Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
47 results about "Cross-linked enzyme aggregate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In biochemistry, a cross-linked enzyme aggregate is an immobilized enzyme prepared via cross-linking of the physical enzyme aggregates with a difunctional cross-linker. They can be used as stereoselective industrial biocatalysts.
Thermostable sucrose phosphorylase
InactiveUS20130029384A1TransferasesEnzyme stabilisationCross-linked enzyme aggregateSucrose phosphorylase
The present invention relates to a sucrose phosphorylase from Bifidobacterium adolescentis which is useful as a biocatalyst in carbohydrate conversions at high temperatures. Indeed, the biocatalysts of the present invention are enzymatically active for a time period of at least 16 h and up to 1 to 2 week(s) at a temperature of at least 60° C. The biocatalysts of the present invention are: a) immobilized on an enzyme carrier, or b) are part of a cross-linked enzyme aggregate (CLEA), and / or c) are mutated, and / or d) are enzymatically active in the continuous presence of their substrate.
Owner:UNIV GENT
Magnetic immobilized cross-linked lipase aggregate and preparation method and application thereof
ActiveCN102505008AIncrease vitalityImprove the problem of poor operabilityOn/in organic carrierEnzyme reactorCross-linked enzyme aggregate
The invention discloses a magnetic immobilized cross-linked lipase aggregate and a preparation method and application thereof. The preparation method comprises the following steps of: putting a cross-linked lipase aggregate into phosphate buffer, and fully oscillating to ensure that the cross-linked lipase aggregate is uniformly distributed in the phosphate buffer; adding a glutaraldehyde activated magnetic immobilized cross-linked lipase aggregate, and cross-linking; and separating liquid from a solid, washing the solid, and performing vacuum freeze drying to obtain the magnetic immobilized cross-linked lipase aggregate with the relative enzyme activity of over 80 percent. The preparation method is low in cost and easy to operate. A magnetic immobilized enzyme technology is organically combined with a cross-linked enzyme aggregate (CLEA) technology, so that the activity of a magnetic immobilized enzyme is improved, the problem of low operating performance of CLEAs is solved, and the obtained magnetic immobilized cross-linked lipase aggregate with high enzyme activity and operating performance is stable in property, can be used as a bio-enzyme catalyst to be widely applied, and is particularly suitable for a large-scale enzyme reactor.
Owner:SOUTH CHINA UNIV OF TECH
Solid phase support crosslinking enzyme aggregation and method for preparing the same
InactiveCN101492665ASolve the shortcomings that cannot be recycled and reusedOpen up the prospect of industrial applicationHydrolasesOxidoreductasesCross-linked enzyme aggregateEnzyme protein
The invention discloses immobilized crosslinked enzyme aggregates (CLEAs) and a preparation method thereof. The crosslinked enzyme aggregates are fixed on a porous microsphere; the preparation method thereof comprises: dispersing detached enzyme needing to be immobilized into the porous microsphere; carrying out depositing and crosslinking on zymoprotein in the porous microsphere in sequence, and finally rinsing the porous microsphere, thus preparing the immobilized crosslinked enzyme aggregates. The method of the immobilized enzyme can be used for immobilizing the enzyme of various types or sources; the immobilized method is simple and easy to be carried out, is easy to be operated, does not need special devices and has low production cost; the prepared crosslinked enzyme aggregates have high stability and strong capacity for resisting trypsinase, has a certain physical form and a stable structure, can be recycled and repeatedly used and can be used for continuous industrial production.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
Preparation method of cross-linked oxalate decarboxylase aggregates (CLEAs)
InactiveCN102492683AHigh purityImprove separation efficiencyImmobilised enzymesMicroorganism based processesDiseaseCross-link
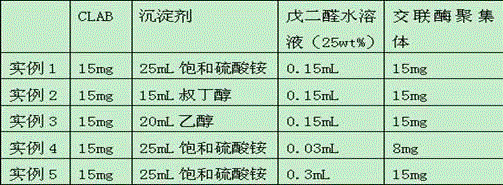
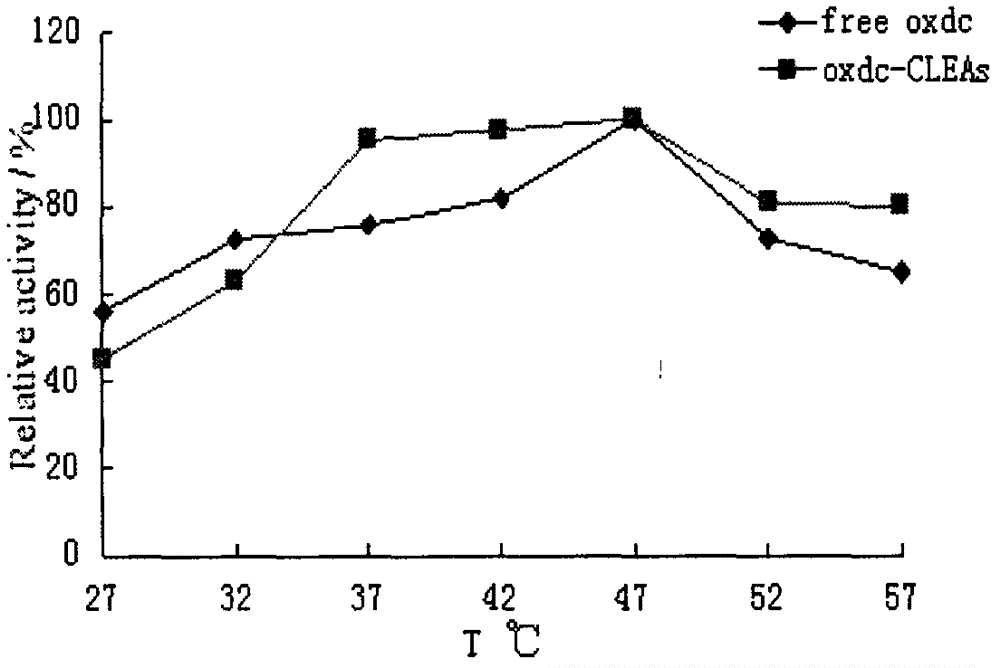
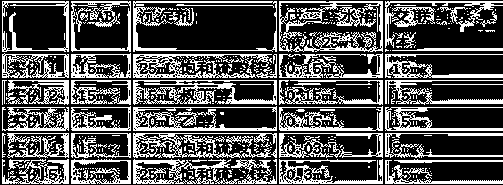
The invention provides a method for cross-linking and fixing oxalate decarboxylase by cross-linked oxalate decarboxylase aggregates (CLEAs), belonging to the technical field of biochemical engineering. The method comprises the following steps: induced expression of recombinant oxalate decarboxylase, separation and purification of oxalate decarboxylase, and preparation of CLEAs. The invention is characterized in that ethanol, acetone or ammonium sulfate are utilized to carry out precipitation separation on oxalate decarboxylase in a crude enzyme liquid; ethanol is utilized to prepare the oxalate decarboxylase aggregates; 0.01-0.15% glutaric dialdehyde is utilized to carry out cross-linking reaction for 0.5-3.5 hours under the conditions of adding 0.1-1.5mg / L BSA (bovine serum albumin), pH value 3-9 and 4 DEG C, thereby preparing the CLEAs; and the enzyme activity recycling rate of the cross-linked immobilized enzyme is up to more than 90%. The invention has the advantages of simple experimental operations and low cost; the obtained CLEAs have obviously higher stability than free oxalate decarboxylase; and thus, the invention can be used for developing enzyme preparations for oxalate calculus disease.
Owner:南宁奕德环境科技有限公司
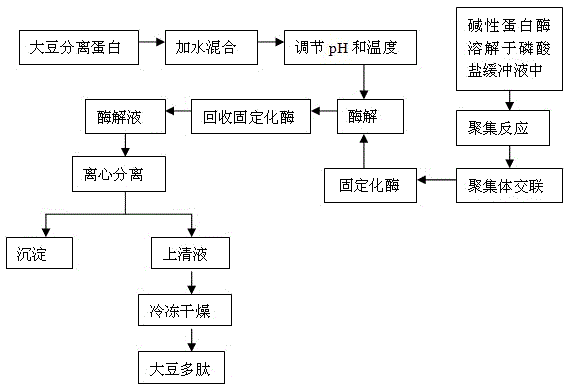
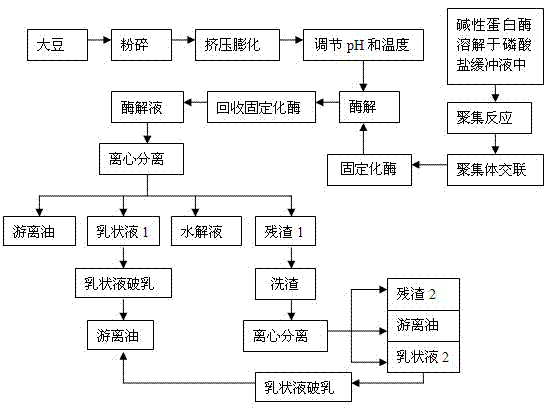
Method for preparing soybean peptides from carrier-free immobilized enzyme
A method for preparing soybean peptides from carrier-free immobilized enzyme belongs to an extract technology of soybean peptides. The method comprises the following steps of: (1) dissolving alkaline protease into phosphate buffer, adding ethanol to precipitate and gather, and then adding glutaraldehyde to carry out crosslinking, so as to obtain cross-linked enzyme aggregate, namely immobilized alkaline protease; and (2) adding water into isolated soy protein solution to be mixed and prepared into the isolated soy protein solution, adjusting the pH and the temperature, and then adding the immobilized enzyme obtained from the step (1) to carry out enzymolysis, recovering the immobilized enzyme after enzymolysis, centrifugally separating the enzymatic hydrolysate to obtain clear solution, and drying the clear solution to obtain the soybean peptides by freezing and drying. The carrier-free immobilized alkaline protease prepared by the method is high in activity, and does not need a high-cost carrier, and meanwhile, the isolated soy protein is subjected to enzymolysis by the immobilized enzyme to prepare the soybean peptides; the method is simple in required processing equipment, and low in energy consumption; and the immobilized enzyme can be recycled and reused. Thus, waste of resources is reduced, and the cost is saved.
Owner:黑龙江珍选食品有限公司
Method for entrapping and cross-linking phosphatidase A1 aggregates
InactiveCN102337256AGood chemical stabilityImprove mechanical propertiesOn/in organic carrierCross-linkCross-linked enzyme aggregate
The invention provides a method for immobilizing phosphatidase A1. The method is improved on the technical basis of cross-linked enzyme aggregates and comprises the steps of: preparing free phosphatidase A1 into cross-linked phosphatidase aggregates; and then entrapping the obtained cross-linked phosphatidase aggregates to obtain immobilized phosphatidase A1. The influences of concentration of a phosphatidase liquid, saturation degree of a precipitating agent, precipitation Ph value, precipitation time, concentration of a cross-linking agent, cross-linking time, concentration of sodium alginate, concentration of calcium ions and other different immobilizing conditions on the immobilizing efficiency and the catalytic effect of the phosphatidase are researched, and the enzymology nature of an immobilized phosphatidase membrane is discussed. The advantage of immobilizing the phosphatidase by adopting the method for entrapping and cross-linking aggregates is further explained through an SEM (Scanning Electron Microscope) image of the immobilized phosphatidase membrane. The phosphatidase is immobilized by utilizing the excellent chemical stability, mechanical property and other properties of the entrapped and cross-linked phosphatidase aggregates, the activity and the stability of the phosphatidase in a reaction system are improved, the activity and the selectivity of the phosphatidase are adjusted and controlled, so as to be favorable for the recovery of the phosphatidase and the maintenance of higher activity recovery rate of the phosphatidase; and the activity recovery rate of the finally obtained immobilized phosphatidase is 80.2 percent, and the relative activity of the phosphatidase after being repeatedly used for seven times is kept at 65 percent above.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Noncellular synthetic biology based preparation method of 2-phenylethanol and application
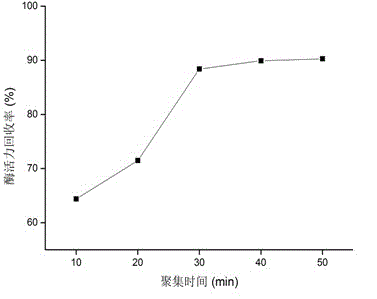
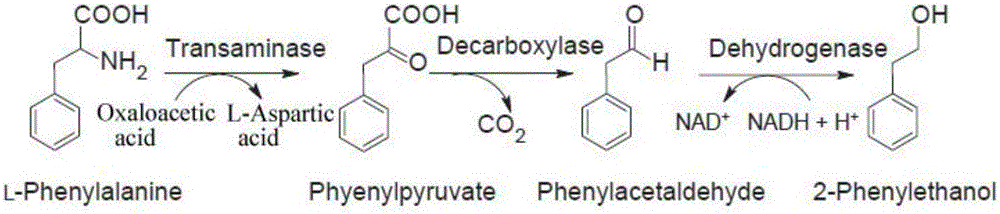
ActiveCN106011184ANo purification requiredHigh catalytic efficiencyFermentationMulti-enzyme systemsCross-linkCross-linked enzyme aggregate
The invention discloses a noncellular synthetic biology based preparation method of 2-phenylethanol and an application. According to the preparation method and the application, three recombinases including ARO8, ARO10 and ADH1 are utilized to constitute a co-reaction system, and a complicated metabolic process of an Ehrlich pathway requiring multi-step continuous reaction and multi-enzyme participation for synthesis of 2-phenylethanol in microorganisms is simplified to be performed in the same reaction system in vitro, so that the environmental pollution is reduced and the reaction process is shortened. Furthermore, the three recombinases are subjected to carrier-free co-immobilization with glutaraldehyde serving as a cross-linking agent, Combi-CLEAs (Combi-Cross-linked Enzyme Aggregates) are prepared, a target product is synthesized in vitro, accordingly, the enzymes are further recycled on the basis of the beneficial effects, and the production cost is reduced; the preparation method fills up the technical blank of multi-enzyme preparation of 2-phenylethanol in vitro, and important reference is provided for biological preparation of 2-phenylethanol.
Owner:SOUTH CHINA AGRI UNIV
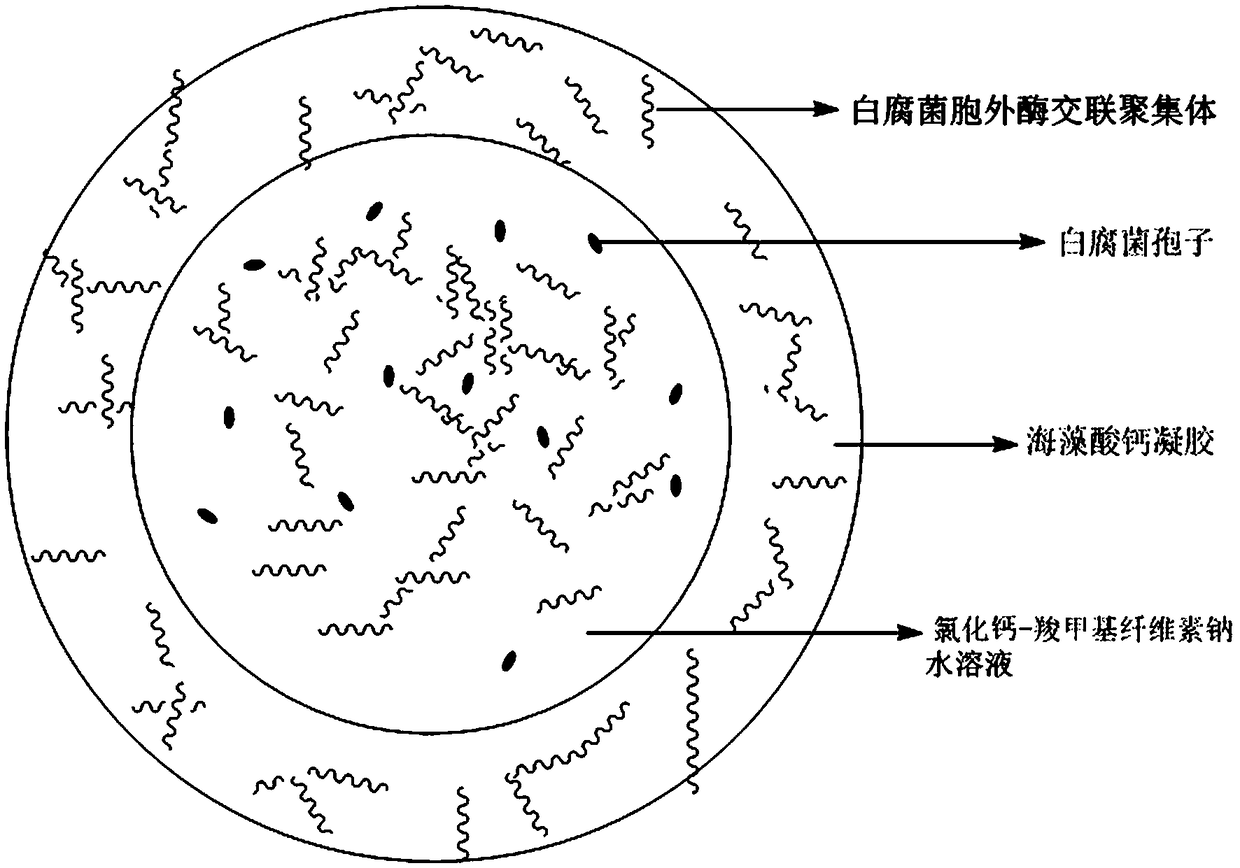
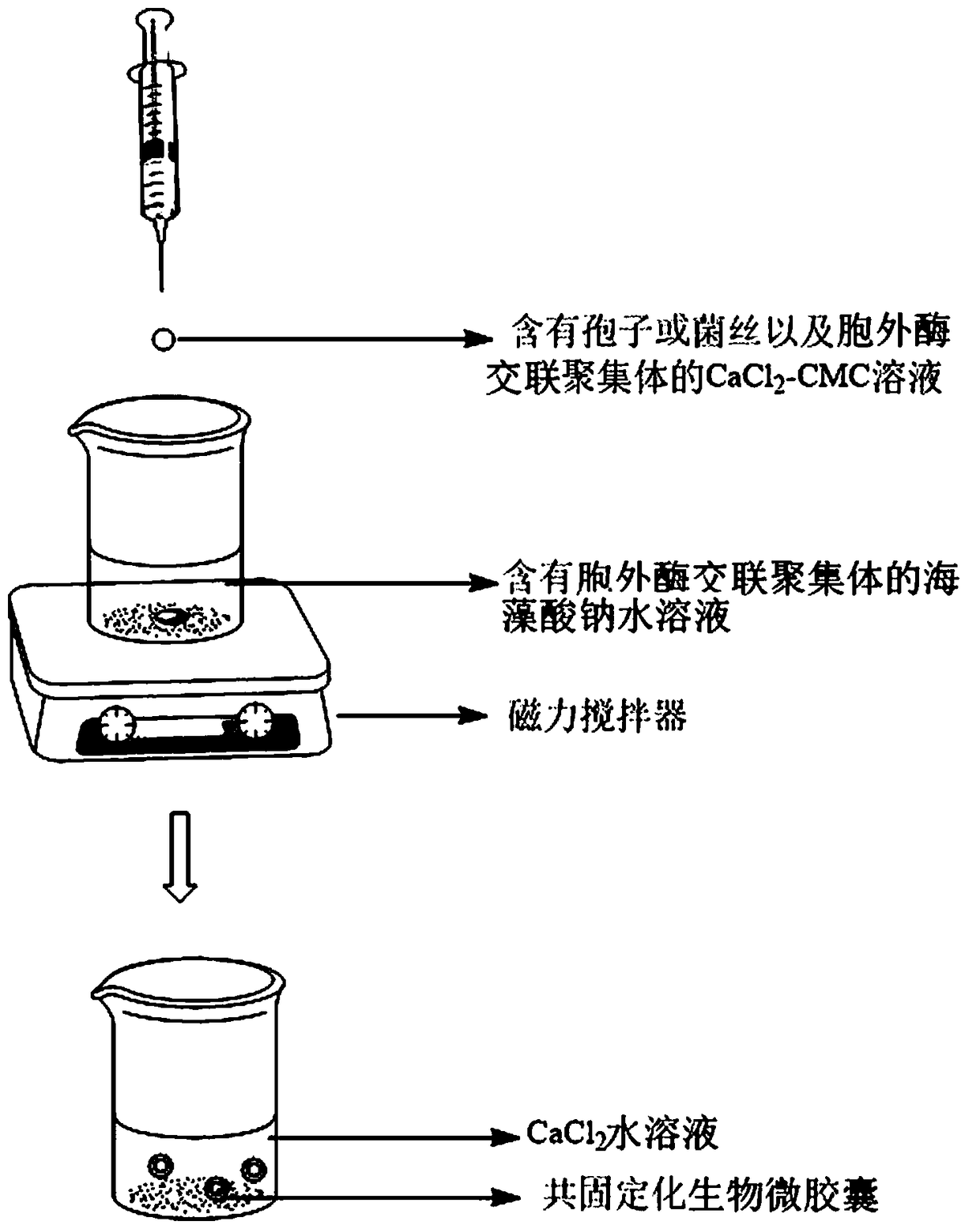
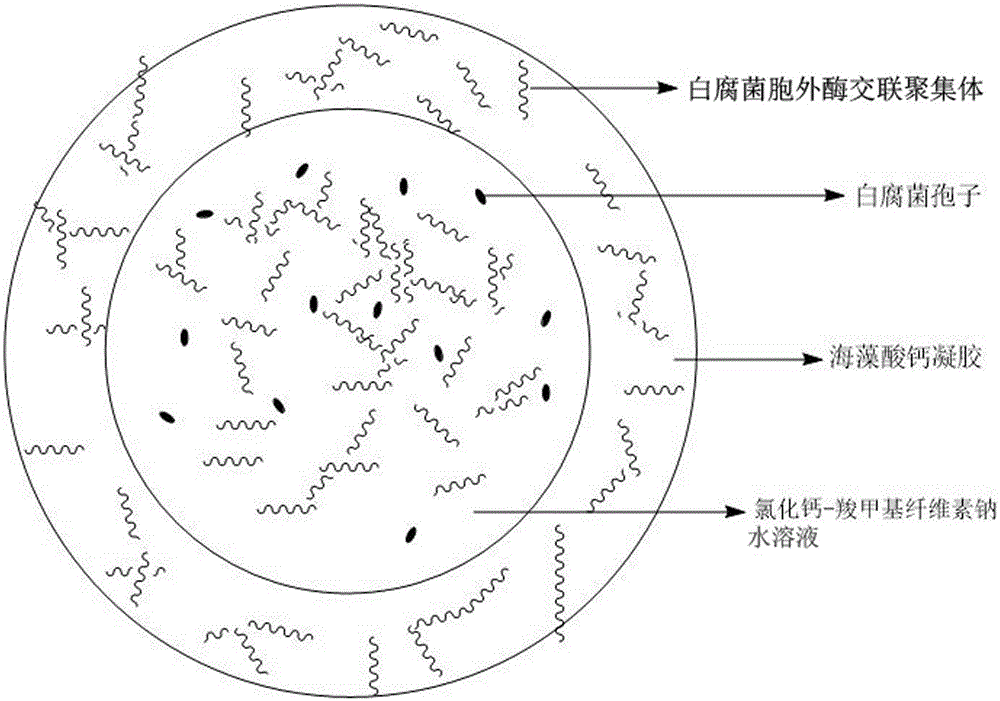
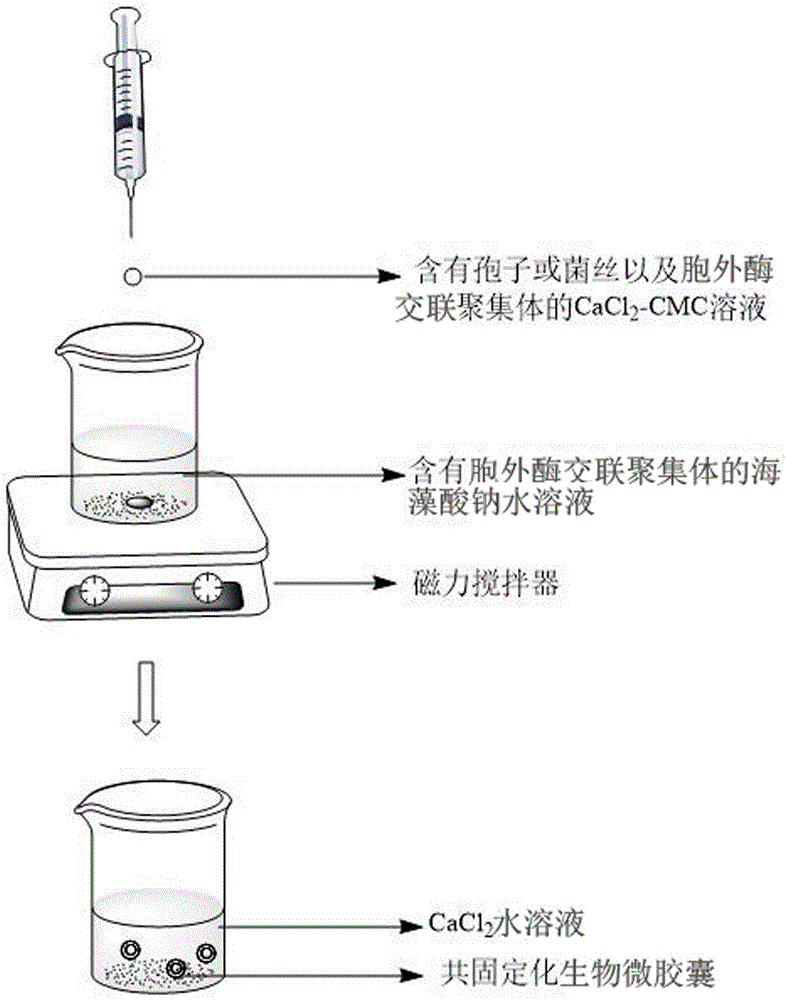
Calcium alginate-sodium carboxymethyl cellulose co-immobilized Phanerochaete chrysosporium strain and biological microcapsule of exoenzyme thereof, and preparation method of strain
ActiveCN105176963AEliminate the purification stepReduce manufacturing costMicroorganism based processesOn/in organic carrierCarboxymethyl celluloseCross-linked enzyme aggregate
The invention relates to a calcium alginate-sodium carboxymethyl cellulose co-immobilized Phanerochaete chrysosporium strain and a biological microcapsule of exoenzyme thereof, and a preparation method of the strain. The biological microcapsule comprises a shell and a shell inside core, wherein the shell material is a calcium alginate gel, and a Phanerochaete chrysosporium exoenzyme crosslinking aggregate is embedded inside the calcium alginate gel; and the shell inside core is a calcium chloride-sodium carboxymethyl cellulose water solution, and Phanerochaete chrysosporium cells and an extracellular crosslinking enzyme aggregate are dispersed inside the calcium chloride-sodium carboxymethyl cellulose water solution. In the biological microcapsule preparation process, the Phanerochaete chrysosporium exoenzyme is directly prepared into the crosslinking enzyme aggregate, thereby omitting the step of purification of the lignin degrading enzyme, effectively lowering the production cost and enhancing the degradation efficiency since the crosslinking enzyme aggregate and Phanerochaete chrysosporium form a close relation.
Owner:山东济清科技服务有限公司
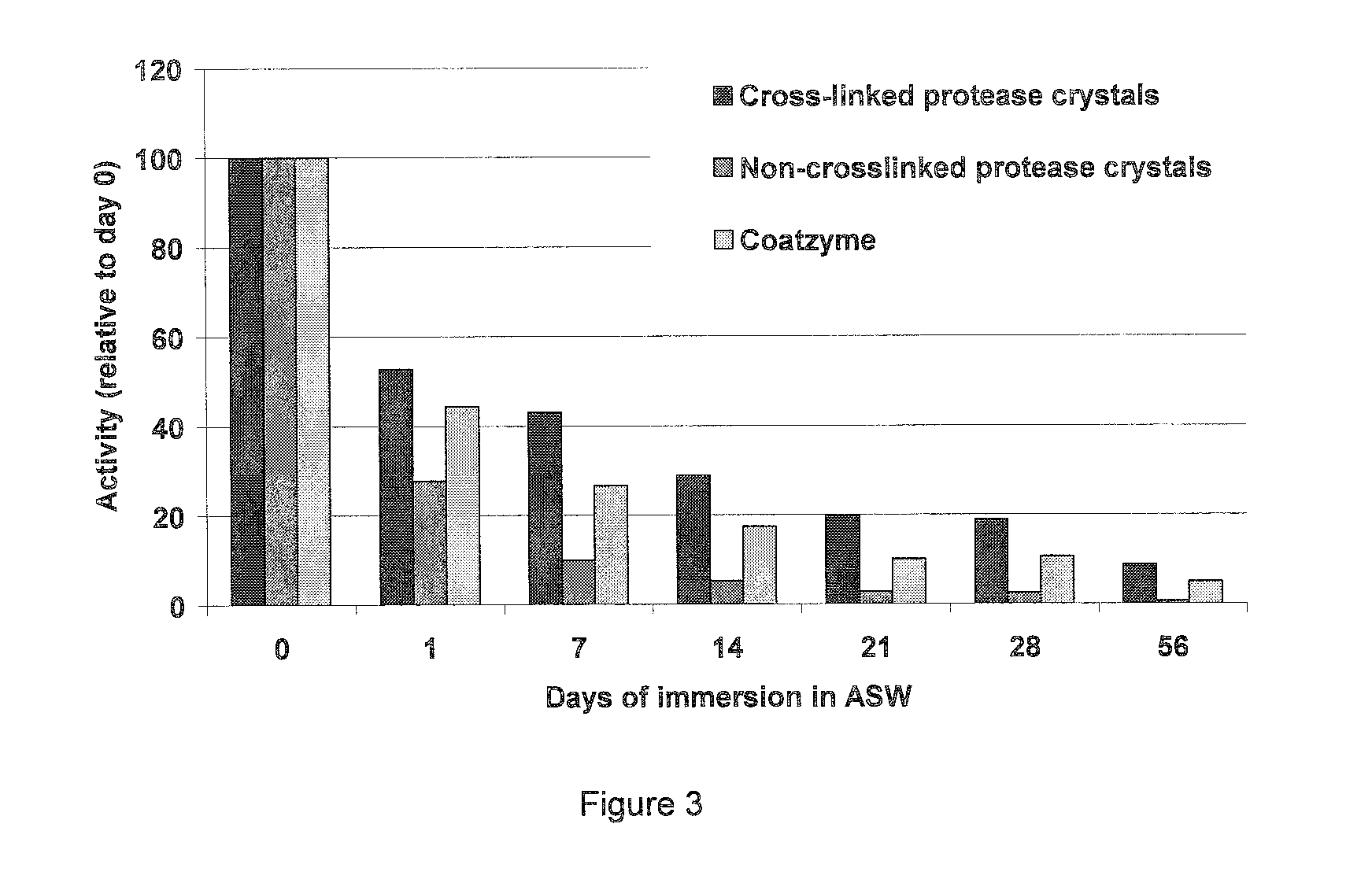
Process for preparing high stability, high activity coatings and processes for using same
InactiveUS20070077568A1Improve stabilityHigh activitySolid waste disposalMicrobiological testing/measurementFiberCross-linked enzyme aggregate
A process for preparing high stability, high activity biocatalytic coatings is disclosed attachable to various materials and fibers, and processes for using same. The process involves attaching crossslinked enzyme aggregates to various materials and fibers forming a biocatalytic coating characterized by high biocatalytic activtity and high stability. The coated materials and fibers are useful in heterogeneous environments. The process creates a useful new biocatalytic immobilized enzyme system with potential applications in bioconversion, bioremediation, biosensors, and biofuel cells.
Owner:BATTELLE MEMORIAL INST
Cross-linked enzyme aggregate prepared in water-in-oil emulsion and preparation method thereof
InactiveCN102304498ALow purity requirementHigh enzyme activity per unitEnzymesCross-linkCross-linked enzyme aggregate
The invention discloses a method for preparing a cross-linked enzyme aggregate prepared in water-in-oil emulsion, which comprises the following steps: (1) preparing enzyme solution; (2) adding a precipitator capable of aggregating and precipitating enzyme in enzyme solution, and preparing an enzyme-containing dispersion phase; (3) dispersing the enzyme-containing dispersion in hydrophobic liquid to form water-in-oil emulsion; (4) cross-linking the aggregate in the water-in-oil emulsion by using a cross-linking agent; (5) collecting the cross-linked enzyme aggregate; and the like. The cross-linked enzyme aggregate prepared by the method is carrier-free immobilized enzyme, the unit enzyme activity of the cross-linked enzyme aggregate is high, and the cross-linked enzyme aggregate can be used in corresponding enzymatic reactions.
Owner:天津市林业果树研究所 +1
Magnetic crosslinking lipase aggregation as well as preparation method and application thereof
InactiveCN103320420AOn/in organic carrierOn/in inorganic carrierCross-linked enzyme aggregateMagnetite Nanoparticles
The invention discloses a magnetic crosslinking lipase aggregation as well as a preparation method and application thereof. The magnetism crosslinking lipase aggregation is prepared from functional group magnetic nanoparticles with dendritic polyamine on the surface. The preparation method integrates a crosslinking enzyme aggregation technology and a magnetic immobilized enzyme technology, not only are the enzyme immobilization efficiency and enzyme activity recovery rate of the magnetism immobilized enzyme improved, but also the problem of the poor operational stability of the crosslinking enzyme aggregation is also greatly improved. The prepared magnetic crosslinking lipase aggregation has high activity and excellent operational stability, and can be used as a biocatalyst widely.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Magnetic combined crosslinked enzyme aggregate biocatalyst and preparation method and application thereof
InactiveCN107227301AIncreased Enzyme LoadingIncrease loadOxidoreductasesFermentationCross-linked enzyme aggregateEnantioselective synthesis
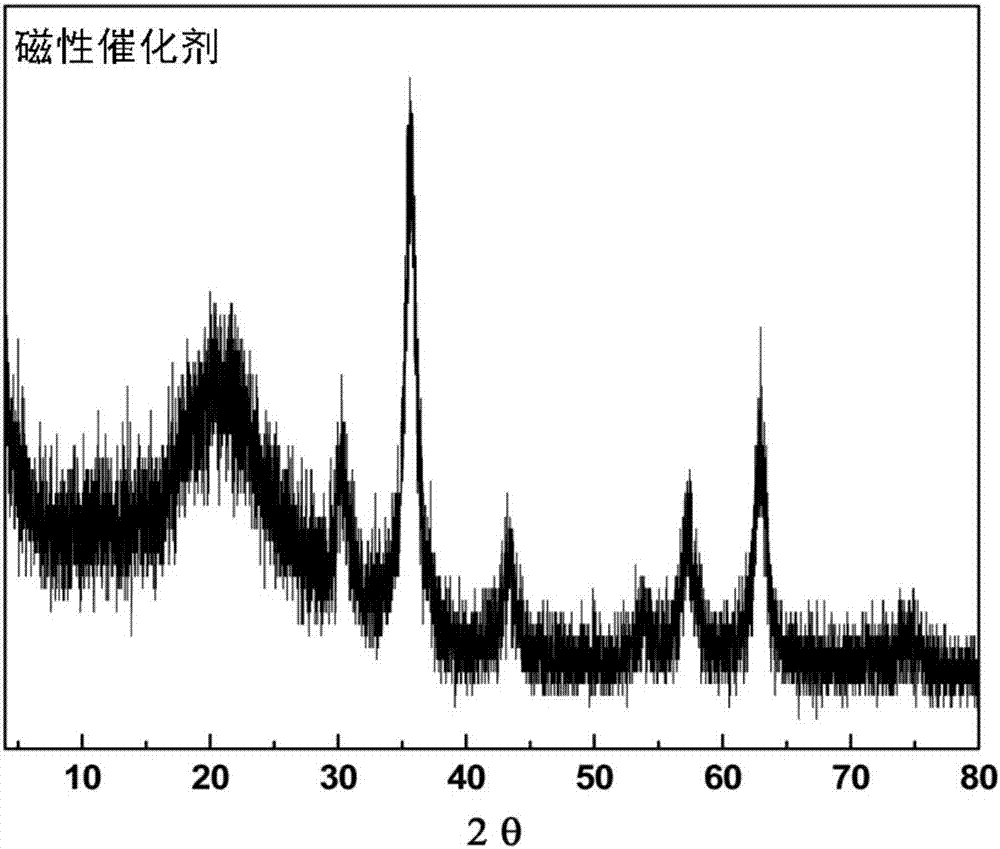
The invention provides a magnetic combined crosslinked enzyme aggregate biocatalyst. The biocatalyst can produce diffraction peaks at diffraction angles 2 theta of 30.5+ / -0.2 degrees, 35.4+ / -0.2 degrees, 44.7+ / -0.2 degrees, 57.3+ / -0.2 degrees and 63.4+ / -0.2 degrees. A preparation method of the biocatalyst is simple, does not need special equipment, needs mild process conditions, is easy to operate, realizes a low cost and is suitable for industrialization. The biocatalyst is used for asymmetric synthesis of (R)-3-quinuclidinol, realizes synchronous carbonyl reduction and coenzyme NADH / NAD<+> in-situ regeneration, prevents diffusional limitation to a substrate , a coenzyme and a product, has good catalytic activity and high catalytic efficiency, greatly shortens reaction time and has obvious economic values.
Owner:CHONGQING MEDICAL UNIVERSITY
Magnetic cross-linked enzyme aggregate, preparation method and application thereof
InactiveCN103937775AImprove efficiencyHigh recovery rateOn/in organic carrierOn/in inorganic carrierCross-linkCrystallography
The present invention relates to a magnetic cross-linked enzyme aggregate, a preparation method and an application thereof. The magnetic cross-linked enzyme aggregate comprises an enzyme aggregate and surface-functionalized magnetic particles, wherein the enzyme aggregate and the surface-functionalized magnetic particles are integratedly cross-linked through p-benzoquinone. The preparation method comprises: uniformly mixing an enzyme solution and surface-functionalized magnetic particles, adding a precipitating agent of the enzyme to make the enzyme form an enzyme aggregate, and adding p-benzoquinone as a cross-linking agent to integratedly cross-link the enzyme aggregate and the surface-functionalized magnetic particles so as to form the magnetic cross-linked enzyme aggregate. The magnetic cross-linked enzyme aggregate has characteristics of high immobilization efficiency, high enzyme activity recovery rate and good operation stability, and can be reused as a biocatalyst.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Method for preparing optically active (S)-bufuralol by enzyme catalysis
InactiveCN102649973AHigh optical purityHigh yieldFermentationCross-linked enzyme aggregateReaction step
The invention provides a method for preparing optically active (S)-bufuralol by enzyme catalysis. By taking 7-ethylbenzofuran-2-formaldehyde as a raw material, the (S)-bufuralol is obtained through the catalysis asymmetric cyanation of cross-linked enzyme aggregates (CLEA for short) of (R)-hydroxynitrile lyase [(R)-Oxynitrilase], hydroxyl protection, Ritter reaction and amide reduction reaction. The preparation method for the (S)-bufuralol has the advantages that the reaction steps are simple, the reaction condition is mild, the yield is high and the optical purity of the obtained (S)-bufuralol is high.
Owner:NANJING UNIV OF TECH
Method for preparing soybean oil through enzymatic hydrolysis of immobilized enzyme
InactiveCN103074152AReduce wasteHigh oil extraction rateFatty-oils/fats productionAlkaline proteaseEnzymatic hydrolysis
The invention discloses a method for preparing soybean oil through enzymatic hydrolysis of an immobilized enzyme, and belongs to a plant oil extracting technology. The method comprises the following steps: (1) dissolving alkaline protease into a phosphate buffer solution, adding ethanol, precipitating and aggregating, adding glutaraldehyde, and crosslinking to obtain a crosslinked enzyme aggregate, namely immobilized alkaline protease; and (2) crushing soybeans, performing extrusion preprocessing to obtain an extruded material, crushing the extruded material, mixing the extruded material with water, regulating pH and temperature, adding the immobilized enzyme obtained in the step (1) for enzymatic hydrolysis, recycling the immobilized enzyme after the enzymatic hydrolysis, and centrifuging enzymatic hydrolysate to obtain the soybean oil. Processing equipment needed in the method is simple; the prepared immobilized enzyme is high in activity; the immobilizing time is short; a high-cost carrier is not required, so that the cost is greatly reduced; when the immobilized enzyme is applied to extraction of the soybean oil through the enzymatic hydrolysis, oil extracting rate is high; the immobilized enzyme can be recycled for reuse, so that resource waste is reduced; therefore, the method has very good economic benefit and development prospect.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Method for preparing optical pure 1-(1-naphthyl)ethylamine through resolution with immobilized enzyme process
InactiveCN104630322AExtended reaction timeStrong operational stabilityFermentationCross-linked enzyme aggregateOrganosolv
The invention discloses a method for preparing optical pure 1-(1-naphthyl)ethylamine through resolution with immobilized enzyme process. The method comprises the following steps of selectively esterifying (R)-1-(1-naphthyl)ethylamine in 1-(1-naphthyl)ethylamine racemate, then separating (R)-1-(1-naphthyl)ethylamine esterified compound from (S)-1-(1-naphthyl)ethylamine to obtain (S)-1-(1-naphthyl)ethylamine, and finally hydrolyzing the (R)-1-(1-naphthyl)ethylamine compound to obtain the (R)-1-(1-naphthyl)ethylamine. The catalyst for selective esterification is crosslinked Candida antarctica lipase B aggregate which is prepared through the following steps of dissolving Candida antarctica lipase B in water to obtain enzyme solution, depositing zymoprotein from the enzyme solution with a precipitator, then adding a difunctional crosslinking agent for crosslinking, and drying. The crosslinked enzyme aggregate is high in repeated utilization rate, and has relatively strong operation stability in a pure organic solvent.
Owner:南京普瑞特生物科技有限公司
Method for immobilizing saccharifying enzyme
InactiveCN102154255AIncreased particle hardness activityEasy to operateImmobilised enzymesCross-linkCross-linked enzyme aggregate
The invention relates to a method for immobilizing saccharifying enzyme and belongs to the technical field of bioengineering. The method is improved on a cross-linked enzyme aggregate immobilization method and the improvements of the method include: a, an enzyme substrate protector is used for protection; b, room-temperature crosslinking is adopted, and glutaraldehyde is used as a cross-linking agent; and c, a low-temperature static crosslinking process is adopted. Compared with other immobilization methods, the method for immobilizing the saccharifying enzyme has the advantages that: the operation is simpler; the dextrin hydrolysis capacity of a cross-linked saccharifying enzyme aggregate compares with that of primary enzyme solution; the external environment-resistance stability is much higher than free enzyme solution; and the saccharifying enzyme can be used repeatedly for more than 2 months. Improvement and optimization both improve the hardness activity of the crosslinked enzyme particles greatly, and recycling is promoted. The method has the advantages that: the operation is simple; the cost is low; the thermal stability and storage stability of the immobilized saccharifying enzyme are high; the immobilized saccharifying enzyme has broad-spectrum pH adaptability; and the activity of the immobilized saccharifying enzyme is more than 60 percent of the activity of free enzyme.
Owner:YUNNAN UNIV
Process for double-enzyme coupling-chemical synthesis of epsilon-caprolactone
InactiveCN106701859AHigh activityHigh selectivityImmobilised enzymesHydrolasesHigh concentrationChemical synthesis
The invention relates to a process for double-enzyme coupling-chemical synthesis of epsilon-caprolactone; hydrogen peroxide generated in situ from GOD oxidation of glucose by a GOD / CALB or GOD / ROL double-enzyme cross-linked enzyme aggregate is used as an oxidant, at the same time, peroxy acid is generated in situ through catalyzing oxidation of an acyl donor by CALB or ROL in the cross-linked enzyme aggregate, and then epsilon-caprolactone (epsilon-CL) is generated through oxidation of cyclohexanone by peroxy acid. The yield of the epsilon-caprolactone reaches 97% or more, and the selectivity of the epsilon-caprolactone is 100%. The process reduces enzyme inactivation caused by too high concentration of hydrogen peroxide, avoids an easily explosiveness risk possibly caused by direct addition of peroxy acid, reduces the influence of decrease of enzyme micro-environment pH on the enzyme activity, and can reduce the inhibition of a substrate on an enzyme.
Owner:QINGDAO UNIV OF SCI & TECH
Method For The Preparation Of Cross-Linked Enzyme Aggregates With Improved Properties
InactiveUS20120149082A1Enzyme loading is rather lowHydrolasesEnzyme stabilisationCross-linkCross-linked enzyme aggregate
Owner:CLEA TECH
Process for production of dyed fabric using enzyme aggregates
ActiveCN107287946ADo not destroyDo not damageBiochemical treatment with enzymes/microorganismsDyeing processFiberYarn
Provided is a process for the production of a dyed fabric using enzyme aggregates. In particular, provided is a process that comprises a step of providing a woven fabric that comprises a base layer and an additional layer which is located on at least one side of the fabric, wherein the yarns of the additional layer comprise fibers that are at least partially dyed, and a step of contacting the woven fabric with enzyme aggregates such as cross-linked enzyme aggregates (CLEAs), to remove at least part of the dye from at least the yarns of said additional layer. The disclosure also provides a fabric obtained with the process and garments including the fabric.
Owner:SANKO TEKSTIL ISLETMELERI SANAYI & TICARET A S
Preparation method of cross-linked oxalate decarboxylase aggregates (CLEAs)
InactiveCN102492683BHigh purityImprove separation efficiencyImmobilised enzymesMicroorganism based processesDiseaseCross-link
The invention provides a method for cross-linking and fixing oxalate decarboxylase by cross-linked oxalate decarboxylase aggregates (CLEAs), belonging to the technical field of biochemical engineering. The method comprises the following steps: induced expression of recombinant oxalate decarboxylase, separation and purification of oxalate decarboxylase, and preparation of CLEAs. The invention is characterized in that ethanol, acetone or ammonium sulfate are utilized to carry out precipitation separation on oxalate decarboxylase in a crude enzyme liquid; ethanol is utilized to prepare the oxalate decarboxylase aggregates; 0.01-0.15% glutaric dialdehyde is utilized to carry out cross-linking reaction for 0.5-3.5 hours under the conditions of adding 0.1-1.5mg / L BSA (bovine serum albumin), pH value 3-9 and 4 DEG C, thereby preparing the CLEAs; and the enzyme activity recycling rate of the cross-linked immobilized enzyme is up to more than 90%. The invention has the advantages of simple experimental operations and low cost; the obtained CLEAs have obviously higher stability than free oxalate decarboxylase; and thus, the invention can be used for developing enzyme preparations for oxalate calculus disease.
Owner:南宁奕德环境科技有限公司
Method for preparing optically pure 1-(1-naphthyl)ethylamine by resolution of immobilized enzyme
InactiveCN104630322BExtended reaction timeStrong operational stabilityFermentationOrganic solventCross-linked enzyme aggregate
The invention discloses a method for splitting and preparing optically pure 1-(1-naphthyl)ethylamine by an immobilized enzyme method. Selective esterification of ‑1‑(1‑naphthyl)ethylamine followed by separation of (R)‑1‑(1‑naphthyl)ethylamine from (S)‑1‑(1‑naphthyl)ethylamine , to obtain (S)‑1‑(1‑naphthyl) ethylamine, and finally (R)‑1‑(1‑naphthyl) ethylamine ester hydrolysis to obtain (R)‑1‑(1‑naphthyl) Ethylamine. The catalyst used for selective esterification is the cross-linked Candida antarctica lipase B aggregate, which first dissolves Candida antarctica lipase B in water to obtain an enzyme solution, and then uses a precipitant to precipitate the enzyme protein from the enzyme solution Come out, then add a bifunctional cross-linking agent for cross-linking, and finally dry it. The cross-linked enzyme aggregate of the invention has high reutilization rate and strong operation stability in pure organic solvent.
Owner:南京普瑞特生物科技有限公司
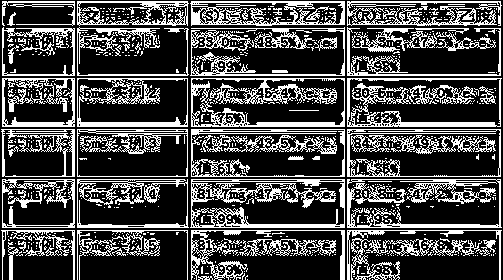
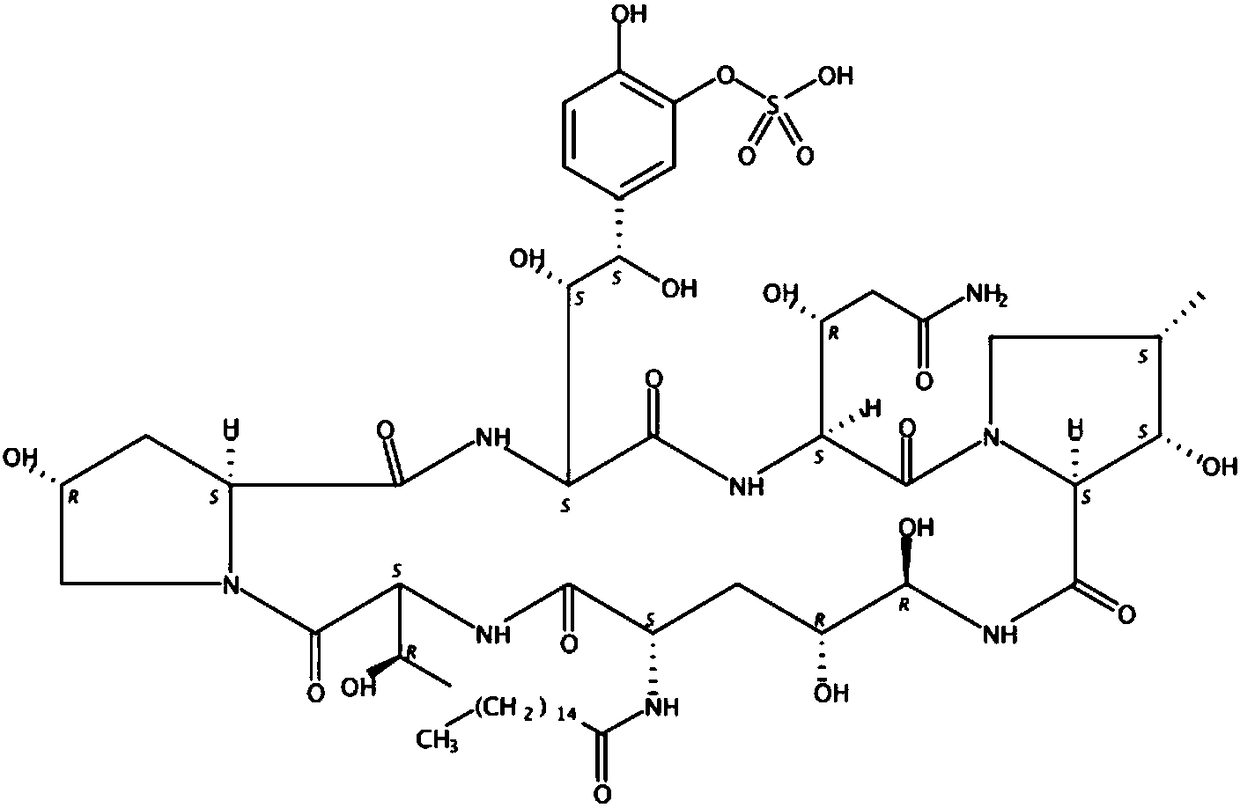
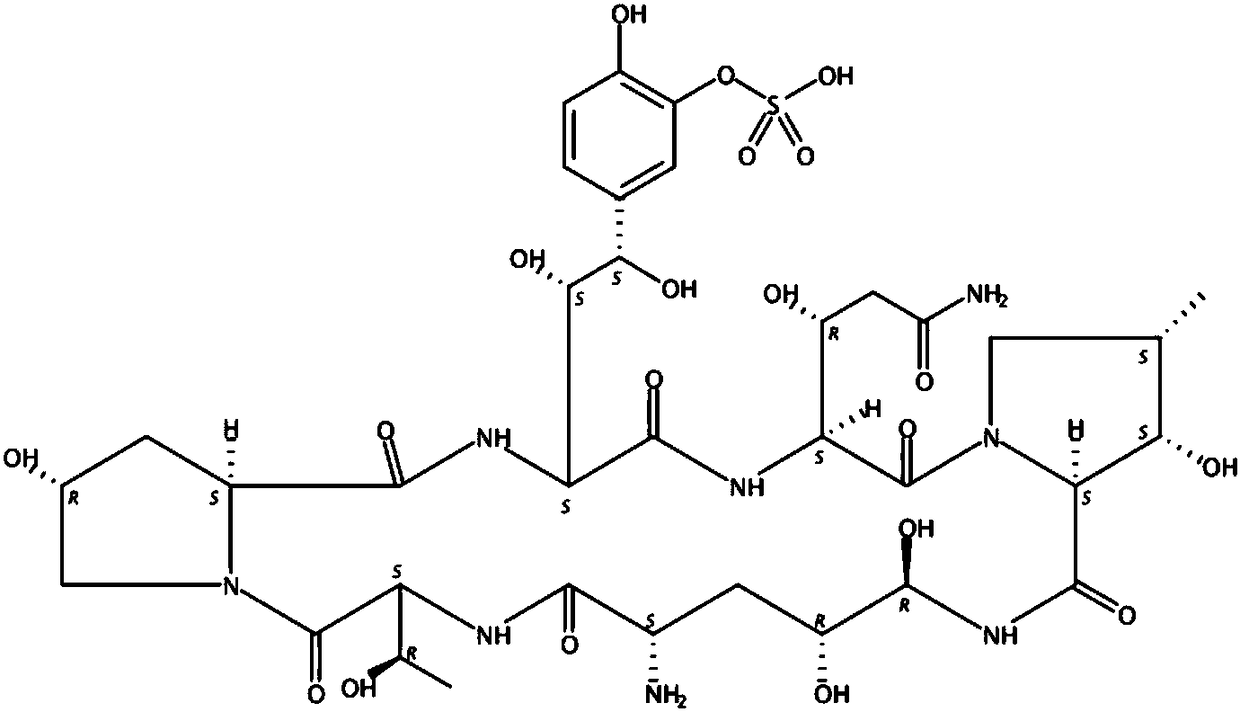
Method for preparing micafungin sodium precursor
PendingCN108467880AImprove stabilityAvoid inactivationPeptidesFermentationCross-linkCross-linked enzyme aggregate
The invention discloses a brand new method for transforming FR901379 into FR179642. According to the method, a cross-linked enzyme aggregation technology is applied to a process of transforming FR901379 into FR179642. The method comprises the following steps: preparing deacylase into deacylase cross-linked enzyme aggregate, and transforming the FR901379 into FR179642 by the cross-linked enzyme aggregate. According to the method, a molar conversion ratio of transforming the FR901379 into FR179642 can reach 92% or higher, the production process is simplified, and the cost is reduced.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
Fermentation method of micafungin sodium precursor FR179642
InactiveCN108441529AImprove stabilityAvoid inactivationPeptidesFermentationCross-linkCross-linked enzyme aggregate
The invention discloses a new method of converting FR901379 into FR179642. In the method, the cross-linked enzyme aggregation technology is applied to the process of converting FR901379 into FR179642.According to the method, deacylase is prepared into a deacylase cross-linked enzyme aggregate, and the cross-linked enzyme aggregate converts FR901379 into FR179642. By means of the method, the FR901379 substrate feed concentration in the reaction system can reach 20 g / L, and the molar conversion rate can reach 90-96%; meanwhile, the production process is simplified, and the cost is reduced.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD +1
Method for catalytic synthesis of (R)-1-[3, 5-bis (trifluoromethyl)] phenethyl alcohol through light-enzyme system
ActiveCN113388646AEasy to operateReduce pollutionSugar derivativesOrganic-compounds/hydrides/coordination-complexes catalystsTio2 nanotubePhotocatalytic water splitting
The invention relates to a method for catalytic synthesis of (R)-1-[3, 5-bis (trifluoromethyl)] phenethyl alcohol by a light-enzyme system, which is characterized in that NADPH is catalytically regenerated by combining a TiO2 nanotube with [Cp*Rh(bpy)(H2O)]<2+>, and the generated NADPH is used for catalytic synthesis of (R)-1-[3, 5-bis (trifluoromethyl)] phenethyl alcohol. The method provided by the invention is low in cost, the TiO2 nanotube is used for supplying hydrogen through photocatalytic water splitting, the used enzyme is a cross-linked enzyme aggregation, which is insoluble in an organic solvent and can be recycled for multiple times, and the method has the advantages of high yield, safe, reliable, less environmental pollution and is suitable for industrial production.
Owner:HANGZHOU NORMAL UNIVERSITY
Composition
InactiveUS20110293591A1Poor inhibitionPrevent and reduce accumulationBiocideAntifouling/underwater paintsCross-linkCross-linked enzyme aggregate
The present invention provides a composition, and a process for preparing and method for using such a composition. The composition comprises (i) a surface coating material; and (ii) (ii) a cross-linked enzyme crystal or cross-linked enzyme aggregate wherein the enzyme is cross-linked with a multifunctional cross-linking agent and wherein the cross-linked enzyme crystal or cross-linked enzyme aggregate has an antifouling activity or generates an antifouling compound. Suitably the composition may be used to inhibit biofilm formation.
Owner:DUPONT NUTRITION BIOSCIENCES APS
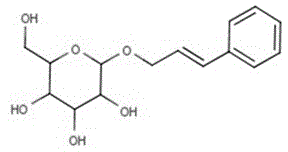
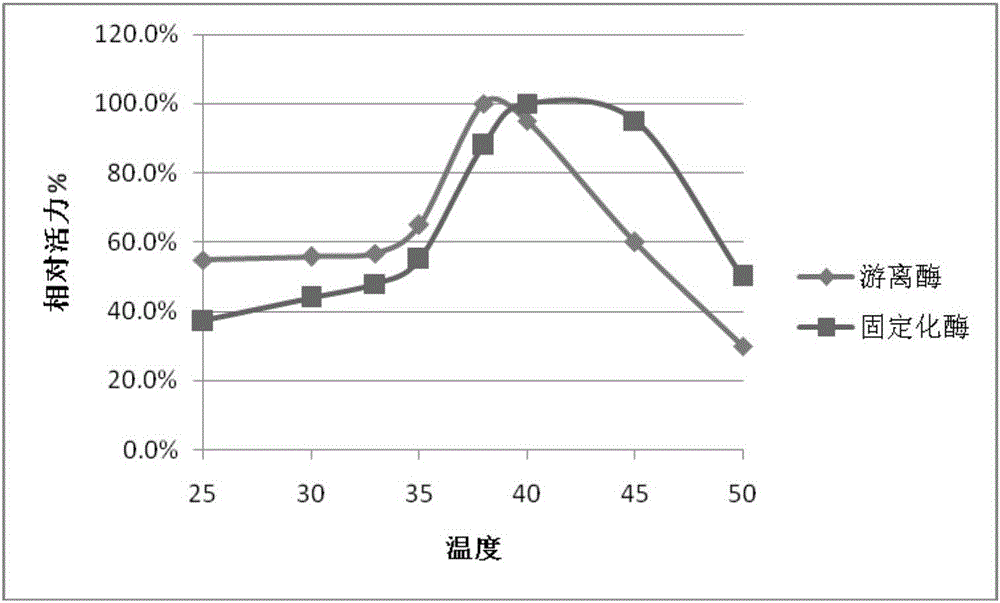
Method for catalytic synthesis of phenylallyl beta-D-glucoside from magnetic cross-linked enzyme aggregates
The invention provides a method for catalytic synthesis of phenylallyl beta-D-glucoside from magnetic cross-linked enzyme aggregates and belongs to the field of biocatalysis. A substrate phenylallyl alcohol and D-glucose are subjected to glycosylation in the presence of an enzyme to synthesize phenylallyl beta-D-glucoside and a reaction system is a non-aqueous-phase system. A report on catalytic glycosylation synthesis of phenylallyl beta-D-glucoside by utilizing magnetic cross-linked beta-glucosidase aggregates is not seen, the method disclosed by the invention has unique and innovative independent innovation and in the non-aqueous-phase system, the activity and stability of the enzyme can be ensured and the thermodynamic equilibrium moves towards the hydrolysis reaction direction. The system is simple, phenylallyl beta-D-glucoside can be directly synthesized in one step and products are easily separated and purified so that high purity is achieved. The substrate selected in the reaction is an inexpensive chemical raw material, the cost is significantly saved compared to the traditional method for extracting phenylallyl beta-D-glucoside from Rhodiola plants and the phenylallyl beta-D-glucoside is suitable for industrial production.
Owner:FUZHOU UNIV
Liquid detergency composition comprising protease and non-protease enzyme
InactiveUS20180245023A1Improved stability and activitySimple and cost-effectiveNon-ionic surface-active compoundsEnzyme stabilisationProteinase activityCross-linked enzyme aggregate
Owner:CONOPCO INC D B A UNILEVER
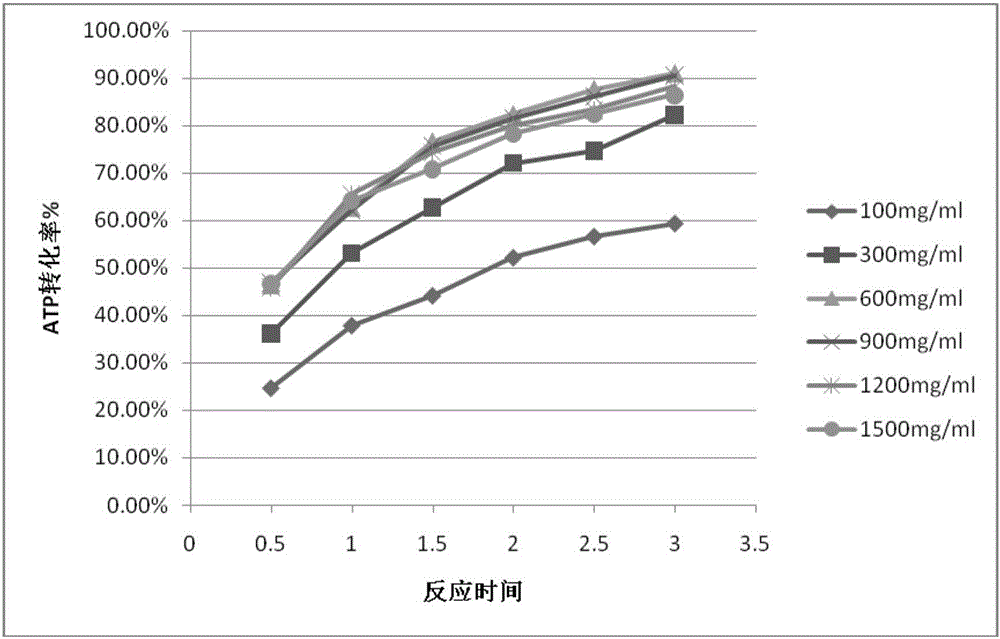
Preparation method of S-adenosylmethionine (SAMe) by enzyme-catalyzed method
InactiveCN106191174AFast conversionIncrease cumulativeChemical industryTransferasesS-Adenosyl-l-methionineCross-linked enzyme aggregate
The invention relates to a method for producing S-adenosylmethionine (or adenosyl methionine) by enzyme catalysis. SAMe synthases are prepared to form cross-linked enzyme aggregates, and the cross-linked enzyme aggregates form immobilized enzymes which are not soluble in water, therefore, the immobilized enzymes are prepared by the method to catalyze adenosine triphosphate (ATP) and methionine to synthesize adenosyl methionine. The preparation method of S-adenosylmethionine (SAMe) by the enzyme-catalyzed method disclosed by the invention is high in synthesis speed because the reaction can be completed by about 3 hours, high in synthetic quantity, stable in catalytic performance and not decreased in conversion capability after 60 times of reaction.
Owner:BEIJING KAWIN TECH SHARE HLDG
Calcium alginate-sodium carboxymethyl cellulose co-immobilized white rot fungus and its extracellular enzyme biological microcapsules and its preparation method
ActiveCN105176963BEliminate the purification stepReduce manufacturing costMicroorganism based processesOn/in organic carrierCarboxymethyl celluloseCross-linked enzyme aggregate
The invention relates to a calcium alginate-sodium carboxymethyl cellulose co-immobilized Phanerochaete chrysosporium strain and a biological microcapsule of exoenzyme thereof, and a preparation method of the strain. The biological microcapsule comprises a shell and a shell inside core, wherein the shell material is a calcium alginate gel, and a Phanerochaete chrysosporium exoenzyme crosslinking aggregate is embedded inside the calcium alginate gel; and the shell inside core is a calcium chloride-sodium carboxymethyl cellulose water solution, and Phanerochaete chrysosporium cells and an extracellular crosslinking enzyme aggregate are dispersed inside the calcium chloride-sodium carboxymethyl cellulose water solution. In the biological microcapsule preparation process, the Phanerochaete chrysosporium exoenzyme is directly prepared into the crosslinking enzyme aggregate, thereby omitting the step of purification of the lignin degrading enzyme, effectively lowering the production cost and enhancing the degradation efficiency since the crosslinking enzyme aggregate and Phanerochaete chrysosporium form a close relation.
Owner:山东济清科技服务有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com





















![Method for catalytic synthesis of (R)-1-[3, 5-bis (trifluoromethyl)] phenethyl alcohol through light-enzyme system Method for catalytic synthesis of (R)-1-[3, 5-bis (trifluoromethyl)] phenethyl alcohol through light-enzyme system](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/537e399d-9256-4c38-8a07-f1aa8e98100c/HDA0003115098540000011.png)
![Method for catalytic synthesis of (R)-1-[3, 5-bis (trifluoromethyl)] phenethyl alcohol through light-enzyme system Method for catalytic synthesis of (R)-1-[3, 5-bis (trifluoromethyl)] phenethyl alcohol through light-enzyme system](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/537e399d-9256-4c38-8a07-f1aa8e98100c/HDA0003115098540000012.png)
![Method for catalytic synthesis of (R)-1-[3, 5-bis (trifluoromethyl)] phenethyl alcohol through light-enzyme system Method for catalytic synthesis of (R)-1-[3, 5-bis (trifluoromethyl)] phenethyl alcohol through light-enzyme system](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/537e399d-9256-4c38-8a07-f1aa8e98100c/HDA0003115098540000021.png)