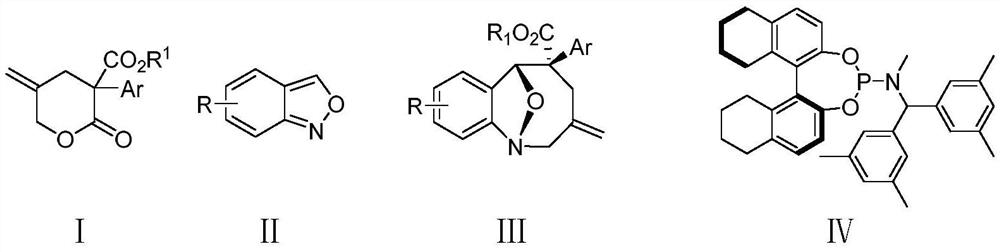
A kind of method for synthesizing eight-membered bridged ring compound by palladium-catalyzed asymmetric cycloaddition reaction
A compound and cycloaddition technology, which is applied to compounds of Group 5/15 elements of the periodic table, chemical instruments and methods, physical/chemical process catalysts, etc., to achieve the effects of low cost, cheap and easy-to-obtain reaction raw materials, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] (1R,5S,6R)-9-Bromo-3-methylene-5-phenyl-3,4,5,6-tetrahydro-2H-1,6-epoxybenzo[b]azacycle Synthesis of Octene-5-carboxylate (Ⅲaa)
[0046] Under nitrogen atmosphere, to a 5 mL round bottom flask was added methyl 5-methylene-2-oxo-3-phenyltetrahydro-2H-pyran-3-carboxylate (Ia) (59 mg), 6-bromo Benzo[c]isoxazole (IIa) (39.4mg), palladium catalyst (Pd 2 (dba) 3 ·CHCl 3 ) (5.2mg), chiral ligand IV (11.5mg) and triethylboron (1mol / L triethylboron in THF solution, 40uL), then added p-xylene 0.6mL, then stirred at 10°C The reaction was carried out for 40h; the crude product after the reaction was directly separated and purified by column chromatography (the eluent was ethyl acetate: petroleum ether=1:10~1:30, v / v) to obtain a white solid (1R, 5S, 6R)- 9-Bromo-3-methylene-5-phenyl-3,4,5,6-tetrahydro-2H-1,6-epoxybenzo[b]azetioctene-5-carboxylate (IIIaa) 65mg, yield 82%, ee value 95%.
[0047] The reaction scheme of the present embodiment is as follows:
[0048]
[0049] ...
Embodiment 2
[0056] (1R,5S,6R)-3-methylene-5-phenyl-3,4,5,6-tetrahydro-2H-1,6-epoxybenzo[b]azetioctene-5 -Synthesis of Carboxylic Ester (IIIab)
[0057] Under nitrogen atmosphere, to a 5 mL round bottom flask was added methyl 5-methylene-2-oxo-3-phenyltetrahydro-2H-pyran-3-carboxylate (Ia) (59 mg), benzo[ c] Isoxazole (IIb) (24 mg), palladium catalyst (Pd 2 (dba) 3 ·CHCl 3 ) (5.2mg), chiral ligand IV (11.5mg) and triethylboron (1mol / L triethylboron in THF solution, 40uL), then added p-xylene 0.6mL, then stirred at 10°C The reaction was carried out for 40h; the crude product after the reaction was directly separated and purified by column chromatography (the eluent was ethyl acetate: petroleum ether=1:10~1:30, v / v) to obtain a white solid (1R, 5S, 6R)- 3-Methylene-5-phenyl-3,4,5,6-tetrahydro-2H-1,6-epoxybenzo[b]azepin-5-carboxylate (IIIab) 60mg , the yield is 80%, and the ee value is 93%.
[0058] The reaction scheme of the present embodiment is as follows:
[0059]
[0060] The c...
Embodiment 3
[0067] (1R,5S,6R)-9-Bromo-5-(4-methoxyphenyl)-3-methylene-3,4,5,6-tetrahydro-2H-1,6-epoxybenzene Synthesis of Methyl [b]azacyclooctene-5-carboxylate (Ⅲba)
[0068] Under nitrogen atmosphere, add 3-(4-methoxyphenyl)-5-methylene-2-oxytetrahydro-2H-pyran-3-carboxylate methyl ester (i.e. Ib)( 66mg), 6-bromobenzo[c]isoxazole (IIa) (39.4mg), palladium catalyst (Pd 2 (dba) 3 ·CHCl 3 ) (5.2mg), chiral ligand IV (11.5mg) and triethylboron (1mol / L triethylboron in THF solution, 40uL), then added p-xylene 0.6mL, then stirred at 10°C The reaction was carried out for 40h; the crude product after the reaction was directly separated and purified by column chromatography (the eluent was ethyl acetate: petroleum ether=1:10~1:30, v / v) to obtain a white solid (1R, 5S, 6R)- 9-Bromo-5-(4-methoxyphenyl)-3-methylene-3,4,5,6-tetrahydro-2H-1,6-epoxybenzo[b]azepin Methyl alkene-5-carboxylate (IIIba) 75 mg, yield 74%, ee value 94%.
[0069] The reaction scheme of the present embodiment is as follo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com