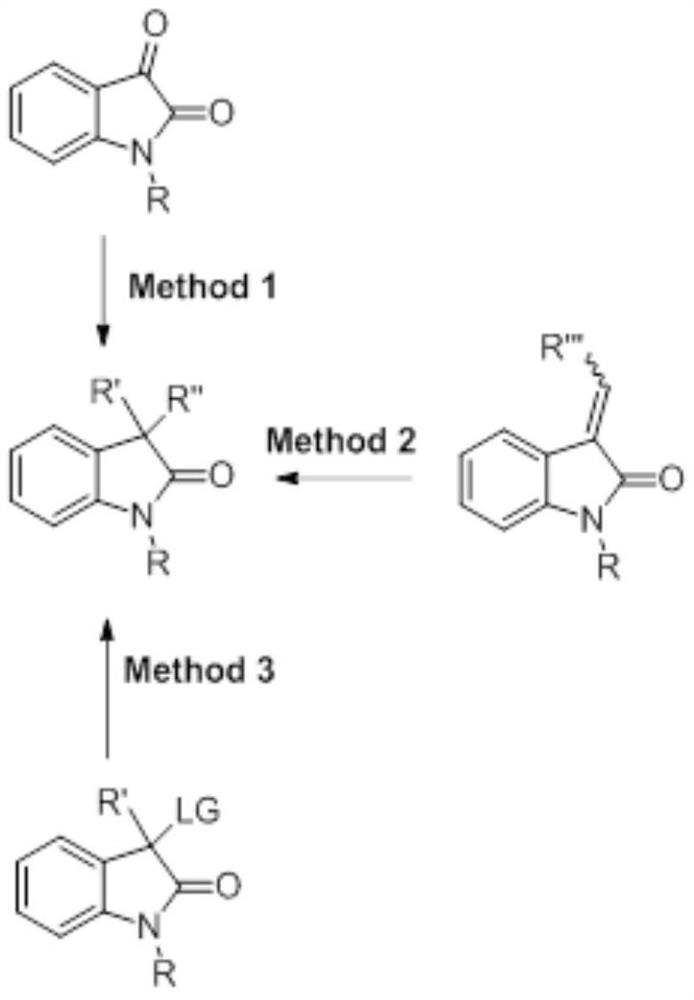
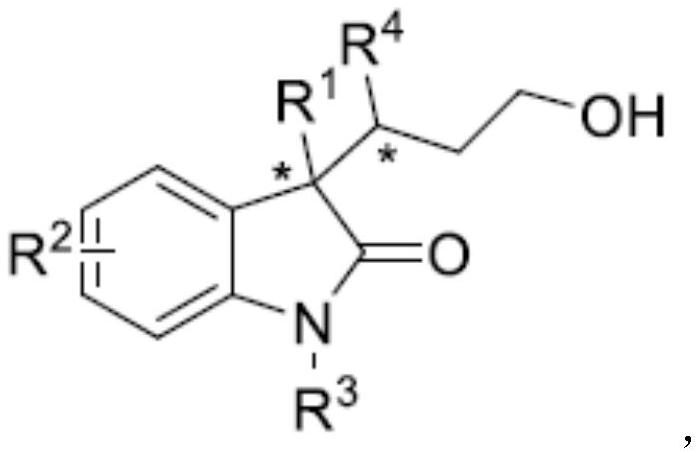
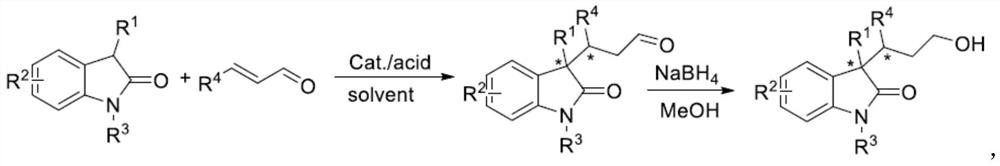
A kind of synthetic method of chiral 3,3-disubstituted indol-2-one derivatives
A synthetic method and a disubstitution technique, which are applied in the field of synthesis of chiral 3,3-disubstituted indol-2-one derivatives, can solve the problems of insufficient universality of substrates and insufficient enantioselectivity. Achieve the effects of cheap and easy-to-obtain reaction reagents, high enantioselectivity, and wide adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1
[0032]
[0033] Add catalyst V-a (9.56 mg, 0.04 mmol), trichloroacetic acid (6.56 mg, 0.04 mmol), 3-methylindol-2-one I-a (29.4 mg, 0.2 mmol), trans -2-butenal II-a (42mg, 0.6mmol), toluene (0.4mL), stirred at room temperature for 18h, quenched with water, extracted 3 times with dichloromethane, washed with saturated brine, and dried the organic layer with anhydrous sodium sulfate , after concentration, add 0.8mL methanol, add NaBH in batches under ice-cooling 4 (15.2mg, 0.4mmol), reacted for 30min, added 1mL saturated ammonium chloride solution, extracted 3 times with ethyl acetate, washed with saturated sodium chloride, dried the organic layer with anhydrous sodium sulfate, concentrated, and the crude product was purified by column chromatography Product 1, IV-a (39.0 mg, 89%) was obtained, 1 HNMR (400MHz, CDCl3) δ8.60 (s, 1H), 7.24-7.14 (m, 2H), 7.02 (t, J = 8.0Hz, 1H), 6.91.
[0034] (d,J=8.0Hz,1H),3.72(m,1H),3.59(m,1H),2.15–2.03(m,1H),1.90–1.82 (...
Embodiment 2
[0050]
[0051] The difference from Example 1 is that the substrate used to replace trans-2-butenal is trans-2-hexenal II-b (0.6mmol, 59mg), other reaction conditions and operating steps and examples 1, the white solid product IV-b (33.1 mg, 67%) was obtained, 1 H NMR (400MHz, CDCl3) δ8.15(s, 1H), 7.21(t, J=8.0Hz, 2H), 7.02(t, J=8.0Hz, 1H), 6.89(d, J=4.0Hz, 1H ),3.70–3.65(m,1H),3.61–3.54(m,1H),1.93-1.89(m,2H),1.60–1.50(m,1H),1.42(s,3H),1.32-1.26(m ,3H), 1.11-1.04(m,1H),0.81(t,J=4.0Hz,3H).13C NMR(100MHz,CDCl3)δ182.9,140.3,134.3,127.7,123.6,122.4,109.6,62.4,52.0, 41.5, 33.9, 33.6, 22.5, 21.7, 14.4. HPLC analysis conditions: Daicel CHIRALPAK ICcolumn, 254nm, n-hexane / i-PrOH=85 / 15, 1.0 ml / min, 10.4min(minor), 18.8min(major) , 82% ee.
Embodiment 3
[0053]
[0054] The difference from Example 1 is that the substrate used to replace 3-methylindolin-2-one is 3-benzylindolin-2-one I-b (0.2mmol, 44.6mg), and other reactions The conditions and operating steps were the same as in Example 1, and the white solid product IV-d (44.9 mg, 76%) was obtained, 1H NMR (400MHz, CDCl3) δ7.46 (s, 1H), 7.30 (d, J=8.0Hz, 1H),7.14-7.09(m,1H),7.04(dd,J=8.0,4.0Hz,1H),7.02–6.96 (m,3H),6.82(dd,J=8.0,4.0Hz,2H),6.60 (d,J=8.0Hz,1H),3.79-3.73(m,1H), 3.67-3.61(m,1H),3.21(q,J=12.0Hz,2H),2.31-2.20(m,1H), 2.02–1.93(m,1H), 1.55-1.46(m,1H),0.92(d,J=8.0Hz,3H).13C NMR(100MHz,CDCl 3 )δ180.4, 141.0, 136.1, 130.8, 129.9, 127.8, 127.5, 126.2, 124.4, 121.9, 109.1, 61.2, 58.3, 41.4, 36.9, 34.2, 14.4. PrOH=82 / 18, 1.0ml / min, 7.5min(minor), 15.0min(major), 86%ee.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com