloro Iron chloride, magnesium chloride, cobalt chlo...
Embodiment 1-44
[0141] Example 1-44: Preparation of heterobridged ring neonicotinoid compounds
Embodiment 1
[0142] Example 1 1-((6-chloropyridin-3-yl)methyl)-8-nitro-1,2,3,5,6,7-hexahydro-5,7-cycloimidoimidazo[ 1,2-a] the synthesis of pyridine (compound A-1):
[0143]
[0144] 1.270g (5.0mmol) of 2-chloro-5-((2-(nitromethylene) imidazolin-1-yl) methyl) pyridine and 0.531g (10.0mmol) of ammonium chloride were added to 20ml Acetonitrile, slowly drop 0.360g (5.0mmol) of malondialdehyde. After reacting for 24 hours, the solvent was removed, and the pure product was obtained as light yellow powder through column chromatography separation, with a yield of 42%. 1 H NMR (400MHz, DMSO-d 6 )δ8.71(d, J=2.9Hz, 1H), 8.05(dd, J 1 =8.0Hz,J 2 =2.0Hz, 1H), 7.34(d, J=8.0Hz, 1H), 4.84(s, 2H), 4.00(t, J=7.6Hz, 1H), 3.90(t, J=9.5Hz, 1H), 3.04(m, 4H), 2.41(m, 1H), 1.84(m, 1H)ppm; 13 C NMR (100MHz, DMSO-d 6 ): δ150.02, 149.48, 148.61, 138.84, 130.35, 123.76, 54.68, 50.86, 50.62, 37.44ppm; HRMS(ES+)C 13 h 14 35 ClN 5 o 2 (M+H) + , calculated value: 308.0836; measured value: 308.0756; C 13 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com