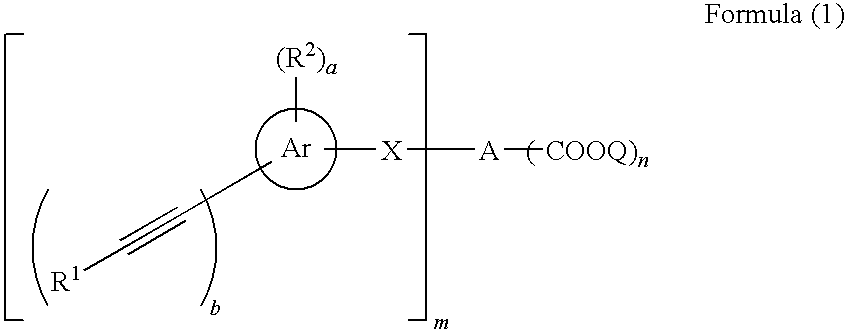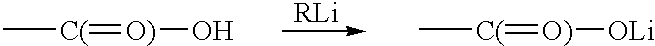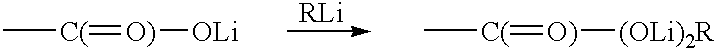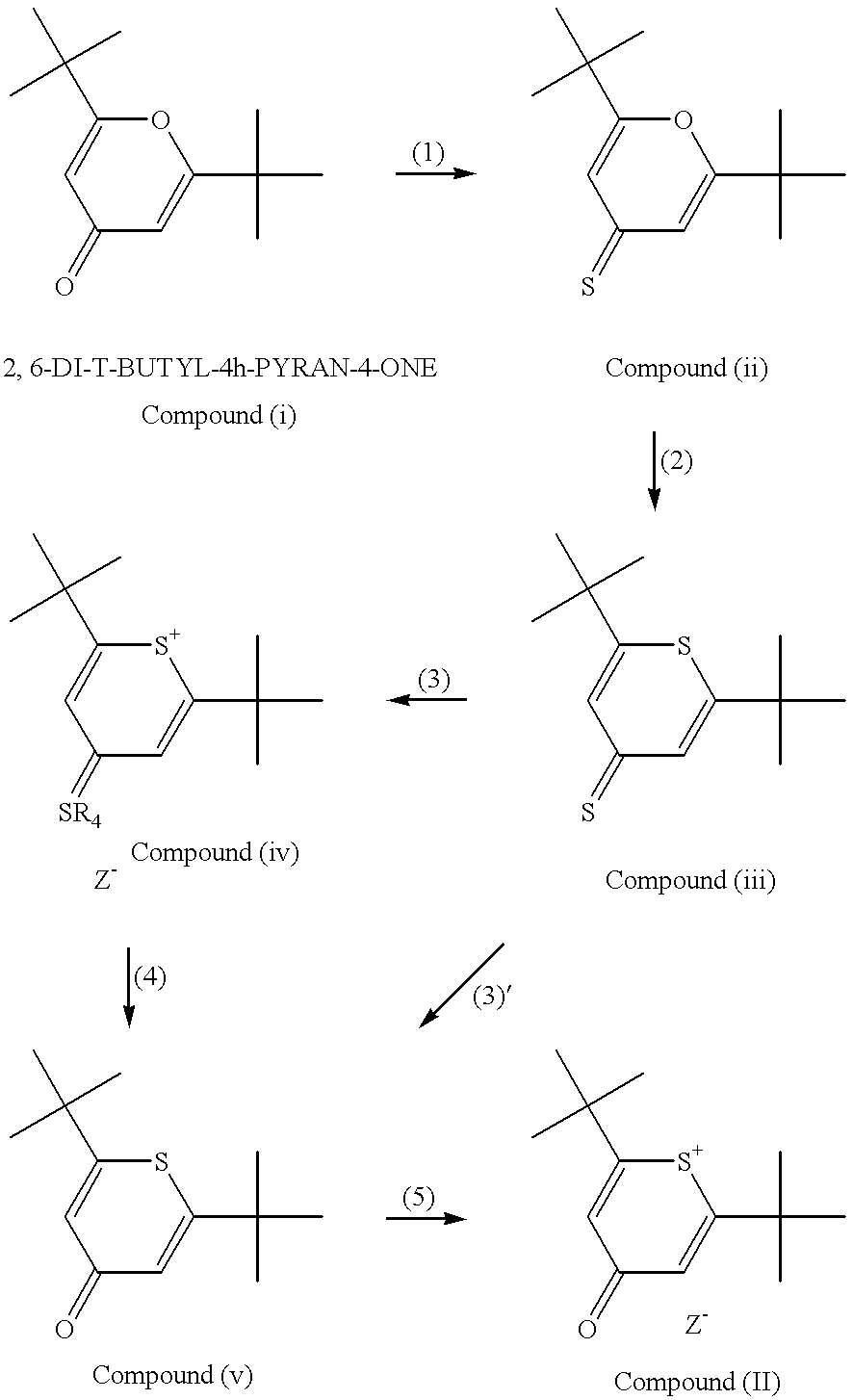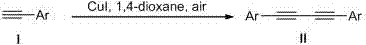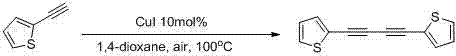Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
83 results about "Acetylene compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Acetylene (əˈsɛtɪˌliːn) n 1. (Elements & Compounds) a colourless flammable gas used in the manufacture of organic chemicals and in cutting and welding metals. Formula: C2H2. a•cet•y•lene (əˈsɛt lˌin, -ɪn) n. a colorless gas, C2H2, used esp. in metal cutting and welding, as an illuminant, and in organic synthesis.
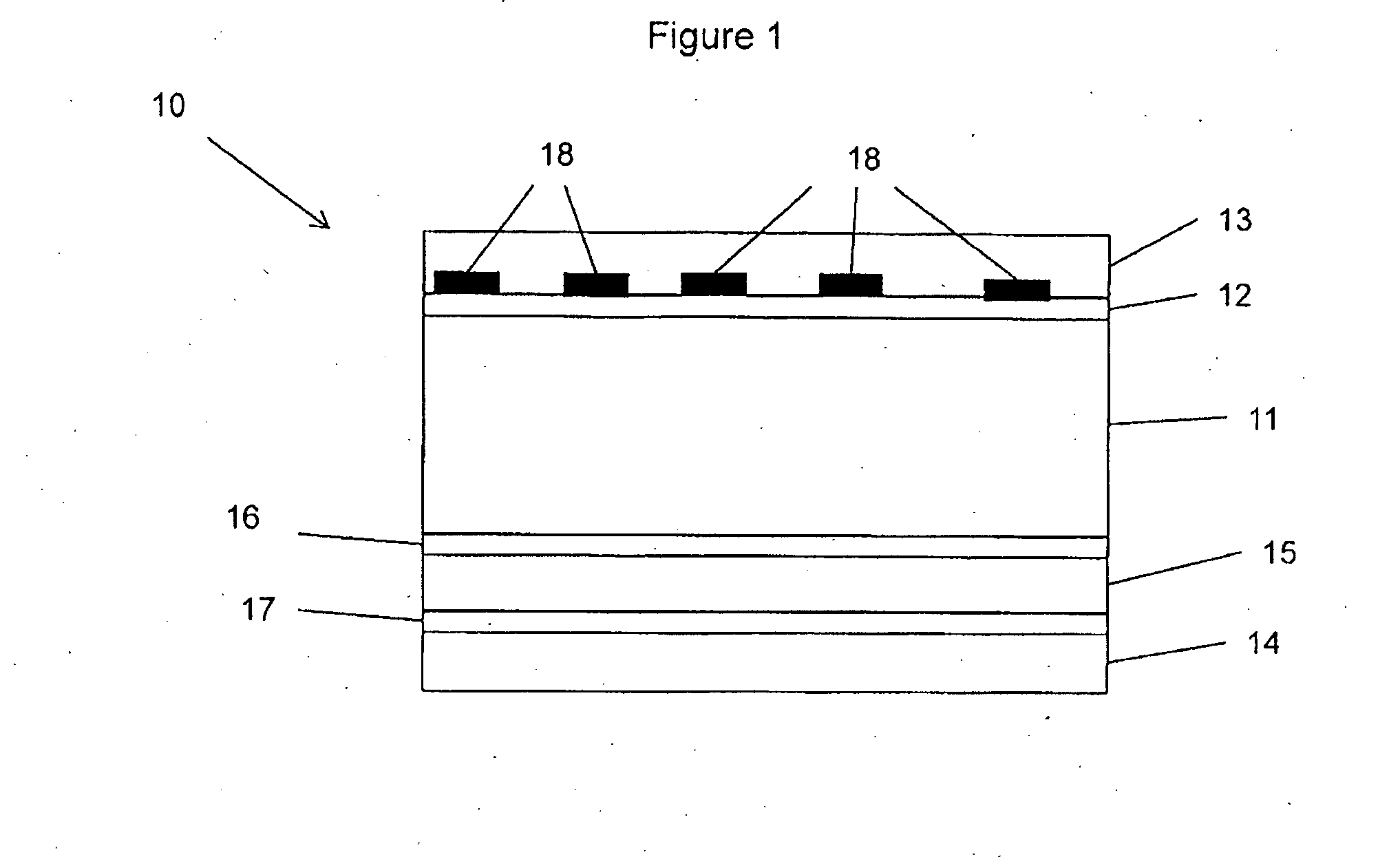
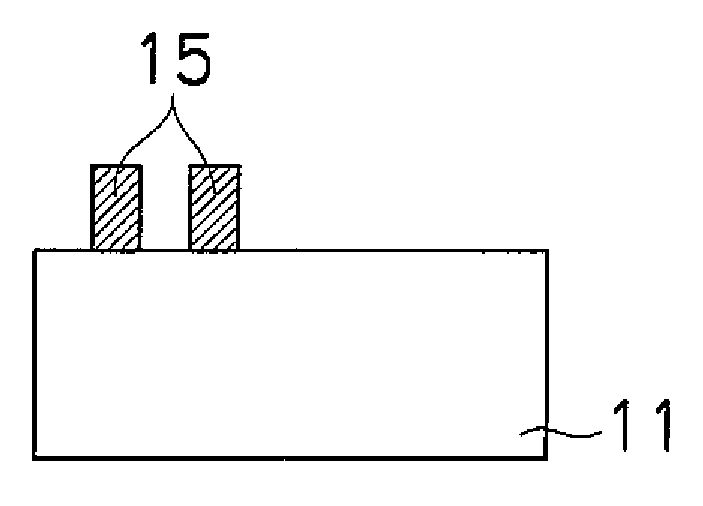
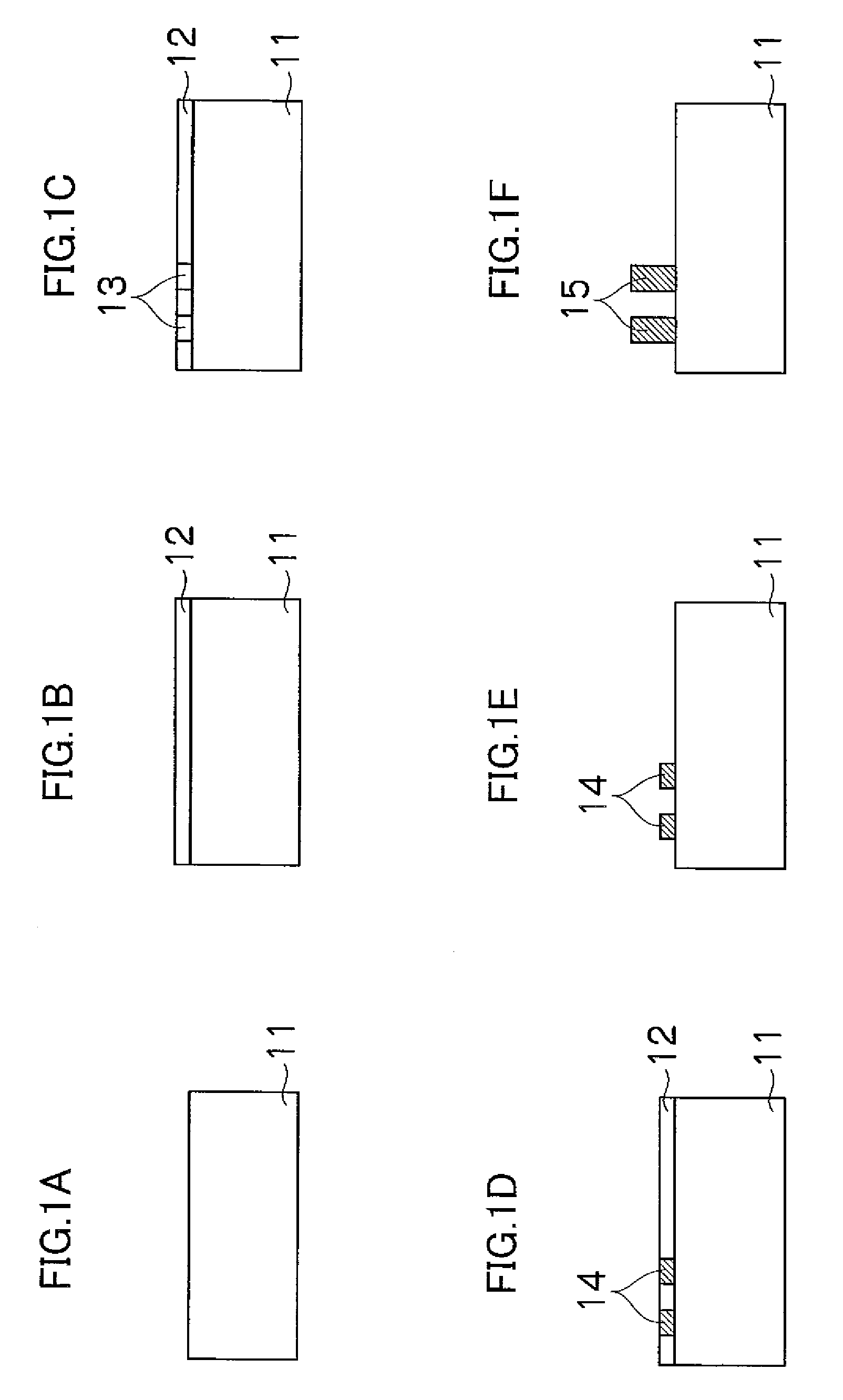
Crystallized diacetylenic indicator compounds and methods of preparing the compounds
ActiveUS20090131718A1Improve abilitiesExpand the scope ofUrea derivatives preparationThermometers using mean/integrated valuesAcetylene compoundsCrystallization
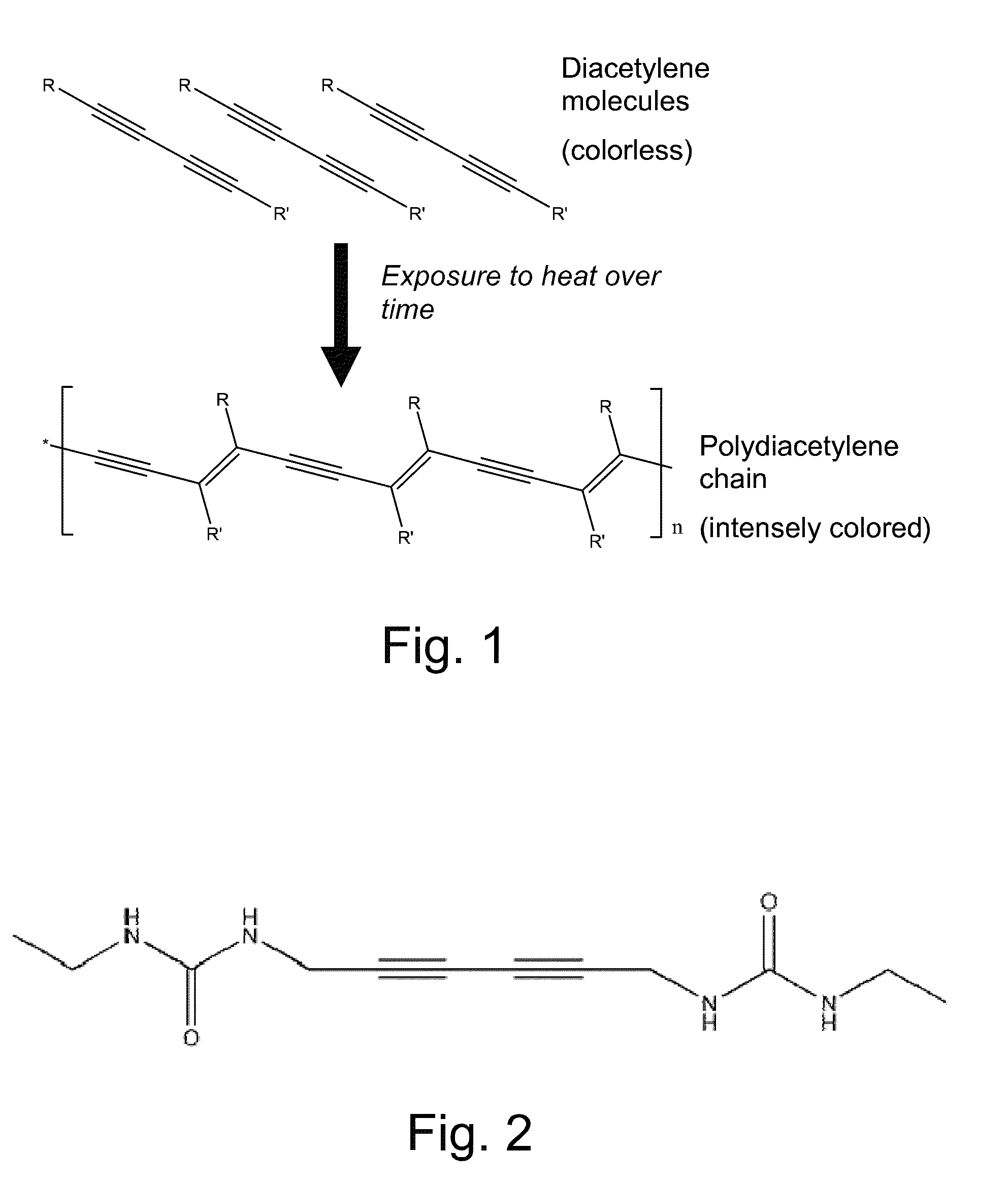
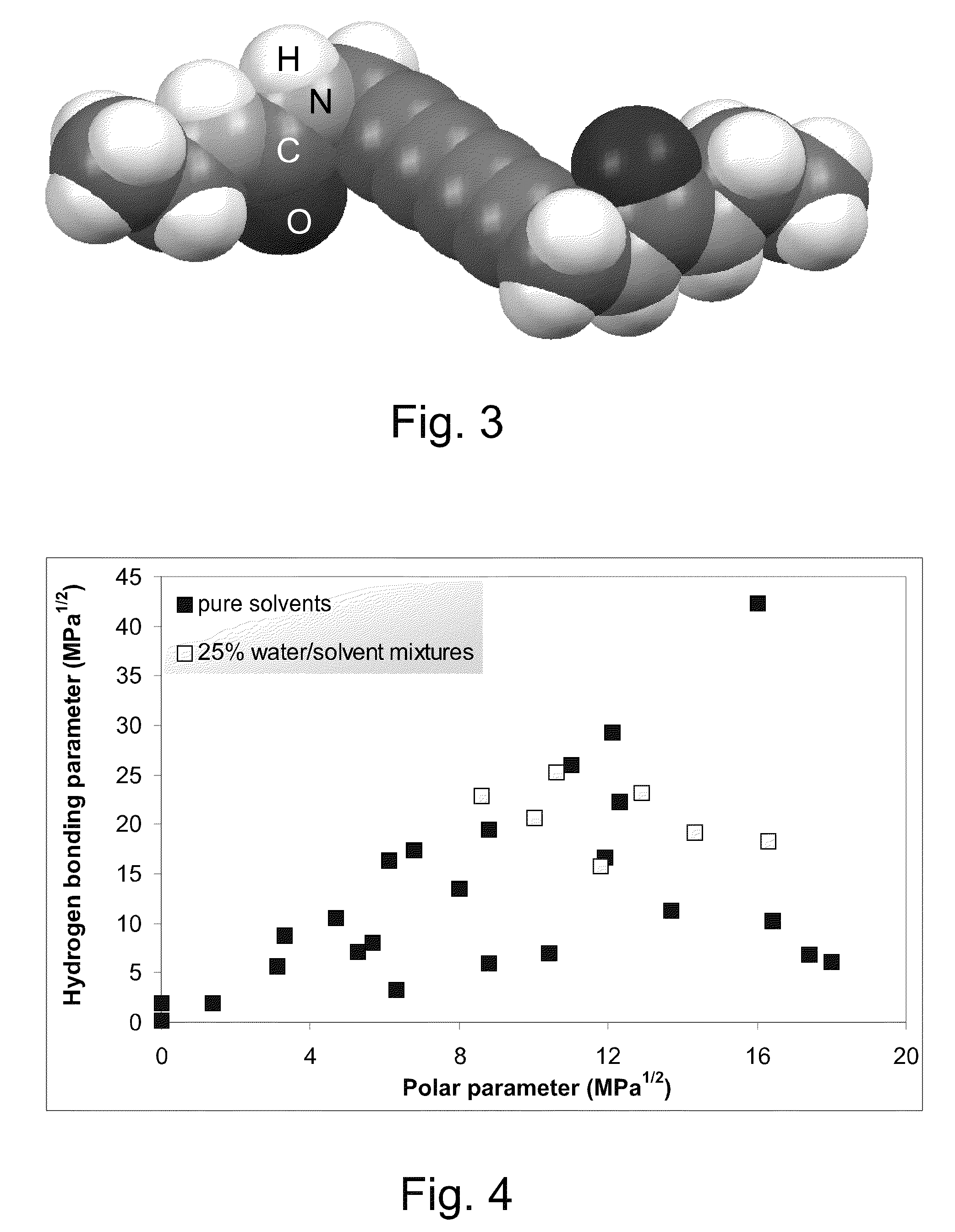
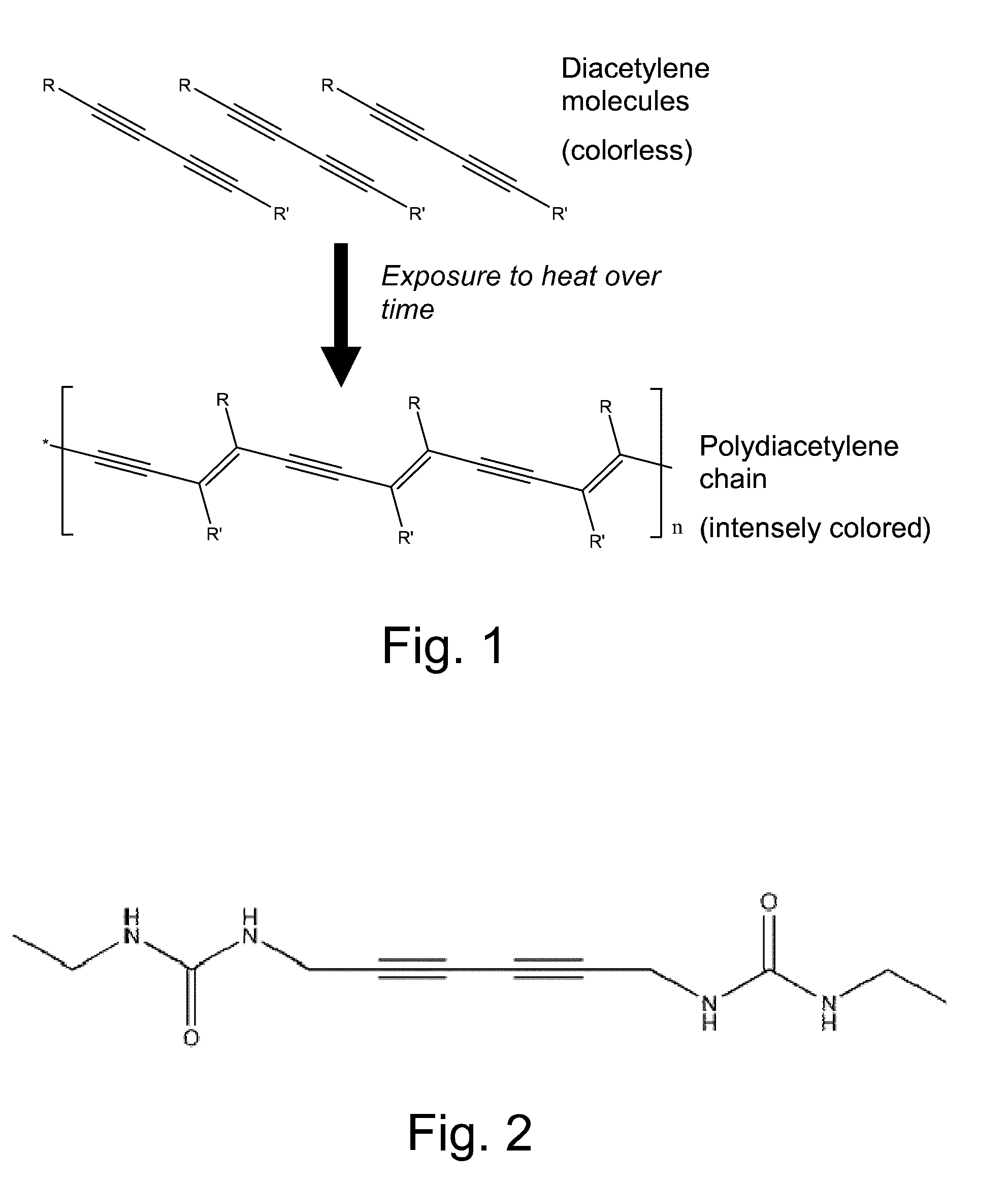
Crystallized diacetylenic compounds having certain crystallographic and other characteristics; diacetylenic compounds and mixtures crystallized from diacetylenic solutions; methods of preparing and identifying solvent systems for dissolving diacetylenic compounds; diacetylenic solutions; methods of recrystallizing diacetylenic compounds; crystals of 2,4-hexadiyn-1,6-bis(alkylurea) compounds; and ambient condition indicators and time-temperature condition indicators comprising crystallized diacetylenic compounds.
Owner:TEMPTIME CORP
Preparation method for polymer dispersion liquid crystal thin film
InactiveCN101121887AFacilitated DiffusionIncrease the driving voltageLiquid crystal compositionsPolymer networkBright spot
The invention pertains to liquid crystal material application and relevant fields, a method to prepare polymer dispersed liquid crystal (PDLC) film. 1,2-bipositive olefin butyl phenylacetylene compound, rigid double functional group acrylic acid ester polymerizable monomer and methyl metharcrylate are evenly mixed according to a mass ratio 8:2:3 to 8:3:2, and the mixture (taken as polymerized monomer of PDLC film) is evenly mixed with nematic liquid crystal according to a mass ratio 2:8 to 8:2, and a isotropic liquid is achieved. The isotropic liquid, photoinitiator and nano micro-beads are mixed evenly and laid between two transparent conducting films coated with ITO, and rolled evenly to form a membranous layer; the bright spots of isotropic liquid are irradiated with 365nm UV-lights under a temperature within 0.5 to 3 DEG C to achieve a PDLC film. The invention is capable to improve the driving voltage of PDLC materials, increase the mesh strength of polymers; as a result, the electro-optical stability of PDLC film is improved.
Owner:JIANGSU SENRAN CHEM
Diacetylenic materials for sensing applications
InactiveUS6963007B2Carboxylic acid nitrile preparationOrganic compound preparationAnalyteSensing applications
Diacetylenic materials for the colorimetric detection of an analyte or exposure to certain environmental factors are disclosed as well as the polymerization reaction products of these diacetylenic compounds.
Owner:3M INNOVATIVE PROPERTIES CO
Processless diacetylenic salt films capable of developing a black image
InactiveUS6177578B1High densityThe process is feasibleCarbamic acid derivatives preparationOrganic compound preparationDiacetyleneCarboxylic acid
This invention relates to a mixture of imageable polyacetylenic compounds which have similar photosensitivities and which are visually imageable in complementary colors combinable to provide a black image, which mixture contains at least one polyacetylenic metal salt which produces a color, preferably a metal salt of a diacetylene C.sub.6 to C.sub.48 mono- or dicarboxylic acid, which is complementary to a color produced by another polyacetylenic metal salt or non-metallic polyacetylenic compound contained in the mixture or in an another integral color forming layer. The invention also pertains to the use of said mixture and the manner of its preparation.
Owner:ISP CAPITAL
Triphenyl diacetylene compound with reaction and liquid crystal polymer containing the compound
InactiveCN1413969AHigh optical anisotropyLarge pitchLiquid crystal compositionsOrganic chemistryHalogenLiquid-crystal display
The present invention relates to a reactive triphenyl diethyne compound, which can take part in the copolymerization reaction with cholesterol-type liquid crystal to prepare cholesterol-type reflecting polaring plate.
Owner:IND TECH RES INST
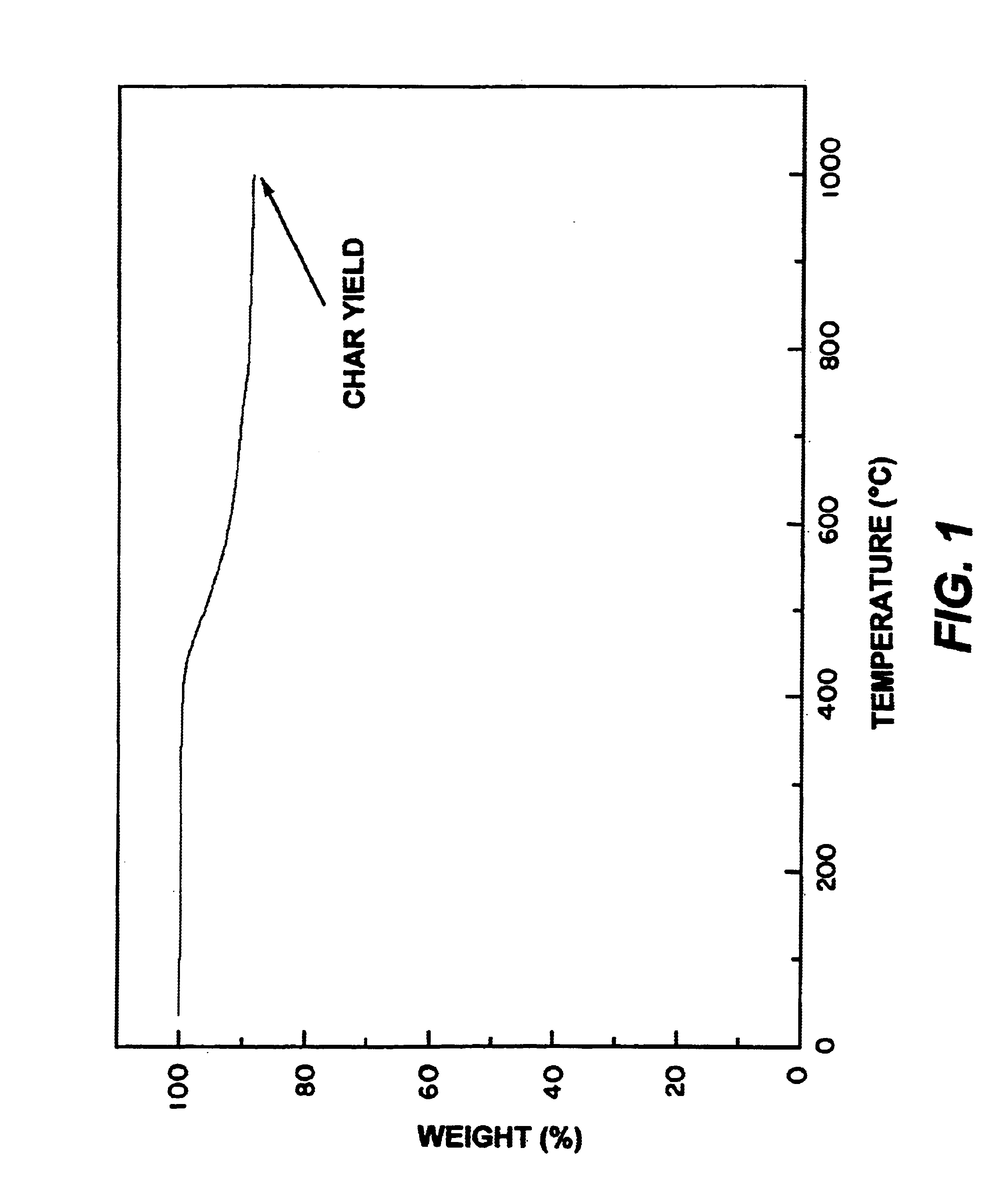
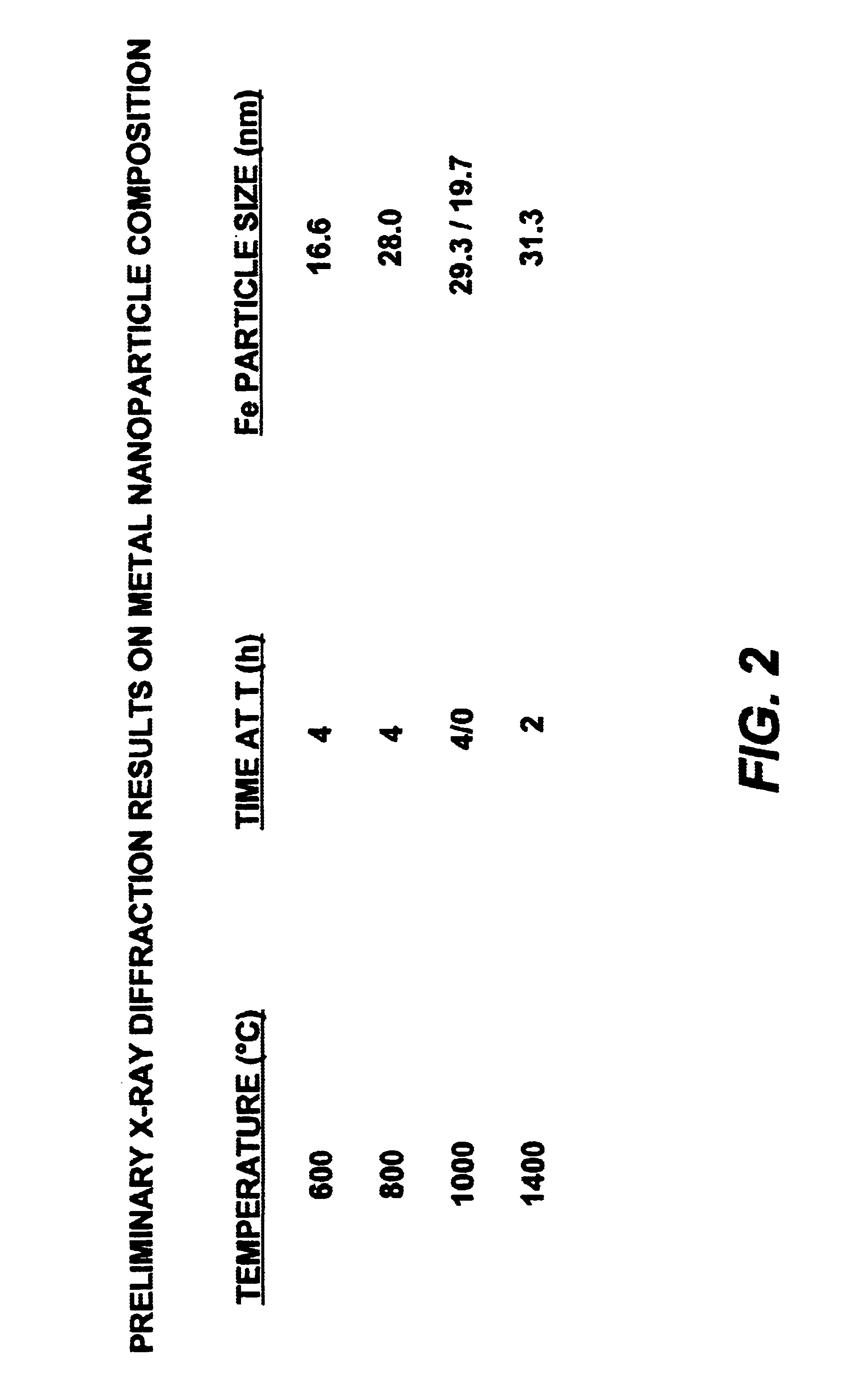
Metal nanoparticle thermoset and carbon compositions from mixtures of metallocene-aromatic-acetylene compounds
The present invention provides for a composition comprising:a composition formed by heating to a temperature of from about 300° C. and above a mixture of:an organometallic composition and an aromatic-acetylene containing compound; andwherein said organometallic composition comprises the formula: wherein A is selected from the group consisting of H, and wherein M is a metal;wherein Rx and Ry are independently selected from the group consisting of an aromatic, a substituted aromatic group and combinations thereof;wherein m, s and z are ≧0;wherein m and s are independently determined in each repeating unit;wherein said aromatic-acetylene containing composition is 1,2,4,5-tetrakis(phenylethynyl)benzene, 1,2,4-tris(phenylethynyl)benzene or 1,3,5-tris(phenylethynyl)benzene; andwherein said organometallic composition and said aromatic-acetylene composition are molar mix proportions of between 1 and 99 of said organometallic composition and between 99 and 1 of said aromatic-acetylene composition.
Owner:THE UNITED STTE OF AMERICA AS REPRESENTED BY THE SEC OF THE NAVY
Aromatic ether and alkynyl containing phthalonitriles
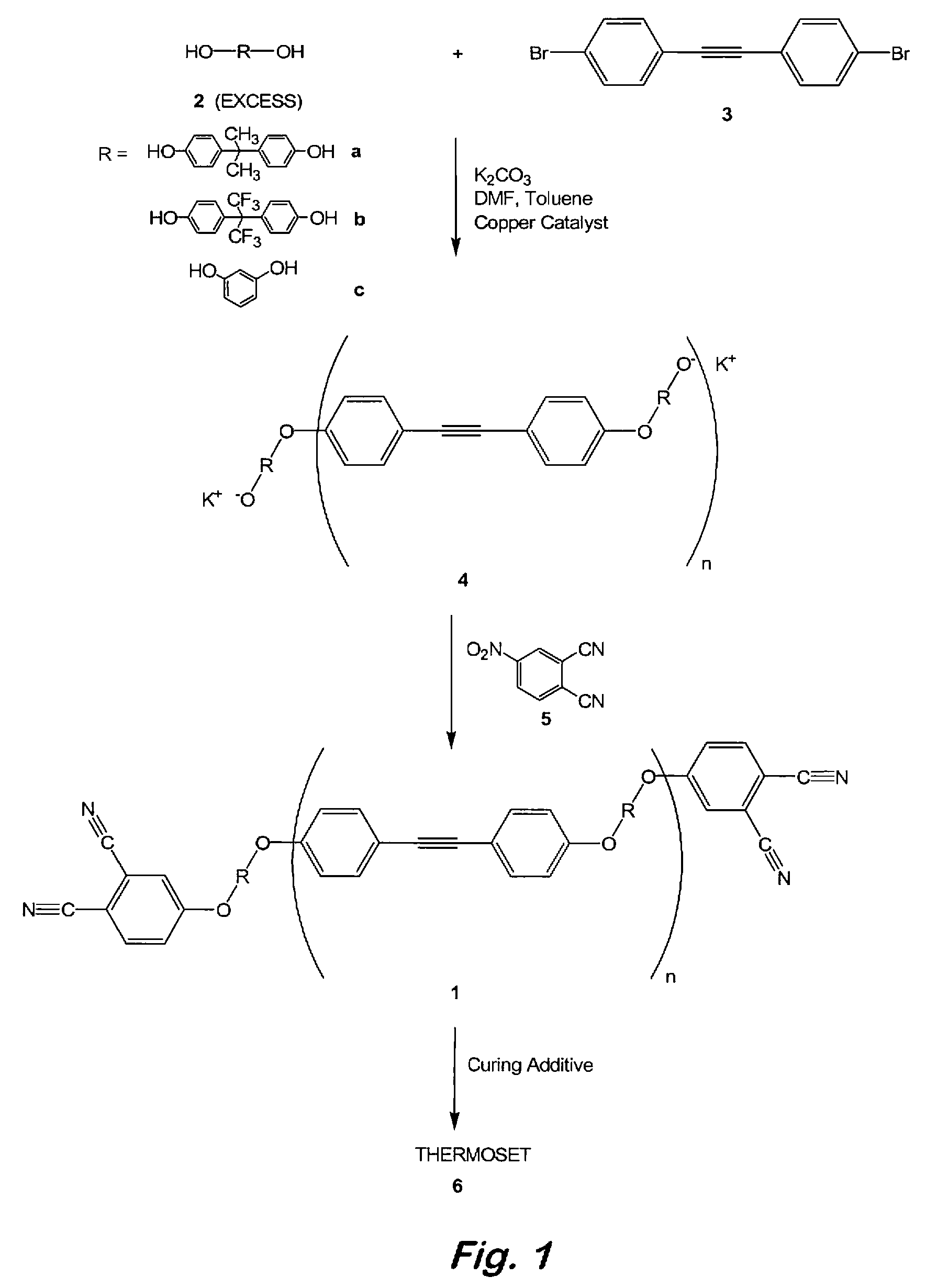
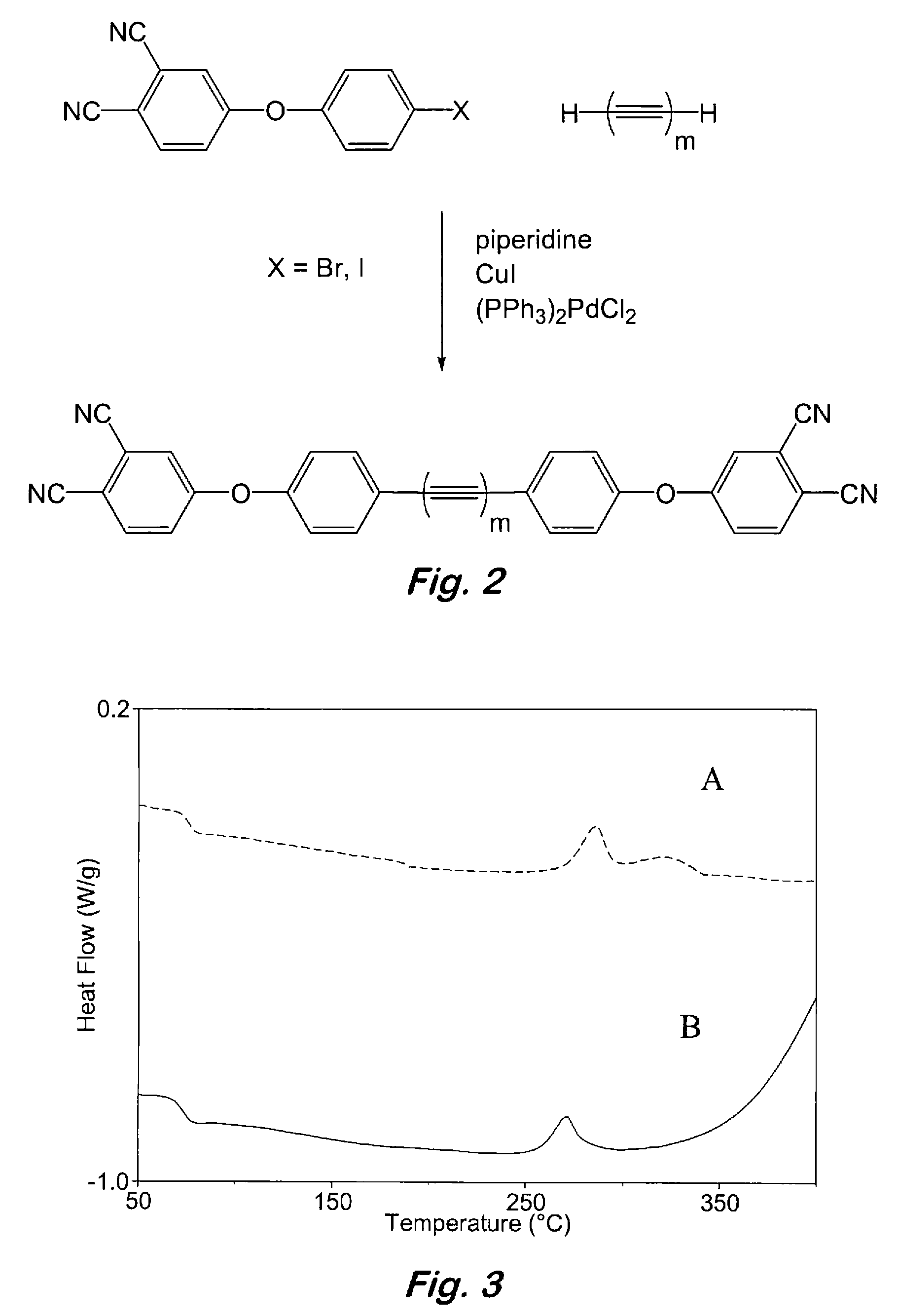
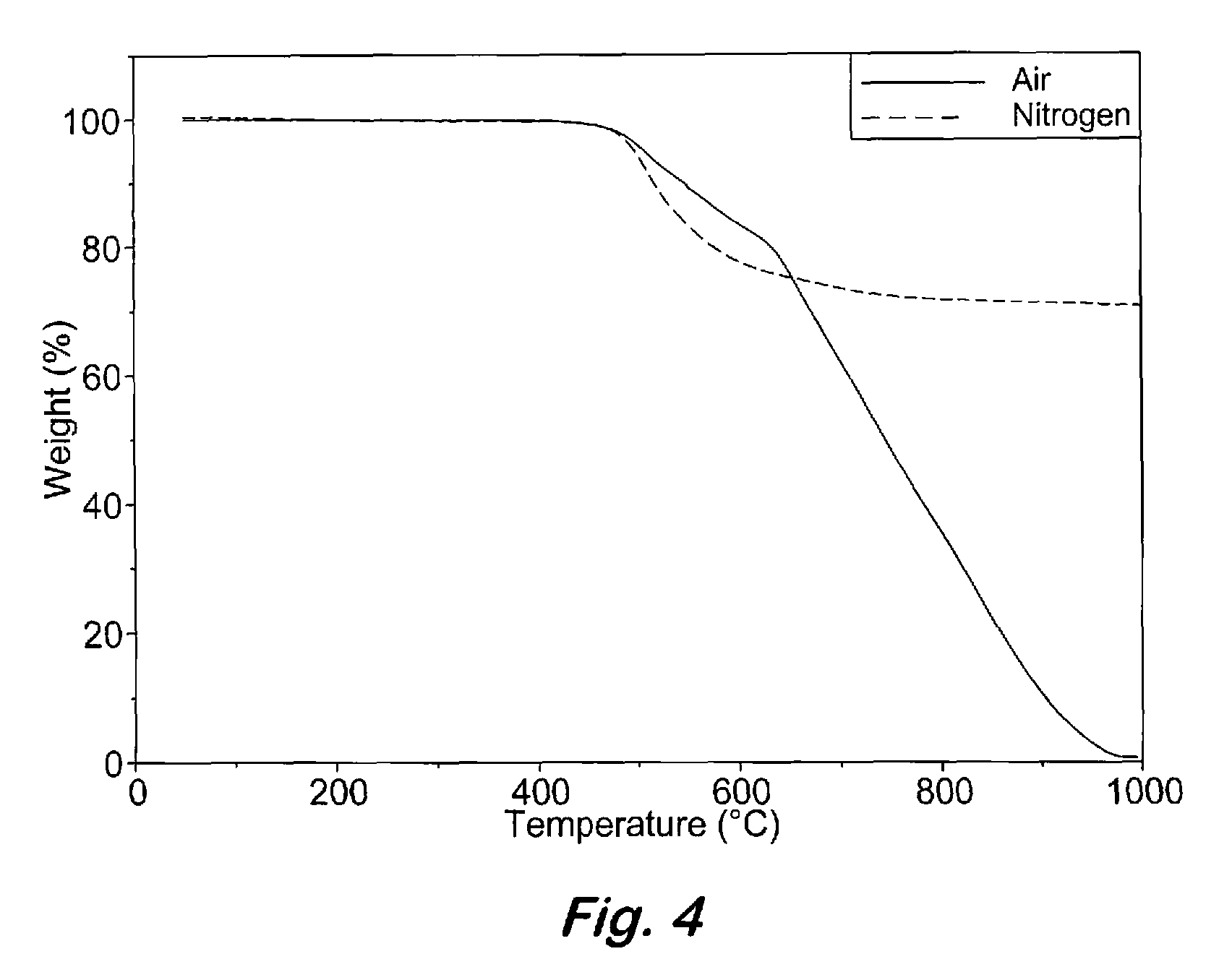
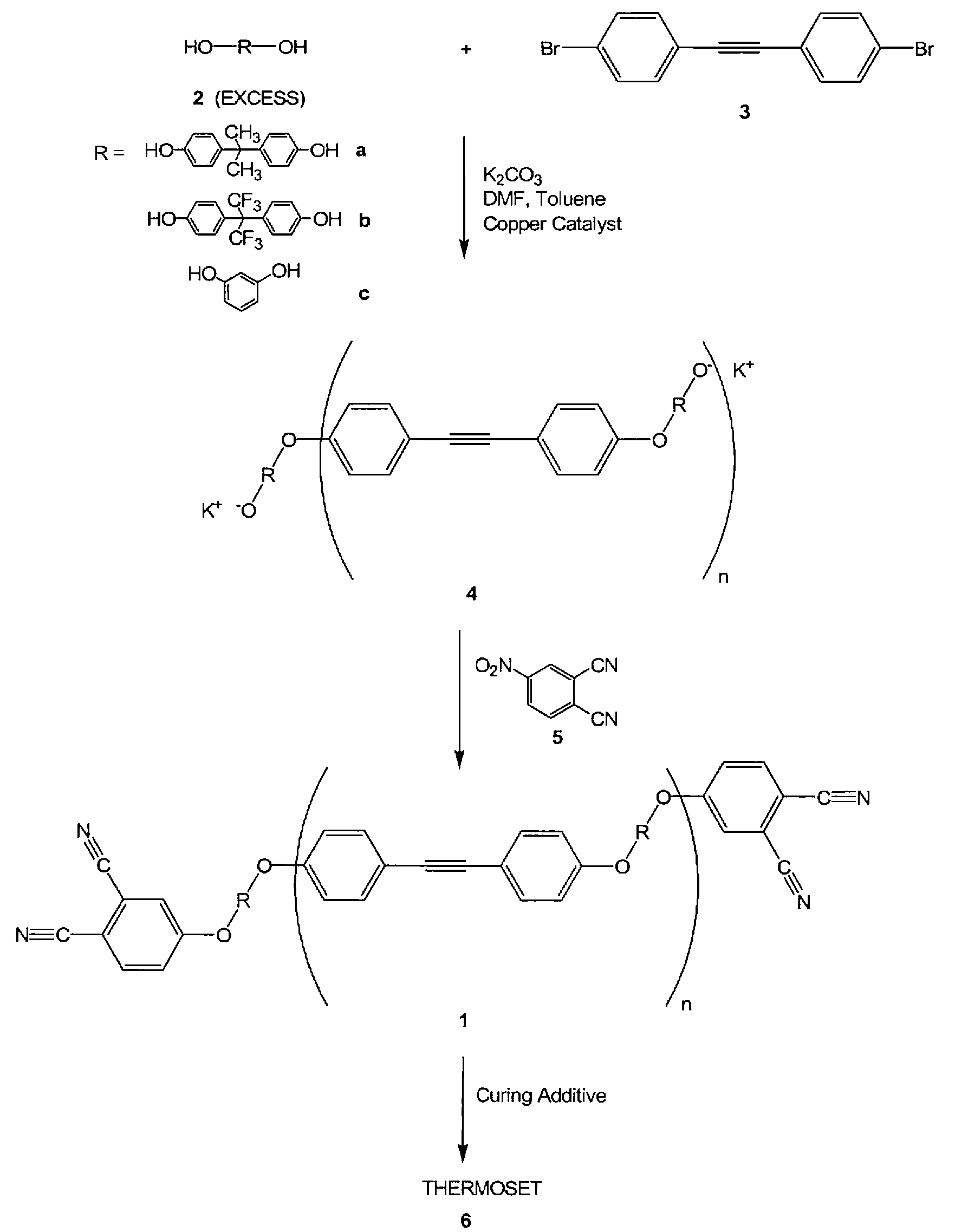
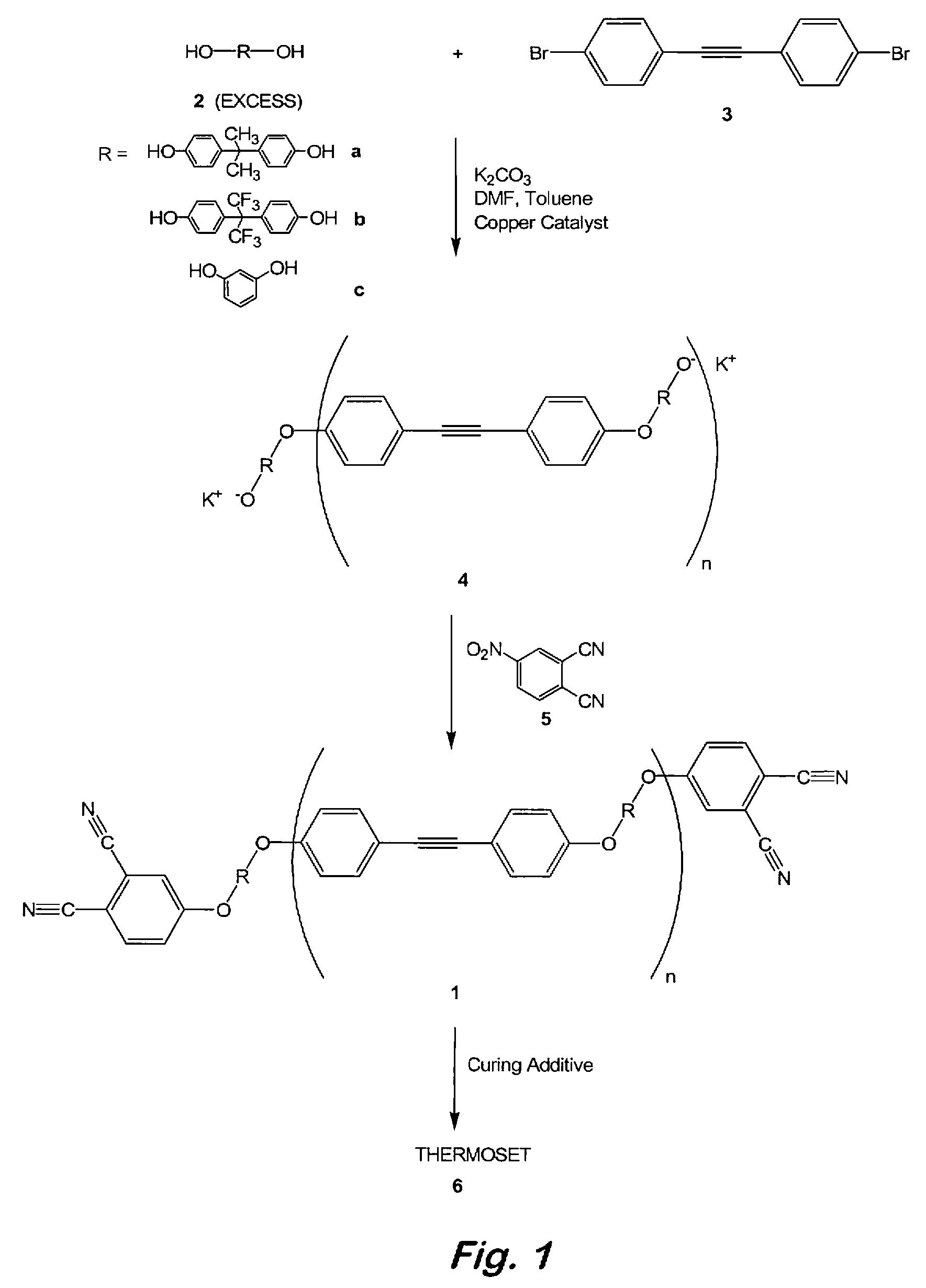
Compounds having the formulas below. R is an aromatic-containing group. Each M is an alkali metal. Each m is a positive integer. The value of n is a positive integer. The value p is 0 or 1. If p is 0 then n is 1. A thermoset made by curing a composition containing the below phthalonitrile monomers.A method of reacting a diphenyl acetylene compound with an excess of an aromatic diol in the presence of an alkali metal carbonate to form the above oligomer. A method of reacting a phenoxyphthalonitrile with an acetylene compound to form the phthalonitrile monomer below.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Aromatic ether and alkynyl containing phthalonitriles
Compounds having the formulas below. R is an aromatic-containing group. Each M is an alkali metal. Each m is a positive integer. The value of n is a positive integer. The value p is 0 or 1. If p is 0 then n is 1. A thermoset made by curing a composition containing the below phthalonitrile monomers.A method of reacting a diphenyl acetylene compound with an excess of an aromatic diol in the presence of an alkali metal carbonate to form the above oligomer. A method of reacting a phenoxyphthalonitrile with an acetylene compound to form the phthalonitrile monomer below.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Synthetic method of coumarin-pyrrole compound
InactiveCN103232462AHigh yieldFacilitate deep expansionOrganic chemistryAminocoumarinsCombinatorial chemistry
The invention discloses a synthetic method of a coumarin-pyrrole compound. The synthetic method comprises the following step of: in a reactive solvent, with 4-amino coumarin compounds and disubstituted acetylene compounds as raw materials, under the catalysis of palladium (II) and action of an oxidant, carrying out a reaction to obtain the coumarin-pyrrole compound. The synthetic method is mild in reaction conditions, simple and convenient in route and high in atom economy and has a favorable industrial application prospect; and raw materials are low in cost and easy to obtain.
Owner:HUBEI UNIV OF SCI & TECH
Structure and preparation method for heterocyclic aryl acetylene compounds containing benzoxazole and benzothiazole groups
The invention discloses a structure and a preparation method for heterocyclic aryl acetylene compounds containing benzoxazole and benzothiazole groups. The preparation method comprises the following steps: reacting heterocyclic aryl acetylene compounds containing benzoxazole and benzothiazole groups with bromine in an organic solvent to obtain brominized products; and reacting with alkali in the organic solvent, and removing hydrogen bromide to obtain the heterocyclic aryl acetylene compounds.
Owner:BEIJING UNIV OF CHEM TECH
Resin composition for laser engraving, relief printing plate precursor for laser engraving, relief printing plate and method of producing the same
The present invention provides a resin composition for laser engraving containing at least an acetylene compound and a binder polymer, a relief printing plate precursor for laser engraving using the same, a relief printing plate, and a method for producing a relief printing plate.
Owner:FUJIFILM CORP
Reversibly activatable diacetylenes and their use as colour-formers
A method of forming a coloured substrate, comprising applying to or incorporating within the substrate a diacetylene compound, and exposing the substrate to (i) a first, activating stimulus that converts the diacetylene compound from an unreactive to a reactive form, and (ii) a second stimulus that causes the reactive form of the diacetylene compound to polymerize and form the coloured substrate, wherein the diacetylene compound reverts to its unreactive form on removal of the activating stimulus and is of formula I: Y—C≡C—C≡C—(CH2)n-T-Q-Z (I) wherein: n=an odd integer; T=CO, CS or a bond; Q=NH, S, O, OCONH, NHCONH, NH—CHE-CONH, NHCOO or NHCSNH wherein E is H or a C1-20 alkyl group; Z=H or a hydrocarbon group containing 1 to 20 carbon atoms; Y=H or any group comprising at least one carbon atom.
Owner:DATALASE
Method for producing binder for inkjet printing ink, inkjet printing ink, and printed material
ActiveUS20120219769A1Good ink dischargeabilityImprove blend stabilityDecorative surface effectsLayered productsOrganic solventPolyol
An object of the present invention is to provide a method for producing a binder for inks and for use in producing an inkjet printing ink that has both good ink dischargeability and blend stability. A method for producing a binder for inkjet printing inks, the binder containing a hydrophilic-group-containing urethane resin (A), an acetylene compound (B), and an aqueous medium (C), includes step (1) of allowing Ga polyol (a1) containing a hydrophilic-group-containing polyol to react with a polyisocyanate (a2) in an organic solvent or in the absence of a solvent and feeding the organic solvent as needed to prepare an organic solvent solution [I], step (2) of mixing the organic solvent solution [I] with the acetylene compound (B) and the aqueous medium (C) to prepare a mixture [II], and step (3) of removing the organic solvent contained in the mixture [II].
Owner:DAINIPPON INK & CHEM INC
Metal pattern forming method
InactiveUS20090087570A1Avoid cloggingStable ejectionPretreated surfacesLiquid/solution decomposition chemical coatingArylHydrogen
A metal pattern forming method includes the steps of: applying one of a metal salt solution and an acetylene compound solution onto the substrate, the acetylene compound solution containing an acetylene compound expressed by a general formula of:(R·(C≡C)l)k−(L)−(A)m,where R is one of a metal element, hydrogen, a carboxyl group or salt thereof, an alkyl group, a cycloalkyl group, an alkenyl group, an alkynyl group, an aryl group, an aralkyl group and a heterocyclic group, L is one of a compound linking A with a carbon-carbon triple bond and a group having (k+m) valency, A is one of a polyoxyether group, a polyaminoether group and polythioether group, k and l are integers not less than 1, and m is an integer not less than 0; and then applying the other of the metal salt solution and the acetylene compound solution onto the substrate so that the metal salt solution reacts with the acetylene compound solution to form a metal precipitate on the substrate, wherein at least one of the applying steps is performed by using an inkjet apparatus to directly form the metal pattern composed of the metal precipitate on the substrate.
Owner:FUJIFILM CORP
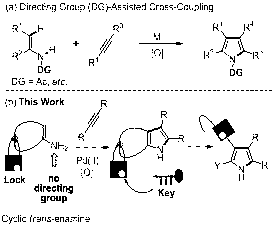
Preparation method of heteroaromatic iminazole [1,2-Alpha]pyridine compounds
InactiveCN102838597AOxidative cyclization reactionSimple reaction conditionsOrganic chemistryOrganic solventNatural product
The invention relates to a preparation method of heteroaromatic iminazole [1,2-Alpha]pyridine compounds. The method comprises the steps that under the existence of univalent silver salt, 2-amino pyridine compounds and acetylene compounds are dissolved in organic solvents to be reacted for 10 to 24 hours at the reacting temperature of 100 to 130 DEG C, and then the compounds are separated and purified to prepare the heteroaromatic iminazole [1,2-Alpha]pyridine compounds. The reacting compounds used in the method of the utility model can be simply prepared at a low cost, the reacting condition is very simple, atom economy is very high, and the oxidation cyclization reaction of the 2-amino pyridine compounds and the acetylene compounds can be realized in a highly selective mode, so as to prepare the heteroaromatic iminazole [1,2-Alpha]pyridine compounds. The method can prepare the prodrug of a drug with adone zoli pyridine simply, and the drug is widely applied to treating gastric ulcers and gastroesophageal reflux diseases. The preparation method provided by the invention has great application potential for synthetizing medicine, natural products and the like.
Owner:WUHAN UNIV
N-conjugated organic material of polycyclic fused ring type,intermediate therefor, and process for producing n-conjugated organic material of polycyclic fused ring type
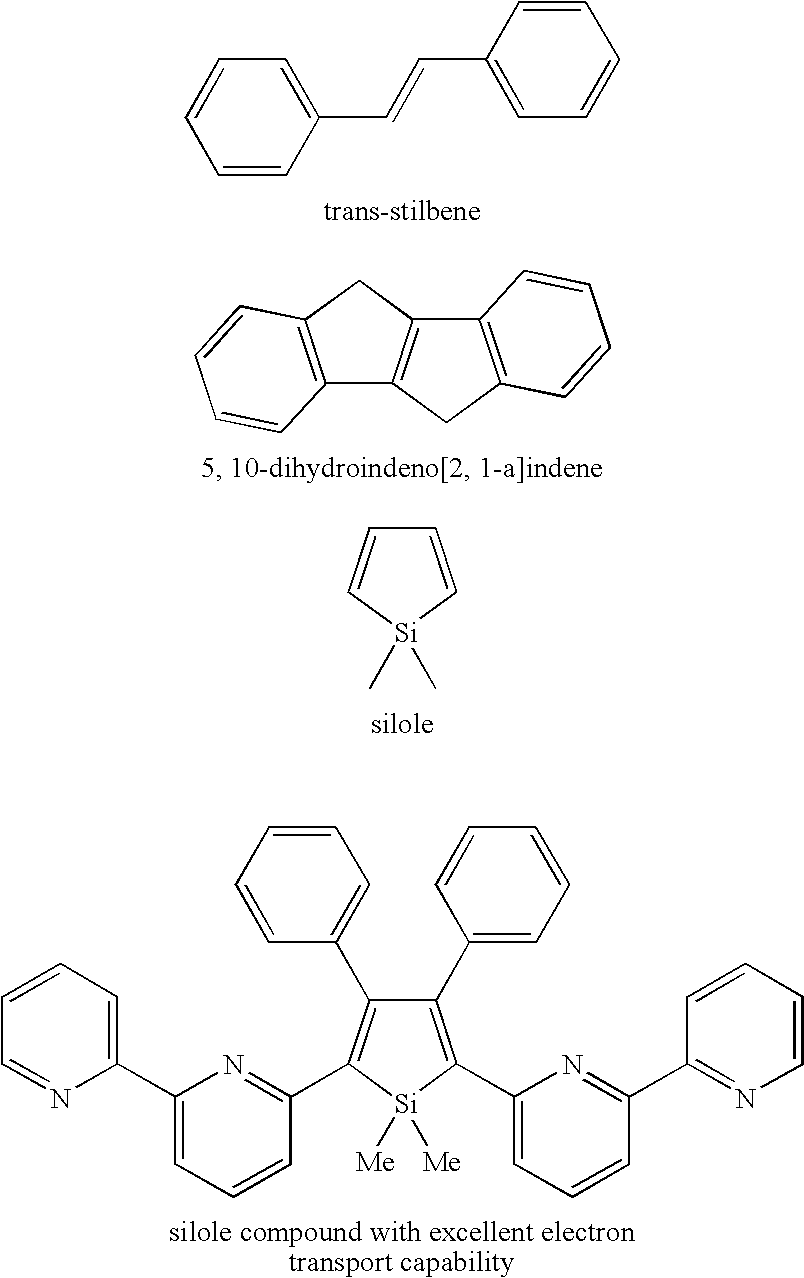
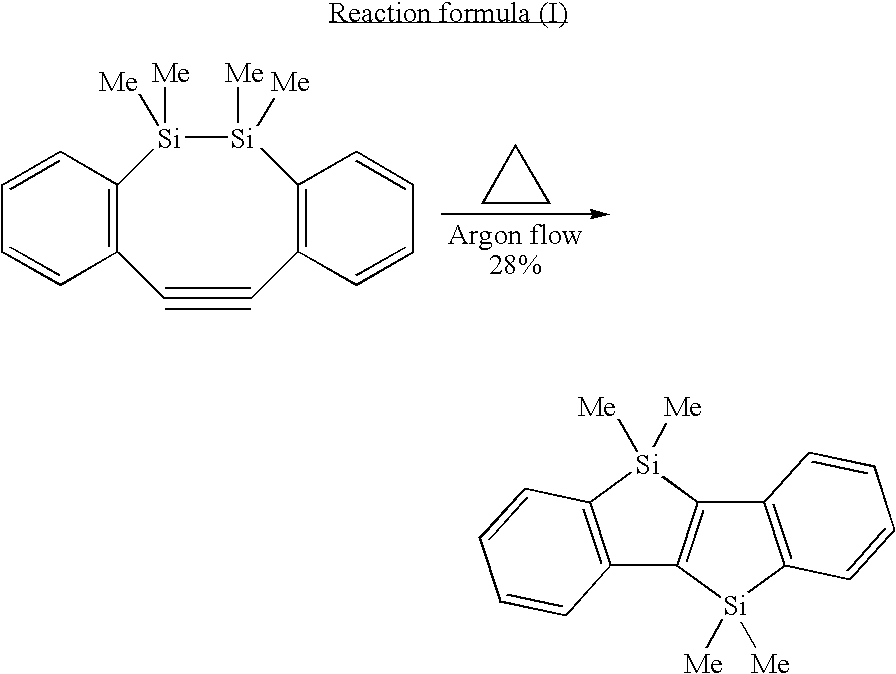
InactiveUS20060100433A1Improve luminous performanceEnhanced charge transport capabilitySilicon organic compoundsPhosphorus organic compoundsArylPhenyl-acetylene
The invention provides condensed polycyclic π-conjugated organic materials and manufacturing methods for the materials. A metal reducing agent is reacted with a straight-chain, triple bond-containing hydrocarbon (aryl acetylene compound, phenyl acetylene compound), the hydrocarbon being a benzene ring with an organic silicon as a substituent, so as to allow an intramolecular reductive cyclization reaction to proceed between the silicon and the triple-bond carbon. The reaction produces condensed polycyclic π-conjugated organic materials of the invention. The invention provides light-emitting materials applicable for organic electroluminescent devices, condensed polycyclic π-conjugated organic materials applicable for charge transport materials, their intermediate products, and a manufacture method for condensed polycyclic π-conjugated organic materials.
Owner:JAPAN SCI & TECH CORP
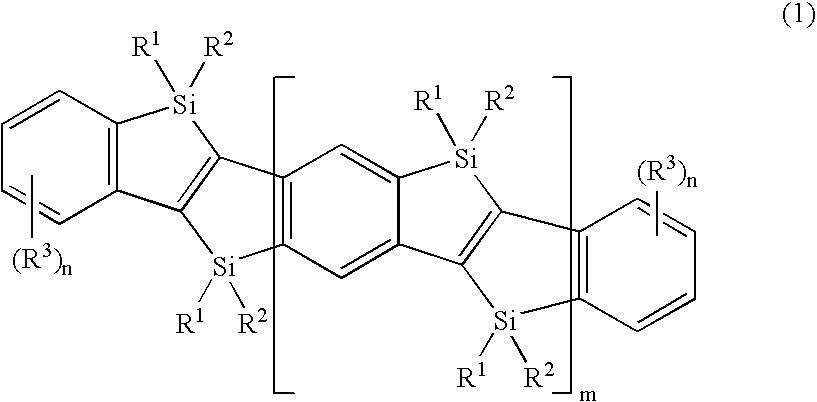
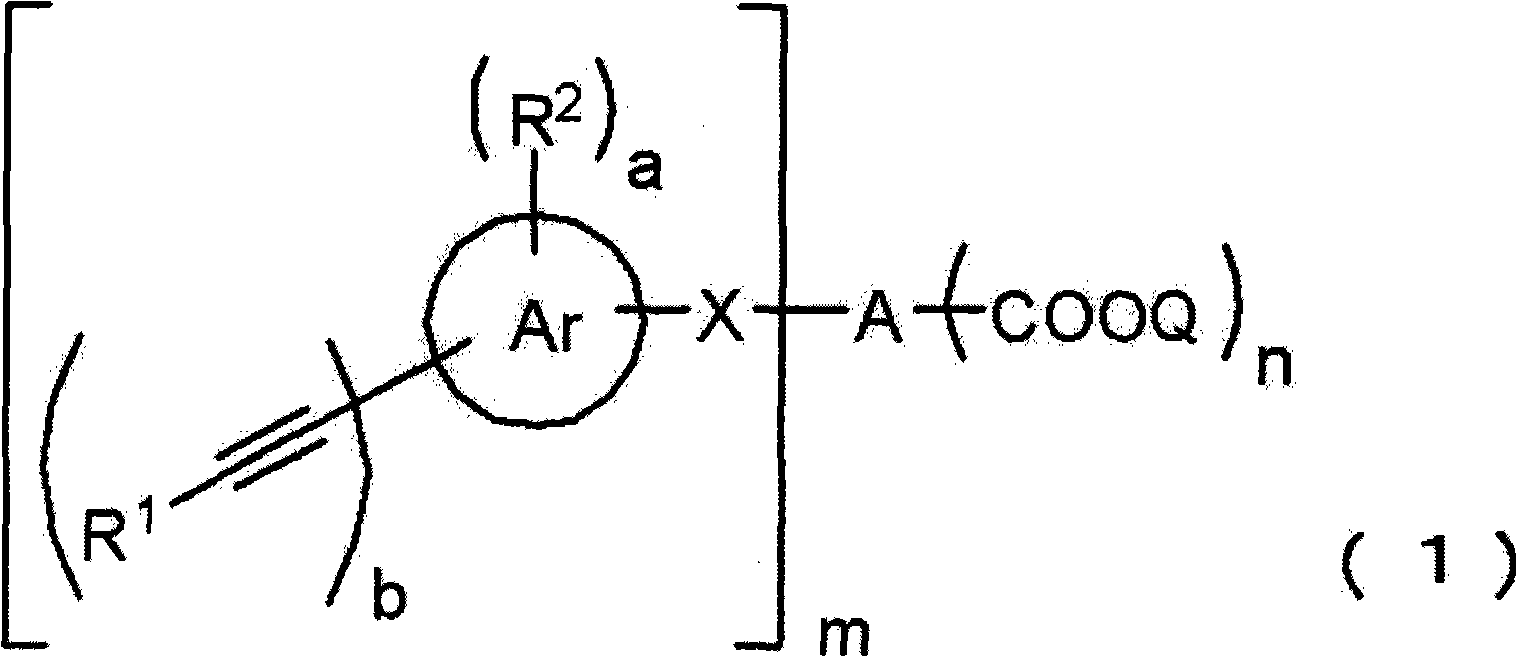
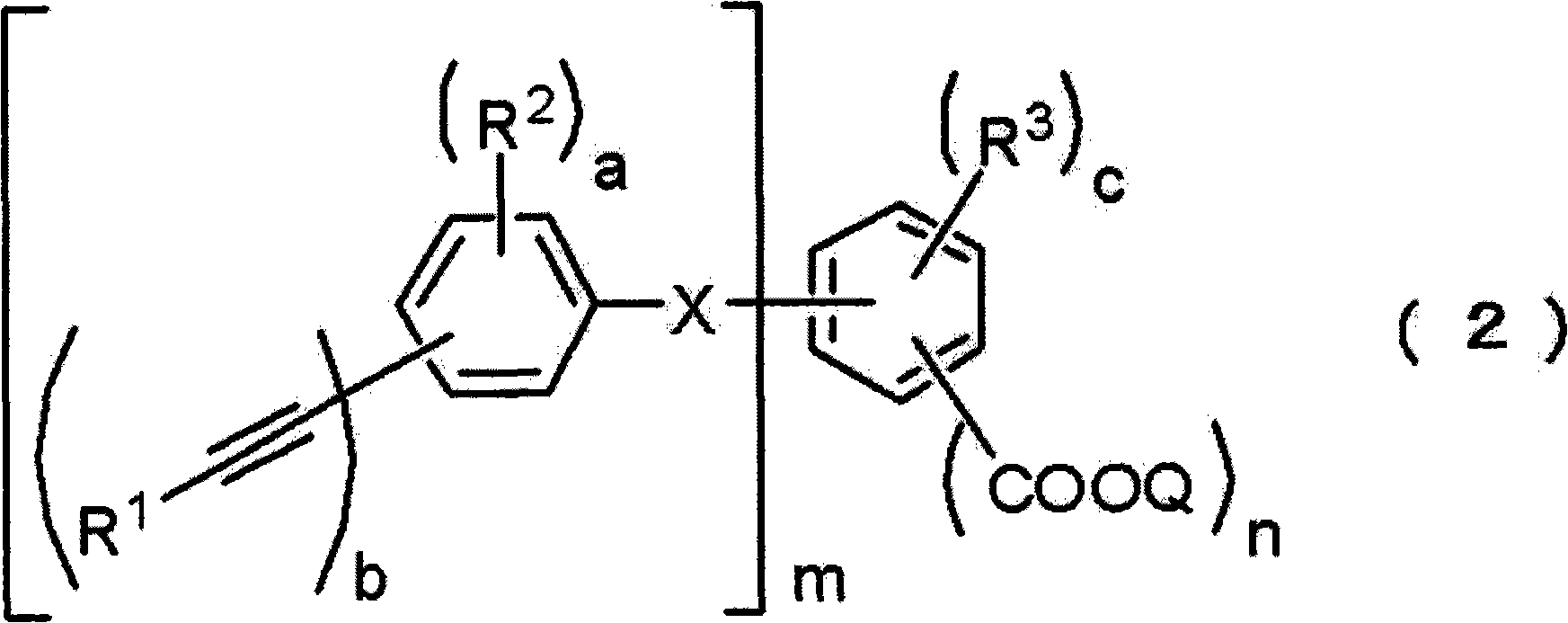
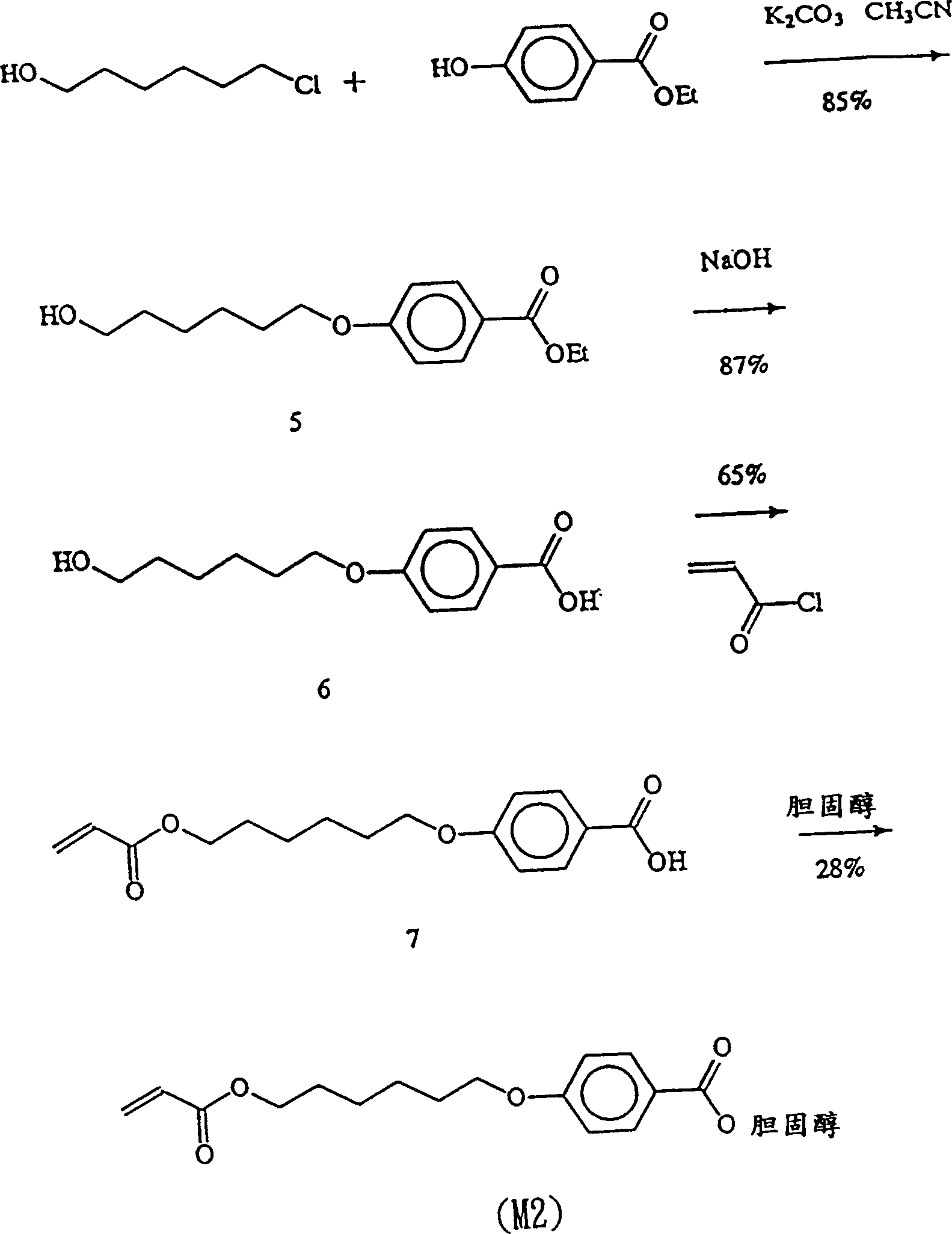
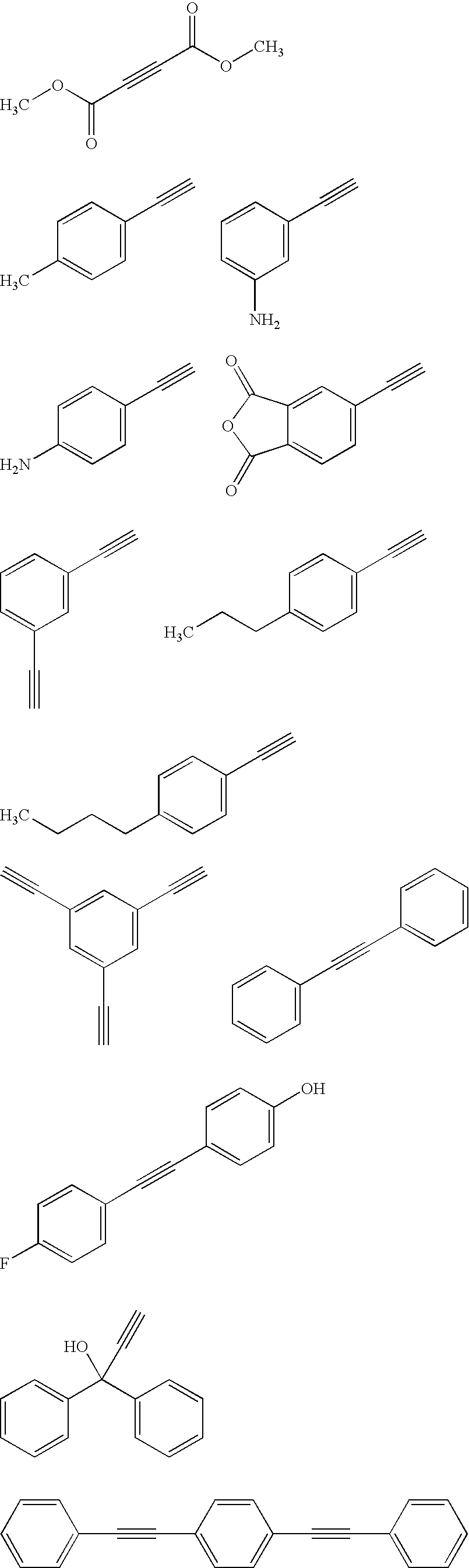
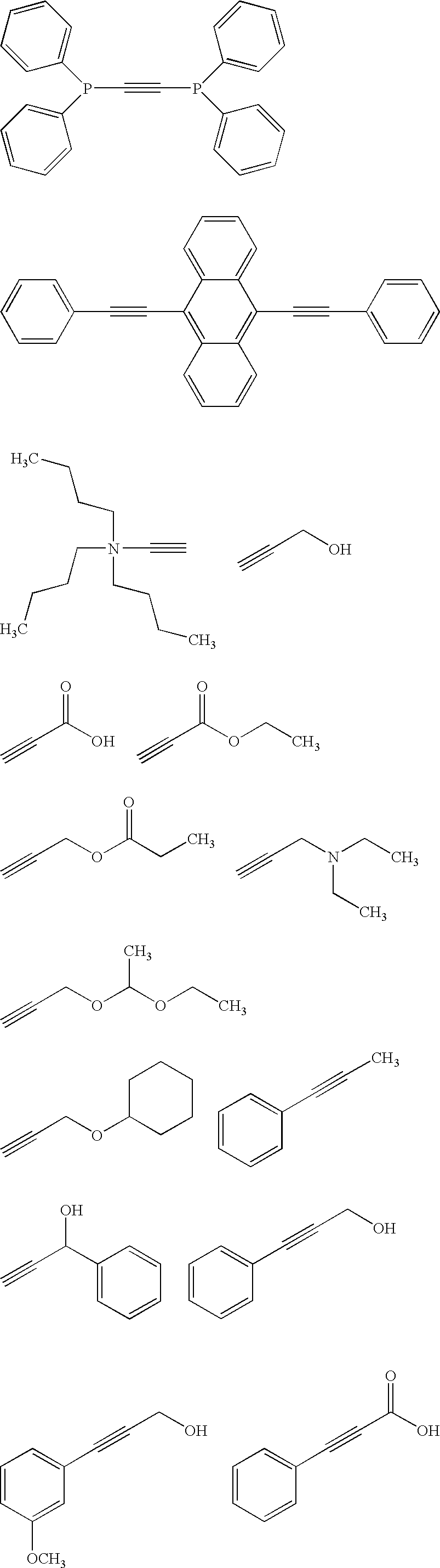
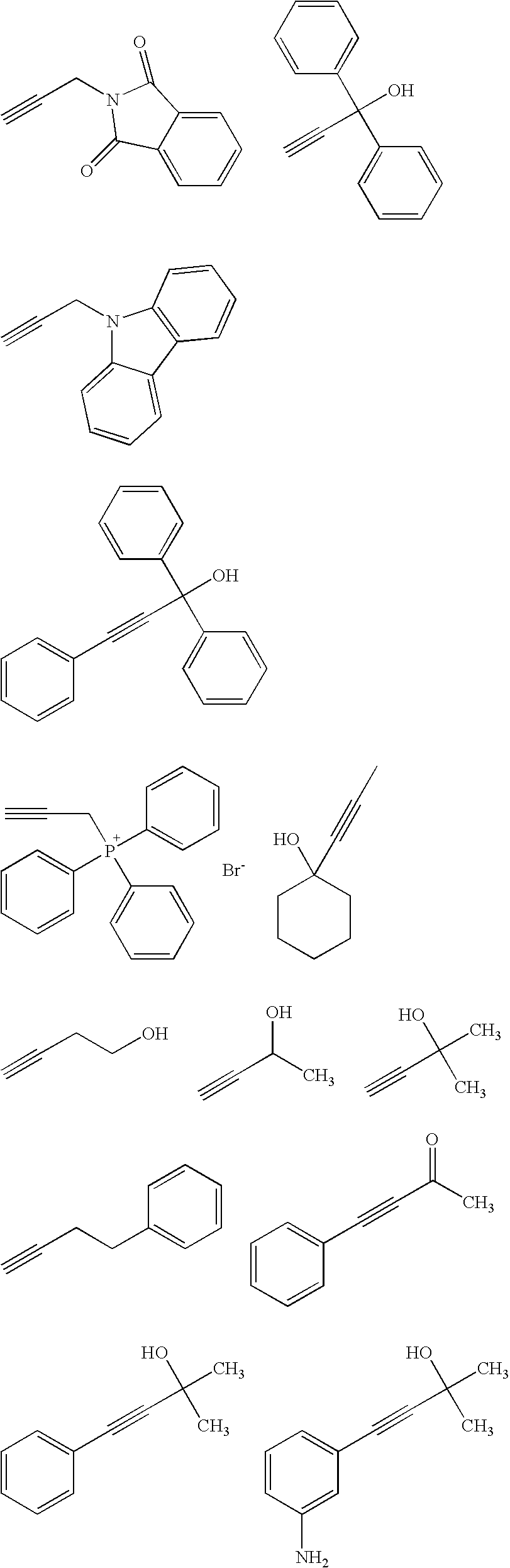
Acetylene compound
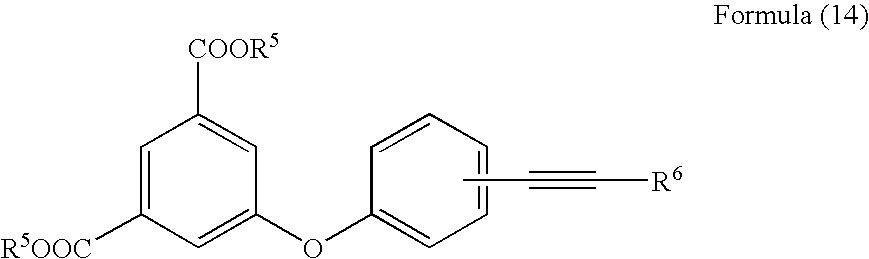
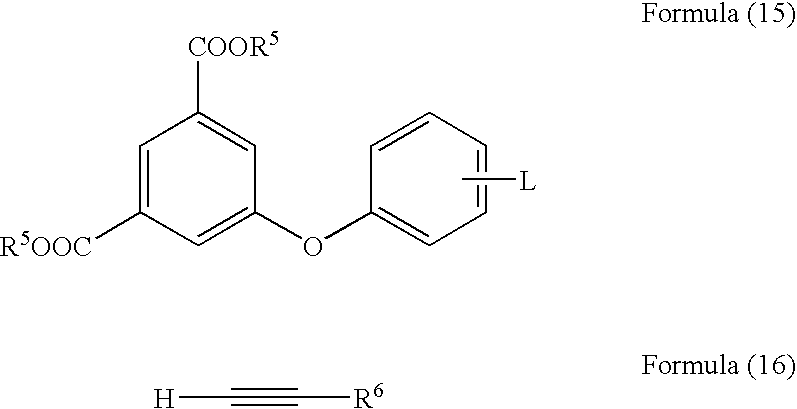
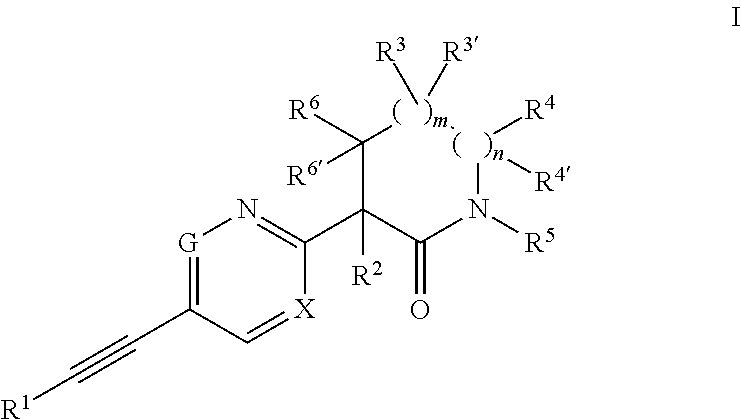
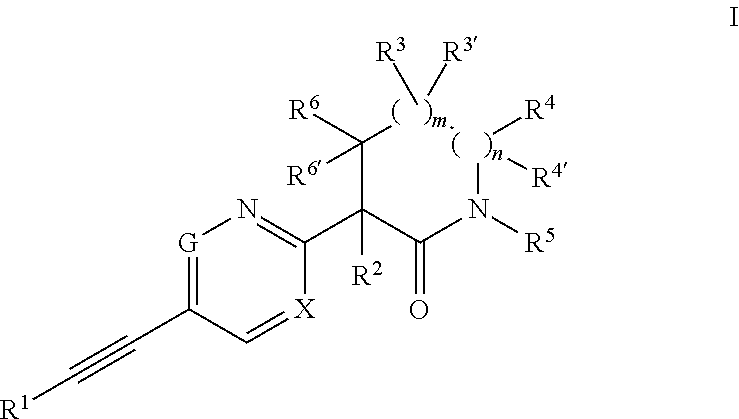
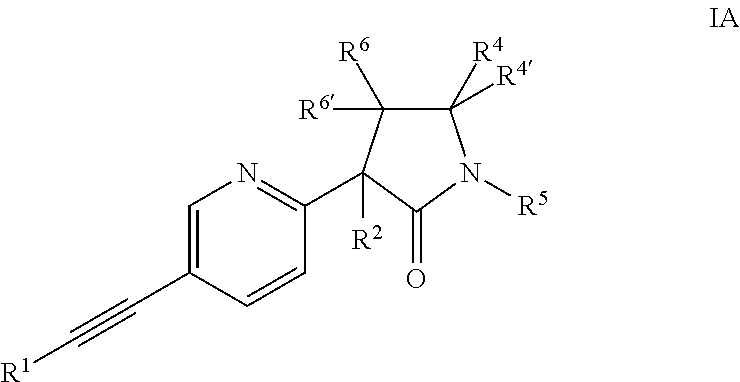
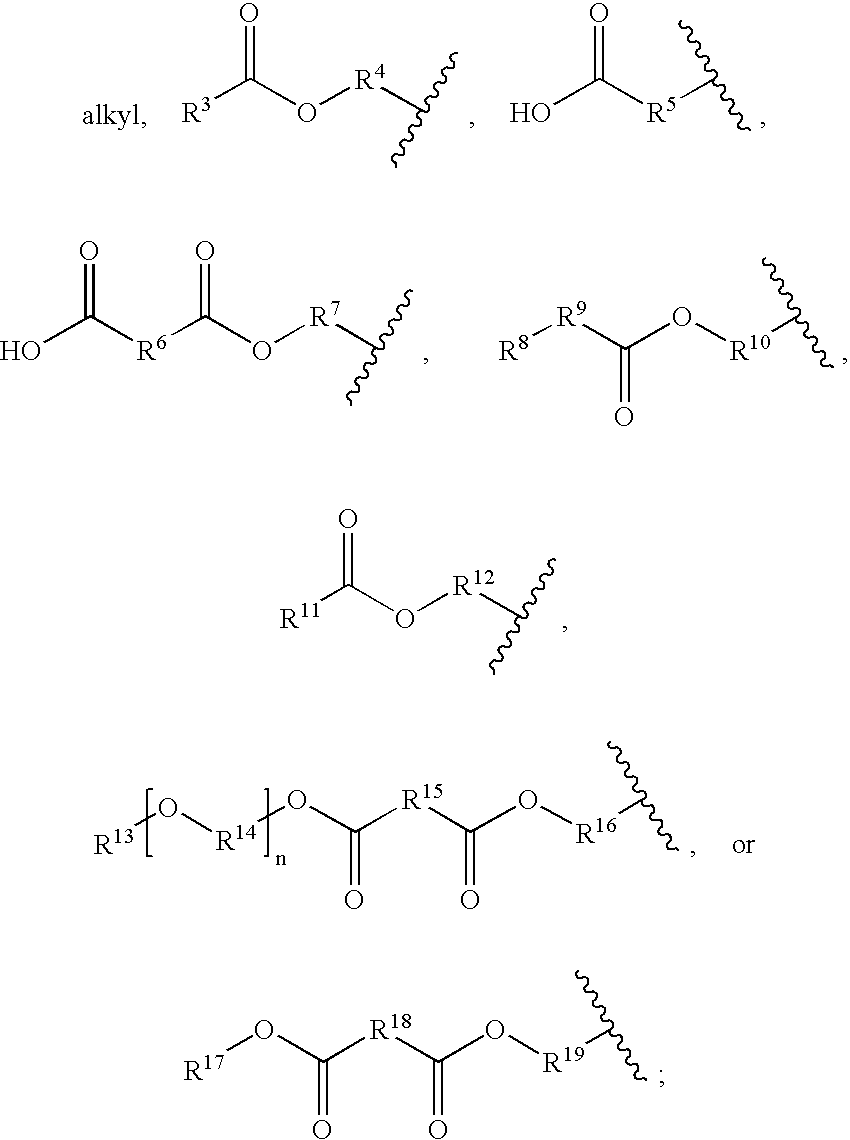
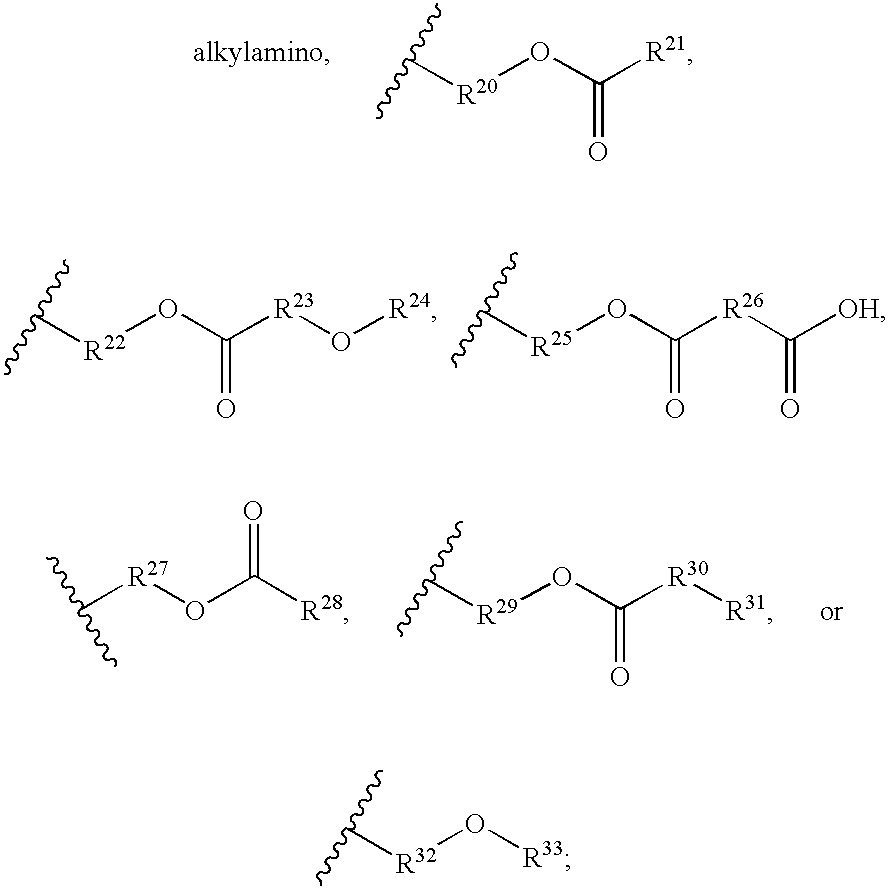
The invention provides an acetylene compound represented by the following Formula (1), which is useful as a raw material for a polymer achieving high thermal resistance;wherein, in Formula (1), the encircled Ar represents an aryl group or a heteroaryl group; X represents a divalent linking group; R, R′ and R1 each independently represent a hydrogen atom, a hydrocarbon group or a heterocyclic group; R2 represents a hydrogen atom or a substituent substitutable on a benzene ring; A represents a hydrocarbon group or a heterocyclic group; Q represents a hydrogen atom, a hydrocarbon group, or a metal element capable of forming a monovalent metal salt; a represents an integer from 0 or more; b, m and n each independently represent an integer of 1 or more; when n, m and b are 1 at the same time, X is not —(C═O)O—; and when n is 2, and both m and b are 1, X is not —O—.
Owner:FUJIFILM CORP
Inkless printing apparatus
A substrate marking apparatus for use in combination with a substrate comprising a multi-colour change diacetylene compound is disclosed. The substrate marking apparatus comprises: at least two radiation sources operable to emit radiation of different wavelengths, optical transformation elements and a control system. The control system takes digital file information and converts this to a set of emission instructions for the radiation sources. The radiation sources are then applied to the substrate in sequence and intensity determined by the control system such that the substrate is activated to change from a colourless state to any one of a range of multiple permanent colours.
Owner:DATALASE
Diacetylenics containing adjacent triple bonds
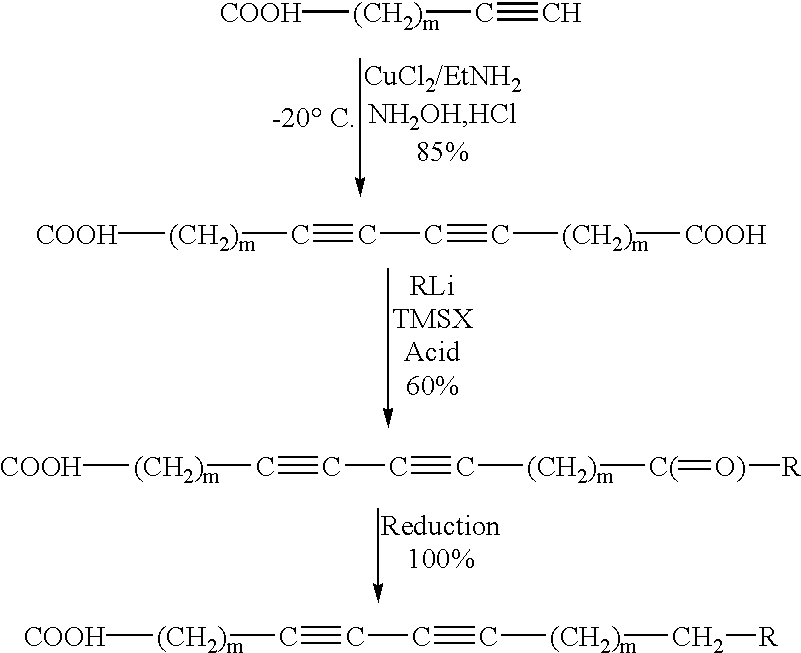
InactiveUS6440056B1Use minimizedPrepared cost-effectivelyFatty acid chemical modificationFatty acids production/refiningTrimethylsilyl chlorideAryl
This invention pertains to a process for preparing diacetylenics, to diacetylenic compounds and to reduced diacetylenic compounds. The process includes the steps of reacting coupling an acetylenic acid in presence of cupric chloride in Ethylamine and hydroxylamine hydrochloride to form a diacetylenic diacid; reacting the diacetylenic diacid with a lithium compound, trimethylsilyl chloride and hydrochloric acid to form a diacetylenic compound; and reducing the diacetylenic compound to a reduced diacetylenic compound. The diacetylenic compounds have the formula COOH-(CH2)m-C=C-C=C-(CH2)m-C(=O)-R or R-C(=O)-(CH2)m-C=C-C=C-(CH2)m-C(=O)-R and the reduced cyclic diacetylenic compounds have the formula COOH-(CH2)m-C=C-C=C-(CH2)m-CH2-R or R-CH2-(CH2)m-C=C-C=C-(CH2)m-CH2-R, where m is 1-18 and R is selected from alkyl groups of 1-10 and cyclic groups containing 6-35 carbon atoms and aryl moieties
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
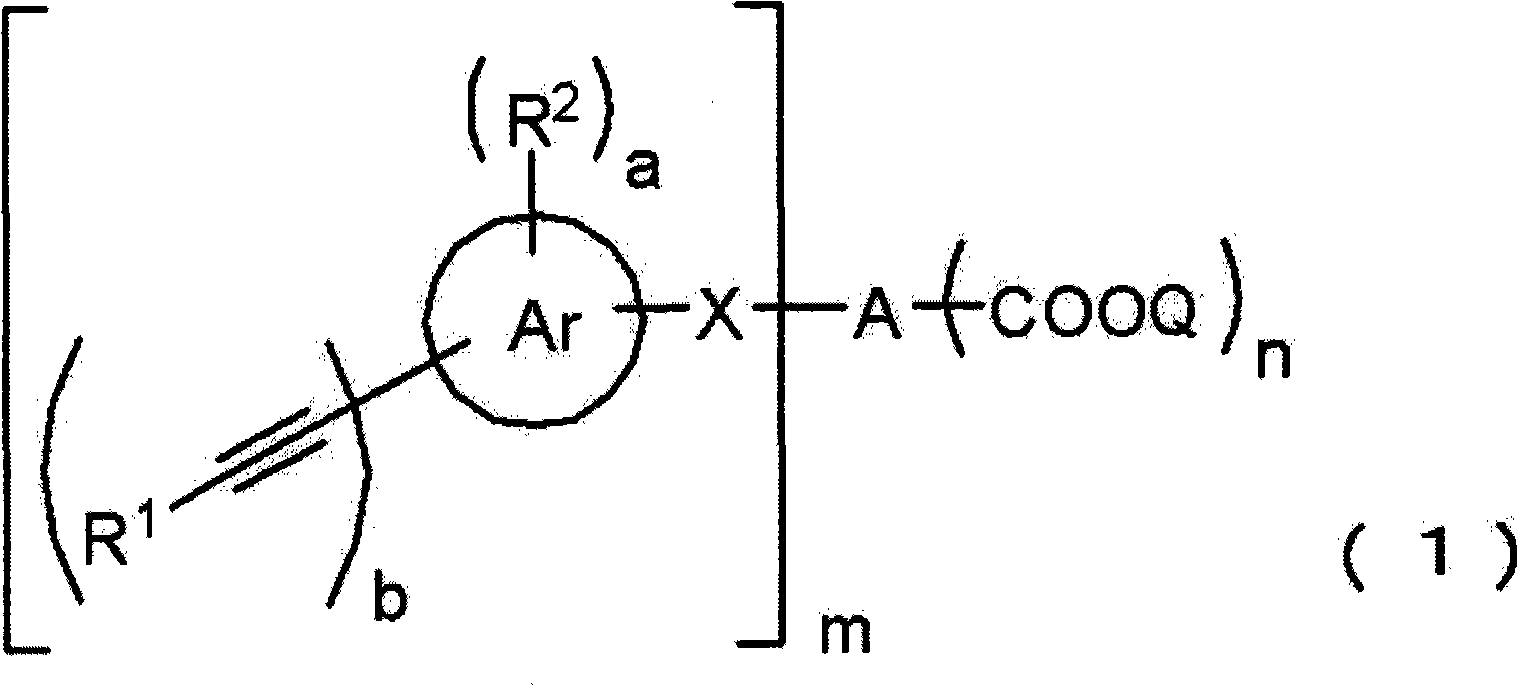
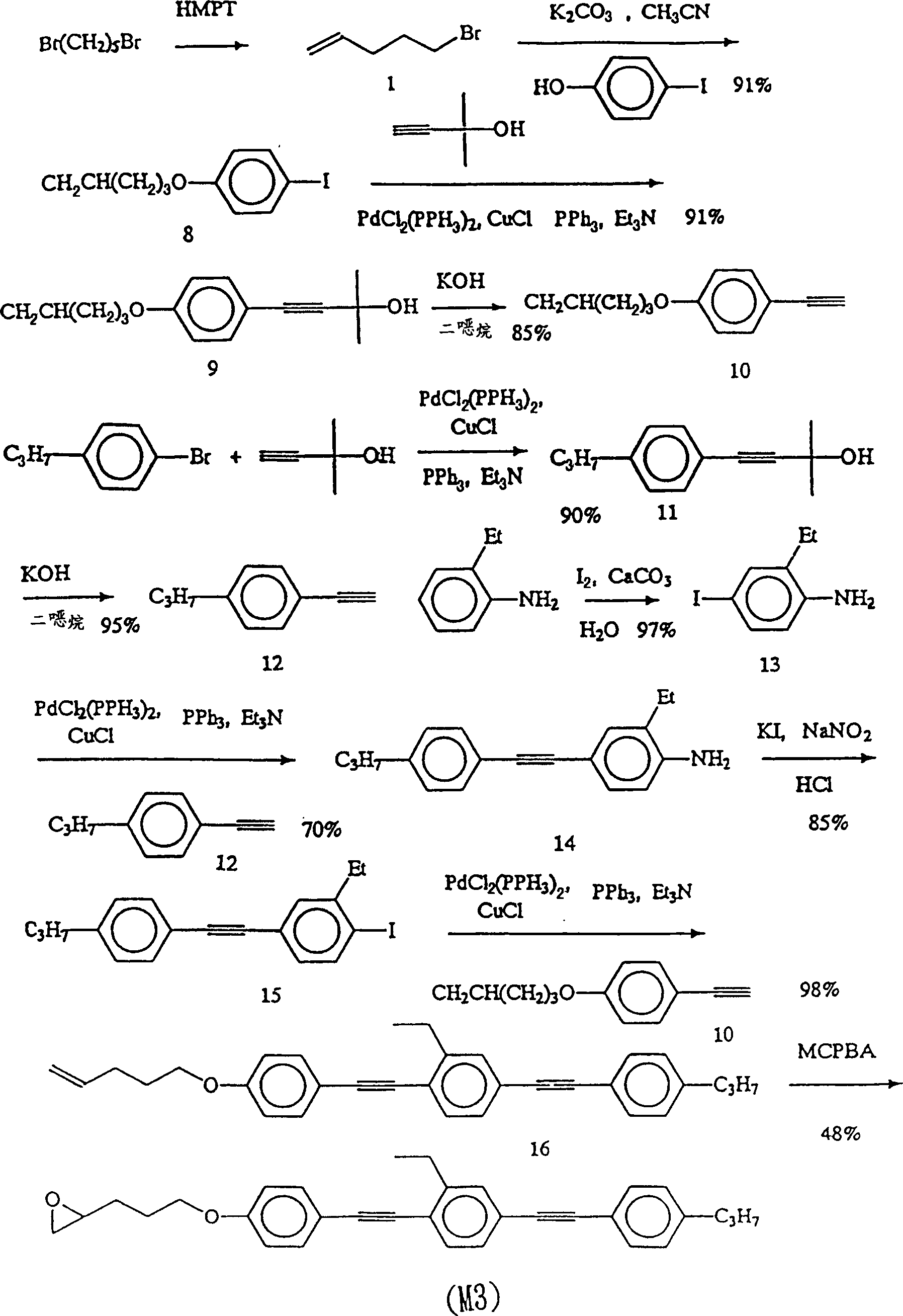
Acetylene compound
Disclosed is an acetylene compound represented by the general formula (1) below, which is useful as a raw material for fused polymers having high heat resistance. (In the formula (1), the encircled Ar represents an aryl group or a heteroaryl group; X represents a divalent linking group; R, R' and R1 each represents a hydrogen atom, a hydrocarbon group or a heterocyclic group; R2 represents a hydrogen atom or a substituent which may be substituted by a benzene ring; A represents a hydrocarbon group or a heterocyclic group; Q represents a hydrogen atom, a hydrocarbon group or a metal element capable of forming a monovalent metal salt; a represents an integer of not less than 0; and b, m and n each represents an integer of not less than 1. When all of n, m and b are 1 at the same time, X is not -(C=O)O-; and when n is 2 and both m and b are 1, X is not -O-).
Owner:FUJIFILM CORP
Crystallized diacetylenic indicator compounds and methods of preparing the compounds
ActiveUS8269042B2Improve solubilityImprove abilitiesUrea derivatives preparationThermometers using mean/integrated valuesAcetylene compoundsCrystallization
Crystallized diacetylenic compounds having certain crystallographic and other characteristics; diacetylenic compounds and mixtures crystallized from diacetylenic solutions; methods of preparing and identifying solvent systems for dissolving diacetylenic compounds; diacetylenic solutions; methods of recrystallizing diacetylenic compounds; crystals of 2,4-hexadiyn-1,6-bis(alkylurea) compounds; and ambient condition indicators and time-temperature condition indicators comprising crystallized diacetylenic compounds.
Owner:TEMPTIME CORP
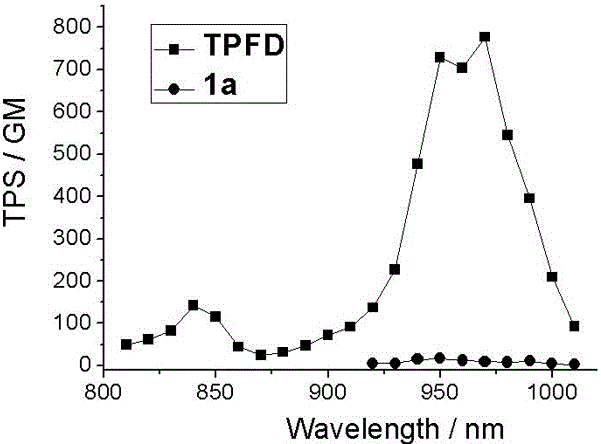
Phenyl-substituted bodipy and diphenylamine fluorene based two-photon fluorescent dye and synthesis method therefor
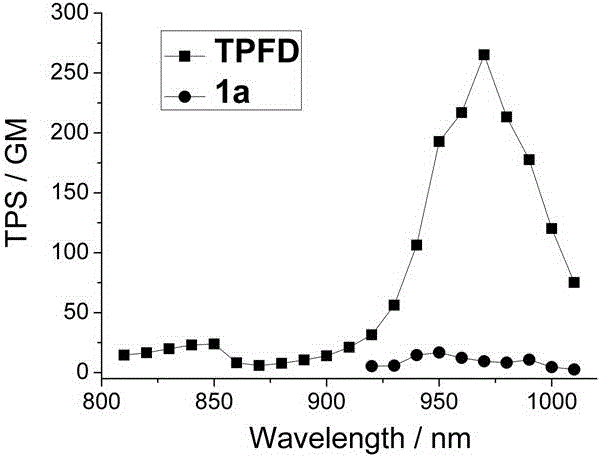
ActiveCN106478706AIncreased absorption cross sectionStrong two-photon fluorescence performanceAzo dyesGroup 3/13 element organic compoundsQuantum yieldBenzaldehyde
The invention discloses a phenyl-substituted bodipy and diphenylamine fluorene based two-photon fluorescent dye. A structural general formula of the fluorescent dye is represented by a formula shown in the description, wherein R is C1-C18 alkyl. Steps for synthesizing the fluorescent dye are as follows: producing 5-phenyl-substituted and 2,6-iodinated bodipy from 2,4-dimethyl pyrrole and benzaldehyde, which serve as raw materials; subjecting fluorene, which serves as a raw material, to bromination, alkylation, Pd(0) catalyzed amination, a Pd(0) and CuI catalyzed Sonogashira cross-coupling reaction and trimethylsilyl removal, so as to produce a diphenylamine-fluorene-acetylene compound; and subjecting the 2,6-iodinated bodipy and the diphenylamine-fluorene-acetylene compound to a reaction, thereby obtaining the target product. The target compound has relatively high two-photon fluorescence performance, the maximum two-photon absorption cross section in toluene reaches 265GM, and the fluorescent quantum yield is 0.35, so that a new design way of think is provided for the synthesis and application of bodipy-based two-photon fluorescent dyes.
Owner:ZHENGZHOU UNIV
Method for preparation of an intermediate dye product
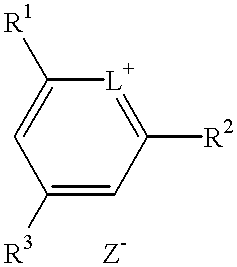
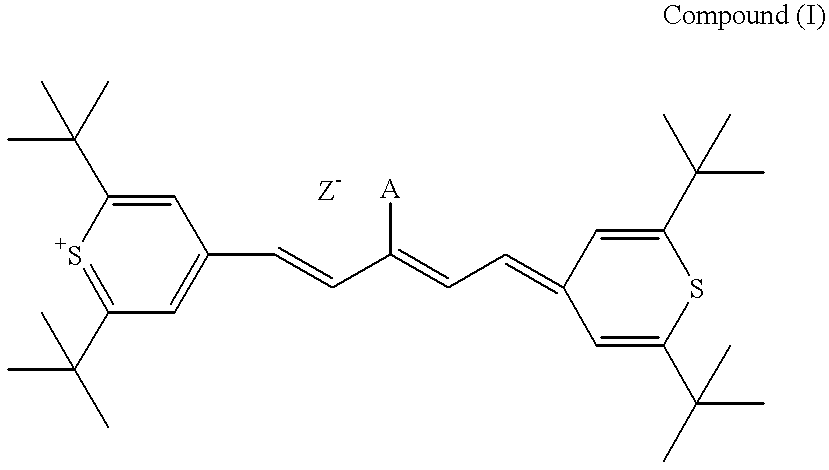
The present invention provides a novel method for the synthesis of an intermediate dye product having the following formula:whereinL is S, Te, or Se;R1 and R2 are either the same or different aryl or alkyl compounds;R3 is hydrogen or a short chain alkyl group; andZ is an anion.The process to formulate this intermediate compound entails reacting an R1-acetylene compound with an R2-acetylene compound (compounds A) into an enol ether compound with the R1 and / or R2 constituents (compound D). And from compound D, it forms into an intermediate dye compound having an L-based cyclic ring with the R1 and / or R2 constituents (compound F). With compound F the desired dye can be made with a greater overall yield for mass production.
Owner:RES FOUND OF STATE UNIV OF NEW YORK THE STATE UNIV OF NEW YORK AT BUFFALO
Process for the selective hydrogenation of alkynes
InactiveUS20060155154A1Good choiceHigh yieldHydrocarbon by hydrogenationHydrocarbon purification/separationHydrogen concentrationDistillation
A process for removal of acetylenic compounds from hydrocarbon streams in which a hydrocarbon feed having a target fraction which contains a first concentration of acetylenic compounds and olefins is contacted with a catalyst selective for the hydrogenation of acetylenic compounds in the presence of hydrogen and a solvent having a boiling point higher than the boiling point of the target fraction in a distillation reaction zone under conditions of temperature, pressure and hydrogen concentration favoring the hydrogenation of acetylenic compounds in which the target fraction is recovered as overheads having a second concentration of acetylenic compounds lower than said first concentration and the solvent is recovered as bottoms.
Owner:CHEM RES & LICENSING CO
Ethynyl compounds
Owner:F HOFFMANN LA ROCHE & CO AG
Preparation method of 1,4-disubstituted-1,3-butadiyne
InactiveCN107513003AConvenient sourceReduce manufacturing costOrganic compound preparationHydrocarbon by hydrocarbon condensationAcetic acidOrganic synthesis
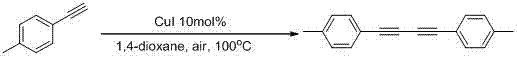
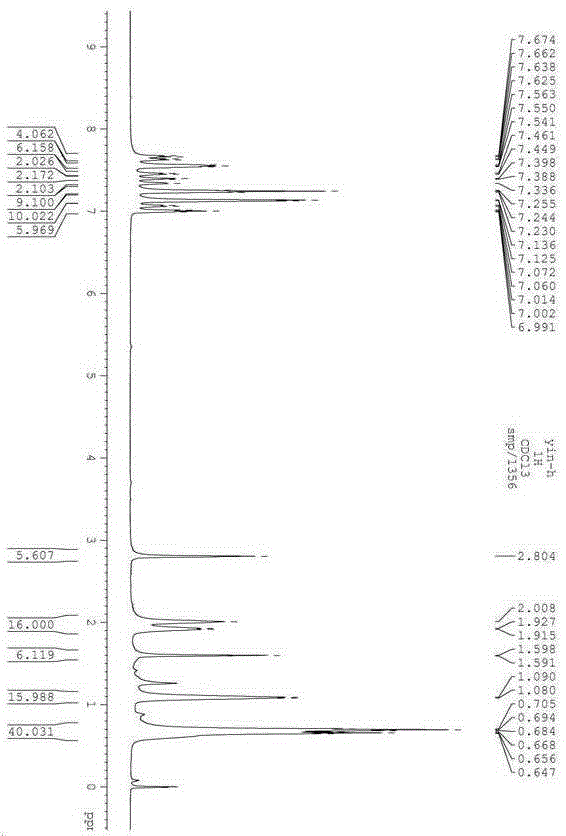
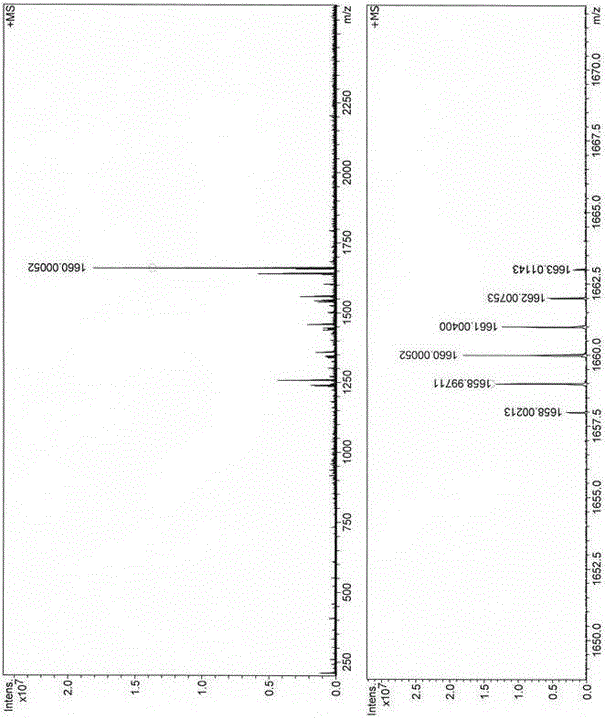
The invention belongs to the field of organic synthesis, and particularly relates to a preparation method of 1,4-disubstituted-1,3-butadiyne. The preparation method comprises the following steps: adding a substituted acetylene compound as shown in Formula I, cuprous iodide and a solvent 1,4-dioxane into a dried reactor, and performing a reaction with stirring in air at a certain temperature; and after the reaction is completed, filtering the reaction solution through a silica gel-containing glass dropper, performing rinsing with ethyl acetate, performing centrifugal drying on the filtrate, and performing separation through column chromatography to obtain the 1,4-disubstituted-1,3-butadiyne compound as shown in Formula II, wherein the reaction formula is shown in the specification.
Owner:佛山煜新科技有限公司
Two-photon fluorescent dye based on phenyl-substituted boron-dipyrromethene and diphenyl indenofluorene and synthetic method thereof
ActiveCN106632435AIncreased absorption cross sectionHigh fluorescence quantum yieldAzo dyesGroup 3/13 element organic compoundsQuantum yieldBenzaldehyde
The invention discloses two-photon fluorescent dye based on phenyl-substituted boron-dipyrromethene and diphenyl indenofluorene; the two-photon fluorescent dye has a structural general formula shown in the description, wherein R is C1-C18 alkyl; the synthetic steps include: using 2,4-dimethylpyrrole and benzaldehyde as raw materials to generate 5-phenyl-substituted and 2,6-iodide boron-dipyrromethene; reducing indenofluorene dione, alkylating, brominating, carrying out Pd(0) catalytic amination, carrying out Pd(0) and CuI catalyzed Sonogashira cross-coupling reaction, and removing trimethylsilyl to generate diphenylamine-indenofluorene-ethynyl compound; enabling the 2,6-iodide boron-dipyrromethene to react with the diphenylamine-indenofluorene-ethynyl compound to obtain the two-photon fluorescent dye; the two-photon fluorescent dye has high two-photon fluorescence, maximum two-photon absorption section in toluene reaches 776 GM, fluorescent quantum yield reaches 0.46, and a new idea to synthesize and apply two-photon fluorescent dyes based on boron-dipyrromethene is provided.
Owner:ZHENGZHOU UNIV
Low-workfunction photocathodes based on acetylide compounds
A low-workfunction photocathode includes a photoemissive material employed as a coating on the photocathode. The photoemissive material includes AnMC2, where A is a first metal element, the first element is an alkali metal, an alkali-earth element or the element Al; n is an integer that is 0, 1, 2, 3 or 4; M is a second metal element, the second metal element is a transition metal or a metal stand-in; and C2 is the acetylide ion C22−. The photoemissive material includes a crystalline structure or non-crystalline structure of rod-like or curvy 1-dimensional polymeric substructures with MC2 repeating units embedded in a matrix of A.
Owner:THE UNITED STATES AS REPRESENTED BY THE DEPARTMENT OF ENERGY
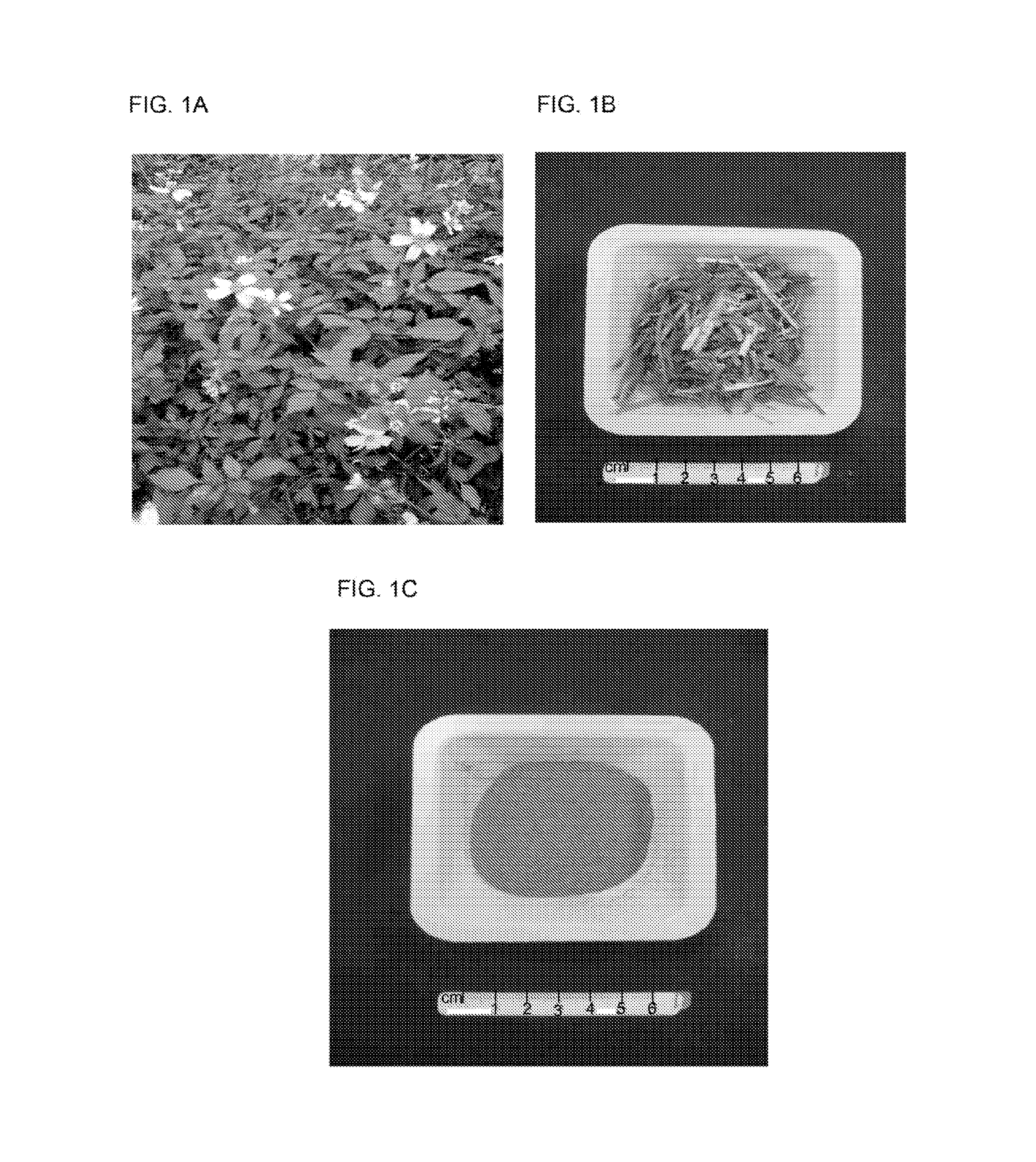
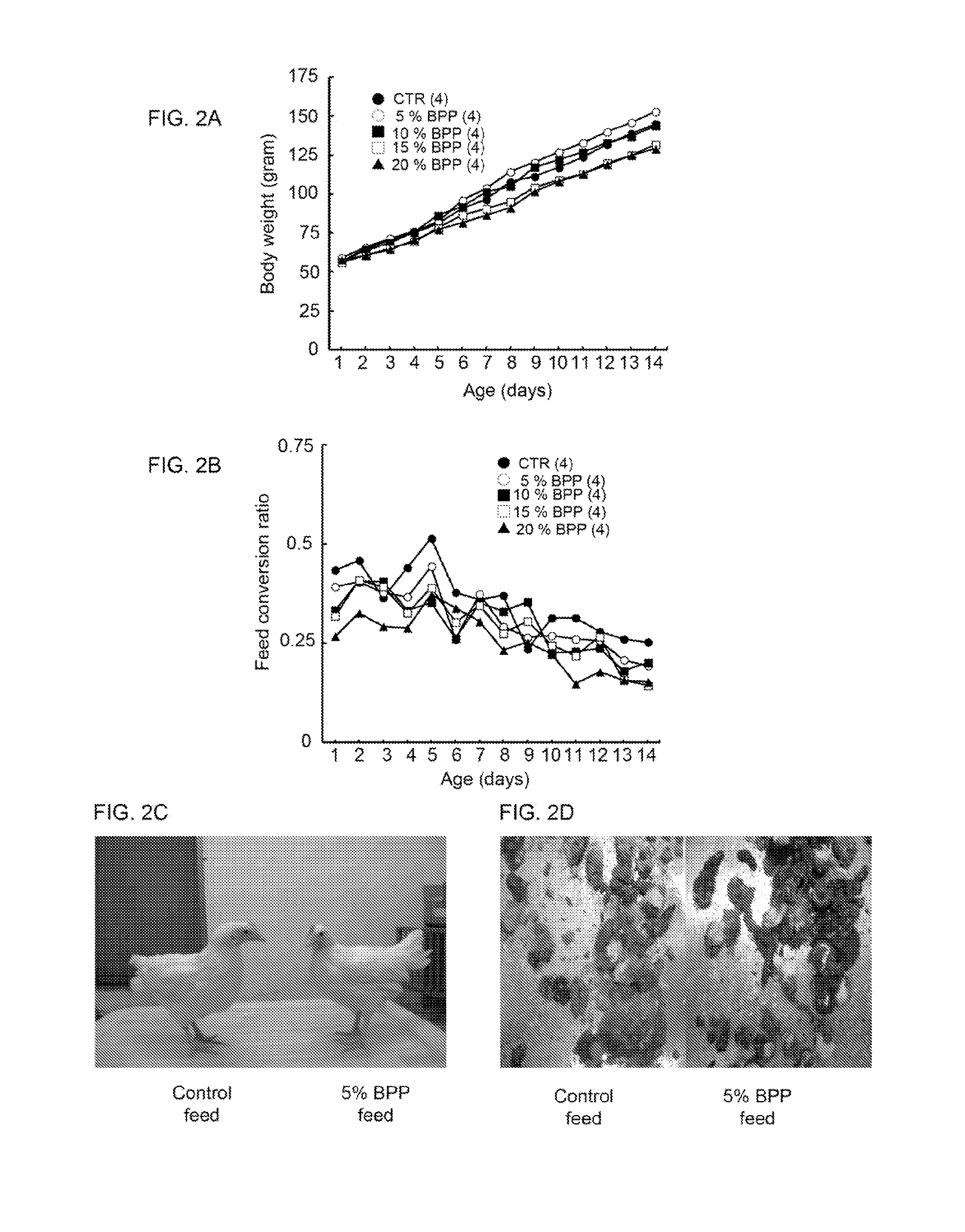
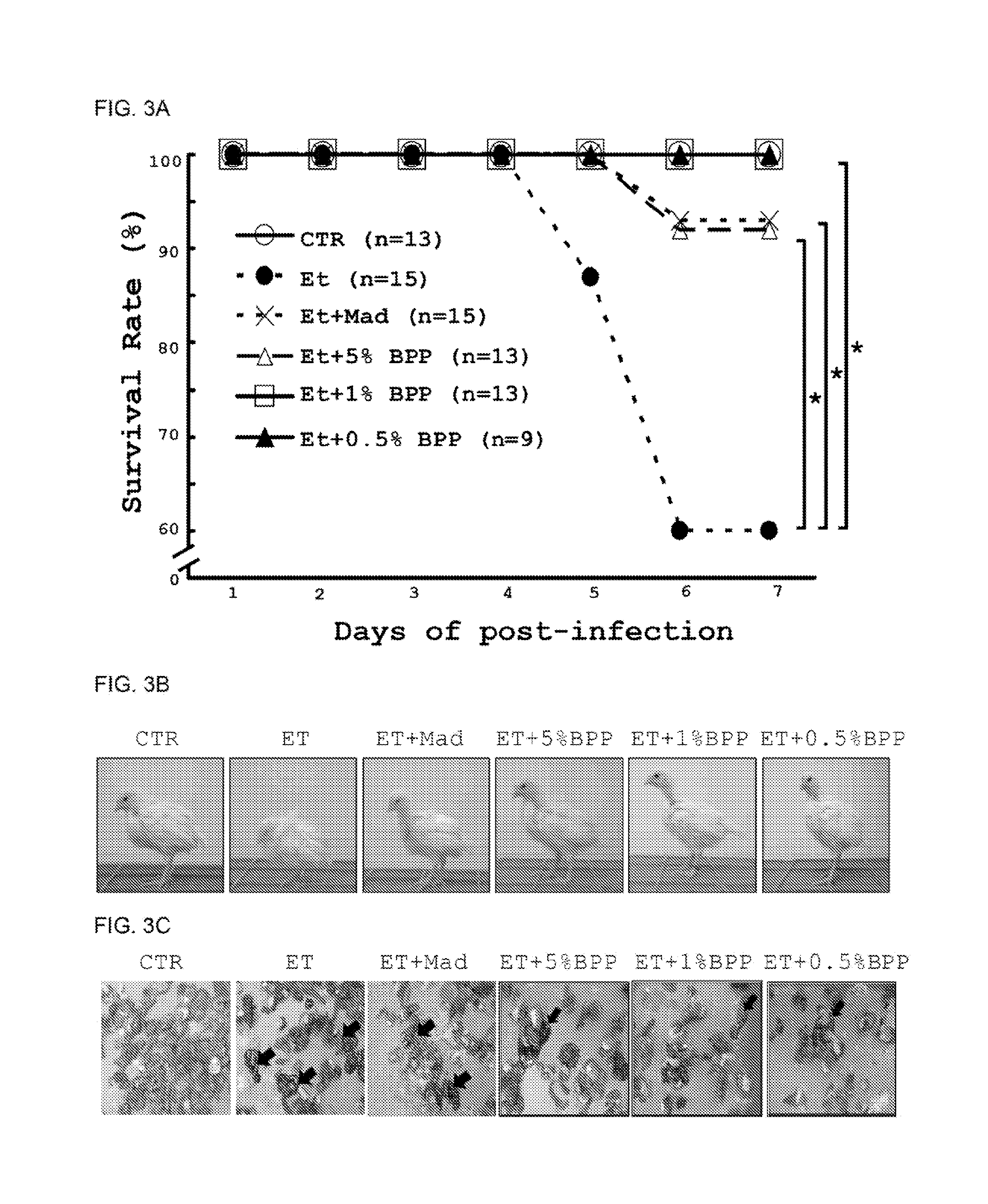
Bidens pilosa and polyacetylenic compounds for prevention and treatment of coccidiosis
A composition for use in prevention, inhibition and / or treatment of coccidiosis in an animal is disclosed. The composition comprises an effective amount of Bidens pilosa, an active constituent thereof, or an active compound isolated therefrom. In another aspect, a composition for use in preventing and / or treating coccidiosis, and / or enhancing growth in an animal is disclosed, the composition comprising an animal feed and an effective amount of Bidens pilosa, or an isolated active constituent comprising a polyacetylenic compound.
Owner:ACAD SINIC
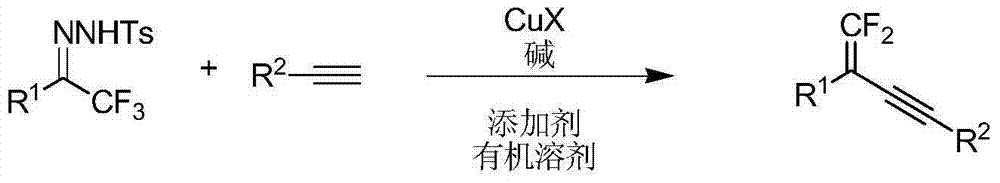
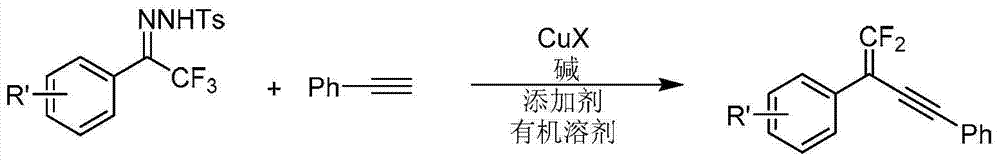
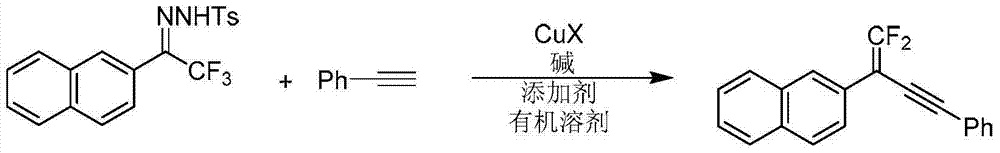
Synthetic method of 1, 1-2 fluorine - 1, 3 - acetylene compounds
ActiveCN104844410AStable and cheap to useImprove universalityGroup 4/14 element organic compoundsAmino preparation from aminesOrganic solventCopper iodide
The invention discloses a synthetic method of 1, 1-2 fluorine - 1, 3 - acetylene compounds. The synthetic method enable tosylhydrazones and acetylene compounds of Alpha, alpha, alpha, 3 methyl ketone to react in organic solvent. 1, 1-2 fluorine - 1, 3 - acetylene compounds is obtained through the catalysis of cuprous CuX and the existing of soda and annexing agent. The synthetic method of 1, 1-2 fluorine - 1, 3 - acetylene compounds preferentially uses copper iodide which is cheap and stable as catalyst, and the operation is relatively convenient and easy. Also the copper iodide has good tolerance and universality to functional groups. The invention has the advantages that the raw materials are easy to obtain, the reaction cost is relative low, and the reaction time is relative short. The invention is suitable for the preparation of 1, 1-2 fluorine - 1, 3 - acetylene compounds.
Owner:PEKING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

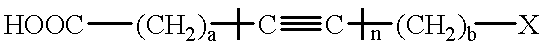

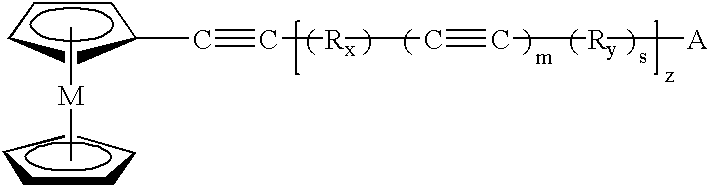

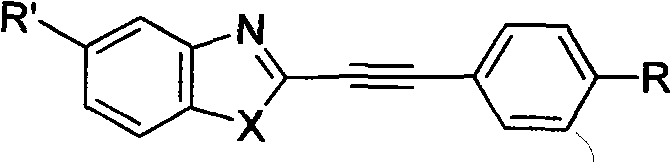



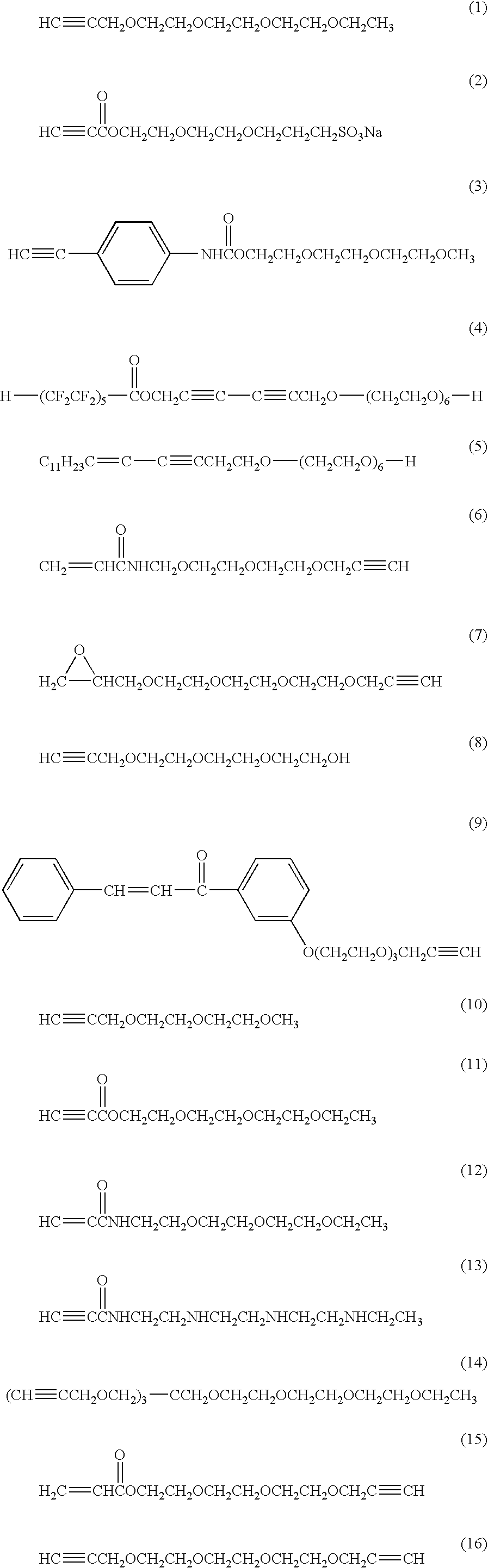
![Preparation method of heteroaromatic iminazole [1,2-Alpha]pyridine compounds Preparation method of heteroaromatic iminazole [1,2-Alpha]pyridine compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/3789bf44-9019-426e-9f6a-46c3f41466a6/BDA00002121136700011.PNG)
![Preparation method of heteroaromatic iminazole [1,2-Alpha]pyridine compounds Preparation method of heteroaromatic iminazole [1,2-Alpha]pyridine compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/3789bf44-9019-426e-9f6a-46c3f41466a6/BDA00002121136700031.PNG)
![Preparation method of heteroaromatic iminazole [1,2-Alpha]pyridine compounds Preparation method of heteroaromatic iminazole [1,2-Alpha]pyridine compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/3789bf44-9019-426e-9f6a-46c3f41466a6/FDA00002121136600011.PNG)