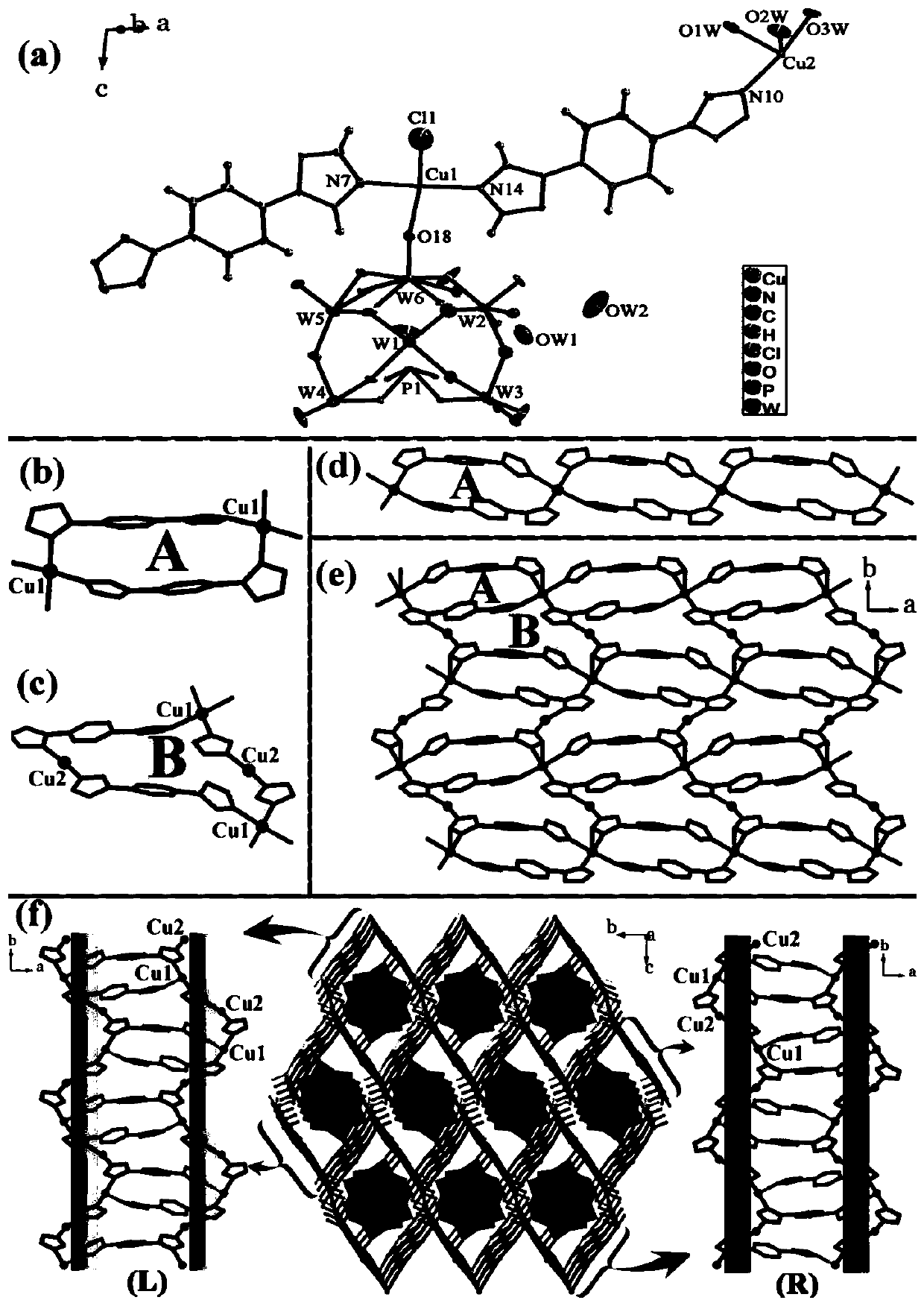
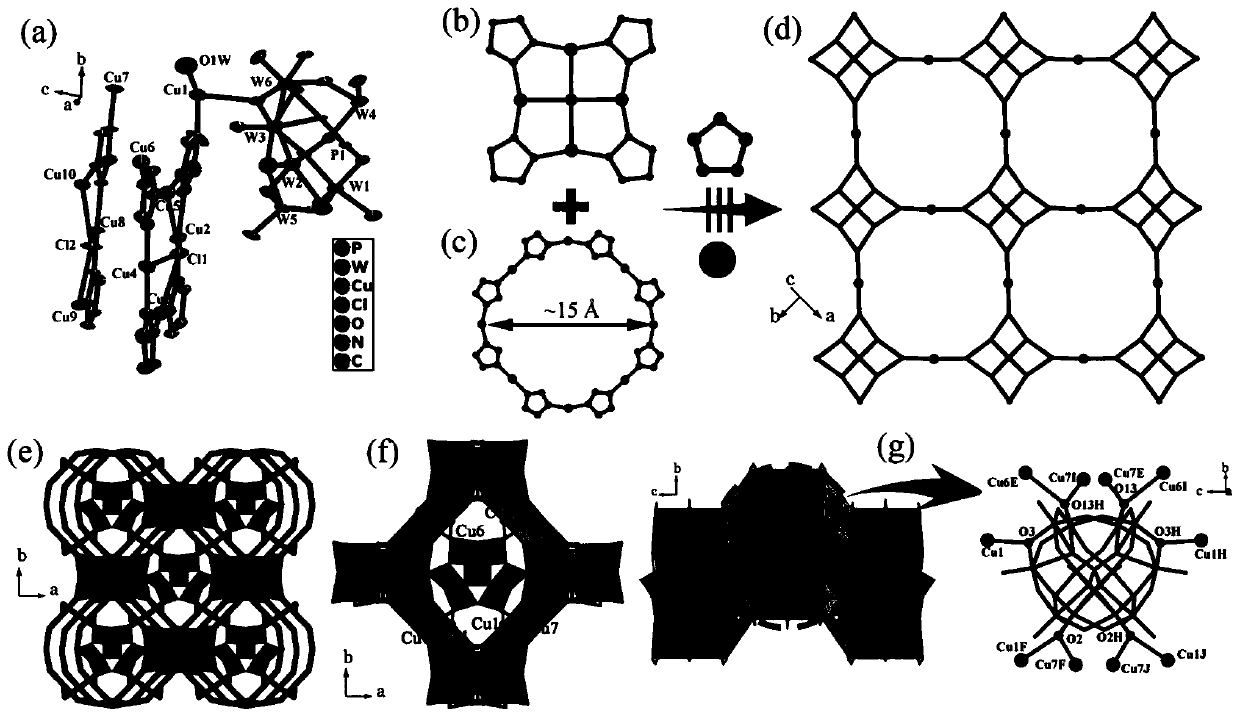
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
41 results about "2-methylnaphthalene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
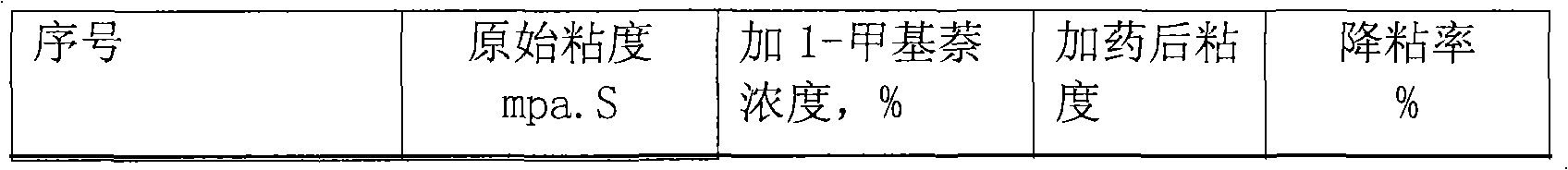
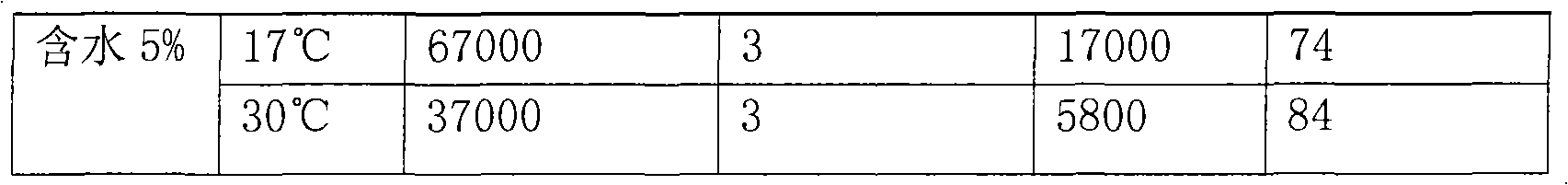
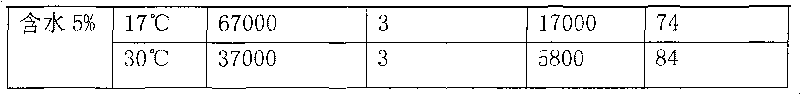
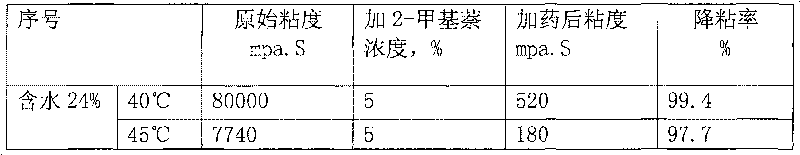
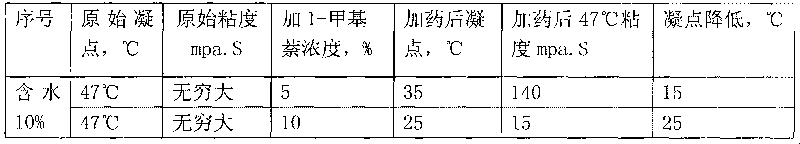
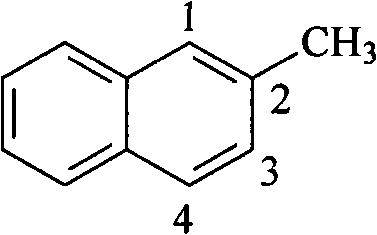
Application of methylnaphthalene in lowering viscosity of thickened oil
ActiveCN101717626ALow viscositySpeed up the flowFluid dynamicsFluid removalOil viscosityHigh surface
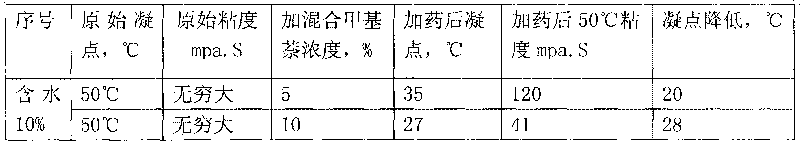
The invention discloses application of methylnaphthalene in lowering viscosity of thickened oil, which comprises application of 1-methylnaphthalene in lowering viscosity of thickened oil and the specific implementation steps, 2-methylnaphthalene in lowering viscosity of thickened oil and the specific implementation steps and mixed methylnaphthalene in lowering viscosity of thickened oil and the specific implementation steps. The invention enlarges the application method of methylnaphthalene, clearly demonstrates the specific application method of using methylnaphthalene to lower viscosity of thickened oil, solves the problems of great load and power consumption, frequent machinery accidents, high surface line return pressure, and the like of an oil extractor during extracting and outwards transporting thickened oil in the background art, and can effectively lower viscosity of thickened oil and reduce extraction and outward transportation cost of thickened oil.
Owner:盘锦河升大地石油科技有限公司
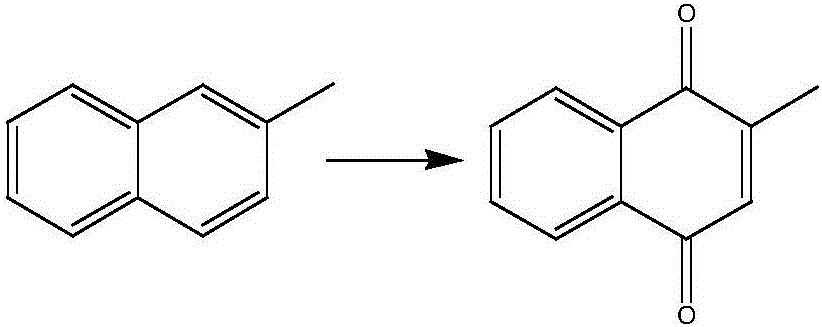
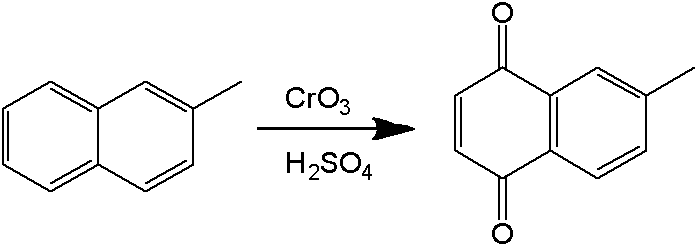
Process for preparation of 2-Methyl-1,4-naphthoquinone
InactiveUS6579994B2Low costReduce usageOrganic compound preparationQuinone preparation by oxidationAcetic acidNaphthoquinone
The present invention describes a process for the preparation of 2-Methyl-1,4-naphthoquinone by oxidizing 2-methylnaphthalene with hydrogen peroxide in the presence of acetic acid.
Owner:COUNCIL OF SCI & IND RES
Novel synthesis method of 2-methyl-1,4-naphthoquinone
InactiveCN103833541AReduce consumptionEasy to separateQuinone preparation by oxidationSynthesis methodsDistillation
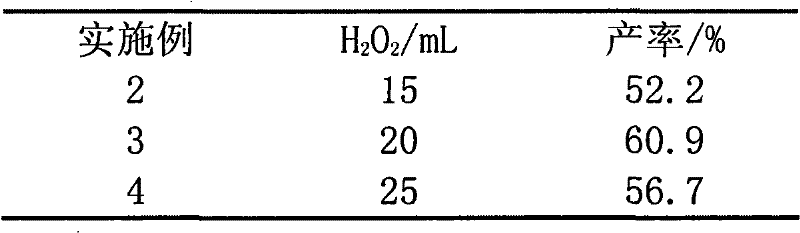
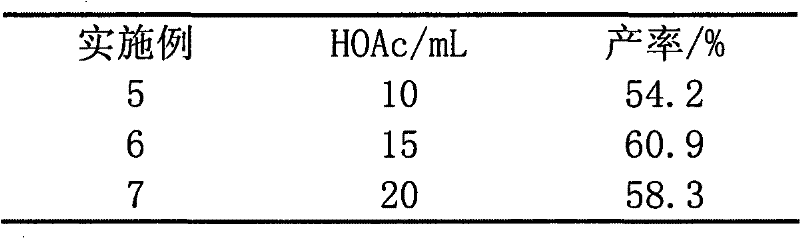
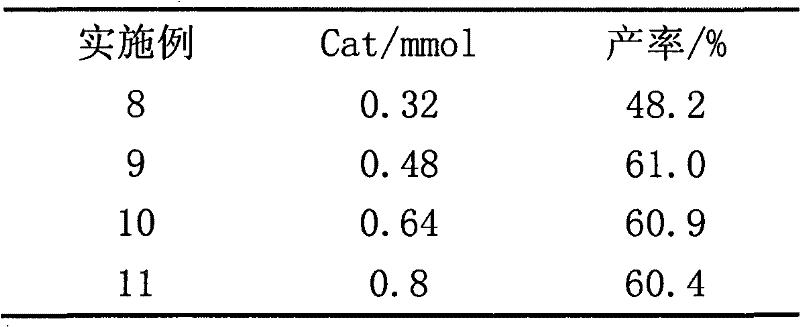
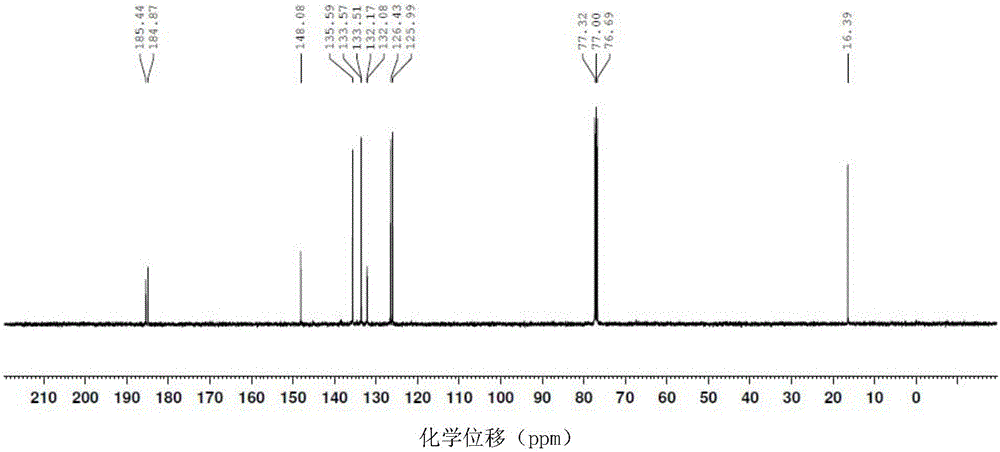
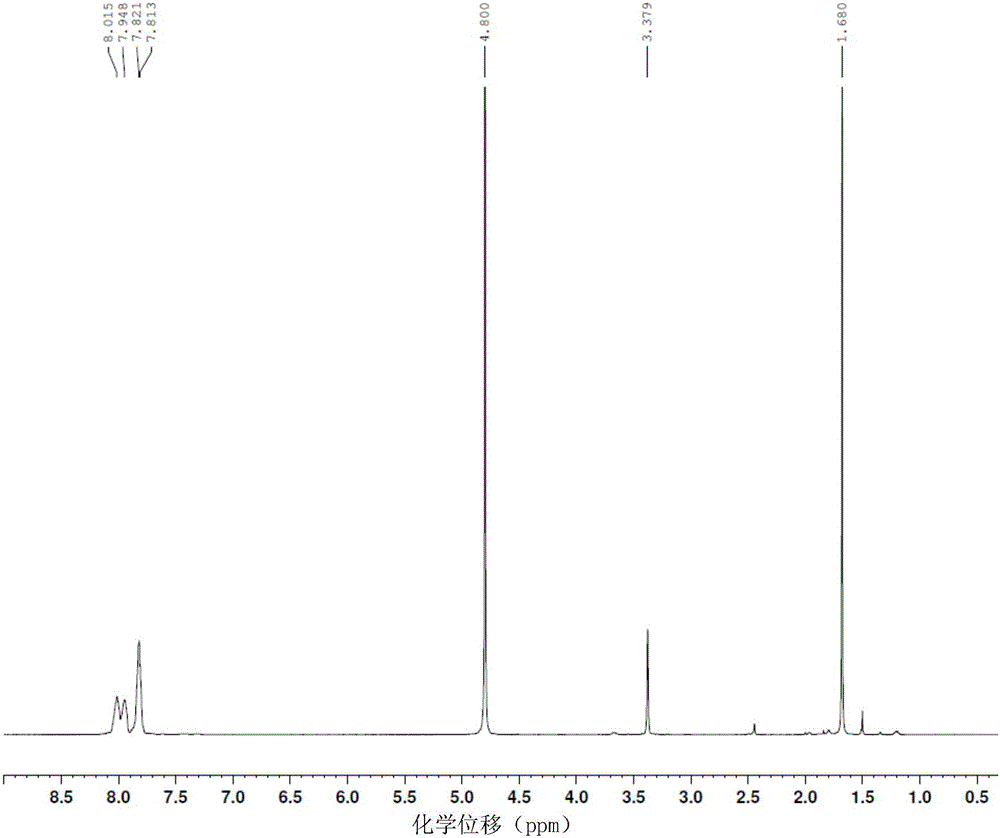
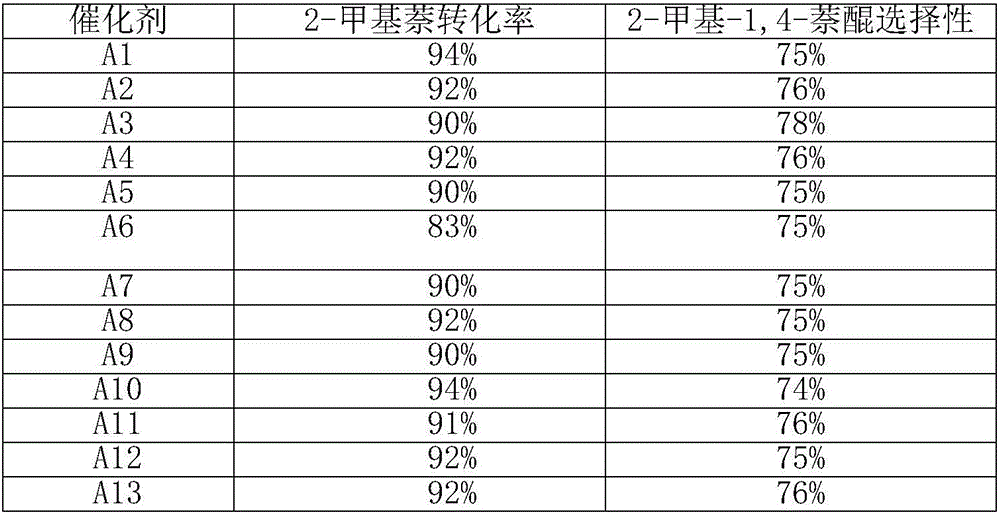
The invention relates to a novel synthesis method of 2-methyl-1,4-naphthoquinone. The novel synthesis method comprises the steps: respectively dissolving a defined amount of 2-methylnaphthalene and metachloroperbenzoic acid in moderate glacial acetic acid to prepare solutions (1) and (2), dropwise adding the solution (2) in the solution (1) under the stirring, after reacting for a period of time at a certain temperature, extracting a reaction solution by using chloroform, washing an extraction solution with a saturated NaHCO3 solution and water, after anhydrous Na2SO4 is dried, performing reduced pressure distillation to prepare a crude product of the 2-methyl-1,4-naphthoquinone, and recrystalizing by using alcohol as a solvent to obtain a pure product of the 2-methyl-1,4-naphthoquinone. According to the novel synthesis method, the conversion rate of the 2-methylnaphthalene can reach 80 percent, and the yield of the 2-methyl-1,4-naphthoquinone can reach 31 percent. The method disclosed by the invention has the characteristics of short reaction time, mild conditions, and the like, and products are easily separated; and no any catalyst is used in the reaction, thus the energy consumption and the environment pollution can be reduced.
Owner:QILU UNIV OF TECH
Pour point depressant for crude oil
ActiveCN101735788ALower the freezing point of crude oilSatisfy depressing needsDrilling compositionDepressantMethyl group
The invention discloses a pour point depressant for crude oil. The depressant is prepared from methylnaphthalene, wherein the methylnaphthalene is 1-methylnaphthalene, any one in mixed methylnaphthalene or a mixture of 1-methylnaphthalene and mixed methylnaphthalene in any ratio; or the methylnaphthalene is a mixture prepared from 2-methylnaphthalene dissolved in 1-methylnaphthalene or mixed methylnaphthalene at normal temperature and pressure or a mixture prepared from 2-methylnaphthalene dissolved in a mixed solution of 1-methylnaphthalene and mixed methylnaphthalene in any ratio under normal temperature and pressure, and the maximum content of the 2-methylnaphthalene in the mixture is not more than the saturation concentration of the 2-methylnaphthalene in the solution at normal temperature and pressure. The invention can greatly decrease the pour point of crude oil in different oil fields, can be widely used for solving the problem of depressing the pour point of crude oil in various phases of oil recovery, gathering and storage and transportation in different oil fields, can lower the cost for depressing the pour point of crude oil, and has the advantages of less toxic and side effects, high safety, simple production, low cost, strong applicability, easy popularization and application and the like.
Owner:盘锦河升大地石油科技有限公司
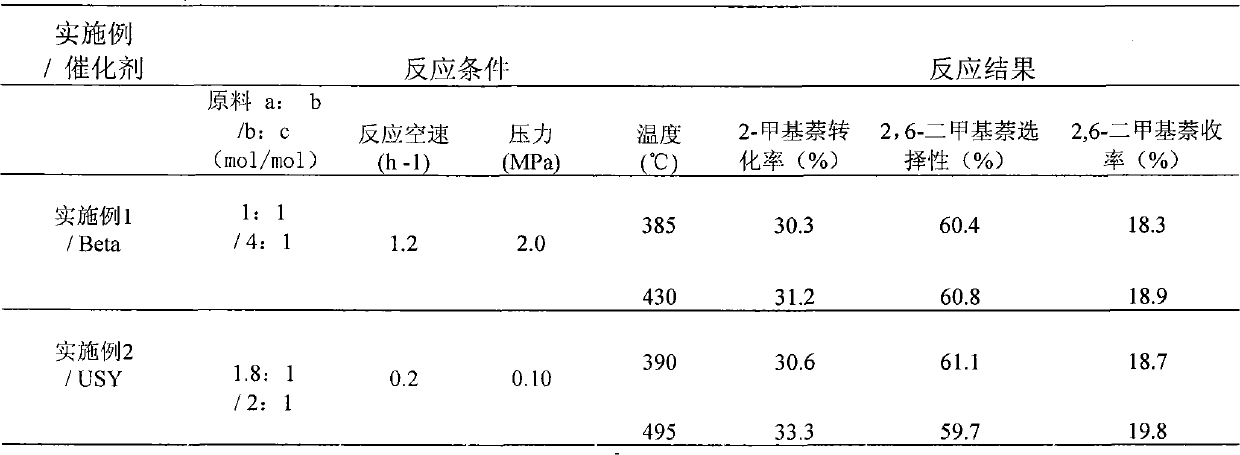
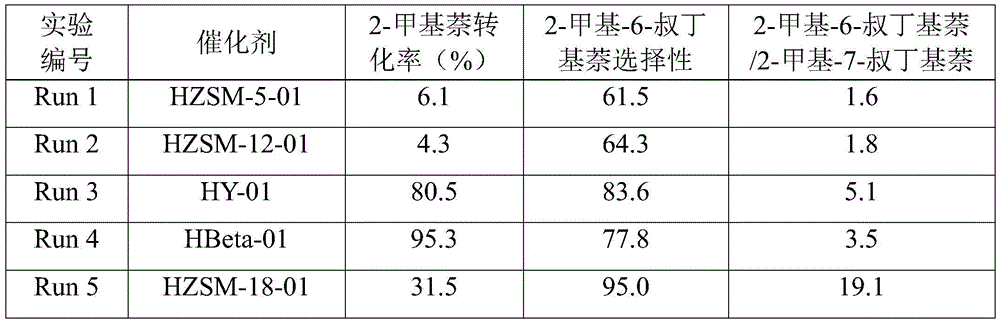
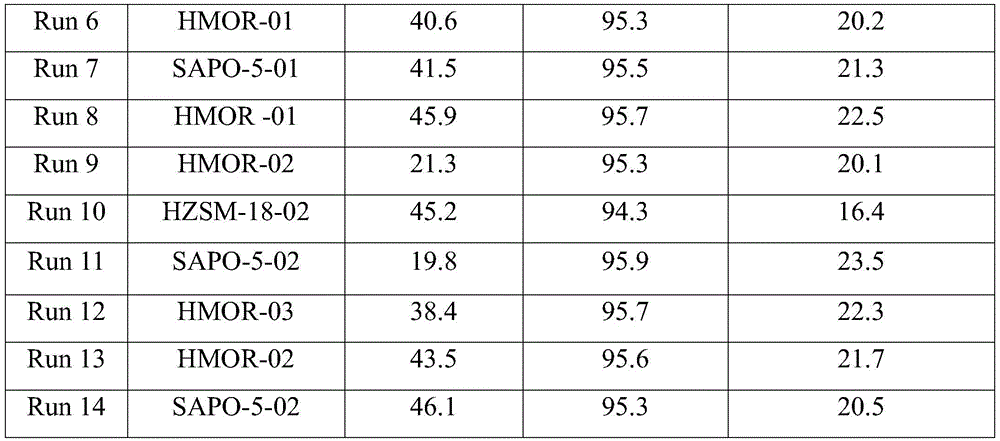
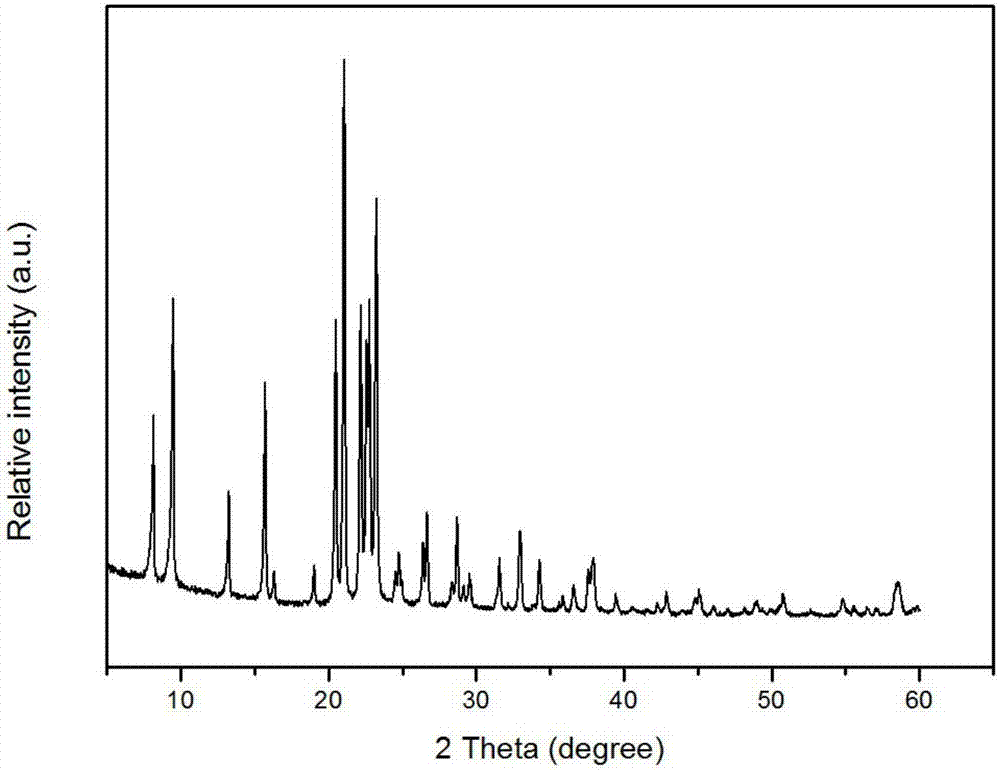
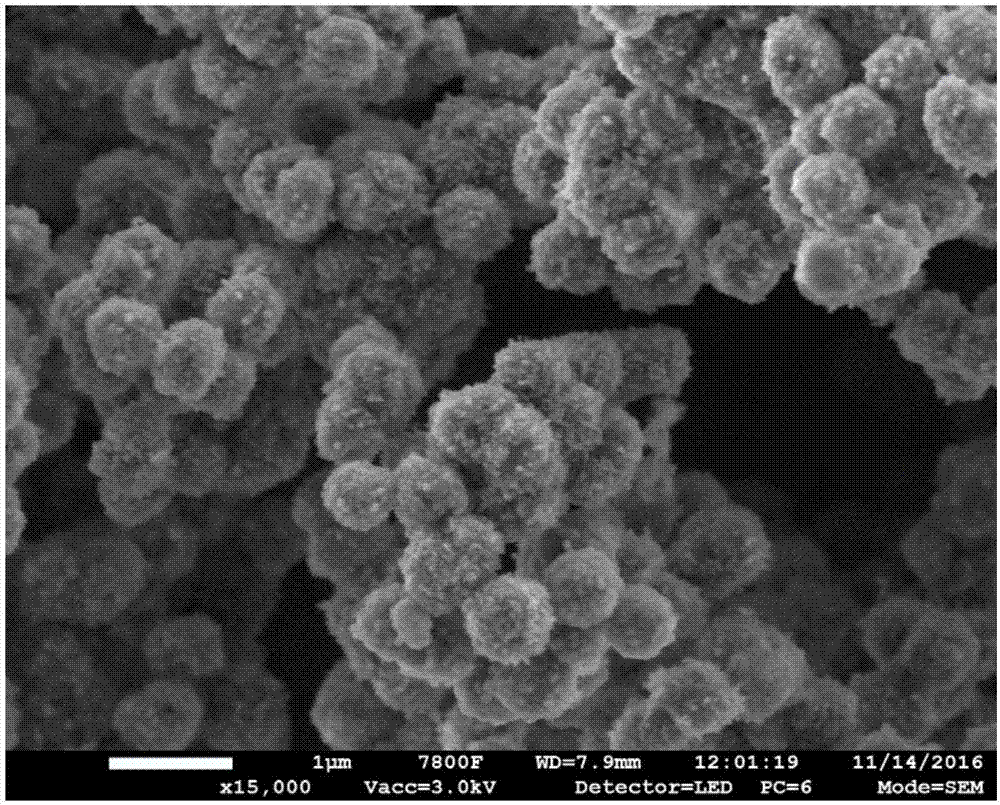
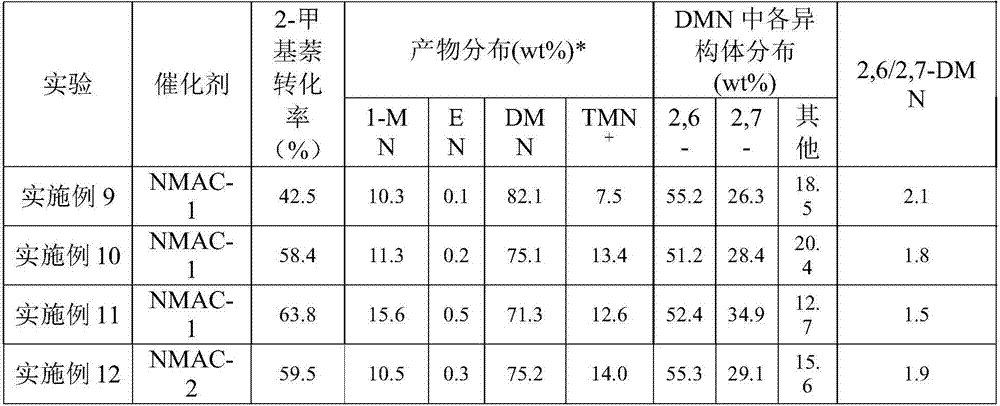
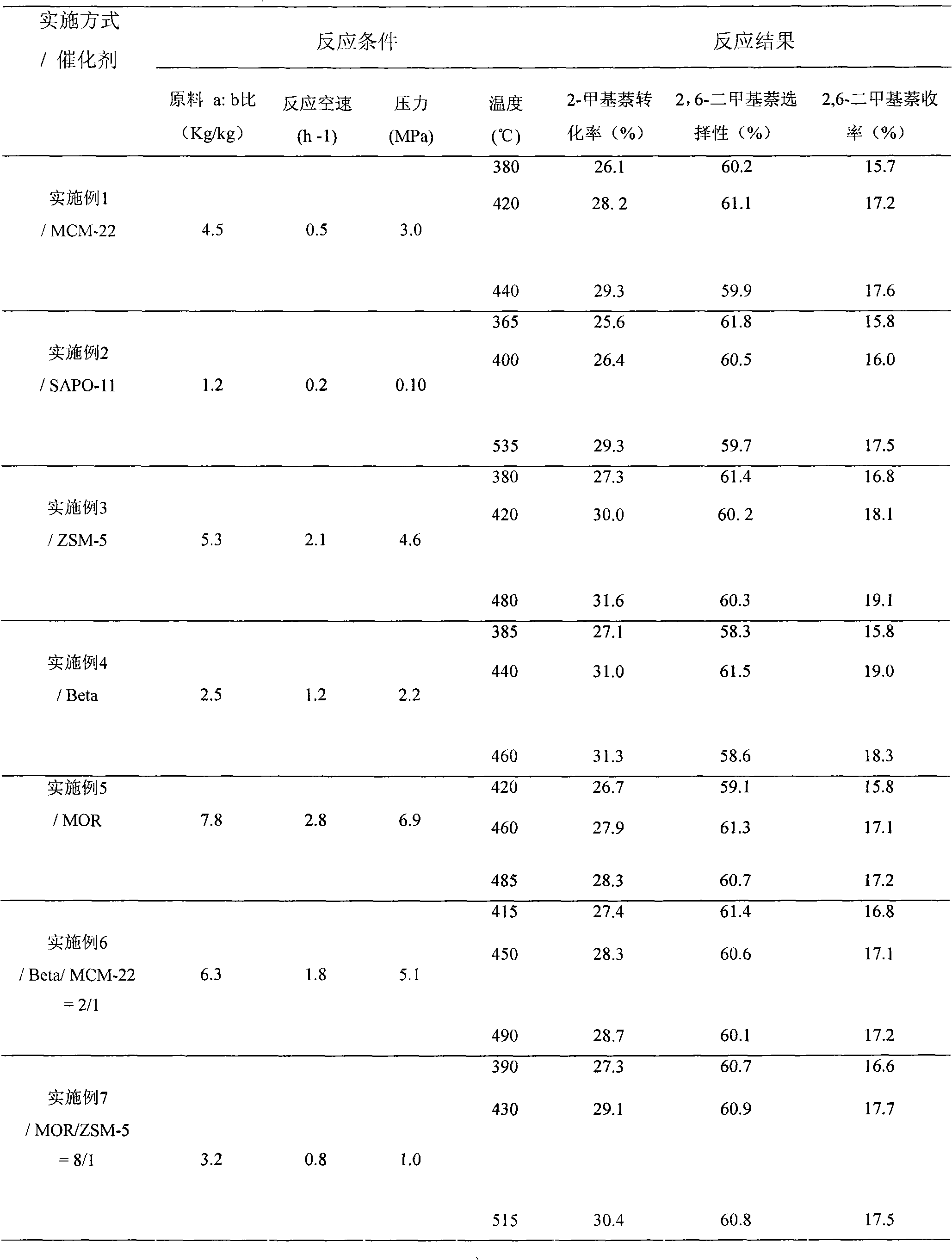
Catalyst used for alkylation of methanol, C10 aromatic hydrocarbons and 2-methylnaphthalene for synthesizing 2,6-dimethylnaphthalene
InactiveCN101972667AHigh catalytic activity for alkylationInhibition of isomerizationMolecular sieve catalystsHydrocarbons2-methylnaphthaleneOxide
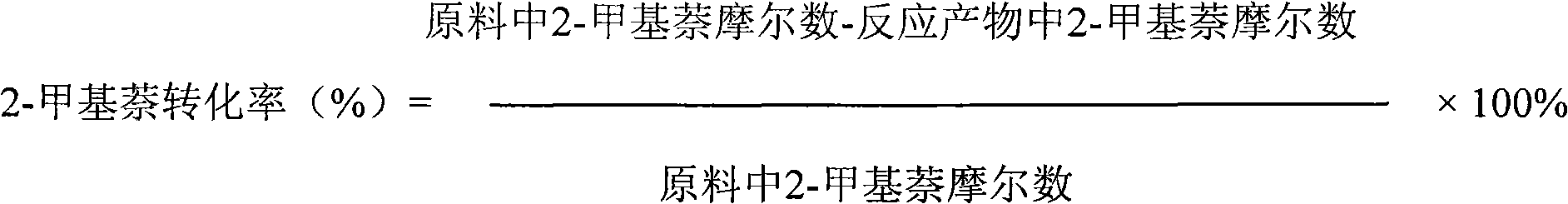
The invention relates to a catalyst used for alkylation of methanol, C10 aromatic hydrocarbons and 2-methylnaphthalene for synthesizing 2,6-dimethylnaphthalene, and mainly solves the problems of low conversion rate of the 2-methylnaphthalene, low selectivity of the 2,6-dimethylnaphthalene and low stability of the catalyst in the conventional synthesis process technology. In the technical scheme of the invention, the catalyst comprises the following components in part by weight: 50 to 95 parts of one or a mixture of any two of hydrogen-type silicon-aluminum zeolites, 5 to 50 parts of binder and 1.0 to 15 parts of metal oxide of at least one of transition metal, rare-earth metal and alkaline-earth metal; and the reaction conditions for synthesizing the 2,6-dimethylnaphthalene are that: temperature is between 360 and 500 DEG C, pressure is between 0 and 7.5MPa, the molar ratio of the methanol to the 2-methylnaphthalene is between 1.0 and 3.0, the weight ratio of the C10 aromatic hydrocarbons to the 2-methylnaphthalene is between 1.0 and 6.0, and air speed is between 0.1 and 3.5 hour<-1>. The catalytic reaction ensures relatively high synthesis yield of the 2,6-dimethylnaphthalene, and the problems in the prior art are well solved.
Owner:TONGJI UNIV
Method for detecting polycyclic aromatic hydrocarbon content in smokeless tobacco products
ActiveCN106645444AGood effectLow requirements for matrix purityComponent separationAcenaphthyleneGas chromatography–mass spectrometry
The invention discloses a method for detecting polycyclic aromatic hydrocarbon content in smokeless tobacco products. The method includes the steps: extracting the smokeless tobacco products by the aid of extraction agents; detecting filtered extraction solution by the aid of a GC-MS / MS (gas chromatography-mass spectrometry). The method can simultaneously measure content of indene, naphthalene, 1-methylnaphthalene, 2-methylnaphthalene, acenaphthylene, acenaphthene, fluorine, phenanthrene, anthracene, fluoranthene, pyrene, benzo [a] anthracene, chrysene, benzo [b] fluoranthene, benzo [k] fluoranthene, benzo [j] fluoranthene, benzo [e] pyrene, benzo [a] pyrene, perylene, indene [1, 2, 3-cd] pyrene, diphenyl [a, h] anthracene, benzo [g, h, i] pyrene and the like in the smokeless tobacco products. The method fills in gaps of the prior art and has the advantages that the method is simple, convenient and rapid in sample pretreatment, goof in detection accuracy and repeatability and high in analytical test flux, and the method is applicable to rapid analysis of large-batch samples.
Owner:YUNNAN TOBACCO QUALITY SUPERVISION MONITORING STATION
Method for simultaneously detecting content of 22 polycyclic aromatic hydrocarbon matters in cigarette smoke
ActiveCN106248835AGood effectLow requirements for matrix purityComponent separationAcenaphthyleneTrapping
The invention discloses a method for simultaneously detecting content of 22 polycyclic aromatic hydrocarbon matters in cigarette smoke. The method comprises the following steps: trapping total particular matters in cigarette main stream smoke by adopting a filter; extracting the trapping filter by utilizing an extraction agent; and detecting the filtered extract liquor by adopting gas chromatography-tandem mass spectrum (GC-MS / MS), and simultaneously detecting the contents of 22 polycyclic aromatic hydrocarbon matters, including indene, naphthalene, 1-methylnaphthalene, 2-methylnaphthalene, acenaphthylene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo [a] anthracene, chrysene, benzo [b] fluoranthene, benzo [k] fluoranthene, benzo [j] fluoranthene, benzo [e] pyrene, benzo [a] pyrene, perylene, indene [1,2,3-cd] perylene, dibenzo [a, h] anthracene, benzo [g, h, i] pyrene, and the like, in cigarette main stream smoke. The method fills the blank of the prior art, has the characteristics of simple, convenient and quick sample pretreatment, high detection accuracy and repeatability and large analysis test flux and is fit for quick analysis of mass samples.
Owner:YUNNAN TOBACCO QUALITY SUPERVISION MONITORING STATION
Method for extracting naphthalene, 1-methylnaphthalene and 2-methylnaphthalene from ethylene tar
ActiveCN102134500AIncrease contentImprove protectionTar working-up by distillationBenzeneBoiling point
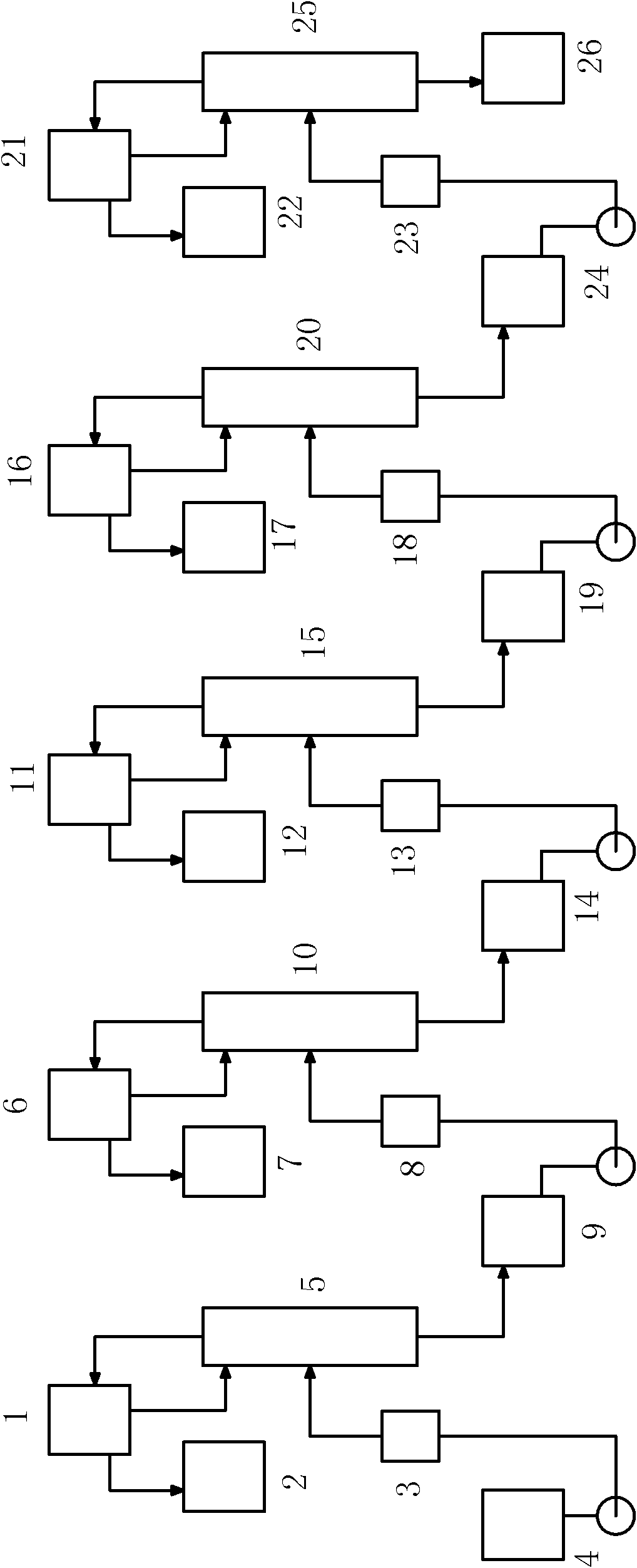
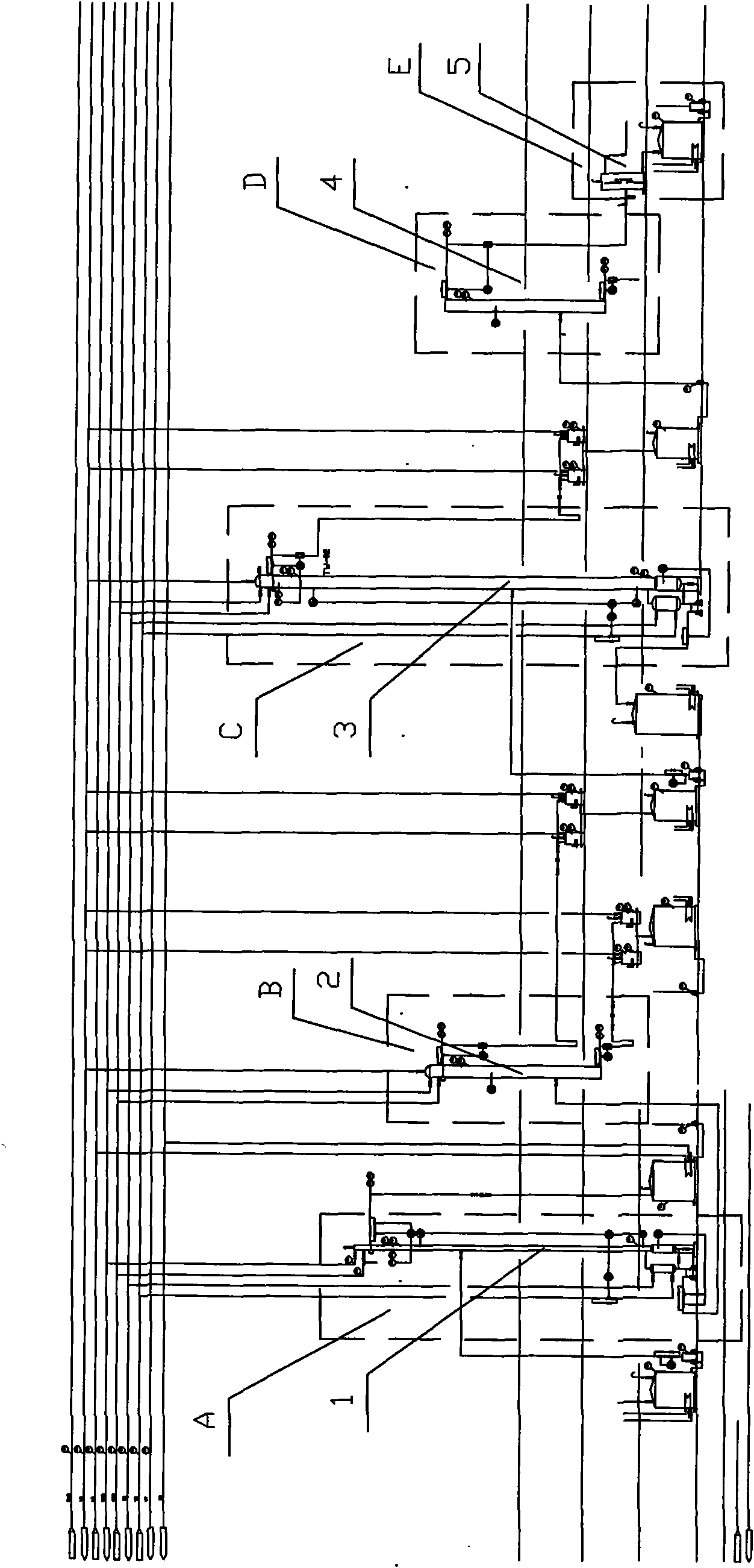
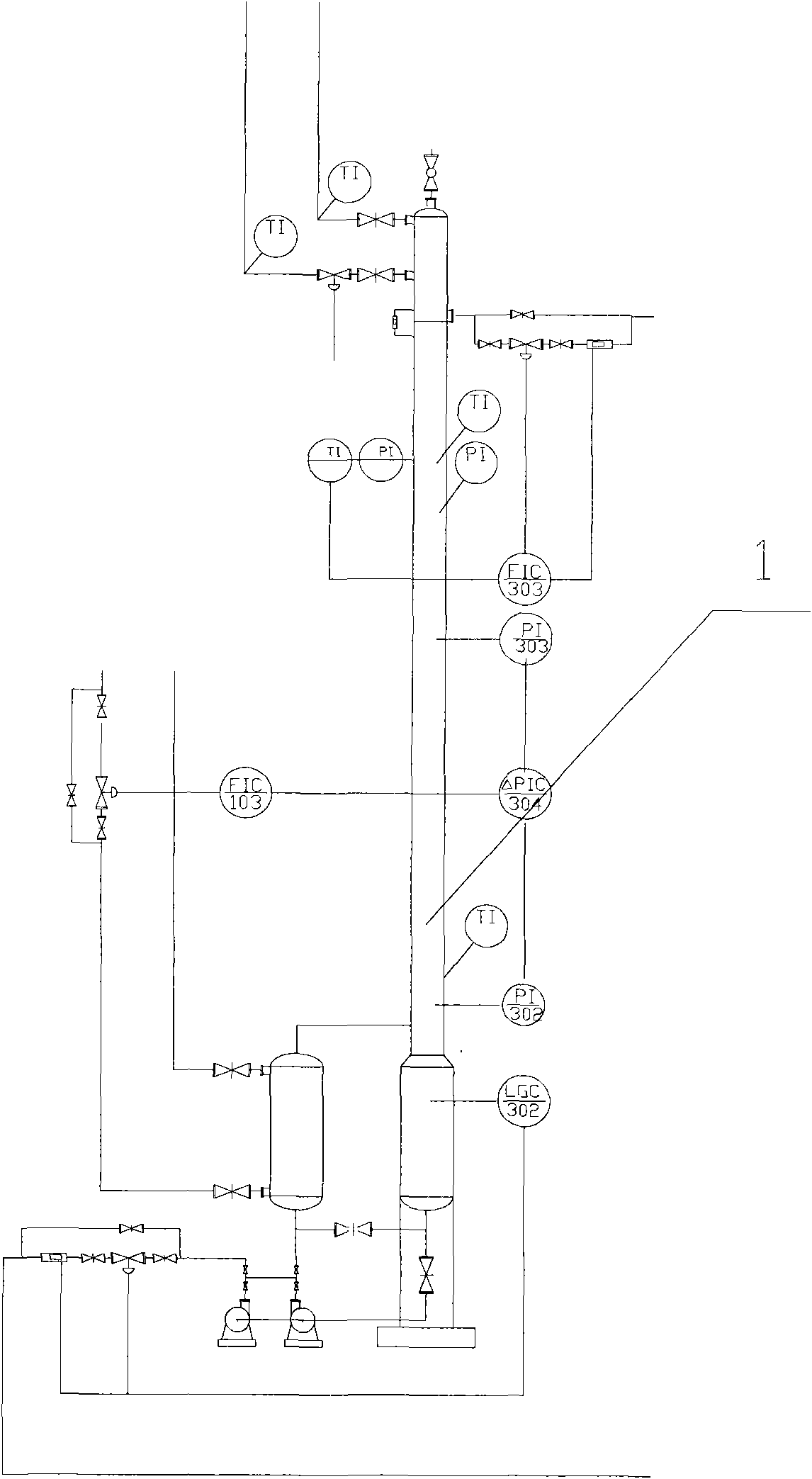
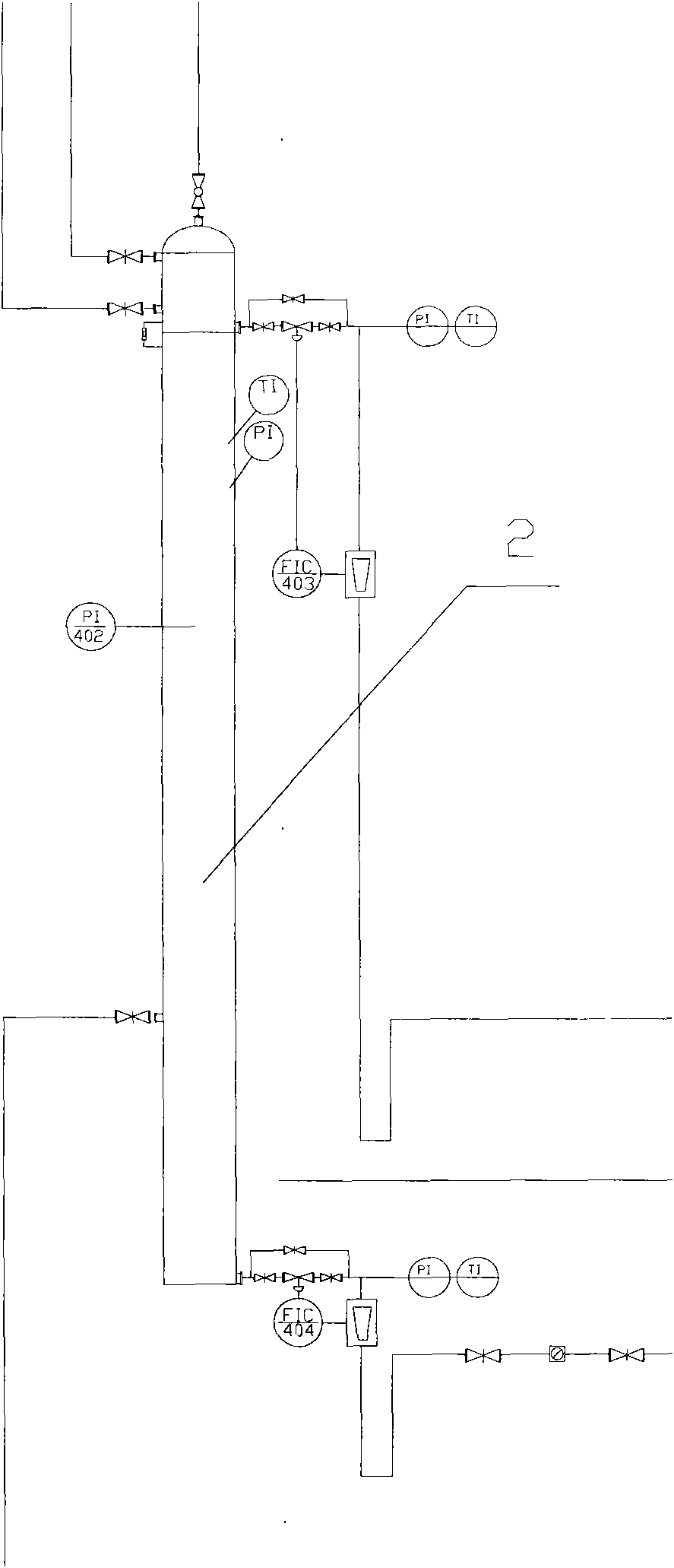
The invention discloses a method for extracting naphthalene, 1-methylnaphthalene and 2-methylnaphthalene from ethylene tar. The method comprises the following steps of: adding ethylene cracking tar with the total content of naphthalene and methylnaphthalene of not less than 30 percent from the middle of a first rectifying tower (5) under certain conditions; recovering materials of which light components are removed by the first rectifying tower at the bottom of the tower; adding from the middle of a second rectifying tower (10), and obtaining the naphthalene with the purity of not less than 95 percent on the top of the tower; adding the materials of which the naphthalene is removed at the bottom of the second rectifying tower from the middle of a third rectifying tower (15), and removing tetramethyl benzene and other components of which the boiling point is lower than that of the 2-methylnaphthalene at the top of the tower; recovering the materials of which the light fraction is removed by the third rectifying tower at the bottom of the tower, adding from the middle of a fourth rectifying tower (20), and obtaining the 2-methylnaphthalene with the purity of not less than 98 percenton the top of the tower; and recovering the materials of which the 2-methylnaphthalene is removed by the fourth rectifying tower at the bottom of the tower, adding from the middle of a fifth rectifying tower (25), and obtaining the 1-methylnaphthalene with the purity of not less than 98 percent. By the method, wastewater and waste residue are not generated and extraction efficiency is high.
Owner:广东新华粤石化集团股份公司 +2
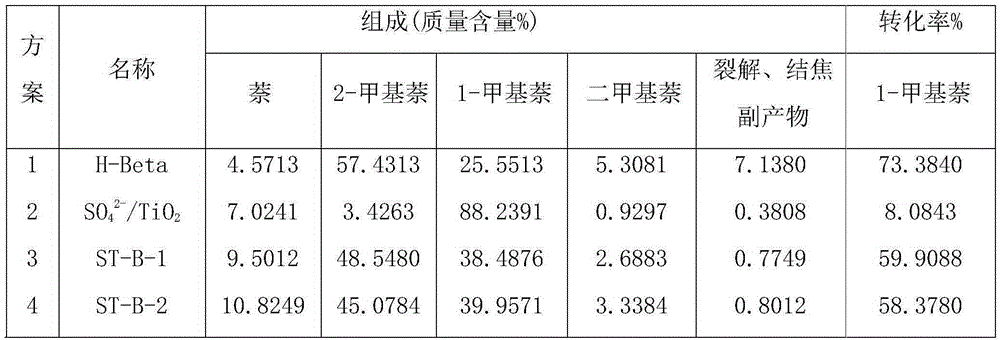
Preparation method of modified Beta molecular sieve catalyst and application thereof
InactiveCN105396612ALarge specific surface areaEasy to makeHydrocarbon by isomerisationMolecular sieve catalystsMolecular sieveIsomerization
The invention discloses a preparation method of a titanous sulfate modified Beta molecular sieve catalyst and application thereof. The method comprises the following steps: (1) putting a roasted and activated Beta molecular sieve into a hydrothermal kettle; (2) preparing a titanous sulfate solution with the mole concentration of 0.1-0.3mol / l; (3) soaking the Beta molecular sieve completely in the titanous sulfate solution at constant temperature and constant pressure, carrying out vacuum drying, and roasting so as to prepare the modified Beta molecular sieve catalyst. The catalyst disclosed by the invention is applied to isomerization and disproportionation of 1-methylnaphthalene; the conversion rate of 1-methylnaphthalene is about 60%, and the selectivity of active products (2-methylnaphthalene, naphthalene and dimethylnaphthalene) is not less than 98%; the modified Beta molecular sieve catalyst can be synthesized by a one-step process; the technology is simple, and the preparation method is suitable for large-scale industrial production; the prepared catalyst can be used for multiple times repeatedly, is high in catalytic activity and selectivity, is long in service life, and is low in cost.
Owner:NANJING NORMAL UNIVERSITY
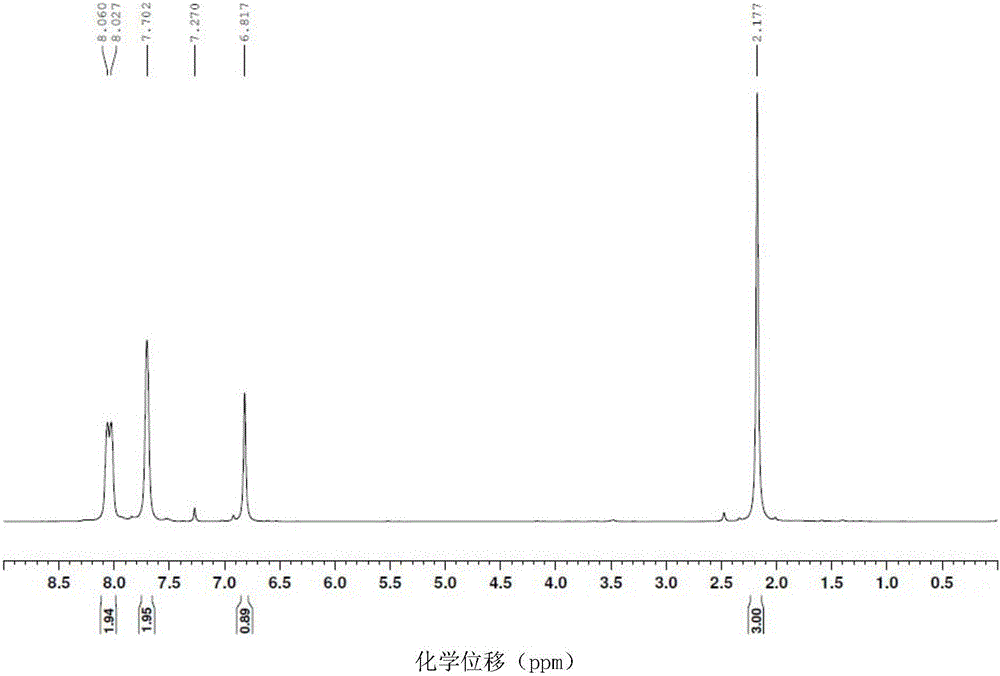
Novel synthetic method of 1,2-dihydro cyclobutene [alpha] naphthalene
ActiveCN103626621ALow priceLow costHydrocarbon from halogen organic compoundsAcetic acidDistillation
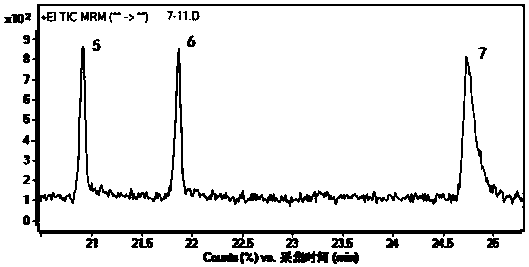
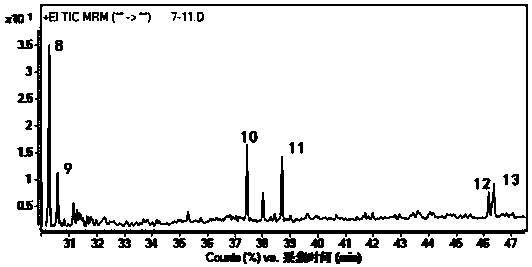
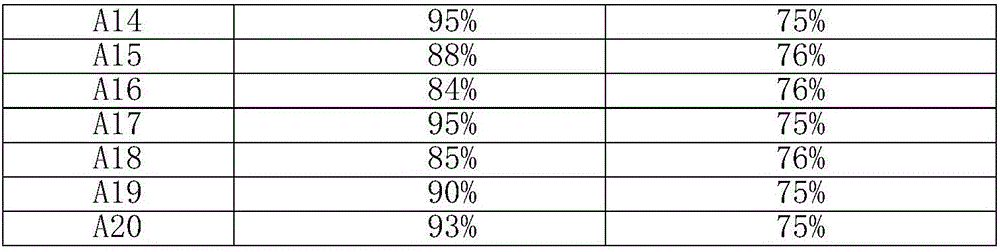
The invention discloses a novel synthetic method of 1,2-dihydro cyclobutene [alpha] naphthalene, which comprises the following steps that (1) 2-methylnaphthalene, paraformaldehyde, glacial acetic acid and hydrochloric acid are directly fed; heating and reaction are performed at 60-70 DEG C; a 1-chloromethane-2-methylnaphthalene crude product is prepared; (2) the obtained 1-chloromethane-2-methylnaphthalene crude product is subjected to reduced pressure distillation; unconverted 2-methylnaphthalene is removed; high-purity 1-chloromethane-2-methylnaphthalene is obtained; (3) high-purity 1-chloromethane-2-methylnaphthalene is added to a pyrolyzer for pyrolysis; a 1,2-dihydro cyclobutene [alpha] naphthalene crude product is obtained; and (4) the 1,2-dihydro cyclobutene [alpha] naphthalene crude product is dried, distilled, subjected to column chromatography and condensed; and a target product is obtained. Compared with the prior art, the method takes 2-methylnaphthalene as a basic raw material to prepare 1,2-dihydro cyclobutene [alpha] naphthalene by chloromethylation and pyrolysis cyclization, and is mild in synthetic condition, high in yield, very high in production value and good in industrial prospect.
Owner:绵阳高新区达高特科技有限公司
Polyacid based metal organic framework crystal material, preparation method and application thereof in catalytic synthesis of p-benzoquinone compounds
ActiveCN110358102AEfficient synthesisAchieve conversionQuinone preparation by oxidationOrganic-compounds/hydrides/coordination-complexes catalystsBenzoquinone2-methylnaphthalene
Belonging to the technical field of catalytic chemistry, the invention in particular relates to a polyacid based metal organic framework crystal material, a preparation method and application thereofin catalytic synthesis of p-benzoquinone compounds. The material can be used as a heterogeneous catalyst, and has excellent catalytic performance in the reaction of catalyzing oxidation of alkylphenol, alkoxybenzene and 2-methylnaphthalene to synthesize corresponding p-benzoquinone, especially in the reaction of catalyzing the oxidation of 2, 3, 6-trimethylphenol to synthesize 2, 3, 5-trimethylbenzoquinone, the substrate almost can be completely converted in 10-20min, the yield of benzoquinone is as high as 96%-99%, and the conversion frequency is also as high as 300-600h<-1>. In addition, the catalyst can be reused without change of the structure and catalytic activity. The material has the characteristics of simple preparation process and high product purity, and has potential application prospects in the catalysis field.
Owner:DALIAN UNIV OF TECH
Method for synthetizing 2,6-dimethylnaphthalene with methanol, C10 arene and 2-methylnaphthalene through alkylation
InactiveCN102001907AReduce the introductionRich sourcesMolecular sieve catalystsHydrocarbon by hydrocarbon and non-hydrocarbon condensationMolecular sieveAlkyl transfer
The invention relates to a method for synthetizing 2,6-dimethylnaphthalene with methanol, C10 arene and 2-methylnaphthalene through alkylation. The method uses the mixture of 2-methylnaphthalene, methanol and C10 arene which is C10 aromatic mixture mainly containing tetramethylbenzene and is a byproduct obtained from the aromatic device, as raw material to prepare 2,6-dimethylnaphthalene through alkylation in the existence of a molecular sieve catalyst. In particular, methanol is used as the raw material a, C10 arene is used as the raw material b and 2-methylnaphthalene is used as the raw material c; the raw materials are used to prepare the raw material mixture, wherein the ratio of a to c is 1-3:1(mol / mol) and the ratio of b to c is 1-6:1(mol / mol); and 2,6-dimethylnaphthalene is synthetized through alkylation in the existence of the molecular sieve catalyst under the conditions that the reaction temperature is 380-500 DEG C, the reaction pressure is 0-6.0MPa and the reaction air speed is 0.1-2h<-1>. By adopting the method, the C10 arene resource which is the byproduct with low value can be utilized, methanol with low price and rich source is introduced in the reactant and the reaction activity, selectivity and reaction stability can be effectively increased.
Owner:TONGJI UNIV
Separation purification process for 2-methylnaphthalene
InactiveCN101293808AMeet production needsReduce energy consumptionCrystallisation purification/separationBeta-methylnaphthaleneEnergy consumption
The invention relates to a method for separating and purifying 2-methylnaphthalene (Beta-methylnaphthalene). The method comprises the main steps: 70 wt% of 2-methylnaphthalene fraction is used as original material, and is subjected to fused crystallization at 34 DEG C to -15 DEG C for at least four times to obtain a target product. The method has the advantages of easily-accessible material, simple operation, low energy consumption, environment friendliness, etc.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Catalyst for compounding 2, 6-dimethylnaphthalene and preparing method thereof
ActiveCN102513146AMolecular sieve catalystsHydrocarbon by hydrocarbon and non-hydrocarbon condensationMolecular sieveAmmonium bromide
The invention discloses a catalyst for compounding 2, 6-dimethylnaphthalene and a preparing method thereof. The weight ratio of main components of the catalyst is: Na 2O 0.25-0.5 parts, SiO 2 1.0 parts, tetrapropyl ammonium bromide (TPABr) 0.05-0.2 parts, Al2O3 0.01-0.03 parts, Fe2O3 0.01-0.03 parts, H2PO4- 0.03-0.06 parts and carbon aerogel 22-45 parts. The catalyst for compounding the 2, 6-dimethylnaphthalene and the preparing method of the catalyst are used in an alkylation reaction of 2-methylnaphthalene and methanol, the selectivity and yield of the 2, 6-dimethylnaphthalene respectively reach 50% and 17% and are obviously better than those of a common molecular sieve catalyst.
Owner:KAILUAN ENERGY CHEM
Method for preparing vitamin K3
InactiveCN105037125AAvoid sublimationInhibitory reactivityQuinone preparation by oxidationQuinone separation/purificationVitamin K3Sodium bisulfate
The invention provides a method for preparing vitamin K3, and relates to vitamin K3. The method for preparing vitamin K3 with the chemical name of 2-methyl-1,4-naphthoquinone includes the following steps: dissolving 2-methylnaphthalene into a hydrocarbon solvent; adding sodium dodecyl sulfate and chromium ion oxidation liquid for an oxidation reaction; after completion of the reaction, purifying the reaction solution to obtain vitamin K3 with the chemical name of 2-methyl-1,4-naphthoquinone. The method for preparing vitamin K3 with the chemical name of 2-methyl-1,4-naphthoquinone sodium bisulfate includes the following steps: dissolving 2-methyl-1,4-naphthoquinone into a mixed solvent of water and ethyl alcohol; then adding sodium bisulfite for a sulfonation reaction; after completion of the reaction, purifying the reaction solution to obtain vitamin K3 with the chemical name of 2-methyl-1,4-naphthoquinone sodium bisulfate. According to the invention, the method is high in reaction selectivity, mild in reaction conditions, short in operation steps, green and environment-friendly, and stable in process; the product purity is 97% or above, and the total yield is 79% or above.
Owner:COMPREHENSIVE TECH SERVICE CENT OF QUANZHOU ENTRY EXIT INSPECTION & QUARANTINE BUREAU OF P R C
Rhenium catalyst and method for catalyzed synthesis of 2-methyl-1,4-naphthoquinone by rhenium catalyst
ActiveCN106622325AEasy to prepareImprove stabilityQuinone preparation by oxidationChemical recyclingActivated carbonOrganic solvent
The invention relates to a rhenium catalyst and a method for catalyzed synthesis of 2-methyl-1,4-naphthoquinone by the rhenium catalyst. The rhenium catalyst is characterized in that a rhenium compound is supported on activated carbon and is coated with a carbon-nitride material; then the rhenium compound is calcined in the atmosphere of inert gas to obtain a coated supported rhenium catalyst, wherein the supporting mass of rhenium accounts for 1 to 3 percent of total mass of the catalyst. The method for the catalyzed synthesis of the 2-methyl-1,4-naphthoquinone by the rhenium catalyst comprises the following specific steps: adding 2-methylnaphthalene, an oxidizing agent and a catalyst into an organic solvent; controlling the reaction temperature and the time to obtain a product, namely the 2-methyl-1,4-naphthoquinone. The catalyst is simple in preparation method, good in stability and suitable for industrial production; in addition, the rhenium catalyst shows good activity and selectivity for the reaction and is easy to recycle and reutilize.
Owner:NANJING UNIV OF TECH
Direct distillation technology for 2-methylnaphthalene
ActiveCN102078699ASimple processHigh yieldDistillation purification/separationVacuum distillation separationDistillationTower
The invention discloses a direct distillation technology for 2-methylnaphthalene, which comprises the following steps: A. removing naphthalene fractions from the mixed methylnaphthalene at the top of the primary distillation tower (1) and then directly introducing a tower bottom liquid into a negative pressure flash evaporator (2) for separation; B. extracting the mixed methylnaphthalene from thebottom of the negative pressure flash evaporator (2) and 2-methylnaphthalene fractions from the tower top of the negative pressure flash evaporator (2); and C. extracting the 2- methylnaphthalene fractions from the tower top of the negative pressure flash evaporator (2) to enter into a negative pressure rectifying tower (3) for carrying out the negative pressure rectification, and finally extracting the mixed methylnaphthalene from the tower bottom of the negative pressure rectifying tower (3) and the 2-methylnaphthalene from the tower top of the negative pressure rectifying tower (3). The amount of the extracted 2-methylnaphthalene in the invention reaches 97%, and the annual output of a single device reaches 2400 tons. What is more, the emission amount of the three wastes is less and can be eliminated automatically, thus being a clean and zero release production technology.
Owner:BAOSHUN TECH CO LTD
Method for preparing 2-methyl-6-tert-butylnaphthalene from 2-methylnaphthalene through alkylation
ActiveCN105601459AEasy to purifyReduce lossesHydrocarbon by hydrocarbon and non-hydrocarbon condensationMolecular sieveAlkyl transfer
The invention provides a method for preparing 2-methyl-6-tert-butylnaphthalene from 2-methylnaphthalene through alkylation, belonging to the field of chemistry and chemical engineering. The method uses 2-methylnaphthalene (beta-methylnaphthalene) as a raw material and highly selectively prepares 2-methyl-6-tert-butylnaphthalene by subjecting 2-methylnaphthalene (beta-methylnaphthalene) and tert-butyl alcohol or isobutene to alkylation. A siloxane-based compound modified molecular sieve catalyst is used for catalyzing a reaction of 2-methylnaphthalene with tert-butyl alcohol or isobutene to prepare 2-methyl-6-tert-butylnaphthalene, and the selectivity of 2-methyl-6-tert-butylnaphthalene in products and a ratio of 2-methyl-6-tert-butylnaphthalene to 2-methyl-7-tert-butylnaphthalene can be effectively increased. The method is simple to operate and low in cost and has potential application value.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Separation and purification technology of 2-methylnaphthalene
InactiveCN106316753AThe separation and purification process is simple and convenientHigh purityDistillation purification/separationHydrocarbonsFiberCrystallization
The invention relates to a separation and purification technology of 2-methylnaphthalene. The technology comprises the following steps: adding mixed methylnaphthalene barren liquid serving as a start raw material into a reactor with an acid solution; adding an alkaline solution into the reactor, and heating to 60-70 DEG C while stirring; with total duration of at least 4h, preserving heat for 1h, and standing for layering; after that, taking the upper-layer mixed 2-methylnaphthalene and introducing into a filter with an active carbon fiber filling layer; then introducing the mixed methylnaphthalene into a negative-pressure rectifying tower for negative-pressure rectification; extracting the mixed methylnaphthalene from the bottom of the negative-pressure rectifying tower, and extracting 2-methylnaphthalene fraction from the top of the negative-pressure rectifying tower, wherein the vacuum degree of the top pressure of the negative-pressure rectifying tower is over 95%, the top temperature is 150-170 DEG C, and the reflux ratio is 10-15; and performing freezing, crystallization and suction filtration on the extracted 2-methylnaphthalene. The separation and purification technology of 2-methylnaphthalene, provided by the invention, is simple and convenient while the energy consumption is low; and the purity of the separated 2-methylnaphthalene is up to 99.8wt%, and the yield reaches 99.5%.
Owner:PENG CHEN NEW MATERIALS TECH CO LTD
Process for Industrial Production of 2-Methyl-1,4-Naphthaquinone
InactiveUS20130324747A1Low yieldReduce yieldQuinone preparation by oxidationAcetic acidNaphthoquinone
Owner:TUBITAK
Catalyst for preparation of 2,6-dimethylnaphthalene as well as preparation and application of catalyst
ActiveCN107262146AImprove accessibilityInnovativeMolecular sieve catalystsMolecular sieve catalystAlkyl transferMolecular sieve
The invention discloses a high-performance catalyst for preparing 2,6-dimethylnaphthalene through alkylation of 2-methylnaphthalene as well as a preparation method and an application of the catalyst. The catalyst is a CeAPSO-11 molecular sieve with a spongy porous structure, contains micropores and rich mesoporous structures and has large external specific surface area, and the accessibility to acid sites in macromolecular reactions is effectively improved. The catalyst has relatively high activity and 2,6-DMN selectivity as well as relatively high carbon deposition resistance when used for a reaction for preparing 2,6-dimethylnaphthalene through alkylation of 2-methylnaphthalene and methanol or dimethyl ether.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI +1
Process for extracting 1-methylnaphthalene and 2-methylnaphthalene from tar
InactiveCN100393677CSimple processEasy to automateDistillation purification/separationAlkyl transferDistillation
The method of extracting 1-methyl naphthalene and 2-methyl naphthalene from tar includes adopting the production apparatus comprising one alkylating polymerization reactor and three serially connected rectification towers, and the production process, which comprises the alkylating polymerization reaction of material with methyl naphthalene content not lower than 60 %, solid acid and / or liquid acid catalyst and alkylation agent in 70-90 deg.c and 95-115 deg.c separately; the separation of the alkylating polymerization product in the first rectification tower to eliminate light distillation fraction from the tower top; the further separation in the second rectification tower to obtain 2-methyl naphthalene of 95 % over purity from the tower top; and the further separation in the third rectification tower to obtain 1-methyl naphthalene of 95 % over purity from the tower top. The present invention makes it possible to treat both tar scrubbing oil and coal tar scrubbing oil in the same apparatus.
Owner:TIANJIN UNIV
Method used for effective separation and purification of 2-methylnaphthalene from wash oil
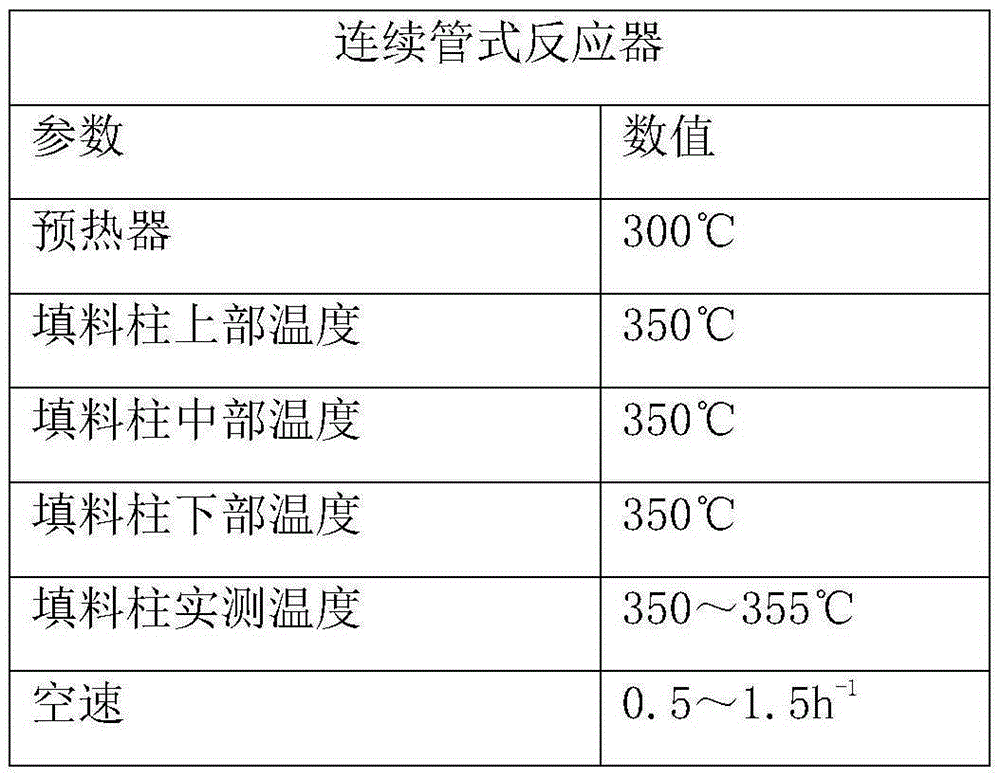
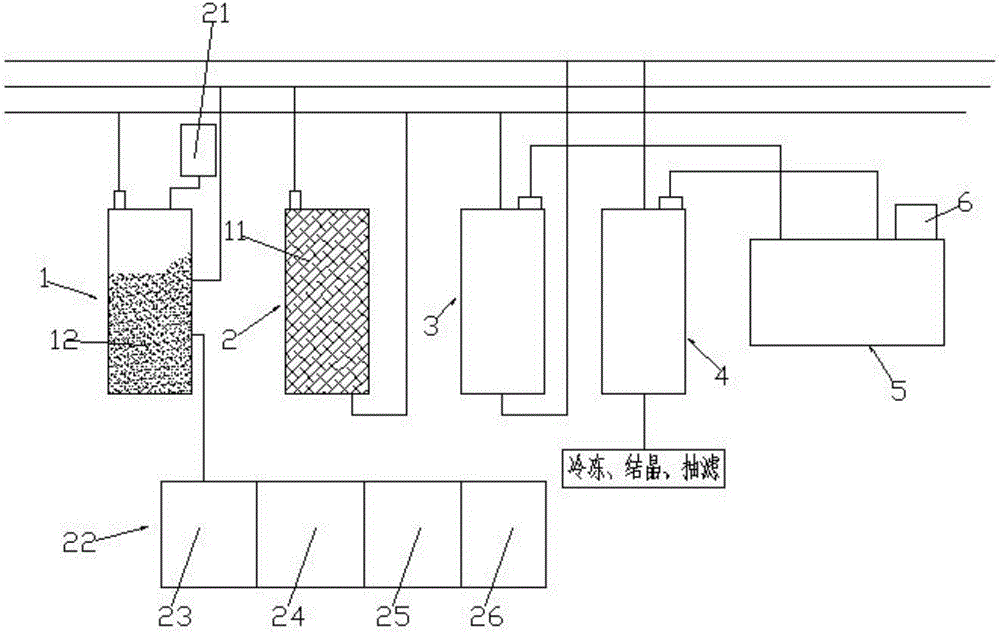
ActiveCN109293464AEffective adjustment of specific surface areaAdjust the specific surface areaDistillation purification/separationCatalyst activation/preparationIsomerizationDistillation
The invention discloses a method used for effective separation and purification of 2-methylnaphthalene from wash oil. The method comprises following steps: firstly, a catalyst is prepared; a device composed of a rectifying tower A, an isomerization reactor, and a rectifying tower B is adopted to prepare a target product; wash oil fractions are introduced into the rectifying tower A for reduced pressure distillation so as to obtain a methylnaphthalene enriched solution, and the methylnaphthalene enriched solution is introduced into the isomerization reactor, the self-made catalyst is added forreaction, a liquid obtained through filtering is introduced into the rectifying tower B for reduced pressure distillation; and at last refrigeration crystallization, and squeezing are carried out so as to obtain object products. The method is simple in operation, and low in cost; the prepared solvent is high in storage stability, boiling point, and yield.
Owner:PENG CHEN NEW MATERIALS TECH CO LTD
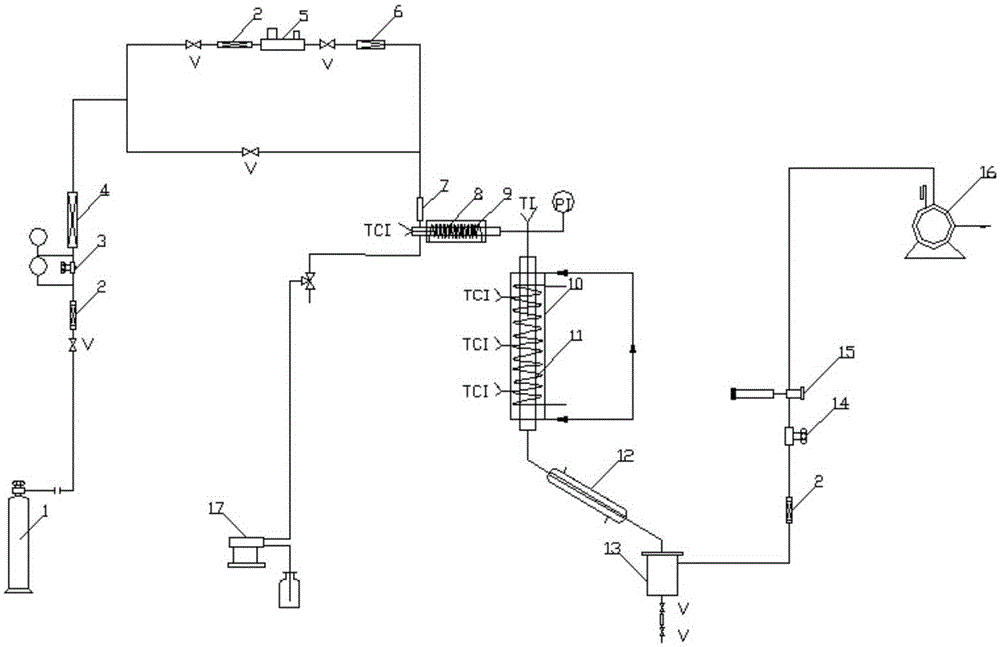
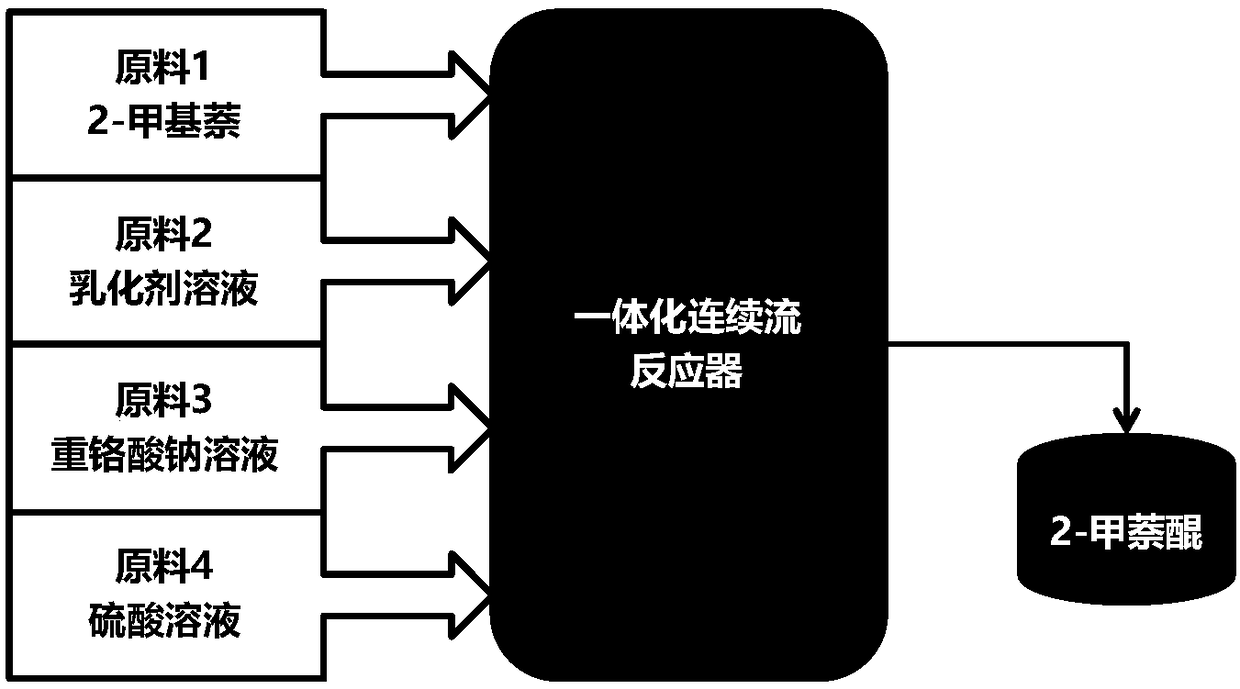
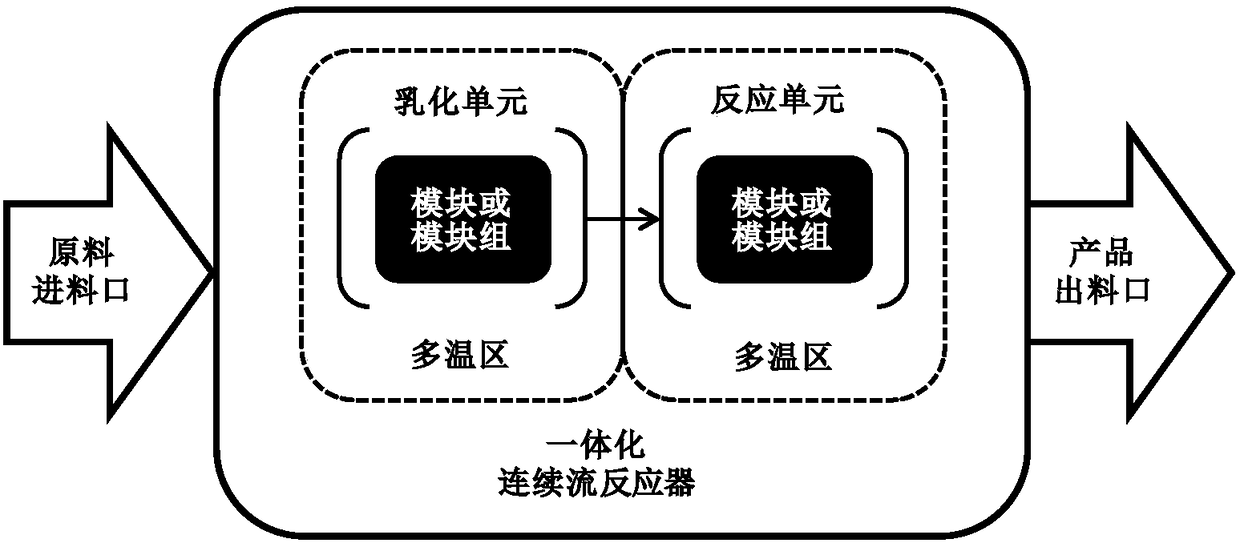
Full continuous flow synthesis process for 2-methyl-1,4-naphthoquinone
ActiveCN108299177ATotal reaction time shortenedIncrease productivityQuinone preparation by oxidationEmulsionNaphthoquinone
The invention relates to a full continuous flow synthesis process for 2-methyl-1,4-naphthoquinone and an integrated continuous flow reactor for implementing the process. According to the synthesis process, 2-methylnaphthalene, an emulsifying agent solution, a sodium dichromate solution and a sulphuric acid solution are taken as raw materials; the synthesis process is performed in the integrated continuous flow reactor; the 2-methylnaphthalene, the emulsifying agent solution, the sodium dichromate solution and the sulphuric acid solution are added into a feeding port of the integrated continuous flow reactor uninterruptedly; continuously performing transient emulsion process and oxidation process, then the 2-methyl-1,4-naphthoquinone is uninterruptedly obtained at a discharging port of theintegrated continuous flow reactor; the reaction time is less than or equal to 700 seconds. The process is a continuous synthesis process for the 2-methyl-1,4-naphthoquinone, which is quick, safe, high in efficiency and high in universality, and facilitates large-scale production.
Owner:SHANGHAI HYBRID CHEM TECH
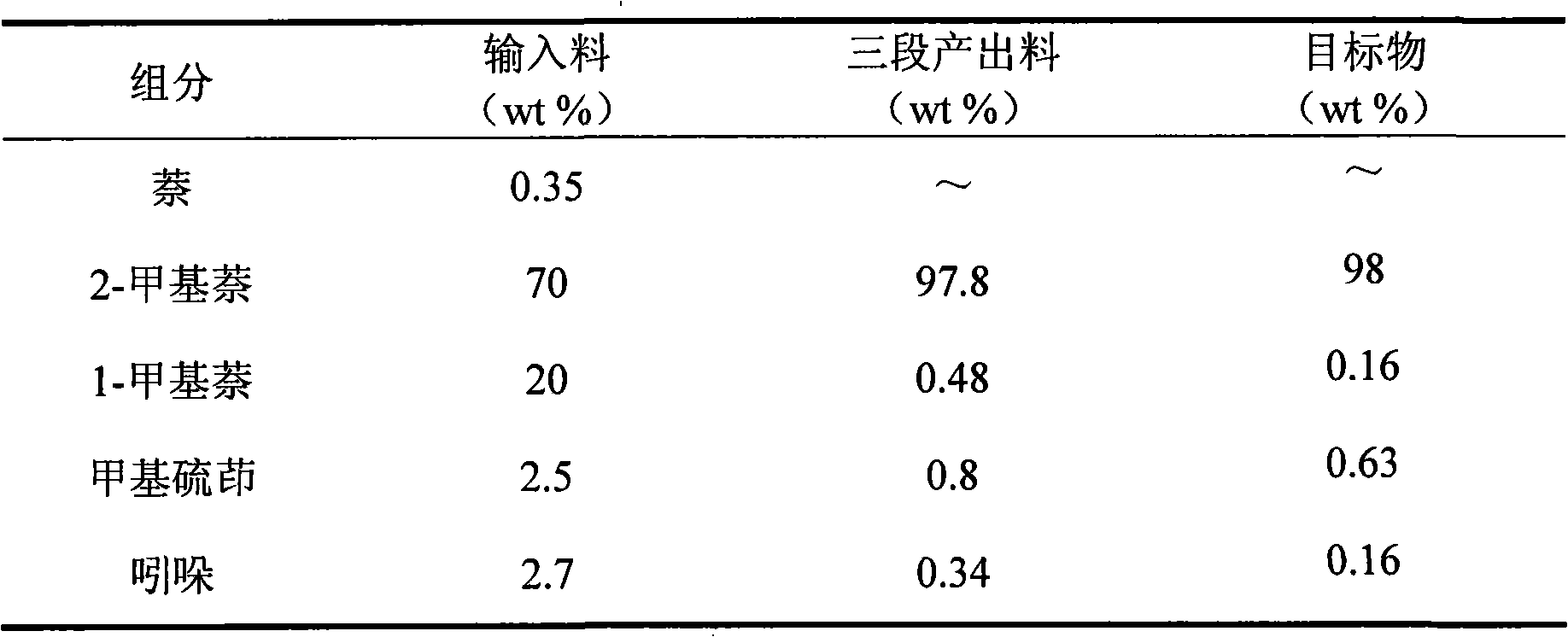
Method for reducing content of impurities in 2-methylnaphthalene
ActiveCN101575262AQuality improvementHigh recovery rateCrystallisation purification/separationMetallurgyCoal tar
The invention discloses a method for reducing the content of impurities in 2-methylnaphthalene. 2-methylnaphthalene which is extracted from coal tar and has the content not lower than 96 percent is used as the raw material. A melt crystallization method is adopted, and the method comprises the following steps: (1) adding the 2-methylnaphthalene raw material in a crystallizer, and gradually raising the temperature of the 2-methylnaphthalene raw material by heating and raising the temperature of the crystallizer to 60-70 DEG C so that the 2-methylnaphthalene raw material in the crystallizer is thoroughly melted;(2) slowly lowering the temperature of the 2-methylnaphthalene raw material after the 2-methylnaphthalene raw material in the crystallizer is thoroughly melted, and cooling the crystallizer to 30-31 DEG C, wherein the whole cooling crystallization process lasts for 3-4.5h; and (3) discharging substances which are not crystallized in the crystallizer, wherein the remaining crystal is the purified 2-methylnaphthalene. The method can effectively reduce the content of such impurities as 1-methylnaphthalene, indole, 2-methyldibenzothiophene, 5-methyldibenzothiophene, and the like in the 2-methylnaphthalene, achieves higher recovery rate and is clean and environment-friendly.
Owner:BAOWU CHARCOAL MATERIAL TECH CO LTD
Method for synthesizing 2,6-dimethylnaphthalene with 2-methylnaphthalene and C10 aromatics by transferring alkyl group
InactiveCN101973841AHigh yieldDelay inactivationHydrocarbonsHydrocarbon preparationMolecular sievePetrifaction
The invention relates to a method for synthesizing 2,6-dimethylnaphthalene with 2-methylnaphthalene and C10 aromatics by transferring an alkyl group, in particular to a method for synthesizing the 2,6-dimethylnaphthalene by transferring the alkyl group through a molecular sieve based catalyst by using the 2-methylnaphthalene and the C10 aromatics (which is an aromatics mixture using tetramethylbenzene as a main component, wherein the carbon atom number of the mixture is 10, and the mixture is a by-product of a petrifaction aromatics device) as raw materials. The method comprises the following steps of: blending the C10 aromatics a and the 2-methylnaphthalene b which are used as the raw materials into a mixture raw material by a weight ratio of a:b=1-5:1; keeping the reaction airspeed to be 0.1-3 h<-1> at a reaction temperature of 360-540 DEG C and a reaction pressure of 0-7.0 MPa; and realizing an alkyl group transfer reaction through the molecular sieve based catalyst to synthesize the 2,6-dimethylnaphthalene. In the invention, the catalyst has low inactivation speed and longer service life, and meanwhile, the reaction selectivity and the yield of the 2,6-dimethylnaphthalene can be effectively improved; in addition, the mixture of the C10 aromatics is used as the raw material, and thus the production process of the 2,6-dimethylnaphthalene is effectively simplified; and the utilization of the C10 aromatics resource which is a byproduct and has low carbon value is also beneficial to the reducing of the production cost.
Owner:TONGJI UNIV
A new method for the synthesis of 1,2-dihydrocyclobuteno[α]naphthalene
ActiveCN103626621BLow priceLow costHydrocarbon from halogen organic compoundsAcetic acidDistillation
The invention discloses a novel synthetic method of 1,2-dihydro cyclobutene [alpha] naphthalene, which comprises the following steps that (1) 2-methylnaphthalene, paraformaldehyde, glacial acetic acid and hydrochloric acid are directly fed; heating and reaction are performed at 60-70 DEG C; a 1-chloromethane-2-methylnaphthalene crude product is prepared; (2) the obtained 1-chloromethane-2-methylnaphthalene crude product is subjected to reduced pressure distillation; unconverted 2-methylnaphthalene is removed; high-purity 1-chloromethane-2-methylnaphthalene is obtained; (3) high-purity 1-chloromethane-2-methylnaphthalene is added to a pyrolyzer for pyrolysis; a 1,2-dihydro cyclobutene [alpha] naphthalene crude product is obtained; and (4) the 1,2-dihydro cyclobutene [alpha] naphthalene crude product is dried, distilled, subjected to column chromatography and condensed; and a target product is obtained. Compared with the prior art, the method takes 2-methylnaphthalene as a basic raw material to prepare 1,2-dihydro cyclobutene [alpha] naphthalene by chloromethylation and pyrolysis cyclization, and is mild in synthetic condition, high in yield, very high in production value and good in industrial prospect.
Owner:绵阳高新区达高特科技有限公司
Application of methylnaphthalene in lowering viscosity of thickened oil
ActiveCN101717626BEfficient developmentGood viscosity reductionFluid dynamicsFluid removalHigh surfaceOil viscosity
Owner:盘锦河升大地石油科技有限公司
Method for simultaneous detection of 22 kinds of polycyclic aromatic hydrocarbons in cigarette smoke
ActiveCN106248835BLow requirements for matrix purityGood choiceComponent separationAcenaphthyleneTrapping
Owner:YUNNAN TOBACCO QUALITY SUPERVISION MONITORING STATION
Method for synthesizing 2-methyl-1,4-naphthaquinone by taking ionic liquid as catalyst
InactiveCN101575276BNo pollutionQuick responseQuinone preparation by oxidationOrganic-compounds/hydrides/coordination-complexes catalystsPhosphoric acidMethyl group
The invention discloses a method for synthesizing 2-methyl-1,4-naphthaquinone by taking ionic liquid as a catalyst. The method comprises the following steps of: adding 2-methylnaphthalene, acetic acid and ionic liquid acidified by phosphoric acid into a reaction container provided with a magnetic stirrer and a thermometer, heating for reaction; adding H2O2 (30%) in a dripping way when the reactant is heated to the temperature of 60-100 DEG C; reacting for 2h after finishing dripping; and directly washing and filtering the reaction solution, thus obtaining 2-methyl-1,4-naphthaquinone. The method adopts ionic liquid as the catalyst for the first time, has the advantages of high conversion rate of 2-methylnaphthalene, less using amount of the catalyst, fast reaction speed, no chromic waste liquor pollution, and the like.
Owner:EAST CHINA NORMAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


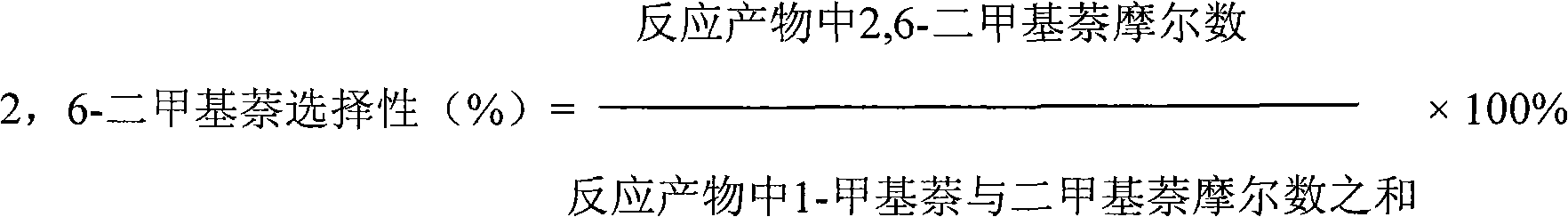







![Novel synthetic method of 1,2-dihydro cyclobutene [alpha] naphthalene Novel synthetic method of 1,2-dihydro cyclobutene [alpha] naphthalene](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5da7fe45-cff5-4f62-991e-d1095961f881/131210154327.PNG)
![Novel synthetic method of 1,2-dihydro cyclobutene [alpha] naphthalene Novel synthetic method of 1,2-dihydro cyclobutene [alpha] naphthalene](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5da7fe45-cff5-4f62-991e-d1095961f881/763077DEST_PATH_IMAGE003.PNG)


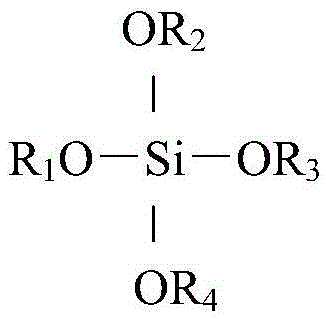
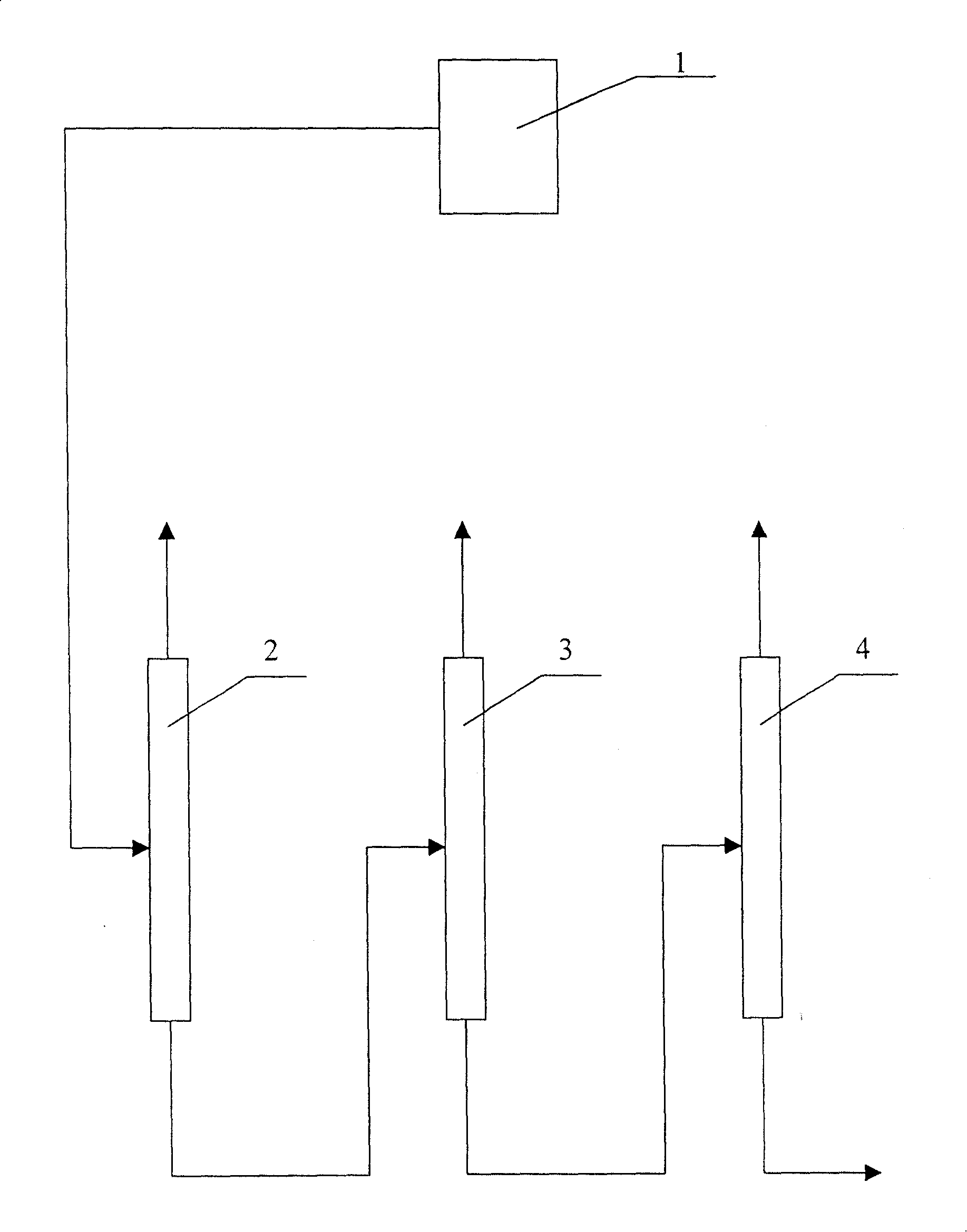


![A new method for the synthesis of 1,2-dihydrocyclobuteno[α]naphthalene A new method for the synthesis of 1,2-dihydrocyclobuteno[α]naphthalene](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0f2a058f-60a2-4a5e-9351-67b4db8927f7/131210154327.PNG)
![A new method for the synthesis of 1,2-dihydrocyclobuteno[α]naphthalene A new method for the synthesis of 1,2-dihydrocyclobuteno[α]naphthalene](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0f2a058f-60a2-4a5e-9351-67b4db8927f7/763077DEST_PATH_IMAGE003.PNG)