Chiral 2, 3-disubstituted indoleamine compound and preparation method thereof
A two-substituted, compound technology, applied in the field of organic chemical pharmaceutical intermediates, can solve problems such as unfavorable industrialization promotion, and achieve the effects of mild conditions, environmental friendliness and simple process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
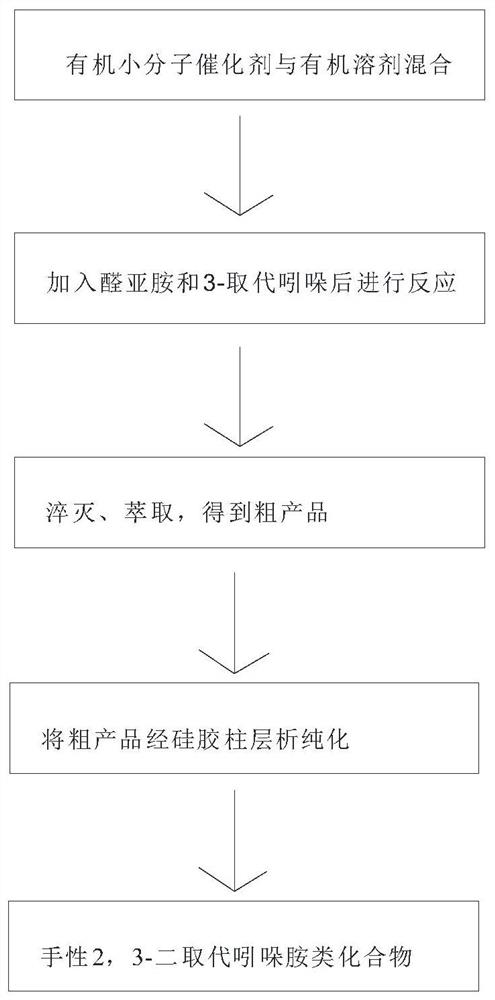
[0041] The synthesis method includes the following steps: mixing an organic small molecule catalyst chiral bissulfonimide and an organic solvent, stirring uniformly, then adding aldimine and 3-substituted indole in sequence, and performing the reaction while stirring until the reaction ends, and the steps are carried out in sequence. Quenching and extraction to obtain a crude product, which is purified by silica gel column chromatography to obtain the chiral disubstituted indoleamines, wherein,
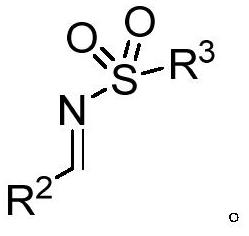
[0042] The structure of aldimine is
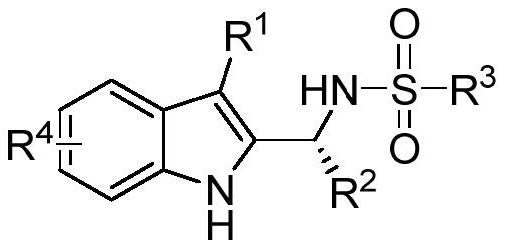
[0043] The structure of 3-substituted indole is
[0044] R 1 is selected from aryl or C1-C8 alkyl, R 2 One of the alkyl or aryl groups selected from C1-C5, R 3 One selected from alkyl or aryl, R 4 Selected from C1-C2 alkyl or halogen.
[0045] The general reaction equation of the present invention is as follows:
[0046]
[0047] The structural formula of chiral bissulfonimide is as follows:
[0048]
[0049] where the R 5 One se...
Embodiment 1
[0052]
[0053] Under nitrogen protection, imine II-a (30.1 mg, 0.1 mmol) and catalyst IV-a (82 mg, 0.01 mmol) were placed in a 10 mL dry reaction tube, 1.0 mL of anhydrous toluene was injected through a syringe, and 3- Substituted indole I-a (26.2 mg, 0.2 mmol) was heated to 35 °C for 2 h. After the reaction was completed, 1.0 mL of water was added to quench, extracted with ethyl acetate three times, washed with saturated brine, and the organic phase was washed with anhydrous Na 2 SO 4 After drying, concentration, the crude product was purified by column chromatography to obtain product 1, namely III-a (32.5 mg, 75%), 1 H NMR (400MHz, CDCl 3)δ8.00(br,s,1H),7.63-7.53(m,2H),7.42(d,J=7.7Hz,1H),7.18-7.24(m,6H),7.15-7.03(m,3H) , 5.87(q, J=7.0Hz, 2H), 2.02(s, 3H), 1.22(s, 9H). 13 CNMR (101MHz, CDCl 3 )δ156.4, 138.8, 136.4, 135.7, 131.3, 128.7, 128.6, 127.9, 127.0, 126.8, 125.6, 122.2, 119.3, 118.7, 110.9, 109.5, 53.9, 35.0, 31.0, 8.2. HPLC analysis conditions: Daicel CHIR c...
Embodiment 2
[0068]
[0069] The difference from Example 1 is: the imine II-a of the tert-butyl substituted substrate used is 4-bromo-substituted imine II-b (0.1mmol, 32.4mg), other reaction conditions and operation steps and implementation Same as Example 1, white solid product III-b (30.0 mg, 68%) was obtained, 1 H NMR (400MHz, CDCl 3 )δ7.83(s,1H),7.46(d,J=7.6Hz,1H),7.41(d,J=8.6Hz,2H),7.29-7.21(m,7H),7.19-7.06(m,3H) ), 5.91(s, 1H), 5.72(br, s, 1H), 2.10(s, 3H). 13 C NMR (101MHz, CDCl 3 )δ138.6, 138.213, 135.6, 131.7, 130.6, 128.9, 128.5, 128.2, 128.2, 127.6, 127.0, 122.7, 119.6, 118.8, 110.8, 110.1, 54.1, 8.4. HPLC analysis conditions: DaicelCHIRALPAK AD-H column, 25nm, 4nm -hexane / i-PrOH=90 / 10, 1.0 mL / min, 12.54 min (minor), 17.66 min (major), 90% ee.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com