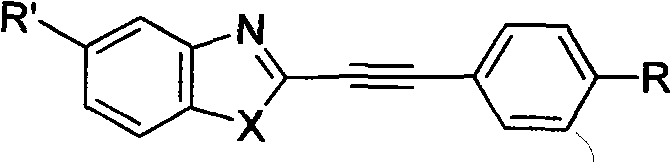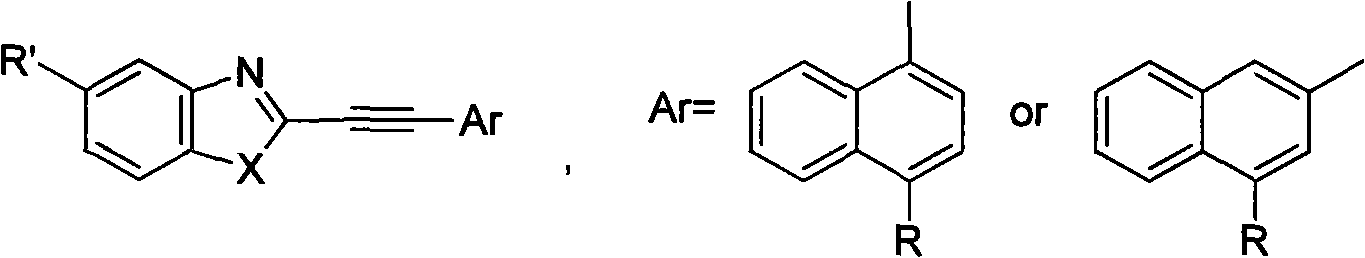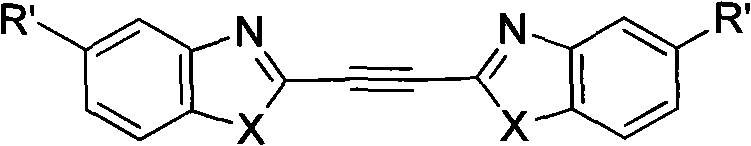Structure and preparation method for heterocyclic aryl acetylene compounds containing benzoxazole and benzothiazole groups
A technology of benzothiazolyl and benzoxazole, applied in the field of heteroaryl acetylene compounds, can solve problems such as difficulty in obtaining raw materials, high price and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1, the synthesis of 1-phenyl-2-(2-benzoxazolyl)acetylene and its derivatives
[0047] A. Synthesis of Heteroaryl Vinyl Compounds
[0048] The raw material heteroaryl vinyl compound of the heteroaryl acetylene compound containing benzoxazole group is synthesized according to the method in the literature. [W. Q. Zhang, J. P. Zhuang, et al., Chinese J. Chem., 2001, 19 (7): 695-701] using substituted aromatic aldehydes as raw materials and 2-methylbenzoxazole as raw materials, with N, N-dimethylformamide is used as a solvent and potassium hydroxide is used as a base catalyst to obtain the target compound.
[0049]
[0050] Weigh 10.61g (0.10mol) of benzaldehyde, 13.32g (0.10mol) of 2-methylbenzoxazole, 150mL of DMF, and 15g of KOH, and add them to a 500mL round-bottomed flask, and stir at room temperature for 2 hours before TLC detection , the reaction is complete. The solution was added to 800mL of water, and a large amount of yellow precipitate was precip...
Embodiment 2
[0069] Embodiment two, the synthesis of 1-phenyl-2-(2-benzothiazolyl) acetylene and its derivatives
[0070] A. Synthesis of benzothiazole-containing heteroaryl vinyl compounds
[0071]
[0072] The synthesis of heteroaryl vinyl compounds containing benzothiazole groups is similar to the synthesis method of heteroaryl vinyl compounds containing benzoxazole groups in Example 1, and the yields are as follows.
[0073] 1-Phenyl-2-(2-benzothiazolyl)ethene, yield: 87.2%
[0074] 1-(4-chlorophenyl)-2-(2-benzothiazolyl)ethylene, yield: 95.4%
[0075] 1-(4-bromophenyl)-2-(2-benzothiazolyl)ethylene, yield: 90.6%
[0076] 1-(4-methylphenyl)-2-(2-benzothiazolyl)ethylene, yield: 95.4%
[0077] B, synthesis of benzothiazole-containing heteroarylethene compound brominated product
[0078]
[0079] Weigh 12.37g (0.052mol) of 1-phenyl-2-(2-benzothiazolyl)ethylene into a 250mL round bottom flask, add 100mL of dichloromethane and stir to dissolve, add dropwise 8.90g of liquid bromine ...
Embodiment 3 1
[0095] Embodiment three, the synthesis of 1,2-two (2-benzoxazolyl) acetylene
[0096] The synthesis of 1,2-bis(2-benzoxazolyl)ethylene refers to the literature, and it is prepared by reacting o-aminophenol and butenedioic acid under the condition of polyphosphoric acid as a solvent. [E.V. Paul, H. Julia, et al. WO 02 / 32886A1]
[0097]
[0098] A, 1, the synthesis of 2-bis(2-benzoxazolyl)-1-bromoethylene
[0099] Take the above 5.00g (0.019mol) 1,2-bis(2-benzoxazolyl)ethylene in a 500°C round bottom flask, add 250ml of dichloromethane, heat to reflux, cool to room temperature, take 3.07g (0.019mol ) liquid bromine into the constant pressure dropping funnel, the dropwise addition was completed in about one hour, stirred at room temperature for 5 hours, and then refluxed overnight, a large amount of precipitate was formed, the heating device was removed, and the crude product was obtained by spinning. Due to the poor solubility of the crude product, only a small amount was t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com