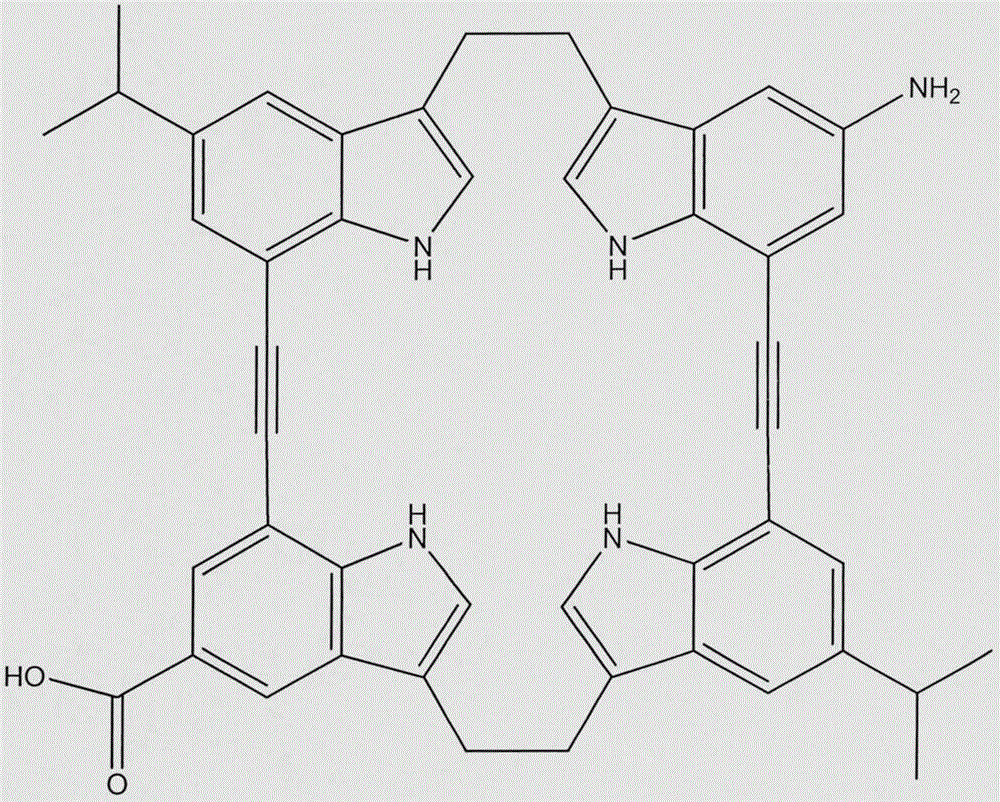
Preparation of macrocyclic molecule-modified nano silica capillary chromatographic column
A nano-silica, capillary chromatographic column technology, applied in ion exchange, other chemical processes, ion-exchange regeneration, etc., can solve the problems of reports on the preparation of nano-silica capillary chromatographic columns without macrocyclic molecules, and achieve separation. Simple and convenient, good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] 1. Weigh 5.0g of nano-silica (40-60nm) into a glass sand core funnel, rinse with 1.0mol / L NaOH, deionized water, 1.0mol / L HCl, and high-purity water for 20 minutes each, and take it out at 110°C Dry for 10h. Then add 42mL of silylating reagent 3-aminopropyltriethoxysilane (APTS) methanol solution (1:1, V / V), and stir electromagnetically at a constant temperature of 55°C for 24h.
[0015] 2. Acylation modification of macrocyclic molecule (MCM): Weigh 3.42g macrocyclic molecule into a 150mL round-bottomed distillation flask, add 30mL absolute ethanol to dissolve it, then add 1.5mL dichlorooxalic acid, 1 drop of N , N-dimethylformamide, electromagnetic stirring (200 rpm) reflux for 4 hours, cooled to 0 ° C, filtered, washed with absolute ethanol to obtain macrocyclic acid chlorides.
[0016] 3. Add 3.0 g of macrocyclic molecular acid chloride, 20 mL of absolute ethanol, and 1 drop of glacial acetic acid to the product of step 1, and stir electromagnetically (rotating at 2...
Embodiment 2
[0018] 1. Weigh 10g of nano-silica (40-60nm) into a glass sand core funnel, wash with 1.0mol / L NaOH, deionized water, 1.0mol / L HCl, and high-purity water for 20 minutes each, and take it out at 110°C Dry for 10h. Then add 42mL of silylating reagent 3-aminopropyltriethoxysilane (APTS) methanol solution (1:1, V / V), and stir electromagnetically at a constant temperature of 55°C for 24h.
[0019] 2. Acylation modification of macrocyclic molecule (MCM): Weigh 6.84g of macrocyclic molecule and put it into a 150mL round-bottomed distillation flask, add 60mL absolute ethanol to dissolve it, then add 3.0mL dichlorooxalic acid, 1 drop of N , N-dimethylformamide, electromagnetic stirring (200 rpm) reflux for 4 hours, cooled to 0 ° C, filtered, washed with absolute ethanol to obtain macrocyclic acid chlorides.
[0020] 3. Add 6.0 g of macrocyclic acid chloride, 40 mL of absolute ethanol, and 1 drop of glacial acetic acid to the product of step 1, and stir it electromagnetically (rotating...
Embodiment 3
[0022] 1. Weigh 20g of nano-silica (40-60nm) into a glass sand core funnel, rinse with 1.0mol / L NaOH, deionized water, 1.0mol / LHCl, and high-purity water for 20 minutes, take it out and dry it at 110°C 10h. Then add 42mL of silylating reagent 3-aminopropyltriethoxysilane (APTS) methanol solution (1:1, V / V), and stir electromagnetically at a constant temperature of 55°C for 24h.
[0023] 2. Acylation modification of macrocyclic molecule (MCM): Weigh 13.68g of macrocyclic molecule and put it into a 150mL round bottom distillation flask, add 120mL absolute ethanol to dissolve it, then add 6.0mL dichlorooxalic acid, 1 drop of N , N-dimethylformamide, electromagnetic stirring (200 rpm) reflux for 4 hours, cooled to 0 ° C, filtered, washed with absolute ethanol to obtain macrocyclic acid chlorides.
[0024] 3. Add 12.0 g of macrocyclic molecular acid chloride, 40 mL of absolute ethanol, 1 drop of glacial acetic acid to the product of step 1, and stir electromagnetically (rotating a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com