Macrocyclic molecule, preparation method and application thereof
A technology for macrocyclic molecules and reactions, applied in the field of macrocyclic molecules and their preparation, can solve the problems of complex preparation methods and inability to use chiral molecular recognition, and achieve simple and easy preparation methods, high yields, and increased recognition efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] This embodiment provides a macrocyclic molecule with the following structure:
[0086]
[0087]
[0088]The preparation method is as follows:
[0089] (1) Synthetic symmetrical rigid bridged bis-naphthalene, the reaction process is as follows:
[0090]
[0091] Intermediate Compound S 1 Synthesis of: anhydrous NaH (12g, 281.3mmol) was added into a solution of 2,6-dihydroxynaphthalene (50g, 312.5mmol) in DMF (500mL) and stirred for 1 hour, then the bromoacetic acid was added through a constant pressure dropping funnel Ethyl ester (31.5mL, 296.9mmol) was slowly added to the reaction solution; the reaction was stirred at room temperature for 12 hours, after the reaction was completed, the reaction solution was poured into 2L of ice water, and a small amount of dilute hydrochloric acid (to pH=7) was added to stir, and a large amount of insoluble solids Precipitate. Filter and wash the filter residue with a large amount of water, dissolve the filter residue in 2L...
Embodiment 2
[0114] This embodiment provides a chiral macrocyclic molecule with the following structure:
[0115]
[0116] The preparation method is as follows:
[0117] (1) Referring to Example 1, compound A was synthesized.
[0118] (2) Synthesis of chiral macrocyclic molecules rac-6-1 and rac-6-2, the process is as follows:
[0119]
[0120] Perform chiral resolution on compound A:
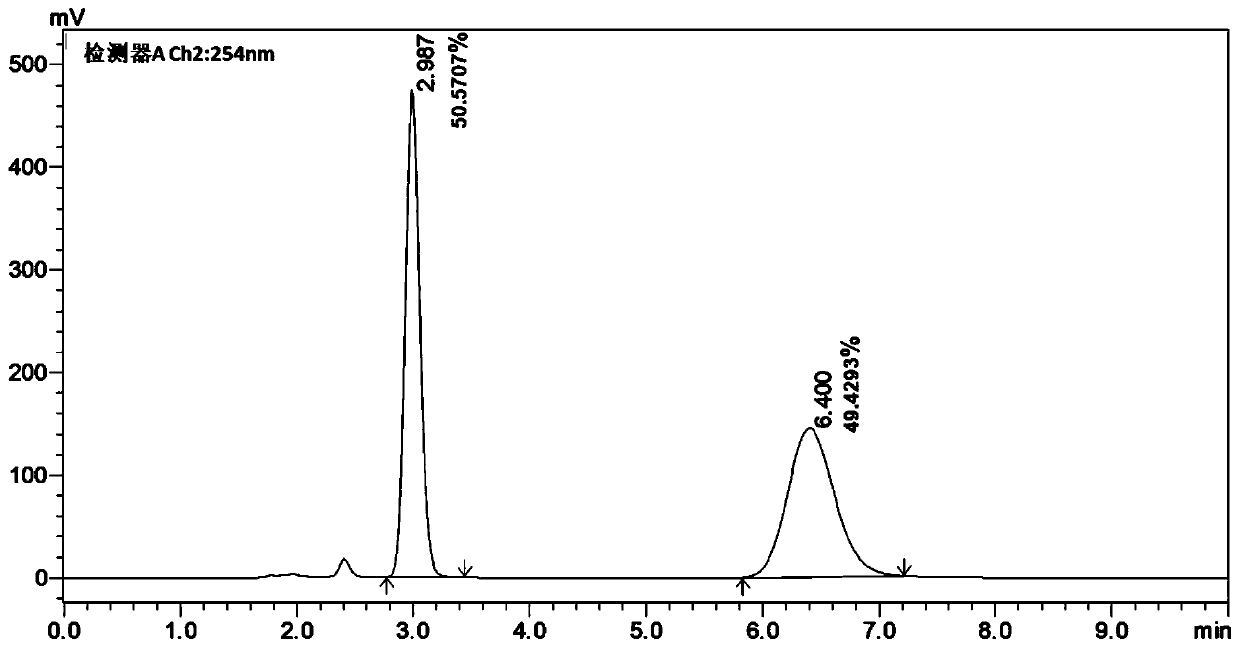
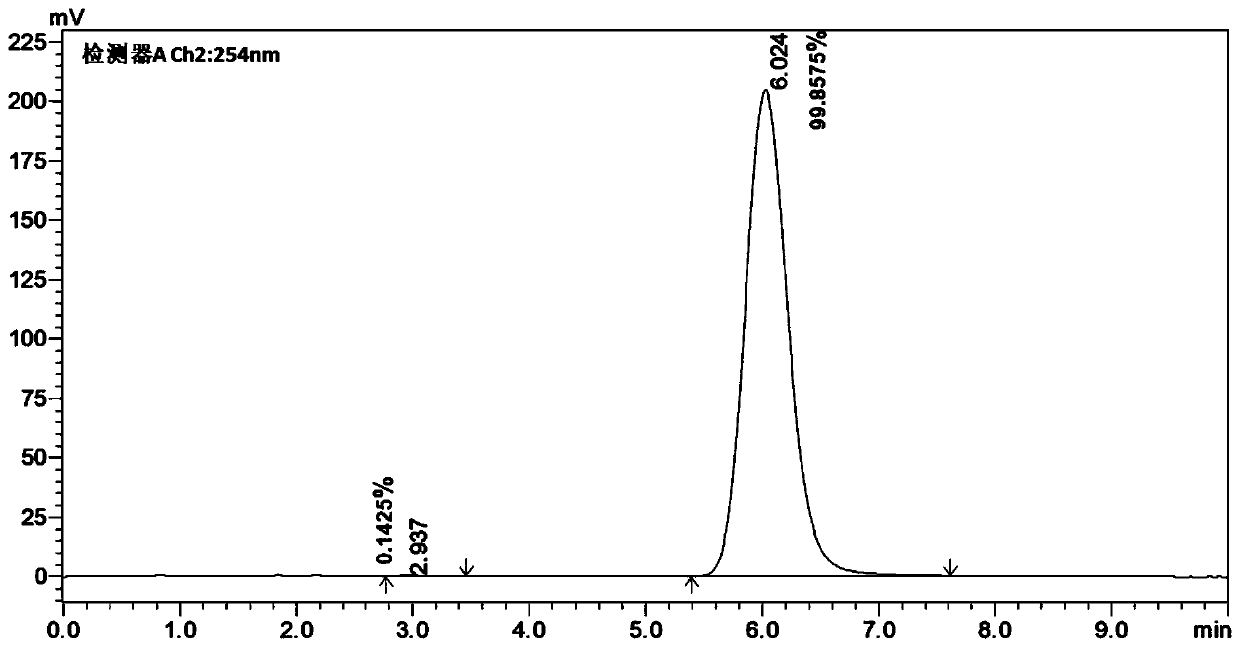
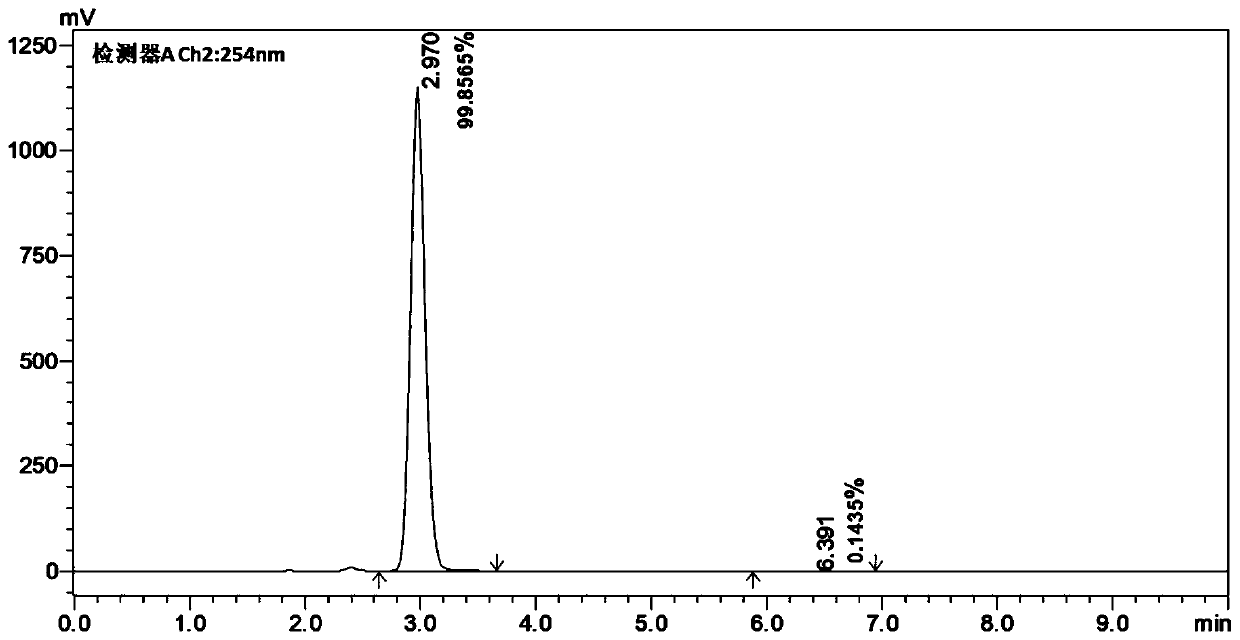
[0121] A pair of enantiomers R, S-A and S, R-A were obtained by chiral resolution of racemic compound A by high performance liquid chromatography, and the purity ee%>97% was confirmed by HPLC, and the detection spectrum was as follows Figure 4-Figure 6 shown.
[0122] Chromatographic conditions:
[0123] Shimadzu LC-20AD high performance liquid chromatography (Shimadzu, Japan); chiral column: CHIRALPAK IG (ID00CD-UF004, 0.46cm I.D.×25cm L, Daicel Pharmaceutical Chiral Technology (Shanghai) Co., Ltd.); mobile phase : Dichloromethane / methanol / diethanolamine / acetic acid=90 / 10 / 0.1 / 0.3(V / V / V / V); flow...
Embodiment 3
[0127] This embodiment provides a chiral macrocyclic molecule with the following structure:
[0128]
[0129] The preparation method is as follows:
[0130] (1) react using the rac-6-1 and rac-6-2 obtained prepared in Example 1, the method is as follows:
[0131] Synthesis of Compound 1: Sodium hydroxide aqueous solution (3.0mL, 6.0N) was added into a mixed solution of methanol (5mL) and tetrahydrofuran (5mL) dissolved with rac-6-1 (234mg, 0.2mmol), reacted at room temperature overnight, and the reaction After completion, add 10% hydrochloric acid (5mL), filter the precipitate, dissolve the filter residue in aqueous sodium hydroxide solution (20mg / mL, 1.6mL), and stir for a few minutes, spin off the solvent, and dry to obtain compound 1 (white solid ).
[0132] The obtained compound 1 was characterized by proton nuclear magnetic spectrum, carbon nuclear magnetic spectrum and mass spectrometry, and the data results are as follows:
[0133] 1 H NMR (500MHz,D 2 O, 298K) δ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com