3-chloro-2-chloromethyl propylene, preparation method and application thereof
A technology of chloromethylpropene and dichloroacetone is applied in the preparation of carbon-based compounds, the preparation of halogenated hydrocarbons, chemical instruments and methods, etc., can solve the problems of complicated steps, expensive raw materials, low yield and the like, and achieves simple steps, Environmentally friendly, high-yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The present embodiment provides a kind of preparation method of 3-chloro-2-chloromethylpropene, specifically as follows:
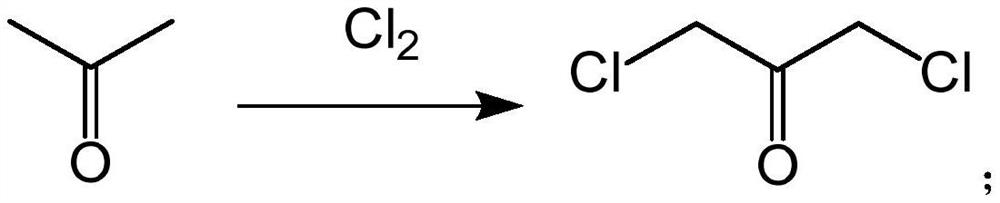
[0054] (1) Add 100g of acetone to 100g of water, then add 100g of calcium carbonate, heat to 70°C, then introduce 140g of chlorine gas, react for 4h under a pressure of 1MPa, filter, and vacuum distill, collect the fractions at 70-80°C to obtain 1,3-dichloroacetone 84g, yield is 84%;
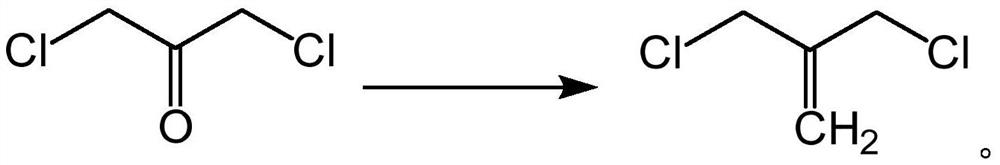
[0055] (2) 1,3-dichloroacetone obtained in step (1) is mixed with 85g of silica-loaded activated carbon catalyst (the mass ratio of silica and activated carbon is 10:0.8) and placed in a pressure tube, at 3MPa, The reaction was carried out at 480° C. for 1 h. After the reaction, distillation was carried out to collect fractions at 140°C to 160° C. to obtain 61 g of 3-chloro-2-chloromethylpropene with a yield of 61%.
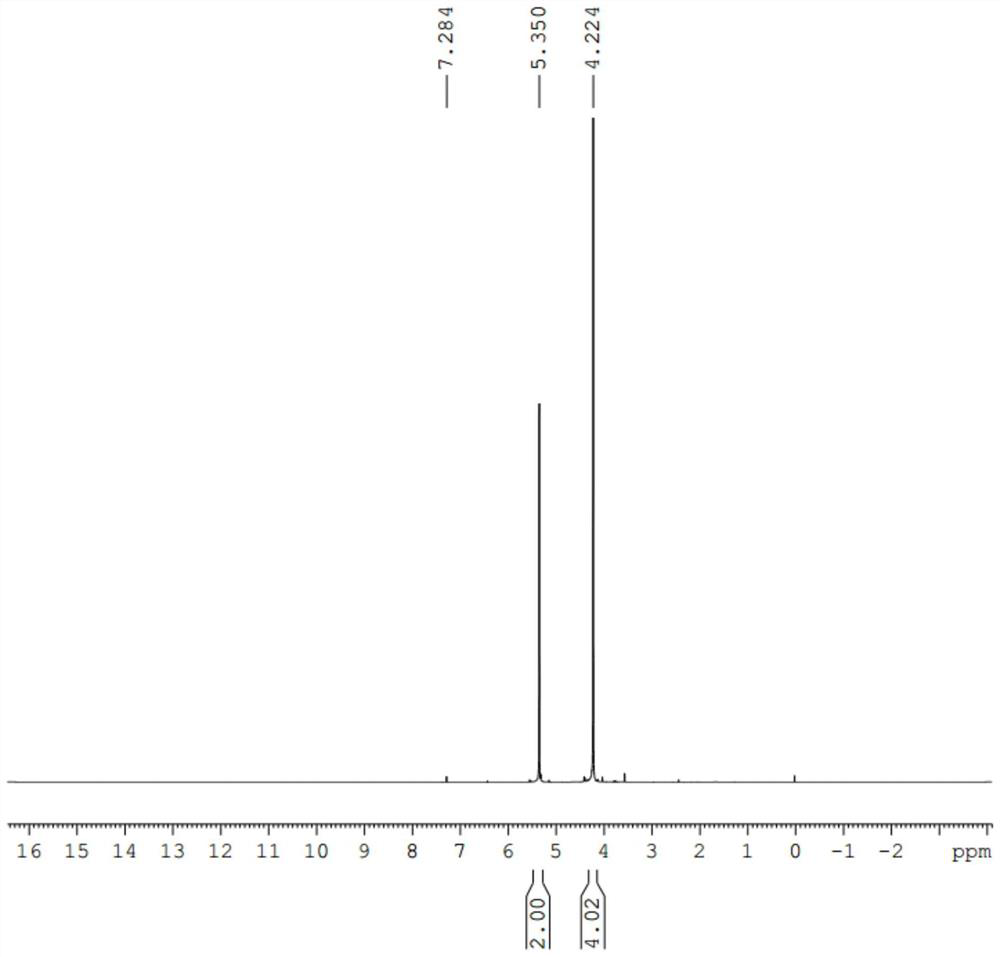
[0056] The results of H NMR spectroscopy were as follows: figure 1 As shown, characteristic peaks: 4.22 (4H), 5.35 (2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com