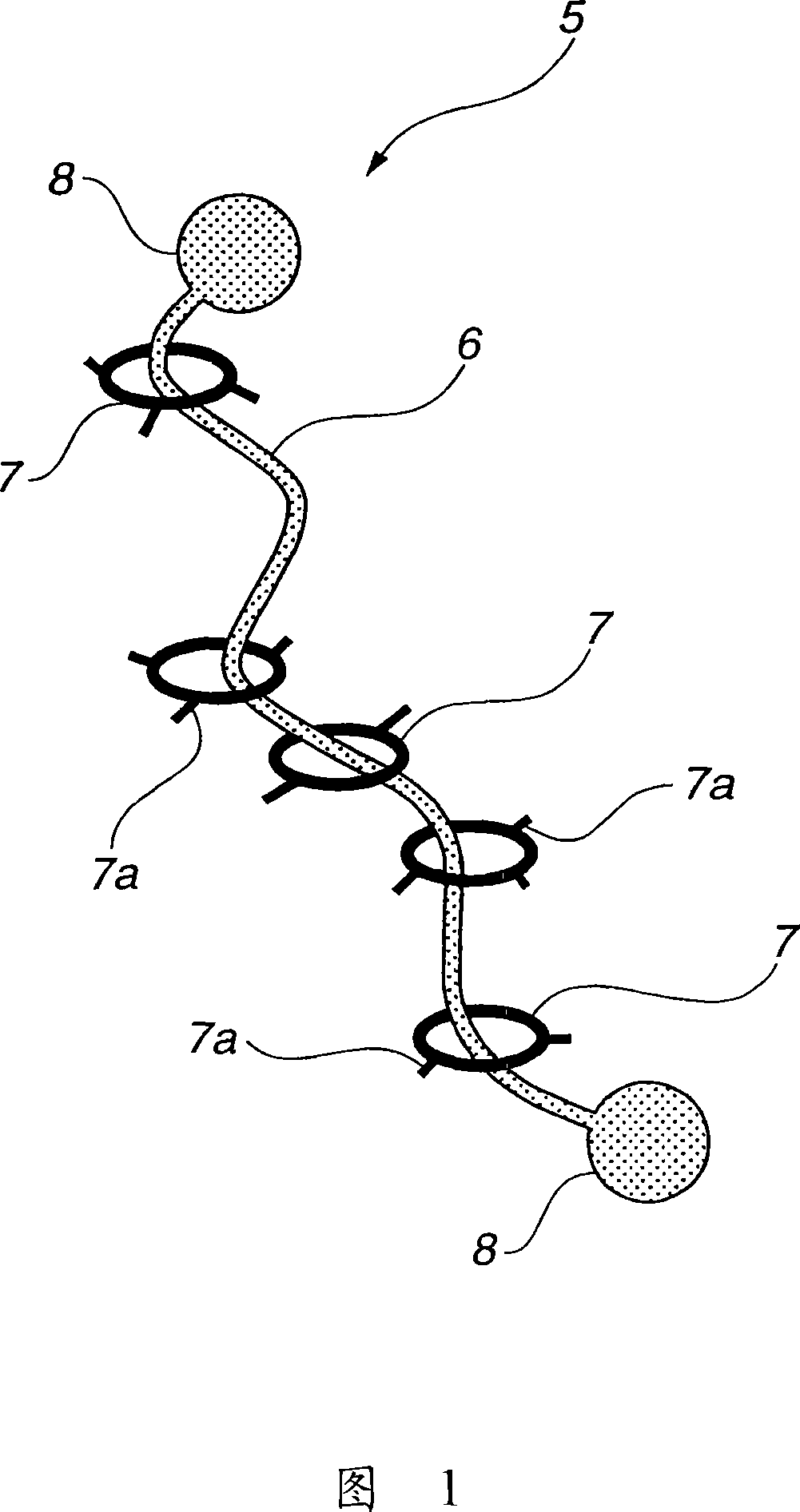
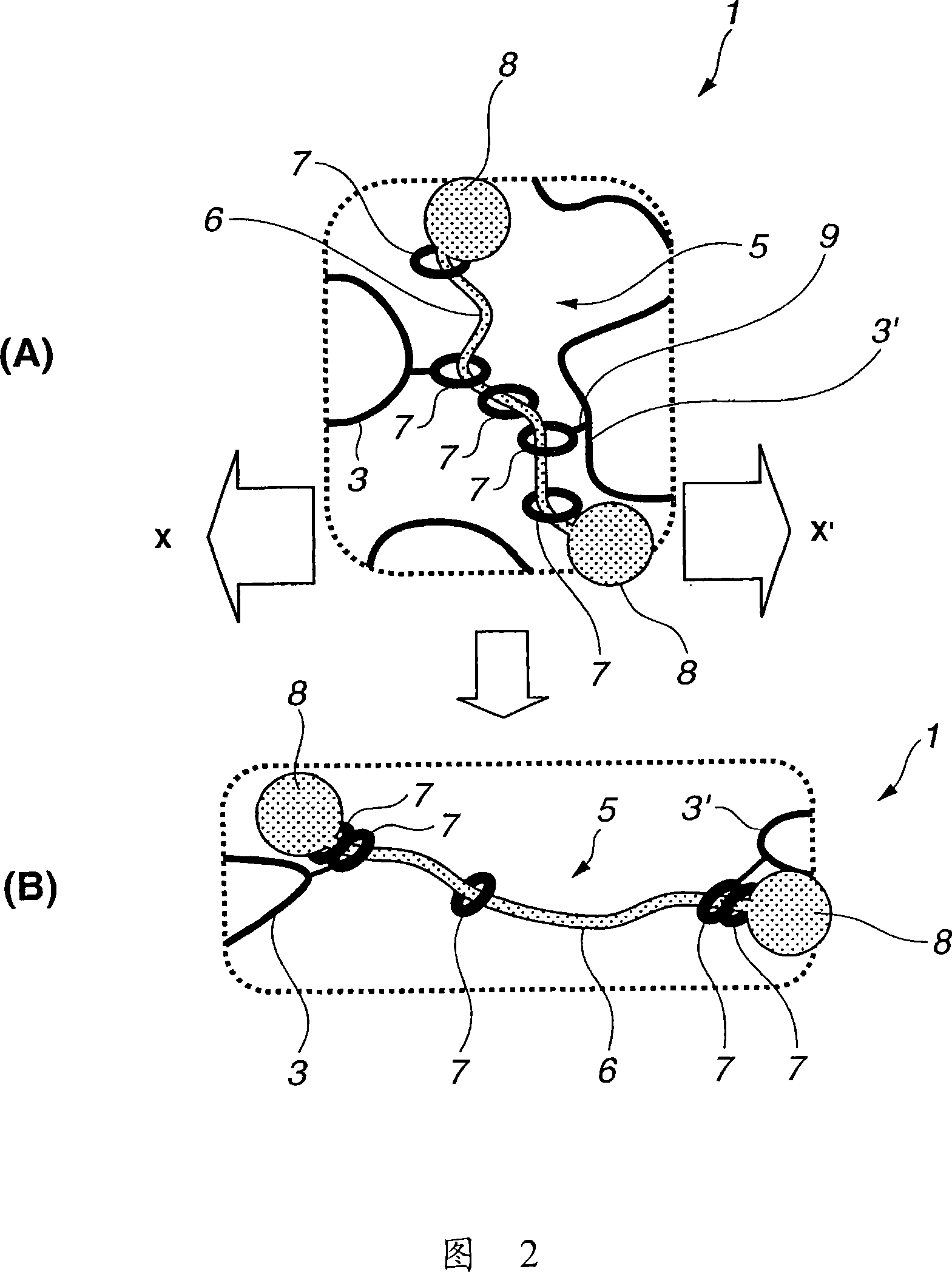
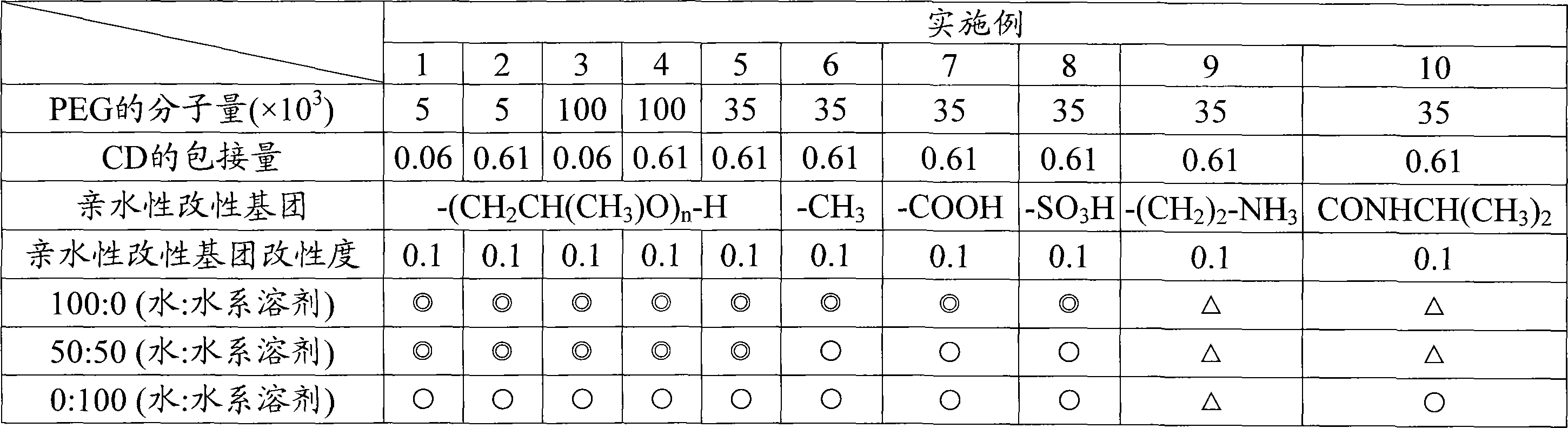
Modified hydrophilic polyrotaxane and cross-linked polyrotaxane
A hydrophilic polyrotaxane technology, applied in the field of hydrophilic modified polyrotaxane, can solve the problems of low mechanical strength and uneven structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0132] (1) Preparation of PEG-carboxylic acid by TEMPO oxidation of PEG
[0133] 10 g of polyethylene glycol (PEG) (molecular weight: 5000), 100 mg of TEMPO (2,2,6,6-tetramethyl-1-piperidinyl-oxyl radical) and 1 g of sodium bromide were dissolved in 100 ml of water. A commercially available sodium hypochlorite aqueous solution (available chlorine concentration: 5%) was added in an amount of 5 ml, and stirred at room temperature for 10 minutes. To decompose excess sodium hypochlorite, ethanol in the range of up to 5 ml was added to terminate the reaction.
[0134] Extraction with 50 ml of dichloromethane was repeated three times, thereby extracting components other than inorganic salts. Thereafter dichloromethane was distilled off from the extracted fraction using an evaporator. Then, the fractions were dissolved in hot ethanol and then allowed to stand overnight in a freezer (-4°C) to extract only PEG-carboxylic acid, which was then recovered and dried.
[0135] (2) Prepara...
Embodiment 2
[0146] (1) Preparation of PEG-carboxylic acid by TEMPO oxidation of PEG
[0147] 10 g of polyethylene glycol (PEG) (molecular weight: 5000), 100 mg of TEMPO (2,2,6,6-tetramethyl-1-piperidinyl-oxyl radical) and 1 g of sodium bromide were dissolved in 100 ml of water. A commercially available sodium hypochlorite aqueous solution (available chlorine concentration: 5%) was added in an amount of 5 ml, and stirred at room temperature for 10 minutes. To decompose excess sodium hypochlorite, ethanol in the range of up to 5 ml was added to terminate the reaction.
[0148] Extraction with 50 ml of dichloromethane was repeated three times, thereby extracting components other than inorganic salts. Thereafter dichloromethane was distilled off from the extracted fraction using an evaporator. Then, the fractions were dissolved in hot ethanol and then allowed to stand overnight in a freezer (-4°C) to extract only PEG-carboxylic acid, which was then recovered and dried.
[0149] (2) Prepara...
Embodiment 3
[0160] (1) Preparation of PEG-carboxylic acid by TEMPO oxidation of PEG
[0161] 10 g of polyethylene glycol (PEG) (molecular weight: 100,000), 100 mg of TEMPO (2,2,6,6-tetramethyl-1-piperidinyl-oxyl radical) and 1 g of sodium bromide were dissolved in 100 ml of water. A commercially available sodium hypochlorite aqueous solution (available chlorine concentration: 5%) was added in an amount of 5 ml, and stirred at room temperature for 10 minutes. To decompose excess sodium hypochlorite, ethanol in the range of up to 5 ml was added to terminate the reaction.
[0162] Extraction with 50 ml of dichloromethane was repeated three times, thereby extracting components other than inorganic salts. Thereafter dichloromethane was distilled off from the extracted fraction using an evaporator. Then, the fractions were dissolved in hot ethanol and then allowed to stand overnight in a freezer (-4°C) to extract only PEG-carboxylic acid, which was then recovered and dried.
[0163] (2) Prep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com