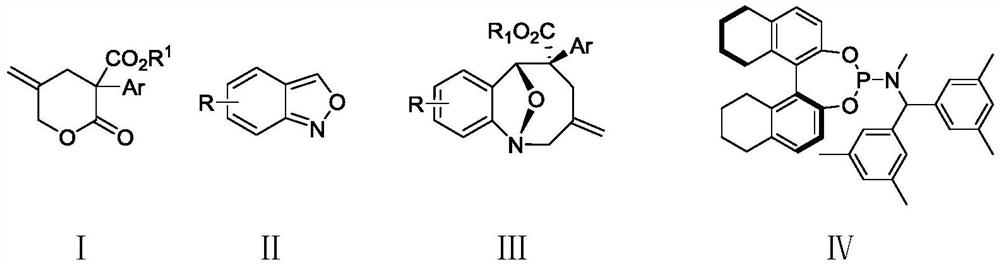
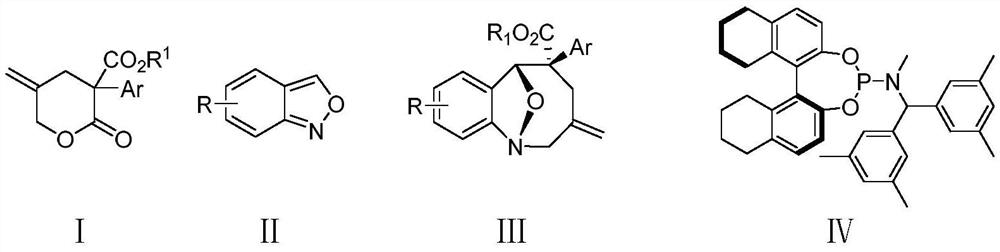
Method for synthesizing eight-membered bridged ring compound through palladium-catalyzed asymmetric cycloaddition reaction
A compound and cycloaddition technology, applied in the fields of compounds of Group 5/15 elements of the periodic table, chemical instruments and methods, catalysts for physical/chemical processes, etc., to achieve the effects of high efficiency, low price, and a wide range of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] (1R,5S,6R)-9-Bromo-3-methylene-5-phenyl-3,4,5,6-tetrahydro-2H-1,6-epoxybenzo[b]azacyclocycle Synthesis of Octene-5-carboxylate (Ⅲaa)
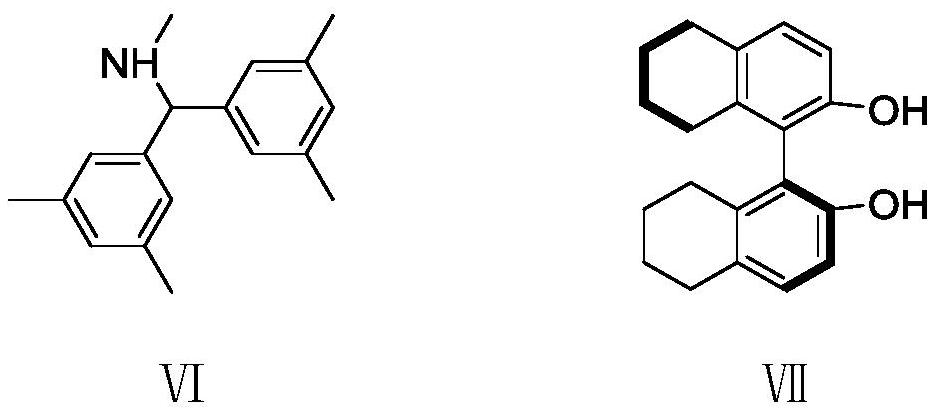
[0046] Under a nitrogen atmosphere, add 5-methylene-2-oxo-3-phenyltetrahydro-2H-pyran-3-carboxylic acid methyl ester (Ia) (59 mg) into a 5 mL round bottom flask, 6-bromo Benzo[c]isoxazole (IIa) (39.4 mg), palladium catalyst (Pd 2 (dba) 3 · CHCl 3 ) (5.2mg), chiral ligand IV (11.5mg) and triethylboron (THF solution of 1mol / L triethylboron, 40uL), then add p-xylene 0.6mL, then stir at 10°C Reacted for 40h; the reacted crude product was directly separated and purified by column chromatography (eluent: ethyl acetate:petroleum ether=1:10~1:30, v / v) to obtain a white solid (1R, 5S, 6R)- 9-Bromo-3-methylene-5-phenyl-3,4,5,6-tetrahydro-2H-1,6-epoxybenzo[b]azacycloctene-5-carboxylate (Ⅲaa) 65mg, yield 82%, ee value 95%.
[0047] The reaction scheme of the present embodiment is as follows:
[0048]
[0049] The characterization data of t...
Embodiment 2
[0056] (1R,5S,6R)-3-Methylene-5-phenyl-3,4,5,6-tetrahydro-2H-1,6-epoxybenzo[b]azacycloctene-5 -Synthesis of carboxylate (Ⅲab)
[0057] Under a nitrogen atmosphere, add 5-methylene-2-oxo-3-phenyltetrahydro-2H-pyran-3-carboxylic acid methyl ester (Ia) (59 mg) into a 5 mL round bottom flask, benzo[ c] isoxazole (Ⅱb) (24mg), palladium catalyst (Pd 2 (dba) 3 · CHCl 3 ) (5.2mg), chiral ligand IV (11.5mg) and triethylboron (THF solution of 1mol / L triethylboron, 40uL), then add p-xylene 0.6mL, then stir at 10°C Reacted for 40h; the reacted crude product was directly separated and purified by column chromatography (eluent: ethyl acetate:petroleum ether=1:10~1:30, v / v) to obtain a white solid (1R, 5S, 6R)- 3-Methylene-5-phenyl-3,4,5,6-tetrahydro-2H-1,6-epoxybenzo[b]azacycloctene-5-carboxylate (Ⅲab) 60mg , yield 80%, ee value 93%.
[0058] The reaction scheme of the present embodiment is as follows:
[0059]
[0060] The characterization data of the resulting product (Ⅲab) are ...
Embodiment 3
[0067] (1R,5S,6R)-9-Bromo-5-(4-methoxyphenyl)-3-methylene-3,4,5,6-tetrahydro-2H-1,6-epoxybenzene Synthesis of a[b]azacyclooctene-5-carboxylate methyl ester (Ⅲba)
[0068] Under a nitrogen atmosphere, add 3-(4-methoxyphenyl)-5-methylene-2-oxytetrahydro-2H-pyran-3-carboxylic acid methyl ester (i.e. Ib) into a 5mL round bottom flask ( 66mg), 6-bromobenzo[c]isoxazole (Ⅱa) (39.4mg), palladium catalyst (Pd 2 (dba) 3 · CHCl 3 ) (5.2mg), chiral ligand IV (11.5mg) and triethylboron (THF solution of 1mol / L triethylboron, 40uL), then add p-xylene 0.6mL, then stir at 10°C Reacted for 40h; the reacted crude product was directly separated and purified by column chromatography (eluent: ethyl acetate:petroleum ether=1:10~1:30, v / v) to obtain a white solid (1R, 5S, 6R)- 9-Bromo-5-(4-methoxyphenyl)-3-methylene-3,4,5,6-tetrahydro-2H-1,6-epoxybenzo[b]azepine Methyl ene-5-carboxylate (Ⅲba) 75 mg, yield 74%, ee value 94%.
[0069] The reaction scheme of the present embodiment is as follows: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com