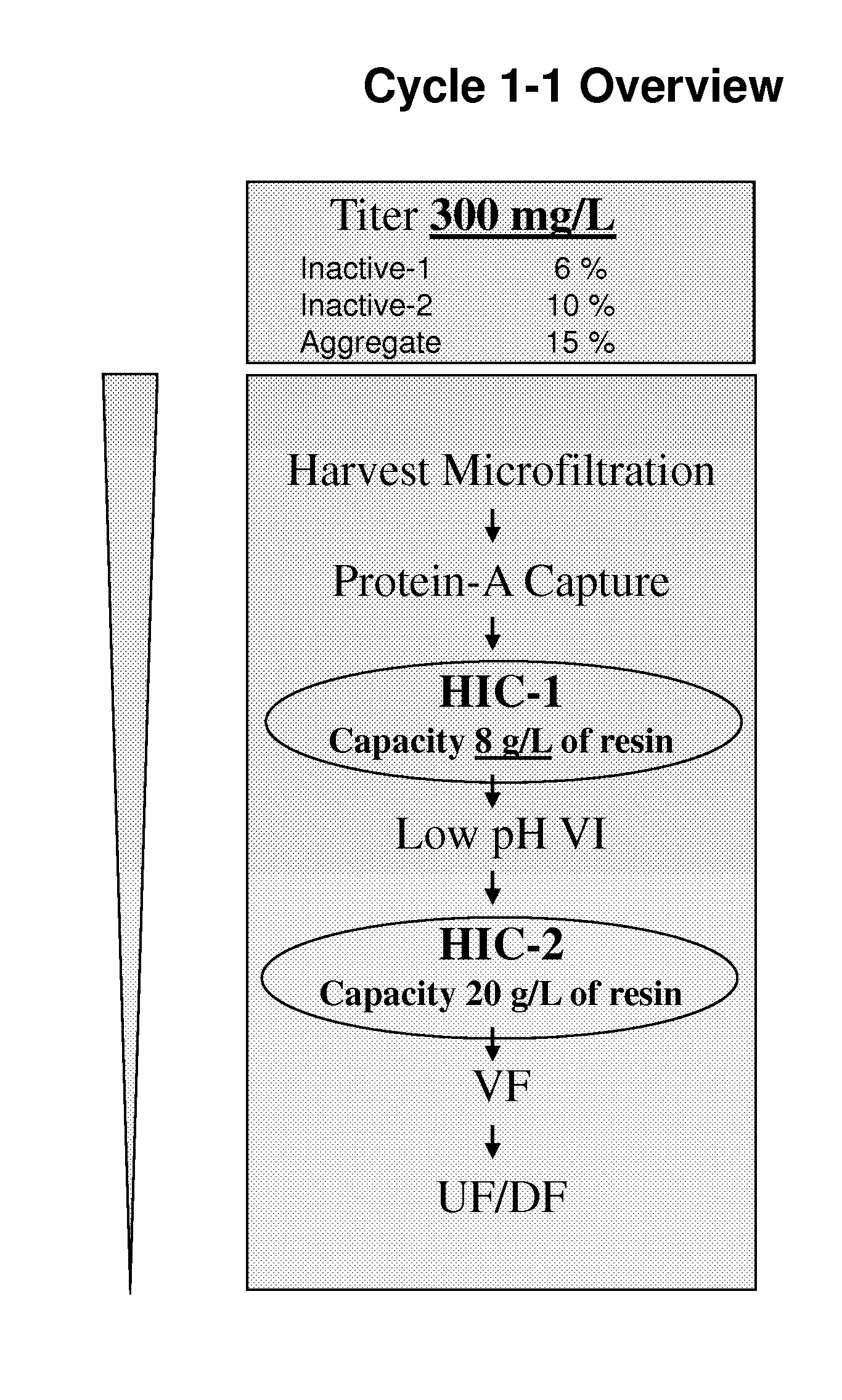
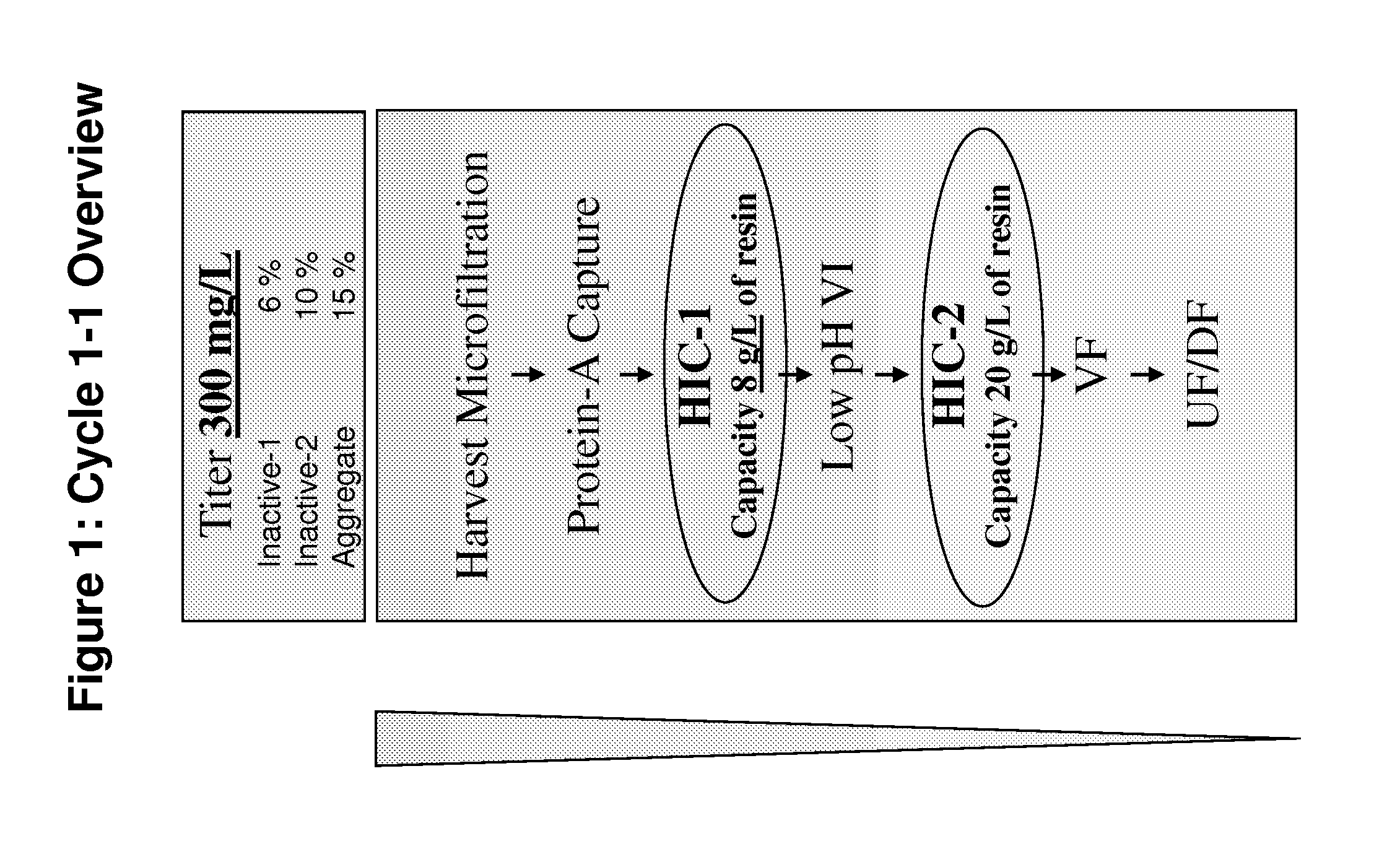
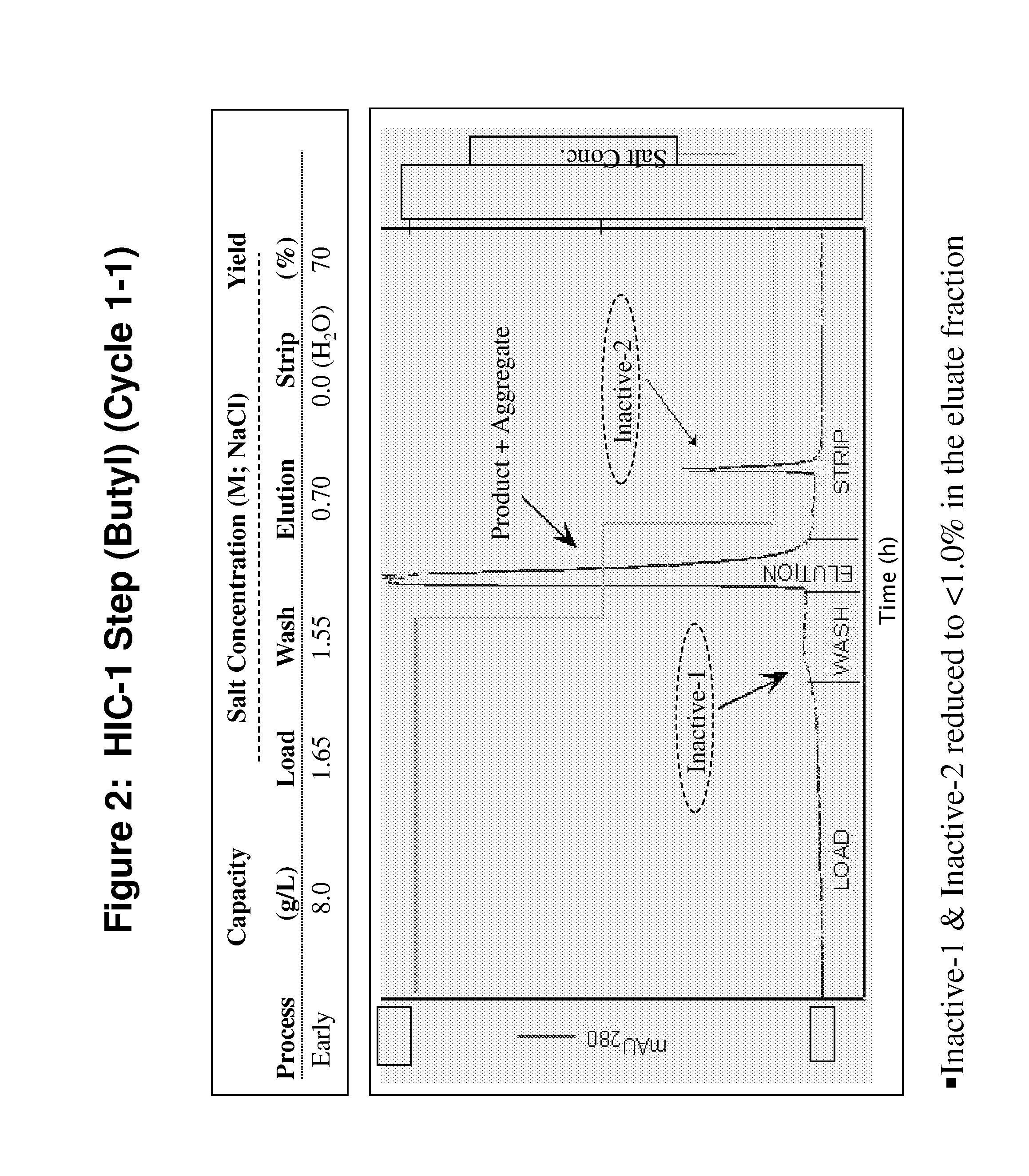
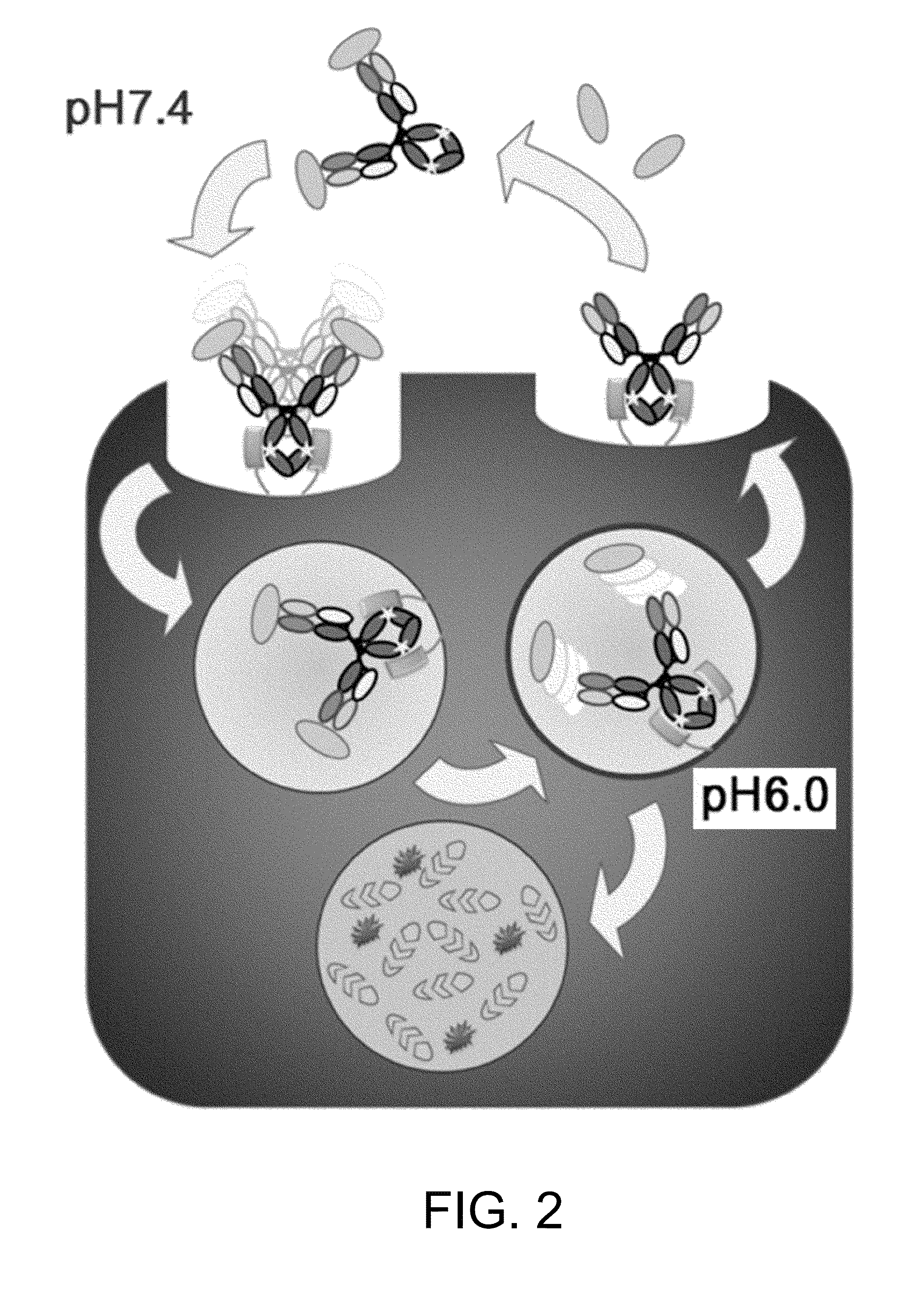
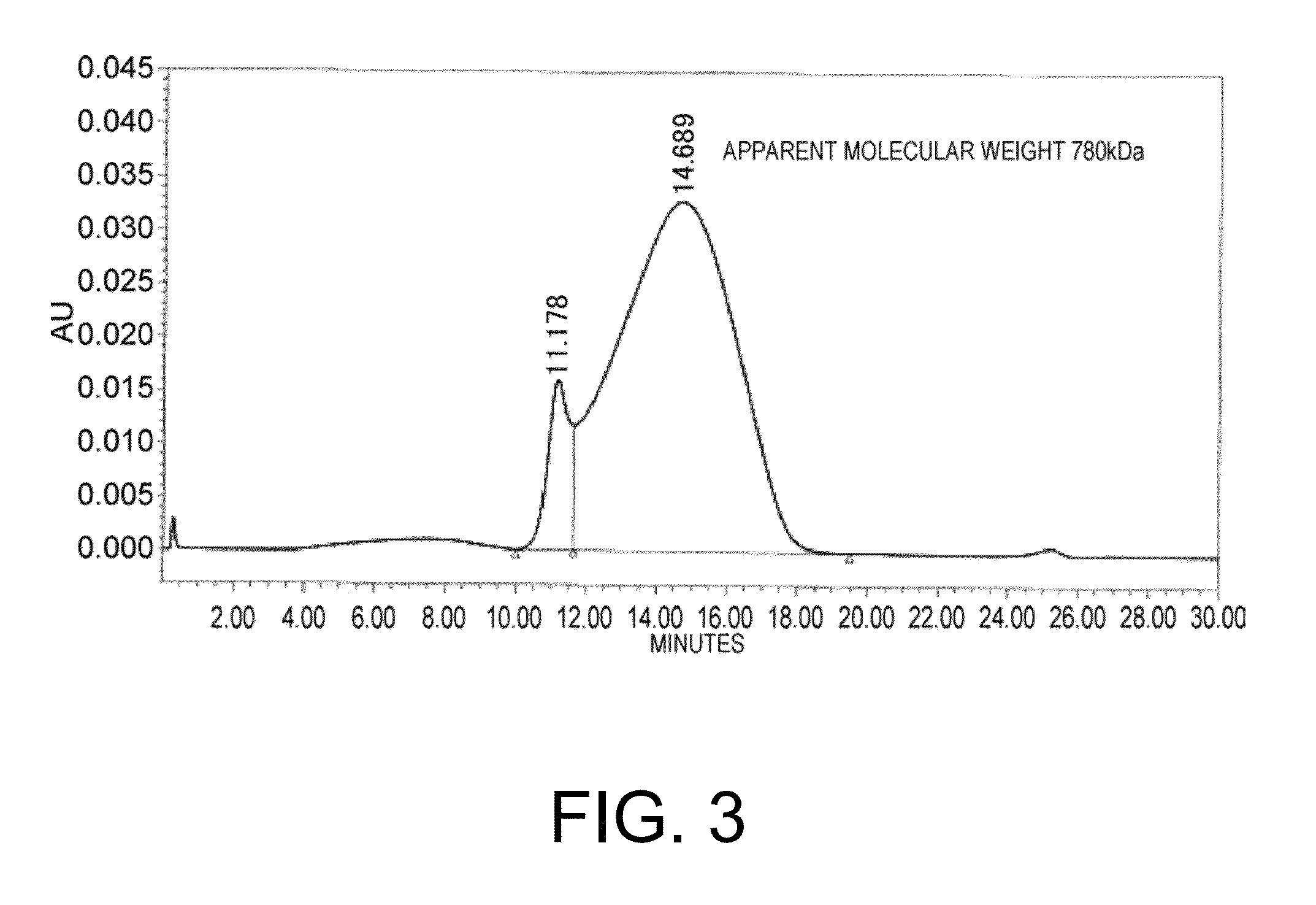
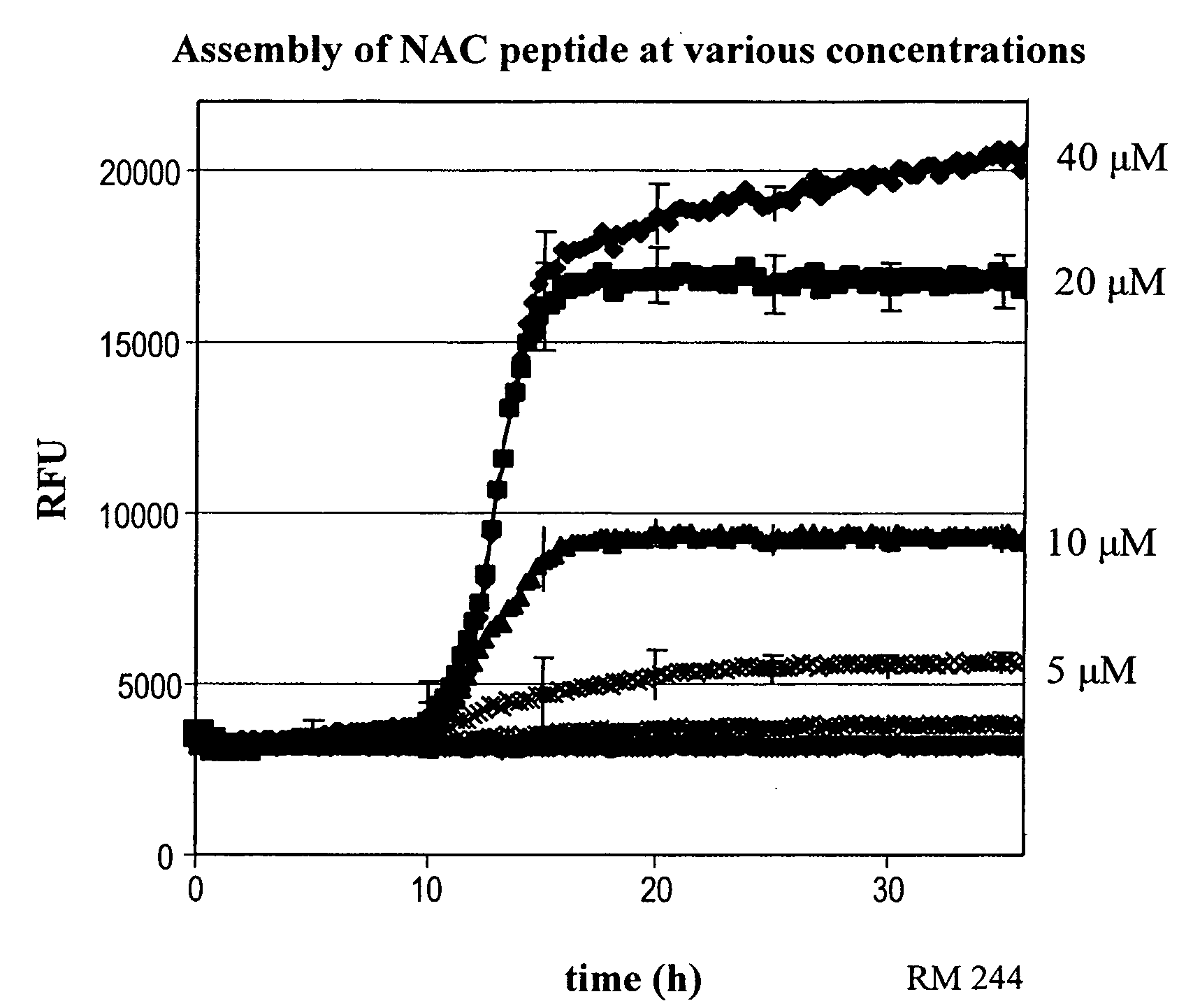
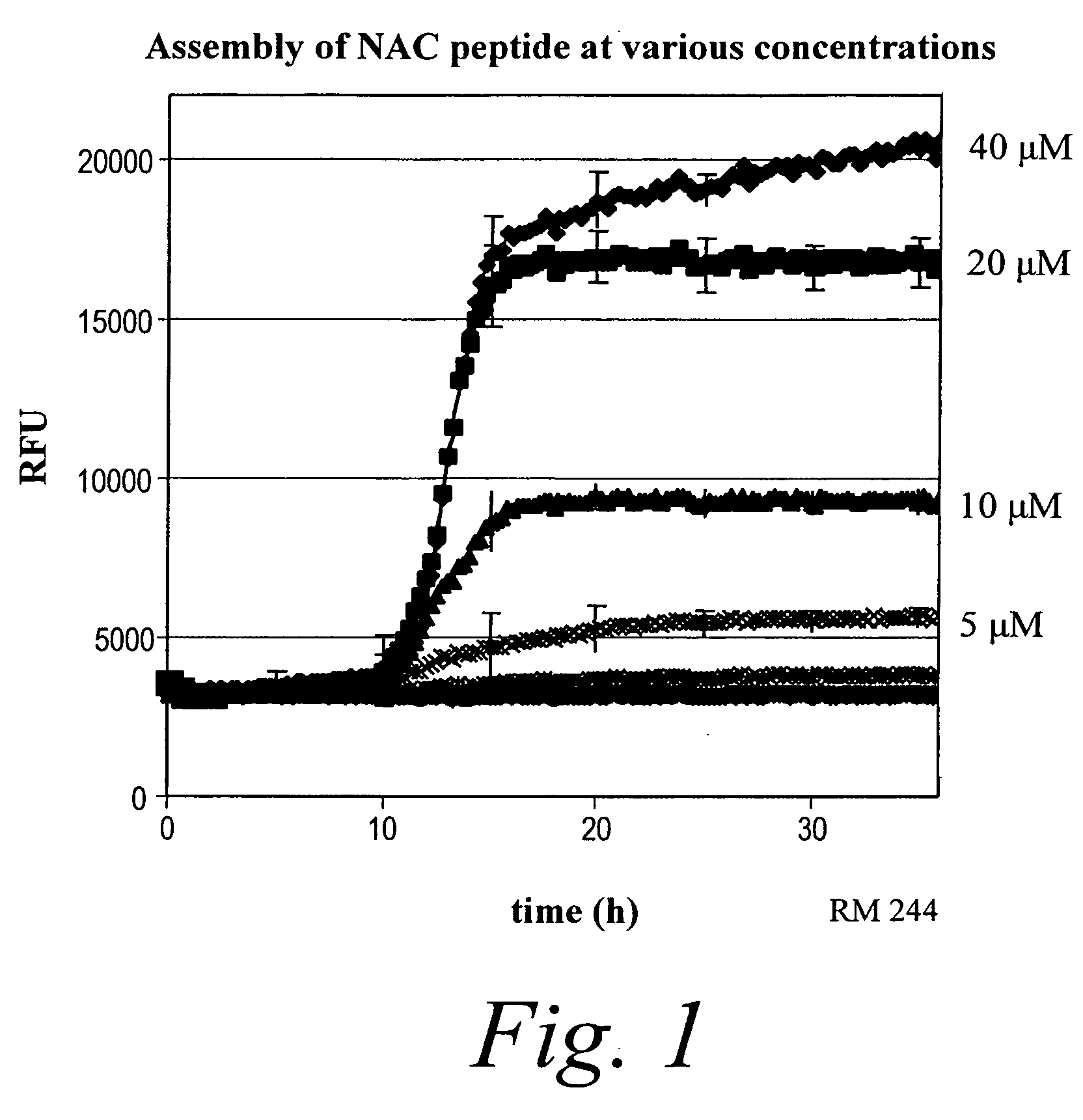
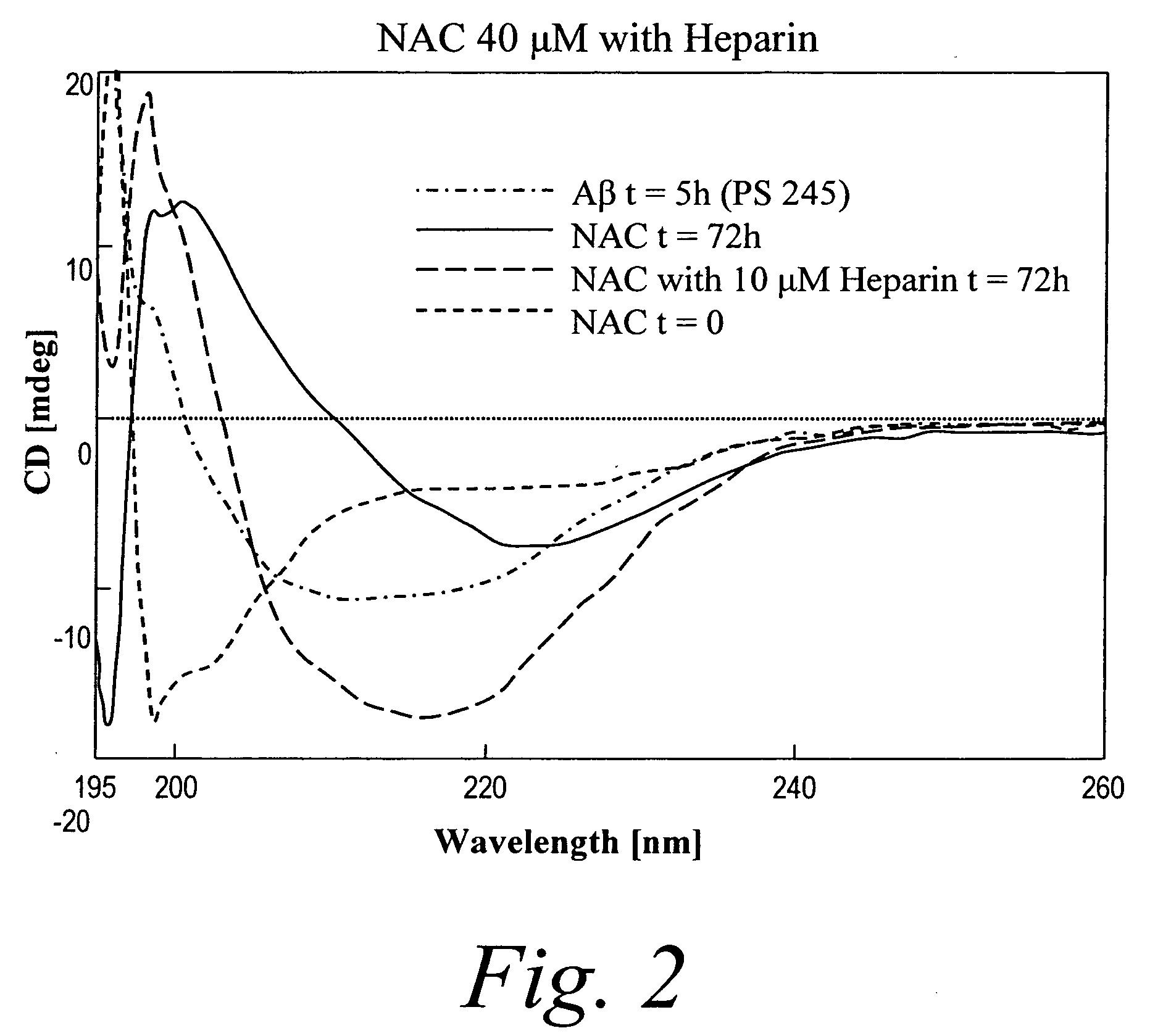
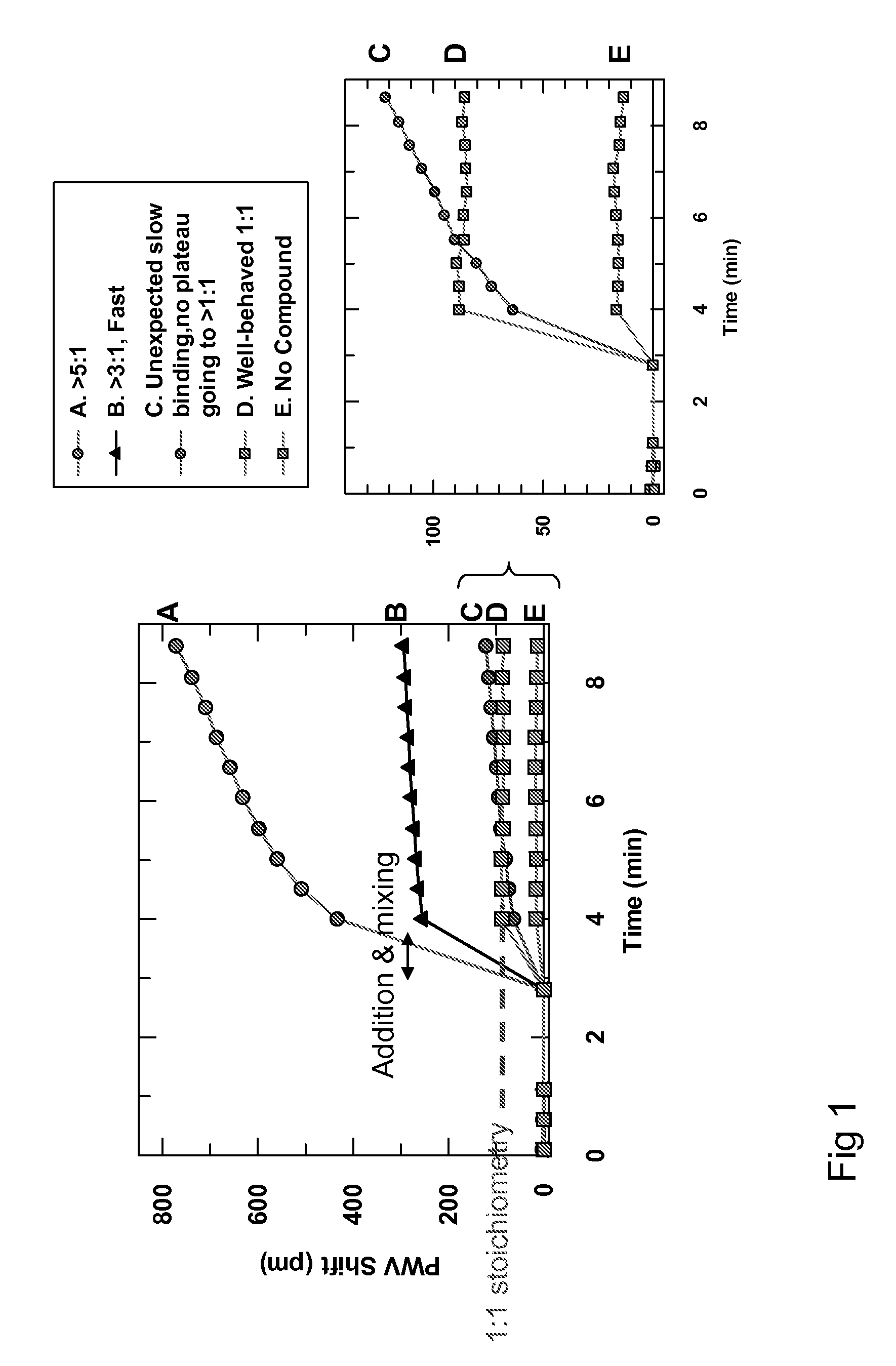
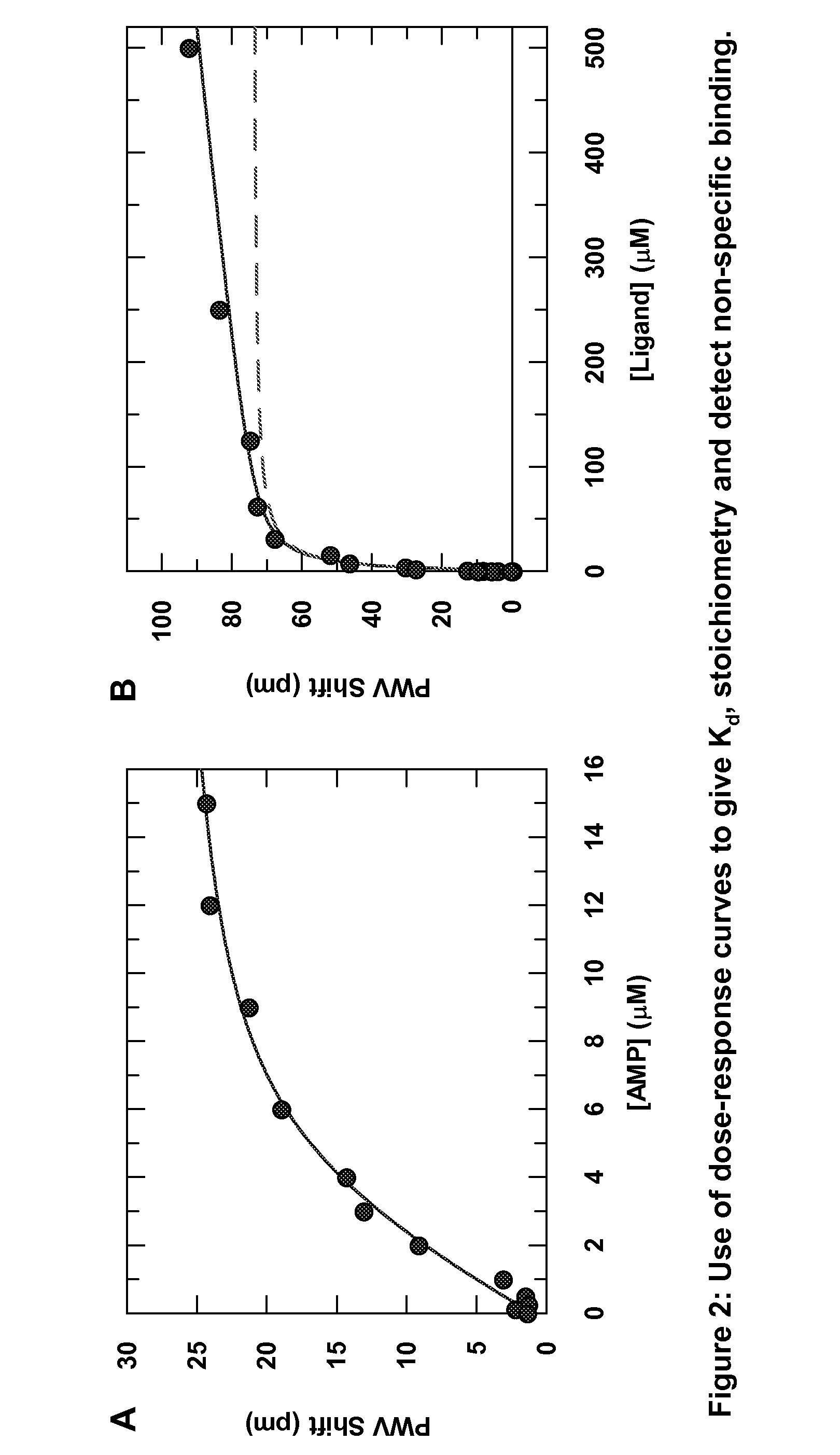
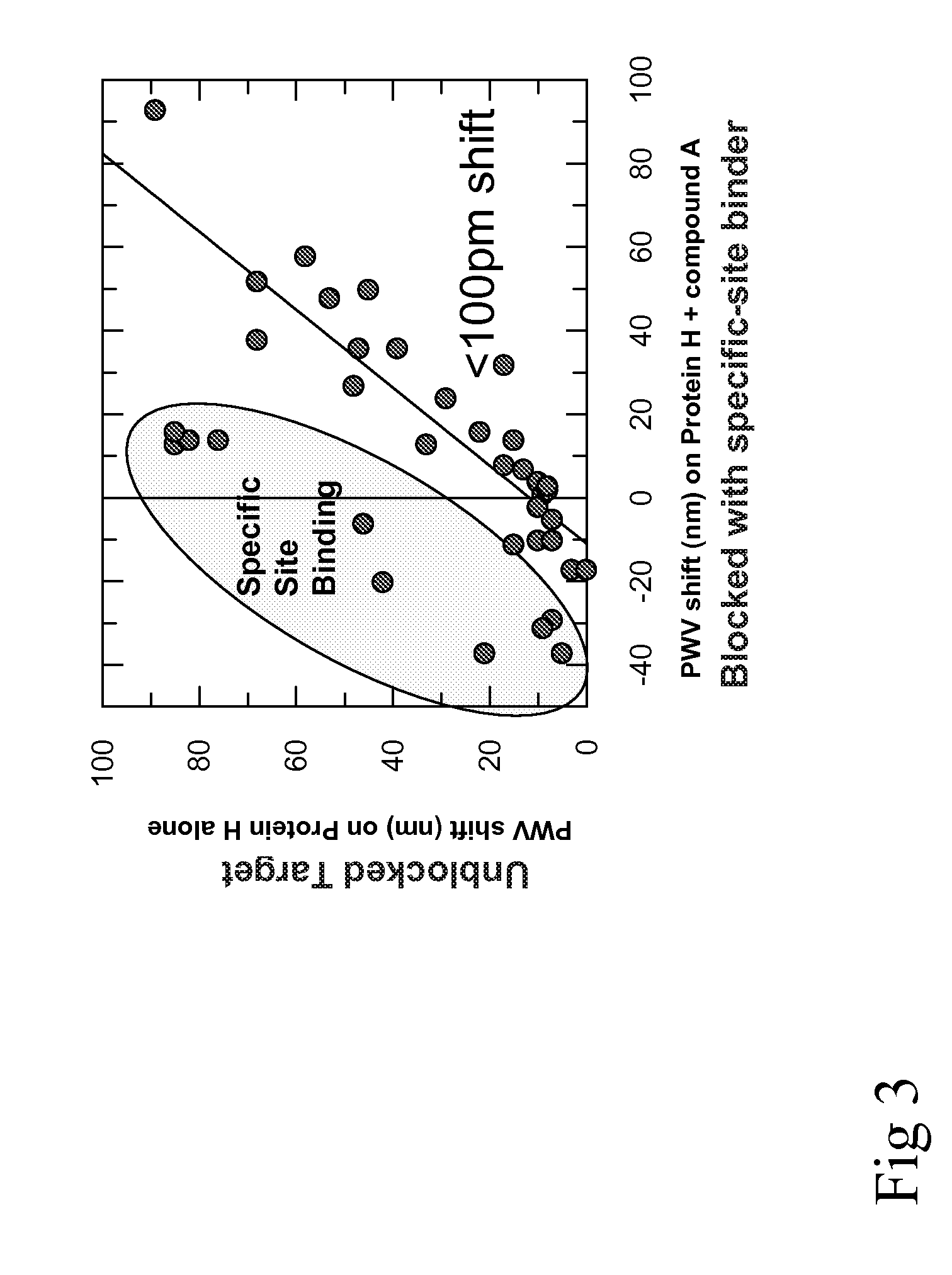
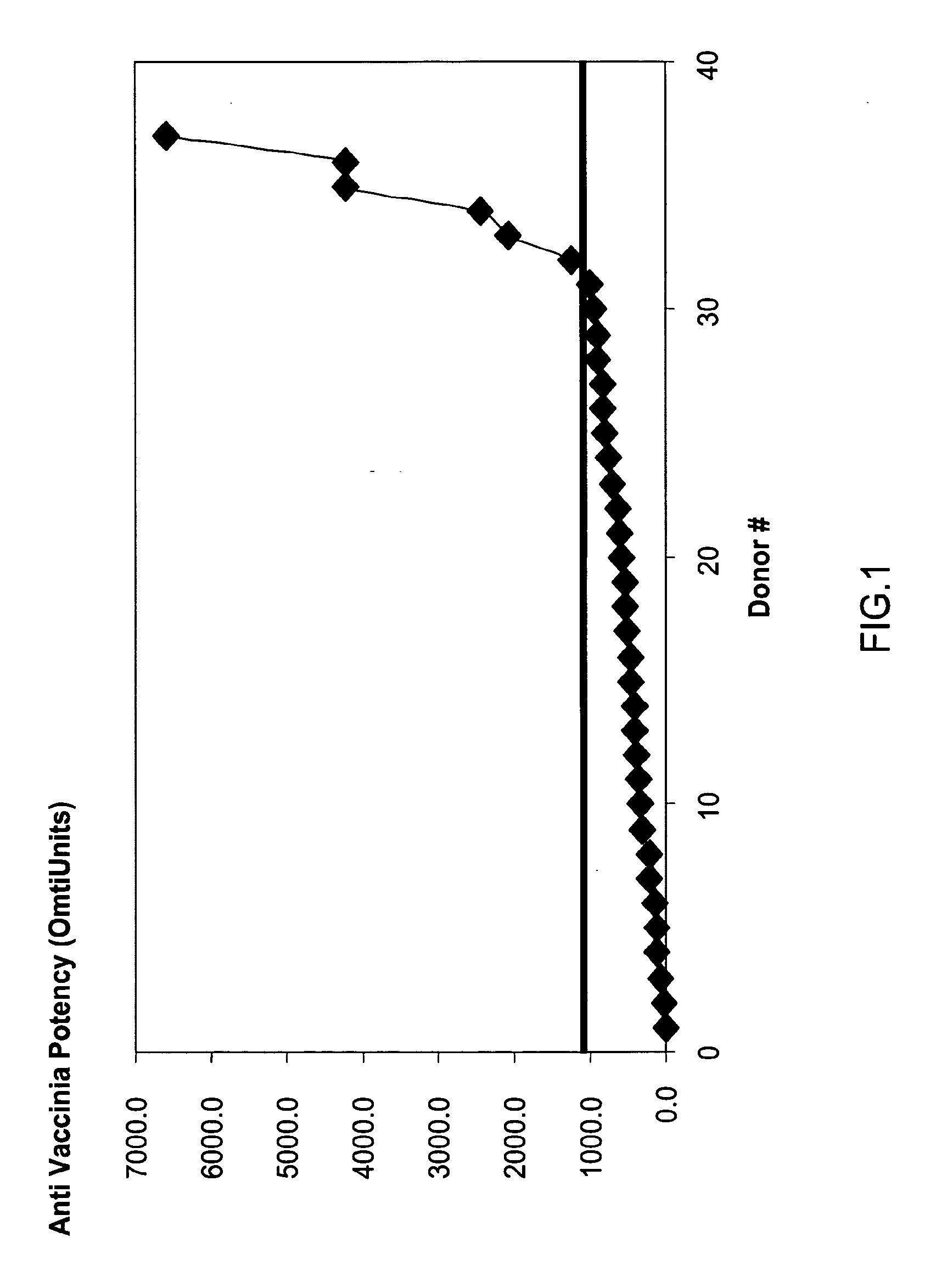
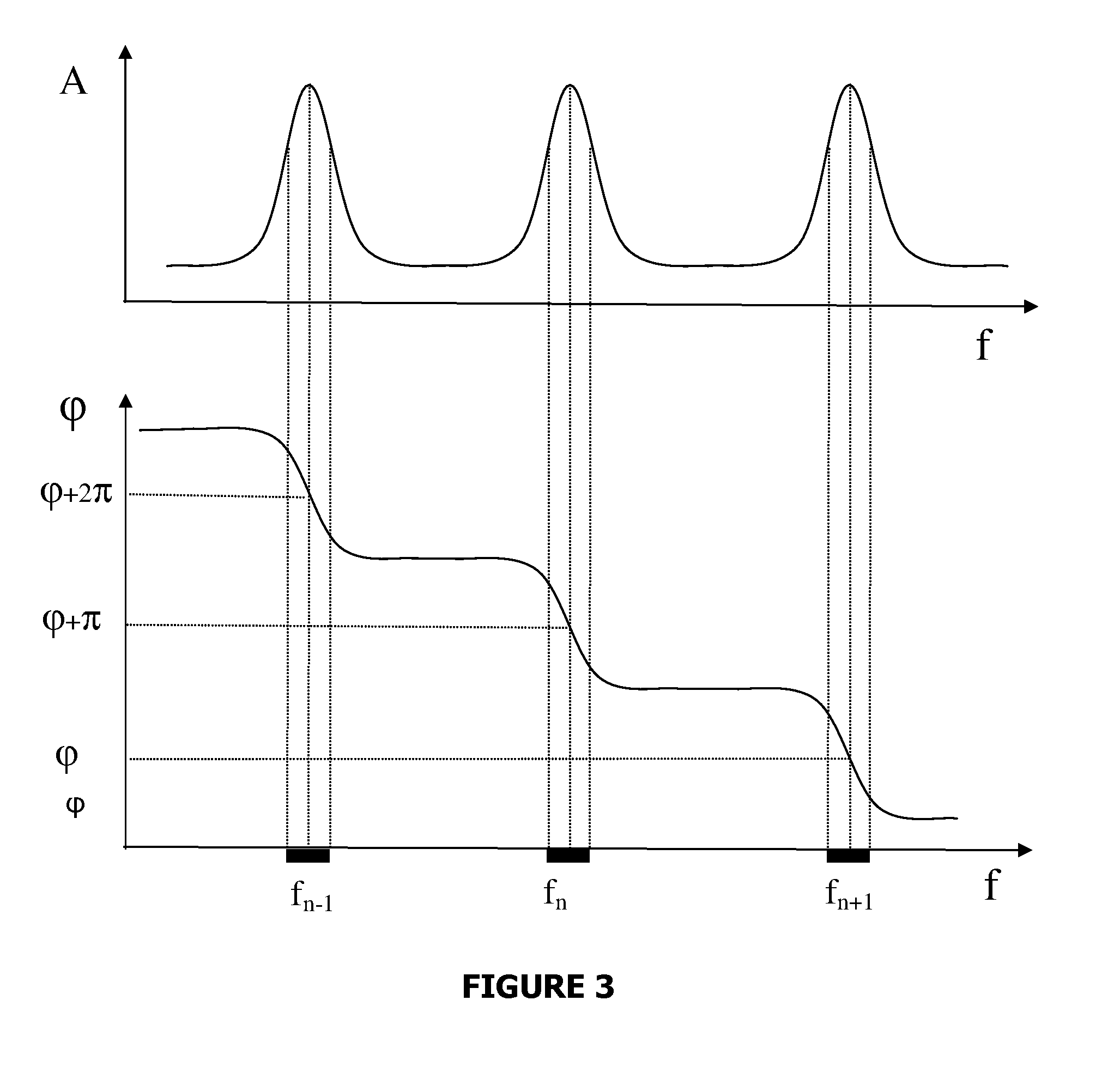
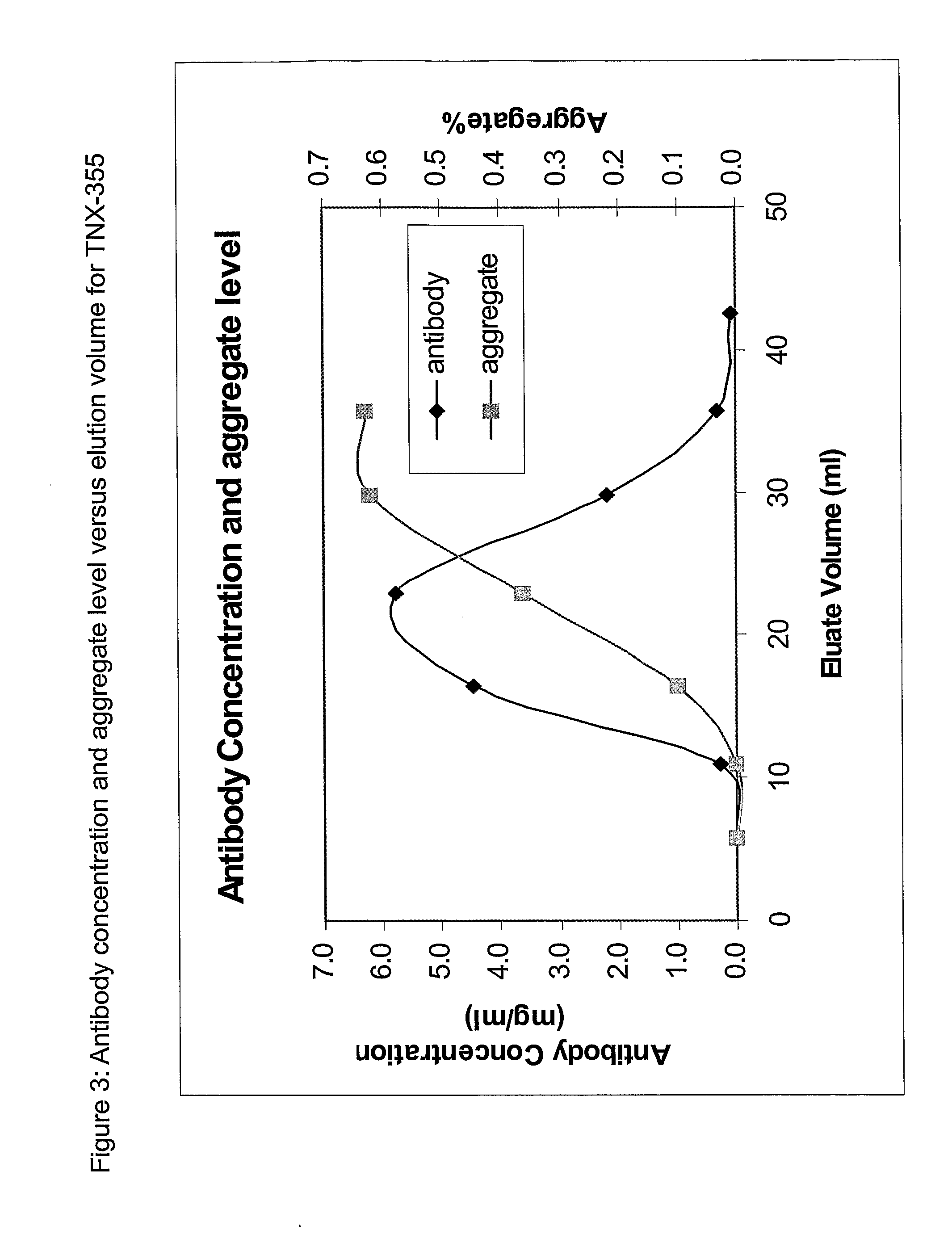
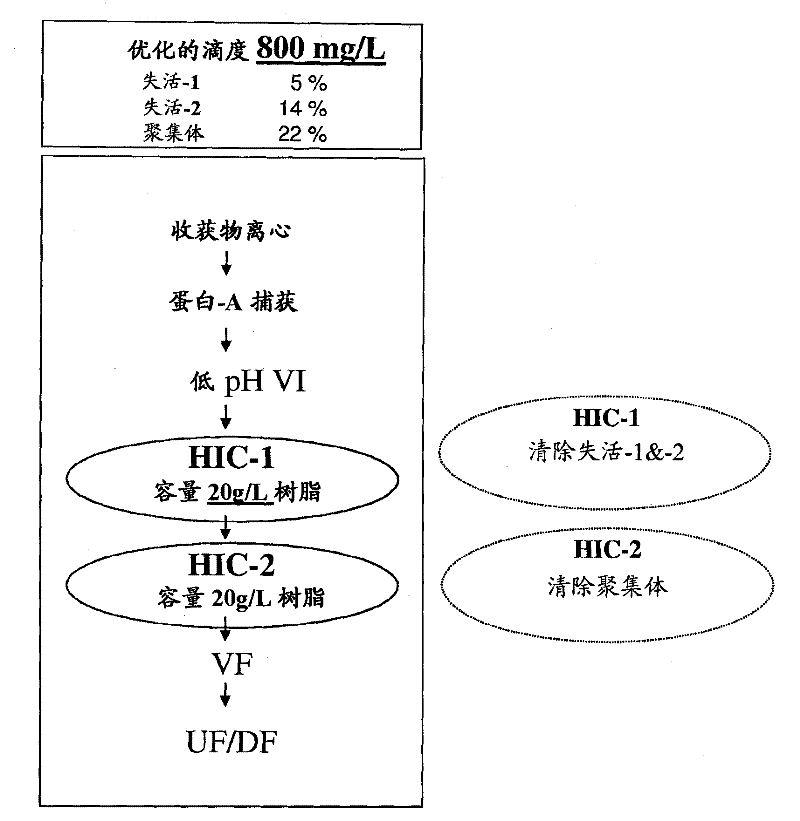
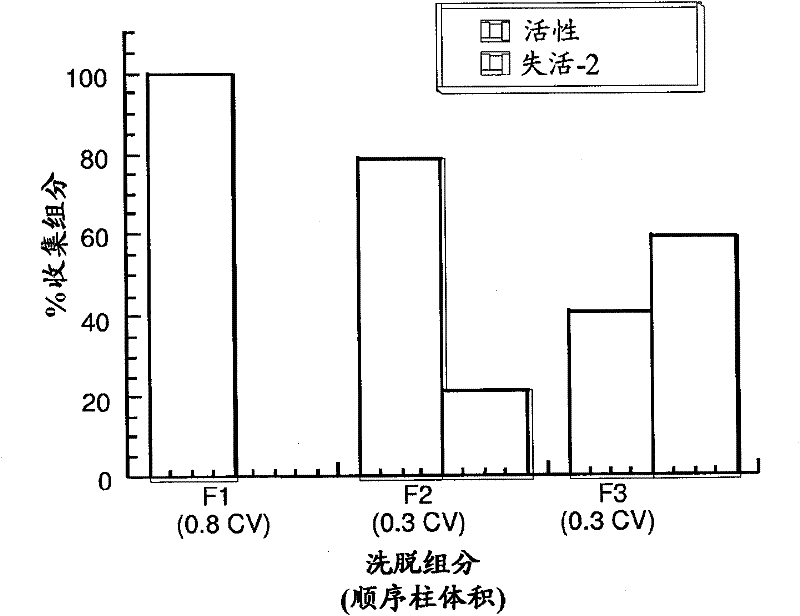
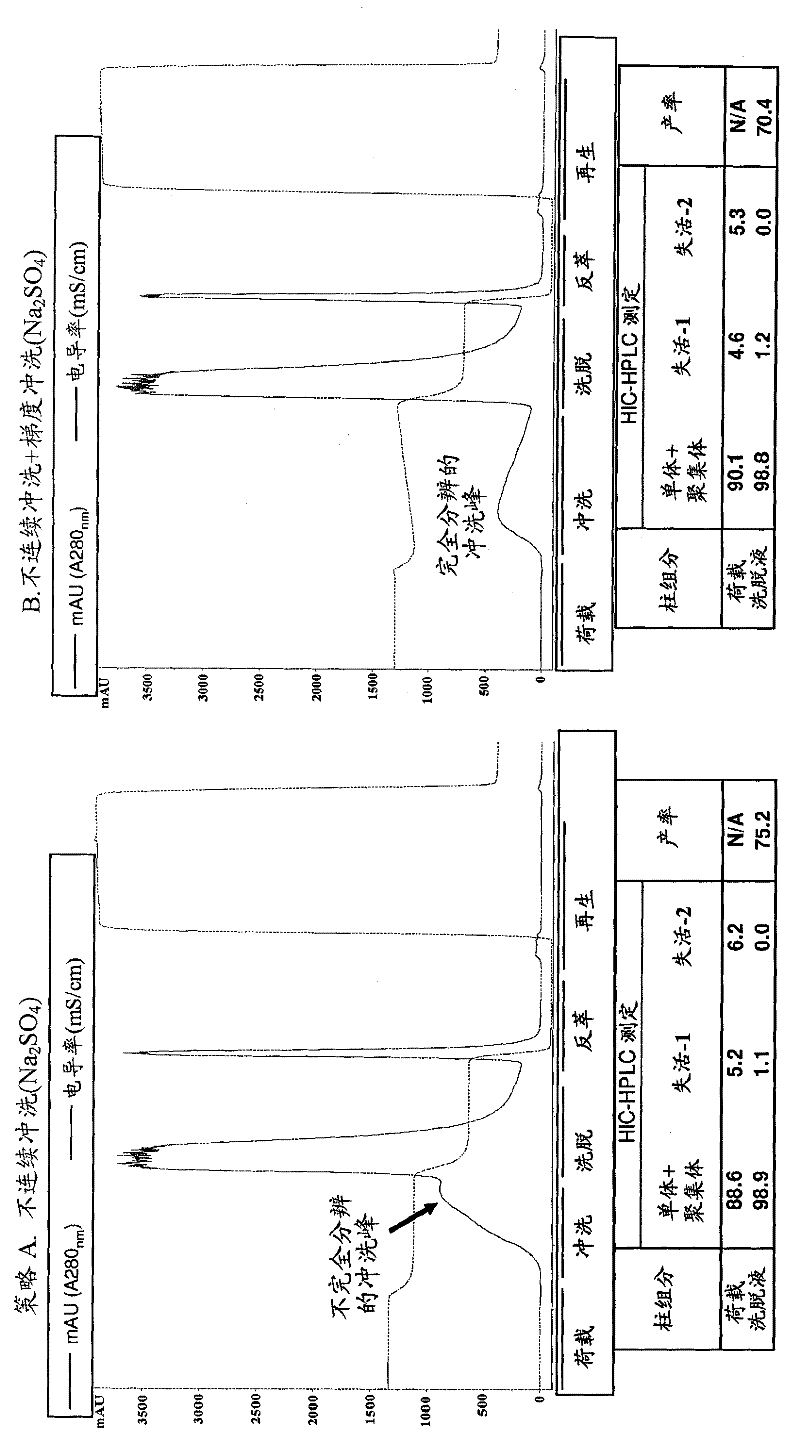
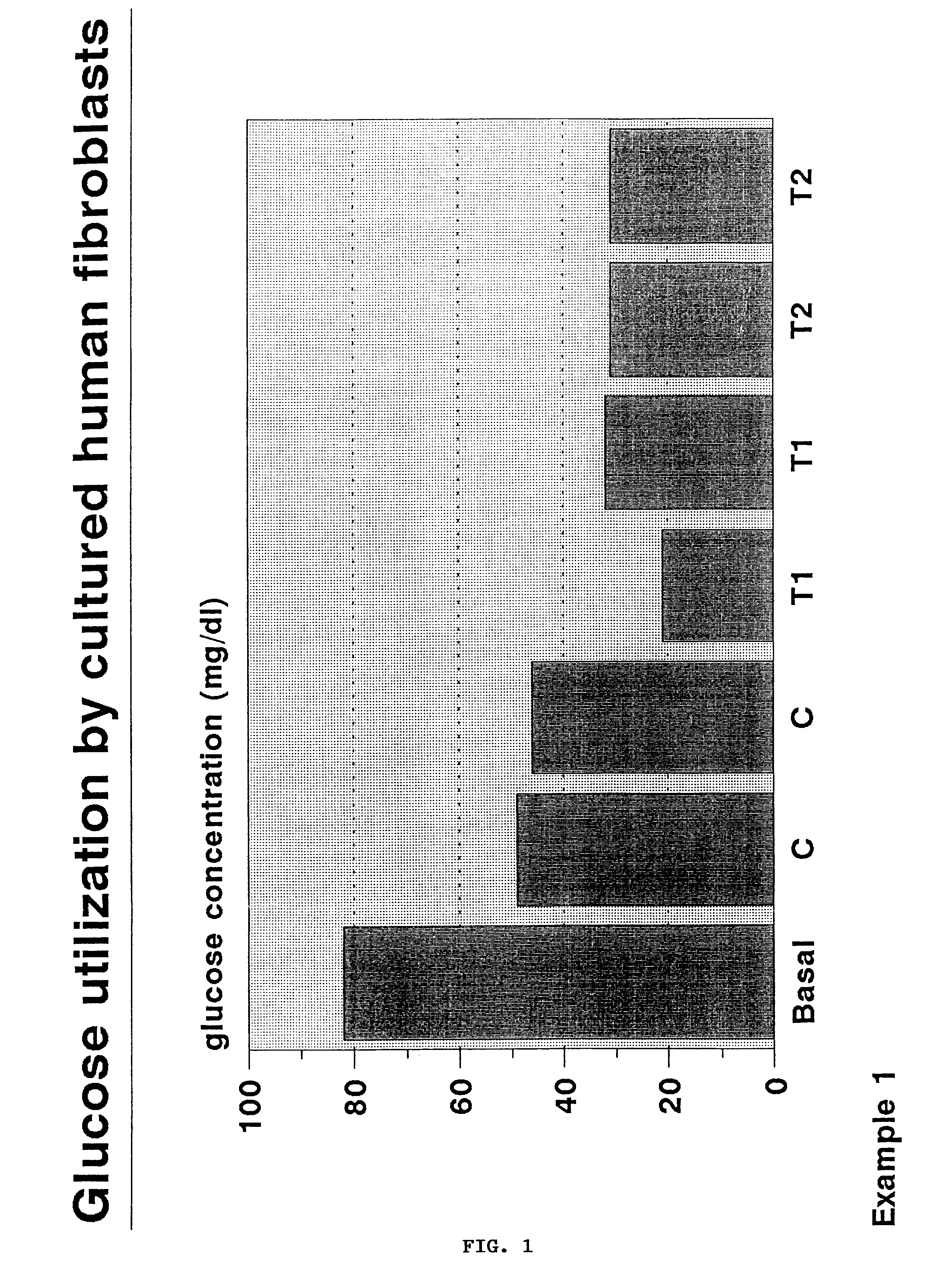
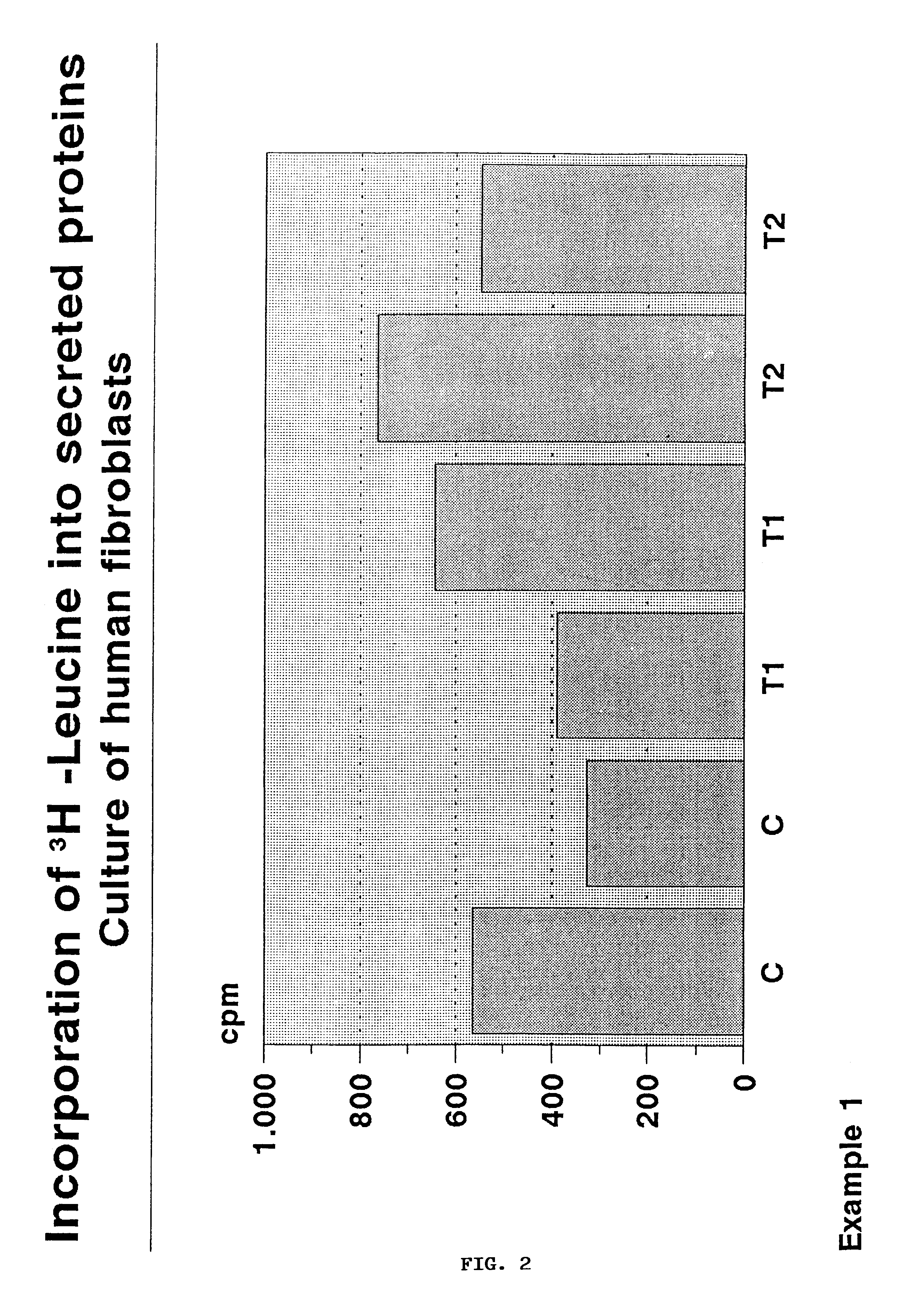
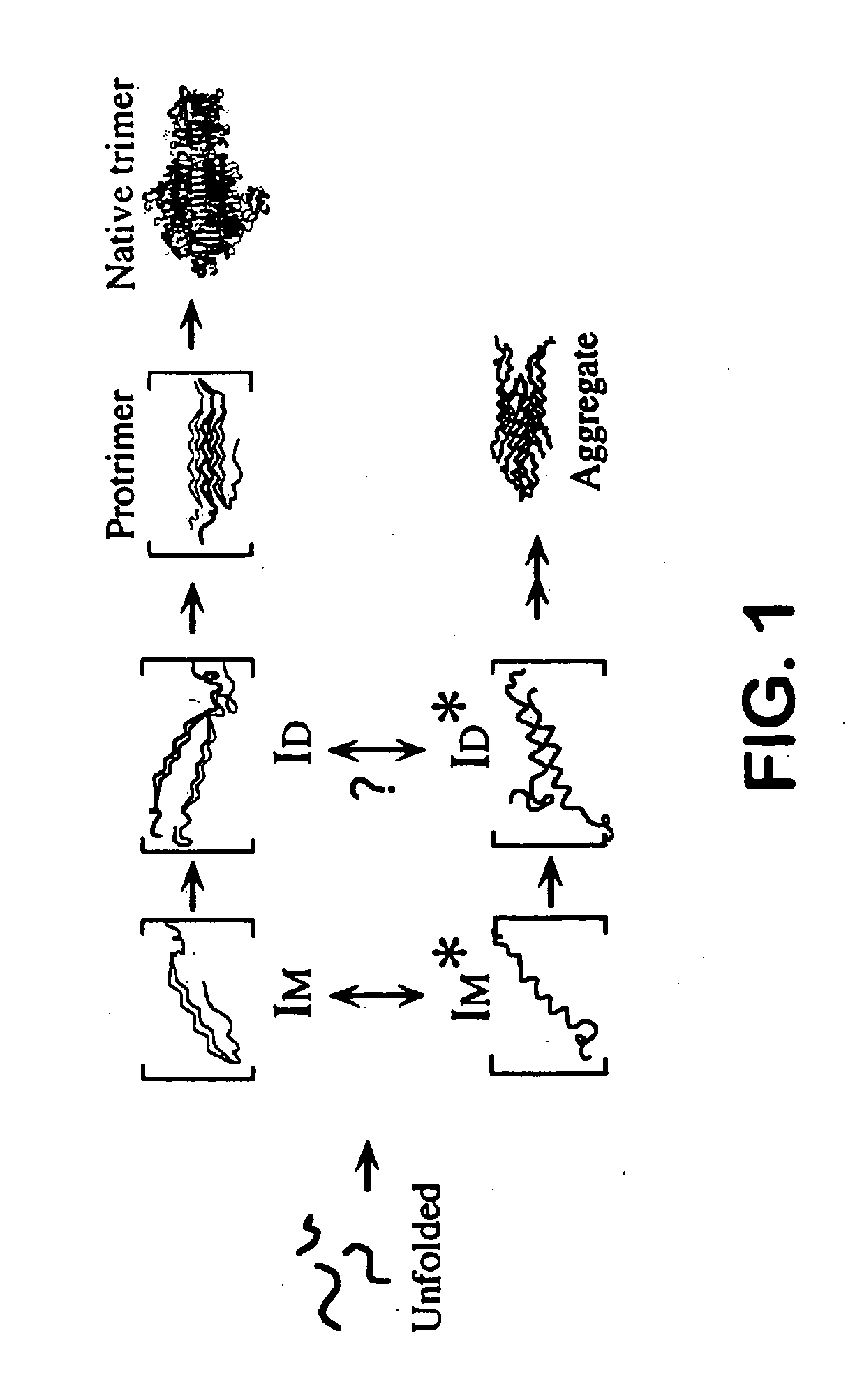
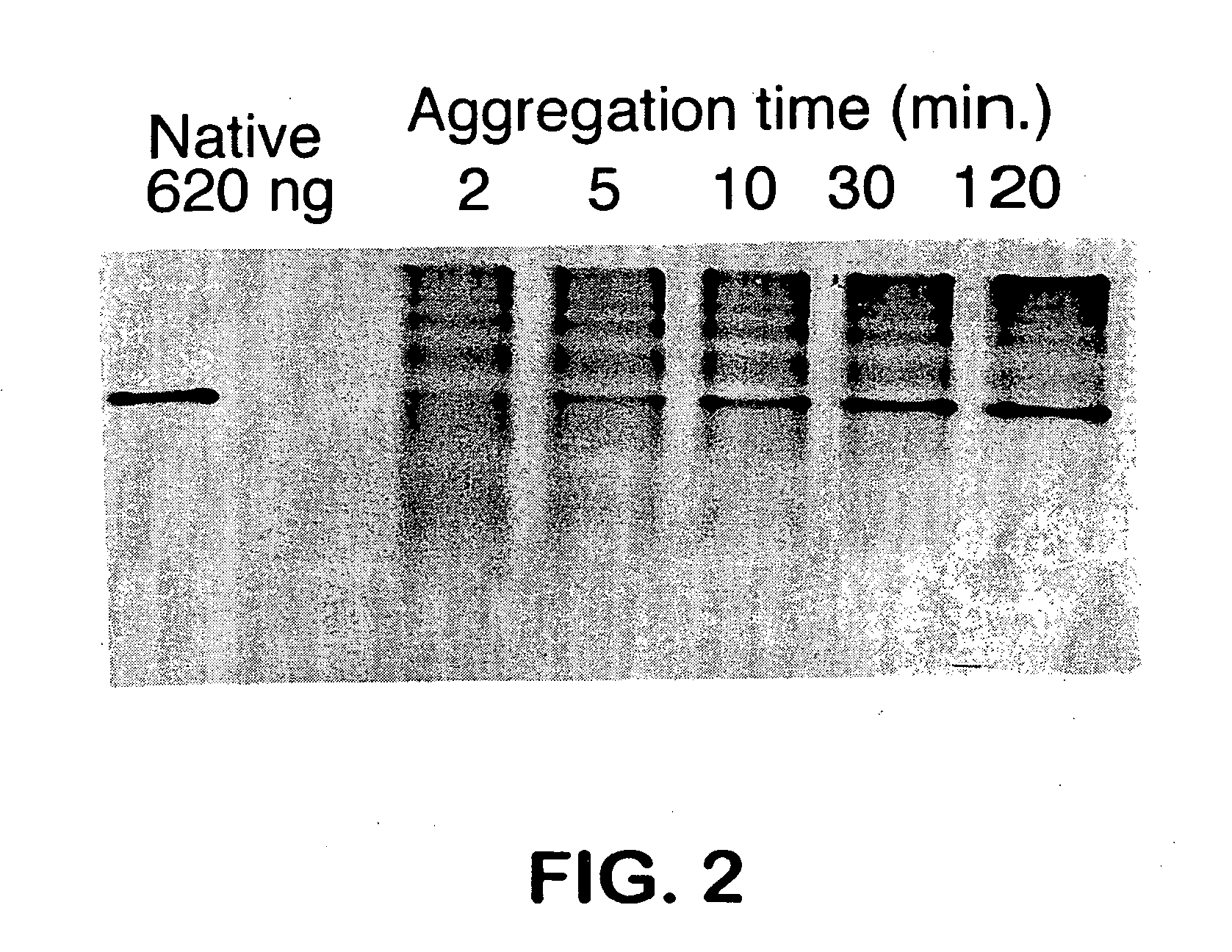
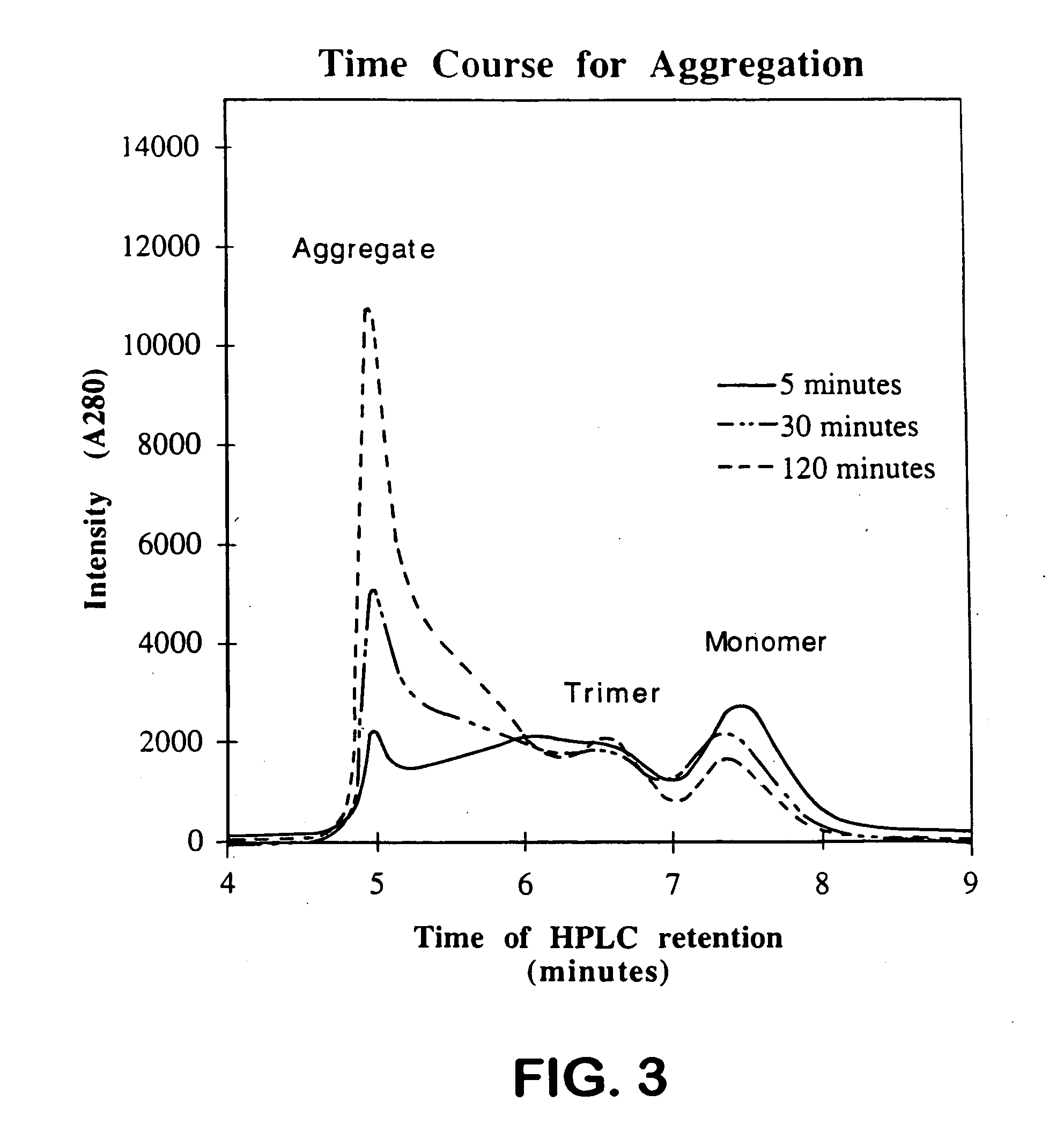
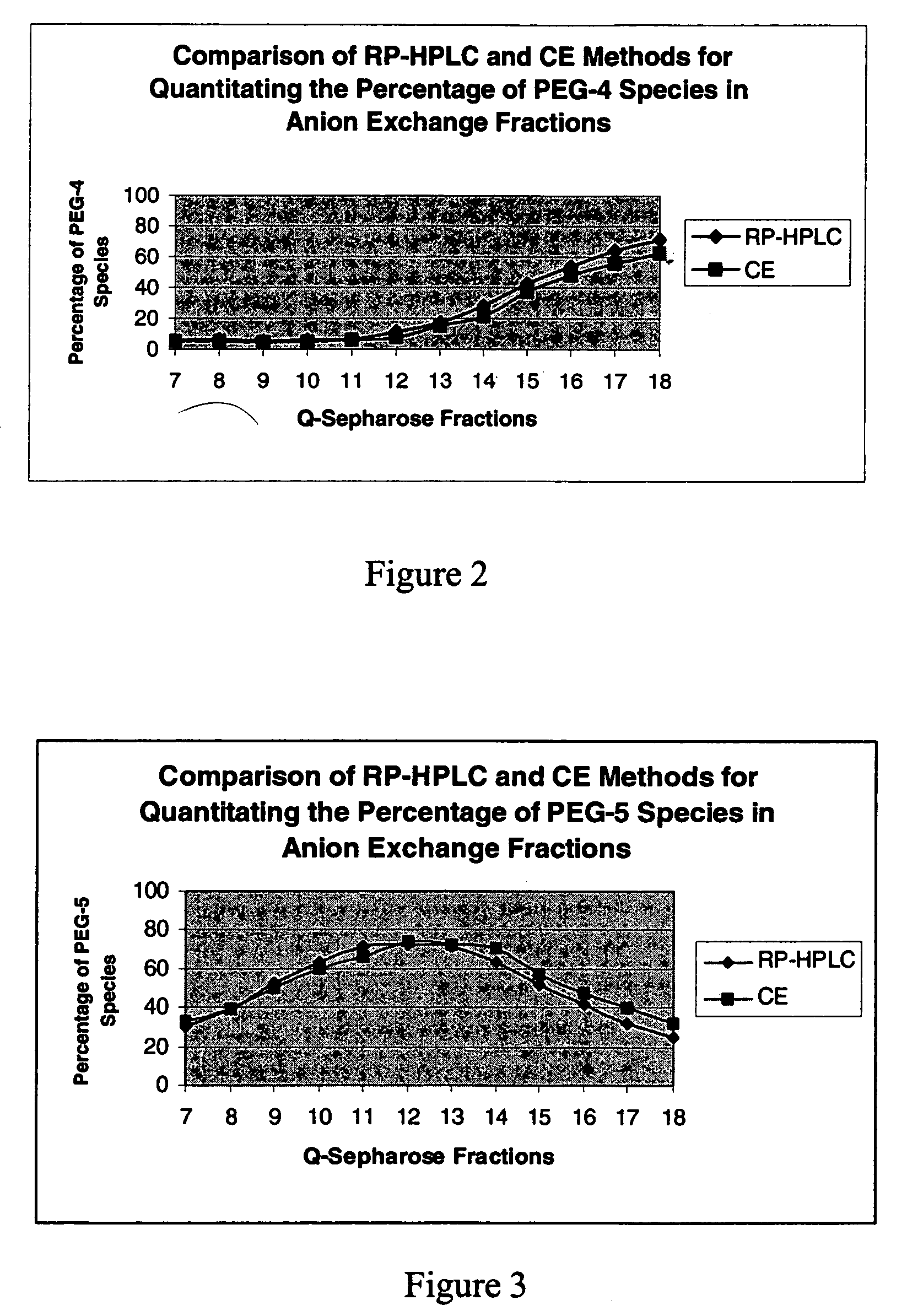
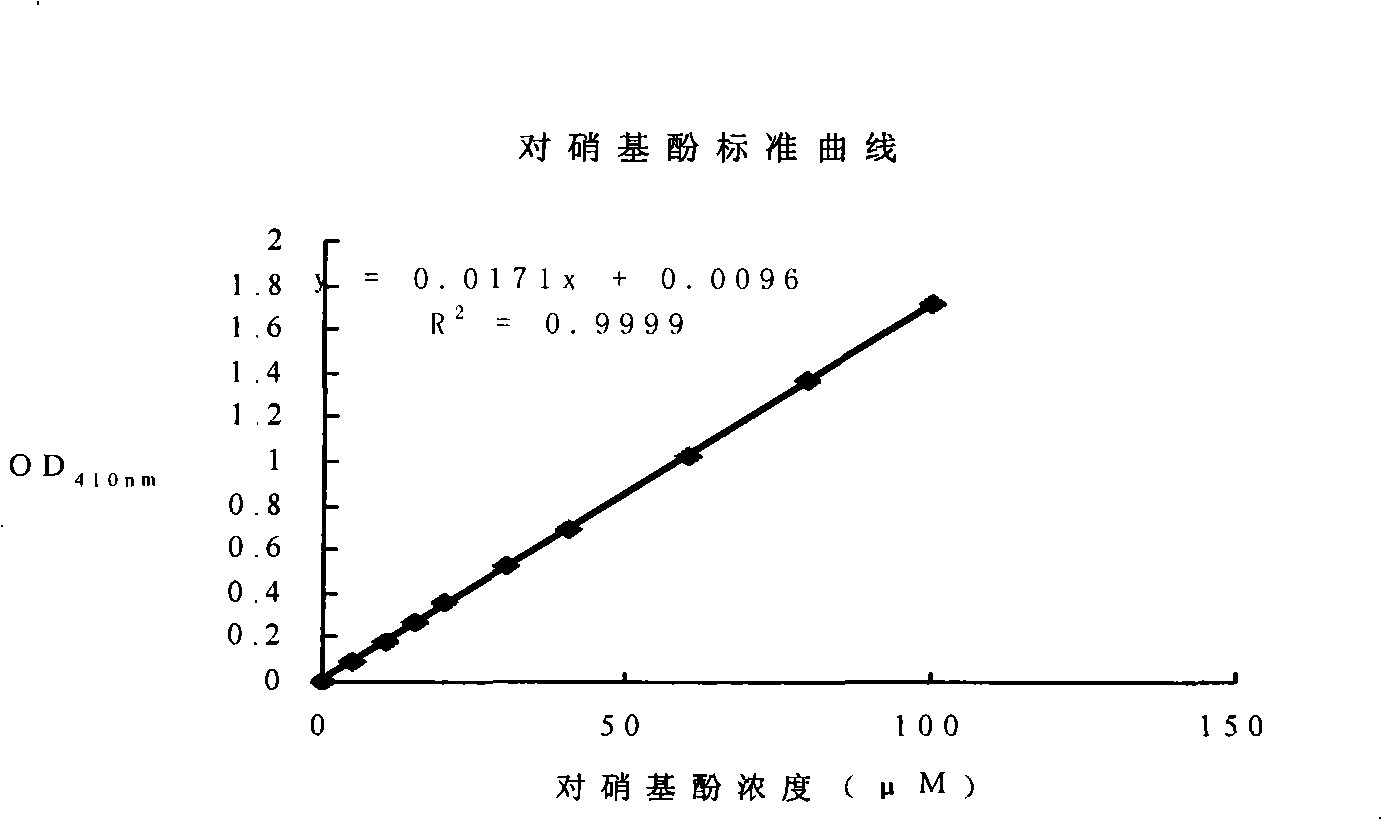
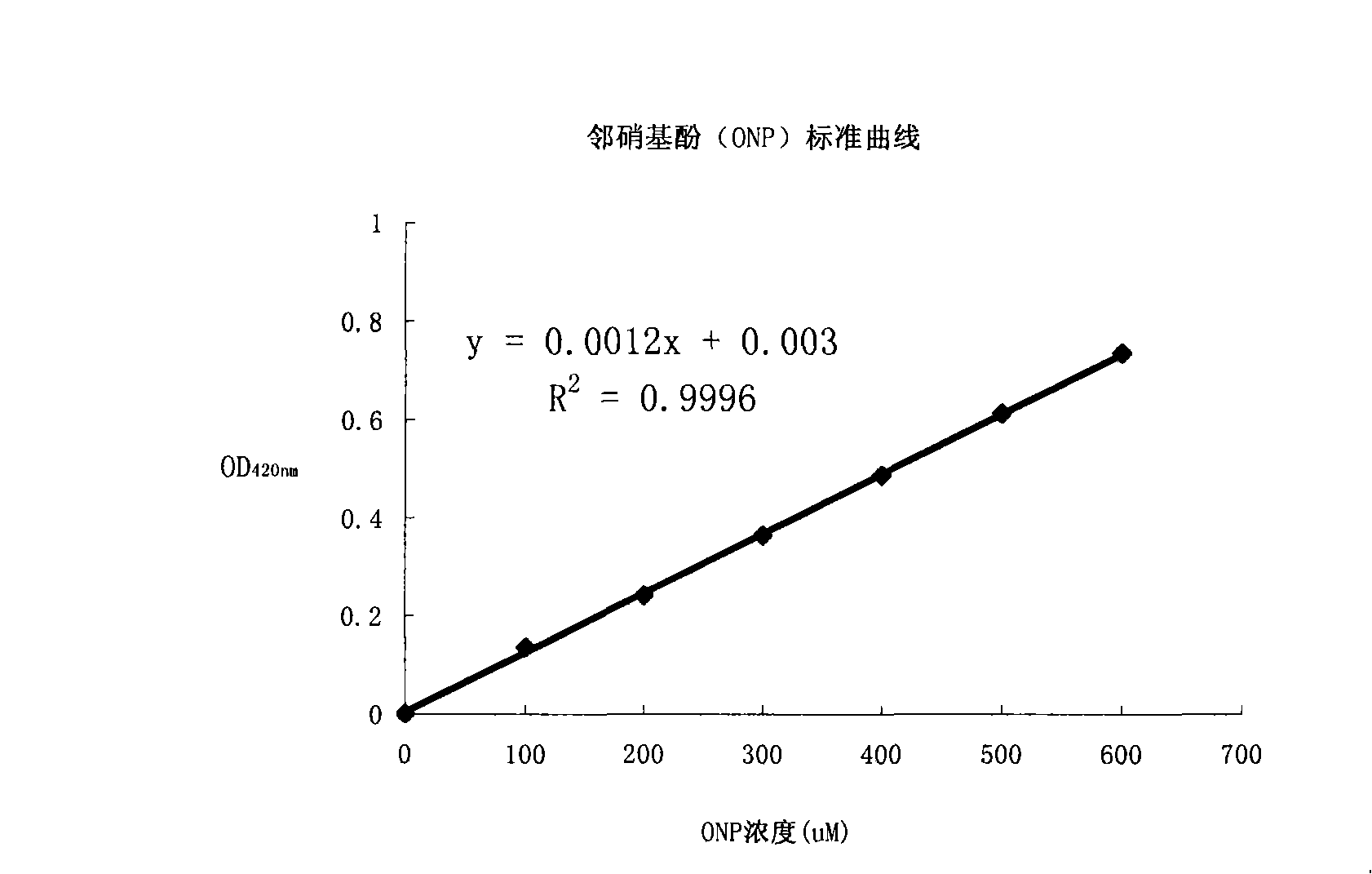
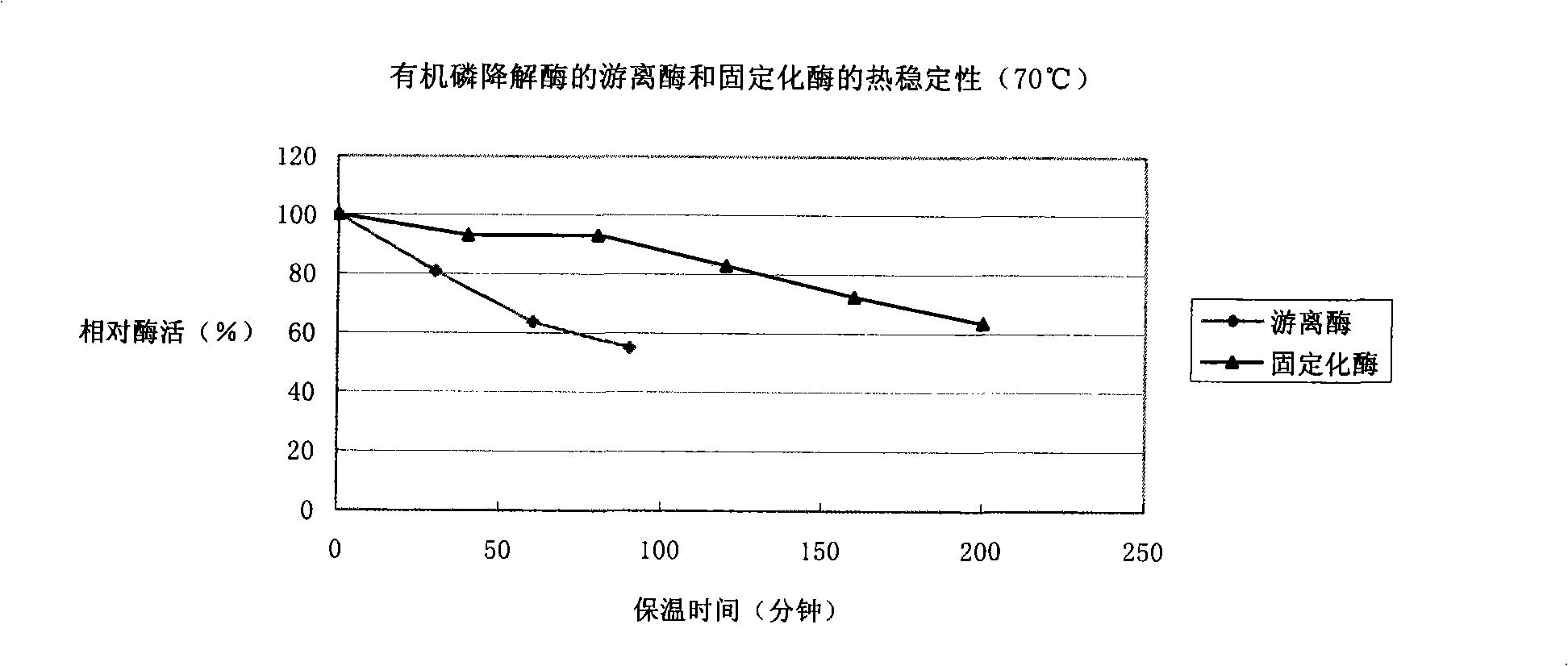
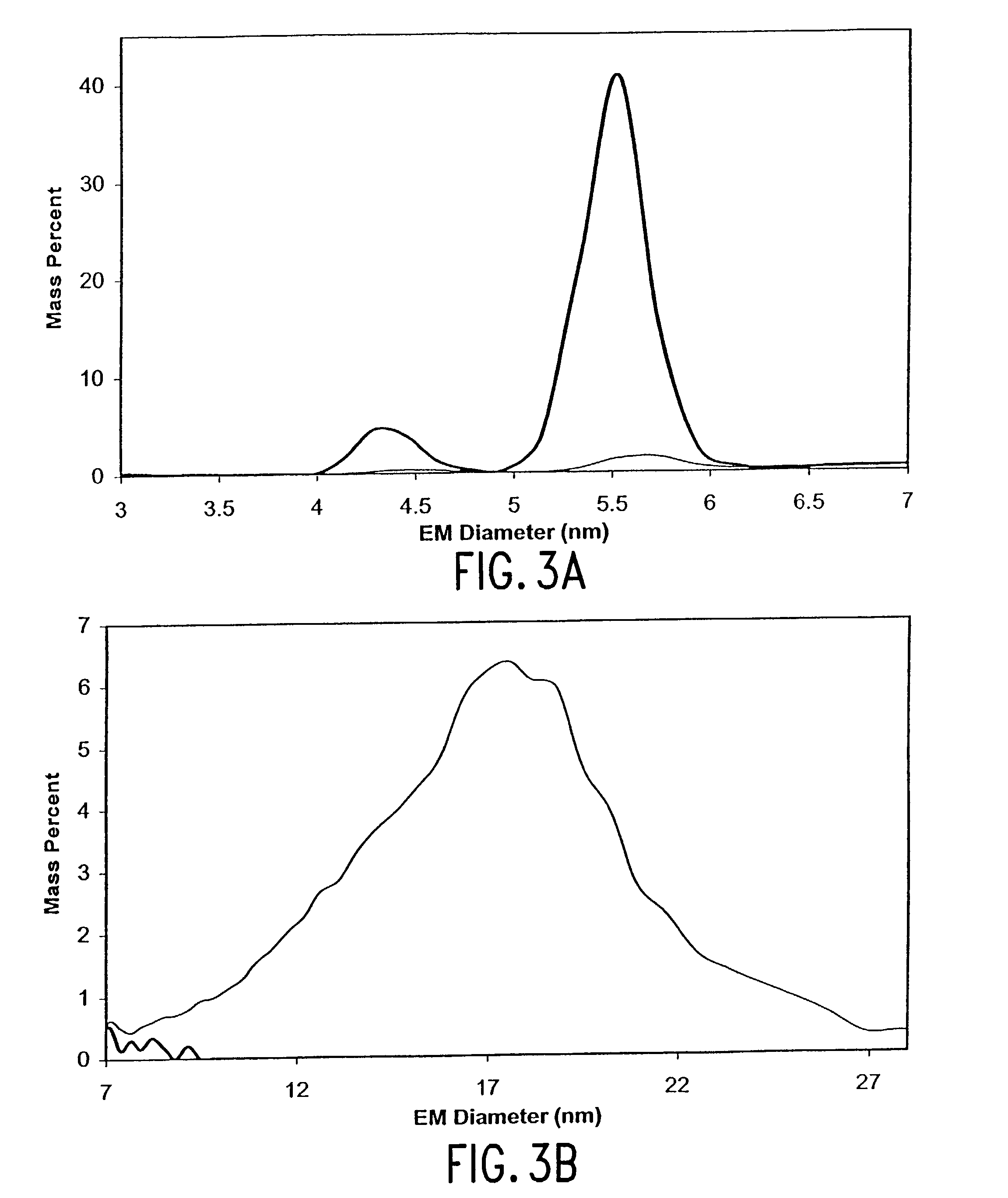
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
260 results about "Aggresome" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
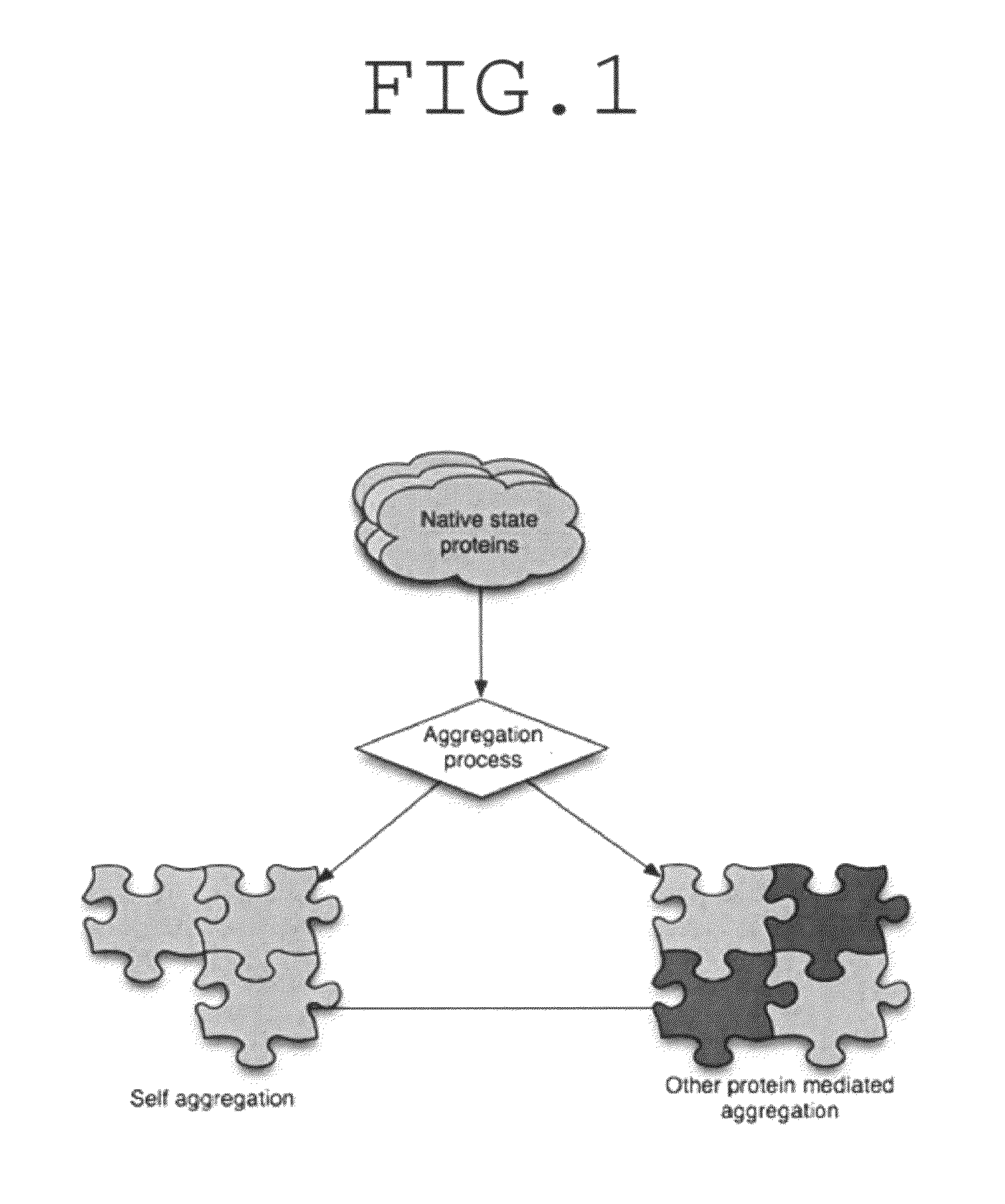
In eukaryotic cells, an aggresome refers to an aggregation of misfolded proteins in the cell, formed when the protein-degradation system of the cell is overwhelmed. Aggresome formation is a highly regulated process that possibly serves to organize misfolded proteins into a single location.
Immunogens and corresponding antibodies specific for high molecular weight aggregation intermediates common to amyloids formed from proteins of differing sequence
ActiveUS20060280733A1Low toxicityNervous disorderPeptide/protein ingredientsHigh molecular massAmyloid disease
Compositions of matter that comprise one or more conformational epitopes found on amyloid peptide aggregates, antibodies to such epitopes and methods for making and using the compositions, eptitopes and / or antibodies. The invention includes synthetic or isolated compositions that contain or consist of certain conformational epitopes that are found on peptide aggregates (e.g., toxic peptide aggregates) present in human or veterinary patients who suffer from, or who are likely to develop, amyloid diseases (e.g., Alzheimer's Disease). The invention includes methods for the detection, treatment and prevention of diseases in humans or animals, using such compositions. The invention further includes antibodies which bind to the conformational epitopes as well as methods for making such antibodies and methods for the detection, treatment and prevention of diseases and / or identification of potential therapies (e.g., drug screening) using such antibodies.
Owner:RGT UNIV OF CALIFORNIA
Removal of protein aggregates from biopharmaceutical preparations in a flow-through mode
InactiveUS20130245139A1Improve conductivityReduce conductivityIon-exchange process apparatusChromatographic cation exchangersBiochemistryProtein aggregation
The present invention provides novel compositions and methods for removal of protein aggregates from a sample in a flow-through mode.
Owner:MERCK PATENT GMBH
Purification of proteins using hydrophobic interaction chromatography
ActiveUS8946395B1Great purification and recovery of proteinGreat purification and recoveryVirus peptidesImmunoglobulins against cytokines/lymphokines/interferonsProtein proteinChemistry
The present invention is directed to methods for purifying a protein of interest, e.g., an antibody, from a sample comprising the protein of interest and at least one impurity, e.g., an aggregate, by employing a hydrophobic interaction chromatography (HIC) method that allows for binding of both the protein of interest and the at least one impurity under strong binding conditions. The present invention is based, at least in part, on the finding that both flow through and bind-elute techniques can be combined to achieve greater purification and recovery of a protein of interest, e.g., an antibody, under isocratic wash conditions and strong binding conditions.
Owner:ABBVIE INC
High pressure refolding of protein aggregates and inclusion bodies
The present disclosure provides an effective method for the refolding of denatured proteins in solution so that properly folded, biologically active protein in solution is recovered in high yield. The refolding takes place at pressures between about 0.25 kbar to about 3.5 kbar, advantageously at about 1.5 kbar to about 3 kbar. Typically a chaotropic agent is present at a concentration which is not effective for denaturing protein at atmospheric pressure, and optionally, oxidation-reduction reagents can be incorporated in the refolding solution so that native intramolecular disulfide bonds can be formed where that is desired. The method is applicable to substantially all proteins, especially after solubilization and / or denaturation of insoluble protein aggregates, inclusion bodies, or abnormal oligomeric (soluble) aggregates.
Owner:BARFOLD INC
Purified immunoglobulin fusion proteins and methods of their purification
InactiveUS20110129468A1Avoid insufficient purityIncrease productionAntibody mimetics/scaffoldsFusions with soluble cell surface receptorImpurityChemistry
The invention provides methods and compositions for separating impurities during the manufacture of immunoglobulin (Ig) fusion proteins. Examples of impurities which may be removed in accordance with the methods of the invention include inactive forms of the Ig fusion protein and / or aggregates.
Owner:BIOGEN MA INC
Antigen-binding molecule for eliminating aggregated antigens
InactiveUS20150353630A1Improve concentrationStrong cytotoxicityLibrary screeningImmunoglobulins against animals/humansProtein aggregationAntigen binding
The present inventors discovered that incorporating an Fc region and an antigen-binding domain whose antigen-binding activity varies depending on ion concentration into an antigen-binding molecule that binds to an aggregate-forming antigen produces an antigen-binding molecule that can preferentially clear protein aggregates in comparison to protein monomers from plasma. Use of antigen-binding molecules of the present invention allows various diseases stemming from target tissues to be treated target-tissue-specifically. Use of antigen-binding molecules of the present invention enables treatment of diseases caused by protein aggregates.
Owner:CHUGAI PHARMA CO LTD
Methods for treating protein aggregation disorders
InactiveUS20050215562A1Easy clearanceIncreased toxicityBiocideAnimal repellantsCell AggregationsChemical compound
The present invention is based, at least in part on the discovery of therapeutic agents capable of preventing, inhibiting or modulating abnormal processing, misfolding or aggregation of protein. The therapeutic agents of the invention may prevent, inhibit or modulate the formation of inclusions. The therapeutic agents of the invention may also be capable of facilitating clearance and / or blocking the cellular toxicity of inclusions to treat or ameliorate disorders characterized by protein aggregation. Compounds which bind to structural motifs commonly found in protein aggregates, such as β-sheets, would represent strong candidates for such compounds and are therefore desirable.
Owner:NEUROCHEM INT
Intravenous immunoglobulin composition
ActiveUS20070037170A1High potencyLow volumePeptide/protein ingredientsAntibody mimetics/scaffoldsPopulationSmallpox virus
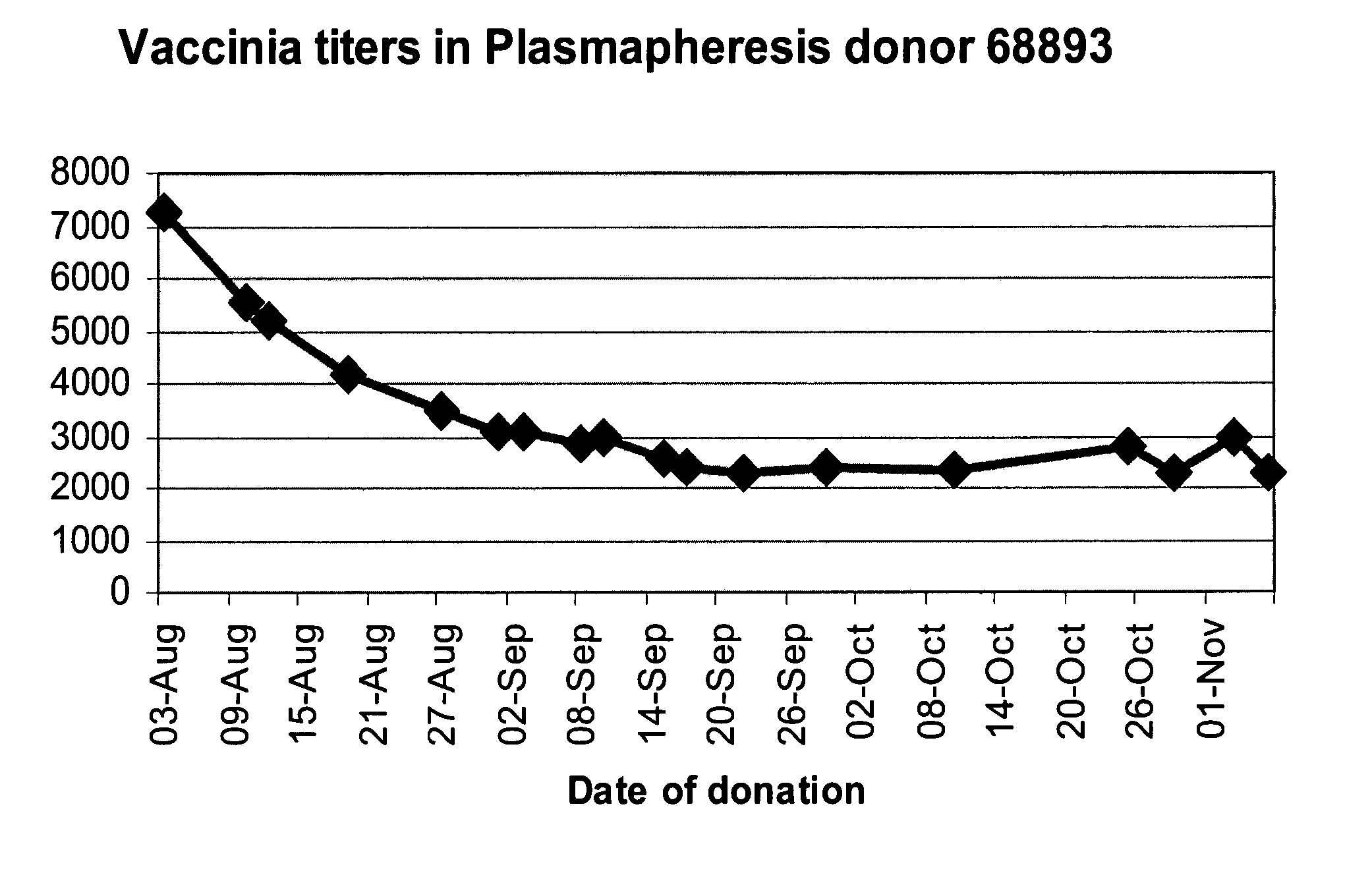
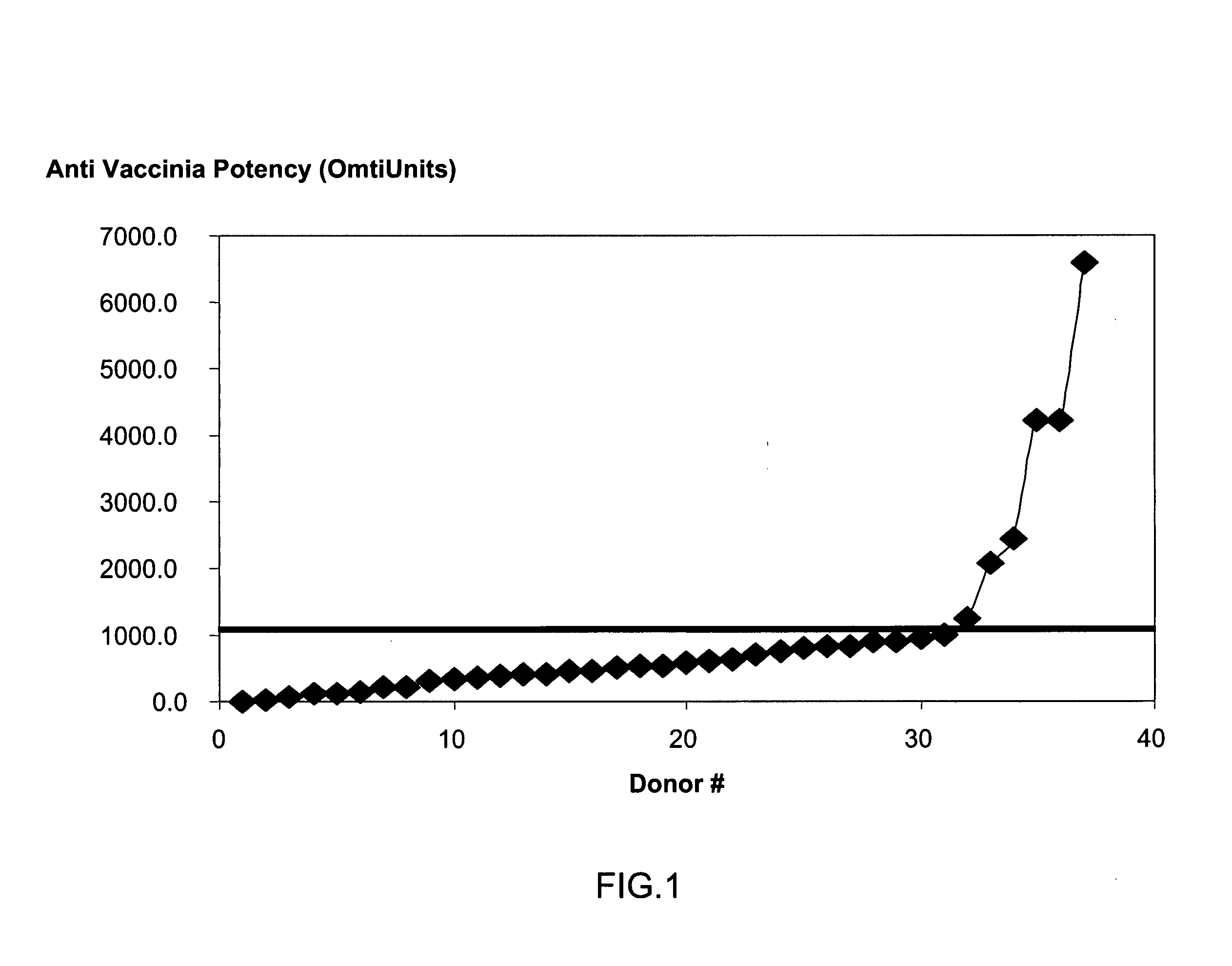
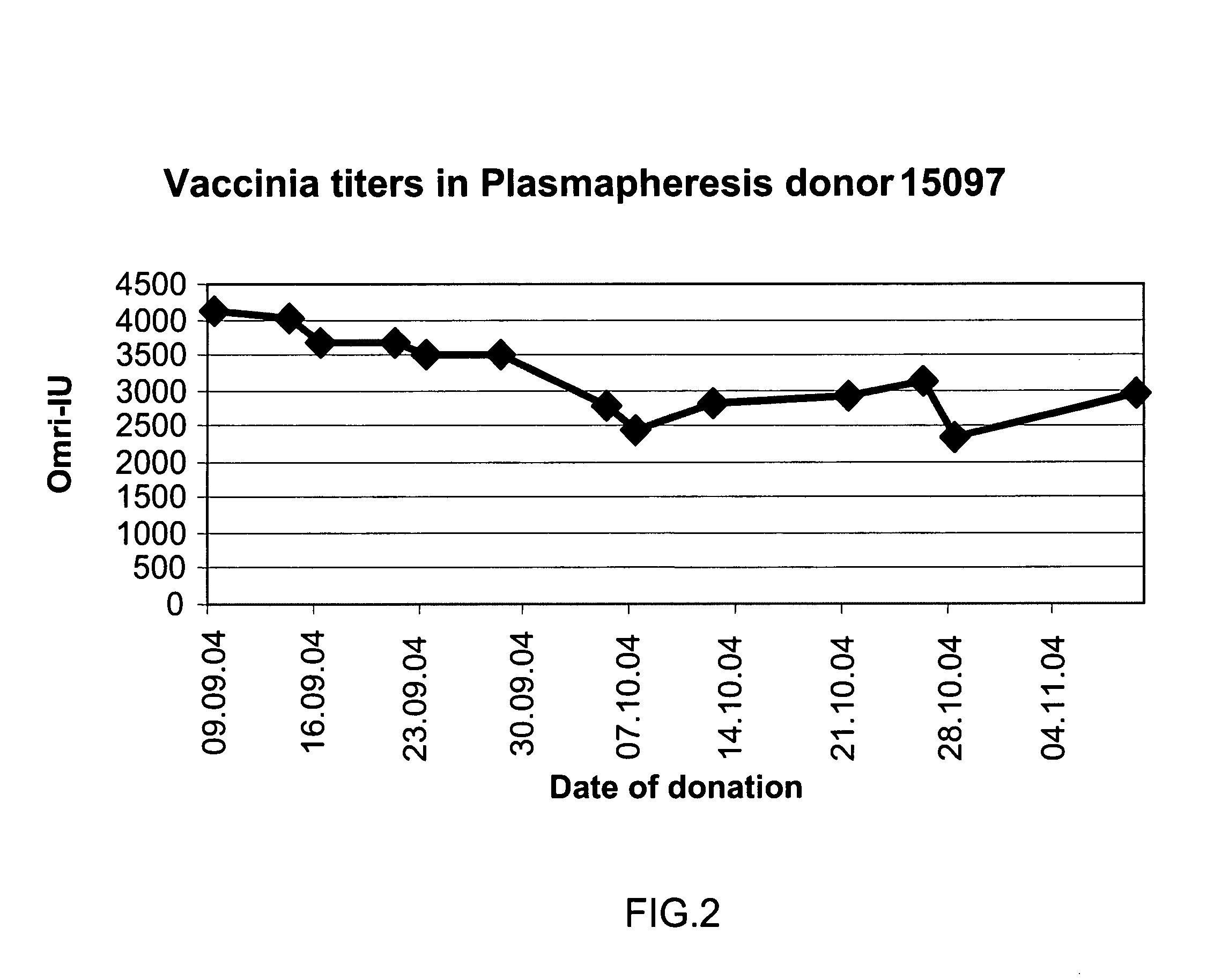
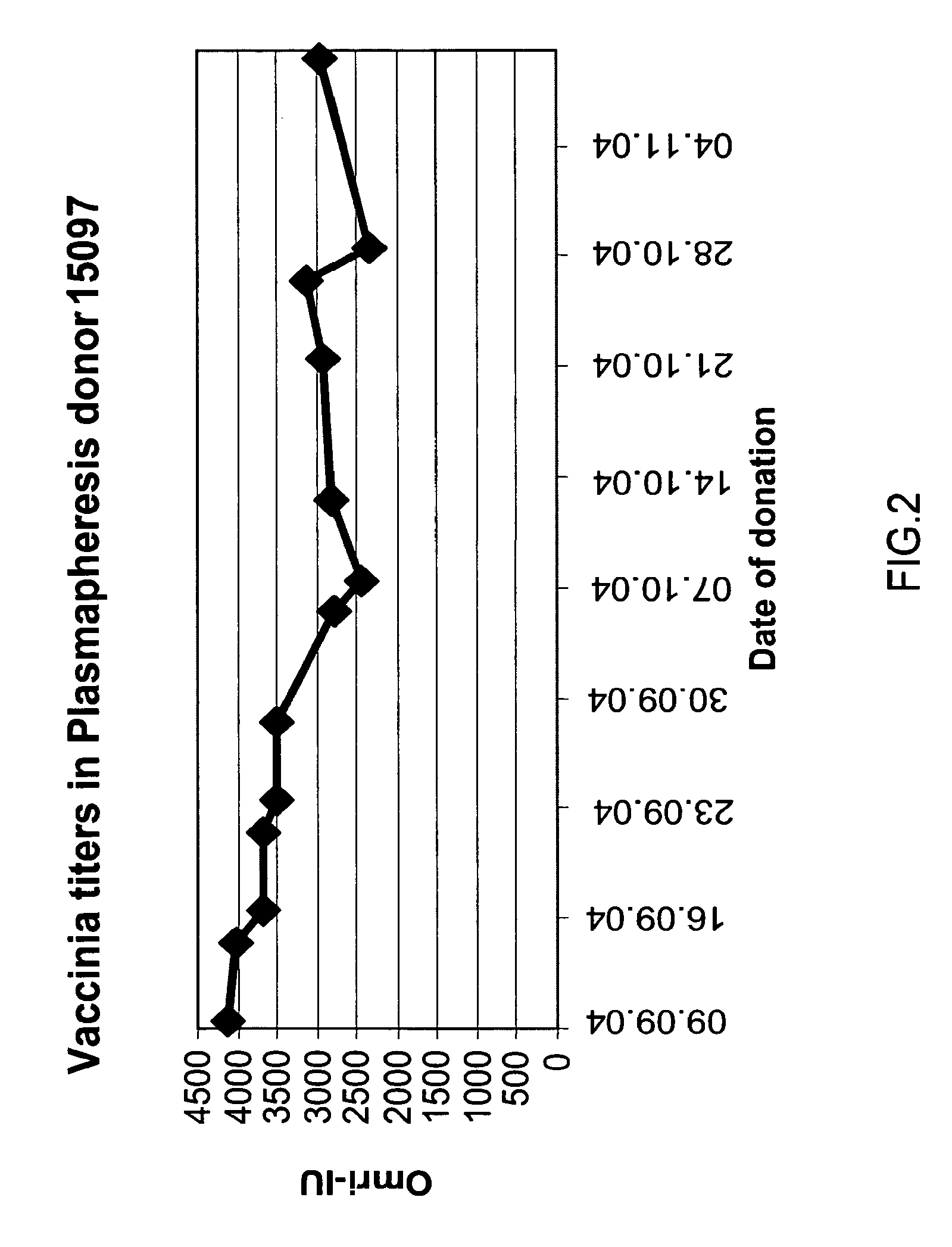
A method for preparing a concentrated, immunoglobulin composition for treating subjects vaccinated against or infected with a pathogenic microorganism, comprising: (a) selecting a population of individuals previously vaccinated against one or more antigens associated with the pathogenic microorganism; (b) determining the level of specific antibodies immunoreactive with the pathogenic microorganism in a blood or blood component of the individuals to identify very high titre individuals having a very high titre of the specific antibodies; (c) combining blood or blood components comprising immunoglobulins from the very high titre individuals; and (d) purifying and / or concentrating the product of step (c), thereby obtaining a concentrated immunoglobulin composition. Also disclosed is a concentrated immunoglobulin composition comprising specific antibodies immunoreactive with a pathogenic microorganism, characterized in that the titre of specific antibodies of the composition is at least 5 times higher than the average titre of specific antibodies of a population of individuals previously vaccinated against one or more antigens associated with the pathogenic microorganism. The composition has a relatively high protein concentration and a low percentage of protein aggregates, and is therefore suitable for both iv and im administration. In a preferred embodiment, the pathogenic microorganism is smallpox virus or vaccinia virus.
Owner:OMRIX BIOPHARM
Novel solid lipid nanoparticle medicament delivery system for protein-loaded medicaments
ActiveCN102106821AHigh activityGuaranteed stabilityPowder deliveryAerosol deliveryActive agentBULK ACTIVE INGREDIENT
The invention provides novel solid lipid nanoparticles for protein medicaments and a preparation method thereof. Each solid lipid nanoparticle consists of two parts, namely a core and a shell, wherein the core is that a biosurfactant forms a micelle or an aggregate to wrap active ingredients, or / and the biosurfactant and the active ingredients form a synergic aggregate; and the shell is that a solid lipid material is adopted for encapsulating the core. A novel solid lipid nanoparticle medicament delivery system has extremely high safety, can effectively protect the biological activity of polypeptides and polypeptide medicaments and remarkably improve the stability of preparations of the polypeptides and the polypeptide medicaments at the same time, can be applied in multiple ways such as injection, non-injection and the like, and achieves better bioavailability.
Owner:SICHUAN UNIV
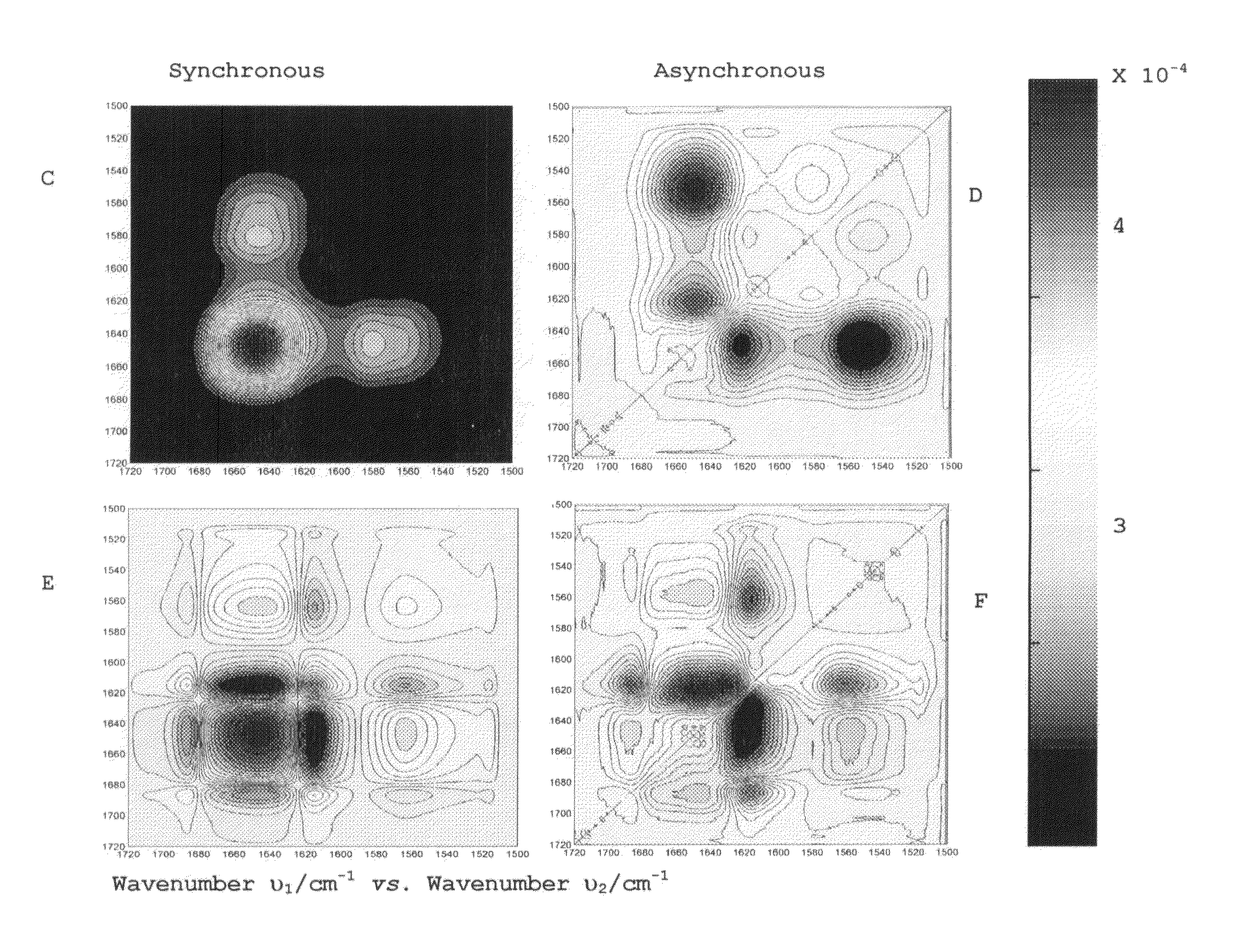
Method for determination of protein, peptide or peptoid aggregation, stability, and viability and system using the same
ActiveUS8268628B1High resolutionEasy to understandMaterial analysis by observing effect on chemical indicatorAnalysis by material excitationCorrelation analysisPeptoid
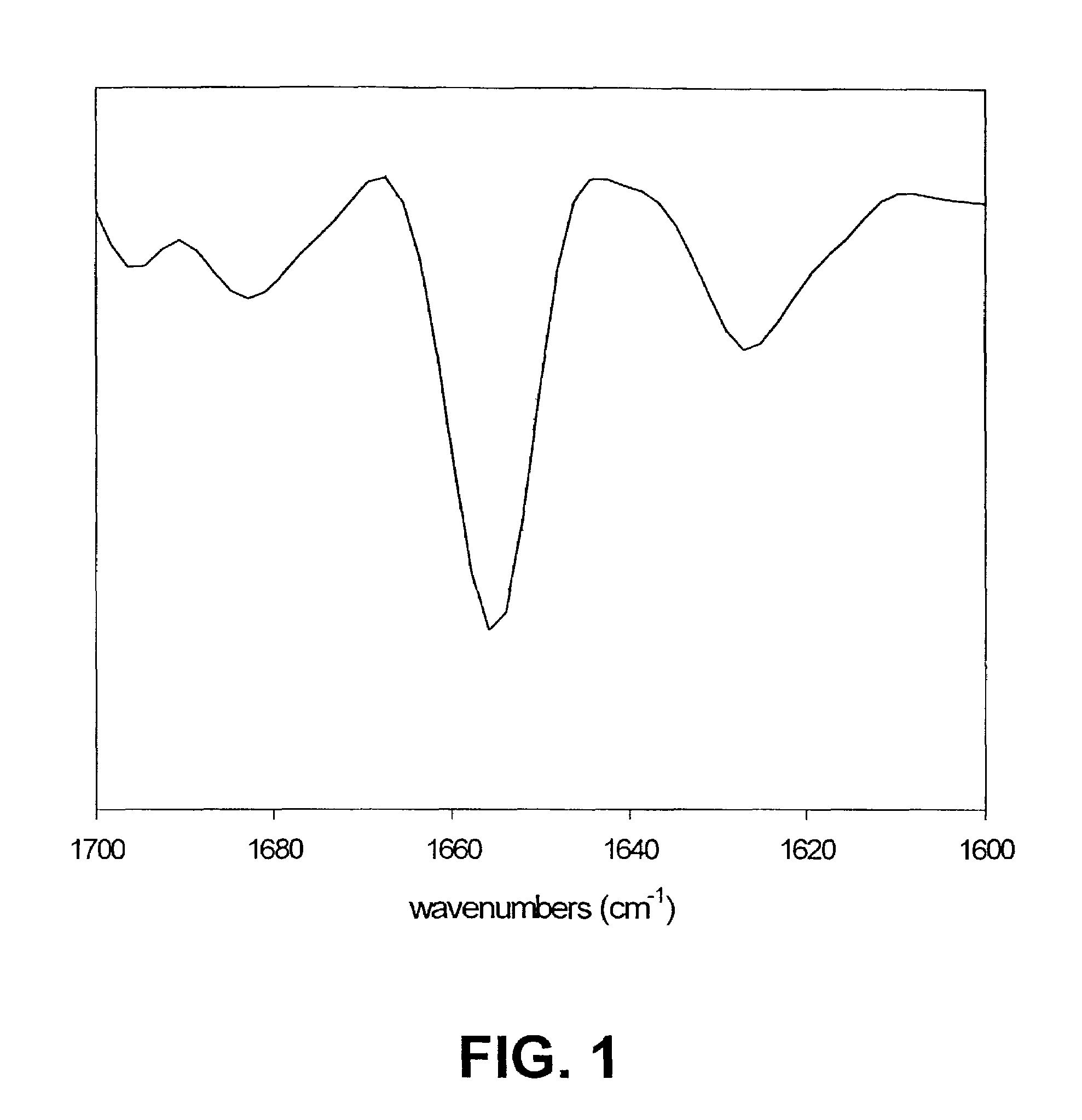
The invention describes a method for determining aggregation in protein, peptide or peptoid formulation, without the use of probes or additives. The method uses FTIR spectroscopy combined with the two-dimensional correlation analysis (2DCOS) which allows for the determination of the presence of aggregates, the determination of the mechanism of aggregation, allowing for correction in the pipeline manufacturing process of the protein to once again generate viable protein. In addition, the thermal transition of the protein can also be determined and a 2DCOS plot generated to compare with the established viable protein, allowing for quality control, stability and viability of the desired protein product. The ease of sample preparation and data analysis allows for the automation of this method.
Owner:UNIVERSITY OF PUERTO RICO +1
Detection of Promiscuous Small Submicrometer Aggregates
InactiveUS20100015721A1Material analysis by observing effect on chemical indicatorChemiluminescene/bioluminescenceNon specificProtein formation
The invention provides methods for the detection of aggregating molecules that are capable of promiscuous or non-specific binding to proteins in a time efficient manner without the use of labels.
Owner:SRU BIOSYST
Intravenous immunoglobulin composition
ActiveUS20130011388A1Reduce riskEasy to managePeptide/protein ingredientsAntibody mimetics/scaffoldsSpecific antibodyHigh titre
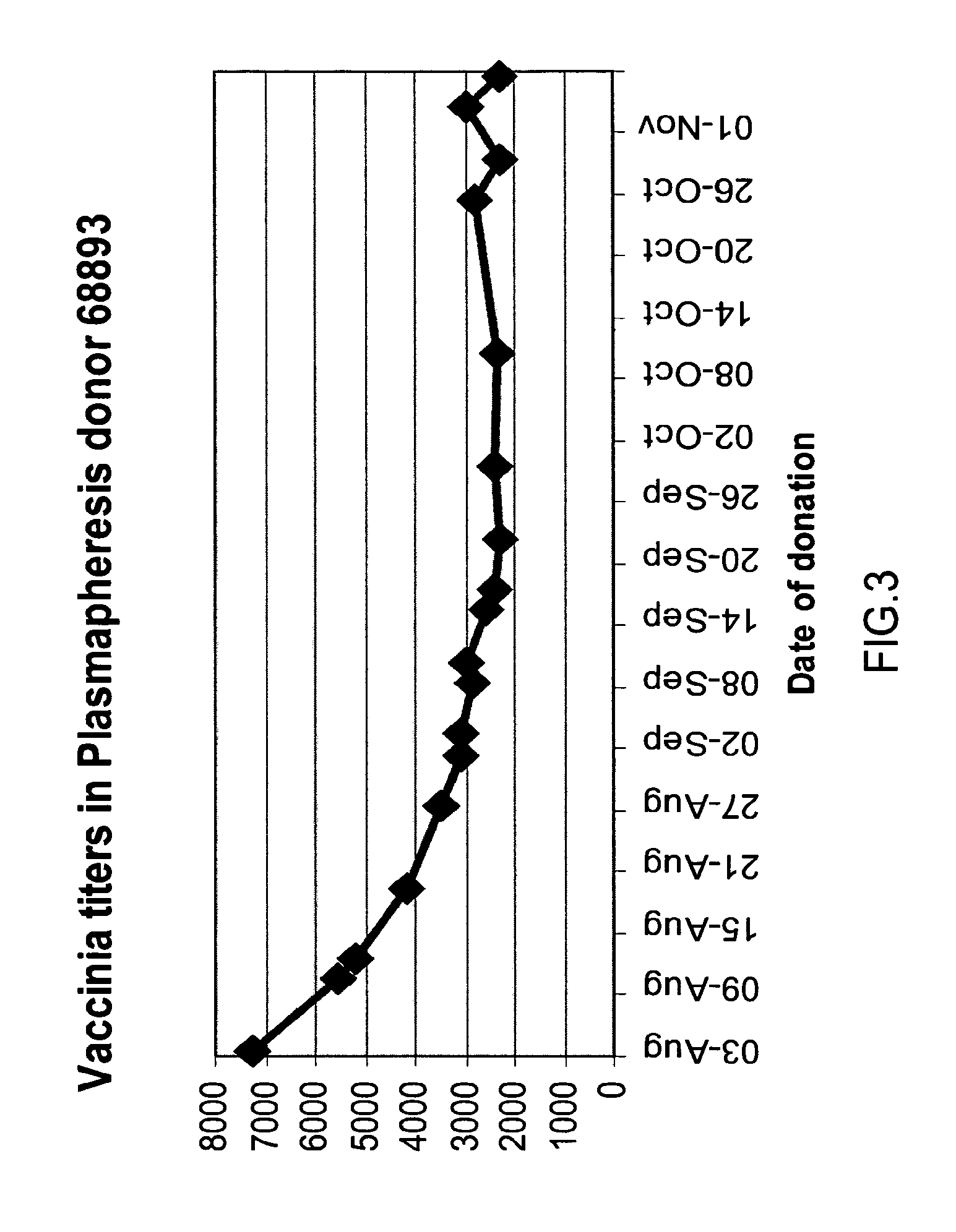
A concentrated, immunoglobulin composition for treating subjects vaccinated against or infected with a pathogenic microorganism, is made by (a) selecting a population of individuals previously vaccinated against antigens associated with the pathogenic microorganism; (b) identifying very high titre individuals by determining the level of specific antibodies immunoreactive with the pathogenic microorganism in the blood of the individuals; (c) combining blood from the very high titre individuals; and (d) purifying and / or concentrating the product of step (c). A concentrated immunoglobulin composition can include specific antibodies immunoreactive with a pathogenic microorganism, wherein the titre of specific antibodies is at least 5 times higher than the average titre of specific antibodies of a population of individuals previously vaccinated against antigens associated with the pathogenic microorganism. The composition has a relatively high protein concentration and a low percentage of protein aggregates. The pathogenic microorganism is preferably smallpox virus or vaccinia virus.
Owner:OMRIX BIOPHARM
Method and device for ultrasound assisted particle agglutination assay
InactiveUS20090053688A1Accurate assessmentSensitive detectionBioreactor/fermenter combinationsBiological substance pretreatmentsActive matterAgglutination assay
Ultrasound-assisted particle agglutination assay methods and apparatuses are described based on first providing a standing wave ultrasound field at a resonance frequency of a test liquid in a resonator cell containing microparticles covered with a binding agent with high affinity to an analyte sought to be detected by the assay test. Formation of the specifically-bound and nonspecifically-bound aggregates of these microparticles is then followed by effective stirring of the liquid with swept-frequency sonication causing disintegration of nonspecifically-bound aggregates and leaving specifically-bound aggregates in place for further detection and measurement. The methods and devices of the invention allow significant improvement in the sensitivity and specificity of agglutination tests and are advantageously applicable to detecting various proteins, DNA, RNA and other biologically active substances. Specific examples are provided.
Owner:ALLIED INNOVATIVE SYST
Method for purifying protein of enzyme aggregate based on self-aggregation short-peptide induction
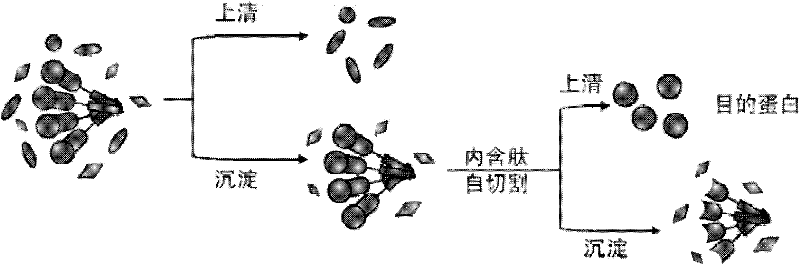
ActiveCN102226172AHigh purityOvercoming Application BottlenecksHydrolasesMicroorganism based processesProtein targetIntein
The invention provides a method for purifying protein of enzyme aggregate based on self-aggregation short-peptide induction. The method comprises the following steps of: fusing and expressing a target protein, a peptide section (preferably intein) containing a cleavage site and a short peptide with self-aggregation function into a triplet in a sequence from left to right or from right to left, forming an enzyme aggregate in an expressing process, and separating from most soluble impurities in cell lysate by adopting a centrifuging or filtering method; and then releasing the target protein into solution by inducing cutting at a cleavage site so as to achieve the purpose of purification. The method has the characteristics of low cost and simple flow, can be used for laboratory-scale high-throughput protein purification and is also beneficial to industrial-scale protein production.
Owner:TSINGHUA UNIV
Method For The Removal Of Aggregate Proteins From Recombinant Samples Using Ion Exchange Chromatography
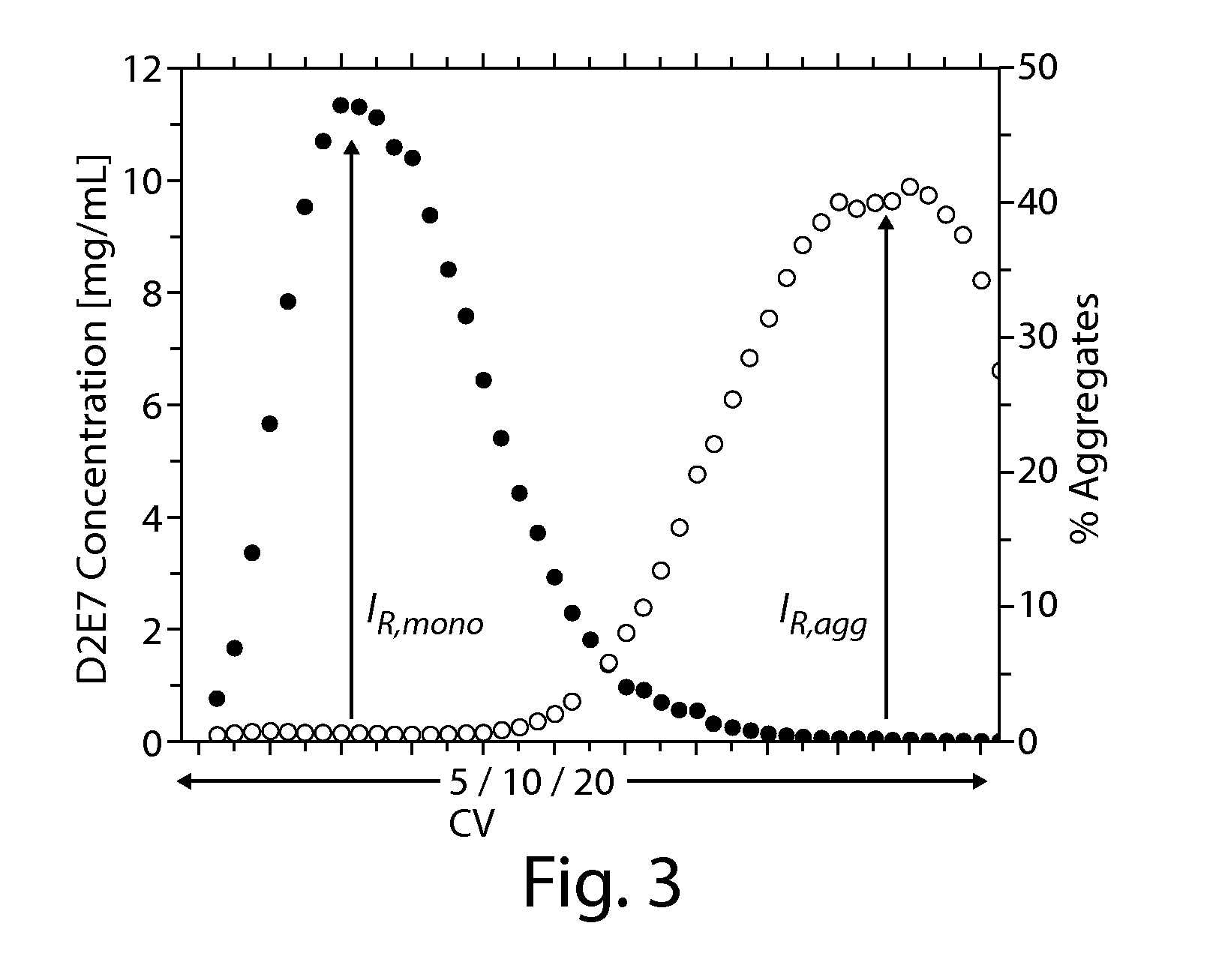
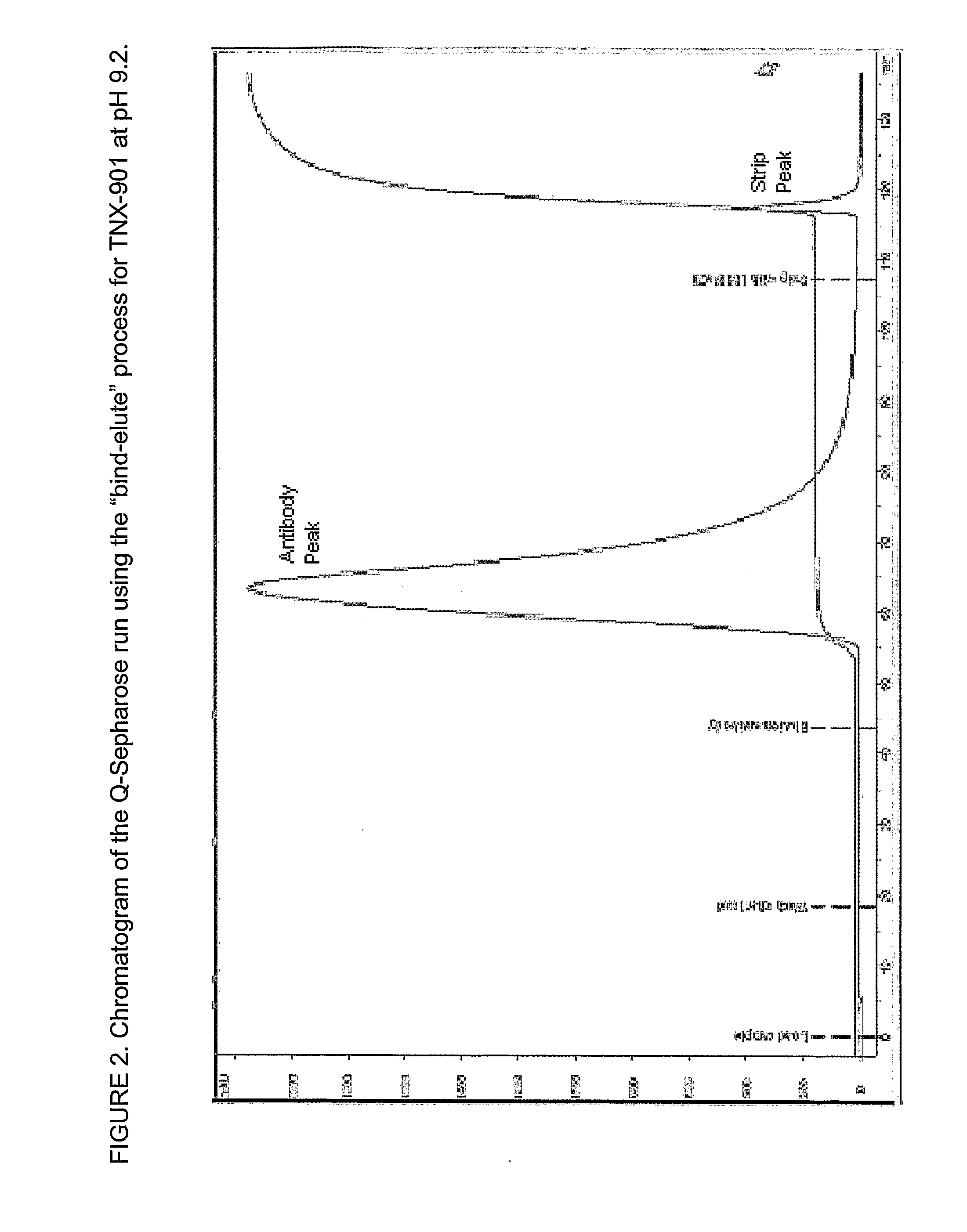
InactiveUS20080058507A1Efficient removalEfficient separationSerum immunoglobulinsDepsipeptidesRetention timeProtein aggregation
The present invention relates to processes for the removal of unwanted protein aggregates from antibody preparations. One process involves removal when the aggregate and antibody are very close in pI value (“Bind-Elute” process). Another process involves the removal when the aggregate and antibody are very close in net electric charge and retention time on ion exchange resin (“Bind-Washout” process). Using either process, at least 90% of the antibody is recovered and 70-90% of the aggregate is removed.
Owner:GENENTECH INC
Soluble cytoplasmic expression of heterologous proteins in escherichia coli
InactiveUS20130273585A1Specific activityPeptide/protein ingredientsMicrobiological testing/measurementEscherichia coliHeterologous
Soluble variants of recombinant proteins produced in a prokaryotic host cell, where the high expression levels often cause the original proteins to aggregate into insoluble inclusion body aggregates. The variant polypeptides retain biological function while increasing protein solubility with comparable or higher recoverable levels of biologically active protein when expressed in a suitable expression host. Methods of identifying critical residues and substituting them are provided to produce the variants.
Owner:GANGAGEN
Purified immunoglobulin fusion proteins and methods of their purification
InactiveCN102239177AAntibody mimetics/scaffoldsFusions with soluble cell surface receptorIntravenous gammaglobulinBiochemistry
The invention provides methods and compositions for separating impurities during the manufacture of immunoglobulin (Ig) fusion proteins. Examples of impurities which may be removed in accordance with the methods of the invention include inactive forms of the Ig fusion protein and / or aggregates.
Owner:BIOGEN MA INC
Encapsulation of supported animal cells using gas-phase inorganic alkoxides
InactiveUS6214593B1Avoiding antibodyAvoiding immune-cell invasive actionBiocidePharmaceutical delivery mechanismCell adhesionReactive gas
A suspension of animal cells is incubated with supports to adhere the cells to the supports. Preferably, the supports have pores that provide pore volume, and the cells are grown during incubation until most of the available pore volume is filled with cells. An encapsulating layer is then formed around the supported cells by exposing the cells on the supports to a reactive gas composed of a carrier gas such as sterile air saturated with an inorganic alkoxide followed by treatment with steam to hydrolyze residual alkoxide groups. The encapsulated cells are stored by immersion in culture media. The cells may be in the form of cell aggregates, and the supports can be sterilized. The supports and encapsulating layer can have pores of a size that permit free exchange nutrients and metabolic products, and excludes the cells from contacting antibodies or immune cells when implanted. The encapsulated cells can used in an extracorporeal device or implanted directly.
Owner:SILBIOTEC DUE
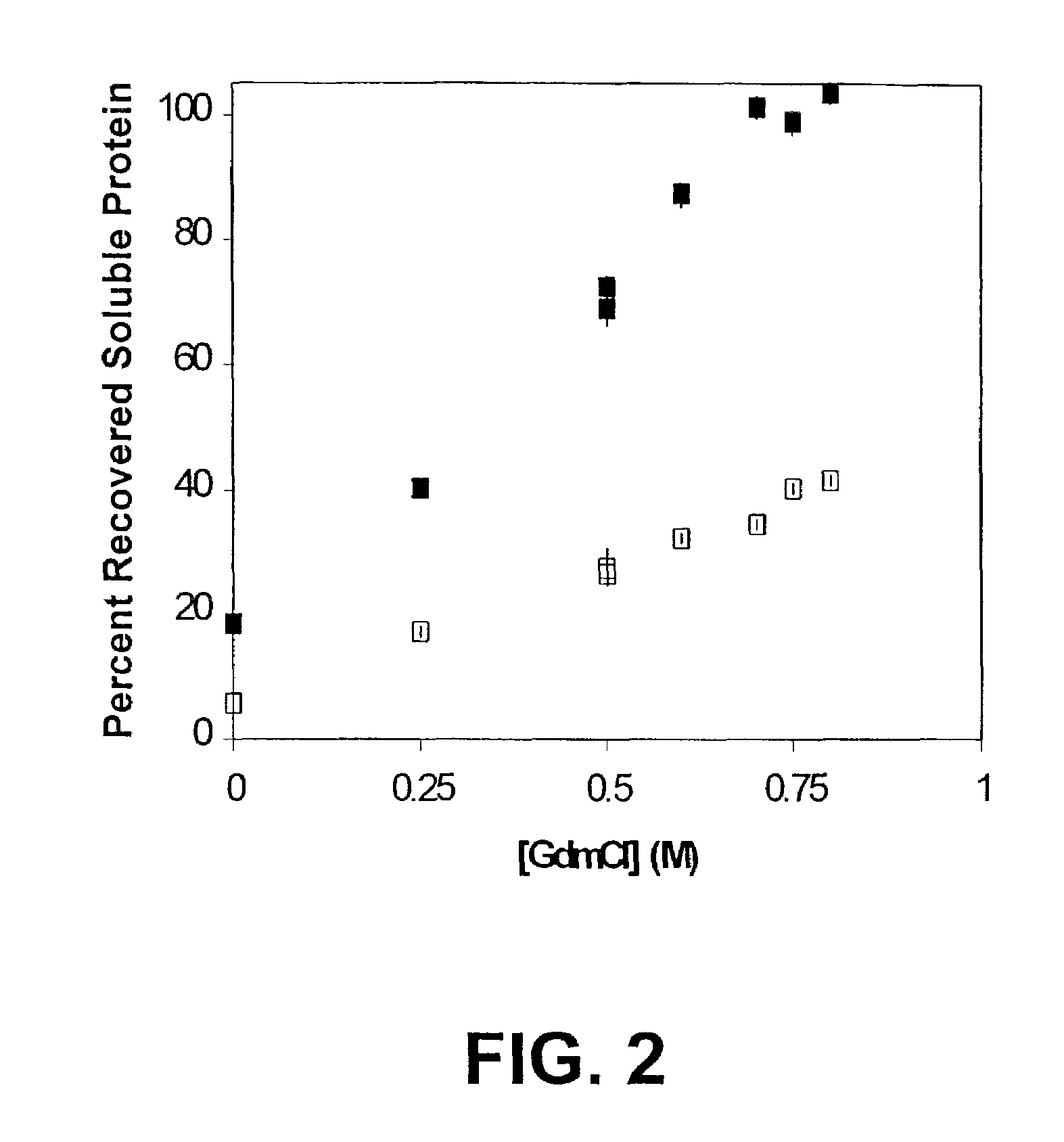
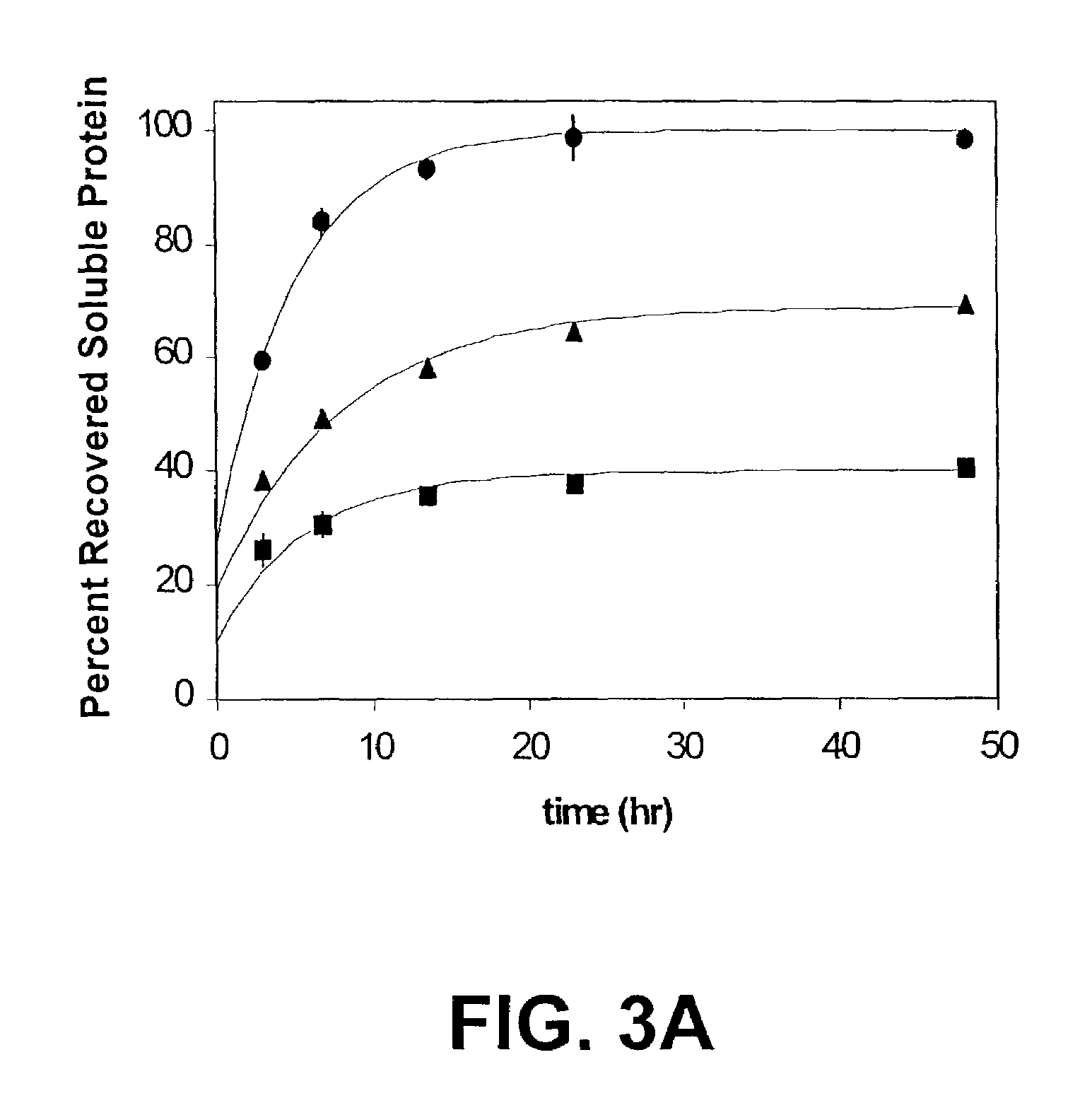
Use of hydrostatic pressure to inhibit and reverse protein aggregation and facilitate protein refolding
InactiveUS20050020818A1High protein concentrationSuitable for useDepsipeptidesPeptide preparation methodsInclusion bodiesCell Aggregations
A novel approach is described for reversing aggregation and increasing refolding by application of hydrostatic pressure. A protein of interest in an aggregated, or inclusion body, or other non-native or inactive state is subjected to high hydrostatic pressure. This treatment denatures the protein to states (or conformations) competant for refolding and results in increased formation of native protein once pressure is released. The technique can facilitate conversion non-native proteins, including inclusion bodies and aggregates to native proteins without addition of chaotropic agents, changes in buffer, or large-scale dilution of reagents required for traditional refolding methods.
Owner:BARFOLD INC +1
Process for the purification of interleukin-4 and its muteins
InactiveUS20060134690A1Efficiently disruptedRefold efficientlyBiological material analysisBiological testingEscherichia coliInsoluble protein
This invention relates generally to a method for purifying Interleukin-4 or related variants from an Escherichia coli culture medium, where Interleukin-4 and its derivatives are expressed as insoluble protein aggregates, so-called inclusion bodies.
Owner:BAYER SCHERING PHARMA AG
Solid phase support crosslinking enzyme aggregation and method for preparing the same
InactiveCN101492665ASolve the shortcomings that cannot be recycled and reusedOpen up the prospect of industrial applicationHydrolasesOxidoreductasesCross-linked enzyme aggregateEnzyme protein
The invention discloses immobilized crosslinked enzyme aggregates (CLEAs) and a preparation method thereof. The crosslinked enzyme aggregates are fixed on a porous microsphere; the preparation method thereof comprises: dispersing detached enzyme needing to be immobilized into the porous microsphere; carrying out depositing and crosslinking on zymoprotein in the porous microsphere in sequence, and finally rinsing the porous microsphere, thus preparing the immobilized crosslinked enzyme aggregates. The method of the immobilized enzyme can be used for immobilizing the enzyme of various types or sources; the immobilized method is simple and easy to be carried out, is easy to be operated, does not need special devices and has low production cost; the prepared crosslinked enzyme aggregates have high stability and strong capacity for resisting trypsinase, has a certain physical form and a stable structure, can be recycled and repeatedly used and can be used for continuous industrial production.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
Methods for protein refolding
InactiveUS7538198B2Extended shelf lifeInactivation/attenuationDepsipeptidesDissolutionImproved method
The present invention discloses improved methods of disaggregating protein aggregates, and refolding denatured proteins, using high pressure. In particular, the present invention provides for the use of agitation, high temperature, “stepped” depressurization, dialysis and dilution under pressure to increase the speed and extent of aggregate dissolution and protein refolding.
Owner:BARFOLD INC
Dendrimer conjugates for selective of protein aggregates
Dendrimer conjugates are presented, which are formed between a dendrimer and a protein solubilising substance. Such dendrimer conjugates ar effective in the treatment of protein aggregate-related diseases (e.g. prion-related diseases). The protein solubilising substance and the dendrimer together show a protein aggregate solubilising effect higher than a physical mixture of the dendrimer and the protein solubilising substance (i.e. a synergistic effect). Such dendrimer conjugates are useful in the treatment or prevention of protein aggregate-relates diseases, in disinfection / decontamination processes and in classifying or identifying protein aggregates. The synthesis of such dendrimer conjugates from readily-available starting materials is described.
Owner:TECHN UNIV OF DENMARK NAT VETERINARY INST
Methods of purifying small modular immunopharmaceutical proteins
InactiveUS20120141497A1Reduce numberReduce processing timeImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsIon exchangeChemistry
The present invention provides, among other things, methods of purifying or recovering proteins, in particular, small modular immunopharmaceutical (SMIPs™) proteins, from protein preparations containing high molecular weight (HMW) aggregates and other impurities based on hydroxyapatite chromatography. In some embodiments, the hydroxyapatite chromatography is used in combination with affinity chromatography and / or ion exchange chromatography. In some embodiments, inventive methods according to the invention involve no more than three chromatography steps. The present invention also provides proteins such as SMIPs™ purified according to the invention and pharmaceutical compositions containing the same.
Owner:WYETH LLC
Aggregate-free urate oxidase for preparation of non-immunogenic polymer conjugates
InactiveUS20080057048A1Reduce immunogenicityIncrease persistencePeptide/protein ingredientsSkeletal disorderMutant proteinImmunogenicity
A naturally occurring or recombinant protein, especially a mutein of porcine urate oxidase (uricase), that is essentially free of large aggregates can be rendered substantially non-immunogenic by conjugation with a sufficiently small number of strands of polymer such that the bioactivity of the protein is essentially retained in the conjugate. Such conjugates are unusually well suited for treatment of chronic conditions because they are less likely to induce the formation of antibodies and / or accelerated clearance than are similar conjugates prepared from protein preparations containing traces of large aggregates.
Owner:HORIZON THERAPEUTICS USA INC +1
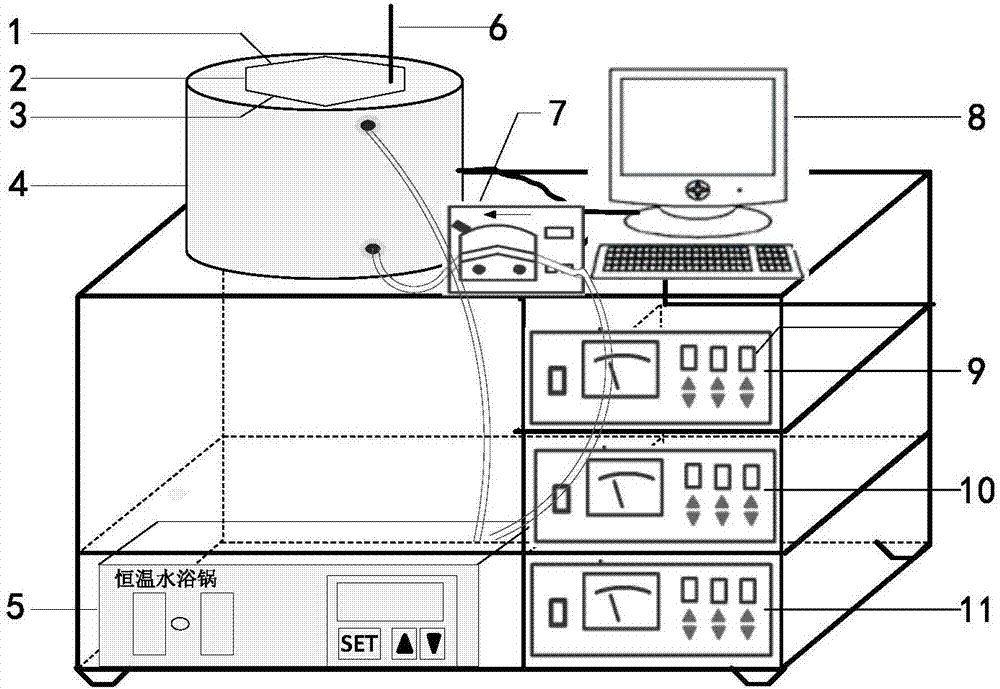
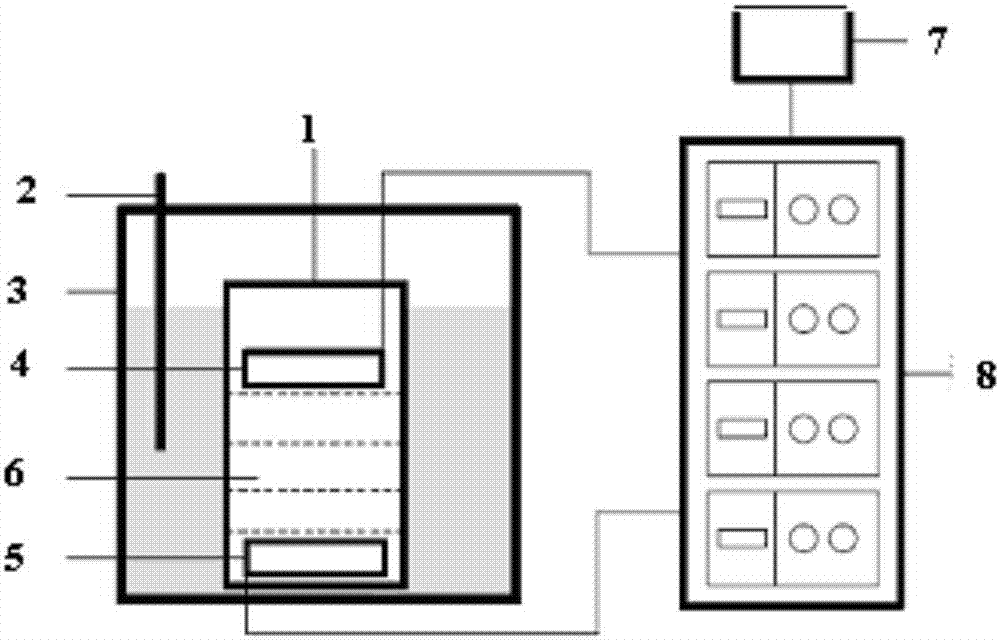
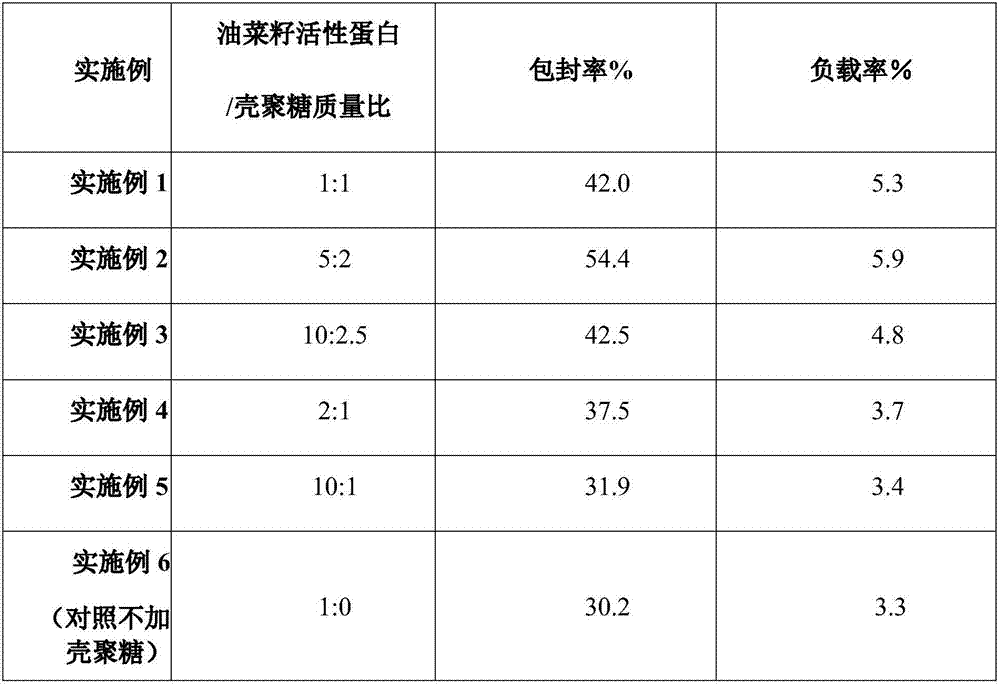
Frequency-sweeping ultrasonic preparation method of rapeseed protein-chitosan nanoparticles
InactiveCN107298773AChange the high-level structurePromote cross-linking intoMaterial nanotechnologyChitosan nanoparticlesRapeseed
The invention discloses a frequency-sweeping ultrasonic preparation method of rapeseed protein-chitosan nanoparticles, and relates to the technical field of composite nanoparticles of functional food. The rapeseed protein-chitosan nanoparticles are prepared from the following raw materials in parts by weight: 2-10 parts of active rapeseed protein and 1-2.5 parts of chitosan. The chitosan is added into the active rapeseed protein to change the advanced structure of the active rapeseed protein, so that the structure thereof is opened to expose active groups; meanwhile, the protein and polysaccharide form small aggregates, so that formation of the nanoparticles by the aggregates through hydrophobic interaction is promoted, and a foundation is laid for embedding a bioactive component. In the preparation process of the curcumin-embedded active rapeseed protein-chitosan composite nanoparticles, by a frequency-sweeping ultrasonic processing technology, cross-linking of the protein and the polysaccharide into the aggregates is promoted through the physical force of ultrasonic, so that the formation of the nanoparticles by the aggregates through the hydrophobic interaction is promoted and the foundation is laid for embedding the bioactive component.
Owner:JIANGSU UNIV
Lyophilized formulations for small modular immunopharmaceuticals
The present invention provides, among other things, stable formulations for small modular immunopharmaceutical (SMIPTM) proteins. In some embodiments, the present invention provides a formulation containing a lyophilized mixture of a small modular immunopharmaceutical protein, wherein less than 7% of the lyophilized small modular immunopharmaceutical protein exists in aggregated form. Formulations according to the invention may contain buffering agents, stabilizers, bulking agents, surfactants and / or other excipients. The present invention also provides formulations for lyophilization, reconstitution and methods of use thereof.
Owner:WYETH LLC
High pressure refolding of protein aggregates and inclusion bodies
The present disclosure provides an effective method for the refolding of denatured proteins in solution so that properly folded, biologically active protein in solution is recovered in high yield. The refolding takes place at pressures between about 0.25 kbar to about 3.5 kbar, advantageously at about 1.5 kbar to about 3 kbar. Typically a chaotropic agent is present at a concentration which is not effective for denaturing protein at atmospheric pressure, and optionally, oxidation-reduction reagents can be incorporated in the refolding solution so that native intramolecular disulfide bonds can be formed where that is desired. The method is applicable to substantially all proteins, especially after solubilization and / or denaturation of insoluble protein aggregates, inclusion bodies, or abnormal oligomeric (soluble) aggregates.
Owner:BARFOLD INC
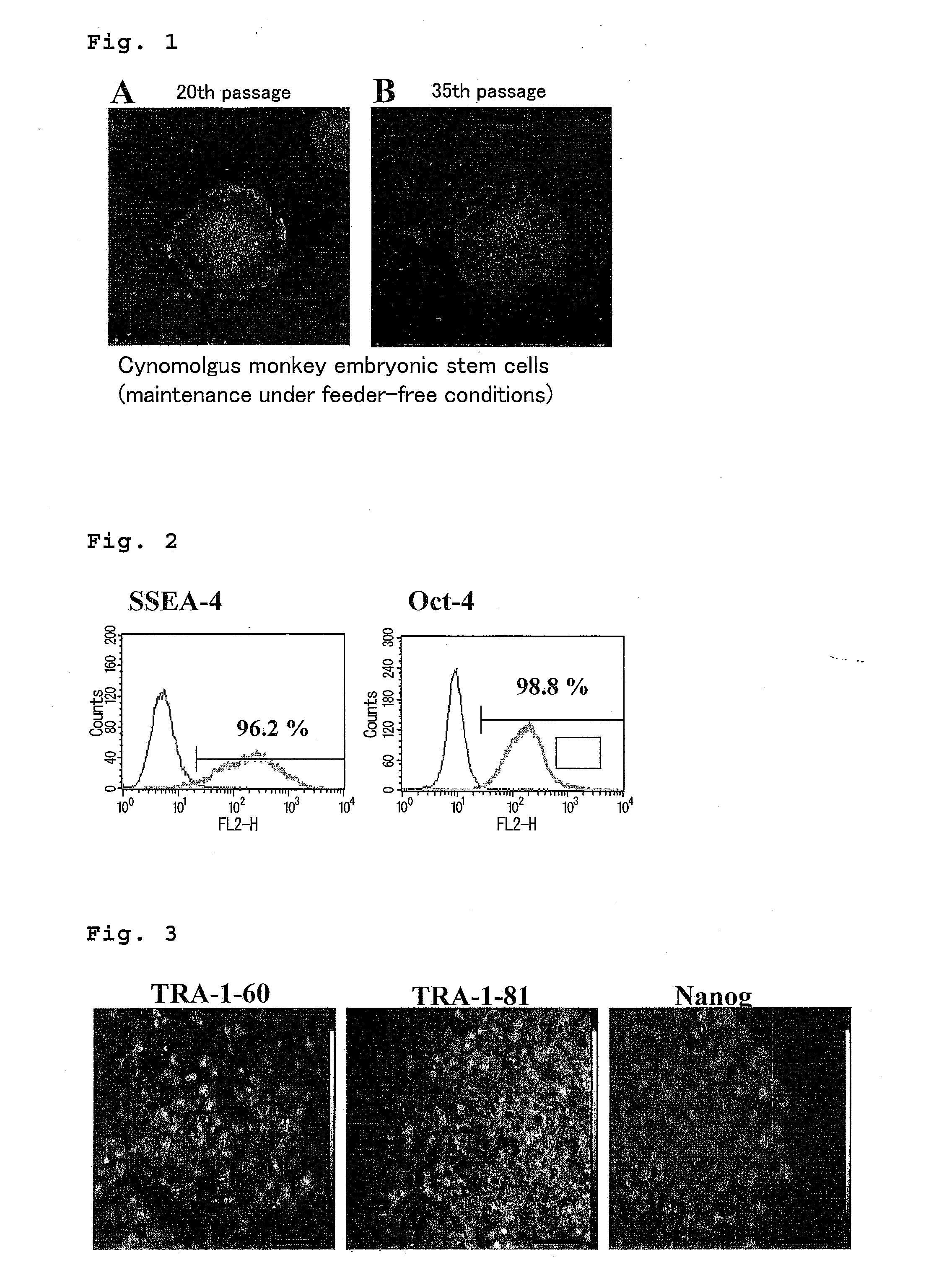
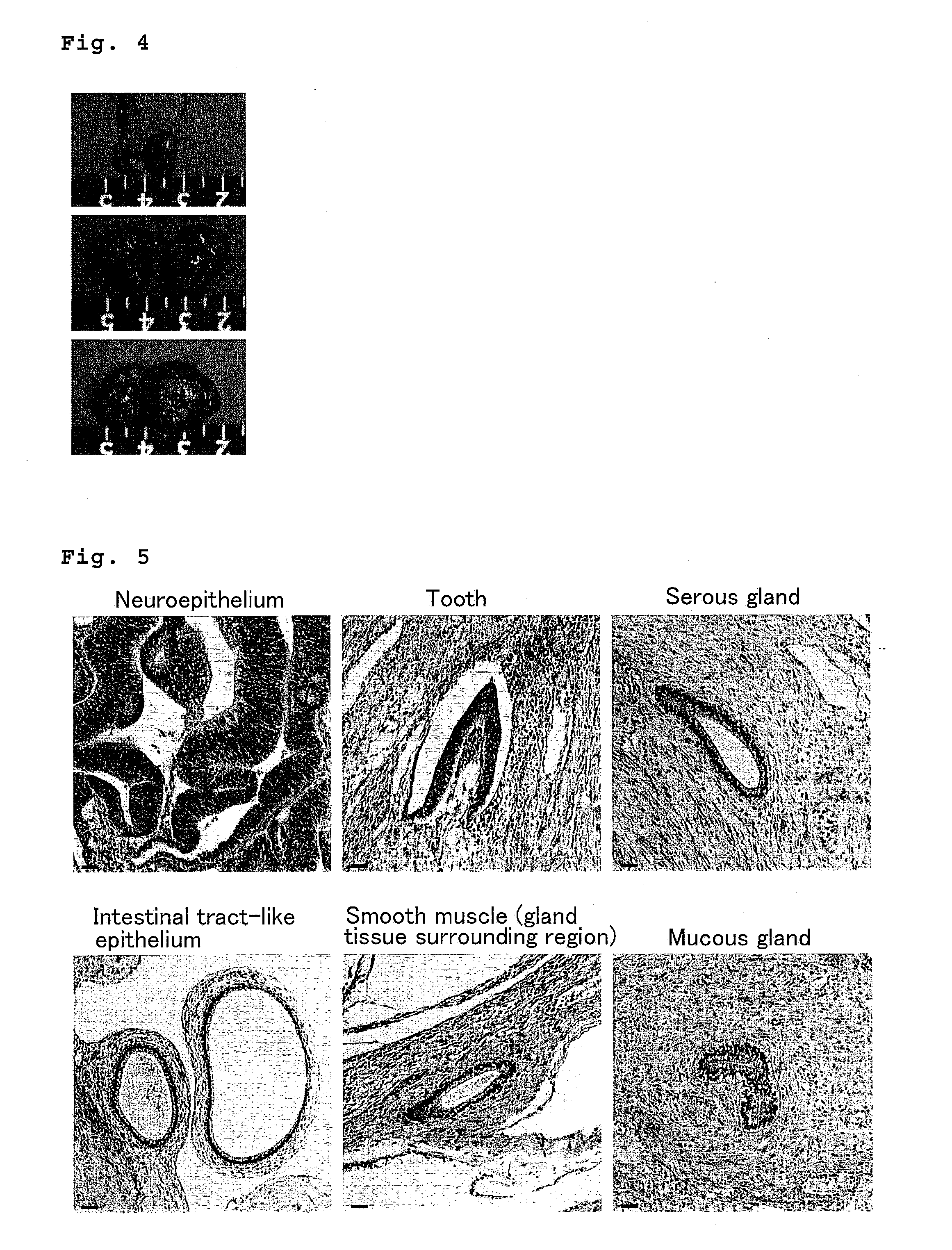
Method for culturing and subculturing primate embryonic stem cell, as well as method for inducing differentiation thereof
InactiveUS20110151554A1Safely culturedLow costCell dissociation methodsCulture processCell-Extracellular MatrixVascular endothelium
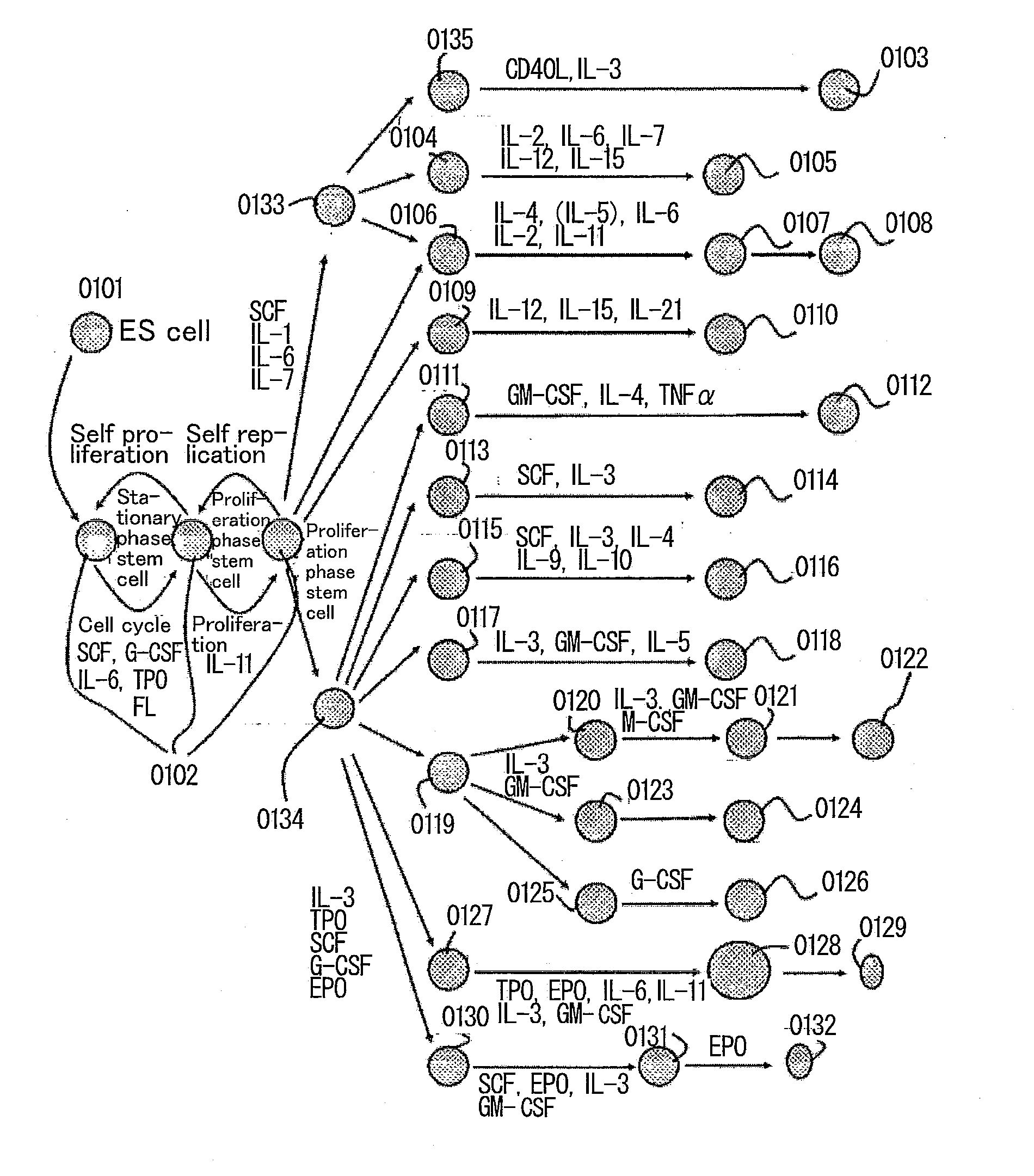
The present invention provides a method for subculturing primate embryonic stem cells, and a method for inducing differentiation of the same cell into a vascular endothelial cell and a blood cell. The present invention provides a method comprising culturing primate embryonic stem cells in a medium containing a protein component without using feeder cells and cytokines in a container coated with an extracellular matrix, detaching colonies of the resulting embryonic stem cells in the presence of a cytodetachment agent, and plating the colonies in the similar medium, and a method comprising culturing primate embryonic stem cells in a serum-containing or not containing medium in the presence of cytokine, adhesion-culturing the resulting embryoid body or embroyid body-analogous cellular aggregate in the presence of a cytokine to obtain specific precursor cells, and separating non-adherent cells and adherent cells from the specific precursor cells to obtain blood cells and vascular endothelial precursor cells.
Owner:NAT CENT FOR GLOBAL HEALTH & MEDICINE +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com