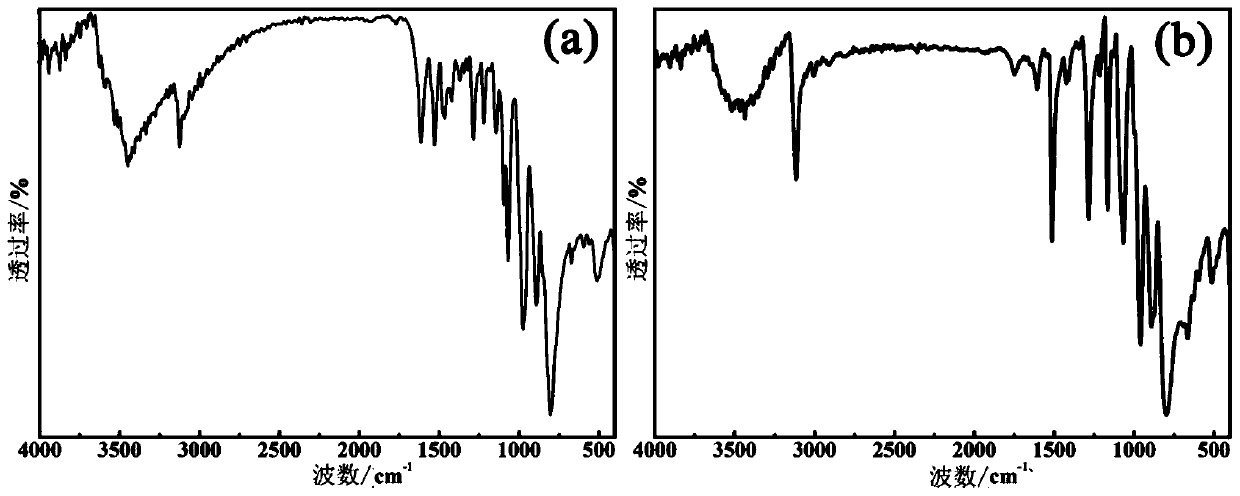
Polyacid based metal organic framework crystal material, preparation method and application thereof in catalytic synthesis of p-benzoquinone compounds
A technology of organic frameworks and crystal materials, applied in the field of catalytic chemistry, to achieve the effects of novel structure, excellent catalytic activity, and excellent catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
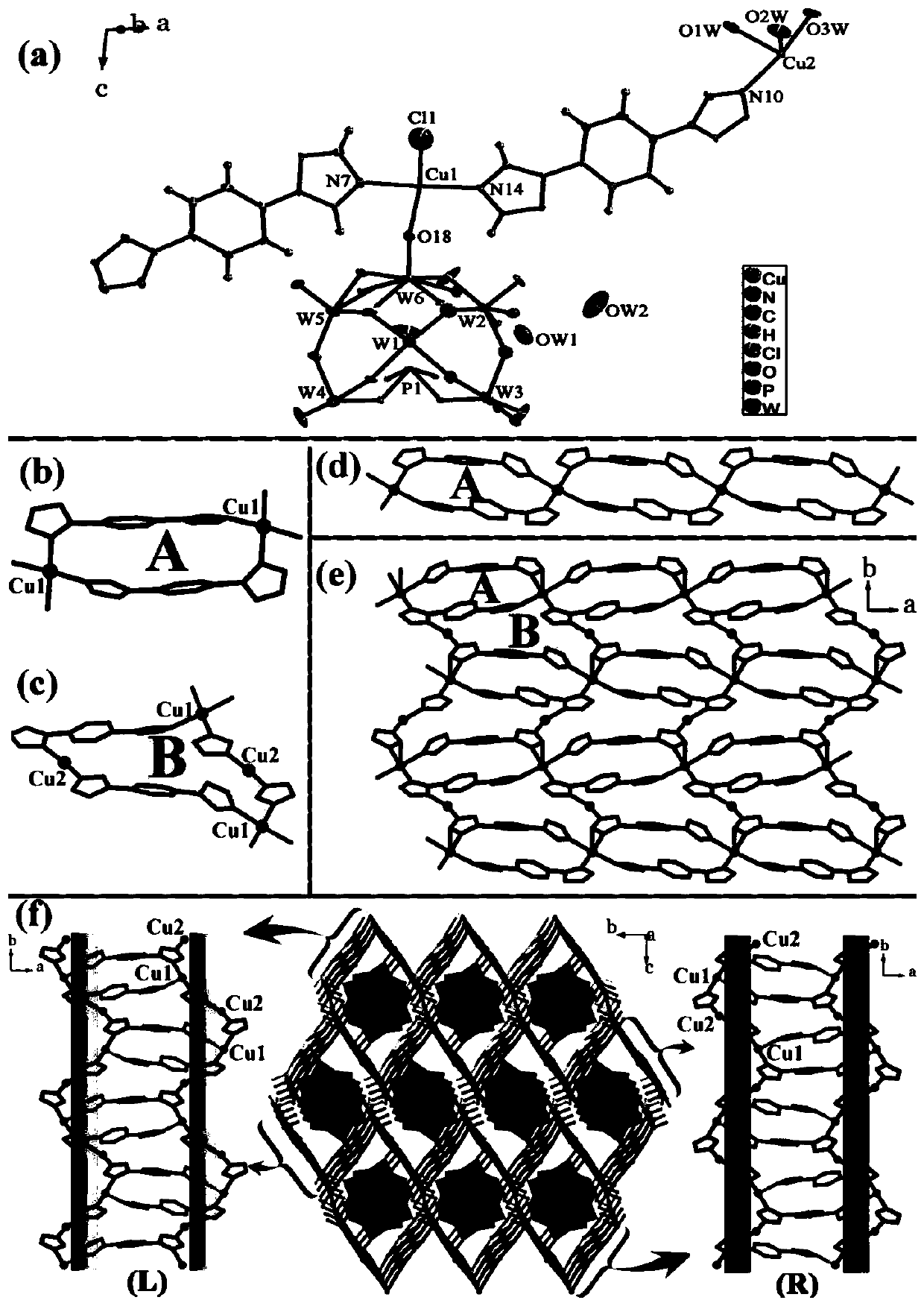
[0040] Polyacid-based metal-organic framework crystal material H[Cu II (ttb)(H 2 O) 3 ] 2 [Cu II (ttb)Cl] 2 [PW 12 o 40 ]·4H 2 The preparation method of O, concrete steps are as follows:
[0041] 1) Synthesize precursor K by conventional methods 4 [PW 11 V V o 40 ]·xH 2 O (Domaille, P.J., Inorg. Synth., John Wiley & Sons.: 1990; Vol.27, 96-104);
[0042] 2)K 4 [PW 11 V V o 40 ]·xH 2 O (0.3g, ~0.1mmol) was dissolved in 10mL water, then CuCl 2 2H 2 O (0.0682g, 0.40mmol) and Httb (0.0491g, 0.23mmol), continued to stir for 0.5h, adjusted the pH to 2.0-2.8 with dilute hydrochloric acid, and continued to stir for 0.5h, then transferred to a polytetrafluoroethylene reactor. After heating at 180°C for about 6 days in an oven, slowly cool to room temperature to obtain a green bulk crystalline material with a yield of about 52% (based on K 4 [PW 11 V V o 40 ]·xH 2 O).
[0043] The 0.0682g CuCl 2 2H 2 O can be prepared from 0.0767g CuCl 2 2H 2 O or 0.0998~0....
Embodiment 2
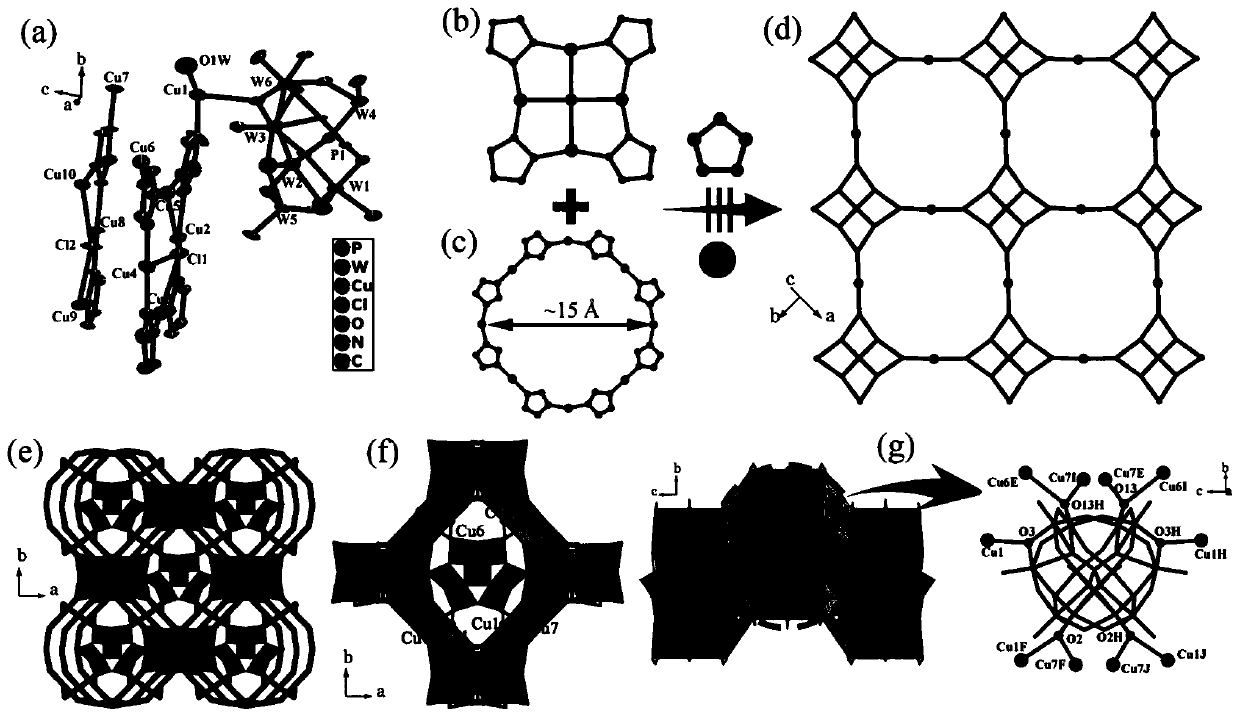
[0045] Polyacid-based metal-organic framework crystalline materials [ClCu 6 I (trz) 4 ][ClCu 5 I (trz) 4 ] 2 [Cu II (H 2 O)][PW 12 o 40 ] The preparation method, concrete steps are as follows:
[0046] 1) Synthesize precursor K by conventional methods 4 [PW 11 V V o 40 ]·xH 2 O (Domaille, P.J., Inorg. Synth., John Wiley & Sons.: 1990; Vol.27, 96-104);
[0047] 2)K 4 [PW 11 V V o 40 ]·xH 2 O (0.35g, ~0.12mmol) was dissolved in 10mL water, then Cu(OAc) was added 2 ·H 2 O (0.1497g, 0.75mmol) and trz (0.0794g, 1.15mmol), continue to stir for 0.5h, adjust the pH to 1.4-1.6 with dilute hydrochloric acid, after continuing to stir for 0.5h, transfer to a polytetrafluoroethylene reactor After heating at 180° C. for about 5 days, slowly cool to room temperature to obtain a brown-black bulk crystalline material with a yield of about 61% (based on K4 [PW 11 V V o 40 ]·xH 2 O).
[0048] The 0.1497g Cu(OAc) 2 ·H 2 O can be obtained from 0.1398g Cu(OAc) 2 ·H 2 ...
Embodiment 3
[0050] Polyacid-based metal-organic framework crystalline materials [ClCu 6 I (trz) 4 ][ClCu 5 I (trz) 4 ] 2 [Cu II (H 2 O)][PW 12 o 40 ] The preparation method, concrete steps are as follows:
[0051] 1) Synthesis of precursor H by conventional methods 3 PW 12 o 42 ·xH 2 O (John, C.B.J., Inorg. Synth., John Wiley & Sons.: 1939; Vol.1, 132-133);
[0052] 2)H 3 PW 12 o 42 ·xH 2 O (0.32g, ~0.12mmol) was dissolved in 10mL water, then Cu(OAc) was added 2 ·H 2 O (0.1497g, 0.75mmol) and trz (0.0794g, 1.15mmol), continue to stir for 0.5h, adjust the pH to 1.4-1.6 with dilute hydrochloric acid, after continuing to stir for 0.5h, transfer to a polytetrafluoroethylene reactor After heating at 180°C for about 5 days, slowly cool to room temperature to obtain a brown-black bulk crystalline material with a yield of about 31% (based on K 4 [PW 12 o 40 ]·xH 2 O).
[0053] The 0.1497g Cu(OAc) 2 ·H 2 O can be obtained from 0.1398g Cu(OAc) 2 ·H 2 O or 0.1194~0.1279g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com